Abstract
BACKGROUND:
Vitiligo, although asymptomatic, highly compromises patients' quality of life (QoL). Therefore, an adequate evaluation of QoL is essential.
OBJECTIVES:
Translation, cultural adaptation and validation of VitiQol (Vitiligo-specific health-related quality of life instrument) into Brazilian Portuguese.
METHODS:
The study was conducted in two stages; the first stage was the translation and cultural/linguistic adaptation of the instrument; the second stage was the instrument's validation.
RESULTS:
The translated VitiQol showed high internal consistency (Cronbach alpha = 0.944) and high test-retest reliability and intraclass correlation coefficient=0.95 (CI 95% 0.86 - 0.98), p<0.001. There was no statistically significant difference between the means of the first completion of the VitiQoL questionnaire and the retest, p = 0.661. There was a significant correlation between VitiQoL and DLQI (r = 0.776, p <0.001) and also between VitiQoL-PB and subjects' assessment of the severity of their disease (r = 0.702, p <0.001).
CONCLUSIONS:
The impact of vitiligo on the QoL of Brazilian patients can be assessed by a specific questionnaire.
Keywords: Indicators of quality of life, Quality of life, Sickness impact profile, Vitiligo
INTRODUCTION
Vitiligo is an acquired pigmentary disorder, characterized by achromatic spots due to a loss of melanocytes. Its prevalence ranges between 0.5% and 1% worldwide. Gujarat, India, is considered to be the place with the highest prevalence of vitiligo: approximately 8.8%.1 The average age of onset is 22 years in the United States and India, 24 years in Brazil and 25 years in England, and half of the patients develop the disease before 20 years of age.1,2,3
Adults and children of both genders are equally affected, but some studies indicate a slight preponderance of cases among females, possibly because women seek medical care more often than men due to the secondary psychosocial consequences.3,4,5,6 There is no predilection for skin type or race.1
There are genetic factors involved in its etiopathogenesis, and the relative risk of vitiligo in first-degree relatives is estimated as seven to 10 times greater than in the general population.3,7 Other aspects involved include autoimmunity, intrinsic defects of melanocytes and T-cells, and oxidative stress.8-11
Many dermatological diseases are characterized by compromising patients' physical and relational well-being. For this reason, the assessment of quality of life (QoL) is of fundamental importance.12,13 Vitiligo, although asymptomatic, highly compromises QoL. It affects patients' lives in many ways, being sometimes psychologically devastating. The condition compromises self-esteem, body image and social life. Individuals with vitiligo suffer social discrimination and stigmatization, which result in significant changes in their lifestyles: from the choice of clothing, use of sunscreen and cosmetic camouflage of the lesions to the avoidance of social events or outdoor activities.14-17
Recently, a specific questionnaire for vitiligo has been developed and validated in the English language: the Vitiligo-specific health-related quality of life instrument (VitiQoL).17 To this date, there were no instruments in Brazilian Portuguese to assess the QoL in vitiligo.
The VitiQoL is a questionnaire of 15 items, with item scores from 0 (never) to 6 (all the time). It yields a total score from 0 to 90. Moreover, it presents a personal assessment of the severity of vitiligo, using a scale ranging from 0 (no skin involvement) to 6 (worst case), which corresponds to the 16th question of the VitiQoL questionnaire. The questionnaire shows to be a promising clinical and epidemiological study instrument, and a powerful marker of outcome.17
The aim of this study was to perform the translation, cross-cultural adaptation and validation of the VitiQoL into Brazilian Portuguese (VitiQoL-PB).
METHODS
The author of the VitiQoL questionnaire authorized its translation, cross-cultural adaptation and validation. The study was conducted in two stages: the first stage was the translation and cultural/linguistic adaptation of the instrument; the second stage was the instrument's validation. This is an observational study. Subjects were divided into two groups: cross-sectional and follow-up (retest). Subjects were selected from a Dermatological Outpatient Clinic from Hospital de Clínicas de Porto Alegre. In this study, we used convenience sampling of consecutive cases. Inclusion criteria were: older than 18 years of age; able to read and understand Brazilian Portuguese; diagnosis of vitiligo confirmed by a dermatologist. Exclusion criteria were: presence of chronic, non-dermatological disease or other concomitant dermatological disease.
In the first stage (translation and cross-cultural adaptation), two independent translators performed the literal translation of the VitiQoL instrument from English into Portuguese. This version was later reviewed by a bilingual group, composed of health professionals. Next, 10 subjects with vitiligo were asked to answer the questionnaire and make suggestions for its clarity and understanding. After repeat review by the same bilingual group, this new version of the questionnaire was translated into English and sent to the author of the original questionnaire for approval. This stage took place from January to June 2013.
Based on the original study and the WHO recommendations for the development of quality of life questionnaires, a specific questionnaire on demographic data, the VitiQoL questionnaire and a generic instrument for dermatological diseases previously validated for Portuguese, the DLQI (Dermatology Life Quality Index), were administered to 74 subjects. In addition, subjects made a personal assessment of their severity of vitiligo, using a scale ranging from 0 (no skin involvement) to 6 (most severe cases), which corresponded to the 16th question of the VitiQoL-PB questionnaire.
For test-retest reliability, the sample was calculated based on the studies reliability graph, using a reliability coefficient (Cronbach's coefficient) of 0.80 and a confidence interval of 0.05. Statistical analysis was performed using SPSS (SPSS Inc. Chicago, II, version 18.0 for Windows). The reliability of the instrument was demonstrated through the analysis of internal consistency using Cronbach's coefficient of reliability. Test-retest reliability was assessed by intraclass correlation coefficient (ICC) and Student t test for paired samples.
The PB-VitiQoL and DLQI were compared using the Pearson correlation coefficient. General data were analyzed using descriptive statistics (mean and standard deviation for quantitative variables with symmetric distribution; median and interquartile range for variables with asymmetric distribution; and frequencies and percentages for categorical variables). The study project was approved by the Research Ethics Committee and all subjects signed an informed consent.
RESULTS
The questionnaire was completed by 74 subjects. For analysis of reproducibility (test-retest reliability), two to four weeks after the first interview, a subgroup of subjects, corresponding to 20% of the sample, completed the VitiQol-PB questionnaire again.
Subjects' demographic and clinical characteristics are described in table 1. The translated VitiQol showed high internal consistency (Cronbach alpha = 0.944) and high test-retest reliability, with an intraclass correlation coefficient of 0.95 (CI 95% 0.86 - 0.98), p<0.001. There was no statistically significant difference between the means of the first completion of the VitiQoL questionnaire and the retest, p = 0.661 (Graph 1)|. The 95% interval of agreement between the test and retest was -25.46 to 22.71.
TABLE 1.
Clinical and demographic data
| Mean | ||
|---|---|---|
| (standard deviation) | ||
| Age | 44.70 (17.107) | |
| Age of onset | 31 (median) | |
| Duration of the disease | 11 (median) | |
| N | (%) | |
| Gender | ||
| Male | 24 | (32.4) |
| Female | 50 | (67.6) |
| Skin type | ||
| II | 12 | (16.2) |
| III | 31 | (41.9) |
| IV | 26 | (35.1) |
| V | 5 | (6.8) |
| Educational level | ||
| Incomplete primary education | 21 | (28.4) |
| Complete primary education | 3 | (4.1) |
| Incomplete secondary education | 13 | (17.6) |
| Complete secondary education | 12 | (16.2) |
| Incomplete higher education | 12 | (16.2) |
| Complete higher education | 13 | (17.6) |
| Marital status | ||
| Married | 27 | (36.4) |
| Divorced/ Separated | 12 | (16.2) |
| Widowed | 6 | (8.3) |
| Single | 29 | (39.1) |
| Income | ||
| up to 500.00 | 3 | (4.1) |
| 500.00-1,000.00 | 20 | (27) |
| 1,000.00-3,000.00 | 33 | (44.6) |
| >3,000.00 | 16 | (21.6) |
| Was unable to report | 2 | (2.7) |
GRAPH 1.
Clinical and demographic data Bland and Altman’s graph showing agreement between test and retest scores. The solid line shows the difference between the means of the test and the retest; the dotted line shows the 95% limits of agreement (mean difference ± 1.96SD)
The single item that most contributed to the total score of VitiQoL-PB was related to the frustration about the skin condition (question 2). Other items that significantly contributed to the final scores were: feeling bothered by the appearance of the skin (question 1), worry about what other people think (question 5), fear of criticism (question 6), embarrassment or inhibition because of the skin (question 7), social or leisure activities (question 9), emotional well-being (question 10). In general, we found that all items contributed to the final score (Table 2).
TABLE 2.
Correlation between items and between the items with the final score
| Item | Correlation between items | Correlation between the items and the final score |
|---|---|---|
| 1- Bother | 0.27-0.84 | 0.83 |
| 2 - Frustration | 0.28-0.85 | 0.85 |
| 3 - Difficulty showing affection | 0.25-0.67 | 0.69 |
| 4- Daily activities | 0.29-0.75 | 0.77 |
| 5 - Worry about what other people think | 0.17-0.84 | 0.81 |
| 6 - Fear of criticism | 0.17-0.84 | 0.82 |
| 7 - Embarrassment or inhibition | 0.35-0.85 | 0.83 |
| 8 - Type of clothing | 0.25-0.68 | 0.72 |
| 9 - Social and leisure activities | 0.30-0.75 | 0.81 |
| 10- Emotional well-being | 0.21-0.75 | 0.83 |
| 11- Physical health as a whole | 0.24-0.71 | 0.70 |
| 12 - Care with personal appearance | 0.24-0.59 | 0.66 |
| 13 - Sun protection care | 0.12-0.35 | 0.41 |
| 14- Making new friends | 0.12-0.67 | 0.75 |
| 15- Disease progression | 0.24-0.59 | 0.62 |
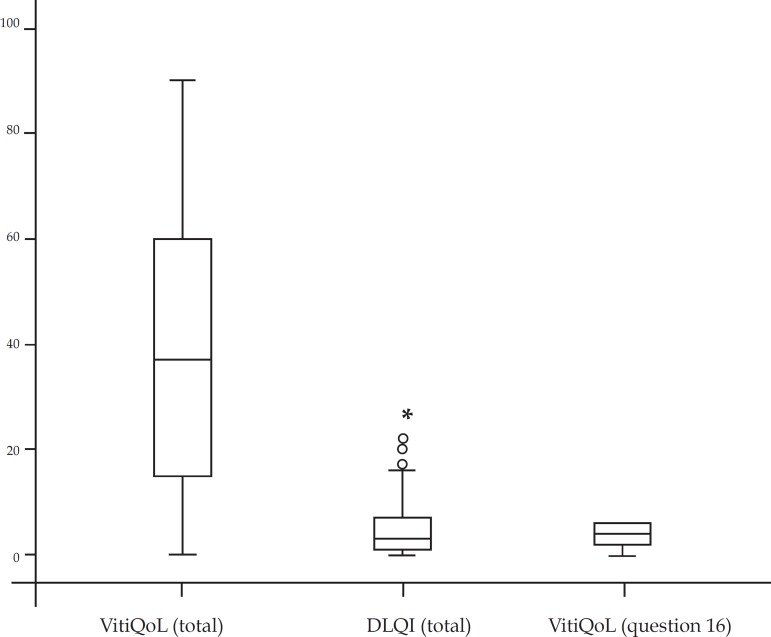
The average VitiQoL-PB score was 40.04+27.32; while the median DLQI was 3 (interquartile range P25 = 1 and P75 = 7). The average patient assessment score for severity of illness was 3.64 + 1.87 (Figure 1).
FIGURE 1.
VitiQoL PB
Reproduced with permission of the authors ALL RIGHTS RESERVED © Lilly E, Kundu RV 2012. Any unauthorized use or reproduction of this document is strictly prohibited.
There was a significant correlation between VitiQoL and DLQI (r = 0.776, p <0.001) and also between VitiQoL-PB and subjects' assessment of the severity of their disease (r = 0.702, p <0.001). We also found a good correlation between the total DLQI and subjects' assessment of the severity of their disease (r = 0.673, p <0.001) (Graph 2).
GRAPH 2.
Correlation between VitiQoL-PB, DLQI and question 16 of VitiQoL (self-reported severity of vitiligo)
DISCUSSION
The development and validation of dermatology-specific QoL questionnaires is recent.18,19 For the evaluation of a specific dermatosis, there is a tendency to combine two questionnaires: one generic questionnaire and another disease-specific dermatologic questionnaire, comparing both scores.20 Disease-specific instruments are already available for several chronic dermatoses, such as atopic dermatitis, acne and melasma.21->23
The absence of a vitiligo-specific questionnaire for the assessment of QoL led to the use of the general dermatology questionnaires, such as DLQI and Skindex-16. However, they do not seem to be sensitive enough for the assessment of an asymptomatic disease such as vitiligo.24 For conditions like acne and psoriasis, it has been shown that specific questionnaires better reflect QoL.25,26 In addition, they make it possible to correlate clinical and demographic aspects of a specific population with QoL.27,28,29
The VitiQoL-PB Cronbach's alpha coefficient was similar to the coefficient of the original questionnaire (original VitiQoL Cronbach's alpha = 0.935), which demonstrates the reliability of the instrument. Additionally, it showed a strong correlation with both the subjects' assessment of disease severity and the DLQI. In the original VitiQoL, one of the limitations highlighted by the authors was the fact that no assessment of test-retest reliability had been carried out. In our study, 16 subjects (21% of the sample) completed the VitiQoL-PB a second time, confirming the high test-retest reliability with an intraclass correlation coefficient (ICC) of 0.95 (95% CI, 0.86 to 0.98). The intraclass correlation coefficient ensures the reproducibility of the questionnaire, and ICC values ≥ 0.75 are considered excellent.
CONCLUSION
There are little data on the quality of life of patients with vitiligo in Brazil. Most studies have been conducted in children, by using the Children's Dermatology Life Quality Index.30,31 Studies conducted with adults generally use the DLQI and the SF-36 (Medical Outcomes Study 36-item Short-Form Health Survey).32,33 Moving forward, the impact of vitiligo on the quality of life of Brazilian patients can be assessed using a disease-specific questionnaire, which will contribute to a more complete and reliable evaluation of these patients.
Vitiligo is a chronic disease and, although it is asymptomatic, it highly compromises patients' quality of life (QoL). The translation, cross-cultural adaptation and validation of the VitiQoL-PB will not only assist in treatment evaluation and in the comparison of results of studies conducted in different regions of Brazil, but also allow the correlation with international data
Footnotes
Financial Support: None.
Conflict of Interest: None.
How to cite this article: Boza JC, Kundu RV, Fabbrin A, Horn R , Giongo N, Cestari TF. Translation, cross-cultural adaptation and validation of the vitiligo-specific health-related quality of life instrument (VitiQoL) into Brazilian Portuguese. An Bras Dermatol. 2015;90(3):358-62.
Study conducted at the Dermatology Service of the Hospital de Clínicas de Porto Alegre (HCPA), Postgraduate Program - Children’s and Adolescent’s Health- School of Medicine, Universidade Federal do Rio Grande do Sul (UFRGS) – Porto Alegre (RS), Brazil.
Reference
- 1.Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473–491. doi: 10.1016/j.jaad.2010.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Barona MI, Arrunátegui A, Falabella R, Alzate A. An epidemiologic case-control study in a population with vitiligo. J Am Acad Dermatol. 1995;33:621–625. doi: 10.1016/0190-9622(95)91282-7. [DOI] [PubMed] [Google Scholar]
- 3.Tarlé RG, Nascimento LM, Mira MT, Castro CC. Vitiligo--part 1. An Bras Dermatol. 2015;90:461–470. doi: 10.1590/abd1806-4841.20142573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sehgal VN, Srivastava G. Vitiligo: compendium of clinico-epidemiological features. Indian J Dermatol Venereol Leprol. 2007;73:149–156. doi: 10.4103/0378-6323.32708. [DOI] [PubMed] [Google Scholar]
- 5.Liu JB, Li M, Yang S, Gui JP, Wang HY, Du WH, et al. Clinical profiles of vitiligo in China: an analysis of 3742 patients. Clin Exp Dermatol. 2005;30:327–331. doi: 10.1111/j.1365-2230.2005.01813.x. [DOI] [PubMed] [Google Scholar]
- 6.Nunes DH, Esser LM. Vitiligo epidemiological profile and the association with thyroid disease. An Bras Dermatol. 2011;86:241–248. doi: 10.1590/s0365-05962011000200006. [DOI] [PubMed] [Google Scholar]
- 7.Nath SK, Majumder PP, Nordlund JJ. Genetic epidemiology of vitiligo: multilocus recessivity cross-validated. Am J Hum Genet. 1994;55:981–990. [PMC free article] [PubMed] [Google Scholar]
- 8.Nejad SB, Qadim HH, Nazeman L, Fadaii R, Goldust M. Frequency of autoimmune diseases in those suffering from vitiligo in comparison with normal population. Pak J Biol Sci. 2013;16:570–574. doi: 10.3923/pjbs.2013.570.574. [DOI] [PubMed] [Google Scholar]
- 9.Ingordo V1, Cazzaniga S, Raone B, Digiuseppe MD, Musumeci ML, Fai D, et al. Circulating Autoantibodies and Autoimmune Comorbidities in Vitiligo Patients: A Multicenter Italian Study. Dermatology. 2014;228:240–249. doi: 10.1159/000357807. [DOI] [PubMed] [Google Scholar]
- 10.Taieb A, Picardo M. Clinical practice: vitiligo. N Engl J Med. 2009;360:160–169. doi: 10.1056/NEJMcp0804388. [DOI] [PubMed] [Google Scholar]
- 11.Laddha NC, Dwivedi M, Mansuri MS, Singh M, Gani AR, Yeola AP, et al. Role of oxidative stress and autoimmunity in onset and progression of vitiligo. Exp Dermatol. 2014;23:352–353. doi: 10.1111/exd.12372. [DOI] [PubMed] [Google Scholar]
- 12.Wachholz PA, Masuda PY, Nascimento DC, Taira CM, Cleto NG. Quality of life profile and correlated factors in chronic leg ulcer patients in the mid-west of São Paulo State, Brazil. An Bras Dermatol. 2015;90:73–81. doi: 10.1590/abd1806-4841.20142156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamamoto Cde S, Hassun KM, Bagatin E, Tomimori J. Acne-specific quality of life questionnaire (Acne- QoL): translation, cultural adaptation and validation into Brazilian-Portuguese language. An Bras Dermatol. 2015;90:83–90. doi: 10.1590/abd1806-4841.20142172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159–164. doi: 10.1001/jamadermatol.2013.927. [DOI] [PubMed] [Google Scholar]
- 15.Teovska Mitrevska N, Eleftheriadou V, Guarneri F. Quality of life in vitiligo patients. Dermatol Ther. 2012;25:S28–S31. doi: 10.1111/dth.12007. [DOI] [PubMed] [Google Scholar]
- 16.Eleftheriadou V, Thomas KS, Whitton ME, Batchelor JM, Ravenscroft JC. Which outcomes should we measure in vitiligo? Results of a systematic review and a survey among patients and clinicians on outcomes in vitiligo trials. Br J Dermatol. 2012;167:804–814. doi: 10.1111/j.1365-2133.2012.11056.x. [DOI] [PubMed] [Google Scholar]
- 17.Lilly E, Lu PD, Borovicka JH, Victorson D, Kwasny MJ, West DP, et al. Development and validation of a vitiligo-specific quality-of-life instrument (VitiQoL) J Am Acad Dermatol. 2013;69:e11–e18. doi: 10.1016/j.jaad.2012.01.038. [DOI] [PubMed] [Google Scholar]
- 18.Both H, Essink-Bot ML, Busschbach J, Nijsten T. Critical review of generic and dermatology-specific health-related quality of life instruments. J Invest Dermatol. 2007;127:2726–2739. doi: 10.1038/sj.jid.5701142. [DOI] [PubMed] [Google Scholar]
- 19.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI)--a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–216. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 20.De Korte J, Mombers FM, Sprangers MA, Bos JD. The suitability of quality-of-life questionnaires for psoriasis research: a systematic literature review. Arch Dermatol. 2002;138:1221–1227. doi: 10.1001/archderm.138.9.1221. [DOI] [PubMed] [Google Scholar]
- 21.Whalley D, McKenna SP, Dewar AL, Erdman RA, Kohlmann T, Niero M, et al. A new instrument for assessing quality of life in atopic dermatitis: international development of the Quality of Life Index for Atopic Dermatitis (QoLIAD) Br J Dermatol. 2004;150:274–283. doi: 10.1111/j.1365-2133.2004.05783.x. [DOI] [PubMed] [Google Scholar]
- 22.Fehnel SE, McLeod LD, Brandman J, Arbit DI, McLaughlin-Miley CJ, Coombs JH, et al. Responsiveness of the Acne-Specific Quality of Life Questionnaire (AcneQoL) to treatment for acne vulgaris in placebo-controlled clinical trials. Qual Life Res. 2002;11:809–816. doi: 10.1023/a:1020880005846. [DOI] [PubMed] [Google Scholar]
- 23.Balkrishnan R, McMichael AJ, Camacho FT, Saltzberg F, Housman TS, Grummer S, et al. Development and validation of a health-related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572–577. doi: 10.1046/j.1365-2133.2003.05419.x. [DOI] [PubMed] [Google Scholar]
- 24.Gawkrodger DJ, Ormerod AD, Shaw L, Mauri-Sole I, Whitton ME, Watts MJ, et al. Guideline for the diagnosis and management of vitiligo. Br J Dermatol. 2008;159:1051–1076. doi: 10.1111/j.1365-2133.2008.08881.x. [DOI] [PubMed] [Google Scholar]
- 25.McKenna SP, Cook SA, Whalley D, Doward LC, Richards HL, Griffiths CE, et al. Development of the PSORIQoL, a psoriasis- specific measure of quality of life designed for use in clinical practice and trials. Br J Dermatol. 2003;149:323–331. doi: 10.1046/j.1365-2133.2003.05492.x. [DOI] [PubMed] [Google Scholar]
- 26.Gupta MA, Johnson AM, Gupta AK. The development of an Acne Quality of Life scale: reliability, validity, and relation to subjective acne severity in mild to moderate acne vulgaris. Acta Derm Venereol. 1998;78:451–456. doi: 10.1080/000155598442773. [DOI] [PubMed] [Google Scholar]
- 27.Boza JC, Basra MK, Vanin RC, Carvalho RR, Weber MB, Cestari TF. Translation into Brazilian Portuguese and validation of the psoriasis family index. An Bras Dermatol. 2013;88:484. doi: 10.1590/abd1806-4841.20131911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freitag FM, Cestari TF, Leopoldo LR, Paludo P, Boza JC. Effect of melasma on quality of life in a sample of women living in southern Brazil. J Eur Acad Dermatol Venereol. 2008;22:655–662. doi: 10.1111/j.1468-3083.2007.02472.x. [DOI] [PubMed] [Google Scholar]
- 29.Cestari TF, Hexsel D, Viegas ML, Azulay L, Hassun K, Almeida AR, et al. Validation of a melasma quality of life questionnaire for Brazilian Portuguese language: the MelasQoL-BP study and improvement of QoL of melasma patients after triple combination therapy. Australas J Dermatol. 2006;156:13–20. doi: 10.1111/j.1365-2133.2006.07591.x. [DOI] [PubMed] [Google Scholar]
- 30.Weber MB, Lorenzini D, Reinehr CP, Lovato B. Assessment of the quality of life of pediatric patients at a center of excellence in dermatology in southern Brazil. An Bras Dermatol. 2012;87:697–702. doi: 10.1590/s0365-05962012000500004. [DOI] [PubMed] [Google Scholar]
- 31.Manzoni AP, Pereira RL, Townsend RZ, Weber MB, Nagatomi AR, Cestari TF. Assessment of the quality of life of pediatric patients with the major chronic childhood skin diseases. An Bras Dermatol. 2012;87:361–368. doi: 10.1590/s0365-05962012000300002. [DOI] [PubMed] [Google Scholar]
- 32.Ludwig MW, Oliveira Mda S, Muller MC, Moraes JF. Quality of life and site of the lesion in dermatological patients. An Bras Dermatol. 2009;84:143–150. doi: 10.1590/s0365-05962009000200007. [DOI] [PubMed] [Google Scholar]
- 33.Ciconelli RM, Ferraz MB, Santos W, Meinão I, Quaresma MR. Tradução para a língua portuguesa e validação do questionário genérico de avaliação de qualidade de vida SF-36 (Brasil SF-36) Rev Bras Reumatol. 1999;39:143–150. [Google Scholar]