Abstract
What makes us different from one another? Why does one person jump out of airplanes for fun while another prefers to stay home and read? Why are some babies born with a predisposition to become anxious? Questions about individual differences in temperament have engaged the minds of scientists, psychologists, and philosophers for centuries. Recent technological advances in neuroimaging and genetics provide an unprecedented opportunity to answer these questions. Here we review the literature on the neurobiology of one of the most basic individual differences—the tendency to approach or avoid novelty. This trait, called inhibited temperament, is innate, heritable, and observed across species. Importantly, inhibited temperament also confers risk for psychiatric disease. Here, we provide a comprehensive review of inhibited temperament including neuroimaging and genetic studies in human and non-human primates. We conducted a meta-analysis of neuroimaging findings in inhibited humans that points to alterations in a fronto-limbic-basal ganglia circuit; these findings provide the basis of a model of inhibited temperament neurocircuitry. Lesion and neuroimaging studies in non-human primate models of inhibited temperament highlight roles for the amygdala, hippocampus, orbitofrontal cortex, and dorsal prefrontal cortex. Genetic studies highlight a role for genes that regulate neurotransmitter function, such as the serotonin transporter polymorphisms (5-HTTLPR), as well as genes that regulate stress response, such as corticotropin-releasing hormone (CRH). Together these studies provide a foundation of knowledge about the genetic and neural substrates of this most basic of temperament traits. Future studies using novel imaging methods and genetic approaches promise to expand upon these biological bases of inhibited temperament and inform our understanding of risk for psychiatric disease.
Keywords: Anxious temperament, Behavioral inhibition, Social anxiety disorder, Neuroimaging, Serotonin, Amygdala
1. Introduction
Why are some babies happy while others are fussy? Why do some individuals seek out new experiences while others prefer things that are familiar? What is the nature of these differences? Philosophers and scientists have been curious about these individual differences for centuries. The Greeks recognized four different temperaments—the sanguine, choleric, melancholic, and phlegmatic—and in the 5th century B.C.E., Hippocrates proposed that the behavioral differences reflected underlying differences in the bodily fluids of blood, yellow bile, black bile, and phlegm. In the modern era, we continue to be interested in describing how individuals differ and identifying the biological causes of these differences. Recent advances in neuroscience methods have provided an unprecedented ability to examine both the neural and genetic bases of temperament. In this review we will detail and integrate our current understanding of the neurobiological bases of one of the most basic individual differences –the tendency to approach or avoid new people, objects, and experiences.
Temperament is defined as innate individual differences in behavioral and emotional tendencies that appear in infancy and are relatively stable across context and time. In the past fifty years, there have been numerous theories of temperament, with each proposing different constructs to best capture individual differences in emotion and behavior. One of the most consistently included constructs is the tendency to approach or avoid novelty. This trait has been referred to as behavioral inhibition to the unfamiliar (Kagan et al., 1984; Kagan and Moss, 1962), fear and distress to novelty (Rothbart, 1981), and approach/withdrawal (Thomas and Chess, 1977). For this review we will use the general term inhibited temperament. At the extremely inhibited end of the trait are individuals who are shy, quiet, and cautious; on the other extreme are individuals who are outgoing, bold, and risk-seeking. Because novel stimuli are ubiquitous, we propose that how one reacts to new people, objects, and environments forms a person’s basic behavioral pattern for interacting with the world. Finally, approach and avoidance of novelty has a clear behavioral component (approach/avoidance) that can be assessed in other species, providing the translational foundation needed to identify the shared neural and genetic substrates.
Individual differences in responses to novelty are observable very early in life (Calkins et al., 1996; Kagan et al., 1998). The developmental precursor to inhibited temperament—high/low reactivity to novelty—is observed as high motor activity and crying in response to novel olfactory, visual, and auditory stimuli as early as 4 months of age. These differences during infancy persist across early development, with 4-month reactivity predicting inhibited behaviors in early childhood (Calkins et al., 1996; Fox et al., 2001; Kagan et al., 1998). However, the infant measures do not predict inhibited behaviors at 4 years (Fox et al., 2001; Kagan et al., 1988a) or anxiety in adolescents (Chronis-Tuscano et al., 2009), which is not surprising given that both emotions and emotion regulation appear at different stages across development (Rothbart et al., 2000). In contrast, assessments performed between 2 and 4 years of age predict inhibited behaviors between 4 and 11 years of age (Asendorpf, 1994; Kagan et al., 1988b; Scarpa et al., 1995).
Importantly, these childhood differences in temperament also predict long-term psychiatric outcomes. Inhibited children have increased rates of anxiety disorders (Biederman et al., 2001, 1993; Chronis-Tuscano et al., 2009; Clauss and Blackford, 2012; Essex et al., 2010; Hirshfeld-Becker, 2010; Hirshfeld et al., 1992; Schwartz et al., 1999). The increased risk is substantial; in a recent meta-analysis, we found that inhibited temperament was associated with a seven-fold increase in odds and a four-fold increase in risk for developing social anxiety disorder (Blackford and Clauss, 2013; Clauss and Blackford, 2012). Almost half of inhibited young children (43%; 107/246 children) developed social anxiety disorder by adolescence, compared to about 1 in 10 control children (see Figure 1; 13%; 57/446). Not all inhibited children develop anxiety and risk is highest in children who remain highly inhibited throughout childhood (Chronis-Tuscano et al., 2009; Essex et al., 2010). The link between inhibited temperament and anxiety has been most commonly investigated--possibly because anxiety disorders occur quite early in development--however inhibited temperament also confers heightened risk for a broad spectrum of psychopathology, including depression (Caspi et al., 1996; Gladstone et al., 2005; Gladstone and Parker, 2006), substance-use problems (Lahat et al., 2012; Williams et al., 2010), and schizophrenia (Goldberg and Schmidt, 2001; Jetha et al., 2013). As such, we propose that inhibited temperament is one of the strongest predictors of later psychopathology.
Figure 1.
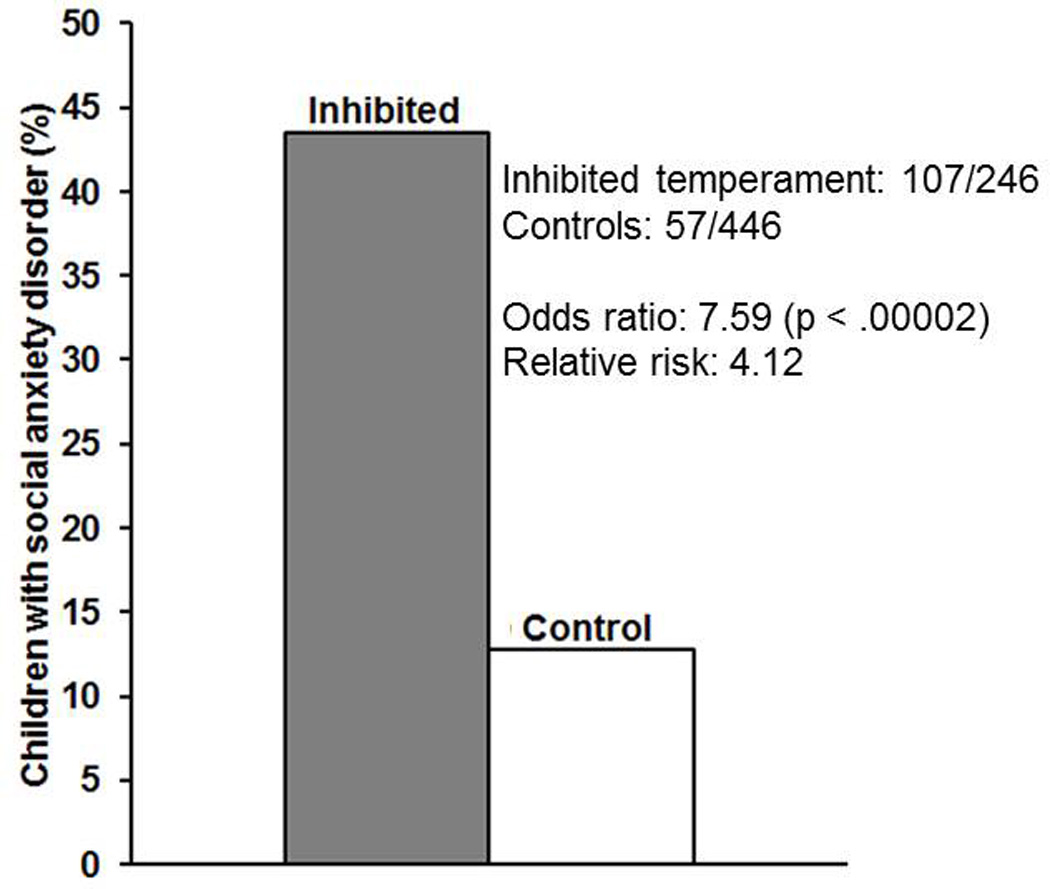
Approximately 43% of children characterized as having an inhibited temperament (107/246) develop social anxiety disorder by mid-adolescence. In contrast, only about 13% of control children (57/446) develop social anxiety disorder by the same age. Data adapted from (Clauss and Blackford, 2012).
1.1. Temperament Measurements and Constructs
Temperament reflects emotional and behavioral tendencies that are relatively stable across time and context. Inhibited temperament has been measured using one or more of the following measurements: behavioral observations, parent report, or self-report. In infants and young children, behavioral assessments of inhibited behavior are most common and developmental stages typically guide both the type of novel stimuli and the behaviors measured; for example, crying or clinging to the mother in the presence of an unfamiliar adult in toddlers (Garcia-Coll et al., 1984), latency to speak to an unfamiliar experimenter in preschool children (Kagan et al., 1998), and shy behavior with peers in older children (Kagan et al., 1988b). However, laboratory measures of temperament are limited to specific time points and contexts, which may not capture the child’s behavior in daily life. Parents, who observe their children across time and in many contexts, have advantages in reporting on their child’s behavior, motivating most researchers to collect parent information in addition to behavioral measures (Chronis-Tuscano et al., 2009; Garcia-Coll et al., 1984; Kagan and Snidman, 2004). Parent report measures typically show moderate correlations with behavioral measures, although these correlations are much higher for children in extreme temperament groups (Bishop et al., 2003; Garcia-Coll et al., 1984), and at least one study found that parent report of inhibition, but not behavior, predicted development of social anxiety disorder in adolescents (Chronis-Tuscano et al., 2009). Most longitudinal studies characterize temperament using composite measures that include behavior and parent ratings across multiple times points (Bar-Haim et al., 2009; Essex et al., 2010; Guyer et al., 2006; Hardee et al., 2013; Helfinstein et al., 2011; Jarcho et al., 2014, 2013; Lahat et al., 2012; Pérez-Edgar et al., 2007). Many of the children in these longitudinal studies are now adolescents or young adults and are being studied using neuroimaging methods. While these current studies have limited ability to address the role of development because inhibition was not assessed again at later developmental stages, future studies using longitudinal imaging methods will be critical for addressing fundamental topics in the field (see section 4).
An alternative approach is to identify adolescents and young adults who are currently inhibited. In this age group, self-report measures are most common, consistent with a long tradition of self-report of personality in adults. Two groups have developed questionnaires to assess current inhibition and a retrospective self-report of childhood inhibition (Gladstone and Parker, 2005; Reznick et al., 1992). To mitigate concerns about self-report bias, the questionnaires use objective questions about behaviors. All of these measures have strong psychometric properties (Gladstone and Parker, 2005; Reznick et al., 1992), but bias due to retrospective distortions cannot be completely ruled out. Our lab is specifically interested in the adolescents and young adults who are stably inhibited; therefore we study individuals who are currently inhibited and also report being inhibited as a child, based on evidence that children who remain inhibited through development are at highest risk for developing psychopathology (Chronis-Tuscano et al., 2009; Essex et al., 2010; Hirshfeld et al., 1992). This approach has the benefit of providing sufficiently large samples of inhibited individuals without the time, expense, and attrition of a longitudinal study, although it carries the limitation of reporter bias. Studying this group also provides a unique opportunity to compare inhibited young adults with and without an anxiety disorder to inform our understanding of risk and resilience in this high-risk group.
Throughout this review, we have included studies which have used both approaches for recruiting individuals with an inhibited temperament—longitudinal studies of infants and young children and cohort studies of currently inhibited young adults who were inhibited as children. As detailed above, each of these methods have advantages and disadvantages. Here, we integrate and combine across methods and species to identify and highlight converging evidence across physiology, neurobiology, and genetics. Another important point is that most studies compare individuals with an inhibited temperament to those with an uninhibited temperament. while this extreme groups approach provides statistical power, it does raise questions about whether the uninhibited control group is the same as a standard “healthy control group”, since uninhibited children are at risk for developing externalizing disorders (Hirshfeld-Becker et al., 2007, 2003, 2002). To understand the potential impact of using extreme temperament groups, we have recently examined two different datasets. First, we have recruited a group of “average” 8–10 year old children to compare to inhibited and uninhibited children, and found that brain function of the average children was statistically similar to the uninhibited children (Clauss and Blackford, unpublished findings). Second, we compared temperament scores from a large group of healthy controls recruited for a neuroimaging study with adults recruited on the basis of extreme temperament and found that 52% of the healthy controls were extremely uninhibited (Blackford and Heckers, unpublished finding), suggesting that many typical healthy controls recruited for research are in fact, uninhibited. More research is needed to understand how inhibited temperament compares with "average" temperament on measures of neurobiology and behavior.
Another issue in the field is the conceptualization of the basic trait of approach/avoidance of novelty. The studies in this review encompass a range of terms including inhibited temperament, behavioral inhibition, high reactivity, temperamental reactivity, temperamental shyness, shyness, and social reticence. These differences in naming convention are related to both measurement—for example, measures of inhibition and reactivity usually include responses to both social and non-social stimuli, whereas shyness and social reticence measure only responses to social stimuli—and to developmental differences—for example, reactivity is the most measured behavior in pre-ambulatory infants, whereas behavioral approach/avoidance is readily observable in ambulatory toddlers and children. We use the general term “inhibited temperament” to represent the broad construct and view each of the specific terms as reflecting this broader trait. Although some take a more narrow approach, we believe there is merit in combining findings across these constructs; converging findings observed across labs provides a strong validation and moves our field forward.
2. Neural Basis of Inhibited Temperament
Are there underlying differences in neurobiology which produce these early behavioral differences? Initial attempts to characterize physiological differences between inhibited and uninhibited children led to the discoveries that inhibited children have a specific physiological profile characterized by heightened arousal and stress responses (Garcia-Coll et al., 1984; Kagan et al., 1988a, 1987; Marshall and Stevenson-Hinde, 1998; Schmidt et al., 1999) and elevated baseline and stress-related cortisol (Kagan et al., 1988b, 1987; Pérez-Edgar et al., 2008; Schmidt et al., 1997). Given alterations in the sympathetic response and cortisol release, inhibited temperament likely has a neural basis.
In the 1980s Kagan proposed that inhibited and uninhibited temperament groups differed in reactivity of limbic brain regions, including the amygdala and hypothalamus, (1988a; 2004, 1991). The amygdala and hypothalamus project to the sympathetic nervous system, hypothalamic-pituitary-adrenal(HPA) axis, and skeletal muscle system (via projections from the reticular formation), where physiological and motor differences in inhibited temperament have been observed. We and others have begun explorations of the neural and genetic bases of inhibited temperament. Our field has made significant strides in elucidating the role of brain function, brain structure, and genes on this fundamental temperament trait. Here, we review the neuroimaging, electroencephalogram (EEG), and genetic findings from studies in humans with an inhibited temperament. We combine findings from human fMRI studies using a coordinate-based meta-analysis. We also review lesion, neuroimaging, and genetic studies from non-human primates with the analogous anxious temperament. We integrate these findings and discuss how the underlying neurobiology may contribute to risk for psychiatric disease, and conclude with future directions for the field.
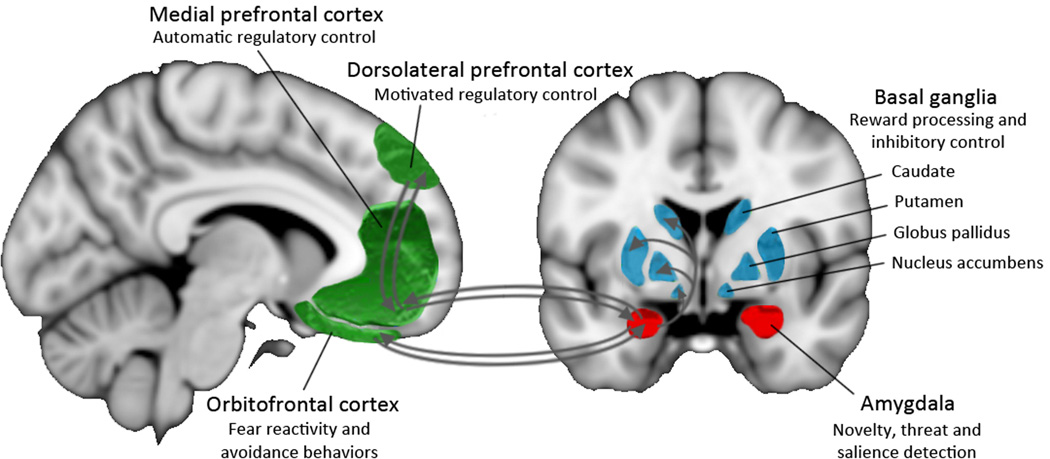
To provide the reader with a background, here we describe the brain regions that have been implicated in human and non-human primate studies of inhibited temperament. These regions, shown in Figure 2, include the amygdala, multiple regions of the prefrontal cortex (orbitofrontal cortex, dorsolateral prefrontal cortex, and dorsal anterior cingulate cortex), and multiple regions of the basal ganglia (caudate, putamen, globus pallidus, and nucleus accumbens). Each component of this circuit has a unique contribution to the inhibited temperament phenotype. The amygdala rapidly detects novel, fearful, or salient stimuli (Blackford et al., 2010; Davis, 1992; Davis and Whalen, 2001; Whalen, 1998). The amygdala is also centrally connected to many brain regions involved in preparing a response to novel stimuli, with efferent connections to limbic cortex, sensory regions, prefrontal cortex, hypothalamic-pituitary-adrenal axis, and descending motor cortex (Aggleton et al., 1980; Freese and Amaral, 2009; Ghashghaei et al., 2007; Price and Amaral, 1981; Russchen et al., 1985). Amygdala interactions across the brain are linked with fear and anxiety behaviors in animal models (Bocchio and Capogna, 2014; Stujenske et al., 2014) and humans (Admon et al., 2013; Buhle et al., In press; Frank et al., 2014). The PFC plays an important role in emotion regulation and expression, cognitive control, attention, and working memory processes. The PFC has both direct and indirect connections with the amygdala (Carmichael and Price, 1995; Freese and Amaral, 2009; Ghashghaei et al., 2007; Ray and Zald, 2012) and can inhibit amygdala responses (Quirk et al., 2003; Rosenkranz and Grace, 2002). Finally, The basal ganglia are involved in reward processing (Haber and Knutson, 2009; Nestor et al., 2010; Pizzagalli, 2014; Treadway and Zald, 2013; Volkow et al., 2013) and cognitive functioning, including inhibitory control(Leisman et al., 2014). Importantly, the amygdala, prefrontal cortex, and basal ganglia are structurally and functionally interconnected; therefore, it is likely that these three regions form the basis of a neural circuit subserving inhibited temperament (see section 2.4.).
Figure 2.
Inhibited temperament is associated with alterations in a complex neurocircuit, including the amygdala, basal ganglia, and prefrontal regions.
2.1. Human Studies of Brain Function
The first studies of brain function in inhibited temperament used EEG, a readily available non-invasive method for measuring cortical activity. The EEG findings in inhibited temperament have been well-covered by several recent reviews (i.e. Fox, 2010; Fox et al., 2005a; Henderson et al., 2014; Kagan et al., 2007; Kagan and Snidman, 2004; Lahat et al., 2011); therefore, we will only briefly summarize those findings here. The early EEG studies in inhibited temperament focused on frontal lobe asymmetry, given a link between right frontal lobe asymmetry and negative affect and avoidance behaviors (Tomarken et al., 1992, 1990). Right EEG asymmetry was observed in both high reactive infants (Calkins et al., 1996) and in inhibited children (Schmidt et al., 1999). Furthermore, greater right frontal asymmetry at 9 months was associated with children who stayed inhibited from 9 – 48 months (Fox et al., 2001). Together these studies show an association between inhibited temperament and a measure of EEG frontal lobe activity during a rest (“non-task”) state. Later studies used event-related potential (ERP), a measure of cortical activity in response to a stimulus, which is used to test for automatic and voluntary attention (Luck et al., 2000). The earliest ERP study in inhibited temperament tested for differences in auditory novelty detection, as measured by mismatch negativity (MMN; Marshall et al., 2009; Reeb-Sutherland et al., 2009). Infants with an inhibited temperament had larger slow wave ERPs to novel stimuli in regions associated with the orienting network, suggesting that inhibited children have a more sensitive orienting network (Marshall et al., 2009). Inhibited children also had enhanced sensitivity to novelty that was in turn associated heightened risk for anxiety disorders (Reeb-Sutherland et al., 2009). Later ERP studies focused on cognitive control and response to incorrect choices (error-related negativity; ERN). Inhibited children have greater response monitoring and ERN (McDermott et al., 2009) and a follow-up study showed that higher ERN predicted later anxiety disorders and social anxiety symptoms (Lahat et al., 2014). Two recent studies found that among inhibited children, those with greater N2 amplitude, which is associated with more attentional control, had more social anxiety symptoms (Henderson, 2010; Lamm et al., 2014). Lamm and colleagues (2014) found that the relationship between inhibited temperament and social anxiety symptoms was moderated by increased activity of the dorsolateral prefrontal cortex (dlPFC) and anterior cingulate cortex (ACC). The prefrontal cortex plays a key role in cognitive control and the flexible allocation of attention (Ochsner and Gross, 2005); the function of the prefrontal cortex is likely critical for the development of anxiety disorders in inhibited children.
While EEG methods provided important early evidence for neural alterations in inhibited temperament, the low spatial resolution and lack of signal from subcortical regions precluded the study of alterations in the amygdala and hypothalamus. Magnetic resonance imaging (MRI), which provides both excellent spatial resolution and whole brain coverage, quickly became the method of choice for studying neural function in inhibited temperament. However, MRI is not without limitations; for example, scanning young children can be challenging. Thus, while the majority of the EEG studies were performed on young children, the vast majority of the MRI studies have been performed on adults and to date, no neuroimaging study findings have been published on children younger than 13 (although we have recently completed a study of inhibited 8–10 year olds). The first fMRI study of inhibited temperament (Schwartz et al., 2003a) confirmed that the amygdala is hyperactive in inhibited temperament and subsequent studies have clarified the nature of amygdala hyperactivity. The past decade of neuroimaging studies has also broadened our perspective on the neural substrates of inhibited temperament, from a narrow focus on the amygdala, to a broader focus on other brain regions with recognition of the importance of connectivity between brain regions and neural circuits.
2.1.1. Amygdala and Hippocampus
In the first neuroimaging study in inhibited temperament, Schwartz and colleagues (2003a) examined brain responses to novel faces in young adults who had participated in a longitudinal study of inhibited temperament as toddlers (Garcia-Coll et al., 1984). The young adults were first familiarized to one set of faces and were shown groups of novel or familiar faces. The inhibited group had a selective increase in amygdala activation to novel faces. In contrast, the uninhibited group showed similar responses to both face types (Schwartz et al., 2003a). This seminal study suggested that the early childhood temperament had a lasting biological profile that could be observed 20 years later, and provided initial evidence for the amygdala’s involvement in inhibited temperament. Following this finding, Blackford and colleagues (2009) tested for temperament differences in the temporal dynamics—the latency, duration, and peak—of the amygdala’s responses to novel and familiar faces in inhibited young adults. Inhibited young adults showed a faster amygdala response to novel faces and a longer amygdala response (duration) to both novel and familiar faces, but no differences in peak response. The faster latency in inhibited individuals suggests a lower threshold for detecting or responding to novel stimuli, while the longer duration may reflect either failure of inhibitory responses to suppress the response or a failure of the amygdala to habituate.
In a subsequent study, Blackford and colleagues (2011) examined the magnitude of amygdala responses to novel and familiar faces in inhibited and uninhibited young adults in a sample twice the size of the two previous studies. Consistent with findings that the human amygdala responds to novelty (Blackford et al., 2010; Schwartz et al., 2003b), novel faces produced amygdala activation in uninhibited individuals whereas familiar faces did not. In contrast, the inhibited individuals showed heightened amygdala activation to both the novel and familiar faces, which suggested that the familiar faces may not become familiar, at least to the amygdala. To examine this possibility, Blackford and colleagues (2013) tested for temperament differences in amygdala habituation to faces during the familiarization period. The uninhibited group initially showed a robust amygdala response to novel faces that declined rapidly with repeated presentations, consistent with evidence that the amygdala rapidly habituates to repeated stimuli (Breiter et al., 1996; Pedreira et al., 2010). In the inhibited group, there was a robust amygdala response to the initial presentation of faces; however, the amygdala failed to habituate. Around the same time, Schwartz and colleagues (2012) reported that young adults who were highly reactive infants also showed a failure of amygdala habituation and a heightened amygdala response to novel stimuli, although their finding was specific to males. In another study, Pérez-Edgar examined the impact of attention on amygdala response during emotional face viewing in adolescents with a history of inhibition (Pérez-Edgar et al., 2007). Inhibited adolescents showed heightened amygdala activation to all faces (regardless of emotion) when attention was directed internally (e.g., “How afraid are you of this face?”), but not during directed attention or passive viewing.
In contrast to the number of studies of the amygdala, the hippocampus, another brain region in the medial temporal lobe, has been largely ignored in human imaging studies of inhibited temperament. The hippocampus has a prominent role in memory and is sensitive to the effects of stress. Two findings from our lab suggest that hippocampal function is altered in inhibited temperament. First, in our study of habituation during familiarization to faces described above, we observed a similar failure to habituate in the hippocampus of inhibited individuals. In a second study we examined effects of child maltreatment on brain function in inhibited adults (Edmiston and Blackford, 2013), based on evidence of hippocampal sensitivity to stress, heightened amygdala and hippocampal activation to faces in adults with a history of maltreatment (Maheu et al., 2010; van Harmelen et al., 2012) and heightened sensitivity to environmental effects in inhibited individuals (Aron and Aron, 1997; Hofmann and Bitran, 2007). We found that history of child maltreatment, measured by the Childhood Trauma Questionnaire (Bernstein et al., 1994), positively correlated with activation to novel faces in the hippocampus and that the correlation was strongest in those that developed an anxiety disorder. Interestingly, there was no correlation between childhood maltreatment and amygdala response to faces, which may reflect the generally heightened amygdala response to faces in inhibited temperament that is not further moderated by negative events. Increased sensitivity to environmental stressors, such as childhood maltreatment and negative parenting styles (Degnan et al., 2010; Williams et al., 2009) may impact normative development in inhibited children and confer additional risk for developing psychiatric disease.
2.1.2. Prefrontal Cortex
The initial fMRI studies discussed above were crucial for determining the importance of the amygdala in inhibited temperament. However, the amygdala does not operate in isolation; for example, observed temperament differences in amygdala activation may reflect either hyperactivity of the amygdala or reduced suppression by inhibitory regions. Therefore it is critical to also investigate brain regions that modulate amygdala responses, such as the prefrontal cortex. EEG studies demonstrated that inhibited temperament is associated with alterations in attention and prefrontal cortex activity (Henderson, 2010; Lahat et al., 2014; Lamm et al., 2014; McDermott et al., 2009); however, EEG has poor cortical localization. FMRI tasks that specifically probe PFC function—such as preparation for an aversive event, emotion regulation, or attentional control—can be used to test for PFC alterations.
One important role of the PFC is anticipatory processing and planning for an upcoming event, which is mediated by two regions of the PFC, the ACC and dlPFC (Grupe and Nitschke, 2013). In the first fMRI investigation of prefrontal cortical function in inhibited adults, Clauss and colleagues (2011) manipulated the expectation of viewing fear faces between groups; half of the participants knew that fear faces would be shown and thus had an opportunity to prepare for viewing the faces, whereas the other half had no prior expectation. Significant effects of expectation by temperament were observed in two prefrontal control regions, the dorsal ACC and dlPFC, and the amygdala. Consistent with findings of heightened PFC activation during anticipation (Nitschke et al., 2006), when forewarned to expect fear faces, the uninhibited participants had increased activation in the PFC and decreased activation in the amygdala, relative to those who weren’t forewarned. In contrast, inhibited individuals who were forewarned had reduced PFC activation and increased amygdala activation compared to those who weren’t forewarned. These findings suggest that inhibited individuals fail to respond adaptively to an upcoming aversive event, similar to patients with an anxiety disorder (Grupe and Nitschke, 2013), and may in fact have a heightened response or sensitization to the upcoming event.
To explicitly test for temperament differences in anticipatory processing (Clauss et al., 2014a), we examined brain activation during a cued anticipation task in a new sample. In this task and one cue signaled an upcoming fear face and another cue signaled an upcoming neutral face. Importantly, the time between the cue and face was sufficiently long to provide a reliable measure of anticipation. We hypothesized that inhibited adults would have less anticipatory PFC activation than uninhibited adults, and were surprised to discover that inhibited temperament was associated with increased PFC activation during anticipation of viewing fear faces. Additional analyses revealed that in inhibited individuals, stronger PFC activation during anticipation predicted both lower social anxiety and better coping skills, demonstrating that inhibited individuals who can effectively engage prefrontal cortical resources during fear anticipation are resilient to developing social anxiety. This finding has important implications for prevention of psychopathology in high-risk inhibited children. Adolescence is both a peak time for the onset of social anxiety disorder and a time of rapid PFC development; therefore, strategies that enhance PFC engagement during anticipation provide a promising strategy for enhancing resilience to psychiatric disorders.
Another important function of the PFC is cognitive control, which has been investigated in inhibited temperament in two recent studies. First, Jarcho and colleagues (2013) examined emotional conflict in young adults with a history of inhibited temperament using a variant of the Stroop task with emotional facial expressions and congruent or incongruent emotional labels. During conflict detection—incongruent relative to congruent faces/labels—inhibited individuals had greater activation in the dorsomedial PFC (dmPFC). Interestingly, presence of lifetime psychopathology (across both temperament groups) was associated with less dlPFC activation. Next, in a largely overlapping sample, Jarcho and colleagues (2014) examined attentional control using a variant of the Stroop task with emotional expressions and congruent or incongruent gender labels. When viewing fear faces with incongruent labels, inhibited individuals had greater activation of the dmPFC, dlPFC, ACC, suggesting that inhibited individuals need higher levels of PFC activation to enlist attentional control. In this task, brain function was not modulated by psychopathology.
Together, these findings suggest that inhibited individuals engage the PFC in an attempt to dampen amygdala reactivity. These findings also highlight a role for PFC activation in the development of psychopathology. Inhibited subjects who are able to flexibly engage the prefrontal cortex during emotion regulation, have fewer anxiety symptoms or disorders, consistent with behavioral and EEG data in inhibited children that show that increased risk for anxiety is associated with stronger inhibitory control, heightened response monitoring, and deficits in attention shifting (Cavanagh and Shackman, 2014; McDermott et al., 2009; White et al., 2011).
2.1.3. Basal Ganglia
Although early studies provide compelling evidence that inhibited individuals have heightened neural reactivity to novel or threatening stimuli, several studies have also investigated the opposite side of the valence spectrum—response to positive stimuli which is mediated by the basal ganglia. In fMRI studies, the neural basis of response to reward and loss of potential reward is commonly investigated using the monetary incentive delay task. In this task, participants are taught to associate different cues with receiving money, losing money, or no effect. The outcome (receiving money or avoiding losing money) is contingent on pressing a button within a narrow time window when a target is detected. In an initial study, adolescents with a history of inhibited temperament had increased basal ganglia activity (caudate, putamen, and nucleus accumbens; Guyer et al., 2006), during anticipation of both loss and reward. A second study clarified that the observed increased basal ganglia activity was specific to conditions where loss or reward was contingent on the participant’s behavioral response (Bar-Haim et al., 2009). A third study found heightened caudate activation in inhibited individuals during notification of a “wrong” response and subsequent loss of reward (Helfinstein et al., 2011). Therefore, although these tasks were initially designed to probe reward processing, the observed basal ganglia hyperactivity in inhibited temperament more likely reflects performance monitoring and sensitivity to feedback. In support of this hypothesis, in a recent study, inhibited children had increased activation of the putamen after response selection and during anticipation of positive feedback from peers (Guyer et al., 2014). However, it should be noted that one recent study reported that basal ganglia (caudate, putamen, and nucleus accumbens) activation to reward cues moderated substance use, not abuse, in young adults with a history of inhibited temperament (Lahat et al., 2012). Additional studies will be needed to clarify the role of the basal ganglia in inhibited temperament and to determine whether the basal ganglia are involved in the heightened risk for substance abuse.
More recently, temperament differences in basal ganglia function have been observed during cognitive control tasks that traditionally engage the PFC. First, in their emotional conflict task, Jarcho and colleagues (2013) found that inhibited individuals had increased activation in the putamen during emotional conflict adaptation. Second, in their attentional control task, Jarcho and colleagues (2014) found that inhibited individuals had increased activation during attentional control to fear stimuli in the caudate and ventral striatum, and decreased activation during attention control in the happy condition in the putamen. While the basal ganglia have traditionally been ascribed a role in motor control and reward processing, these subcortical brain regions are strongly connected to multiple PFC regions (Di Martino et al., 2008; Haber, 2003; Lehéricy et al., 2004), forming fronto-striatal loops that subserve attention, learning, executive control and cognition (Leisman et al., 2014). Thus, increased activation of basal ganglia regions during both reward anticipation and cognitive control tasks may reflect inhibitory control functions of both the basal ganglia and PFC.
2.1.4. Cerebellum
Emerging evidence suggests that alterations in cerebellar activity may also contribute to inhibited temperament. Blackford et al. (2009) first reported cerebellar hyperactivity in inhibited young adults during face viewing. This finding has been replicated and extended by another group who found that highly inhibited adults had heightened cerebellar activation to both novel faces and novel scenes (Caulfield and Servatius, 2013). Finally, in an independent sample we have replicated our original finding, showing sustained activation to familiar faces in the cerebellum, similar to the pattern observed in the amygdala (Blackford et al., 2011). These findings may be driven by structural and functional connectivity with the amygdala (Clauss et al., 2014b; Roy et al., 2009); however, the cerebellum may also make a distinct contribution to inhibited temperament through its role in learning and attention. The cerebellum, Latin for “little brain”, has received the most attention for its role in motor function. However, similar to emerging understanding of the basal ganglia, it is now recognized that the cerebellum contributes broadly to cognition and emotion including learning, attention, emotion processing, and emotion control. A recent review provides intriguing evidence that the alterations in cerebellar function confers risk for anxiety disorders through enhanced avoidance learning (Caulfield and Servatius, 2013). Thus, alterations in cerebellar function observed in inhibited temperament may be one neurobiological path towards heightened risk for anxiety.
2.1.5. Functional Connectivity
To date, neuroimaging studies of inhibited temperament have clearly demonstrated differences in brain function in specific regions. However, connections between these regions have remained largely unstudied. In order to fully understand the inhibited brain, it is critical to investigate neural circuits because activity in one brain region influences activity in many other regions. Functional MRI can be used to assess functional connectivity, a measure of the correlation or coherence of two brain regions during a state of rest (i.e., resting state connectivity or intrinsic connectivity) or during a task (i.e., task-based connectivity). Resting state connectivity assesses intrinsic networks that exist in the absence of a task. Importantly, intrinsic networks are reliable over time and across individuals (Biswal et al., 2010), can reflect underlying structural connectivity (Shen et al., 2012), but can also reflect polysynaptic connectivity (Buckner et al., 2013; Honey et al., 2009; Vincent et al., 2007). Three studies have tested for temperament differences in functional connectivity; one examined resting state connectivity and the other two examined task-based connectivity.
Temperament differences in intrinsic, or resting state, connectivity were examined by Blackford and colleagues (2014). Because the amygdala is actually comprised of multiple subnuclei that each have distinct connectivity patterns (Roy et al., 2009), we examined connectivity separately for the basolateral amygdala, centromedial amygdala, and superficial amygdala (Amunts et al., 2005). Across all three amygdala subnuclei, a pattern emerged; inhibited young adults had reduced amygdala connectivity with regions that regulate the amygdala, such as the PFC, and with regions that have bidirectional amygdala connections, such as the hippocampus, visual cortex, and insula. To determine if these temperament differences would be observed in broader networks, we also examined several resting state networks. Consistent with the amygdala findings, inhibited individuals had reduced connectivity between regions of the default mode network and dorsal attention network. However, in two of the networks—the executive control network and salience network—a different pattern emerged; inhibited individuals had increased connectivity between nodes within each network. Reduced amygdala connectivity may contribute to the amygdala habituation failure observed in prior studies (Blackford et al., 2013; Schwartz et al., 2012) and may underlie avoidance behaviors. Increased connectivity in the executive control and salience networks may reflect heightened inhibitory control, the ability to activate or inhibit and override emotional responses, which is associated with social withdrawal and social anxiety in inhibited children (McDermott et al., 2009; White et al., 2011).
Hardee and colleagues (2013) examined functional connectivity during an attentional bias task in young adults with or without a history of inhibited temperament. The dotprobe task measures attention bias to threat stimuli by showing a pair of faces (a neutral face and an angry face, a neutral face and a happy face, or two neutral faces), followed by a dot stimulus in the position of one of the faces. Speed in responding to the dots in the position of the neutral or emotional face is measured; differences in reaction time when the dot is in the location of the emotional face, relative to neutral face, indexes threat bias. During the angry trials, the inhibited subjects had negative connectivity between the amygdala and both the insula and the dlPFC; whereas during neutral trials, inhibited subjects had positive connectivity between those regions. Furthermore, decreased amygdala-insula connectivity mediated the relationship between increased childhood inhibition and increased internalizing symptoms in adulthood in inhibited subjects. These differences were limited to the angry trials, suggesting a role of emotion in moderating the relationship between temperament and functional connectivity.
Clauss and colleagues (2014a) tested for temperament differences in functional connectivity during a task—anticipation of viewing fear faces—in young adults. Inhibited subjects had more negative connectivity in three different circuits during anticipation of viewing fear faces: amygdala and rostral ACC; insula and rostral and dorsal ACC; and insula and dlPFC. In addition to the between group differences, positive connectivity between the rACC and amygdala correlated with both lower social anxiety and higher emotion regulation. Both the insula and rACC are structurally connected to the amygdala, send inputs to the amygdala, and modulate outputs of the amygdala (Carmichael and Price, 1995; Ghashghaei et al., 2007; Mufson et al., 1981). Thus, increased connectivity between the rACC and emotional brain regions, such as the insula and amygdala, likely contribute to resilience against developing social anxiety disorder.
These three connectivity studies show that inhibited individuals have alterations in amygdala-prefrontal connectivity and amygdala-insula connectivity. One critical challenge in the interpretation of connectivity studies is to understand what “negative connectivity” represents: Does it reflect lower or reduced levels of excitatory connectivity or higher levels of inhibitory connectivity? Further research using animal models is needed to answer this question. We can, however, infer that prefrontal cortex modulation of amygdala activity impacts development of anxiety symptoms in inhibited individuals.
2.1.6. Summary of Human Studies of Brain Function
Studies of brain function in inhibited temperament have consistently pointed to alterations in amygdala, with a faster latency, longer duration, higher magnitude, and habituation failure observed in inhibited individuals. Additional findings include elevated responses in the hippocampus, basal ganglia and cerebellum, combined with decreased PFC regulatory control over the amygdala. Together, these findings suggest alterations in a critical limbic-prefrontal-basal ganglia circuit broadly responsible for novelty detection, emotion regulation, and attention, which may govern decision making and behavior through complex inter-regional feedback. Limitations of the previous functional studies included small sample sizes and region of interest approaches; larger studies including whole brain analyses are needed to determine whether other brain regions are involved.
2.2. Meta-Analysis of fMRI Studies of Inhibited Temperament
The findings reviewed above include studies from multiple laboratories using different fMRI tasks and different methods for measuring inhibited temperament. While many studies have found consistent patterns in brain activity in inhibited temperament, differences in methodology make direct comparisons between findings difficult. Meta-analysis provides a method for combining these studies to identify regions commonly activated. Here, we conduct the first meta-analysis of fMRI studies of inhibited temperament in humans.
Studies were selected by searching Web of Knowledge (http://www.webofknowledge.com) for fMRI studies of inhibited temperament available through April 2014. Search terms were: (“inhibited temperament” or “behavioral inhibition” or “behaviorally inhibited”) AND (MRI OR neuroimaging OR “brain activation” OR “brain activity”). We also included studies found by searching reference lists and manuscripts in press. Studies were eligible for inclusion if they included a comparison between individuals with an inhibited temperament and individuals with an average or uninhibited temperament. Studies had to include coordinates of their findings in either Montreal Neurological Institute (MNI) or Talairach space. Two hundred and eighty-nine (289) articles were identified. Of the articles, 98 were excluded for not being an empirical study, 19 were excluded for non-human data, 85 were excluded for not including functional imaging data, 71 were excluded for not including a measure of inhibited temperament, two were excluded for containing only correlations within an inhibited group, and one study was excluded for reporting no group differences in functional activation. Data from 13 studies were included (Bar-Haim et al., 2009; Blackford et al., 2013, 2011, 2009; Clauss et al., 2014a, 2011; Guyer et al., 2006; Helfinstein et al., 2011; Jarcho et al., 2014, 2013; Pérez-Edgar et al., 2007; Schwartz et al., 2012, 2003a). Coordinates were included for the contrast of inhibited temperament group versus control group or the interaction (e.g. temperament × emotion) if reported. Meta-analyses were conducted using GingerALE software (version 2.3.2; http://www.brainmap.org/ale), which uses an activation likelihood estimation (ALE) to test for overlap between foci across studies (Eickhoff et al., 2009). A random-effects model was used. All data were transformed to MNI space using the tal2icbm function within the GingerALE software and peak coordinates were modeled with a 3D Gaussian kernel with a FWHM of 8.5–9.5 (larger FWHM for studies with fewer subjects). Convergence across all studies was calculated as the union of each individual study, with a voxel p-value of .01 and a cluster size of 25 voxels (200 mm3).
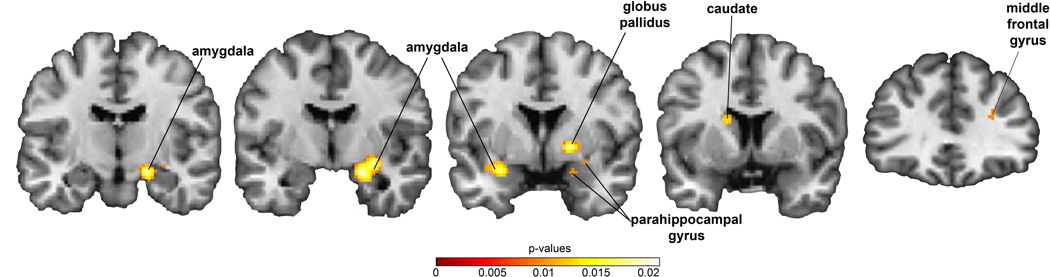
There were four clusters where the inhibited temperament group had significantly greater activation than the uninhibited temperament group. The inhibited group had greater activation in the amygdala, three regions of the basal ganglia (globus pallidus, putamen, and caudate), and the middle frontal gyrus (see Table 1 and Figure 3). There were no regions where the uninhibited group had greater activation. Thus, inhibited temperament was associated with heightened activation in brain regions involved in both novelty/threat processing and reward processing/inhibitory control.
Table 1.
Brain regions activated across studies of inhibited temperament.
| peak voxel | |||||
|---|---|---|---|---|---|
| Region | Volume (mm3) |
Corrected p-value |
x | y | z |
| Amygdala, parahippocampal gyrus, globus pallidus, putamen (R) | 3056 | 0.021008 | 22 | −6 | −16 |
| Amygdala, parahippocampal gyrus, temporal lobe (L) | 808 | 0.016605 | −28 | 4 | −16 |
| Caudate (L) | 320 | 0.016504 | −14 | 10 | 18 |
| Middle frontal gyrus (R) | 128 | 0.010215 | 30 | 36 | 24 |
Note: coordinates in Montreal Neurologic Institute space; L = left; R = right.
Figure 3.
Based on a meta-analysis of 13 functional MRI studies of inhibited temperament, inhibited temperament is associated with increased activity in the bilateral amygdala, parahippocampal gyrus, globus pallidus, caudate, and medial frontal gyrus.
Of note, these three regions—the amygdala, basal ganglia, and PFC—have been proposed to form an interconnected circuit responsible for governing motivated behaviors in healthy adults (Ernst et al., 2006; see: Ernst and Fudge, 2009). In this Triadic Model, approach behaviors are governed by the basal ganglia (reward) while avoidance is governed by the amygdala (fear); approach/avoidance behavior is regulated through complex PFC feedback loops with both of these regions. Balanced regulation of the basal ganglia and amygdala is hypothesized to be important to adaptive behavior in a variety of situations, while imbalances in regulation of the basal ganglia and amygdala are hypothesized to result in excessive reward-seeking or avoidance behavior, impacting both normal development and propensity for psychiatric illness.
It should be noted that most studies included in the meta-analysis used a region of interest approach, although whole brain data were included when available; thus the meta-analysis is inherently limited by the available data and tasks used. Similarly, GingerALE models observed findings to identify common regions of group differences; however, this approach does not include null findings. The studies included come from three different laboratories (Schwartz, Fox, and Blackford) and some of these studies represent overlapping samples of subjects (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011; Jarcho et al., 2014, 2013; Pérez-Edgar et al., 2007) and (Blackford et al., 2013, 2011; Clauss et al., 2011); however, each study used a different task, thus findings represent common brain activation across cognitive processes, subjects, laboratories, and ages. Finally, there was heterogeneity in psychopathology/psychotropic medication use across samples; thus, the findings may represent both inhibited individuals with psychopathology and resilient individuals. In future studies, separating out these two groups will be critical to understanding how inhibited temperament leads to risk for psychopathology.
2.3. Human Studies of Brain Structure
2.3.1. Amygdala
Inhibited temperament is characterized by an avoidance of novelty, which fMRI studies have shown is primarily subserved by increased amygdala activation. However, the underlying cause of increased amygdala activity is unknown. For example, increased amygdala activity may result from more neurons, more synapses, or alterations in neuron function. To determine whether inhibited individuals had differences in amygdala volume, we used three measures (manual tracing, voxel-based morphometry, and surface mapping) to measure amygdala volume in inhibited and uninhibited individual (Clauss et al., 2014b). Inhibited individuals had larger amygdala volume across all three measures, and the volume differences were most prominent in the basolateral subnucleus. Importantly, larger amygdala volume correlated with stronger activation to faces, providing an initial link between structure and function. In rodent models, chronic immobilization stress increases the number of spines on the basolateral subregion (Mitra et al., 2005; Vyas et al., 2002); inhibited adults may be particularly vulnerable to stress or may experience more stress as a result of their social inhibition, suggesting a possible mechanism for increased basolateral amygdala volume in inhibited adults.
In a second study investigating amygdala volume, larger amygdala volumes were found in adolescents who spent more time close to their mother at age 5 during a peer-play task (Hill et al., 2010). Larger amygdala volume has also been associated with traits similar to inhibited temperament, including fearfulness (van der Plas et al., 2010), sensitivity to punishment (Barros-Loscertales et al., 2006), and harm avoidance (Iidaka et al., 2006). Several recent studies suggest that amygdala volume may be related to certain genes associated with anxiety vulnerability (Haaker et al., 2014; Mueller et al., 2013; Stjepanović et al., 2013); however, more studies are needed to replicate these findings. The causes of larger amygdala volumes in inhibited individuals remain unclear, although increased synaptogenesis, increased neuron number, or increased glia number are all likely mechanisms. Studies of amygdala structure in non-human primates can help to shed light onto the findings of increased amygdala volume in humans with inhibited temperament. First, it will be important to test to see if increased amygdala volume is present in monkeys with anxious temperament, and then to examine which cell types might contribute to increased volume in anxious temperament.
2.3.2. Orbitofrontal Cortex
The OFC (or ventral PFC) has also been proposed as a substrate of inhibited temperament (Kalin et al., 2007). Schwartz and colleagues (2010) found that young adults who had been categorized as high-reactive at 4 months of age had thinner left lateral OFC, but thicker right medial OFC. The ventrolateral PFC has inhibitory connections with the amygdala; therefore, thinner ventrolateral PFC may result in less amygdala inhibition and increased amygdala activity. Inhibited temperament, and its precursor high-reactive temperament, are associated with right frontal EEG asymmetry (Davidson and Fox, 1989; McManis et al., 2002; Schmidt et al., 1999; Schmidt and Fox, 1994), which, in non-human primates, was reduced following OFC lesions (Kalin et al., 2007). Interestingly, one study found that inhibited temperament was associated with right asymmetry in OFC thickness (Hill et al., 2010), paralleling previous EEG findings. Thicker right OFC may produce more brain activity on the right side, and thus more EEG activation; however, no studies in inhibited temperament have examined the relationship between EEG asymmetry and structural MRI.
2.3.3. Basal Ganglia
We recently found that inhibited adults have a larger caudate volume (Clauss et al., 2014b). This finding is intriguing in light of functional MRI studies that show inhibited temperament is associated with increased activation of the caudate and other regions of the basal ganglia (Bar-Haim et al., 2009; Guyer et al., 2006; Helfinstein et al., 2011); however, in this study larger caudate volume was an unexpected finding based on an exploratory whole brain analysis, and was not based on an a priori hypothesis. We also found that larger caudate volume correlated with greater caudate activation to faces (Clauss et al., 2014b), consistent with the caudate’s role in emotional processing. However, until these results have been replicated in other studies of inhibited temperament, these findings should be considered preliminary.
2.3.4. Summary of Human Studies of Brain Structure
Structural MRI findings point to alterations in brain regions that mediate response to novelty, attention, and sensitivity to the environment: the amygdala, OFC, and caudate. One caveat of these structural findings is that studies have been limited by relatively small sample sizes and a priori selection of regions of interest. Although both Clauss et al (2014b) and Schwartz and colleagues (2010) also conducted whole brain (or whole cerebrum) analyses, statistical power was limited by small samples. Structural MRI studies in larger samples of inhibited adults, samples of inhibited children, and non-human primates, are needed to advance our knowledge of the structural brain correlates of inhibited temperament.
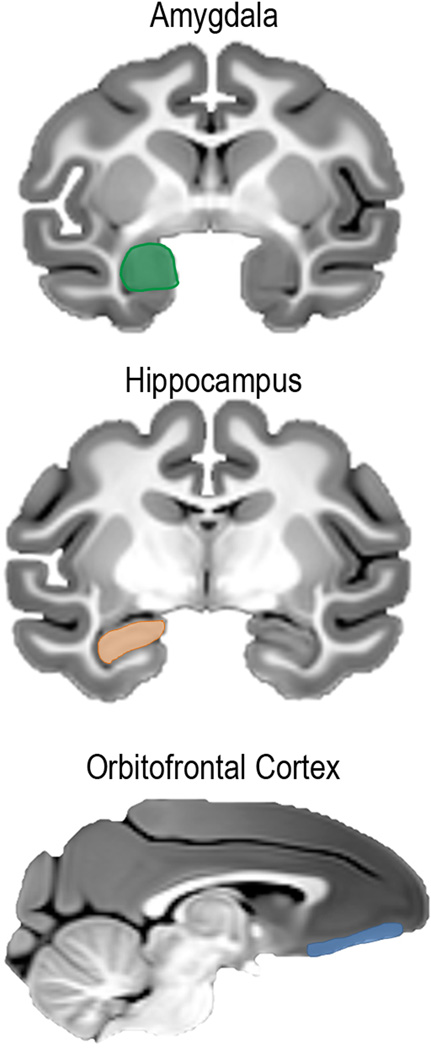
2.5. Non-Human Primate Lesion Studies
Non-human primates provide an excellent model for studying inhibited temperament, and rhesus monkeys in particular are similar to humans in socialization, neuroendocrine responses, and brain structure (Fox and Kalin, 2014; Kalin and Shelton, 2000; Oler et al., In press). One distinct advantage to the use of non-human primates is the ability to test directly for causation by lesioning individual brain regions (Figure 4). Additionally, the most common dimensions of inhibited temperament in young humans—freezing and spontaneous vocalizations—can be readily measured in monkeys (Kalin and Shelton, 1989). As in humans, these measures are stable and reliable over time (Fox et al., 2008; Kalin and Shelton, 2003, 1989; Shackman et al., 2013), providing strong evidence that inhibited temperament is a trait observed across species.
Figure 4.
The effects of individual brain regions on anxious temperament in rhesus monkeys have been demonstrated by lesioning the amygdala, hippocampus, and orbitofrontal cortex.
In non-human primates, inhibited temperament has been coined “anxious temperament” (Kalin and Shelton, 2003). Anxious temperament is measured using behavioral and neuroendocrine assessments that parallel inhibited temperament assessment in humans. Monkeys with an anxious temperament have a complex phenotype that includes decreased cooing, increased freezing, and increased cortisol concentration (Kalin et al., 1998, 2000). Kalin and Shelton (1989) pioneered the use of a behavioral task, known as the human intruder paradigm, to test for individual differences in anxious temperament. The human intruder paradigm includes three conditions which are used to measure response to novelty: the alone in a new cage (ALN) condition, in which monkeys are separated from their cage mates and put into a novel cage; the no eye contact intruder (NEC) condition, in which monkeys are placed in a novel cage and are exposed to a novel human intruder who presents his profile to the without making eye contact and most closely parallels measurement of inhibited temperament in humans; and the stare (ST) condition, in which monkeys are placed in a novel cage and again are exposed to a novel human intruder who stares directly at the monkey (Kalin and Shelton, 2003, 1989; Machado and Bachevalier, 2008). During each of these conditions, behavior and activity of the HPA axis can be measured.
2.5.1. Amygdala Lesions
One way to test if a brain region is critical for inhibited temperament is to lesion that region. Studies in humans have shown that the amygdala is hyperactive in inhibited temperament; however, human studies cannot demonstrate causation through elective amygdala lesions. Several studies, however, have used lesions to examine the role of the amygdala in non-human primate anxious temperament. When the amygdala is lesioned in young adult monkeys, monkeys exhibit less fear of snakes and less submissive behavior to threat from an unfamiliar monkey; however, it should be noted that in this study the lesions did not affect behavior during other types of threat (the NEC and ST conditions; Kalin et al., 2001). Similarly, in a study by another lab, amygdala lesions in young adult monkeys reduced the duration of freezing and reduced cortisol levels in response to a new environment (Machado and Bachevalier, 2008). In contrast to behavioral effects of adult lesions, recent studies have shown that neonatal amygdala lesions in monkeys result in increased freezing during threat (NEC condition; Raper et al., 2013b, 2013c). Typically, monkeys display a modulation of fearful, hostile, and defensive behavior across the human intruder paradigm; however, monkeys with neonatal amygdala lesions displayed similar levels of fearful, hostile, and defensive behavior across the conditions (ALN, NEC, and ST) of the human intruder paradigm at two to four months of age (Raper et al., 2013b, 2013c). Neonatal amygdala lesions also resulted in a flattened diurnal cortisol rhythm in monkeys at 5 months of age (Raper et al., 2013a) and blunted cortisol response to stress in young adult monkeys (Raper et al., 2013c). Finally, neonatal amygdala lesions resulted in significantly less freezing in adulthood (Raper et al., 2013c).
Together, these mixed findings support the notion that developmental processes continue to influence the amygdala throughout childhood and into adolescence. The amygdala undergoes extensive postnatal development in macaques (Chareyron et al., 2012; Payne et al., 2010), and plays a critical role in the development of normative fear, such as fear of strangers, heights, and other dangerous situations (Blackford and Pine, 2012). Therefore, opposing findings may depend on the age at which the amygdala was lesioned may be the result of developmental differences.
Early amygdala lesion findings may also have been influenced by heterogeneity in the extent of the amygdala subnuclei that were lesioned. The amygdala has a complex neurocircuitry. Input from sensory and prefrontal regions enters through the basolateral regions and flows both directly to the CeA and through inhibitory intercalated cells to the CeA (Paré and Smith, 1993; Sah et al., 2003; Tye et al., 2011). Initial amygdala lesion studies used large, nonspecific lesions; however, the amygdala is a heterogeneous structure and is composed of multiple subnuclei, including the CeA, basal nucleus, and lateral nucleus. Of these, the CeA has been proposed to be critical for anxious temperament. The CeA provides the main amygdala output and projects to descending sympathetic, hypothalamic, and brainstem pathways; the CeA may mediate the behavioral effects of amygdala activation and increased sympathetic response (LeDoux et al., 1988; Sah et al., 2003). To examine the role of the CeA specifically in anxious temperament, Kalin and colleagues (2004) performed CeA lesions in early adolescent monkeys. CeA lesions resulted in a reduction of several components of anxious temperament, including reduced freezing and increased cooing across all three conditions (ALN, NEC, and ST) of the human intruder paradigm. CeA lesions also resulted in less fear of snakes, and less activity of the hypothalamic-pituitary axis, including less cortisol, CRF, and ACTH release. These findings suggest that the CeA is critical for the expression of all three components of anxious temperament and bring up an important question: why do CeA specific lesions ameliorate almost all of the anxious temperament phenotype, but entire amygdala lesions only affect freezing and cortisol response? One answer may be that the entire amygdala lesions were incomplete in many monkeys and may not have lesioned the CeA, which is a small region that is located in the dorsal portion of the primate amygdala.
2.5.2. Hippocampus Lesions
The hippocampus has functions in novelty detection, memory, and emotion processing (Knight, 1996; Phelps, 2004; Scoville and Milner, 1957)--which are critical for anxious temperament--and regulates cortisol release. The hippocampus also regulates cortisol release (Herman et al., 2005). To date, one study has examined the effects of hippocampus lesions on anxious temperament. Machado and Bachevalier (2008) found that hippocampal lesions in young adult monkeys increased tense behaviors, such as cooing and locomotion, across all three conditions of the human intruder paradigm and reduced modulation of tense behaviors between the three conditions. In a follow-up study, hippocampal lesions did not have any effect on cortisol concentrations in response to social isolation or at baseline in the monkey’s home cage (Machado and Bachevalier, 2008). Response to conditioned aversive stimuli, such as equipment associated with capture and restraint, and unconditioned aversive stimuli, such as snakes, was not changed by hippocampus lesions (Machado et al., 2009). The hippocampus, like the amygdala, may be important for modulation of anxious temperament behaviors across situations and may be critical for output of cooing behavior, but is likely not necessary for cortisol reactivity to social or non-social novelty. More studies are needed to replicate these findings.
2.5.3. Orbitofrontal Cortex Lesions
The orbitofrontal cortex (OFC) has been proposed as a primary neural substrate of anxious temperament, given its role in fear reactivity and avoidance behavior, as well as its bidirectional connections with the amygdala (Ghashghaei et al., 2007; Ray and Zald, 2012). In adult monkeys, OFC lesions decreased anxious temperament across several measures, including less freezing during the NEC condition, decreased snake fear, and increased left frontal EEG activity (Kalin et al., 2007). OFC lesions did not alter cortisol, ACTH, or CRH responses during baseline, social threat, or non-social threat conditions (Kalin et al., 2007; Machado and Bachevalier, 2008); however, OFC lesions did reduce cortisol levels in response to exposure to a new environment (Machado and Bachevalier, 2008). One study (Izquierdo and Murray, 2004) examined the effects of combined unilateral amygdala and OFC lesions and found that monkeys with combined amygdala and OFC lesions had fewer non-social fear behaviors, but no differences in duration of defensive behavior exhibited in the ST, ALN, or NEC conditions.
One critical issue in OFC lesion studies is the lesion method. Lesions can be created using aspiration, which removes both gray and white matter and may disrupt fibers of passage to and from the amygdala, or using ibotenic acid, which damages only gray matter and leaves fibers of passage intact (Jarrard, 1989). Kalin and colleagues (2007) used aspiration lesions and Machado and colleagues (2009) used a combination of aspiration and ibotenic acid lesions in their study. The effects of OFC aspiration lesions may be due to disruption of fibers of passage, in addition to disruption of the OFC gray matter. These preliminary studies suggest that OFC lesions may disrupt the maintenance and production of anxious temperament behavior, including mediating freezing, social fear, and EEG asymmetry findings.
The OFC is a heterogeneous structure and can be functionally divided in the anterior/posterior direction or in the medial/lateral direction (Bachevalier et al., 2011; Kringelbach, 2004).The anterior OFC has functions in the representation of complex rewards and reinforcers, whereas the posterior OFC may represent less complex reinforcers. The posterior OFC also has bidirectional connections with the amygdala, some of which terminate in the inhibitory intercalated cell masses of the amygdala; activation of intercalated amygdala cells by the posterior OFC may result in activation of descending hypothalamic and brainstem pathways (Barbas, 2007). The medial OFC has connections to autonomic regions and has the strongest connections with the hippocampus and ACC. The medial OFC appears to mediate selection of rewarded or correct responses (Bachevalier et al., 2011; Elliott et al., 2000). The lateral OFC has connections with sensory regions and medial temporal lobe structures, such as the amygdala and hippocampus. The lateral OFC is involved in responding to changing reward contingencies and suppressing responses to previously rewarded stimuli (Bachevalier et al., 2011; Elliott et al., 2000). The OFC continues to develop throughout childhood (Gogtay et al., 2004), thus timing of lesion may be important. In one study, neonatal OFC lesions resulted in less modulation of aggressive behaviors across the human intruder paradigm and an increase in emotion reactivity (Bachevalier et al., 2011). Future studies should test the specificity of the findings for specific OFC regions and for developmental effects based on age at lesion.
2.5.4. Summary of Non-human Primate Lesion Studies
Anxious temperament, like its human counterpart inhibited temperament, is a complex phenotype and is likely the result of a distributed neural circuit. Lesions in one node of the circuit may be insufficient to change all components of anxious temperament (see Table 2). The CeA mediates freezing behavior, behavioral responses to non-social novelty and non-social threat, and stress-induced cortisol release (Kalin et al., 2004, 2001; Machado et al., 2009; Machado and Bachevalier, 2008). The hippocampus plays a role in regulating cooing behavior (Machado and Bachevalier, 2008). The OFC mediates expression of EEG asymmetry in anxious temperament. The OFC may also mediate freezing behavior and response to non-social threat; however, it remains unknown if the effects of OFC lesions are due to disruption of gray matter or white matter tracts in the region. Lesion studies have several limitations, including that lesions are often heterogeneous and incomplete, and the lesion studies examined here had low sample sizes (6–17 monkeys per group). Studies with larger sample sizes that directly compare lesions of different regions across the same paradigm are needed to determine which brain regions produce individual components of anxious temperament. The timing of lesion studies may also be important—neonatal amygdala lesions are associated with an initial increase in fear behavior, but then decreased fear behavior in adulthood (Raper et al., 2013c); however, to date, few studies have examined developmental effects of lesions on anxious temperament.
Table 2.
Effects of lesions on components of anxious temperament in primates.
| Region | Lesion age |
Freezing | Cooing | HPA axis | Other |
|---|---|---|---|---|---|
| Amygdala | neonatal | ↑ at 2 months - at 2.5 months - at 4 months ↓ at 12 months ↓ as young adults |
↑ at 2.5 months ↑ at 12 months |
- ACTH release at 12 months - ACTH release as young adults ↓ cortisol release at 2.5 months ↓ cortisol afternoon release at 5 months ↑ cortisol release at 12 months ↓ cortisol release as young adults |
|
| Amygdala (central nucleus) | young adult | ↓ | ↑ | ↓ ACTH release ↓ CRF release -↓ cortisol release |
↓ snake fear - left frontal EEG asymmetry |
| Amygdala | Young adult | -↓ | ↓ | ↓ cortisol release | ↓↓↓ snake fear -left frontal EEG asymmetry |
| Orbitofrontal cortex | Young adult | ↓↓↓ | - ACTH release - CRF release ↓ cortisol release |
-↓ snake fear ↑ left frontal EEG asymmetry ↓ BNST and entorhinal cortex metabolism |
|
| Hippocampus | Young adult | - | - cortisol release | - snake fear |
Note: data from (Fox et al., 2010; Izquierdo and Murray, 2004; Kalin et al., 2007, 2004, 2001; Machado et al., 2009; Machado and Bachevalier, 2008; Raper et al., 2013a, 2013b, 2013c). HPA = hypothalamic-pituitary-adrenal axis; ACTH = adrenocorticotropic hormone; CRF = corticotropin-releasing hormone; ↑ = one study showing a significant increase; - = one study showing no effect; ↓ = one study showing a significant decrease
2.6. Non-Human Primate Studies Involving Pharmacological Challenge
Pharmacological challenge studies in non-human primates can interrogate the role of neurotransmitters and neuropeptides in anxious temperament. To date, two systems have been investigated: gamma-aminobutyric acid (GABA) and the opiate system. Inhibited temperament is believed to be associated with a failure of GABA neurocircuitry, as GABA inhibits amygdala output (Kalin, 2003). GABAergic interneurons, known as intercalated cells, are located between the basolateral amygdala—the main input region of the amygdala—and CeA—the main output region. Intercalated cells are required for extinction of learned fear (Likhtik et al., 2008) and stimulation of basolateral neurons that project to inhibitory interneurons results in decreased anxiety behavior (Tye et al., 2011). Additionally, GABA agonists, such as benzodiazepines, are an effective treatment for anxiety disorders. In infant rhesus monkeys, administering diazepam, a GABA agonist, prior to the NEC condition decreased two defensive behaviors, freezing and crouching (Kalin and Shelton, 1989). Diazepam administration increases left-sided frontal EEG activity in monkeys (Davidson et al., 1992) and more inhibited monkeys show the greatest shift (Davidson et al., 1993). The opiate system may regulate features of anxious temperament including vocal distress or cooing (Kalin et al., 1988; Kehoe and Blass, 1986) and freezing, through opioid receptors in the periaqueductal gray, a brainstem region with a critical role in freezing behavior (Pert et al., 1976). Administration of morphine, an opiate agonist, to infant rhesus monkeys, reduced cooing vocalizations across all conditions, and administration of naloxone, an opiate antagonist, increased cooing across all conditions (Kalin and Shelton, 1989). The use of pharmacological manipulations in anxious temperament has been limited thus far, but results from studies of the GABA and opioid systems are promising. Future studies should investigate the effects of novel neurotransmitters and neuropeptides systems, such as endocannabanoids and corticotropin releasing factor.
2.7. Non-Human Primate Studies of Brain Function
Neuroimaging methods provide an ideal platform for identifying the common neural substrates of anxious or inhibited temperament across species (for examples, see: Birn et al., 2014; Oler et al., 2012). The majority of neuroimaging studies in non-human primates have used position emotion tomography (PET); in PET, radioactive ligands are taken up by the brain or bound to different receptors, and ligand uptake or binding is quantified. [18F]-fluorodeoxyglucose (FDG) is a radioactive form of glucose that is taken up by active neurons. Once the FDG is taken up, the FDG molecule is phosphorylated, which prevents the molecule from leaving the neuron, and provides a stable marker of brain activity (Reivich et al., 1979). In FDG-PET studies of anxious temperament, monkeys are injected with FDG before completing a behavioral assessment (outside the scanner), which provides a measure of brain activity in a freely moving animal in its natural environment. Seventy percent (70%) of the FDG is taken up within 30–40 minutes of the injection (Rilling et al., 2001) and after the task is completed, brain activity is measured with a PET scan.
Kalin and colleagues (2005) used FDG-PET to determine whether differences in one measure of inhibition—freezing—correlates with differences in brain activity. Increased freezing duration during both the ALN and NEC conditions was associated with increased activity in the amygdala, BNST, substantia innominata, and nucleus accumbens, and was negatively correlated with activity in the motor cortex. Later, Fox and colleagues (2008) tested for a correlation between anxious temperament scores (composite of freezing, cooing, and cortisol) and brain activity. During the NEC condition, anxious temperament was correlated with activity in the amygdala, hippocampus, and brainstem. These neuroimaging studies confirm data from lesion studies that the amygdala and hippocampus are critical for the expression of anxious temperament phenotype.
One important question is whether the brain regions previously associated with anxious temperament mediate the broad phenotype or whether there are brain regions that mediate each of the individual measures. To investigate this question, Shackman and colleagues (2013) used FDG-PET to test for broad associations between anxious temperament and brain activity, as well as unique associations with each of the three components—cooing, freezing, and cortisol—while controlling for the other components. First, anxious temperament broadly was associated with activation in the right CeA and bilateral anterior hippocampus. Second, there were unique associations with several brain regions: higher cortisol concentration was positively correlated with activation in the lateral anterior hippocampus; longer freezing correlated with decreased activation in the primary motor cortex; and decreased cooing was associated with decreased activity in the ventrolateral PFC. The broad temperament association with increased amygdala and hippocampus activation is consistent with previous studies in both monkeys and humans, providing compelling, translational evidence that these two brain structures mediate inhibited temperament. Within the amygdala, mounting evidence points to the CeA as the key contributor to anxious temperament. Importantly, these findings also highlight roles for other brain regions in mediating specific components of anxious temperament.
Given the critical role of the CeA in mediating anxious temperament (Kalin et al., 2004), a recent study tested for an association between anxious temperament and both CeA activity and CeA functional connectivity (Birn et al., 2014). Consistent with the prior studies by the Kalin lab, higher CeA activity predicted anxious temperament. Anxious monkeys also had decreased CeA functional connectivity with both the medial PFC and dorsolateral PFC. The relationship between anxious temperament and CeA-PFC connectivity was mediated by CeA activity, suggesting that increased CeA metabolism drives both anxious temperament and decreased connectivity with regulatory prefrontal regions. Developmental studies will be crucial for teasing apart these relationships and determining whether early emerging CeA hyperactivity shapes brain connectivity over time.
Neuroimaging provides a unique opportunity to identify the effects of brain lesions on other components of the neural circuit. Fox and colleagues (2010) examined the effect of OFC lesions on other brain regions and anxious temperament. FDG was injected into lesioned and control animals both before and after the lesions, and differences in brain activity during the NEC condition were compared. Consistent with previous lesion studies, OFC lesions produced significant decreases in freezing. OFC lesions also resulted in less activation in the bed nucleus of the stria teminalis (BNST), part of the “extended amygdala” that plays a critical role in anxiety (Davis et al., 1997; Walker and Davis, 2008). Importantly, BNST activation was also correlated with freezing during the NEC condition, providing a link between brain activity and behavior. OFC lesions also produced increased activation in other brain regions—including the parietal cortex, midcingulate cortex, and motor cortex—suggesting that these regions may be part of a more complex circuit underlying behavior during the NEC condition. This study provides the first evidence for the BNST involvement in anxious temperament and suggests that a broader neural circuit, including the OFC and BNST, may mediate anxious temperament.
Based on neuroimaging studies in large samples of non-human primates, anxious temperament broadly is associated with activity in the amygdala, hippocampus, BNST, and brainstem. Cortisol reactivity may be mediated by activity in the anterior hippocampus and freezing behavior may be mediated by the amygdala, BNST, and motor cortex. There are some inconsistencies in findings between lesion studies and neuroimaging studies in non-human primates, such as the role of the hippocampus in cortisol reactivity. These inconsistencies may be because neuroimaging studies are more specific and can pinpoint activity in a particular subregion of a brain region, whereas lesion studies typically encompass the entire region, which may have mixed effects. Additionally, anxious temperament is a complex phenotype based on three individual components—freezing, cortisol, and cooing—which is likely mediated by a network of brain regions, rather than a single brain region.
2.8. Rodent Models of Inhibited Temperament
Several rodent models of inhibited temperament have been developed and have been used to replicate behavioral and physiological findings in humans (for a review on the Wistar Kyoto rat model of inhibited temperament, see: Jiao et al., 2011a, pp. 95–110). Individual differences in rodent inhibited temperament can be elicited by exposing rats to a novel environment (Cavigelli and McClintock, 2003) or to a natural predator (i.e., ferret; Qi et al., 2010). Inhibited temperament in rodent models is stable over time and has been associated with increased anxiety-like behavior throughout development and increased corticosterone response to novelty (Cavigelli and McClintock, 2003; Clinton et al., 2011; Jiao et al., 2011b; Qi et al., 2010). Similar to findings in humans with anxiety disorders, inhibited rats exhibit alterations in avoidance learning. During avoidance learning, more inhibited rats had less activation of the medial prefrontal cortex, but no differences in amygdala activation (Perrotti et al., 2013). Inhibited behaviors in rats can be reduced by diazepam administration, a common treatment for anxiety in humans, (Qi et al., 2010). In rats, inhibited temperament was associated with increased immediate early gene expression in the hippocampus and hypothalamus in response to a predator threat, but not in the amygdala (Qi et al., 2010). Additionally, inhibited temperament in rats was associated with marked differences in hippocampal mRNA expression early in postnatal life, including in genes related to cell function and development, neurodevelopment, and intracellular function, among others (Clinton et al., 2011). More inhibited rats also had larger volume of the rostral and middle hippocampus and more cell proliferation in the dentate gyrus (Clinton et al., 2011). Finally, in one study, inhibited rats were more likely to die earlier as compared to their uninhibited counterparts (Cavigelli and McClintock, 2003). Consistent evidence across studies points to alterations in structure and function of the hippocampus as an underlying neurobiological basis for inhibited temperament. While not all studies tested for differences in amygdala structure and function, the studies that did test for amygdala differences did not find any, suggesting that at least in rodent models, hippocampal function may be more critical for inhibited temperament. Rodent models have the advantage of providing a quickly reproducing model organism that can be easily manipulated using both genetic and neurobiological methods. However, rodent brains are very different from primate brains, and lack critical parts of the prefrontal cortex. Therefore, non-human primate models will be crucial for providing insight into the neurobiology of inhibited temperament.
3. Genetic Basis of Inhibited Temperament
Inhibited temperament is widely observed—both across cultures (Meysamie et al., 2014; Scarpa et al., 1995) and across the majority of species (for a review, see: Gosling and John, 1999). The extreme groups of inhibited and uninhibited temperament are preserved by natural selection, maintaining the genes for both groups. Both groups have an evolutionary advantage, with relative strengths in different contexts (e.g. Biro and Post, 2008; Korte et al., 2005). For example, uninhibited individuals—who are willing to approach novelty—are more likely to be successful in situations where novelty-seeking behavior is rewarded; for example, when resources are scarce and exploration of the environment is necessary. Alternatively, inhibited individuals—who tend to avoid novelty—will have the advantage when resources are plentiful, as they will be less likely to expose themselves to predators. While both extremes are evolutionarily adaptive, at both ends of the temperament spectrum there is also increased risk for psychiatric illness. Identifying the genes that contribute to inhibited temperament can help elucidate the biological mechanisms that give rise to these distinct temperament profiles and the associated risk for psychiatric disease.
While the heritability of inhibited temperament has been firmly established, the exact nature of the genetic basis remains elusive. Multiple approaches have been used to investigate the genetic basis ranging from early linkage studies focused on finding the location of genes on the genome (Smoller et al., 2001a) to later association studies focused on identifying which genes are involved (as detailed below). To date, the majority of genetic studies of inhibited temperament have used association methods to investigate candidate genes—genes that are selected based on prior findings or theoretical links to the phenotype. The most popular candidates have been genes involved in anxiety and stress, for example, genes involved in neurotransmission and hypothalamic-pituitary-adrenal axis function.
3.1. Serotonin
Serotonin Transporter Gene (SERT)
The serotonin transporter gene (SERT) has been most frequently studied in inhibited temperament, consistent with its popularity in psychiatric research. The short (“s”) allele of the serotonin transporter-linked polymorphic region (5-HTTLPR) polymorphism has been associated with a vast number of anxiety vulnerability traits (Canli and Lesch, 2007; Sen et al., 2004), and brain biomarkers (Heinz et al., 2005; Munafò et al., 2008a). Many studies of 5-HTTLPR and inhibited temperament have found the expected association between the short allele and inhibited temperament across multiple samples using different methods for assessing inhibited temperament (Battaglia et al., 2005; Davies et al., 2013; Hayden et al., 2007). However, other studies failed to find an association between 5-HTTLPR and shyness (Schmidt, 2002) or found the opposite pattern—the long allele was associated with more shyness (Arbelle et al., 2003; Jorm et al., 2000). Studies in rhesus monkeys with the rhesus variant (rh-5HTTLPR) are similarly mixed. In a small sample of infant and juvenile rhesus monkeys, the s/s genotype was associated with more inhibited and fearful behaviors (Bethea et al., 2004); however, in two much larger studies of juvenile rhesus monkeys, there was no association between 5-HTTLPR genotype and anxious temperament (Oler et al., 2010; Rogers et al., 2008).
One possible reason for the inconsistencies across studies is that studies did not account for the potential effects of environmental differences. Even if anxiety vulnerability is 100% heritable at birth, epistasis is highly likely to shape the developmental trajectory of inhibited temperament. Support for this perspective comes from longitudinal studies that show that inhibited temperament is quite stable in some individuals, but variable in others (Gest, 1997; Kagan et al., 1984) and that trajectories can be influenced by early environmental differences. For example extremely inhibited infants who experience non-parental child care are less likely to remain inhibited as toddlers (Fox et al., 2001). Likewise, infants born with the high-risk short allele of 5-HTTLPR may be more susceptible to negative environmental influences, including trauma and poor parenting. Fox and colleagues investigated the role of gene × environment interactions in inhibited children and found that 5-HTTLPR and maternal social support interacted to predict later shyness (Fox et al., 2005b). The influence of 5-HTTLPR on inhibited temperament was strongest for children whose mothers reported lower levels of social support. For children with one short allele, lower social support predicted more inhibited temperament, and for children with the l/l genotype, low social support was associated with less inhibited temperament. In another study, Burkhouse and colleagues (2011) tested for gene × environment interactions between 5-HTTLPR and parenting. In their study of young children, the 5-HTTLPR genotype and child’s report of maternal overprotectiveness interacted to predict inhibited temperament; children with the s/s genotype showed the strongest effect of maternal overprotectiveness on inhibited temperament. The findings are consistent with a substantial literature showing that the effects of 5-HTTLPR on depression are moderated by stress and trauma (Caspi et al., 2003; Karg et al., 2011). Together, these findings suggest that 5-HTTLPR is associated with inhibited temperament and the impact of 5-HTTLPR on inhibited temperament is likely moderated by environmental factors, such as low social support or overprotective parenting.
3.2. Dopamine
Dopamine Receptor Gene (DRD4)
Dopamine has a prominent role in reward processing and exploration and has been associated with novelty seeking traits in humans (Zald et al., 2008). Several early reports suggested that novelty-seeking was associated with variation in a polymorphism in the dopamine D4 receptor gene (DRD4); specifically, the presence of a 7-allele repeat in exon 3 was associated with higher scores on a novelty-seeking trait (Ebstein et al., 1996). Based on these findings, it was expected that inhibited individuals would be less likely to have the 7-allele repeat. Several studies investigated this candidate gene in both shy children (Arbelle et al., 2003; Schmidt et al., 2002) and adolescents who were categorized as inhibited as children (Pérez-Edgar et al., 2013) and no studies found evidence for an association. This lack of a relationship is consistent with two recent meta-analyses showing that the initial finding of the 7-allele repeat and novelty seeking did not withstand replication (Kluger et al., 2002; Munafò et al., 2008b). However, the study by Perez-Edgar and colleagues also included brain imaging measures of reward processing. In that study, the 7-allele repeat moderated the relationship between brain activation and inhibited temperament. For adolescents with the 7–allele repeat, greater inhibition correlated with stronger caudate activation to medium amounts of monetary incentives. This study highlights the complexity of genetic effects on temperament and suggests that additional measures, such as brain markers, may be necessary to reveal the underlying influence of genetic variation.
Dopamine Transporter Gene (DAT)
Because many genes are involved in the effective signaling of any given neurotransmitter, it is possible that variations in other parts of the dopamine system are involved in temperament, despite the lack of findings for DRD4. Davies and colleagues (2013) were the first to examine the dopamine transporter gene (DAT) in inhibited temperament. Inhibited children had a DAT composite score (formed from alleles from three different DAT polymorphisms) that reflected less efficient transporter activity.
3.3. GABA
Glutamic acid decarboxylase gene (GAD)
GAD is the rate-limiting enzyme for converting glutamate to gamma-aminobutyric acid (GABA), the primary inhibitory neurotransmitter in the brain. GABA is thought to play a role in anxiety, given the potent effects of GABA agonists on reducing anxiety. The Smoller lab investigated a polymorphism in intron 3 of the GAD2 gene (also known as GAD65; Smoller et al., 2001b). Children with a 168-bp allele in GAD65 were significantly less likely to have an inhibited temperament. A subsequent study in adults, found several single nucleotide polymorphisms (SNPs) that were associated with inhibited temperament in healthy controls and in patients with an anxiety disorder (Unschuld et al., 2009).
3.4. Neurotransmitter Regulation
Monoamine Oxidase A gene (MAOA)
The MAOA gene codes for enzymes that degrade multiple neurotransmitters, including serotonin and dopamine. Allelic differences in MAOA are thought to contribute to differences in serotonin and dopamine function, which may impact stress reactivity and/or reward processing. The single study that examined MAOA in inhibited children failed to find an association (Arbelle et al., 2003)
Catechol-O-methyltransferase gene (COMT)
COMT is an enzyme involved in the degradation of multiple neurotransmitters, including dopamine. The COMT gene codes for the production of this enzyme. Allelic differences in COMT are thought to contribute to differences in dopamine signaling and reward processing. To date, one study investigated COMT in inhibited children and found no evidence for an association (Arbelle et al., 2003).
3.5. Corticotropin Releasing Hormone
Another system frequently investigated in anxiety vulnerability and anxiety disorders is the hypothalamic-pituitary-adrenal (HPA) axis, which regulates stress responses. Within the HPA axis, corticotropin releasing hormone (CRH) stimulates the synthesis of adrenocorticotropic hormone (ACTH), which in turn stimulates the synthesis of cortisol. Elevated cortisol levels have been reported in inhibited children (Kagan et al., 1987; Pérez-Edgar et al., 2008; Schmidt et al., 1997) and non-human primates (Kalin et al., 2000, 1998) and may be one factor contributing to increased prevalence of stress-related illnesses, such as anxiety and depression.
Corticotropin Releasing Hormone gene (CRH)
The CRH gene encodes corticotrophin releasing hormone. Smoller and colleagues investigated the association between allelic variation in CRH and inhibited temperament in two studies (Smoller et al., 2005, 2003). In the first study, Smoller and colleagues (2003) examined a genomic region of CRH (CRH.PCR1) in a sample of children at risk for anxiety based on one parent having panic disorder or major depression. A family based association test revealed an association between a 173-bp allele and inhibited temperament; subgroup analyses identified that the association was specific to the families with panic disorder. In the second study, Smoller and colleagues (2005) examined CHR.PCR1 and multiple SNPs in the CRH gene in an expanded sample of families. The 173-bp allele of CRH.PCR1 was again associated with inhibited temperament in addition to an association with three SNPs in the CRH gene.
Corticotropin Releasing Hormone Receptor 1 gene (CRHR1)
The CRH receptors, CRH1 and CRH2, are another important part of the HPA axis. The Kalin lab recently investigated the relationship between inhibited temperament in monkeys and an evolutionarily conserved region of the CRH1 receptor gene, CRHR1 (Rogers et al., 2012). Three independent polymorphisms were associated with inhibited temperament. Importantly, one of those polymorphisms was also associated with metabolism in several brain regions (amygdala, hippocampus, precuneus, intraparietal sulcus) providing a possible mechanism linking genetic variation in CRHR1 and inhibited temperament. This finding provides an exciting direction for future investigations in humans.
3.6. Other Candidate Genes
More recently, scientists have moved beyond the “usual suspects” and have begun investigating less conspicuous genes, although still largely guided by general principles of investigating genes broadly based in anxiety or related traits. For example, the Smoller lab examined the gene encoding regulator of G protein signaling 2 (RGS2)—a gene on chromosome 1 that is suspected to underlie anxiety traits in mice. They found that polymorphisms in RGS2 predicted: inhibited temperament in a family association study; introverted (NEO-E) personality in a sample of college students; and activation in the amygdala and insula during an emotion face matching task in young adults (Smoller et al., 2008). This multi-sample/multi-modal approach produced compelling findings linking genes to brain function to behavior. However, as is common in the field, a subsequent study with a larger sample failed to replicate the RGS2 association with a set of anxiety vulnerability phenotypes (Hettema et al., 2013).
Another example of exciting new genetic discoveries is contactin-associated protein-like 2 (CNTNAP2), a gene involved in language impairments in autism and selective mutism. Reluctance to speak and latency to initiate speech are common features of inhibited children (Kagan et al., 1987), providing a conceptual link to selective mutism. Stein and colleagues found that variation in the CNTNAP2 gene was associated with selective mutism in a family study and also associated with inhibited temperament in healthy young adults (Stein et al., 2011). These findings illustrate that anxiety risk traits and anxiety disorders likely share a genetic basis.
It should be noted that for each positive finding, there are multiple other genes with negative findings, many of which are not reported. For example, a previous study reported that inhibited temperament is not associated with the adenosine A1A receptor gene (A1AR), adenosine A2A receptor gene (A2AR), or the preproenkephalin gene (PENK) (Smoller et al., 2001b).
3.7. Gene Expression
Another exciting approach to discovering the genetic basis of inhibited temperament is to study inhibited monkeys. The Kalin lab has now phenotyped more than 500 monkeys with known pedigrees. This sample provides a unique window into the link between genes, brain, and behavior. For example, in a recent study (Oler et al., 2010) they showed that although both amygdala and hippocampus metabolism were associated with inhibited temperament, however, only hippocampal metabolism was heritable. Previously, the amygdala had been the focus of many temperament studies, based on its central functions in fear and anxiety. These findings suggest that the hippocampus may play an important role in inhibited temperament and anxiety vulnerability, consistent with Gray’s septo-hippocampal theory of anxiety (Gray, 1982).
The Kalin lab has followed this heritability study with messenger RNA (mRNA) expression studies in tissue from the CeA, a region consistently implicated in inhibited temperament. Importantly, this type of study would be very difficult in humans, since most post-mortem brain samples will not have well-characterized temperament phenotypes. Using this approach, Kalin and colleagues have discovered several novel genetic associations with inhibited temperament including: decreased mRNA expression of neurotrophic tyrosine kinase receptor type 3 (NTRK3) (Fox et al., 2012) and lower mRNA levels of neuropeptide Y receptor 1 (NPY1R) and receptor 5 (NPY5R) (Roseboom et al., 2013).
Another benefit of using non-human primate models to study temperament is that differences in gene expression across the brain can be examined. For example, previous findings have highlighted the importance of the CeA to anxious temperament (Kalin et al., 2004), and Fox and colleagues (2012) tested for an association between anxious temperament and mRNA expression in the CeA. Anxious temperament was associated with altered expression in the CeA of a diverse set of mRNA including genes related to hormone stimulation, developmental growth, and axon growth. Notably, anxious temperament as associated with decreased expression of the NTRK3 (also known as TrkC) gene. The NTRK3 receptor is activated by neurotrophin-3 and activates signaling pathways involved in neuronal survival, differentiation, and synaptic plasticity (Kang and Schuman, 1995; Mart nez et al., 1998). NTRK3 plays a role during development and in adult neuroplasticity, and has been implicated in anxiety disorders (Dierssen et al., 2006); NTRK3 is also downregulated following restraint stress and can be rescued by treatment with a selective-serotonin reuptake inhibitor (fluoxetine) (Barreto et al., 2012). Given findings that alterations in amygdala structure have developmental effects on anxious temperament (Kalin et al., 2001; Machado et al., 2009; Raper et al., 2013a, 2013c), alterations in amygdala neuroplasticity may underlie the development of anxious temperament.
The initial study by Fox and colleagues (2012) also found that anxious temperament was associated with decreased expression of neuropeptide Y (NPY) receptor 1. Roseboom and colleagues (2014) followed-up on these findings in an independent sample of monkeys. NPY has anxiolytic and stress-reducing effects and is expressed throughout the brain, including in the amygdala and hippocampus (Bowers et al., 2012). NPY receptor 1 (NPY1R) and receptor 5 (NPY5R) are believed to be anxiolytic and NPY receptor 2 (NPY2R) is believed to be anxiogenic (Bowers et al., 2012; Heilig et al., 1993; Sørensen et al., 2004). Decreased expression of NPY1R and NPY5R mRNA in the CeA was related to more anxious temperament; however, there was no relationship between anxious temperament and NPY or NPY2R mRNA expression. Increased NPY1R and NPY5R expression was correlated with increased metabolic activity in the dlPFC as measured by FDG-PET during the NEC condition. Increased NPY1R expression was also associated with decreased metabolic activity in the rostral ACC. These findings suggest that decreased NPY receptor expression in the amygdala may underlie some of the behavioral and neuroimaging findings associated with anxious temperament. NPY is believed to play a role in resilience and regulation of the anxiety response (Bowers et al., 2012; Heilig et al., 1993; Sørensen et al., 2004). These findings suggest that a reduction in NPYRexpressing neurons may result in less activation of emotion-regulatory regions such as the dlPFC (Roseboom et al., 2014). Additionally, in a recent study, decreased CeA-dlPFC connectivity was related to more anxious temperament and anxiety disorders (Birn et al., 2014). However, it remains unknown if alterations in NPY1R and NPY5R expression relate to connectivity or the mechanism by which receptor expression may alter connectivity. No correlations were found between NPY expression and anxious temperament and the relationship between NPY1R and NPY5R expression and NPY expression is not known. NPY is not currently explicitly targeted by any conventional anxiety treatments and might represent a novel target for treatment.
4. Summary and Future Directions
During the past decade, scientists have made many exciting discoveries about the neural and genetic basis of inhibited temperament. Inhibited adolescents and adults show alterations predominantly in neural circuits governing novelty detection, fear processing, anticipation, reward processing, and attention. The brain regions that show the most consistent alterations are the amygdala, nucleus accumbens, caudate, and prefrontal cortex. Consistent with the neuroimaging field, most of the earlier studies focused on regions of interest. However, to advance our knowledge, it will be critical for future studies to test for differences across the whole brain and to systematically examine neural circuits using the growing number of sophisticated connectivity methods. We propose the following future directions for the field:
Determine whether the BNST mediates inhibited temperament in humans. To date, most of the lesion and neuroimaging studies have focused on the amygdala and prefrontal cortex. A largely understudied brain region that is likely to be important for inhibited temperament is the BNST. The BNST is a part of the extended amygdala, a macrostructure that includes the central nucleus of the amygdala, BNST, and shell of the nucleus accumbens (Alheid and Heimer, 1988). Importantly, the BNST mediates anxiety and response to potential threat (Alvarez et al., 2011; Davis et al., 1997; Somerville et al., 2010; Walker and Davis, 2008) as well as addiction (Flavin and Winder, 2013; Koob and Volkow, 2009; Silberman and Winder, 2013; Stamatakis et al., 2014). Of interest to us is the link between social anxiety disorder and substance abuse (Kessler et al., 2005b), with specificity for alcohol and cannabis, both central nervous system depressants. We propose that inhibited individuals use drugs for the anxiolytic effects; consistent with this proposal, the BNST is involved in the withdrawal and negative affect state of addiction, not the initial binge/intoxication stage (Koob and Volkow, 2009). Thus, the BNST is well positioned to mediate inhibited behaviors and confer risk for both anxiety and addiction. Non-human primate studies provide preliminary evidence for the BNST’s role (Fox et al., 2010, 2008); however examination of the BNST in humans has been limited by MRI scanner resolution. We have recently developed a BNST mask using an ultra-high field scanner and specific scanning sequence. Using this mask and a high-field scanner, we have provided the first mapping of the human BNST structural and functional connectivity (Avery et al., 2014). The BNST functional connectivity findings have been recently replicated (McMenamin et al., 2014), paving the way for future studies of the BNST.
Determine whether inhibited children show differences in brain structure or function across development. Inhibited temperament was initially identified in infants and toddlers (Garcia-Coll et al., 1984; Kagan et al., 1998); however, all published MRI studies of inhibited temperament have been conducted in adolescents and young adults (see Figure 5). Thus, no studies to date have been able to address two important questions: Do inhibited children show differences in brain structure or function? What are the developmental trajectories of brain structure and function in inhibited children? Future studies are needed to elucidate the early brain endophentoype of inhibited temperament, especially before the onset of psychopathology.
Develop anxiety risk profiles using combined imaging, genetics, behavioral, and psychophysiological measures. Another critical future direction is to understand why children with early inhibited temperament have divergent outcomes, given about 44% of inhibited children go on to develop social anxiety disorder by adolescence (see Figure 1; Clauss and Blackford, 2012), whereas more than half of inhibited children go on to be healthy adults. To date, there are no specific genetic or neural biomarkers to predict which inhibited children will go on to develop anxiety disorders (for a discussion, see: Henderson et al., 2014). We recommend a large scale, multi-site study that combines multiple measures across several system levels (genes, brain, physiology, behavior), as well as environment (e.g., parenting, parent psychopathology), to provide a comprehensive assessment of the phenotype. These data could then be analyzed using cluster analyses or machine learning to identify the combination of measures that are most predictive of later psychopathology. This type of approach can provide valid risk models that can lead to targeted prevention interventions to the highest-risk children.
Conduct a large-scale multi-site genome-wide association study (GWAS) of inhibited temperament. GWAS studies have the potential to uncover novel biological mechanisms that underlie inhibited temperament and guide future research and development of prevention strategies for the highest-risk children. Significant advances have been made in several psychiatric disorders using large scale GWAS studies based on consortia that combine data across many sites (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013; Ripke et al., 2011; Visscher et al., 2012). However, to date, most genetic studies of inhibited temperament have used candidate gene approaches with relatively small sample sizes. While these have been informative and have provided preliminary evidence for a genetic basis of inhibited temperament, this approach is limited to known candidate genes. GWAS studies of inhibited temperament have the potential to broaden our knowledge of the genetics of inhibited temperament from the “usual suspects” and to identify novel genes previously not associated inhibited temperament.
Test for neurobiological differences in children with an uninhibited temperament. Inhibited temperament has been the main focus of studies of temperament given its association with anxiety disorders and depression; however, uninhibited temperament is also associated with psychopathology, including attention deficit hyperactivity disorder (ADHD), bipolar disorder, and substance use disorders (Hirshfeld-Becker et al., 2007, 2003, 2002). Much remains unknown about the genetic and neural basis of uninhibited temperament and how these differences may confer risk for psychopathology.
Broaden the types of tasks used in neuroimaging in inhibited temperament. In neuroimaging studies, the data are only as good as the task performed. Therefore to advance our understanding of the neurocircuitry of inhibited temperament, we must expand the types of tasks used. A natural progression in identifying alterations in fear neurocircuitry is to use well-developed fear conditioning tasks that include conditioning, extinction, and extinction. Given the intriguing recent findings of PFC alterations, we propose that future studies further interrogate prefrontal cortex function using tasks that target cognitive flexibility and implicit and explicit emotion regulation. Finally, we propose that future studies target memory formation and avoidance and appetitive learning to broaden our understanding of the neural substrates of inhibited temperament.
Figure 5.
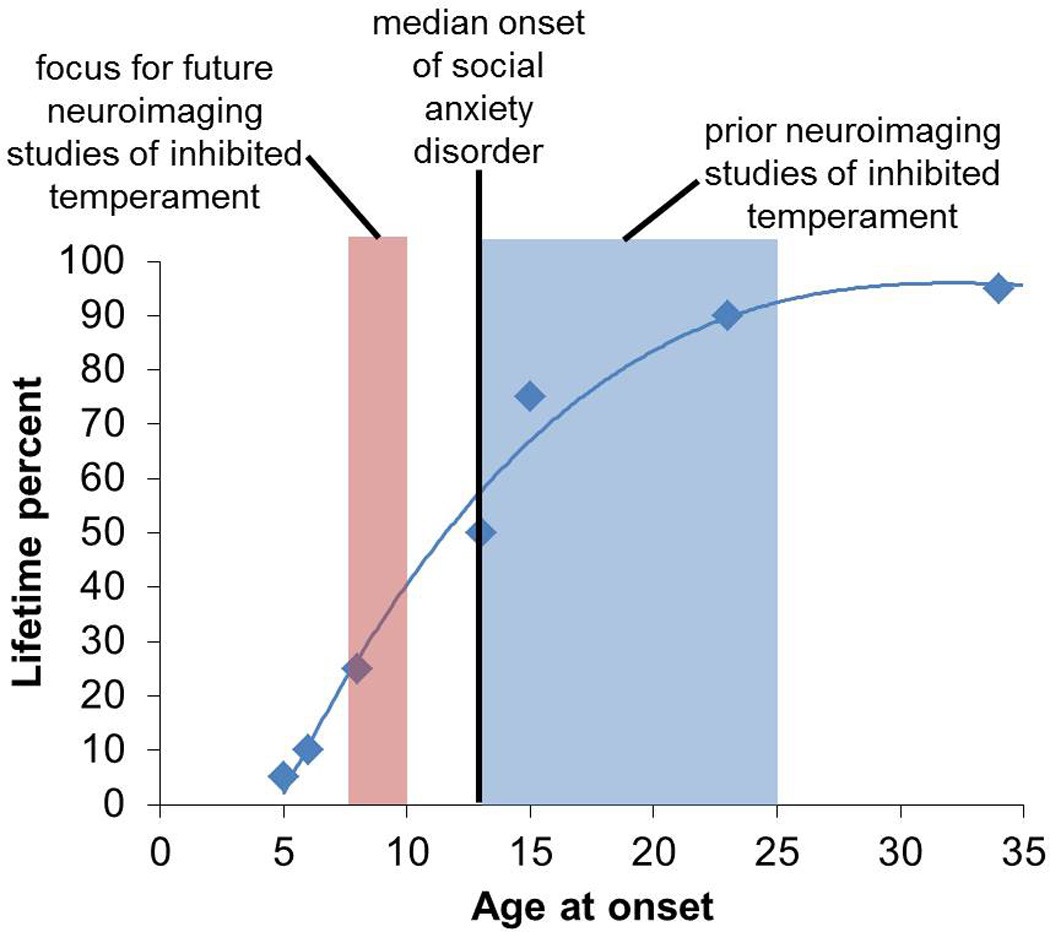
Social anxiety disorder has a median age of onset of 13 years and 75% of patients have an onset by age 15 (Kessler et al., 2005a). Published neuroimaging studies of inhibited temperament have focused on imaging adolescents and young adults (blue), who have already reached the median age of onset for social anxiety disorder. Future studies should focus on younger children, who have yet to reach the age of onset for social anxiety disorder to understand how temperament affects brain function.
Article Highlights.
Inhibited temperament is a heritable trait that emerges early in development.
Inhibited temperament confers heightened risk for multiple psychiatric disorders.
Amygdala hyperactivity plays a central role in inhibited temperament.
Prefrontal cortex and basal ganglia function are altered in inhibited individuals.
Genes regulating neurotransmitter function are implicated in inhibited temperament.
Acknowledgements
This work was supported by funding from the National Institute of Mental Health (F30-MH097344; T32-MH018921; F31-MH102008), the Vanderbilt Medical Scientist Training Program (NIGMS; T32-GM07347). The authors thank Andrew Fox and Ned Kalin for the nonhuman primate brain template. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- 5-HTTLPR
serotonin transporter-linked polymorphic region
- A1AR
adenosine A1A receptor gene
- A2AR
adenosine A2A receptor gene
- ACC
anterior cingulate cortex
- ACTH
adrenocorticotropic hormone
- ADHD
attention-deficit hyperactivity disorder
- ALE
activation likelihood estimation
- ALN
alone in a new cage condition in the human intruder paradigm
- BNST
bed nucleus of the stria terminalis
- CeA
central nucleus of the amygdala
- COMT
catechol-O-methyltransferase gene
- CRH
corticotropin-releasing hormone
- CRH
corticotropin-releasing hormone gene
- CRHR1
corticotropin-releasing hormone receptor 1 gene
- CNTNAP2
contactin-associated protein-like 2
- DAT
dopamine transporter gene
- dlPFC
dorsolateral prefrontal cortex
- dmPFC
dorsomedial prefrontal cortex
- DRD4
dopamine D4 receptor gene
- EEG
electroencephalography
- ERN
error-related negativity
- ERP
event-related potential
- FDG
[18F]-fluorodeoxyglucose
- fMRI
functional magnetic resonance imaging
- GABA
gamma-aminobutyric acid
- GAD
glutamic acid decarboxylase gene
- GWAS
genome-wide association study
- HPA
hypothalamic-pituitary-adrenal axis
- MAOA
monoamine oxidase A gene
- MMN
mismatch negativity
- MRI
magnetic resonance imaging
- mRNA
messenger RNA
- NEC
no eye contact condition in the human intruder paradigm
- NPY
neuropeptide Y
- NPY1R
neuropeptide Y receptor 1
- NPY5R
neuropeptide Y receptor 5
- NTRK3
neurotrophic tyrosine kinase receptor, type 3
- OFC
orbitofrontal cortex
- PET
positron emission tomography
- PFC
prefrontal cortex
- PENK
preproenkephalin gene
- RGS2
regulator of G protein signaling 2
- SERT
serotonin transporter gene
- SNP
single nucleotide polymorphism
- ST
stare condition in the human intruder paradigm
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors report no biomedical financial interests or potential conflicts of interest.
References
- Admon R, Milad MR, Hendler T. A causal model of post-traumatic stress disorder: disentangling predisposed from acquired neural abnormalities. Trends Cogn. Sci. 2013;17:337–347. doi: 10.1016/j.tics.2013.05.005. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Res. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: The striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- Alvarez RP, Chen G, Bodurka J, Kaplan R, Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage. 2011;55:389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ, Habel U, Schneider F, Zilles K. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat. Embryol. (Berl.) 2005;210:343–352. doi: 10.1007/s00429-005-0025-5. [DOI] [PubMed] [Google Scholar]
- Arbelle S, Benjamin J, Golin M, Kremer I, Belmaker RH, Ebstein RP. Relation of shyness in grade school children to the genotype for the long form of the serotonin transporter promoter region polymorphism. Am. J. Psychiatry. 2003;160:671–676. doi: 10.1176/appi.ajp.160.4.671. [DOI] [PubMed] [Google Scholar]
- Aron EN, Aron A. Sensory-processing sensitivity and its relation to introversion and emotionality. J. Pers. Soc. Psychol. 1997;73:345–368. doi: 10.1037//0022-3514.73.2.345. [DOI] [PubMed] [Google Scholar]
- Asendorpf JB. The malleability of behavioral inhibition: a study of individual developmental functions. Dev. Psychol. 1994;30:912–919. [Google Scholar]
- Avery SN, Clauss JA, Winder DG, Woodward N, Heckers S, Blackford JU. BNST neurocircuitry in humans. NeuroImage. 2014;91:311–323. doi: 10.1016/j.neuroimage.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Machado CJ, Kazama A. Behavioral outcomes of late-onset or early-onset orbital frontal cortex (areas 11/13) lesions in rhesus monkeys: Orbital frontal lesions alter behavioral adaptation. Ann. N. Y. Acad. Sci. 2011;1239:71–86. doi: 10.1111/j.1749-6632.2011.06211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Specialized elements of orbitofrontal cortex in primates. Ann. N. Y. Acad. Sci. 2007;1121:10–32. doi: 10.1196/annals.1401.015. [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Fox NA, Benson B, Guyer AE, Williams A, Nelson EE, Perez-Edgar K, Pine DS, Ernst M. Neural correlates of reward processing in adolescents with a history of inhibited temperament. Psychol. Sci. 2009;20:1009–1018. doi: 10.1111/j.1467-9280.2009.02401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto RA, Walker FR, Dunkley PR, Day TA, Smith DW. Fluoxetine prevents development of an early stress-related molecular signature in the rat infralimbic medial prefrontal cortex. Implications for depression? BMC Neurosci. 2012;13:125. doi: 10.1186/1471-2202-13-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Loscertales A, Meseguer V, Sanjuan A, Belloch V, Parcet MA, Torrubia R, Avila C. Behavioral Inhibition System activity is associated with increased amygdala and hippocampal gray matter volume: A voxel-based morphometry study. Neuroimage. 2006;33:1011–1015. doi: 10.1016/j.neuroimage.2006.07.025. [DOI] [PubMed] [Google Scholar]
- Battaglia M, Ogliari A, Zanoni A, Citterio A, Pozzoli U, Giorda R, Maffei C, Marino C, Article O. Influence of the serotonin transporter promoter gene and shyness on children’s cerebral responses to facial expressions. Arch Gen Psychiatry. 2005;62:85–94. doi: 10.1001/archpsyc.62.1.85. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J. Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am. J. Psychiatry. 1994;151:1132–1136. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Streicher JM, Coleman K, Pau FK-Y, Moessner R, Cameron JL. Anxious behavior and fenfluramine-induced prolactin secretion in young rhesus macaques with different alleles of the serotonin reuptake transporter polymorphism (5HTTLPR) Behav. Genet. 2004;34:295–307. doi: 10.1023/B:BEGE.0000017873.61607.be. [DOI] [PubMed] [Google Scholar]
- Biederman J, Hirshfeld-Becker DR, Rosenbaum JF, Herot C, Friedman D, Snidman N, Kagan J, Faraone SV. Further evidence of association between behavioral inhibition and social anxiety in children. Am. J. Psychiatry. 2001;158:1673–1679. doi: 10.1176/appi.ajp.158.10.1673. [DOI] [PubMed] [Google Scholar]
- Biederman J, Rosenbaum JF, Bolduc-Murphy EA, Faraone SV, Chaloff J, Hirshfeld DR, Kagan J. A 3-year follow-up of children with and without behavioral inhibition. J. Am. Acad. Child Adolesc. Psychiatry. 1993;32:814–821. doi: 10.1097/00004583-199307000-00016. [DOI] [PubMed] [Google Scholar]
- Birn RM, Shackman AJ, Oler JA, Williams LE, McFarlin DR, Rogers GM, Shelton SE, Alexander AL, Pine DS, Slattery MJ, Davidson RJ, Fox AS, Kalin NH. Evolutionarily-conserved prefrontal-amygdalar dysfunction in early-life anxiety. Mol. Psychiatry. 2014 doi: 10.1038/mp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biro PA, Post JR. Rapid depletion of genotypes with fast growth and bold personality traits from harvested fish populations. Proc. Natl. Acad. Sci. 2008;105:2919–2922. doi: 10.1073/pnas.0708159105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop G, Spence SH, McDonald C. Can parents and teachers provide a reliable and valid report of behavioral inhibition? Child Dev. 2003;74:1899–1917. doi: 10.1046/j.1467-8624.2003.00645.x. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X-N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski A-M, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li S-J, Lin C-P, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng G-J, Veijola J, Villringer A, Walter M, Wang L, Weng X-C, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang Y-F, Zhang H-Y, Castellanos FX, Milham MP. Toward discovery science of human brain function. Proc. Natl. Acad. Sci. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Allen AH, Cowan RL, Avery SN. Amygdala and hippocampus fail to habituate to faces in individuals with an inhibited temperament. Soc. Cogn. Affect. Neurosci. 2013;8:143–150. doi: 10.1093/scan/nsr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Cowan RL, Shelton RC, Zald DH. Sustained amygdala response to both novel and newly familiar faces characterizes inhibited temperament. Soc. Cogn. Affect. Neurosci. 2011;6:621–629. doi: 10.1093/scan/nsq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Avery SN, Shelton RC, Zald DH. Amygdala temporal dynamics: temperamental differences in the timing of amygdala response to familiar and novel faces. BMC Neurosci. 2009;10:145. doi: 10.1186/1471-2202-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Buckholtz JW, Avery SN, Zald DH. A unique role for the human amygdala in novelty detection. Neuroimage. 2010;50:1188–1193. doi: 10.1016/j.neuroimage.2009.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Clauss JA. Dr. Blackford and Ms. Clauss reply. J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:319–320. doi: 10.1016/j.jaac.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Blackford JU, Clauss JA, Avery SN, Cowan RL, Benningfield MM, VanDerKlok RM. Amygdala–cingulate intrinsic connectivity is associated with degree of social inhibition. Biol. Psychol. 2014;99:15–25. doi: 10.1016/j.biopsycho.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders. Child Adolesc. Psychiatr. Clin. N. Am. 2012;21:501–525. doi: 10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchio M, Capogna M. Oscillatory substrates of fear and safety. Neuron. 2014;83:753–755. doi: 10.1016/j.neuron.2014.08.014. [DOI] [PubMed] [Google Scholar]
- Bowers ME, Choi DC, Ressler KJ. Neuropeptide regulation of fear and anxiety: Implications of cholecystokinin, endogenous opioids, and neuropeptide Y. Physiol. Behav. 2012;107:699–710. doi: 10.1016/j.physbeh.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–887. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Krienen FM, Yeo BTT. Opportunities and limitations of intrinsic functional connectivity MRI. Nat. Neurosci. 2013;16:832–837. doi: 10.1038/nn.3423. [DOI] [PubMed] [Google Scholar]
- Buhle J, Silvers J, Wager T, Lopez R, Onyemekwu C, Kober H, Weber J, Ochsner KN. Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cereb. Cortex. doi: 10.1093/cercor/bht154. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhouse KL, Gibb BE, Coles ME, Knopik VS, McGeary JE. Serotonin transporter genotype moderates the link between children’s reports of overprotective parenting and their behavioral inhibition. J. Abnorm. Child Psychol. 2011;39:783–90. doi: 10.1007/s10802-011-9526-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins SD, Fox NA, Marshall TR. Behavioral and physiological antecedents of inhibited and uninhibited behavior. Child Dev. 1996;67:523–540. [PubMed] [Google Scholar]
- Canli T, Lesch KP. Long story short: the serotonin transporter in emotion regulation and social cognition. Nat. Neurosci. 2007;10:1103–1109. doi: 10.1038/nn1964. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders: longitudinal evidence from a birth cohort. Arch. Gen. Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Caulfield MD, Servatius RJ. Focusing on the possible role of the cerebellum in anxiety disorders. In: Durbano F, editor. New Insights into Anxiety Disorders. 2013. [Google Scholar]
- Cavanagh JF, Shackman AJ. Frontal midline theta reflects anxiety and cognitive control: Meta-analytic evidence. J. Physiol.-Paris. 2014 doi: 10.1016/j.jphysparis.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavigelli SA, McClintock MK. Fear of novelty in infant rats predicts adult corticosterone dynamics and an early death. Proc. Natl. Acad. Sci. 2003;100:16131–16136. doi: 10.1073/pnas.2535721100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareyron LJ, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the amygdala: A stereological study in macaque monkeys. J. Comp. Neurol. 2012;520:1965–1984. doi: 10.1002/cne.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronis-Tuscano A, Degnan KA, Pine DS, Perez-Edgar K, Henderson HA, Diaz Y, Raggi VL, Fox NA. Stable early maternal report of behavioral inhibition predicts lifetime social anxiety disorder in adolescence. J. Am. Acad. Child Adolesc. Psychiatry. 2009;48:928–935. doi: 10.1097/CHI.0b013e3181ae09df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Avery SN, VanDerKlok RM, Rogers BP, Cowan RL, Benningfield MM, Blackford JU. Neurocircuitry underlying risk and resilience to social anxiety disorder. Depress. Anxiety. 2014a doi: 10.1002/da.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Blackford JU. Behavioral inhibition and risk for developing social anxiety disorder: a meta-analytic study. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51:1066–1075. doi: 10.1016/j.jaac.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Cowan RL, Blackford JU. Expectation and temperament moderate amygdala and dorsal anterior cingulate cortex responses to fear faces. Cogn. Affect. Behav. Neurosci. 2011;11:13–21. doi: 10.3758/s13415-010-0007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauss JA, Seay AL, VanDerKlok RM, Avery S, Cao A, Cowan RL, Benningfield MM, Blackford JU. Structural and functional bases of inhibited temperament. Soc. Cogn. Affect. Neurosci. 2014b doi: 10.1093/scan/nsu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinton SM, Stead JDH, Miller S, Watson SJ, Akil H. Developmental underpinnings of differences in rodent novelty-seeking and emotional reactivity: developmental underpinnings of emotionality. Eur. J. Neurosci. 2011;34:994–1005. doi: 10.1111/j.1460-9568.2011.07811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross-Disorder Group of the Psychiatric Genomics Consortium. Identification of risk loci with shared effects on five major psychiatric disorders: a genome-wide analysis. The Lancet. 2013 doi: 10.1016/S0140-6736(12)62129-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson RJ, Fox NA. Frontal brain asymmetry predicts infants’ response to maternal separation. J. Abnorm. Psychol. 1989;98:127–131. doi: 10.1037//0021-843x.98.2.127. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kalin NH, Shelton S. Lateralized response to diazepam predicts temperamental style in rhesus monkeys. Behav. Neurosci. 1993;107:1106–1110. doi: 10.1037//0735-7044.107.6.1106. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Kalin NH, Shelton SE. Lateralized effects of diazepam on frontal brain electric asymmetries in rhesus monkeys. Biol. Psychiatry. 1992;32:438–451. doi: 10.1016/0006-3223(92)90131-i. [DOI] [PubMed] [Google Scholar]
- Davies PT, Cicchetti D, Hentges RF, Sturge-Apple ML. The genetic precursors and the advantageous and disadvantageous sequelae of inhibited temperament: an evolutionary perspective. Dev. Psychol. 2013;49:2285–300. doi: 10.1037/a0032312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annu. Rev. Neurosci. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol. Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Degnan KA, Almas AN, Fox NA. Temperament and the environment in the etiology of childhood anxiety: temperament, anxiety, and environment. J. Child Psychol. Psychiatry. 2010;51:497–517. doi: 10.1111/j.1469-7610.2010.02228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierssen M, Gratacòs M, Sahún I, Martín M, Gallego X, Amador-Arjona A, Martínez de Lagrán M, Murtra P, Martí E, Pujana MA, Ferrer I, Dalfó E, Martínez-Cué C, Flórez J, Torres-Peraza JF, Alberch J, Maldonado R, Fillat C, Estivill X. Transgenic mice overexpressing the full-length neurotrophin receptor TrkC exhibit increased catecholaminergic neuron density in specific brain areas and increased anxiety-like behavior and panic reaction. Neurobiol. Dis. 2006;24:403–418. doi: 10.1016/j.nbd.2006.07.015. [DOI] [PubMed] [Google Scholar]
- Di Martino A, Scheres A, Margulies DS, Kelly AMC, Uddin LQ, Shehzad Z, Biswal B, Walters JR, Castellanos FX, Milham MP. Functional Connectivity of Human Striatum: A Resting State fMRI Study. Cereb. Cortex. 2008;18:2735–2747. doi: 10.1093/cercor/bhn041. [DOI] [PubMed] [Google Scholar]
- Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH. Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat. Genet. 1996;12:78–80. doi: 10.1038/ng0196-78. [DOI] [PubMed] [Google Scholar]
- Edmiston EK, Blackford JU. Childhood maltreatment and response to novel face stimuli presented during functional magnetic resonance imaging in adults. Psychiatry Res. Neuroimaging. 2013;212:36–42. doi: 10.1016/j.pscychresns.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: Evidence from human neuroimaging studies. Cereb. Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Ernst M, Fudge JL. A developmental neurobiological model of motivated behavior: anatomy, connectivity and ontogeny of the triadic nodes. Neurosci. Biobehav. Rev. 2009;33:367–382. doi: 10.1016/j.neubiorev.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychol. Med. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Slattery MJ, Goldsmith HH, Kalin NH. Early risk factors and developmental pathways to chronic high inhibition and social anxiety disorder in adolescence. Am. J. Psychiatry. 2010;167:40–46. doi: 10.1176/appi.ajp.2009.07010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavin SA, Winder DG. Noradrenergic control of the bed nucleus of the stria terminalis in stress and reward. Neuropharmacology. 2013;70:324–330. doi: 10.1016/j.neuropharm.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Kalin NH. A translational neuroscience approach to understanding the development of social anxiety disorder and its pathophysiology. Am. J. Psychiatry. 2014;171:1162–1173. doi: 10.1176/appi.ajp.2014.14040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Oler JA, Shelton SE, Nanda SA, Davidson RJ, Roseboom PH, Kalin NH. Central amygdala nucleus (Ce) gene expression linked to increased trait-like Ce metabolism and anxious temperament in young primates. Proc. Natl. Acad. Sci. 2012;109:18108–18113. doi: 10.1073/pnas.1206723109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Converse AK, Davidson RJ, Kalin NH. Orbitofrontal cortex lesions alter anxiety-related activity in the primate bed nucleus of stria terminalis. J. Neurosci. 2010;30:7023–7027. doi: 10.1523/JNEUROSCI.5952-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox AS, Shelton SE, Oakes TR, Davidson RJ, Kalin NH. Trait-like brain activity during adolescence predicts anxious temperament in primates. PLoS ONE. 2008;3:e2570. doi: 10.1371/journal.pone.0002570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox NA. Factors contributing to the emergence of anxiety among behaviorally inhibited children: The role of attention. New Dir. Child Adolesc. Dev. 2010;2010:33–49. doi: 10.1002/cd.261. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Marshall PJ, Nichols KE, Ghera MM. Behavioral inhibition: linking biology and behavior within a developmental framework. Annu Rev Psychol. 2005a;56:235–262. doi: 10.1146/annurev.psych.55.090902.141532. [DOI] [PubMed] [Google Scholar]
- Fox NA, Henderson HA, Rubin KH, Calkins SD, Schmidt LA. Continuity and discontinuity of behavioral inhibition and exuberance: psychophysiological and behavioral influences across the first four years of life. Child Dev. 2001;72:1–21. doi: 10.1111/1467-8624.00262. [DOI] [PubMed] [Google Scholar]
- Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, Ernst M, Pine DS. Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychol. Sci. 2005b;16:921–926. doi: 10.1111/j.1467-9280.2005.01637.x. [DOI] [PubMed] [Google Scholar]
- Frank DW, Dewitt M, Hudgens-Haney M, Schaeffer DJ, Ball BH, Schwarz NF, Hussein AA, Smart LM, Sabatinelli D. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 2014;45:202–211. doi: 10.1016/j.neubiorev.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The Human Amygdala. New York, NY: The Guilford Press; 2009. pp. 3–42. [Google Scholar]
- Garcia-Coll C, Kagan J, Reznick JS. Behavioral inhibition in young children. Child Dev. 1984;55:1005–1019. [Google Scholar]
- Gest SD. Behavioral inhibition: Stability and associations with adaptation from childhood to early adulthood. J. Pers. Soc. Psychol. 1997;72:467–475. doi: 10.1037//0022-3514.72.2.467. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. NeuroImage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB. Is behavioral inhibition a risk factor for depression? J. Affect. Disord. 2006;95:85–94. doi: 10.1016/j.jad.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Gladstone GL, Parker GB, Mitchell PB, Wilhelm KA, Malhi GS. Relationship between self-reported childhood behavioral inhibition and lifetime anxiety disorders in a clinical sample. Depress. Anxiety. 2005;22:103–113. doi: 10.1002/da.20082. [DOI] [PubMed] [Google Scholar]
- Gladstone G, Parker G. Measuring a behaviorally inhibited temperament style: Development and initial validation of new self-report measures. Psychiatry Res. 2005;135:133–143. doi: 10.1016/j.psychres.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg JO, Schmidt LA. Shyness, sociability, and social dysfunction in schizophrenia. Schizophr. Res. 2001;48:343–349. doi: 10.1016/s0920-9964(00)00143-2. [DOI] [PubMed] [Google Scholar]
- Gosling SD, John OP. Personality dimensions in nonhuman animals: a cross-species review. Curr. Dir. Psychol. Sci. 1999;8:69–75. [Google Scholar]
- Gray JA. The neuropsychology of anxiety - an inquiry into the functions of the septo-hippocampal system. Behav. Brain Sci. 1982;5:469–484. [Google Scholar]
- Grupe DW, Nitschke JB. Uncertainty and anticipation in anxiety: an integrated neurobiological and psychological perspective. Nat. Rev. Neurosci. 2013;14:488–501. doi: 10.1038/nrn3524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Benson B, Choate VR, Bar-Haim Y, Perez-Edgar K, Jarcho JM, Pine DS, Ernst M, Fox NA, Nelson EE. Lasting associations between early-childhood temperament and late-adolescent reward-circuitry response to peer feedback. Dev. Psychopathol. 2014;26:229–243. doi: 10.1017/S0954579413000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Nelson EE, Perez-Edgar K, Hardin MG, Roberson-Nay R, Monk CS, Bjork JM, Henderson HA, Pine DS, Fox NA, Ernst M. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J. Neurosci. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haaker J, Lonsdorf TB, Raczka KA, Mechias M-L, Gartmann N, Kalisch R. Higher anxiety and larger amygdala volumes in carriers of a TMEM132D risk variant for panic disorder. Transl. Psychiatry. 2014;4:e357. doi: 10.1038/tp.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2009;35:4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardee JE, Benson BE, Bar-Haim Y, Mogg K, Bradley BP, Chen G, Britton JC, Ernst M, Fox NA, Pine DS, Pérez-Edgar K. Patterns of neural connectivity during an attention bias task moderates associations between early childhood temperament and internalizing symptoms in young adulthood. Biol. Psychiatry. 2013;74:273–279. doi: 10.1016/j.biopsych.2013.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden ER, Dougherty LR, Maloney B, Durbin CE, Olino TM, Nurnberger JI, Lahiri DK, Klein DN. Temperamental fearfulness in childhood and the serotonin transporter promoter region polymorphism: a multimethod association study. Psychiatr. Genet. 2007;17:135–142. doi: 10.1097/YPG.0b013e3280147847. [DOI] [PubMed] [Google Scholar]
- Heilig M, McLeod S, Brot M, Heinrichs SC, Menzaghi F, Koob GF, Britton KT. Anxiolytic-like action of neuropeptide Y: mediation by Y1 receptors in amygdala, and dissociation from food intake effects. Neuropsychopharmacology. 1993;8:357–363. doi: 10.1038/npp.1993.35. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, Wrase J, Puls I, Hermann D, Klein S, Grusser SM, Flor H, Schumann G, Mann K, Buchel C. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nat Neurosci. 2005;8:20–21. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Helfinstein SM, Benson B, Perez-Edgar K, Bar-Haim Y, Detloff A, Pine DS, Fox NA, Ernst M. Striatal responses to negative monetary outcomes differ between temperamentally inhibited and non-inhibited adolescents. Neuropsychologia. 2011;49:479–485. doi: 10.1016/j.neuropsychologia.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson HA. Electrophysiological correlates of cognitive control and the regulation of shyness in children. Dev. Neuropsychol. 2010;35:177–193. doi: 10.1080/87565640903526538. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Pine DS, Fox NA. Behavioral inhibition and developmental risk: a dual-processing perspective. Neuropsychopharmacology. 2014 doi: 10.1038/npp.2014.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Hettema JM, Sun C, Chen X, Kendler KS. Genetic association study between RGS2 and anxiety-related phenotypes. Psychiatr. Genet. 2013;23:92. doi: 10.1097/YPG.0b013e32835d70b3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Tessner K, Wang SH, Carter H, McDermott M. Temperament at 5 years of age predicts amygdala and orbitofrontal volume in the right hemisphere in adolescence. Psychiatry Res.-Neuroimaging. 2010;182:14–21. doi: 10.1016/j.pscychresns.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR. Familial and temperamental risk factors for social anxiety disorder. New Dir. Child Adolesc. Dev. 2010;2010:51–65. doi: 10.1002/cd.262. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Calltharp S, Rosenbaum ED, Faraone SV, Rosenbaum JF. Behavioral inhibition and disinhibition as hypothesized precursors to psychopathology: implications for pediatric bipolar disorder. Biol. Psychiatry. 2003;53:985–999. doi: 10.1016/s0006-3223(03)00316-0. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Faraone SV, Violette H, Wrightsman J, Rosenbaum JF. Temperamental correlates of disruptive behavior disorders in young children: preliminary findings. Biol. Psychiatry. 2002;51:563–574. doi: 10.1016/s0006-3223(01)01299-9. [DOI] [PubMed] [Google Scholar]
- Hirshfeld-Becker DR, Biederman J, Henin A, Faraone SV, Micco JA, van Grondelle A, Henry B, Rosenbaum JF. Clinical outcomes of laboratory-observed preschool behavioral disinhibition at five-year follow-up. Biol. Psychiatry. 2007;62:565–572. doi: 10.1016/j.biopsych.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshfeld DR, Rosenbaum JF, Biederman J, Bolduc EA, Faraone SV, Snidman N, Reznick JS, Kagan J. Stable behavioral-inhibition and its association with anxiety disorder. J. Am. Acad. Child Adolesc. Psychiatry. 1992;31:103–111. doi: 10.1097/00004583-199201000-00016. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Bitran S. Sensory-processing sensitivity in social anxiety disorder: relationship to harm avoidance and diagnostic subtypes. J. Anxiety Disord. 2007;21:944–954. doi: 10.1016/j.janxdis.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey CJ, Sporns O, Cammoun L, Gigandet X, Thiran J-P, Meuli R, Hagmann P. Predicting human resting-state functional connectivity from structural connectivity. Proc. Natl. Acad. Sci. 2009;106:2035–2040. doi: 10.1073/pnas.0811168106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iidaka T, Matsumoto A, Ozaki N, Suzuki T, Iwata N, Yamamoto Y, Okada T, Sadato N. Volume of left amygdala subregion predicted temperamental trait of harm avoidance in female young subjects. A voxel-based morphometry study. Brain Res. 2006;1125:85–93. doi: 10.1016/j.brainres.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. J. Neurophysiol. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Etkin A, Leibenluft E, Shechner T, Ernst M. The neural correlates of emotion-based cognitive control in adults with early childhood behavioral inhibition. Biol. Psychol. 2013;92:306–314. doi: 10.1016/j.biopsycho.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarcho JM, Fox NA, Pine DS, Leibenluft E, Shechner T, Degnan KA, Perez-Edgar K, Ernst M. Enduring influence of early temperament on neural mechanisms mediating attention-emotion conflict in adults. Depress. Anxiety. 2014;31:53–62. doi: 10.1002/da.22140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrard LE. On the use of ibotenic acid to lesion selectively different components of the hippocampal formation. J. Neurosci. Methods. 1989;29:251–259. doi: 10.1016/0165-0270(89)90149-0. [DOI] [PubMed] [Google Scholar]
- Jetha MK, Goldberg JO, Schmidt LA. Temperament and its relation to social functioning in schizophrenia. Int. J. Soc. Psychiatry. 2013;59:254–263. doi: 10.1177/0020764011433639. [DOI] [PubMed] [Google Scholar]
- Jiao X, Beck KD, Pang KCH, Servatius RJ. Animal models of anxiety vulnerability - the Wistar Kyoto rat. In: Selek S, editor. Different Views on Anxiety Disorders. Rijeka: InTech; 2011a. [Google Scholar]
- Jiao X, Pang KCH, Beck KD, Minor TR, Servatius RJ. Avoidance perseveration during extinction training in Wistar-Kyoto rats: an interaction of innate vulnerability and stressor intensity. Behav. Brain Res. 2011b;221:98–107. doi: 10.1016/j.bbr.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Prior M, Sanson A, Smart D, Zhang Y, Easteal S. Association of a functional polymorphism of the serotonin transporter gene with anxiety-related temperament and behavior problems in children: a longitudinal study from infancy to the mid-teens. Mol. Psychiatry. 2000;5:542–547. doi: 10.1038/sj.mp.4000782. [DOI] [PubMed] [Google Scholar]
- Kagan J, Moss HA. Birth to Maturity: A Study in Psychological Development. London: Wiley; 1962. [Google Scholar]
- Kagan J, Reznick JS, Clarke C, Snidman N, Garcia-Coll C. Behavioral inhibition to the unfamiliar. Child Dev. 1984;55:2212–2225. [Google Scholar]
- Kagan J, Reznick JS, Snidman N. The physiology and psychology of behavioral inhibition in children. Child Dev. 1987;58:1459–1473. [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N. Biological bases of childhood shyness. Science. 1988a;240:167–171. doi: 10.1126/science.3353713. [DOI] [PubMed] [Google Scholar]
- Kagan J, Reznick JS, Snidman N, Gibbons J, Johnson MO. Childhood derivatives of inhibition and lack of inhibition to the unfamiliar. Child Dev. 1988b;59:1580–1589. doi: 10.1111/j.1467-8624.1988.tb03685.x. [DOI] [PubMed] [Google Scholar]
- Kagan J, Snidman N. Infant predictors of inhibited and uninhibited profiles. Psychol. Sci. 1991;2:40–44. [Google Scholar]
- Kagan J, Snidman N. The Long Shadow of Temperament. Cambridge, MA.: Harvard University Press; 2004. [Google Scholar]
- Kagan J, Snidman N, Arcus D. Childhood derivatives of high and low reactivity in infancy. Child Dev. 1998;69:1483–1493. [PubMed] [Google Scholar]
- Kagan J, Snidman N, Kahn V, Towsley S, Steinberg L, Fox NA. The preservation of two infant temperaments into adolescence. Monogr. Soc. Res. Child Dev. 2007;i–95 doi: 10.1111/j.1540-5834.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- Kalin NH. Nonhuman primate studies of fear, anxiety, and temperament and the role of benzodiazepine receptors and GABA systems. J. Clin. Psychiatry. 2003;64:41–44. [PubMed] [Google Scholar]
- Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behav. Neurosci. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Larson C, Shelton SE, Davidson RJ. Asymmetric frontal brain activity, cortisol, and behavior associated with fearful temperament in rhesus monkeys. Behav. Neurosci. 1998;112:286–292. doi: 10.1037//0735-7044.112.2.286. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. The regulation of defensive behaviors in rhesus monkeys. In: Davidson RJ, editor. Anxiety, Depression, and Emotion, Series in Affective Science. New York, NY: Oxford University Press; 2000. pp. 50–68. [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann. N. Y. Acad. Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Barksdale CM. Opiate modulation of separation-induced distress in non-human primates. Brain Res. 1988;440:285–292. doi: 10.1016/0006-8993(88)90997-3. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Cerebrospinal fluid corticotropin-releasing hormone levels are elevated in monkeys with patterns of brain activity associated with fearful temperament. Biol. Psychiatry. 2000;47:579–585. doi: 10.1016/s0006-3223(99)00256-5. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Cerebrospinal fluid corticotropin-releasing hormone levels are elevated in monkeys with patterns of brain activity associated with fearful temperament. Biol. Psychiatry. 2000;47:579–585. doi: 10.1016/s0006-3223(99)00256-5. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. J. Neurosci. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol. Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. J. Neurosci. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Fox AS, Oakes TR, Davidson RJ. Brain regions associated with the expression and contextual regulation of anxiety in primates. Biol. Psychiatry. 2005;58:796–804. doi: 10.1016/j.biopsych.2005.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HJ, Schuman EM. Neurotrophin-induced modulation of synaptic transmission in the adult hippocampus. J. Physiol. 1995;89:11–22. doi: 10.1016/0928-4257(96)80547-x. [DOI] [PubMed] [Google Scholar]
- Karg K, Burmeister M, Shedden K, Sen S. The serotonin transporter promoter variant (5-HTTLPR), stress, and depression meta-analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry. 2011;68:444–54. doi: 10.1001/archgenpsychiatry.2010.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe P, Blass EM. Opioid-mediation of separation distress in 10-day-old rats: Reversal of stress with maternal stimuli. Dev. Psychobiol. 1986;19:385–398. doi: 10.1002/dev.420190410. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005a;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry. 2005b;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluger AN, Siegfried Z, Ebstein RP. A meta-analysis of the association between DRD4 polymorphism and novelty seeking. Mol. Psychiatry. 2002;7:712–717. doi: 10.1038/sj.mp.4001082. [DOI] [PubMed] [Google Scholar]
- Knight RT. Contribution of human hippocampal region to novelty detection. Nature. 1996;383:256–259. doi: 10.1038/383256a0. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2009;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korte SM, Koolhaas JM, Wingfield JC, McEwen BS. The Darwinian concept of stress: benefits of allostasis and costs of allostatic load and the trade-offs in health and disease. Neurosci. Biobehav. Rev. 2005;29:3–38. doi: 10.1016/j.neubiorev.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kringelbach M. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lahat A, Hong M, Fox NA. Behavioural inhibition: Is it a risk factor for anxiety? Int. Rev. Psychiatry. 2011;23:248–257. doi: 10.3109/09540261.2011.590468. [DOI] [PubMed] [Google Scholar]
- Lahat A, Lamm C, Chronis-Tuscano A, Pine DS, Fox NA. Early behavioral inhibition and increased error monitoring predict later social phobia symptoms in childhood. J. Am. Acad. Child Adolesc. Psychiatry. 2014;53:447–455. doi: 10.1016/j.jaac.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahat A, Pérez-Edgar K, Degnan KA, Guyer AE, Lejuez CW, Ernst M, Pine DS, Fox NA. Early childhood temperament predicts substance use in young adults. Transl. Psychiatry. 2012;2:e157. doi: 10.1038/tp.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm C, Walker OL, Degnan KA, Henderson HA, Pine DS, McDermott JM, Fox NA. Cognitive control moderates early childhood temperament in predicting social behavior in 7-year-old children: an ERP study. Dev. Sci. 2014;17:667–681. doi: 10.1111/desc.12158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988;8:2517–2529. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehéricy S, Ducros M, Van De Moortele P-F, Francois C, Thivard L, Poupon C, Swindale N, Ugurbil K, Kim D-S. Diffusion tensor fiber tracking shows distinct corticostriatal circuits in humans. Ann. Neurol. 2004;55:522–529. doi: 10.1002/ana.20030. [DOI] [PubMed] [Google Scholar]
- Leisman G, Braun-Benjamin O, Melillo R. Cognitive-motor interactions of the basal ganglia in development. Front. Syst. Neurosci. 2014;8 doi: 10.3389/fnsys.2014.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–645. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luck SJ, Woodman GF, Vogel EK. Event-related potential studies of attention. Trends Cogn. Sci. 2000;4:432–440. doi: 10.1016/s1364-6613(00)01545-x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Kazama AM, Bachevalier J. Impact of amygdala, orbital frontal, or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9:147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheu FS, Dozier M, Guyer AE, Mandell D, Peloso E, Poeth K, Jenness J, Lau JYF, Ackerman JP, Pine DS, Ernst M. A preliminary study of medial temporal lobe function in youths with a history of caregiver deprivation and emotional neglect. Cogn. Affect. Behav. Neurosci. 2010;10:34–49. doi: 10.3758/CABN.10.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Reeb BC, Fox NA. Electrophysiological responses to auditory novelty in temperamentally different 9-month-old infants. Dev. Sci. 2009;12:568–582. doi: 10.1111/j.1467-7687.2008.00808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall PJ, Stevenson-Hinde J. Behavioral inhibition, heart period, and respiratory sinus arrhythmia in young children. Dev. Psychobiol. 1998;33:283–292. [PubMed] [Google Scholar]
- Martínez A, Alcántara S, Borrell V, Del Río JA, Blasi J, Otal R, Campos N, Boronat A, Barbacid M, Silos-Santiago I, Soriano E. TrkB and TrkC signaling are required for maturation and synaptogenesis of hippocampal connections. J. Neurosci. 1998;18:7336–7350. doi: 10.1523/JNEUROSCI.18-18-07336.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott JM, Perez-Edgar K, Henderson HA, Chronis-Tuscano A, Pine DS, Fox NA. A history of childhood behavioral inhibition and enhanced response monitoring in adolescence are linked to clinical anxiety. Biol. Psychiatry. 2009;65:445–448. doi: 10.1016/j.biopsych.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McManis MH, Kagan J, Snidman NC, Woodward SA. EEG asymmetry, power, and temperament in children. Dev. Psychobiol. 2002;41:169–177. doi: 10.1002/dev.10053. [DOI] [PubMed] [Google Scholar]
- McMenamin BW, Langeslag SJE, Sirbu M, Padmala S, Pessoa L. Network organization unfolds over time during periods of anxious anticipation. J. Neurosci. 2014;34:11261–11273. doi: 10.1523/JNEUROSCI.1579-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meysamie A, Ghalehtaki R, Borjian A, Daneshvar-fard M, Mohammadi MR, Saboohi F. Prevalence of behavioral inhibition among preschool aged children in Tehran, Iran. Acta Med. Iran. 2014;52:298–302. [PubMed] [Google Scholar]
- Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc. Natl. Acad. Sci. U. S. A. 2005;102:9371–9376. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SC, Aouidad A, Gorodetsky E, Goldman D, Pine DS, Ernst M. Gray matter volume in adolescent anxiety: an impact of the brain-derived neurotrophic factor Val66Met polymorphism? J. Am. Acad. Child Adolesc. Psychiatry. 2013;52:184–195. doi: 10.1016/j.jaac.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Munafò MR, Brown SM, Hariri AR. Serotonin transporter (5-HTTLPR) genotype and amygdala activation: a meta-analysis. Biol. Psychiatry. 2008a;63:852–857. doi: 10.1016/j.biopsych.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munafò MR, Yalcin B, Willis-Owen Sa, Flint J. Association of the dopamine D4 receptor (DRD4) gene and approach-related personality traits: meta-analysis and new data. Biol. Psychiatry. 2008b;63:197–206. doi: 10.1016/j.biopsych.2007.04.006. [DOI] [PubMed] [Google Scholar]
- Nestor L, Hester R, Garavan H. Increased ventral striatal BOLD activity during non-drug reward anticipation in cannabis users. NeuroImage. 2010;49:1133–1143. doi: 10.1016/j.neuroimage.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschke JB, Sarinopoulos I, Mackiewicz KL, Schaefer HS, Davidson RJ. Functional neuroanatomy of aversion and its anticipation. NeuroImage. 2006;29:106–116. doi: 10.1016/j.neuroimage.2005.06.068. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Gross J. The cognitive control of emotion. Trends Cogn. Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Oler JA, Fox AS, Shackman AJ, Kalin NH. The central nucleus of the amygdala is a critical substrate for individual differences in anxiety. In: Amaral DG, Bauman MD, Adolphs R, editors. Living Without an Amygdala. In press. [Google Scholar]
- Oler JA, Fox AS, Shelton SE, Rogers J, Dyer TD, Davidson RJ, Shelledy W, Oakes TR, Blangero J, Kalin NH. Amygdalar and hippocampal substrates of anxious temperament differ in their heritability. Nature. 2010;466:864–868. doi: 10.1038/nature09282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oler J, Birn R, Patriat R, Fox AS, Shelton S, Burghy C, Stodola D, Essex M, Davidson R, Kalin NH. Evidence for coordinated functional activity within the extended amygdala of non-human and human primates. NeuroImage. 2012;61:1059–1066. doi: 10.1016/j.neuroimage.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–1090. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: a volumetric magnetic resonance imaging study. Hippocampus. 2010;20:922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedreira C, Mormann F, Kraskov A, Cerf M, Fried I, Koch C, Quiroga RQ. Responses of human medial temporal lobe neurons are modulated by stimulus repetition. J. Neurophysiol. 2010;103:97–107. doi: 10.1152/jn.91323.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Hardee JE, Guyer AE, Benson BE, Nelson EE, Gorodetsky E, Goldman D, Fox Na, Pine DS, Ernst M. DRD4 and striatal modulation of the link between childhood behavioral inhibition and adolescent anxiety. Soc. Cogn. Affect. Neurosci. 2013 doi: 10.1093/scan/nst001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, Ernst M. Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage. 2007;35:1538–1546. doi: 10.1016/j.neuroimage.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Schmidt LA, Henderson HA, Schulkin J, Fox NA. Salivary cortisol levels and infant temperament shape developmental trajectories in boys at risk for behavioral maladjustment. Psychoneuroendocrinology. 2008;33:916–925. doi: 10.1016/j.psyneuen.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrotti LI, Dennis TS, Jiao X, Servatius RJ, Pang KCH, Beck KD. Activation of extracellular signal-regulated kinase (ERK) and ΔFosB in emotion-associated neural circuitry after asymptotic levels of active avoidance behavior are attained. Brain Res. Bull. 2013;98:102–110. doi: 10.1016/j.brainresbull.2013.07.004. [DOI] [PubMed] [Google Scholar]
- Pert CB, Kuhar MJ, Snyder SH. Opiate receptor: autoradiographic localization in rat brain. Proc. Natl. Acad. Sci. 1976;73:3729–3733. doi: 10.1073/pnas.73.10.3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps E. Human emotion and memory: interactions of the amygdala and hippocampal complex. Curr. Opin. Neurobiol. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA. Depression, stress, and anhedonia: toward a synthesis and integrated model. Annu. Rev. Clin. Psychol. 2014;10:393–423. doi: 10.1146/annurev-clinpsy-050212-185606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. J. Neurosci. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi C, Roseboom PH, Nanda SA, Lane JC, Speers JM, Kalin NH. Anxiety-related behavioral inhibition in rats: a model to examine mechanisms underlying the risk to develop stress-related psychopathology. Genes Brain Behav. 2010;9:974–984. doi: 10.1111/j.1601-183X.2010.00636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Bachevalier J, Wallen K, Sanchez M. Neonatal amygdala lesions alter basal cortisol levels in infant rhesus monkeys. Psychoneuroendocrinology. 2013a;38:818–829. doi: 10.1016/j.psyneuen.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wallen K, Sanchez MM, Stephens SBZ, Henry A, Villareal T, Bachevalier J. Sex-dependent role of the amygdala in the development of emotional and neuroendocrine reactivity to threatening stimuli in infant and juvenile rhesus monkeys. Horm. Behav. 2013b;63:646–658. doi: 10.1016/j.yhbeh.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Wilson M, Sanchez M, Machado CJ, Bachevalier J. Pervasive alterations of emotional and neuroendocrine responses to an acute stressor after neonatal amygdala lesions in rhesus monkeys. Psychoneuroendocrinology. 2013c;38:1021–1035. doi: 10.1016/j.psyneuen.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 2012;36:479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeb-Sutherland BC, Vanderwert RE, Degnan KA, Marshall PJ, Pérez-Edgar K, Chronis-Tuscano A, Pine DS, Fox NA. Attention to novelty in behaviorally inhibited adolescents moderates risk for anxiety. J. Child Psychol. Psychiatry. 2009;50:1365–1372. doi: 10.1111/j.1469-7610.2009.02170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reivich M, Kuhl D, Wolf A, Greenberg J, Phelps M, Ido T, Casella V, Fowler J, Hoffman E, Alavi A, Som P, Sokoloff L. The [18F]fluorodeoxyglucose method for the measurement of local cerebral glucose utilization in man. Circ. Res. 1979;44:127–137. doi: 10.1161/01.res.44.1.127. [DOI] [PubMed] [Google Scholar]
- Reznick JS, Hegeman IM, Kaufman ER, Woods SW, Jacobs M. Retrospective and concurrent self-report of behavioral-inhibition and their relation to adult mental-health. Dev. Psychopathol. 1992;4:301–321. [Google Scholar]
- Rilling JK, Winslow JT, O’Brien D, Gutman DA, Hoffman JM, Kilts CD. Neural correlates of maternal separation in rhesus monkeys. Biol. Psychiatry. 2001;49:146–157. doi: 10.1016/s0006-3223(00)00977-x. [DOI] [PubMed] [Google Scholar]
- Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin D-Y, Duan J, Ophoff RA, Andreassen OA, Scolnick E, Cichon S, St. Clair D, Corvin A, Gurling H, Werge T, Rujescu D, Blackwood DHR, Pato CN, Malhotra AK, Purcell S, Dudbridge F, Neale BM, Rossin L, Visscher PM, Posthuma D, Ruderfer DM, Fanous A, Stefansson H, Steinberg S, Mowry BJ, Golimbet V, De Hert M, Jönsson EG, Bitter I, Pietiläinen OPH, Collier DA, Tosato S, Agartz I, Albus M, Alexander M, Amdur RL, Amin F, Bass N, Bergen SE, Black DW, Børglum AD, Brown MA, Bruggeman R, Buccola NG, Byerley WF, Cahn W, Cantor RM, Carr VJ, Catts SV, Choudhury K, Cloninger CR, Cormican P, Craddock N, Danoy PA, Datta S, de Haan L, Demontis D, Dikeos D, Djurovic S, Donnelly P, Donohoe G, Duong L, Dwyer S, Fink-Jensen A, Freedman R, Freimer NB, Friedl M, Georgieva L, Giegling I, Gill M, Glenthøj B, Godard S, Hamshere M, Hansen M, Hansen T, Hartmann AM, Henskens FA, Hougaard DM, Hultman CM, Ingason A, Jablensky AV, Jakobsen KD, Jay M, Jürgens G, Kahn RS, Keller MC, Kenis G, Kenny E, Kim Y, Kirov GK, Konnerth H, Konte B, Krabbendam L, Krasucki R, Lasseter VK, Laurent C, Lawrence J, Lencz T, Lerer FB, Liang K-Y, Lichtenstein P, Lieberman JA, Linszen DH, Lönnqvist J, Loughland CM, Maclean AW, Maher BS, Maier W, Mallet J, Malloy P, Mattheisen M, Mattingsdal M, McGhee KA, McGrath JJ, McIntosh A, McLean DE, McQuillin A, Melle I, Michie PT, Milanova V, Morris DW, Mors O, Mortensen PB, Moskvina V, Muglia P, Myin-Germeys I, Nertney DA, Nestadt G, Nielsen J, Nikolov I, Nordentoft M, Norton N, Nöthen MM, O’Dushlaine CT, Olincy A, Olsen L, O’Neill FA, Ørntoft TF, Owen MJ, Pantelis C, Papadimitriou G, Pato MT, Peltonen L, Petursson H, Pickard B, Pimm J, Pulver AE, Puri V, Quested D, Quinn EM, Rasmussen HB, Réthelyi JM, Ribble R, Rietschel M, Riley BP, Ruggeri M, Schall U, Schulze TG, Schwab SG, Scott RJ, Shi J, Sigurdsson E, Silverman JM, Spencer CCA, Stefansson K, Strange A, Strengman E, Stroup TS, Suvisaari J, Terenius L, Thirumalai S, Thygesen JH, Timm S, Toncheva D, van den Oord E, van Os J, van Winkel R, Veldink J, Walsh D, Wang AG, Wiersma D, Wildenauer DB, Williams HJ, Williams NM, Wormley B, Zammit S, Sullivan PF, O’Donovan MC, Daly MJ, Gejman PV. Genome-wide association study identifies five new schizophrenia loci. Nat. Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler Ja, Cheverud J, Muzny DM, Gibbs Ra, Davidson RJ, Kalin NH. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol. Psychiatry. 2012:1–8. doi: 10.1038/mp.2012.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Shelton SE, Shelledy W, Garcia R, Kalin NH. Genetic influences on behavioral inhibition and anxiety in juvenile rhesus macaques. Genes Brain Behav. 2008;7:463–469. doi: 10.1111/j.1601-183X.2007.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom PH, Nanda Sa, Fox AS, Oler Ja, Shackman AJ, Shelton SE, Davidson RJ, Kalin NH. Neuropeptide Y receptor gene expression in the primate amygdala predicts anxious temperament and brain metabolism. Biol. Psychiatry. 2013:1–8. doi: 10.1016/j.biopsych.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseboom PH, Nanda SA, Fox AS, Oler JA, Shackman AJ, Shelton SE, Davidson RJ, Kalin NH. Neuropeptide Y receptor gene expression in the primate amygdala predicts anxious temperament and brain metabolism. Biol. Psychiatry. 2014 doi: 10.1016/j.biopsych.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenkranz JA, Grace AA. Cellular mechanisms of infralimbic and prelimbic prefrontal cortical inhibition and dopaminergic modulation of basolateral amygdala neurons in vivo. J. Neurosci. 2002;22:324–337. doi: 10.1523/JNEUROSCI.22-01-00324.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbart MK. Measurement of temperament in infancy. Child Dev. 1981;52:569–578. [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. J. Pers. Soc. Psychol. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AMC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russchen FT, Bakst I, Amaral DG, Price JL. The amygdalostriatal projections in the monkey. An anterograde tracing study. Brain Res. 1985;329:241–257. doi: 10.1016/0006-8993(85)90530-x. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber ESL, De Armentia ML, Power J. The amygdaloid complex: anatomy and physiology. Physiol. Rev. 2003;83:803–834. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- Scarpa A, Raine A, Venables PH, Mednick SA. The stability of inhibited/uninhibited temperament from ages 3 to 11 years in Mauritian children. J. Abnorm. Child Psychol. 1995;23:607–618. doi: 10.1007/BF01447665. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA. Patterns of cortical electrophysiology and autonomic activity in adults’ shyness and sociability. Biol. Psychol. 1994;38:183–198. doi: 10.1016/0301-0511(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Hu S, Hamer DH. Molecular genetics of shyness and aggression in preschoolers. Personal. Individ. Differ. 2002;33:227–238. [Google Scholar]
- Schmidt LA, Fox NA, Rubin KH, Sternberg EM, Gold PW, Smith CC. Behavioral and neuroendocrine responses in shy children. Dev. Psychobiol. 1997;30:127–140. doi: 10.1002/(sici)1098-2302(199703)30:2<127::aid-dev4>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Schmidt LA, Fox NA, Schulkin J, Gold PW. Behavioral and psychophysiological correlates of self-presentation in temperamentally shy children. Dev. Psychobiol. 1999;35:119–135. [PubMed] [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, Kagan J, Snidman NC, Bloch RB. A phenotype of early infancy predicts reactivity of the amygdala in male adults. Mol. Psychiatry. 2012;17:1042–1050. doi: 10.1038/mp.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Kunwar PS, Greve DN, Moran LR, Viner JC, Covino JM, Kagan J, Stewart SE, Snidman NC, Vangel MG, Wallace SR. Structural differences in adult orbital and ventromedial prefrontal cortex are predicted by 4-month infant temperament. Arch. Gen. Psychiatry. 2010;67:78–84. doi: 10.1001/archgenpsychiatry.2009.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz CE, Snidman N, Kagan J. Adolescent social anxiety as an outcome of inhibited temperament in childhood. J. Am. Acad. Child Adolesc. Psychiatry. 1999;38:1008–1015. doi: 10.1097/00004583-199908000-00017. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin L, Kagan J, Rauch SL. Inhibited and uninhibited infants “grown up”: adult amygdalar response to novelty. Science. 2003a;300:1952–1953. doi: 10.1126/science.1083703. [DOI] [PubMed] [Google Scholar]
- Schwartz CE, Wright CI, Shin LM, Kagan J, Whalen PJ, McMullin KG, Rauch SL. Differential amygdalar response to novel versus newly familiar neutral faces: a functional MRI probe developed for studying inhibited temperament. Biol. Psychiatry. 2003b;53:854–862. doi: 10.1016/s0006-3223(02)01906-6. [DOI] [PubMed] [Google Scholar]
- Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J. Neurol. Neurosurg. Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Burmeister M, Ghosh D. Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;127:85–89. doi: 10.1002/ajmg.b.20158. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Fox AS, Oler JA, Shelton SE, Davidson RJ, Kalin NH. Neural mechanisms underlying heterogeneity in the presentation of anxious temperament. Proc. Natl. Acad. Sci. 2013;110:6145–6150. doi: 10.1073/pnas.1214364110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen K, Bezgin G, Hutchison RM, Gati JS, Menon RS, Everling S, McIntosh AR. Information processing architecture of functionally defined clusters in the macaque cortex. J. Neurosci. 2012;32:17465–17476. doi: 10.1523/JNEUROSCI.2709-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman Y, Winder DG. Emerging role for corticotropin releasing factor signaling in the bed nucleus of the stria terminalis at the intersection of stress and reward. Front. Psychiatry. 2013:4. doi: 10.3389/fpsyt.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Acierno JS, Rosenbaum JF, Biederman J, Pollack MH, Meminger S, Pava JA, Chadwick LH, White C, Bulzacchelli M, Slaugenhaupt SA. Targeted genome screen of panic disorder and anxiety disorder proneness using homology to murine QTL regions. Am. J. Med. Genet. Neuropsychiatr. Genet. 2001a;105:195–206. doi: 10.1002/ajmg.1209. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Paulus MP, Fagerness JA, Purcell S, Yamaki LH, Hirshfeld-Becker DR, Biederman J, Rosenbaum JF, Gelernter J, Stein MB. Influence of RGS2 on Anxiety-Related Temperament, Personality, and Brain Function. Arch. Gen. Psychiatry. 2008;65:298–308. doi: 10.1001/archgenpsychiatry.2007.48. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Rosenbaum JF, Biederman J, Kennedy J, Dai D, Racette SR, Laird NM, Kagan J, Snidman N, Hirshfeld-Becker DR, Tsuang MT, Sklar PB, Slaugenhaupt SA. Association of a genetic marker at the corticotropin-releasing hormone locus with behavioral inhibition. Biol. Psychiatry. 2003;54:1376–1381. doi: 10.1016/s0006-3223(03)00598-5. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Rosenbaum JF, Biederman J, Susswein LS, Kennedy J, Kagan J, Snidman N, Laird N, Tsuang MT, Faraone SV, Schwarz A, Slaugenhaupt SA. Genetic association analysis of behavioral inhibition using candidate loci from mouse models. Am. J. Med. Genet. 2001b;105:226–235. doi: 10.1002/ajmg.1328. [DOI] [PubMed] [Google Scholar]
- Smoller JW, Yamaki LH, Fagerness JA, Biederman J, Racette S, Laird NM, Kagan J, Snidman N, Faraone SV, Hirshfeld-Becker DR, Tsuang MT, Slaugenhaupt SA, Rosenbaum JF, Sklar PB. The corticotropin-releasing hormone gene and behavioral inhibition in children at risk for panic disorder. Biol. Psychiatry. 2005;57:1485–1492. doi: 10.1016/j.biopsych.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Whalen PJ, Kelley WM. Human bed nucleus of the stria terminalis indexes hypervigilant threat monitoring. Biol. Psychiatry. 2010;68:416–424. doi: 10.1016/j.biopsych.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sørensen G, Lindberg C, Wörtwein G, Bolwig TG, Woldbye DPD. Differential roles for neuropeptide Y Y1 and Y5 receptors in anxiety and sedation. J. Neurosci. Res. 2004;77:723–729. doi: 10.1002/jnr.20200. [DOI] [PubMed] [Google Scholar]
- Stamatakis AM, Sparta DR, Jennings JH, McElligott ZA, Decot H, Stuber GD. Amygdala and bed nucleus of the stria terminalis circuitry: implications for addiction-related behaviors. Neuropharmacology. 2014;76:320–328. doi: 10.1016/j.neuropharm.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Yang B-Z, Chavira Da, Hitchcock Ca, Sung SC, Shipon-Blum E, Gelernter J. A common genetic variant in the neurexin superfamily member CNTNAP2 is associated with increased risk for selective mutism and social anxiety-related traits. Biol. Psychiatry. 2011;69:825–831. doi: 10.1016/j.biopsych.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stjepanović D, Lorenzetti V, Yücel M, Hawi Z, Bellgrove MA. Human amygdala volume is predicted by common DNA variation in the stathmin and serotonin transporter genes. Transl. Psychiatry. 2013;3:e283. doi: 10.1038/tp.2013.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stujenske JM, Likhtik E, Topiwala MA, Gordon JA. Fear and safety engage competing patterns of theta-gamma coupling in the basolateral amygdala. Neuron. 2014;83:919–933. doi: 10.1016/j.neuron.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas A, Chess S. Temperament and Development. New York, NY: Brunner Mazel; 1977. [Google Scholar]
- Tomarken AJ, Davidson RJ, Henriques JB. Resting frontal brain asymmetry predicts affective responses to films. J. Pers. Soc. Psychol. 1990;59:791–801. doi: 10.1037//0022-3514.59.4.791. [DOI] [PubMed] [Google Scholar]
- Tomarken AJ, Davidson RJ, Wheeler RE, Doss RC. Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. J. Pers. Soc. Psychol. 1992;62:676–687. doi: 10.1037//0022-3514.62.4.676. [DOI] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Parsing anhedonia: translational models of reward-processing deficits in psychopathology. Curr. Dir. Psychol. Sci. 2013;22:244–249. doi: 10.1177/0963721412474460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unschuld PG, Ising M, Specht M, Erhardt A, Ripke S, Heck A, Kloiber S, Straub V, Brueckl T, Müller-Myhsok B, Holsboer F, Binder EB. Polymorphisms in the GAD2 gene-region are associated with susceptibility for unipolar depression and with a risk factor for anxiety disorders. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2009;150B:1100–1109. doi: 10.1002/ajmg.b.30938. [DOI] [PubMed] [Google Scholar]
- Van der Plas EAA, Boes AD, Wemmie JA, Tranel D, Nopoulos P. Amygdala volume correlates positively with fearfulness in normal healthy girls. Soc. Cogn. Affect. Neurosci. 2010;5:424–431. doi: 10.1093/scan/nsq009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Harmelen A-L, van Tol M-J, Demenescu LR, van der Wee NJA, Veltman DJ, Aleman A, van Buchem MA, Spinhoven P, Penninx BWJH, Elzinga BM. Enhanced amygdala reactivity to emotional faces in adults reporting childhood emotional maltreatment. Soc. Cogn. Affect. Neurosci. 2012;8:362–369. doi: 10.1093/scan/nss007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent JL, Patel GH, Fox MD, Snyder AZ, Baker JT, Van Essen DC, Zempel JM, Snyder LH, Corbetta M, Raichle ME. Intrinsic functional architecture in the anaesthetized monkey brain. Nature. 2007;447:83–86. doi: 10.1038/nature05758. [DOI] [PubMed] [Google Scholar]
- Visscher PM, Goddard ME, Derks EM, Wray NR. Evidence-based psychiatric genetics, AKA the false dichotomy between common and rare variant hypotheses. Mol. Psychiatry. 2012;17:474–485. doi: 10.1038/mp.2011.65. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Tomasi D, Baler RD. Unbalanced neuronal circuits in addiction. Curr. Opin. Neurobiol. 2013;23:639–648. doi: 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J. Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Davis M. Role of the extended amygdala in short-duration versus sustained fear: a tribute to Dr. Lennart Heimer. Brain Struct. Funct. 2008;213:29–42. doi: 10.1007/s00429-008-0183-3. [DOI] [PubMed] [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: initial neuroimaging studies of the human amygdala. Curr. Dir. Psychol. Sci. 1998;7:177–188. [Google Scholar]
- White LK, McDermott JM, Degnan KA, Henderson HA, Fox NA. Behavioral Inhibition and anxiety: the moderating roles of inhibitory control and attention shifting. J. Abnorm. Child Psychol. 2011;39:735–747. doi: 10.1007/s10802-011-9490-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Degnan KA, Perez-Edgar KE, Henderson HA, Rubin KH, Pine DS, Steinberg L, Fox NA. Impact of behavioral inhibition and parenting style on internalizing and externalizing problems from early childhood through adolescence. J. Abnorm. Child Psychol. 2009;37:1063–1075. doi: 10.1007/s10802-009-9331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LR, Fox NA, Lejuez CW, Reynolds EK, Henderson HA, Perez-Edgar KE, Steinberg L, Pine DS. Early temperament, propensity for risk-taking and adolescent substance-related problems: a prospective multi-method investigation. Addict. Behav. 2010;35:1148–1151. doi: 10.1016/j.addbeh.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Zald DH, Cowan RL, Riccardi P, Baldwin RM, Ansari MS, Li R, Shelby ES, Smith CE, McHugo M, Kessler RM. Midbrain Dopamine Receptor Availability Is Inversely Associated with Novelty-Seeking Traits in Humans. J. Neurosci. 2008;28:14372–14378. doi: 10.1523/JNEUROSCI.2423-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]