Abstract
Sensory systems must represent stimuli in manners dependent upon a wealth of factors, including stimulus intensity and duration. One way the brain might handle these complex functions is to assign the tasks throughout distributed nodes, each contributing to information processing. We sought to explore this important aspect of sensory network function in the mammalian olfactory system, wherein the intensity and duration of odor exposure are critical contributors to odor perception. This is a quintessential model for exploring processing schemes given the distribution of odor information by olfactory bulb mitral and tufted cells into several anatomically distinct secondary processing stages, including the piriform cortex (PCX) and olfactory tubercle (OT), whose unique contributions to odor coding are unresolved. We explored the coding of PCX and OT neuron responses to odor intensity and duration. We found that both structures similarly partake in representing descending intensities of odors by reduced recruitment and modulation of neurons. Additionally, while neurons in the OT adapt to odor exposure, they display reduced capacity to adapt to either repeated presentations of odor or a single prolonged odor presentation compared with neurons in the PCX. These results provide insights into manners whereby secondary olfactory structures may, at least in some cases, uniquely represent stimulus features.
Keywords: olfaction, perception, intensity, learning, adaptation
for normal perception to occur, sensory systems must represent stimuli in manners dependent upon their multifaceted nature. These factors include stimulus identity (e.g., chemical structure, frequency), intensity, and duration. Beyond essential transduction steps, one way the brain might handle these complex functions is to assign tasks throughout distributed, hierarchical nodes that each similarly contribute to the representation and coding of information. Conversely, the brain may route information into nodes that each uniquely process and represent for key features of stimuli (e.g., intensity, duration) in a parallel manner (Galizia and Rössler 2010; Marsat et al. 2012; Nassi and Callaway 2009; Petersen 2007). Major questions remain regarding how the brain accomplishes these tasks.
We sought to explore this important aspect of sensory network function within the olfactory system, wherein the intensity of odors and the duration of odor exposure (and possible subsequent cellular adaptation) are critical contributors to odor perception (Cain 1970; Chaput and Panhuber 1982; Dalton and Wysocki 1996; Doty et al. 1978; Getchell and Shepherd 1978; Laing et al. 1984; Zufall and Leinders-Zufall 2000). This is a powerful model for exploring information processing schemes (e.g., Anderson et al. 2003; Haberly 2001; Kadohisa and Wilson 2006) given the distribution of odor information by olfactory bulb mitral and tufted cells into several secondary anatomically distinct processing stages, including the piriform cortex (PCX) and olfactory tubercle (OT) (Giessel and Datta 2014; Igarashi et al. 2012; Kang et al. 2011; Nagayama et al. 2010; Schwob and Price 1984; Scott 1981; White 1965). It is believed that odor information at this level of processing is further refined in manners that contribute to complex aspects of odor perception (Gottfried 2010; Wilson and Sullivan 2011). Additionally, anatomical and chemical differences in sensory innervation between the OT and PCX (Wesson and Wilson 2011) posit the possibility that these structures contribute differentially to odor processing, including the coding of stimulus features such as odor intensity and odor duration.
Intrinsic and calcium imaging studies have revealed that the PCX is more greatly recruited by odors of strong intensity (Stettler and Axel 2009; Sugai et al. 2005), with larger regions of the PCX activated by high-intensity odors (Sugai et al. 2005). Furthermore, while not the focus of this study, seemingly greater numbers of neurons are recruited with increasing odor intensities (Stettler and Axel 2009). The magnitude of inhibitory and excitatory postsynaptic currents also change considerably with variations in odor intensity (Poo and Isaacson 2009). These studies suggest that olfactory cortex neurons, at least those within the PCX, read-out concentration variant information relayed by mitral and tufted cells (e.g., Duchamp-Viret et al. 2000; Meredith 1986). Importantly, tufted cells respond with higher firing rates than mitral cells to odors (Nagayama et al. 2004). Mitral and tufted cells differentially innervate particular regions of the olfactory cortex, with a greater amount of tufted cells synapsing within the OT compared with the PCX, which receives greater portions of mitral cells (Igarashi et al. 2012; Imamura et al. 2011; Scott et al. 1980; Scott 1981). This anatomical connectivity provides a possible substrate for differential access of low-concentration odor information and the coding of intensity features, likely via tufted cells (Nagayama et al. 2004), into the OT compared with the PCX. It is presently unknown whether neurons in one area display more concentration-invariant coding.
Peripheral mechanisms underlying cellular odor adaptation include depression of olfactory receptor neurons to repeated and prolonged duration stimuli (Kurahashi and Menini 1997; Reisert and Matthews 2001; Zufall and Leinders-Zufall 2000). Centrally, both the olfactory bulb and PCX adapt to odors (Chaudhury et al. 2010; Gray and Skinner 1988; Scott 1977; Wilson 1998a,b), with the PCX demonstrating more rapid and robust adaptation (Wilson 1998b) (for review see Wilson and Linster 2008). Indeed, the PCX displays profound learning-dependent modulations in several intrinsic features and odor coding (e.g., Chapuis and Wilson, 2011; Chen et al. 2011; Cohen et al. 2008; Gire et al. 2013; Li et al. 2008; Mouly et al. 2001; Schoenbaum and Eichenbaum 1995). For example, current stimulation of mitral and tufted cell axons in a manner similar to that evoked by odors yields homosynaptic depression (Best and Wilson 2004), which would be behaviorally advantageous for yielding odor-specific adaptation and habituation to environmental odors. Whether OT neurons display odor adaptation is unknown. The lack of a local association fiber system within the OT (Haberly and Price 1978), in contrast to the extensive and highly plastic association fiber system within the PCX (Barkai et al. 1994; Haberly and Price 1978; Hasselmo et al. 1990; Linster et al. 2009), suggests that the OT's capacity for learning and adaptation may be markedly less than the PCX.
While neurons in both the OT and PCX represent odors similarly, in terms of breadth of tuning and sniff-coupled response latencies (Payton et al. 2012), it is presently unknown in what manners, if any, these structures contribute uniquely to coding schemes for odor stimulus features. Do these structures each represent modulations in odor duration and intensity by changes in neural recruitment or firing magnitudes? Furthermore, do these structures represent these changes in similar manners or is it possible one plays a largely dominant or distinct role in coding for a select odor feature? Here we address these questions by performing in vivo recordings of extracellular OT and PCX multi- and single-unit activity both in mice presented with odors at multiple magnitudes of intensity and in two established odor adaptation paradigms.
MATERIALS AND METHODS
Experimental subjects.
Adult male C57Bl/6 mice (n = 38, 2–4 mo of age) were obtained from Harlan Laboratories (Haslett, MI) and maintained within the Case Western Reserve University School of Medicine animal facility. The mice were utilized for two separate experiments. We used 18 mice for experiments on odor intensity coding and 20 mice for odor adaptation experiments. Food and water were available ad libitum. The mice were on a 12:12-h (light-dark) cycle with all experiments performed during the light phase. All experiments were executed in accordance with the guidelines of the National Institutes of Health and were approved by the Case Western Reserve University's Institutional Animal Care Committee.
In vivo electrophysiology.
Similar electrophysiological methods were utilized for both experiments on odor intensity coding and adaptation. Mice were administered a urethane injection (1.0 mg/kg ip) and then mounted on a stereotaxic frame upon a water-filled heating pad (38°C). Anesthesia depth was verified by the lack of toe-pinch reflex. Following, the wound margin site received a 0.05 ml injection of local anesthesia (1% lidocaine in 5% EtOH sc) before we exposed the dorsal skull by removing the scalp. Two craniotomies were performed for electrode placement. The hole for the reference electrode was first drilled over the cortex contralateral to the recording site. For the recording electrode, a craniotomy was drilled from 0 to 1.5 mm anterior to bregma to record from the PCX and/or OT. Throughout all experiments, OT and PCX recordings were performed in an alternate order, with most mice only being utilized for recordings from one of the structures. Physiological (0.9%, 38°C) NaCl was applied to all craniotomy sites, which was replenished intermittently throughout each recording.
A tungsten electrode (0.01“ OD; A-M Systems, Sequim, WA) was lowered into the reference craniotomy site ∼1.5 mm deep, while the recording electrode (a second tungsten electrode, same as above) was lowered into the PCX or OT. The recording electrode activity was digitized at 28 kHz along with respiration and stimulus presentation events using a Tucker-Davis Technologies amplifier and software (Alachua, FL).
Immediately following the recording session, mice were transcardially perfused with 4°C 0.9% NaCl and then 10% formalin (Fisher Scientific, Waltham, MA). The brains were then removed and stored in 30% sucrose formalin at 4°C.
Electrode placement verification.
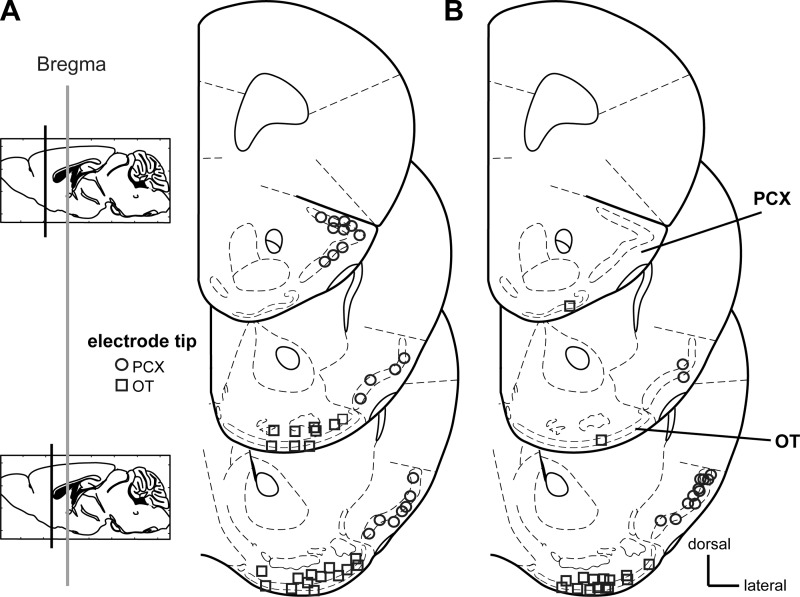
All recording sites were verified by postmortem histological examinations of slide-mounted 40 μm coronal brain sections stained with a 0.1% cresyl violet solution. PCX recording sites (layers i, ii, and/or iii; n = 21 from a total of nine mice for intensity experiments) (Fig. 1A; n = 26 from a total of 13 mice for adaptation experiments, Fig. 1B) were found across all coronal sections of the anterior regions of the PCX, which contained the lateral olfactory tract. OT recording sites (n = 22 from a total of 12 mice for intensity experiments; n = 27 from a total of 14 mice for adaptation experiments) were found across all sections (layers i, ii, and/or iii; Fig. 1, A and B). Electrode tip locations were verified by multiple observers (S. Adjei and C. Xia) with reference to a mouse brain atlas (Paxinos and Franklin 2001).
Fig. 1.
Electrode tip locations from confirmed olfactory tubercle (OT) and piriform cortex (PCX) recordings. Coronal stereotaxic panels showing the approximate location of the electrode tips following histological verification used for recordings of odor intensity coding (A) and adaptation coding (B). See materials and methods for site and animal numbers. Sections span from 2.0 to 0.5 mm anterior of Bregma, in 0.5 mm intervals. Based upon Paxinos & Franklin (Paxinos and Franklin 2001), modified with permission from Elsevier.
Stimulus presentation.
Odors for both odor intensity and adaptation recordings were purchased from Sigma Aldrich (St. Louis, MO). Liquid odors were all diluted in mineral oil to 1 Torr using known chemical vapor pressures near room temperature (EPI Suite v4.11) so that all odors possessed equal vapor pressure. The 1 Torr dilution was utilized as our maximum intensity stimulus to reduce possibility that an odor might also serve as a trigeminal stimulant at higher intensities (Carlson et al. 2013). Odors (and % dilution in mineral oil to achieve 1 Torr vapor pressure) included 1,7-octadiene (98% pure; 0.049%), isopentyl acetate (98% pure; 0.28%), ethyl propionate (99% pure; 0.03%), ethyl butyrate (99% pure; 0.08%), (-)-limonene (96% pure; 0.86%), 2-heptanone (99% pure; 0.46%), and heptanal (99% pure; 0.33%). We aliquoted 2 ml of each diluted odor stimulus into 25 ml glass head space vials capped with Teflon septa.
In the intensity experiments, each 1 Torr odor stock (10°) was then further diluted serially in mineral oil to 10−1, 10−2, 10−3, and 10−4 dilutions. In this design, five liquid dilutions of each odor were employed. Odors were presented for 2 s each in an alternate order, ≥4 trials/stimulus, at a ≥20 s interstimulus interval. Each recording location was probed for responses to all concentrations of two of the odors (either ethyl propionate and heptanal or isopentyl acetate and 1,7-octadiene). These odor pairs were selected given their known modulation of OT and PCX units (Payton et al. 2012). The decision to record from a given recording site was not dependent upon the unit responses to both odors. Air dilution of each individual odor concentration was achieved with medical-grade N2 (100 ml vaporized N2: 900 ml clean N2).
For the adaptation experiments we employed two established and unique paradigms (Chaudhury et al. 2010; Wilson 1998b). At each recording location, an odor was presented for either 10 repeated short 2 s trials at a 30 s interstimulus interval or a single prolonged 50 s trial. The execution of either paradigm upon a given recording site was counterbalanced. At least 2 min intervals were provided between the two paradigms of the same odor. Each odor (at 1 Torr vapor pressure) was diluted with filtered room air (100 ml odorized: 900 ml unodorized) to prevent ventilatory changes that would otherwise occur upon presentation with prolonged N2. Concentration stability of odor for both adaptation paradigms was verified with a photoionization detector (RAE systems, San Jose, CA).
All odors were presented through a Teflon odor-port using a custom air-dilution olfactometer at the total flow rate of 1 l/min in all experiments. For all experiments, the exit of the odor port was held at a distance of 1 cm from the tip of the nose. All stimulus lines were composed of independent Teflon tubing up to the point of immediate entry into the Teflon odor port, eliminating the potential of cross-stimulus contamination. Odor onset (time to exit the odor port) was controlled across all stimuli by means of a vacuum line connected to a three-way solenoid valve. In our design, air-diluted odor flowed toward and through the odor port for 20 s, in which time they were scavenged by the vacuum line, prior to being delivered to the animal. Computer-timed switching of the three-way solenoid valve resulted in rapid stimulus delivery. Thus, this design provided temporally precise and similar air-dilution treatment of all liquid-diluted odors.
Data analysis.
Waveform analysis and k-means cluster cutting were performed utilizing Tucker-Davis Technologies software. Sorted data were verified to be independent units by an interspike interval analysis. To determine a single-unit, no more than 2% of all spikes could occur with an inter-spike interval <2 ms (Carlson et al. 2013). Putative single-units that did not pass this criteria were omitted from further analysis. Local field potential (LFP) spectral power (Kay et al. 2009) was extracted during the long duration odor exposure paradigm. Raw data (0.1–200 Hz) were processed with an offline fast Fourier transform (FFT) analysis to allow classification of data within components of different LFP frequencies (1024 FFT size). A power spectrum was extracted containing the LFP power from 0.1 to 80 Hz (Wesson and Wilson 2010; Wesson et al. 2011).
The number of spikes (within 100 ms time bins for intensity and repeated 2 s trials paradigm in adaptation, within 10 s time bins for 50 s prolonged trial paradigm in adaptation) relative to the time of stimulus onset, were extracted and organized by odor, odor dilution, and trial number. Custom macros written in Microsoft's Excel (Seattle, WA) were used to sort trials by stimulus type and calculate responsivity of neurons. A P value (P < 0.05, 2-tailed paired Student's t-test) was employed when comparing prestimulus (−2-0 s) to during-stimulus (0 to 2 s) epochs of trial 1 to identify stimulus-modulated units in the intensity and repeated 2 s trials paradigm in adaptation studies (Wesson and Wilson 2010; Wilson 2001). For the 50 s trial adaptation paradigm, we identified stimulus-modulated units by comparing −10-0 s to 0–10 s epochs within 1 s time bins that were significantly responsive (P < 0.05, 2-tailed paired Student's t-test). Across region (OT vs. PCX) and stimulus (odor, odor dilution, trial number in repeated 2 s trials, and 10 s time bins in 50 s prolonged trial) comparisons were made by ANOVA with appropriate post hoc tests and/or correction factors as indicated throughout the text. Repeated-measures ANOVA was used to test for dilution-dependent changes, trial number-dependent changes, and prolonged stimulus exposure-dependent changes in unit activity. χ2 tests (2-tailed with Yates correction) were used for within dilution comparisons between regions of the percentage of units modulated. Statistical analyses were performed in Microsoft Excel or Origin (OriginLab, Northampton, MA). All data are reported as means ± SE unless otherwise noted.
RESULTS
All experiments were performed in anesthetized mice to control for influences of behavioral state or active sampling (sniffing) on neural responses (Buonviso et al. 2003; Doucette et al. 2011; Rojas-Líbano and Kay 2012; Verhagen et al. 2007), which may otherwise occur differentially throughout the course of prolonged odor exposure or amid high-intensity odors. To prevent carry-over effects from one experimental paradigm to the other, experiments exploring intensity coding were performed in a separate cohort of animals than those for adaptation coding, with recordings of PCX and OT unit activity counterbalanced in order throughout each.
Concentration-variant representation of odor intensity across the OT and PCX.
We first explored the representation of odor intensity among OT and PCX neurons. Four odors were presented at five different intensities, all of which are known to elicit activity within both the OT and PCX when presented at high intensities (Payton et al. 2012; Wesson and Wilson 2010). Each recording site was probed for responses to two of the odors (5 intensities each, 10 total stimuli). We recorded a total of 60 OT and 77 PCX single units (Fig. 1A). Evoked activity in response to isopentyl acetate and 1,7-octadiene were recorded among 28 OT units and 27 PCX units. Similarly, evoked activity in response to ethyl propionate and 2-heptanal were recorded among 32 OT units and 50 PCX units. Odor intensity-dependent amounts of firing were observed among units in both structures. In the example OT unit shown in Fig. 2A, presentation of the odor 1,7-octadiene elicited a robust response at the 1 Torr dilution, which was markedly reduced in subsequent descending concentrations.
Fig. 2.
Concentration-variant representation of odor intensity by the OT and PCX. A: example traces from a multiunit (MUA) recording in the OT displaying evoked responses to descending intensities of the odor 1,7-octadiene across successive presentations (2 s duration, 30 s intertrial interval, n = 5 trials/intensity). Also shown is a line raster plot from a single unit following spike sorting (unit). This unit ceased responding to 1,7-octadiene at intensities <0.01 Torr dilution. The local field potential (LFP, 100 Hz low-pass filter) and respiration (resp) measured from the piezo foil under the animal's chest are also shown. Gray shaded box represents odor presentation. Line graphs depicting the percentage of cell-odor pairs, across the entire population of single-unit recordings, significantly modulated at each intensity (Bi) and among only those cell-odor pairs significantly modulated by the 1 Torr dilution of an odor (Bii). The OT and PCX displayed similar population-level recruitment of neurons by variant concentrations of odors. *P < 0.05, **P < 0.01, χ2 tests with Yate's correction; n.s., not significant (P > 0.05). C: histogram displaying odor-evoked breadths of tuning among OT and PCX units (same as in Bii) as a function of odor intensity. D: population-level stem plot of response magnitudes of OT and PCX units (same as in Bii) to the odor intensities tested. E: mean response magnitude of excitatory cell-odor pairs (same as in Bii, excluding 2 OT inhibitory units) throughout descending intensities of odor.
To quantify the capacity for each structure to represent odor intensity, we began by identifying units that were significantly modulated by each of the odors at the greatest intensity (1 Torr). Units were considered significantly modulated if the number of spikes during 1 Torr odor presentation (0 to 2 s) was significantly different than the number of spikes preodor (−2-0 s) across all trials (P < 0.05, 2-tailed, paired Student's t-test). We found that units within both structures represent descending odor intensity with decreased amounts of significant responsivity (Fig. 2Bi). Repeated-measures ANOVA revealed an effect of intensity on the percent of cell-odor pairs modulated [F (4,272) 55.182, P < 0.0001] (Fig. 2Bi), but no effect of region on the percent of modulated cell-odor pairs [F (1,272) 2.278, P = 0.1324] (n = 120 OT, 154 PCX cell-odor pairs). An interaction effect was observed between region and intensity [F (4,1088) 4.88, P < 0.0007]. Perhaps underlying this effect, significantly less cell-odor pairs were modulated by the highest concentration of odors within the OT compared with the PCX [χ2 (1) = 7.56, P = 0.006, 2-tailed with Yates correction] (Fig. 2Bi). Interestingly, however, upon presentation with the lowest concentration odor, the OT and PCX possessed similar amounts of modulated cell-odor pairs (2-tailed χ2 tests with Yates correction, P > 0.05). These data, at the entire population level, reflect that similar amounts of single units within the OT and PCX are recruited by low concentrations of odors.
To eliminate possible interregional differences in odor responsivity, we next analyzed only those units significantly modulated by each odor at its 1 Torr dilution (Fig. 2Bii). Again, repeated-measures ANOVA revealed an effect of intensity on the percent of cell-odor pairs modulated [F (4,116) 100.211, P < 0.0001] and a significant interaction between region and intensity [F (4,464) 3.085, P = 0.016], but no effect of region alone on the percentage of modulated units [F (1,116) 2.051, P = 0.155] (n = 40 OT, 78 PCX cell-odor pairs) (Fig. 2Bii). χ2 tests (2-tailed with Yates correction) for the proportion of modulated units at each intensity between regions revealed a significant regional difference at the 0.01 Torr [χ2 (1) = 4.087, P = 0.043] and 0.001 Torr dilutions [χ2 (1) = 5.99, P = 0.0144], but not at other intensities (P > 0.05). Similar distributions of odor-evoked breadths of tuning were observed between structures (P > 0.05, 2-sample Kolmogorov-Smirnov test), with each displaying the widest breadth of tuning at the highest intensity of odor tested (Fig. 2C). Thus, the OT and PCX represent odors among even low intensities by reduced numbers of modulated neurons with only modest differences between structures.
Do OT and PCX units represent descending intensities of odors by reduced spike numbers? Does the amount of spiking to low-intensity odors differ between these regions? To test these questions, we calculated the mean response magnitudes of each cell-odor pair across all odor intensities. A population-level plot of OT and PCX unit response magnitudes across odor intensities is displayed in Fig. 2D. All units except for two in the OT displayed largely excitatory response magnitudes to the 1 Torr stimulus intensity. As expected based upon the data in Fig. 2, A–C, all units with few exceptions displayed their greatest mean response magnitude to the 1 Torr stimulus intensity and their weakest evoked response magnitude to the 0.0001 Torr stimulus intensity (Fig. 2D). Repeated-measures ANOVA revealed an effect of intensity on the response magnitude of cell-odor pairs [F (4,114) 82.58, P < 0.0001] but no effect of region on response magnitude [F (1,114) 0.961, P = 0.33], nor an interaction between region and intensity [F (4,456) 1.203, P = 0.309] (n = 38 OT, 78 PCX excitatory cell-odor pairs) (Fig. 2E). Post hoc tests for the response magnitude within each dilution failed to find any significant effects of region at any of the tested stimuli (P > 0.05, ANOVA, with Bonferroni correction across the five comparisons significance must cross P < 0.01). Taken together, these results demonstrate that single units in the olfactory cortex represent odors with intensity variant changes in firing. We also found evidence that both the OT and PCX represent odors among even low intensities by reduced numbers of modulated neurons.
PCX, but not OT, displays adaptation to repeated odor exposure.
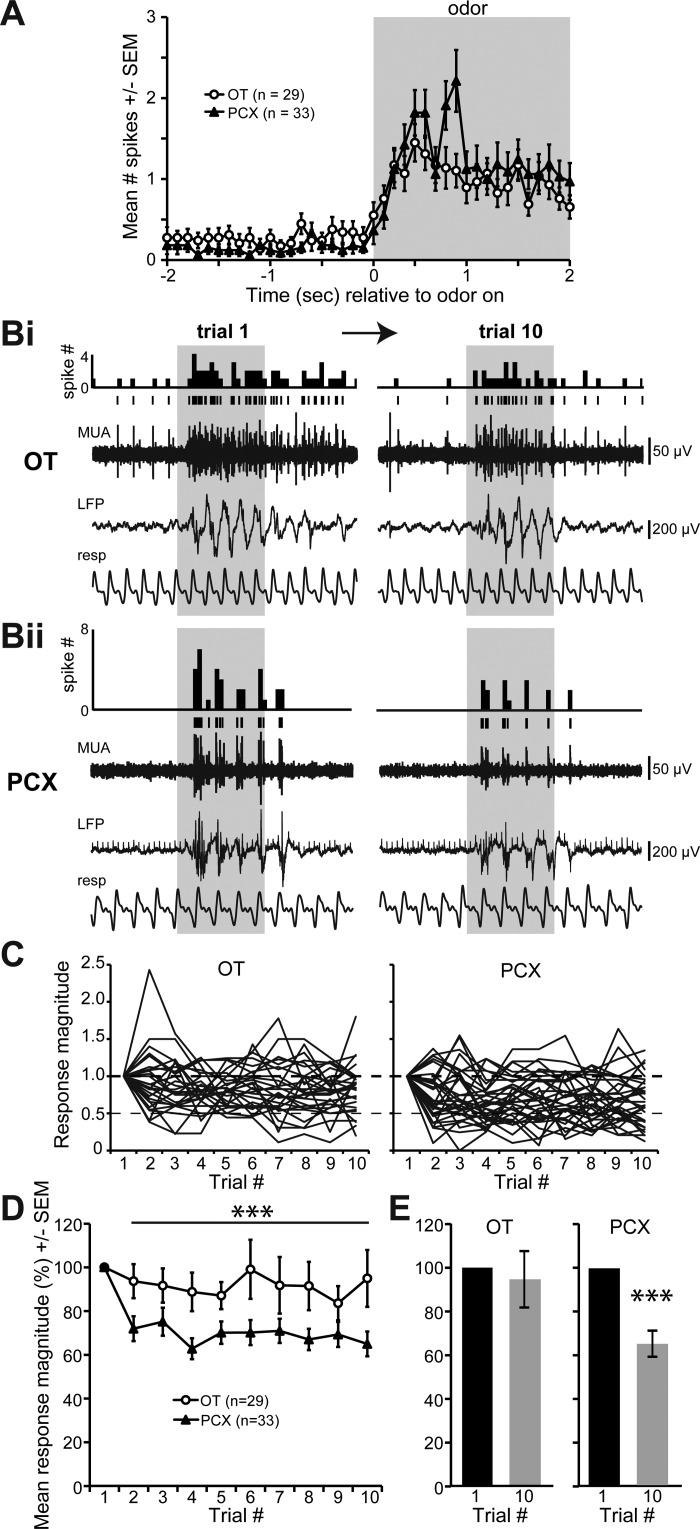
We next explored manners of odor adaptation in the OT and PCX. To test for regional differences in adaptation to repeated presentations of short-duration odors, we first probed recording sites (Fig. 1B) for units significantly excited by one of the tested odors (see materials and methods). The same six odors contributed evoked data among both the OT and PCX. As before, a modulated unit was classified as one that displayed a significantly greater number of spikes when one compared the spike number during odor to the 2 s preodor (P < 0.05, 2-tailed, paired Student's t-test). We found 29 OT units (14 mice) and 33 PCX units (12 mice) that displayed characteristic and significant odor-evoked increases in firing (Fig. 3A). Among these modulated units, we observed that OT units were capable of displaying robust odor-evoked responses across all 10 repeated trials of 2 s odors (Fig. 3Bi). In contrast, units in the PCX displayed adaptation by the 10th trial (Fig. 3Bii), as also previously reported (Wilson 1998b).
Fig. 3.
The PCX, but not the OT, displays adaptation to repeated odor exposure. A: average number of spikes across all PCX and OT units significantly modulated by odor upon the first presentation (”trial 1,“ P < 0.05, 2-tailed paired Student's t-test, # spikes 2 s preodor vs 2 s during). Example traces from a separate OT (Bi) and PCX recording (Bii) throughout presentations with 2 s duration odor on trial 1 (left) and trial 10 (right). Histogram of spike # within 100 ms time bins, single-unit line raster plot, MUA, LFP, and respiration are shown. Gray shaded box represents odor presentation. C: odor-evoked response magnitude during trials 1–10 of all significantly modulated units (same units as in A). Data are expressed as a proportion of magnitudes within trial 1. D: mean odor-evoked response magnitude as in C expressed as a percentage of magnitudes within trial 1. ***P < 0.0001 across trials 2–10. E: mean response magnitudes from trial 1 and 10 in both the OT and PCX (same data as in D) displaying that only the PCX significantly adapted by the 10th trial. ***P < 0.0001.
To quantify these possible interregional differences, for each unit we normalized the response magnitude within each 2 s trial as a percentage of the response in the first trial as described in (Wilson 1998b) (Fig. 3C). Repeated-measures ANOVA of these normalized data revealed an overall effect of trial number on the response magnitude [F (9,60) 4.185, P < 0.0001], an effect of region on the response magnitude [F (1,60) 5.465, P = 0.023], but no significant interaction between trial number and region [F (9,540) 1.693, P = 0.088] (Fig. 3D). When the normalized data within trial 1 was excluded, the significant difference between OT and PCX across all trials increased [F (1,556) 35.767, P < 0.0001]. Within trials, however, ANOVA did not reveal significant differences between the OT and PCX (Fig. 3D, with Bonferroni correction across the nine comparisons significance must cross P < 0.0055). Thus, whereas odor adaptation in this paradigm is rapid in the PCX (occurring as rapidly as trial 2), the OT continues to display robust response magnitudes in face of repeated odor presentations. Indeed, only the PCX (P < 0.0001), but not the OT (P = 0.70), significantly adapted by trial 10 (Fig. 3E).
Differential adaptation to prolonged odor exposure in the OT and PCX.
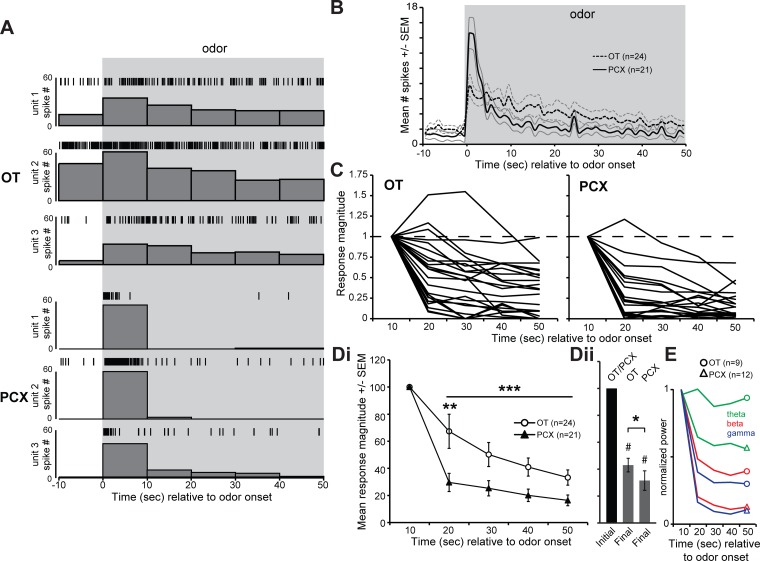
To test for regional differences in adaptation to prolonged presentations of odors (50 s), as performed above for short-duration odors, we first probed recording sites for units excited by 2 s odor presentations. Four odors contributed evoked data in the OT and six odors in the PCX. After isolating a responsive recording site, we allowed a 2 min period void of stimulus before presentation of the prolonged 50 s odor. Off-line analyses revealed that 24 OT units (14 mice) and 21 PCX units (13 mice) were significantly modulated upon presentation with the 50 s odor (# spikes in the 10 s during odor vs. 10 s preodor; P < 0.05, 2 tailed, paired Student's t-test).
To quantify possible interregional differences, we calculated the response magnitude of each unit as the sum of the spiking activity within 10 s time bins, from the first bin onwards relative to odor onset, as a percentage of the response in the first 10 s bin as described by (Wilson 1998b) (Fig. 4C). Repeated-measures ANOVA revealed that there was a significant effect of stimulus time across the 10 s epochs on the mean response magnitude [F (4,43) 87.497, P < 0.0001], a significant effect of region [F (1,43) 6.932, P = 0.012], and a significant interaction between time and region [F (4,172) 4.478, P = 0.002]. Excluding the normalized data within the first 10 s time bin, again we found a significant difference between OT and PCX across the entire prolonged stimulus presentation [F (1,178) 21.075, P < 0.0001]. The interregional differences originate from greater mean response magnitudes in the OT vs. the PCX in all time bins following odor onset (Fig. 4Di). Within bins, the OT was significantly more responsive within the 20 s time bin (P = 0.0052), yet in none other (ANOVA, with Bonferroni correction across the 4 comparisons significance must cross P < 0.0125). Both structures significantly adapted by the end of the 50 s prolonged odor exposure compared with the first 10 s bin (P < 0.0001). We found differences between structures in the magnitude of adaptation by the last time bin (P = 0.022), with the OT adapting less by this time (Fig. 4Dii). These interregional differences in adaptation were also observed at the network level. Indeed, the power of LFP spectral bands from mice that contributed LFP data adapted ≥33% more strongly in the PCX than in the OT by the end of the 50 s prolonged odor (Fig. 4E). Together, these differences in odor adaptation support the concept that the OT and PCX may contribute uniquely to odor learning and memory.
Fig. 4.
Differential adaptation to prolonged odor exposure in the OT and PCX. A: histograms and line raster plots of odor-evoked single-unit activity from 3 different recordings from both the OT (top) and PCX (bottom) in response to 50 s duration odor. Gray box represents odor presentation. B: peri-stimulus time histogram of mean single-unit activity from both OT and PCX recordings throughout presentation with 50 s duration odor. Data only from units significantly modulated by odor (P < 0.05, 2-tailed paired Student's t-test, # spikes 10 s preodor vs. 10 s during). Time bins = 1 s. Boldfaced lines = mean, accompanying lines = SE; n = 1 odor trial/unit. C: same data as in B but plotted as a percentage of responses during the first 10 s of odor, within 10 s bins thereafter. Di: within-region mean data as in C. **P < 0.01, ***P < 0.0001. Dii: mean odor response magnitudes within the first (”initial“) 10 s bin and in the final 10 s bin in both the OT and PCX (same data as in Di). Both structures significantly adapted by the final bin of the 50 s odor compared with the 1st bin (#P < 0.0001), but with differences between structures (*P < 0.05). E: mean normalized LFP spectral power. Data are normalized to the maximum LFP power (in each band) within 10 s bins relative to odor onset. Data are presented for theta (0–10 Hz), beta (15–30 Hz), and gamma bands (35–70 Hz) (Kay et al. 2009; Wesson et al. 2011); n = number of odor trials.
DISCUSSION
We explored coding schemes for stimulus features within the olfactory system, wherein the intensity of odors and the duration of exposure (and thus possible adaptation) are both critical contributors to odor perception. We investigated the coding of PCX and OT neuron responses to odor stimulus features, intensity and duration, and found that both structures partake in specific aspects of coding for odor duration, but not intensity. While reports on the exact proportion of odor-responsive neurons in the olfactory cortex (i.e., PCX) varies depending upon the method of sampling/recording, quantification, and the intensities of odors employed (e.g., Poo and Isaacson 2009; Shakhawat et al. 2014), the proportion of units sampled as being odor responsive in this study was in agreement with previous reports (Payton et al. 2012; Rennaker et al. 2007; Wesson and Wilson 2010; Wilson 2000), and thus we predict these findings are representative of rodent extracellular in vivo physiology in the OT and PCX.
Insights into odor intensity coding within the olfactory cortex.
A fundamental aspect of sensory system function is the ability to represent stimuli in manners dependent upon stimulus intensity. This principle is observed at multiple levels of sensory system function. At the level of receptor neurons, environmental stimulus energy is transduced dependent upon stimulus availability to one and/or multiple receptor neurons (Bhandawat et al. 2005; Kinnamon and Margolskee 1996; Yau and Hardie 2009). This transduction provides action potential propagation to downstream centers, and thus the amount of stimulus available for transduction is a starting point but also a limiting factor for determining the amounts of neural activity to begin the representation and processing of sensory codes. Indeed, some aspects of sensory perception are modulated by stimulus intensity (Cain 1970; Gross-Isseroff and Lancet 1988; Kingdom 2011; Verhagen and Engelen 2006; Wojcik and Sirotin 2014).
Within the olfactory systems of terrestrial vertebrates, extranasal airborne odors are inhaled into the nasal cavity upon each inhalation (Mozell and Jagodowicz 1973; Schoenfeld and Cleland 2006; Scott 2006; Wachowiak 2011), fundamentally limiting the access of odor receptors to odor. Upon binding to odor receptors localized upon olfactory receptor neurons, a G protein-coupled intracellular signaling cascade triggers action potential propagation along the olfactory receptor neuron axons, which then terminate into olfactory bulb glomeruli. Multiple reports have provided evidence that olfactory receptor neuron sensory input into the olfactory bulb is concentration variant (Bozza et al. 2004; Duchamp-Viret et al. 2000; Lecoq et al. 2009; Wachowiak and Cohen 2001). Whereas low-intensity odors evoke distinct olfactory receptor neuron activation resulting in focal chemotopy within olfactory bulb glomeruli, high-intensity odors result in activation of large populations of these neurons, and thus glomeruli and, therefore, result in loss of chemotopy (Wachowiak and Cohen 2001). This level of concentration variant coding provides odor information flow onto secondary neurons in the olfactory bulb, the mitral and tufted cells. Odor-evoked activity among mitral and tufted cells is also concentration dependent (Meredith 1986), including variations in the magnitude of spiking both throughout and within unique temporal aspects of the odor pulse (Meredith 1986).
Whereas considerable evidence for concentration variant coding of odors by olfactory bulb neurons exists, relatively little is understood regarding coding of intensity in secondary olfactory structures (but see Anderson et al. 2003; Onoda et al. 2005; Pause et al. 1997; Stettler and Axel 2009; Sugai et al. 2005). Here, we found that the bulk of OT and PCX neurons represent odors by intensity variant changes in firing. That is, neurons in both structures displayed similar levels of intensity in-variant coding. This finding is in agreement with imaging studies that revealed growing activation of PCX by increases in odor intensity (Sugai et al. 2005). Our finding that the OT also partakes in odor intensity coding demonstrates, as would be expected, that this characteristic is a distributed property of olfactory cortical structures. It is interesting to consider whether intensity-variant representations of odors is a common feature of secondary olfactory structures (as also found in the amygdala, Anderson et al. 2003) and whether other secondary structures might display an even more enhanced representation of low intensity odors than that reported herein.
Insights into odor adaptation and stimulus learning and memory.
Adaptation of a cellular response to repeated or prolonged odor exposure is essential to detect new information in the face of change. In agreement with a wealth of detailed previous studies (for review see Wilson and Linster 2008), here we observed substantial adaptation of both short-duration repeated odors and long-duration odors by PCX units. This supports the view of the PCX as critical for odor learning and its role as an associative structure (Gottfried 2010; Haberly 2001; Wilson and Sullivan 2011). Interestingly, while the OT did adapt throughout long-duration (50 s) odor exposure, it failed to display adaptation to repeated short-duration odors. The OT also displayed substantially less adaptation throughout the 50 s odor exposure at both the single unit and LFP level. This suggests that while the OT is capable of odor learning, it likely partakes in odor learning in a limited manner compared with the PCX. For instance, while interregional differences in adaptation were observed in the present study, both structures are known to partake and display modifications in odor representations due to associative learning (Chen et al. 2011; Gadziola et al. 2015; Gire et al. 2013; Schoenbaum and Eichenbaum 1995). Determining the sources underlying long-duration odor adaptation in the OT will be important in assessing its contributions to odor learning. While a mechanistic understanding for these differences is beyond the scope of the present study, our LFP spectral analysis revealed that LFP spectral power is more preserved in the OT than in the PCX throughout prolonged odor exposure. Based upon this, it will be important for future studies to explore differential aspects of mitral and tufted cell synaptic plasticity, which may innervate the OT and PCX, including feedforward inhibition (Luna and Schoppa 2008; Poo and Isaacson 2009; Suzuki and Bekkers 2011).
CONCLUSION
The results of the present study provide additional evidence that neurons within anatomically distinct olfactory bulb output structures partake in the coding of odors (Kadohisa and Wilson 2006) and odor stimulus features, in unique manners. The capacity for the OT and PCX to code uniquely for adaptation may be perceptually and behaviorally advantageous given the distinct output targets of these structures. While it remains a question of great interest what exact odor information each of these structures receives from mitral and tufted cells (e.g., see Igarashi et al. 2012), the uncovered scheme is novel in that we find the information available in the output from the OT and PCX into higher-order structures is unique. Future research in awake-behaving animals, ideally in combination with measures of sniffing to optimally assess temporal dynamics, will be needed to explore manners whereby these differential sources of information may be utilized.
GRANTS
This work was supported by National Science Foundation Grant IOS-1121471 and Alzheimer's Association Grant NIRG-305847 to D. W. Wesson.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.Z.X. and S.A. performed experiments; C.Z.X., S.A., and D.W.W. analyzed data; C.Z.X., S.A., and D.W.W. prepared figures; C.Z.X. and D.W.W. drafted manuscript; C.Z.X. and D.W.W. edited and revised manuscript; C.Z.X., S.A., and D.W.W. approved final version of manuscript; D.W.W. conception and design of research; D.W.W. interpreted results of experiments.
ACKNOWLEDGMENTS
We thank Dr. Marie Gadziola and Kaitlin Carlson for comments on an earlier version of this manuscript.
REFERENCES
- Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci 6: 196–202, 2003. [DOI] [PubMed] [Google Scholar]
- Barkai E, Bergman RE, Horwitz G, Hasselmo ME. Modulation of associative memory function in a biophysical simulation of rat piriform cortex. J Neurophysiol 72: 659–677, 1994. [DOI] [PubMed] [Google Scholar]
- Best AR, Wilson DA. Coordinate synaptic mechanisms contributing to olfactory cortical adaptation. J Neurosci 24: 652–660, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandawat V, Reisert J, Yau KW. Elementary response of olfactory receptor neurons to odorants. Science 308: 1931–1934, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozza T, McGann JP, Mombaerts P, Wachowiak M. In vivo imaging of neuronal activity by targeted expression of a genetically encoded probe in the mouse. Neuron 42: 9–21, 2004. [DOI] [PubMed] [Google Scholar]
- Buonviso N, Amat C, Litaudon P, Roux S, Royet JP, Farget V, Sicard G. Rhythm sequence through the olfactory bulb layers during the time window of a respiratory cycle. Eur J Neurosci 17: 1811–1819, 2003. [DOI] [PubMed] [Google Scholar]
- Cain WS. Odor intensity after self-adaptation and cross-adaptation. Percept Pyschophysics 7: 271–275, 1970. [Google Scholar]
- Carlson KS, Xia CZ, Wesson DW. Encoding and representation of intranasal CO2 in the mouse olfactory cortex. J Neurosci 33: 13873–13881, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapuis J, Wilson DA. Bidirectional plasticity of cortical pattern recognition and behavioral sensory acuity. Nat Neurosci 15: 155–161, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaput MA, Panhuber H. Effects of long duration odor exposure on the unit activity of olfactory bulb cells in awake rabbits. Brain Res 250: 41–52, 1982. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Manella L, Arellanos A, Escanilla O, Cleland TA, Linster C. Olfactory bulb habituation to odor stimuli. Behav Neurosci 124: 490–499, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CFF, Barnes DC, Wilson DA. Generalized vs stimulus-specific learned fear differentially modifies stimulus encoding in primary sensory cortex of awake rats. J Neurophysiol 106: 3136–3144, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen Y, Reuveni I, Barkai E, Maroun M. Olfactory learning-induced long-lasting enhancement of descending and ascending synaptic transmission to the piriform cortex. J Neurosci 28: 6664–6669, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P, Wysocki CJ. The nature and duration of adaptation following long-term odor exposure. Percept Psychophys 58: 781–792, 1996. [DOI] [PubMed] [Google Scholar]
- Doty RL, Brugger WE, Jurs PC, Ordorff MA, Snyder PJ, Lowry LD. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav 20: 175–185, 1978. [DOI] [PubMed] [Google Scholar]
- Doucette W, Gire David H, Whitesell J, Carmean V, Lucero Mary T, Restrepo D. Associative cortex features in the first olfactory brain relay station. Neuron 69: 1176–1187, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchamp-Viret P, Duchamp A, Chaput MA. Peripheral odor coding in the rat and frog: quality and intensity specification. J Neurosci 20: 2383–2390, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadziola M, Tylicki K, Christian D, Wesson DW. The olfactory tubercle encodes odor valence in behaving mice. J Neurosci 35: 4515–4527, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galizia CG, Rössler W. Parallel olfactory systems in insects: anatomy and function. Annu Rev Entomol 55: 399–420, 2010. [DOI] [PubMed] [Google Scholar]
- Getchell TV, Shepherd GM. Adaptive properties of olfactory receptors analysed with odour pulses of varying durations. J Physiol 282: 541–560, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giessel AJ, Datta SR. Olfactory maps, circuits and computations. Curr Opin Neurobiol 24: 120–132, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gire DH, Whitesell JD, Doucette W, Restrepo D. Information for decision-making and stimulus identification is multiplexed in sensory cortex. Nat Neurosci 16: 991–993, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA. Central mechanisms of odour object perception. Nat Rev Neurosci 11: 628–641, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Skinner JE. Field potential response changes in the rabbit olfactory bulb accompany behavioral habituation during the repeated presentation of unreinforced odors. Exp Brain Res 73: 189–197, 1988. [DOI] [PubMed] [Google Scholar]
- Gross-Isseroff R, Lancet D. Concentration-dependent changes of perceived odor quality. Chem Senses 13: 191–204, 1988. [Google Scholar]
- Haberly LB. Parallel-distributed processing in olfactory cortex: new insights from morphological and physiological analysis of neuronal circuitry. Chem Senses 26: 551–576, 2001. [DOI] [PubMed] [Google Scholar]
- Haberly LB, Price JL. Association and commissural fiber systems of the olfactory cortex of the rat. I. systems originating in the piriform cortex and adjacent areas. J Comp Neurol 178: 711–740, 1978. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Wilson MA, Anderson BP, Bower JM. Associative memory function in piriform (olfactory) cortex: computational modeling and neuropharmacology. Cold Spring Harb Symp Quant Biol 55: 599–610, 1990. [DOI] [PubMed] [Google Scholar]
- Igarashi KM, Ieki N, An M, Yamaguchi Y, Nagayama S, Kobayakawa K, Kobayakawa R, Tanifuji M, Sakano H, Chen WR, Mori K. Parallel mitral and tufted cell pathways route distinct odor information to different targets in the olfactory cortex. J Neurosci 32: 7970–7985, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura F, Ayoub AE, Rakic P, Greer CA. Timing of neurogenesis is a determinant of olfactory circuitry. Nat Neurosci 14: 331–337, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadohisa M, Wilson DA. Separate encoding of identity and similarity of complex familiar odors in piriform cortex. Proc Natl Acad Sci USA 103: 15206–15211, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. Different profiles of main and accessory olfactory bulb mitral/tufted cell projections revealed in mice using an anterograde tracer and a whole-mount, flattened cortex preparation. Chem Senses 36: 251–260, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM, Beshel J, Brea J, Martin C, Rojas-Líbano D, Kopell N. Olfactory oscillations: the what, how and what for. Trends Neurosci 32: 207–214, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdom FAA. Lightness, brightness and transparency: a quarter century of new ideas, captivating demonstrations and unrelenting controversy. Vision Res 51: 652–673, 2011. [DOI] [PubMed] [Google Scholar]
- Kinnamon SC, Margolskee RF. Mechanisms of taste transduction. Curr Opin Neurobiol 6: 506–513, 1996. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Menini A. Mechanism of odorant adaptation in the olfactory receptor cell. Nature 385: 725–729, 1997. [DOI] [PubMed] [Google Scholar]
- Laing DG, Panhuber H, Willcox ME, Pittman EA. Quality and intensity of binary odor mixtures. Physiol Behav 33: 309–319, 1984. [DOI] [PubMed] [Google Scholar]
- Lecoq J, Tiret P, Charpak S. Peripheral adaptation codes for high odor concentration in glomeruli. J Neurosci 29: 3067–3072, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science 319: 1842–1845, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Menon AV, Singh CY, Wilson DA. Odor-specific habituation arises from interaction of afferent synaptic adaptation and intrinsic synaptic potentiation in olfactory cortex. Learn Memory 16: 452–459, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna VM, Schoppa NE. GABAergic circuits control input-spike coupling in the piriform cortex. J Neurosci 28: 8851–8859, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsat G, Longtin A, Maler L. Cellular and circuit properties supporting different sensory coding strategies in electric fish and other systems. Curr Opin Neurobiol 22: 686–692, 2012. [DOI] [PubMed] [Google Scholar]
- Meredith M. Patterned response to odor in mammalian olfactory bulb: the influence of intensity. J Neurophysiol 56: 572–597, 1986. [DOI] [PubMed] [Google Scholar]
- Mouly AM, Fort A, Ben-Boutayab N, Gervais R. Olfactory learning induces differential long-lasting changes in rat central olfactory pathways. Neuroscience 102: 11–21, 2001. [DOI] [PubMed] [Google Scholar]
- Mozell MM, Jagodowicz M. Chromatographic separation of odorants by the nose: retention times measured across in vivo olfactory mucosa. Science 181: 1247–1249, 1973. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Takahashi YK, Yoshihara Y, Mori K. Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol 91: 2532–2540, 2004. [DOI] [PubMed] [Google Scholar]
- Nagayama S, Enerva A, Fletcher ML, Masurkar AV, Igarashi KM, Mori K, Chen WR. Differential axonal projection of mitral and tufted cells in the mouse main olfactory system. Front Neural Circuits 4: 1–8, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassi JJ, Callaway EM. Parallel processing strategies of the primate visual system. Nat Rev Neurosci 10: 360–372, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoda N, Sugai T, Yoshimura H. Odor-intensity coding in the anterior piriform cortex. Chem Senses 30, Suppl 1: i162–i163, 2005. [DOI] [PubMed] [Google Scholar]
- Pause BM, Sojka B, Ferstl R. Central processing of odor concentration is a temporal phenomenon as revealed by chemosensory event-related potentials (CSERP). Chem Senses 22: 9–26, 1997. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates (2nd Ed). San Diego: Academic Press, 2001. [Google Scholar]
- Payton CA, Wilson DA, Wesson DW. Parallel odor processing by two anatomically distinct olfactory bulb target structures. PLoS One 7: e34926, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CCH. The functional organization of the barrel cortex. Neuron 56: 339–355, 2007. [DOI] [PubMed] [Google Scholar]
- Poo C, Isaacson JS. Odor representations in olfactory cortex: ”sparse“ coding, global inhibition, and oscillations. Neuron 62: 850–861, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Responses to prolonged odour stimulation in frog olfactory receptor cells. J Physiol 534: 179–191, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennaker RL, Chen CFF, Ruyle AM, Sloan AM, Wilson DA. Spatial and temporal distribution of odorant-evoked activity in the piriform cortex. J Neurosci 27: 1534–1542, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Líbano D, Kay LM. Interplay between sniffing and odorant sorptive properties in the rat. J Neurosci 32: 15577–15589, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenbaum G, Eichenbaum H. Information coding in the rodent prefrontal cortex. I. Single-neuron activity in orbitofrontal cortex compared with that in pyriform cortex. J Neurophysiol 74: 733–750, 1995. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TA, Cleland TA. Anatomical contributions to odorant sampling and representation in rodents: zoning in on sniffing behavior. Chem Senses 31: 131–144, 2006. [DOI] [PubMed] [Google Scholar]
- Schwob JE, Price JL. The development of axonal connections in the central olfactory system of rats. J Comp Neurol 223: 177–202, 1984. [DOI] [PubMed] [Google Scholar]
- Scott JW. A measure of extracellular unit responses to repeated stimulation applied to observations of the time course of olfactory responses. Brain Res 132: 247–258, 1977. [DOI] [PubMed] [Google Scholar]
- Scott JW. Electrophysiological identification of mitral and tufted cells and distributions of their axons in olfactory system of the rat. J Neurophysiol 46: 918–931, 1981. [DOI] [PubMed] [Google Scholar]
- Scott JW. Sniffing and spatiotemporal coding in olfaction. Chem Senses 31: 119–130, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JW, McBride RL, Schneider SP. The organization of projections from the olfactory bulb to the piriform cortex and olfactory tubercle in the rat. J Comp Neurol 194: 519–534, 1980. [DOI] [PubMed] [Google Scholar]
- Shakhawat AM, Harley CW, Yuan Q. Arc visualization of odor objects reveals experience-dependent ensemble sharpening, separation, and merging in anterior piriform cortex in adult rat. J Neurosci 34: 10206–10210, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron 63: 854–864, 2009. [DOI] [PubMed] [Google Scholar]
- Sugai T, Miyazawa T, Fukuda M, Yoshimura H, Onoda N. Odor-concentration coding in the guinea-pig piriform cortex. Neuroscience 130: 769–781, 2005. [DOI] [PubMed] [Google Scholar]
- Suzuki N, Bekkers JM. Two layers of synaptic processing by principal neurons in piriform cortex. J Neurosci 31: 2156–2166, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhagen JV, Engelen L. The neurocognitive bases of human multimodal food perception: sensory integration. Neurosci Biobehav Rev 30: 613–650, 2006. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci 10: 631–639, 2007. [DOI] [PubMed] [Google Scholar]
- Wachowiak M. All in a sniff: olfaction as a model for active sensing. Neuron 71: 962–973, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachowiak M, Cohen LB. Representation of odorants by receptor neuron input to the mouse olfactory bulb. Neuron 32: 723–735, 2001. [DOI] [PubMed] [Google Scholar]
- Wesson DW, Wilson DA. Smelling sounds: olfactory-auditory sensory convergence in the olfactory tubercle. J Neurosci 30: 3013–3021, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Wilson DA. Sniffing out the contributions of the olfactory tubercle to the sense of smell: hedonics, sensory integration, and more? Neurosci Biobehav Rev 35: 655–668, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DW, Borkowski AH, Landreth GE, Nixon RA, Levy E, Wilson DA. Sensory network dysfunction, behavioral impairments, and their reversibility in an Alzheimer's beta-amyloidosis mouse model. J Neurosci 31: 15962–15971, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE. Olfactory bulb projections of the rat. Anat Rec 152: 465–479, 1965. [Google Scholar]
- Wilson DA. Synaptic correlates of odor habituation in the rat anterior piriform cortex. J Neurophysiol 80: 998–1001, 1998a. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Habituation of odor responses in the rat anterior piriform cortex. J Neurophysiol 79: 1425–1440, 1998b. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Comparison of odor receptive field plasticity in the rat olfactory bulb and anterior piriform cortex. J Neurophysiol 84: 3036–3042, 2000. [DOI] [PubMed] [Google Scholar]
- Wilson DA. Receptive fields in the rat piriform cortex. Chem Senses 26: 577–584, 2001. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Linster C. Neurobiology of a simple memory. J Neurophysiol 100: 2–7, 2008. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Sullivan RM. Cortical processing of odor objects. Neuron 72: 506–519, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik PT, Sirotin Yevgeniy B. Single scale for odor intensity in rat olfaction. Curr Biol 24: 568–573, 2014. [DOI] [PubMed] [Google Scholar]
- Yau KW, Hardie RC. Phototransduction motifs and variations. Cell 139: 246–264, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zufall F, Leinders-Zufall T. The cellular and molecular basis of odor adaptation. Chem Senses 25: 473–481, 2000. [DOI] [PubMed] [Google Scholar]