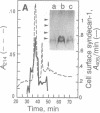
Abstract
Cell surface heparan sulfate proteoglycans, such as the syndecans, are required for cellular responses to heparin-binding growth factors and extracellular matrix components. Expression of syndecan-1 and -4 is induced in mesenchymal cells during wound repair in the mouse, consistent with a role for syndecans in regulating cell proliferation and migration in response to these effectors. Here we show that wound fluid contains inductive activity that mimics the in vivo induction in time of appearance, specificity for mesenchymal cells, and selectivity for syndecan-1 and -4. We have purified and synthesized a 4.8-kDa proline-rich protein from wound fluid that reproduces this induction of syndecan-1 and -4 in cultured cells. This peptide, identical to the antibacterial peptide PR-39, is released into the wound by the cellular infiltrate and induces syndecan expression at the same peptide concentrations that lyse bacteria. These results indicate that wounds contain a multifunctional protein that induces mammalian cells to express cell surface heparan sulfate proteoglycans as part of the wound repair process and that kills bacteria as part of a nonimmune defense mechanism.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agerberth B., Lee J. Y., Bergman T., Carlquist M., Boman H. G., Mutt V., Jörnvall H. Amino acid sequence of PR-39. Isolation from pig intestine of a new member of the family of proline-arginine-rich antibacterial peptides. Eur J Biochem. 1991 Dec 18;202(3):849–854. doi: 10.1111/j.1432-1033.1991.tb16442.x. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Boman H. G. Antibacterial peptides: key components needed in immunity. Cell. 1991 Apr 19;65(2):205–207. doi: 10.1016/0092-8674(91)90154-q. [DOI] [PubMed] [Google Scholar]
- Breuing K., Eriksson E., Liu P., Miller D. R. Healing of partial thickness porcine skin wounds in a liquid environment. J Surg Res. 1992 Jan;52(1):50–58. doi: 10.1016/0022-4804(92)90278-8. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- David G., Bernfield M. R. Collagen reduces glycosaminoglycan degradation by cultured mammary epithelial cells: possible mechanism for basal lamina formation. Proc Natl Acad Sci U S A. 1979 Feb;76(2):786–790. doi: 10.1073/pnas.76.2.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G. Integral membrane heparan sulfate proteoglycans. FASEB J. 1993 Aug;7(11):1023–1030. doi: 10.1096/fasebj.7.11.8370471. [DOI] [PubMed] [Google Scholar]
- David G., Lories V., Decock B., Marynen P., Cassiman J. J., Van den Berghe H. Molecular cloning of a phosphatidylinositol-anchored membrane heparan sulfate proteoglycan from human lung fibroblasts. J Cell Biol. 1990 Dec;111(6 Pt 2):3165–3176. doi: 10.1083/jcb.111.6.3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elenius K., Vainio S., Laato M., Salmivirta M., Thesleff I., Jalkanen M. Induced expression of syndecan in healing wounds. J Cell Biol. 1991 Aug;114(3):585–595. doi: 10.1083/jcb.114.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo R. L., Staszewski R., Sauder D. N., Knisely T. L., Granstein R. D. Regulation of GM-CSF and IL-3 production from the murine keratinocyte cell line PAM 212 following exposure to ultraviolet radiation. J Invest Dermatol. 1991 Aug;97(2):203–209. doi: 10.1111/1523-1747.ep12479676. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K., Hayashi M., Jalkanen M., Firestone J. H., Trelstad R. L., Bernfield M. Immunocytochemistry of cell surface heparan sulfate proteoglycan in mouse tissues. A light and electron microscopic study. J Histochem Cytochem. 1987 Oct;35(10):1079–1088. doi: 10.1177/35.10.2957423. [DOI] [PubMed] [Google Scholar]
- Jalkanen M., Nguyen H., Rapraeger A., Kurn N., Bernfield M. Heparan sulfate proteoglycans from mouse mammary epithelial cells: localization on the cell surface with a monoclonal antibody. J Cell Biol. 1985 Sep;101(3):976–984. doi: 10.1083/jcb.101.3.976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. W., Goldberger O. A., Gallo R. L., Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994 Jul;5(7):797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang O. D., Ascencio F., Fransson L. A., Wadström T. Binding of heparan sulfate to Staphylococcus aureus. Infect Immun. 1992 Mar;60(3):899–906. doi: 10.1128/iai.60.3.899-906.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marikovsky M., Breuing K., Liu P. Y., Eriksson E., Higashiyama S., Farber P., Abraham J., Klagsbrun M. Appearance of heparin-binding EGF-like growth factor in wound fluid as a response to injury. Proc Natl Acad Sci U S A. 1993 May 1;90(9):3889–3893. doi: 10.1073/pnas.90.9.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C. The coordinated regulation of heparan sulfate, syndecans and cell behavior. Curr Opin Cell Biol. 1993 Oct;5(5):844–853. doi: 10.1016/0955-0674(93)90034-n. [DOI] [PubMed] [Google Scholar]
- Rapraeger A., Bernfield M. Cell surface proteoglycan of mammary epithelial cells. Protease releases a heparan sulfate-rich ectodomain from a putative membrane-anchored domain. J Biol Chem. 1985 Apr 10;260(7):4103–4109. [PubMed] [Google Scholar]
- Ruoslahti E., Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991 Mar 8;64(5):867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Sanderson R. D., Hinkes M. T., Bernfield M. Syndecan-1, a cell-surface proteoglycan, changes in size and abundance when keratinocytes stratify. J Invest Dermatol. 1992 Oct;99(4):390–396. doi: 10.1111/1523-1747.ep12616103. [DOI] [PubMed] [Google Scholar]
- Saunders S., Jalkanen M., O'Farrell S., Bernfield M. Molecular cloning of syndecan, an integral membrane proteoglycan. J Cell Biol. 1989 Apr;108(4):1547–1556. doi: 10.1083/jcb.108.4.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder E. Y., Deitcher D. L., Walsh C., Arnold-Aldea S., Hartwieg E. A., Cepko C. L. Multipotent neural cell lines can engraft and participate in development of mouse cerebellum. Cell. 1992 Jan 10;68(1):33–51. doi: 10.1016/0092-8674(92)90204-p. [DOI] [PubMed] [Google Scholar]
- Storici P., Zanetti M. A cDNA derived from pig bone marrow cells predicts a sequence identical to the intestinal antibacterial peptide PR-39. Biochem Biophys Res Commun. 1993 Nov 15;196(3):1058–1065. doi: 10.1006/bbrc.1993.2358. [DOI] [PubMed] [Google Scholar]
- Trautman M. S., Kimelman J., Bernfield M. Developmental expression of syndecan, an integral membrane proteoglycan, correlates with cell differentiation. Development. 1991 Jan;111(1):213–220. doi: 10.1242/dev.111.1.213. [DOI] [PubMed] [Google Scholar]
- Woods A., McCarthy J. B., Furcht L. T., Couchman J. R. A synthetic peptide from the COOH-terminal heparin-binding domain of fibronectin promotes focal adhesion formation. Mol Biol Cell. 1993 Jun;4(6):605–613. doi: 10.1091/mbc.4.6.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Koehler B., Kulesz-Martin M., Hennings H. Clonal growth of mouse epidermal cells in medium with reduced calcium concentration. J Invest Dermatol. 1981 Feb;76(2):144–146. doi: 10.1111/1523-1747.ep12525490. [DOI] [PubMed] [Google Scholar]