Abstract
The relationship between intestinal microbiota composition and acute graft-versus-host disease (GVHD) after allogeneic blood/marrow transplantation (allo BMT) is not well understood. Intestinal bacteria have long been thought to contribute to GVHD pathophysiology, but recent animal studies in non-transplant settings have found that anti-inflammatory effects are mediated by certain subpopulations of intestinal commensals. Hypothesizing that a more nuanced relationship may exist between the intestinal bacteria and GVHD, we evaluated the fecal bacterial composition of 64 patients 12 days after BMT. We found that increased bacterial diversity was associated with reduced GVHD-related mortality. Furthermore, harboring increased amounts of bacteria belonging to the genus Blautia was associated with reduced GVHD lethality in this cohort and was confirmed in another independent cohort of 51 patients from the same institution. Blautia abundance was also associated with improved overall survival. We evaluated the abundance of Blautia with respect to clinical factors and found that loss of Blautia was associated with: 1) treatment with antibiotics that inhibit anaerobic bacteria and 2) receiving total parenteral nutrition (TPN) for longer durations. We conclude that increased abundance of commensal bacteria belonging to the Blautia genus is associated with reduced lethal GVHD and improved overall survival.
Introduction
Despite continuing improvements in outcomes of patients undergoing allo BMT, acute GVHD continues to be a leading cause of mortality [1]. Current immune suppression strategies are only partially effective at preventing GVHD and simultaneously increase the risk for infections and disease recurrence. Strategies that can reduce GVHD but leave immune function intact may thus potentially significantly improve outcomes. One such strategy is to target the complex community of microbes that reside within our intestinal tracts, collectively termed the intestinal microbiota.
A relationship between the microbiota and GVHD has long been suspected but is still not well understood. Mice transplanted in germ-free conditions [2] or treated with gut-decontaminating antibiotics [3] develop less severe GVHD. Clinical studies initially suggested a benefit from near-total bacterial decontamination [4, 5], but later showed no clear benefit [6-8] and this approach was discontinued in the early 1990s [9]. Partial gut decontamination continues to be practiced but little is known regarding optimal selection of antibiotic regimens. One study found the addition of metronidazole to ciprofloxacin led to a significant reduction in acute GVHD, suggesting that anaerobic bacteria may contribute to GVHD pathogenesis [10].
More recent results, however, indicate that this approach may not be ideal. Several studies have found that obligate anaerobes in the intestine, in particular Clostridial species, are important mediators of intestinal homeostasis and prevent inflammation by upregulating intestinal regulatory T cells [11]. Our group recently reported that in a study of 80 allo BMT recipients at our center, increased intestinal bacterial diversity at the time of engraftment was associated with improved overall survival and reduced non-relapse mortality [12]. While we did not find a significant association between bacterial diversity and GVHD in this study, this may have been because the population was underpowered to detect a difference in GVHD. Many of the patients had received a T cell-depleted allograft, which confers a much lower risk of developing GVHD [13, 14] and may have led to insufficient GVHD events to detect an effect of the microbiota.
In this study we focused on the outcome of GVHD by studying patients who were most at risk. Utilizing a prospectively collected fecal specimen bank, we examined a population of 115 allo BMT patients from our institution who received T cell-replete allografts. Here, we describe our finding that bacteria in the intestinal tract from the genus Blautia are associated with reduced mortality from GVHD.
Methods
Study design and oversight
The patients in this study are a subset of patients prospectively enrolled in a fecal collection protocol, where samples were collected during the initial transplant hospitalization and stored in a biospecimen bank. Since 2009, nearly all patients undergoing allogeneic BMT performed by the adult BMT service at our center (age 18 and older) have been approached to enroll, and the vast majority of patients have agreed to participate.
Patients who received conventional grafts (non-T cell depleted) and had a fecal sample collected within 4 days of day 12 following allo BMT were included in this study. Patients who received ex-vivo T cell-depleted grafts were specifically excluded, given their historically low rates of grade II-IV acute GVHD of approximately 15%; notably in this setting patients do not receive any post-transplant GVHD prophylaxis [13, 14]. In comparison, in-vivo T cell depletion with ATG administration is less effective at reducing the incidence of GVHD in recipients of conventional grafts, estimated at 28% in a recent large meta-analysis, with most patients still receiving GVHD prophylaxis with calcineurin inhibitors [15]. Thus in this study we allowed inclusion of patients receiving ATG given their continued substantial risk of developing acute GVHD. No patients in the fecal specimen protocol received alemtuzumab for conditioning or post-transplant cyclophosphamide during the study period.
Stool specimens were requested at least weekly over the course of the transplant hospitalization. After collection, specimens were initially stored at 4°C. Within 24 hours, specimens were aliquoted into cryovials and stored at -80°C. The study was approved by the institutional review board at MSKCC. All study patients provided written informed consent for biospecimen collection and analysis.
Patients included in this study were divided into Cohorts 1 and 2, determined by the sequencing platform utilized to analyze their fecal samples. Selection of sequencing platform resulted from a combination of BMT date, stool collection days and number of samples from each patient. From 2009 through 2012, we sequenced using the Roche 454 platform samples from consecutive patients that met inclusion criteria regarding number and timing of samples for studies previously published [12, 16]; these patients comprised Cohort 1. Samples collected from patients who did not meet inclusion criteria were stored but not sequenced at the time. After 2013, we transitioned to the Illumina MiSeq sequencing platform. Patients with stored samples who met inclusion criteria for the current study were included in Cohort 2, as well as additional patients transplanted in 2013. Primary comparisons performed in this study were within each of the two cohorts, and thus there were no comparisons across cohort groups.
Study definitions
Acute GVHD was diagnosed clinically, confirmed pathologically by biopsy whenever possible, and classified according to International Bone Marrow Transplant Registry criteria [17]. Late-onset acute GVHD, occurring more than 100 days after transplantation [18, 19], was included in the definition of acute GVHD, while chronic GVHD in the absence of acute features was not. Cases of GVHD were further characterized by treatment: a) topical steroids or budesonide, b) systemic steroids (prednisone or methylprednisolone, 0.5 mg/kg daily or higher), and c) second-line therapy in patients with steroid-refractory GVHD. Cause of death was determined using a standard algorithm where competing causes were prioritized in the following order: 1) primary disease recurrence, 2) graft failure, 3) GVHD, 4) infection, and 5) organ failure; thus in patients without disease recurrence or graft failure, those who were being treated for GVHD at the time of death were considered to have succumbed to GVHD-related mortality, including those who died with infections [20]. Deaths classified as non-GVHD treatment-related mortality included patients who succumbed to graft failure, infection (in the absence of acute GVHD therapy), and organ failure. Disease risk was determined according to the ASBMT RFI 2014 Disease Classification [21]. Conditioning intensity was assigned based on previously established working definitions [22].
Antibiotic categories
Antibiotics used during the transplant hospitalization were divided into those that included significant activity against anaerobic bacteria (piperacillin-tazobactam, ticarcillin-clavulanate, imipenem-cilastatin, meropenem, metronidazole, oral vancomycin and clindamycin), and those with reduced anaerobic activity in the intestinal tract (intravenous vancomycin, ceftriaxone, ceftazidime, cefepime, aztreonam, and trimethoprim-sulfamethoxazole) [23].
Transplantation practices
As per our institutional practice, patients whose conditioning was more intense than nonmyeloablative received prophylaxis with ciprofloxacin (400 mg intravenously or 500 mg orally every 12 hours) and vancomycin (1 gram intravenously every 12 hours) starting day -2 relative to BMT infusion [24]. Antibiotic prophylaxis against Pneumocystis carinii (trimethoprim-sulfamethoxazole, aerosolized pentamadine, or atovaquone) was given at the discretion of the transplant physician. Administration of TPN was considered on day 2 following transplant in patients with poor oral intake and was discontinued upon oral recovery. Treatment of GVHD was at the discretion of the transplant physician and generally involved topical corticosteroids for mild to moderate isolated skin GVHD, oral budesonide for mild gastrointestinal (GI) GVHD, and systemic steroids for all other manifestations of GVHD, including liver and more severe skin and GI GVHD. Patients were also maintained on a non-steroid immunosuppressant, typically a calcineurin inhibitor, to allow eventual tapering of systemic steroids. Patients refractory to systemic steroids received second-line therapy at the discretion of the transplant physician.
Analysis of specimens
For each stool specimen, DNA was purified using a phenol-chloroform extraction technique with mechanical disruption (bead-beating) based on a previously described protocol [25]. Samples from the first 64-patient flora cohort were analyzed using the 454 GS FLX Titanium platform to sequence the V1-V3 region of the bacterial 16S rRNA gene. Samples from the second 51-patient flora cohort were subsequently analyzed using the Illumina MiSeq platform to sequence the V4-V5 region of the 16S rRNA gene. Sequence data were compiled and processed using mothur version 1.34 [26], screened and filtered for quality [27] then classified to the species level [28] using a modified form of the Greengenes reference database [29]. Microbial diversity was quantified using the inverse Simpson index [30] and the Shannon diversity index [31] of operational taxonomic units with 97% similarity [32]. Taxonomic abundance comparisons were performed in order to identify biomarkers of GVHD-related mortality using linear discriminant analysis (LDA) effect size (LEfSe) analysis [33], using a logarithmic LDA cutoff of 2.0 as described in the original manuscript by the developers. Data from this study has been stored in the NCBI Sequence Read Archive (http://www.ncbi.nlm.nih.gov/sra). Quantification of 16S rRNA copies was performed using qPCR on DNA extracted from fecal specimens as previously described [34].
Statistical analysis
The incidence of acute GVHD and GVHD-related mortality was estimated using cumulative incidence functions, treating relapse and death unrelated to GVHD as competing events, and compared across factors using Gray's test. Comparison across Blautia abundance was done first in a cohort of patients, and then confirmed in a second cohort. Overall survival probabilities were estimated using Kaplan-Meier methodology and compared using the logrank test. Incidence and overall survival data are presented relative to the time of BMT, but patient cohorts were analyzed relative to the landmark of day 12, the median time of stool collection. We note that no patients in the study died nor developed GVHD prior to day 16. Comparison of the risk of acute GVHD and GVHD-related mortality after adjusting for clinical factors was performed using Cox regression. A receiver operating characteristic (ROC) curve was used to explore other thresholds of Blautia as discriminators for one-year GVHD-related mortality [35]. A Blautia abundance trend curve was constructed using LOESS local regression to estimate the marginal trend of Blautia abundance over time with a corresponding 95% confidence interval. Comparisons of bacterial abundance were performed using the Mann-Whitney U for unpaired 2-way tests, Kruskal-Wallis for 3-way tests, and Wilcoxon signed-rank for paired tests. In all analyses statistical significance was defined as p < 0.05 based on a 2-sided test. Statistical analyses were performed using R version 3.1.0 (The R Foundation for Statistical Computing, Vienna, Austria).
Results
Intestinal flora diversity near day 12 is associated with reduced GVHD-related mortality
Our group recently reported that increased bacterial diversity at the time of engraftment following allo BMT was associated with improved overall survival and reduced transplant-related mortality, but in that heterogeneous patient population we did not find an association between diversity and GVHD [12]. For the current study, we began by asking if bacterial flora diversity was associated with lethal GVHD in a more uniform population of patients at higher risk for developing GVHD. We utilized prospectively banked stool samples collected from patients who underwent allo BMT at our center. We chose to target day 12, reasoning that this time point, 1) was late enough for stool specimens to reflect changes induced by the transplant hospitalization, including antibiotic exposures, and 2) still preceded the development of GVHD in all patients. We identified 64 patients in Cohort 1 who, following conventional allo BMT without T-cell depletion, had provided a stool sample following BMT infusion and prior to hospital discharge (collected day 8-16, median day 12; clinical characteristics summarized in Table 1). We analyzed the flora composition of these stool samples using the 454 platform and followed patients clinically for development of GVHD-related mortality. Importantly, our institutional practice regarding bacterial prophylaxis is to administer ciprofloxacin and intravenous vancomycin prophylaxis starting day -2. We have found that this antibiotic prophylaxis practice has a relatively modest impact on intestinal flora diversity [12], which is in agreement with the pharmacokinetics and biodistribution of intravenous vancomycin [36].
Table 1.
Clinical characteristics of allo BMT patients transplanted at MSKCC with stool samples collected day 12 after BMT included in an identification and validation cohort.
| Cohort 1 | Cohort 2 | |
|---|---|---|
| Dates of transplant | September 2009 to October 2012 | August 2011 to August 2013 |
| Age (years) | 25 to 70, median 53 | 26 to 75, median 50 |
| Gender | Female 38%, male 62% | Female 32%, male 69% |
| Primary malignancy | NHL 38%, AML 38%, ALL 9.4%, Hodgkin disease 6.3%, CLL 6.3%, MDS 3.1% | NHL 35%, AML 37%, ALL 12%, Hodgkin disease 3.9%, CLL 7.8%, MDS 3.9% |
| Disease risk | High 45%, intermediate 36%, low 19% | High 35%, intermediate 27%, low 37% |
| Graft source | Peripheral blood 55%, cord blood 42%, bone marrow 3.1% | Peripheral blood 57%, cord blood 39%, bone marrow 3.9% |
| Donor relationship and HLA | Sibling identical (29%), unrelated identical (22%), unrelated non-identical (48%) | Sibling identical (24%), unrelated identical (29%), unrelated non-identical (47%) |
| Conditioning intensity | Standard intensity myeloablative 23%, reduced intensity myeloablative 44%, nonmyeloablative 33% | Standard intensity myeloablative 14%, reduced intensity myeloablative 57%, nonmyeloablative 29% |
| Stool sample collection day | +8 to +16, median +12 | +8 to +16, median +12 |
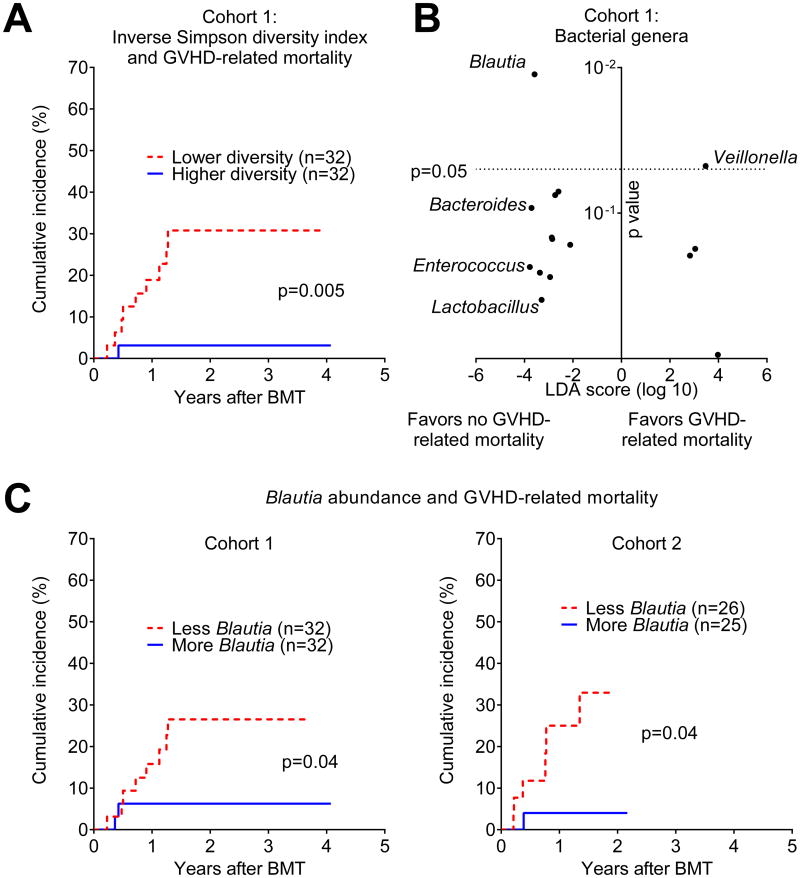
To evaluate the association between flora diversity and GVHD-related mortality, we employed an approach commonly used to evaluate potential biomarkers, where patients are stratified by the median value into two equal-sized groups and the incidence of the outcome of interest is compared [37]. We found that increased bacterial diversity, quantified by the inverse Simpson index, was associated with reduced GVHD lethality (Figure 1A, p=0.005).
Figure 1.
Changes in the intestinal flora are associated with differences in GVHD-related mortality. A) Bacterial diversity was quantified using the inverse Simpson index following composition analysis of stool samples from Cohort 1 performed by 16S gene sequencing. Patients were stratified by the median diversity index value and analyzed for cumulative incidence of GVHD-related mortality. B) Associations of bacterial genera with GVHD-related mortality outcomes were quantified by LEfSe analysis. Position along the vertical axis indicates statistical significance. C) Patients from Cohorts 1 and 2 were stratified by median Blautia abundance (0.05% in both) and analyzed for incidence of GVHD-related mortality.
Abundance of bacteria from the genus Blautia near day 12 is associated with reduced acute GVHD requiring systemic immune suppression
To identify bacterial subsets associated with GVHD-related mortality, we focused on bacterial phylogeny at the genus level, which is generally the most specific level at which 16S deep sequencing still provides reliable classification. Our approach involved first identifying bacterial genera potentially associated with GVHD-related mortality using a taxonomic discovery analysis, then evaluating candidate genera for their association with the incidence of GVHD-related mortality, and finally, evaluating for reproducibility of observed associations in an additional independent cohort of patients from the same institution. Beginning with taxonomic discovery analysis of bacterial genera, we found that bacteria belonging to the genus Blautia were most significantly associated with reduced GVHD-related mortality (Figure 1B, p=0.01). The Blautia genus notably includes anaerobic intestinal commensal organisms within the bacterial class Clostridia [38, 39]. The genus Veillonella, in turn, was associated with increased GVHD-related mortality (p=0.047).
We then evaluated the association of Blautia and Veillonella abundance with GVHD-related mortality, stratifying patients by the median abundance (0.05% and 0.04%, respectively). We found that patients with higher Blautia abundance had reduced GVHD-related mortality (Figure 1C, p=0.04), while Veillonella was not significantly associated with GVHD-related mortality (data not shown, p=0.16). We repeated this analysis in Cohort 2, which included 51 independent patients from the same institution who provided fecal samples analyzed on the MiSeq deep-sequencing platform (clinical characteristics summarized in Table 1). This cohort reproduced our prior results, where a Blautia abundance above the median (which, similar to Cohort 1, was 0.05%) was associated with less GVHD lethality (Figure 1C, p=0.04). In this second cohort, Veillonella again was not associated with GVHD-related mortality (data not shown, p=0.5).
Abundance of bacteria from the genus Blautia near day 12 is associated with improved overall survival
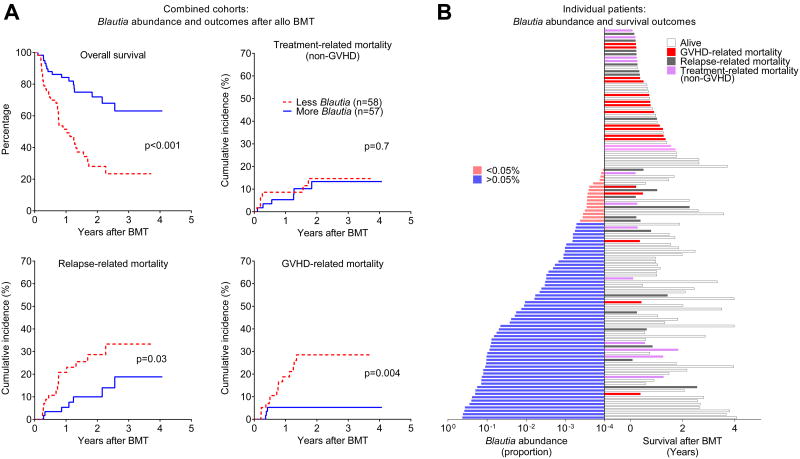
By evaluating the combined cohorts, we found that Blautia abundance was strongly associated with improved overall survival following allo BMT (p<0.001). This could largely be explained by reduced GVHD-related mortality (p=0.004) and to a lesser degree reduced relapse-related mortality (p=0.03), with no difference in non-GVHD treatment-related mortality (Figure 2A). Similar results were observed when cohorts were analyzed separately (data not shown). Blautia abundance, survival, and cause of death are depicted for each individual patient in Figure 2B, demonstrating that many of the GVHD-related deaths occurred in patients with lower Blautia abundance.
Figure 2.
A) Association of Blautia abundance and outcomes after allo BMT. Patients from the two flora cohorts were combined and stratified by Blautia abundance below or above 0.05%, and evaluated for the indicated outcomes; similar results were seen for each individual cohort (not shown). B) Blautia abundance (left), survival, and cause of death (right) are indicated for individual patients from two cohorts combined, ranked by Blauita abundance.
Some of the GVHD-related deaths in this patient population occurred somewhat later than would be expected following allo BMT, with 9 of 17 events occurring after day 180. In these patients, the majority developed acute GVHD during the taper of immune suppression (median day 41 after BMT, range 21-80). Donor lymphocyte infusion, commonly performed for disease relapse and a potential risk factor for late acute GVHD, was administered to 3 patients but did not contribute to acute GVHD in these patients. Chronic GVHD, which can occur following acute GVHD or develop de-novo, was diagnosed in only one patient who experienced GVHD-related mortality, and thus was also not a significant contributor to late GVHD-related mortality in this study.
Having demonstrated an association of Blautia abundance with GVHD-related mortality using median Blautia abundance to stratify patients, we next sought to compare this threshold with other potential cut-offs of Blautia abundance. We generated a receiver operating characteristic (ROC) curve (Figure S1A), which had an estimated AUC of 0.72 (95% CI 0.52-0.93), further suggesting that Blautia abundance may have utility as a biomarker of GVHD-related mortality. With a sensitivity of 0.51 and specificity of 0.84, the ROC curve supported the use of the median to stratify patients at high risk for GVHD-related mortality.
A potential limitation of 16S rRNA deep sequencing is the lack of absolute abundance information regarding bacterial subtypes. To determine the absolute abundance of Blautia 16S rRNA genes in fecal samples, we normalized the relative-abundance measurements from deep sequencing to the absolute abundance of 16S rRNA quantified by real-time PCR. We had adequate DNA to perform this for most of the patients (84 of 115, Table S1) and found that absolute Blautia abundance, similar to relative Blautia abundance, was associated with reduced GVHD-related mortality (p=0.01, Figure S1B).
We also searched for an association of GVHD-related mortality with other bacterial genera. Because increased Enterococcus may be associated with GVHD [40], we evaluated if Enterococcus, or potentially beneficial bacterial genera (Lactobacillus [41, 42] and Bacteroides [43]) were associated with GVHD-related mortality in our patient population. Our results indicated that none of these bacterial genera were associated with GVHD-related mortality in the combined cohorts (p=0.8, p=0.5, p=0.3, respectively, Table 2).
Table 2.
Association of various bacterial taxa with GVHD-related mortality in patients from the cohorts combined.
| Classification level | Bacterial taxon | Median abundance | GVHD-related mortality logrank p value |
|---|---|---|---|
| Genus | Blautia | 0.05% | 0.004 |
| Genus | Enterococcus | 1.32% | 0.8 |
| Genus | Bacteroides | 0.02% | 0.5 |
| Genus | Lactobacillus | 0.23% | 0.3 |
| Genus | Veillonella | 0.03% | 0.8 |
| Family | Lachnospiraceae | 0.21% | 0.02 |
| Order | Clostridiales | 2.92% | 0.02 |
| Class | Clostridia | 3.51% | 0.02 |
| Phylum | Firmicutes | 95.39% | 0.7 |
| Sub-order | Clostridial clusters IV, XIVa, and XVIII | 0.31% | 0.09 |
P values <0.05 are depicted in bold.
We next asked if bacterial subtypes related to Blautia were associated with reduced lethal GVHD. Bacteria from the genus Blautia are classified as follows: family – Lachnospiraceae, order – Clostridiales, class – Clostridia, and phylum – Firmicutes [38]. Stratification of patients by abundance of bacteria from Lachnospiraceae, Clostridiales, and Clostridia all demonstrated associations with a reduced incidence of lethal GVHD (p=0.02, p=0.02, p=0.02, respectively, Table 2), suggesting that members of Blautia, and potentially its close relatives, contribute a protective effect against lethal GVHD. Inclusion of more distant relatives by examining all Firmicutes, however, resulted in loss of the association (p=0.7, Table 2).
Abundance of bacteria from the genus Blautia near day 12 is associated with reduced need for acute GVHD treatment
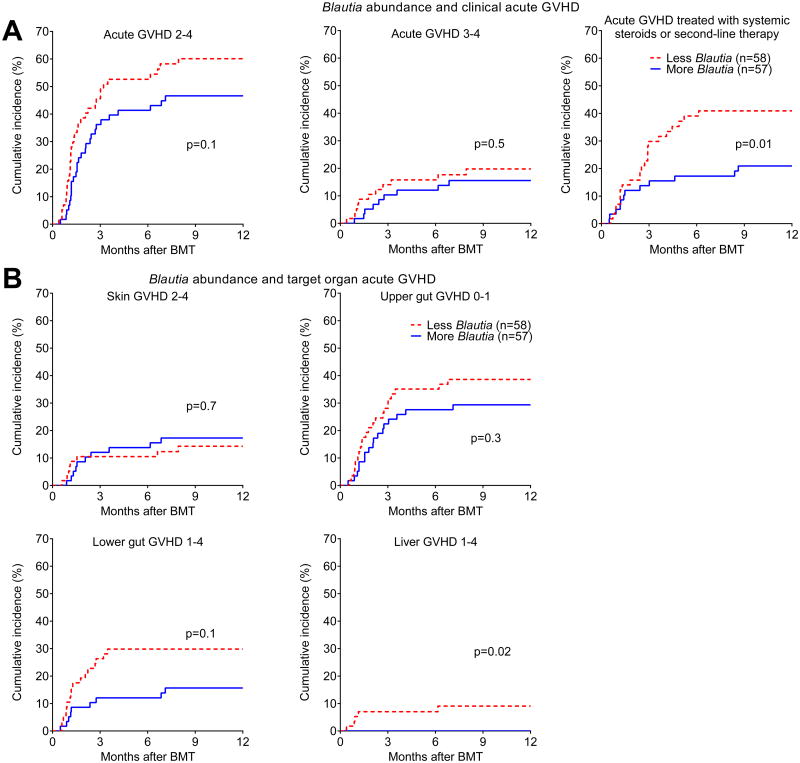
We then asked if Blautia abundance was associated with a reduction in the clinical severity of acute GVHD. We found that the association between Blautia abundance and reduced incidence of acute GVHD grades 2-4 did not reach statistical significance (p=0.1, Figure 3A) and there was no association with acute GVHD grades 3-4 (p=0.5, Figure 3A). Blautia abundance, however, was associated with reduced development of acute GVHD that required treatment with systemic corticosteroids or was steroid refractory (p=0.01, Figure 3A), suggesting that loss of Blautia is associated with acute GVHD that will not respond to topical corticosteroids or budesonide alone. Regarding classical acute GVHD target organs, increased Blautia abundance was not associated with skin GVHD or upper gut GVHD (Figure 3B). The association with reduced lower gut GVHD was not statistically significant (p=0.1, Figure 3B), while that with reduced liver GVHD was significant (p=0.02, Figure 3B) though the number of events was small.
Figure 3.
Association of Blautia abundance with clinical acute GVHD. A) Patients were stratified by Blautia abundance below or above 0.05%, and evaluated for development of the indicated severity grades of acute GVHD, as well as acute GVHD that required systemic therapy with corticosteroids. B) Patients were evaluated for development of acute GVHD in typical target organs.
Abundance of bacteria from the genus Blautia is most associated with reduced GVHD lethality in patients receiving nonmyeloablative conditioning and in patients receiving peripheral blood stem cell (PBSC) allografts
We then evaluated the association between Blautia and lethal GVHD in patients divided into subgroups by conditioning intensity or by graft source. Blautia was most associated with reduced lethal GVHD in patients who received nonmyeloablative conditioning (p=0.02, Table 3) while the association in patients receiving myeloablative and reduced intensity conditioning did not reach statistical significance (p=0.1 and p=0.3, Table 3). In patients divided by graft source, those who received PBSC allografts showed an association between Blautia abundance and reduced lethal GVHD (p=0.006, Table 3), while in those receiving umbilical cord blood stem cell grafts the association did not reach statistical significance (p=0.2, Table 3).
Table 3.
Subgroup analysis of patients from the cohorts combined examining the relationship between Blautia abundance and GVHD-related mortality.
| Subgroup | n (Blautia low) | n (Blautia high) | GVHD-related mortality log-rank p value |
|---|---|---|---|
| Myeloablative conditioning | 13 | 9 | 0.1 |
| Reduced intensity conditioning | 27 | 30 | 0.3 |
| Nonmyeloablative conditioning | 18 | 18 | 0.02 |
| PBSC graft | 31 | 33 | 0.006 |
| Cord blood graft | 24 | 23 | 0.2 |
Abbreviation: peripheral blood stem cell (PBSC). P values <0.05 are depicted in bold.
Blautia abundance is independent of known clinical acute GVHD risk factors
To determine if Blautia abundance provides additional prognostic information to GVHD outcomes, we investigated potential associations between Blautia abundance and known risk factors for acute GVHD [44-46]. We found that patient age, performance status, disease risk, CMV status, graft source, donor/patient gender, conditioning intensity, ATG administration, or GVHD prophylactic regimen were not associated with Blautia abundance (Table S2). While limited by small numbers, patients of an Asian or Hispanic background appeared to have lower abundance of Blautia.
Identifying potential clinical determinants of Blautia abundance during allo BMT hospitalization
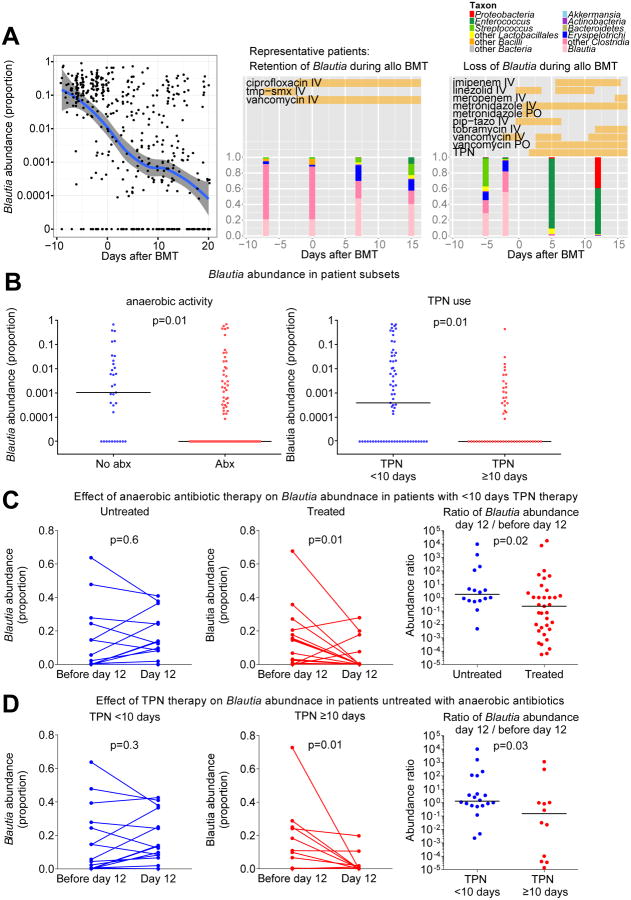
To better understand the heterogeneity in Blautia abundance in our patient population, we attempted to identify determinants of Blautia abundance. An analysis of all stool samples collected from both flora cohorts showed that most patients had relatively abundant amounts of Blautia upon admission for transplant hospitalization, with a median abundance >0.1 (10%) (Figure 4A). Some patients retained their Blautia throughout their hospital course, while others rapidly dropped Blautia levels during hospitalization (Figures 4A and S2). We found that patients not exposed to antibiotics containing increased anaerobic coverage were more likely to have increased levels of Blautia (p=0.01, Figure 4B), a finding that was expected given that Blautia is an obligate anaerobe. Intestinal flora composition analyses for individual patient samples along with antibiotic exposures are provided in Figure S2.
Figure 4.
Identifying potential determinants of Blautia abundance in allo BMT patients. A) Left: Blautia abundances in all available stool samples from both flora cohorts. An abundance trend was constructed using moving average filtering (solid blue line; 95% confidence intervals shown in gray). Middle and Right: Serial fecal sample composition analysis from two representative patients. Vertical bars indicate composition of fecal microbiota by the indicated taxa. Horizontal bars indicate antibiotic and TPN exposures. Flora composition and time courses of antibiotic and TPN exposure for all patients are shown in Figure S2. Abbreviations: intravenous (IV), oral (PO), piperacillin-tazobactam (pip-tazo), trimethoprim-sulfamethoxazole (tmp-smx), total parenteral nutrition (TPN). B) Patients were subsetted by exposure to antibiotics with anaerobic coverage prior to sample collection, and duration of TPN therapy, and Blautia abundance was evaluated. Horizontal bars indicate median Blautia abundance. C) A subset of patients who were not exposed to antibiotics with anaerobic coverage prior to sample collection were further subsetted by duration of TPN therapy and evaluated for Blautia abundance in a stool sample prior to day 12 and from day 12.
We then asked if conditioning intensity was also associated with Blautia abundance, but found that this was not the case (p=0.5, Table S2). Interestingly, however, use of total parenteral nutrition (TPN) was associated with changes in Blautia abundance. Due to nausea and mucositis following conditioning, allo BMT patients commonly experience a prolonged period of significantly reduced oral intake and are treated with supplementary TPN. Because initiation of TPN at our institution is considered at the earliest on day 2 and stool samples were on average collected on day 12, we compared patients with TPN use of less than 10 days duration (indicating delayed, interrupted or discontinued TPN therapy) with those with TPN use of 10 days or more. We found increased levels of Blautia in patients who received shorter courses of TPN therapy (Figure 4B), and TPN duration was associated with loss of Blautia even in patients that avoided treatment with anaerobe-active antibiotics (Figure 4C). TPN exposures for individual patients are provided in Figure S2.
Blautia abundance remains associated with GVHD-related mortality after adjusting for potential clinical confounders
We finally performed univariate and multivariate analyses to determine if the association of Blautia abundance with GVHD outcomes was driven by potential clinical confounders. Given the limited numbers of GVHD-related mortality events, we restricted our multivariate analysis to two factors, in addition to Blautia abundance. To identify potential confounders, we examined a variety of clinical factors previously shown to be linked to GVHD. This approach identified the GVHD prophylaxis regimen as the only statistically significant factor for GVHD-related mortality; specifically patients who received cyclosporine and mycophenolate had higher event rates (p=0.044). This regimen is the standard GVHD prophylaxis for patients receiving cord blood grafts at our center, and indeed, recipients of cord grafts showed a similar trend towards higher GVHD-related mortality (p=0.062). An additional factor that almost reached statistical significance was exposure to antibiotics with anaerobic coverage (p=0.055). We thus generated a model adjusting for two factors, graft source and exposure to anaerobic antibiotics, and found that Blautia abundance remained associated with both GVHD-related mortality (HR (95% CI) 0.18 (0.05-0.63), p=0.007) and clinical GVHD treated with systemic steroids (HR (95% CI) 0.3 (0.14-0.64) p=0.002). Finally, we tested the association of Blautia abundance with relapse-related mortality after adjusting for two factors, disease risk and graft source, and found that the association with Blautia abundance no longer reached statistical significance (p=0.055).
Discussion
In this study we found that in allo BMT recipients intestinal flora diversity is associated with reduced GVHD lethality. Furthermore, the bacterial genus from stool samples most associated with reduced GVHD-related mortality was Blautia, in two independent cohorts from the same institution. Patients with more Blautia also showed a reduced incidence of acute GVHD that required treatment with systemic corticosteroids or was steroid refractory, as well as improved overall survival. Surprisingly, despite the association with GVHD-related mortality, Blautia abundance did not distinguish the incidence of acute GVHD grades 2-4, or even grades 3-4, which has been shown to identify patients less likely to respond to steroids, leading to poorer survival [47]. However, a subpopulation of patients who initially present with grade 2 acute GVHD but nevertheless fare poorly has been described, and these patients may be better identified by novel GVHD grading systems [47] or by novel biomarkers [48]. Further investigations in additional larger patient cohorts may determine if Blautia abundance can similarly supplement the prognostic utility of clinical acute GVHD grading.
This study has several important limitations. First, because our results are from a single center, results may not be generalizable to other centers with different patient populations, and in particular, different antibiotic practices. Additionally, while we have found that Blautia is associated with GVHD-related mortality, this was identified in an exploratory manner and confirmed in a relatively small validation cohort. Furthermore, we have not demonstrated causality, i.e., that the presence of Blautia directly protects against lethal GVHD. It is possible that Blautia is predominant member of a larger group of bacteria that can mediate beneficial anti-inflammatory effects and disappear as a consortium. Honda and colleagues have recently reported that administering a mixture of 17 Clostridia isolates can reduce intestinal inflammation in mice by increasing infiltration of the large intestine with regulatory T cells [11]. Consistent with this, we found that the abundance of bacteria from the class Clostridia, which includes Blautia, was associated with reduced GVHD-related mortality in our patients. A beneficial anti-inflammatory association of Blautia has also been observed in other clinical settings, including colorectal cancer [49], inflammatory pouchitis following ileal pouch-anal anastomosis [49], and liver cirrhosis [50], where Blautia was one of several subtypes of bacteria associated with improved outcomes.
One question that arises is how a patient's Blautia abundance on day 12 after allo BMT could biologically have an impact on whether months later he or she develops symptoms of acute GVHD, or as late as a year later succumbs acute GVHD-related mortality. There are precedents however; low serum cyclosporine concentrations in the first week following allo BMT were found to be associated with later development of acute GVHD with onset largely occurring after day 30 [51]. Additionally, serum levels of the biomarker ST2 levels on day 14 were recently found to be associated with 6-month non-relapse mortality [48]. Together, these studies suggest that clinical conditions early post-BMT may affect the initiation of GVHD and modulate its eventual severity, though this can in some cases take months to fully manifest, perhaps due to partial containment of inflammation by ongoing administration of immune suppressants in the forms of GVHD prophylaxis and therapy.
Interestingly, while our results indicate that Blautia is associated with reduced GVHD suggesting a link with immune suppression, Blautia was not associated with increased relapse-related mortality. Indeed we detected an association of Blautia with a reduction in relapse-related mortality, though this no longer reached significance in our multivariate analysis. One explanation is that Blautia may be associated with a localized anti-inflammatory effect, primarily in the intestinal tract, without systemic immune suppression that could hinder a graft-versus-tumor effect. This possibility is supported by the absence of an association with skin GVHD. Together, these data suggest that targeting the intestinal microbiota may allow for reduced intestinal GVHD without simultaneously increasing the risk for malignant relapse.
In our subgroup analyses, we found associations between Blautia and GVHD outcomes in patients who received nonmyeloablative conditioning and in patients who received PBSC allografts, but these associations did not reach significance in other patient subgroups who received more intense conditioning or received cord blood grafts. Limitations in sample size may have contributed to this, and larger studies are likely required to evaluate the association of Blautia with GVHD outcomes in allo BMT subgroups. In this study, patients undergoing increased conditioning intensity experienced higher rates of treatment with antibiotics and TPN, as did patients receiving cord blood grafts. It is possible that an accumulation of risk factors for microbiota injury led to reduced heterogeneity in certain subgroups, and as a result the ability of Blautia abundance to discriminate patients with GVHD outcomes was reduced.
By characterizing the abundance of Blautia in our patients over the course of their transplant hospitalization, we found that most patients initially had relatively large amounts of Blautia that were dramatically lost during their hospital course. Established risk factors for GVHD were not associated with changes in Blautia abundance, but two clinical risk factors were associated with loss of Blautia: 1) treatment with antibiotics with increased anaerobic coverage, and 2) prolonged administration of TPN. While conditioning intensity and duration of TPN necessity are known to be associated [52], we found no significant association between conditioning intensity and Blautia abundance. This suggests that mucositis and/or poor oral nutrition may contribute to loss of Blautia more directly than intense conditioning. Corroborating this are findings in mouse models that myeloablative conditioning is associated with only mild perturbations in flora composition, in comparison to larger perturbations characterized by loss of Clostridiales seen in both mice and humans with the onset of GVHD, a potent inducer of anorexia [42]. A pattern of loss of members of Clostridiales, including Roseburia, Faecalibacterium, Ruminococcus and Blautia species, can similarly be observed in volunteers placed on high-protein and low-carbohydrate diets [53] or on diets derived entirely from animal products [54]. Alternatively, epithelial damage due to conditioning-induced mucositis could also potentially result in changes in flora composition via changes in expression of antimicrobial molecules produced by intestinal epithelium [55].
A possibility raised by this study is that clinical strategies to prevent damage to the intestinal microbiota, in particular to preserve obligate anaerobic bacteria such as Blautia, may be worth investigating in an effort to improve clinical outcomes for allo BMT patients. Potential strategies include avoidance of antibiotics that suppress anaerobic members of the commensal flora, or encouraging oral nutrition to supporting anaerobic populations. Interestingly, a study from Seguy and colleagues in 2006 reported that treating patients with enteral feeding led to a benefit in overall survival and reduced rates of acute GVHD grade 3-4 and deaths due to infection [56]. In the context of our findings, these results may indicate that intestinal commensals could mediate some of the benefits of enteral nutrition following allo BMT. Choosing less intense conditioning regimens may also help patients maintain oral nutrition and boost Blautia abundance; indeed, multicenter registry data indicate that less intense conditioning regimens are associated with reduced acute GVHD [45].
In conclusion, we have shown that the composition of the intestinal flora, in particular the abundance of anaerobic bacteria from the genus Blautia, can be used as a novel prognostic indicator for GVHD-related mortality. Further studies will need to be performed to determine if this finding holds true at other institutions. Additionally, studies to evaluate the potential impact of Blautia and related bacteria on the pathophysiology of GVHD are warranted, as are efforts to develop strategies to support anaerobic intestinal commensals in patients who undergo allo BMT.
Supplementary Material
Figure S1. A) A receiver operating characteristic (ROC) curve of the association between Blautia abundance and GVHD-related mortality in patients from cohorts 1 and 2 combined. Asterisk (*) indicates the position of the median (0.05%). B) Absolute Blautia abundance was quantified by normalizing relative Blautia abundance data from 16S deep sequencing by quantitative PCR measurements of total 16S DNA from the 84 patients from combined cohorts 1 and 2 from whom DNA was available. Patients were stratified by median absolute Blautia abundance and analyzed for incidence of GVHD-related mortality.
Figure S2. Flora composition analysis results from patients in Cohorts 1 and 2. Vertical bars indicate composition of fecal microbiota by the indicated taxa. Horizontal bars indicate antibiotic and TPN exposures. Abbreviations: intravenous (IV), oral (PO), piperacillin-tazobactam (pip-tazo), trimethoprim-sulfamethoxazole (tmp-smx), total parenteral nutrition (TPN).
Table S1. Patient information. Abbreviations: TBI (total body irradiation), ATG (anti-thymocyte globulin), CSA (cyclosporine), Tac (tacrolimus), Siro (sirolimus), MTX (methotrexate), MMF (mycophenolate), PBSC (peripheral blood stem cells), aGVHD (acute GVHD), cGVHD (chronic GVHD)
Table S2. Quantification of the association of known risk factors for acute GVHD and Blautia abundance. PBSC = peripheral blood stem cell. BM = bone marrow. PS = performance status. *P-value compares Blautia levels across cord blood and PBSC groups. **Missing, equivocal, or other covariate values not included in p-value calculation.
Highlights.
Intestinal bacteria diversity is associated with reduced mortality from GVHD
Biomarker discovery analysis identified bacteria from the genus Blautia
Increased Blautia was associated with reduced GVHD mortality in 2 independent cohorts
Blautia abundance is not associated with known risk factors for GVHD
Blautia is reduced after exposure to antibiotics with increased anaerobic coverage
Blautia is also reduced after prolonged use of total parenteral nutrition
Acknowledgments
This research was supported by National Institutes of Health award numbers R01-HL069929 (M.R.M. van den Brink), R01-AI080455 (M.R.M. van den Brink), R01-AI101406 (M.R.M. van den Brink), P01-CA023766 (R. J. O'Reilly), Project 4 of P01-CA023766 (M.R.M. van den Brink), P30-CA008748 (S. M. Devlin), R01-AI042135 (E. G. Pamer), R01-AI095706 (E. G. Pamer), and K23-AI095398 (Y. Taur). Support was also received from the U.S National Institute of Allergy and Infectious Diseases (NIAID Contract HHSN272200900059C), The Experimental Therapeutics Center of MSKCC funded by Mr. William H. Goodwin and Mrs. Alice Goodwin, The Lymphoma Foundation, Alex's Lemonade Stand, The Geoffrey Beene Cancer Research Center at MSKCC, The Susan and Peter Solomon Divisional Genomics Program, the Lucille Castori Center for Microbes, Inflammation, and Cancer, and the Tow Foundation. This project has received funding from the European Union's Seventh Programme for research, technological development and demonstration under grant agreement No [602587].
Footnotes
Financial Disclosure Statement: The authors have no primary financial relationships with any companies that have a direct financial interest in the subject matter or products discussed in the submitted manuscript, or with a company that produces a competing product.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Robert R. Jenq, Adult BMT, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Ying Taur, Infectious Diseases Service, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Sean M. Devlin, Epidemiology & Biostatistics, Memorial Sloan Kettering Cancer Center, New York, New York.
Doris M. Ponce, Adult BMT, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Jenna D. Goldberg, Adult BMT Service, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Katya F. Ahr, Immunology, Memorial Sloan Kettering Cancer Center
Eric R. Littmann, Infectious Diseases, Memorial Sloan Kettering Cancer Center
Lilan Ling, Immunology, Memorial Sloan Kettering Cancer Center.
Asia C. Gobourne, Immunology, Memorial Sloan Kettering Cancer Center
Liza C. Miller, Immunology, Memorial Sloan Kettering Cancer Center
Melissa D. Docampo, Immunology, Memorial Sloan Kettering Cancer Center
Jonathan U. Peled, Adult BMT, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Nicholas Arpaia, Immunology, Memorial Sloan Kettering Cancer Center.
Justin R. Cross, Cell Metabolism Core, Memorial Sloan Kettering Cancer Center.
Tatanisha K. Peets, Nutrition, Memorial Sloan Kettering Cancer Center.
Melissa A. Lumish, Immunology, Memorial Sloan Kettering Cancer Center
Yusuke Shono, Immunology, Memorial Sloan Kettering Cancer Center.
Jarrod A. Dudakov, Immunology, Memorial Sloan Kettering Cancer Center.
Hendrik Poeck, Immunology, Memorial Sloan Kettering Cancer Center.
Alan M. Hanash, Adult BMT, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Juliet N. Barker, Adult BMT, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Miguel-Angel Perales, Adult BMT, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Sergio A. Giralt, Adult BMT, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Eric G. Pamer, Infectious Diseases, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
Marcel R.M. van den Brink, Adult BMT, Memorial Sloan Kettering Cancer Center, New York, New York, Weill Cornell Medical College, New York, New York.
References
- 1.Pasquini MC, W Z. Current use and outcome of hematopoietic stem cell transplantation. CIBMTR Summary Slides. 2013 Available at: http://www.cibmtr.org.
- 2.Jones JM, Wilson R, Bealmear PM. Mortality and gross pathology of secondary disease in germfree mouse radiation chimeras. Radiat Res. 1971;45:577–88. [PubMed] [Google Scholar]
- 3.van Bekkum DW, Roodenburg J, Heidt PJ, van der Waaij D. Mitigation of secondary disease of allogeneic mouse radiation chimeras by modification of the intestinal microflora. J Natl Cancer Inst. 1974;52:401–4. doi: 10.1093/jnci/52.2.401. [DOI] [PubMed] [Google Scholar]
- 4.Storb R, Prentice RL, Buckner CD, Clift RA, Appelbaum F, Deeg J, et al. Graft-versus-host disease and survival in patients with aplastic anemia treated by marrow grafts from HLA-identical siblings. Beneficial effect of a protective environment. N Engl J Med. 1983;308:302–7. doi: 10.1056/NEJM198302103080602. [DOI] [PubMed] [Google Scholar]
- 5.Vossen JM, Heidt PJ, van den Berg H, Gerritsen EJ, Hermans J, Dooren LJ. Prevention of infection and graft-versus-host disease by suppression of intestinal microflora in children treated with allogeneic bone marrow transplantation. Eur J Clin Microbiol Infect Dis. 1990;9:14–23. doi: 10.1007/BF01969527. [DOI] [PubMed] [Google Scholar]
- 6.Petersen FB, Buckner CD, Clift RA, Nelson N, Counts GW, Meyers JD, et al. Infectious complications in patients undergoing marrow transplantation: a prospective randomized study of the additional effect of decontamination and laminar air flow isolation among patients receiving prophylactic systemic antibiotics. Scand J Infect Dis. 1987;19:559–67. doi: 10.3109/00365548709032423. [DOI] [PubMed] [Google Scholar]
- 7.Passweg JR, Rowlings PA, Atkinson KA, Barrett AJ, Gale RP, Gratwohl A, et al. Influence of protective isolation on outcome of allogeneic bone marrow transplantation for leukemia. Bone Marrow Transplant. 1998;21:1231–8. doi: 10.1038/sj.bmt.1701238. [DOI] [PubMed] [Google Scholar]
- 8.Russell JA, Chaudhry A, Booth K, Brown C, Woodman RC, Valentine K, et al. Early outcomes after allogeneic stem cell transplantation for leukemia and myelodysplasia without protective isolation: a 10-year experience. Biol Blood Marrow Transplant. 2000;6:109–14. doi: 10.1016/s1083-8791(00)70073-5. [DOI] [PubMed] [Google Scholar]
- 9.Martin PJ, McDonald GB, Sanders JE, Anasetti C, Appelbaum FR, Deeg HJ, et al. Increasingly frequent diagnosis of acute gastrointestinal graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2004;10:320–7. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 10.Beelen DW, Elmaagacli A, Muller KD, Hirche H, Schaefer UW. Influence of intestinal bacterial decontamination using metronidazole and ciprofloxacin or ciprofloxacin alone on the development of acute graft-versus-host disease after marrow transplantation in patients with hematologic malignancies: final results and long-term follow-up of an open-label prospective randomized trial. Blood. 1999;93:3267–75. [PubMed] [Google Scholar]
- 11.Atarashi K, Tanoue T, Oshima K, Suda W, Nagano Y, Nishikawa H, et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature. 2013;500:232–6. doi: 10.1038/nature12331. [DOI] [PubMed] [Google Scholar]
- 12.Taur Y, Jenq RR, Perales MA, Littmann ER, Morjaria S, Ling L, et al. The effects of intestinal tract bacterial diversity on mortality following allogeneic hematopoietic stem cell transplantation. Blood. 2014 doi: 10.1182/blood-2014-02-554725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jakubowski AA, Small TN, Kernan NA, Castro-Malaspina H, Collins N, Koehne G, et al. T cell-depleted unrelated donor stem cell transplantation provides favorable disease-free survival for adults with hematologic malignancies. Biol Blood Marrow Transplant. 2011;17:1335–42. doi: 10.1016/j.bbmt.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg JD, Linker A, Kuk D, Ratan R, Jurcic J, Barker JN, et al. T cell-depleted stem cell transplantation for adults with high-risk acute lymphoblastic leukemia: long-term survival for patients in first complete remission with a decreased risk of graft-versus-host disease. Biol Blood Marrow Transplant. 2013;19:208–13. doi: 10.1016/j.bbmt.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theurich S, Fischmann H, Shimabukuro-Vornhagen A, Chemnitz JM, Holtick U, Scheid C, et al. Polyclonal anti-thymocyte globulins for the prophylaxis of graft-versus-host disease after allogeneic stem cell or bone marrow transplantation in adults. The Cochrane database of systematic reviews. 2012;9:CD009159. doi: 10.1002/14651858.CD009159.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, et al. Intestinal Domination and the Risk of Bacteremia in Patients Undergoing Allogeneic Hematopoietic Stem Cell Transplantation. Clin Infect Dis. 2012 doi: 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowlings PA, Przepiorka D, Klein JP, Gale RP, Passweg JR, Henslee-Downey PJ, et al. IBMTR Severity Index for grading acute graft-versus-host disease: retrospective comparison with Glucksberg grade. British journal of haematology. 1997;97:855–64. doi: 10.1046/j.1365-2141.1997.1112925.x. [DOI] [PubMed] [Google Scholar]
- 18.Vigorito AC, Campregher PV, Storer BE, Carpenter PA, Moravec CK, Kiem HP, et al. Evaluation of NIH consensus criteria for classification of late acute and chronic GVHD. Blood. 2009;114:702–8. doi: 10.1182/blood-2009-03-208983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ponce DM, Gonzales A, Lubin M, Castro-Malaspina H, Giralt S, Goldberg JD, et al. Graft-versus-host disease after double-unit cord blood transplantation has unique features and an association with engrafting unit-to-recipient HLA match. Biol Blood Marrow Transplant. 2013;19:904–11. doi: 10.1016/j.bbmt.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Copelan E, Casper JT, Carter SL, van Burik JA, Hurd D, Mendizabal AM, et al. A scheme for defining cause of death and its application in the T cell depletion trial. Biol Blood Marrow Transplant. 2007;13:1469–76. doi: 10.1016/j.bbmt.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 21.Transplantation ASfBaM. ASBMT RFI 2014 - Disease Classifications Corresponding to CIBMTR Classifications. 2014 [Google Scholar]
- 22.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15:1628–33. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, Baron EJ, et al. Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis. 2010;50:133–64. doi: 10.1086/649554. [DOI] [PubMed] [Google Scholar]
- 24.Jaffe D, Jakubowski A, Sepkowitz K, Sebti R, Kiehn TE, Pamer E, et al. Prevention of peritransplantation viridans streptococcal bacteremia with early vancomycin administration: a single-center observational cohort study. Clin Infect Dis. 2004;39:1625–32. doi: 10.1086/425612. [DOI] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009;75:7537–41. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schloss PD, Gevers D, Westcott SL. Reducing the effects of PCR amplification and sequencing artifacts on 16S rRNA-based studies. PLoS One. 2011;6:e27310. doi: 10.1371/journal.pone.0027310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007;73:5261–7. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.DeSantis TZ, Hugenholtz P, Larsen N, Rojas M, Brodie EL, Keller K, et al. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl Environ Microbiol. 2006;72:5069–72. doi: 10.1128/AEM.03006-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magurran AE. Measuring biological diversity. Malden, MA: Blackwell Publishing; 2004. [Google Scholar]
- 31.Shannon CE. The mathematical theory of communication. 1963. MD computing : computers in medical practice. 1997;14:306–17. [PubMed] [Google Scholar]
- 32.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nature methods. 2010;7:335–6. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome biology. 2011;12:R60. doi: 10.1186/gb-2011-12-6-r60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clifford RJ, Milillo M, Prestwood J, Quintero R, Zurawski DV, Kwak YI, et al. Detection of bacterial 16S rRNA and identification of four clinically important bacteria by real-time PCR. PLoS One. 2012;7:e48558. doi: 10.1371/journal.pone.0048558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saha P, Heagerty PJ. Time-dependent predictive accuracy in the presence of competing risks. Biometrics. 2010;66:999–1011. doi: 10.1111/j.1541-0420.2009.01375.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moellering RC., Jr Pharmacokinetics of vancomycin. The Journal of antimicrobial chemotherapy. 1984;14(Suppl D):43–52. doi: 10.1093/jac/14.suppl_d.43. [DOI] [PubMed] [Google Scholar]
- 37.Ahmad T, Fiuzat M, Neely B, Neely ML, Pencina MJ, Kraus WE, et al. Biomarkers of myocardial stress and fibrosis as predictors of mode of death in patients with chronic heart failure. JACC Heart failure. 2014;2:260–8. doi: 10.1016/j.jchf.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu C, Finegold SM, Song Y, Lawson PA. Reclassification of Clostridium coccoides, Ruminococcus hansenii, Ruminococcus hydrogenotrophicus, Ruminococcus luti, Ruminococcus productus and Ruminococcus schinkii as Blautia coccoides gen. nov., comb. nov., Blautia hansenii comb. nov., Blautia hydrogenotrophica comb. nov., Blautia luti comb. nov., Blautia producta comb. nov., Blautia schinkii comb. nov. and description of Blautia wexlerae sp. nov., isolated from human faeces. International journal of systematic and evolutionary microbiology. 2008;58:1896–902. doi: 10.1099/ijs.0.65208-0. [DOI] [PubMed] [Google Scholar]
- 39.Park SK, Kim MS, Bae JW. Blautia faecis sp. nov., isolated from human faeces. International journal of systematic and evolutionary microbiology. 2013;63:599–603. doi: 10.1099/ijs.0.036541-0. [DOI] [PubMed] [Google Scholar]
- 40.Holler E, Butzhammer P, Schmid K, Hundsrucker C, Koestler J, Peter K, et al. Metagenomic Analysis of the Stool Microbiome in Patients Receiving Allogeneic Stem Cell Transplantation: Loss of Diversity Is Associated with Use of Systemic Antibiotics and More Pronounced in Gastrointestinal Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2014;20:640–5. doi: 10.1016/j.bbmt.2014.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gerbitz A, Schultz M, Wilke A, Linde HJ, Scholmerich J, Andreesen R, et al. Probiotic effects on experimental graft-versus-host disease: let them eat yogurt. Blood. 2004;103:4365–7. doi: 10.1182/blood-2003-11-3769. [DOI] [PubMed] [Google Scholar]
- 42.Jenq RR, Ubeda C, Taur Y, Menezes CC, Khanin R, Dudakov JA, et al. Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J Exp Med. 2012;209:903–11. doi: 10.1084/jem.20112408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–5. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 44.Weisdorf D, Hakke R, Blazar B, Miller W, McGlave P, Ramsay N, et al. Risk factors for acute graft-versus-host disease in histocompatible donor bone marrow transplantation. Transplantation. 1991;51:1197–203. doi: 10.1097/00007890-199106000-00010. [DOI] [PubMed] [Google Scholar]
- 45.Jagasia M, Arora M, Flowers ME, Chao NJ, McCarthy PL, Cutler CS, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119:296–307. doi: 10.1182/blood-2011-06-364265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hahn T, McCarthy PL, Jr, Zhang MJ, Wang D, Arora M, Frangoul H, et al. Risk factors for acute graft-versus-host disease after human leukocyte antigen-identical sibling transplants for adults with leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008;26:5728–34. doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.MacMillan ML, DeFor TE, Weisdorf DJ. What predicts high risk acute graft-versus-host disease (GVHD) at onset?: identification of those at highest risk by a novel acute GVHD risk score. British journal of haematology. 2012;157:732–41. doi: 10.1111/j.1365-2141.2012.09114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vander Lugt MT, Braun TM, Hanash S, Ritz J, Ho VT, Antin JH, et al. ST2 as a marker for risk of therapy-resistant graft-versus-host disease and death. N Engl J Med. 2013;369:529–39. doi: 10.1056/NEJMoa1213299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W, Liu F, Ling Z, Tong X, Xiang C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One. 2012;7:e39743. doi: 10.1371/journal.pone.0039743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bajaj JS, Hylemon PB, Ridlon JM, Heuman DM, Daita K, White MB, et al. Colonic mucosal microbiome differs from stool microbiome in cirrhosis and hepatic encephalopathy and is linked to cognition and inflammation. American journal of physiology Gastrointestinal and liver physiology. 2012;303:G675–85. doi: 10.1152/ajpgi.00152.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Malard F, Szydlo RM, Brissot E, Chevallier P, Guillaume T, Delaunay J, et al. Impact of cyclosporine-A concentration on the incidence of severe acute graft-versus-host disease after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2010;16:28–34. doi: 10.1016/j.bbmt.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 52.Barrell C, Dietzen D, Jin Z, Pinchefsky S, Petrillo K, Satwani P. Reduced-intensity conditioning allogeneic stem cell transplantation in pediatric patients and subsequent supportive care. Oncology nursing forum. 2012;39:E451–8. doi: 10.1188/12.ONF.E451-E458. [DOI] [PubMed] [Google Scholar]
- 53.Russell WR, Gratz SW, Duncan SH, Holtrop G, Ince J, Scobbie L, et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. The American journal of clinical nutrition. 2011;93:1062–72. doi: 10.3945/ajcn.110.002188. [DOI] [PubMed] [Google Scholar]
- 54.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gallo RL, Hooper LV. Epithelial antimicrobial defence of the skin and intestine. Nat Rev Immunol. 2012;12:503–16. doi: 10.1038/nri3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Seguy D, Berthon C, Micol JB, Darre S, Dalle JH, Neuville S, et al. Enteral feeding and early outcomes of patients undergoing allogeneic stem cell transplantation following myeloablative conditioning. Transplantation. 2006;82:835–9. doi: 10.1097/01.tp.0000229419.73428.ff. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. A) A receiver operating characteristic (ROC) curve of the association between Blautia abundance and GVHD-related mortality in patients from cohorts 1 and 2 combined. Asterisk (*) indicates the position of the median (0.05%). B) Absolute Blautia abundance was quantified by normalizing relative Blautia abundance data from 16S deep sequencing by quantitative PCR measurements of total 16S DNA from the 84 patients from combined cohorts 1 and 2 from whom DNA was available. Patients were stratified by median absolute Blautia abundance and analyzed for incidence of GVHD-related mortality.
Figure S2. Flora composition analysis results from patients in Cohorts 1 and 2. Vertical bars indicate composition of fecal microbiota by the indicated taxa. Horizontal bars indicate antibiotic and TPN exposures. Abbreviations: intravenous (IV), oral (PO), piperacillin-tazobactam (pip-tazo), trimethoprim-sulfamethoxazole (tmp-smx), total parenteral nutrition (TPN).
Table S1. Patient information. Abbreviations: TBI (total body irradiation), ATG (anti-thymocyte globulin), CSA (cyclosporine), Tac (tacrolimus), Siro (sirolimus), MTX (methotrexate), MMF (mycophenolate), PBSC (peripheral blood stem cells), aGVHD (acute GVHD), cGVHD (chronic GVHD)
Table S2. Quantification of the association of known risk factors for acute GVHD and Blautia abundance. PBSC = peripheral blood stem cell. BM = bone marrow. PS = performance status. *P-value compares Blautia levels across cord blood and PBSC groups. **Missing, equivocal, or other covariate values not included in p-value calculation.