Abstract
Numerous human diseases are caused by excessive signaling of mutant G protein-coupled receptors (GPCRs) or receptors that are overstimulated due to upstream signaling imbalances. The feasibility of functional compensation by arrestins with enhanced ability to quench receptor signaling was recently tested in the visual system. The results showed that even in this extremely demanding situation of rods that have no ability to phosphorylate rhodopsin, enhanced arrestin improved rod morphology, light sensitivity, survival, and accelerated photoresponse recovery. Structurally distinct enhanced mutants of arrestins that bind phosphorylated and non-phosphorylated active GPCRs with much higher affinity than parental wild-type (WT) proteins have been constructed. These “super-arrestins” are likely to have the power to dampen the signaling by hyperactive GPCRs. However, most cells express 5–20 GPCR subtypes, only one of which would be overactive, while nonvisual arrestins are remarkably promiscuous, binding hundreds of different GPCRs. Thus, to be therapeutically useful, enhanced versions of nonvisual arrestins must be made fairly specific for particular receptors. Recent identification of very few arrestin residues as key receptor discriminators paves the way to the construction of receptor subtype-specific nonvisual arrestins.
Keywords: Congenital disorders, Gain-of-function mutants, GPCRs, Receptor-specific arrestins, Functional compensation, Gene therapy, Protein-based therapeutics
1 The Case for Nonvisual Arrestins with High Receptor Specificity
The quenching of G protein-coupled receptor (GPCR) signaling was the first arrestin (arr) function described (Kühn et al. 1984; Lohse et al. 1990; Attramadal et al. 1992; Gurevich and Benovic 1995; Barak et al. 1997; Gurevich and Gurevich 2006b). After more than 30 years since rod arrestin (modern systematic name arrestin-11) was first discovered (Kühn 1978; Kühn et al. 1984), receptor desensitization is still the best-characterized function of the members of this protein family. Vertebrate evolution created only one truly receptor-specific arrestin family member, visual arrestin-1, with high preference for rhodopsin (Gurevich et al. 1993, 1995; Vishnivetskiy et al. 2004, 2011), reasonable affinity for cone pigments (Sutton et al. 2005; Chan et al. 2007), and fairly low binding to nonvisual GPCRs (Gurevich et al. 2011). Even arr-4 expressed exclusively in cone photoreceptors (Craft et al. 1994; Nikonov et al. 2008) binds several GPCRs essentially as well as nonvisual arrestins (Sutton et al. 2005). Arr-1 is also the most selective: it binds to light-activated and phosphorylated rhodopsin (P-Rh*) with an affinity orders of magnitude higher than to non-phosphorylated light-activated (Rh*) or dark phosphorylated rhodopsin (P-Rh) (Gurevich and Benovic 1993; Zhuang et al. 2013). Arr-1 demonstrates high preference for P-Rh* over other GPCRs in vitro (Gurevich et al. 1993, 1995; Vishnivetskiy et al. 2004) and in live cells (Vishnivetskiy et al. 2011; Gimenez et al. 2012a). In contrast, nonvisual arrestins (arr-2 and arr-3 in vertebrates) are ubiquitously expressed and bind numerous GPCR subtypes (Gurevich et al. 1995; Barak et al. 1997; Gimenez et al. 2012b). Nearly 800 different genes encoding GPCRs have been identified in humans (Lagerstrom and Schioth 2008; Almen et al. 2009; Nordstrom et al. 2011; Suwa et al. 2009) and the two nonvisual arrestins apparently bind most of these receptors (Gurevich and Gurevich 2006b). Although differences in the interactions between nonvisual arrestins and different receptors have led to a GPCR classification based on the stability of the complex (Oakley et al. 2000), the differences in arr-2 and arr-3 recruitment to various GPCRs do not create a significant selectivity that can be exploited experimentally or therapeutically (Vishnivetskiy et al. 2011; Gimenez et al. 2012a). Thus, if one intends to “tweak” the selectivity of nonvisual arrestins for different receptors, two questions must be answered. First, whether is it even possible to build into a nonvisual arrestin, high selectivity for a specific receptor? Second, in what context would arrestins with enhanced receptor selectivity be beneficial? These two questions define the scope of this chapter.
It is hard to overestimate the importance of GPCRs in general homeostasis. GPCRs are key receptors in most sensory systems, detecting light, odorants, and taste molecules. About 400 GPCRs in every mammal respond to hormones, neurotransmitters, and autacoids. Also known as seven-transmembrane domain receptors, or 7TMRs, GPCRs regulate a myriad of critical functions in unicellular and multicellular organisms (Dohlman et al. 1991). For example, yeast haploid cell types express Ste2 and Ste3, which respond to α and a-factor pheromones, promoting cell cycle arrest and fusion with cells of opposite mating type (Versele et al. 2001). Also in yeast, glucose triggers the shift towards the anaerobic conversion of the sugar into ethanol. This process is initiated by the activation of another GPCR, the glucose receptor Gpr1 (Kraakman et al. 1999).
In multicellular organisms, GPCR signaling is required to maintain homeostasis and to ensure coordinated cellular function. Novel functions of GPCRs are constantly being identified. For example, in Drosophila melanogaster, the product of mth encodes a secretin receptor-like GPCR called Methuselah (Mth). Mth regulates life span in flies (Lin et al. 1998) by modulating the oxidative stress resistance response (Araujo et al. 2013; Gimenez et al. 2013) through mechanisms that involve controlling secretion of insulin-like peptides from a restricted population of insulin-producing cells (IPCs) in the brain (Gimenez et al. 2013). Unexpectedly, both expression of dominant negative mutants of Mth and overexpression of this protein in the IPCs result in a prolonged fly life span (Gimenez et al. 2013). Thus, normal longevity is only observed when fly IPCs receive strictly calibrated signaling from Mth.
In vertebrates, GPCRs mediate constant hormonal control of organ function, as well as tissue growth and cell proliferation, during normal and pathological adaptation. In most cases, prolonged uncontrolled stimulation of any GPCR leads to pathology. In the heart, neuroendocrine stimulation initiated by cardiac adrenergic receptors induces hypertrophic changes of the myocardium (Dorn and Force 2005). Under persistent stimulation, excessive cardiac remodeling can lead to heart failure, as has been shown in a murine model of persistent muscarinic receptor stimulation by antibodies with agonist-like action (Gimenez et al. 2005). Agonist-like autoantibodies mediating prolonged receptor stimulation were found in patients with Chagas' disease and other dilated cardiomyopathies (Ribeiro et al. 2007; Hernandez et al. 2008). Their deleterious effects highlight the importance of balanced GPCR signaling.
Several human disorders are caused by activating mutations in various GPCRs (Schipani et al. 1995; Paschke 1996; Khoo et al. 1999; Claus et al. 2005; reviewed in Schöneberg et al. 2004; Vassart and Costagliola 2011) or genetic errors eliminating GRK phosphorylation sites (Apfelstedt-Sylla et al. 1993; Kim et al. 1993; Restagno et al. 1993) necessary for timely signal shutoff (Chen et al. 1995). These gain-of-function mutations are dominant, i.e., the other allele encoding a normal protein cannot reduce the signaling by an overactive mutant. An even greater variety of disorders are associated with excessive GPCR signaling caused by pharmacological therapeutic interventions (Ahmed et al. 2010). It stands to reason that arrestins with greater than normal ability to quench GPCR signaling, which can be constructed in several ways (see Chap. 7), can functionally compensate (Song et al. 2009). It is very likely that when excessive GPCR signaling underlies the pathology, bringing the balance back to normal will cure the disease.
However, virtually every cell in the body expresses between 5 and 20 different GPCRs, only one of which is a mutant or signals too much for some other reason. Both nonvisual arrestins bind many GPCRs with similar affinity (Gurevich et al. 1995; Barak et al. 1997; Gimenez et al. 2012b), and activating mutations make them even less discriminating (Gurevich et al. 1997; Kovoor et al. 1999; Celver et al. 2002). Thus, an enhanced mutant constructed on the basis of promiscuous nonvisual arrestins will reduce the signaling by the overactive GPCR, while simultaneously dampening the signaling by all other receptors expressed in the same cell. This is likely to cause side effects that could be even worse than the disease itself. Thus, therapeutic use of enhanced nonvisual arrestins requires the construction of mutants with narrow receptor selectivity, better yet with a strict specificity for an individual GPCR subtype that needs to be targeted.
2 Identification of an Extensive Receptor-Binding Arrestin Surface
Before the discovery of the arrestin–clathrin interaction (Goodman et al. 1996), GPCRs were the only known class of arrestin-binding proteins. Considerable effort by many groups was invested into the identification of arrestin residues directly engaged by receptors and mapping of the receptor “footprint” on arrestin. In fact, many arrestin elements involved in receptor binding were identified before the first crystal structure became available (Gurevich and Benovic 1993, 1995, 1997; Gurevich et al. 1993, 1995; Ohguro et al. 1994; Gray-Keller et al. 1997). The residues identified in these studies were later mapped onto the structure of the basal conformation of bovine arr-1 (Granzin et al. 1998; Hirsch et al. 1999) and found to be localized on the concave sides of both arrestin domains.
Interestingly, every arrestin element identified by subsequent studies using peptide competition (Pulvermuller et al. 2000), epitope insertion (Dinculescu et al. 2002), element swapping (Vishnivetskiy et al. 2004), site-directed mutagenesis (Vishnivetskiy et al. 1999, 2000, 2010, 2011; Hanson and Gurevich 2006), site-directed spin labeling/EPR (Hanson et al. 2006; Vishnivetskiy et al. 2010, 2011; Kim et al. 2012), and NMR (Zhuang et al. 2013) was also found to localize to the same concave sides of the two arrestin domains (Fig. 1). Thus, we can be fairly confident that regardless of the arrestin–receptor combination, the entire receptor “footprint” is localized within these concave surfaces, and it likely covers a considerable fraction of them.
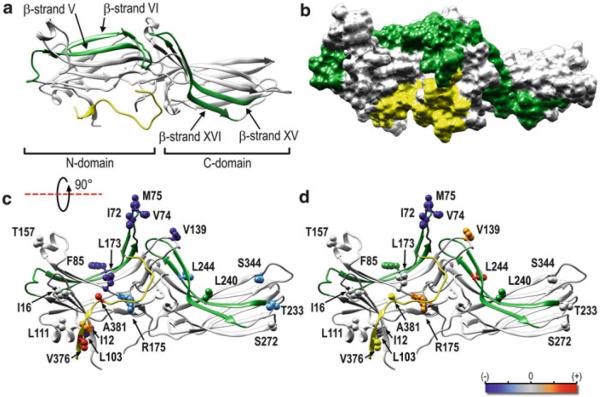
Fig. 1.
The receptor-binding interface has been mapped to the concave side of both domains in all arrestin subtypes. (a) Ribbon representation of bovine arr-1 based on PDB: 1CF1 (Hirsch et al. 1999) as viewed from the receptor “viewpoint.” Arrestins consist of two domains linked by a flexible hinge and the C-tail that comes back from the C-domain and makes a strong contact with the β-strand I and α-helix I in the N-domain (see Chap. 7, Fig. 1). The β-strands V–VI and XV–XVI with adjacent loops, identified as key elements that determine receptor specificity (Vishnivetskiy et al. 2004), are shown in green; the C-tail (including the parts not resolved in crystal structures) is shown in yellow. (b) Space-filling model of arr-1, oriented and color coded as in panel (a). (c, d) Side view of arr-1 [90° rotation from the perspective shown in panel (a)] with spin-labeled residues (Hanson et al. 2006) shown as ball-and-stick models. The magnitude of the detected changes in spin-label mobility upon receptor binding is color coded as follows: gray (or green/yellow), no change; pink/red, small and large increases in mobility, respectively; lightblue/dark blue, small and large decreases in mobility, respectively. (c) Changes upon binding to dark (inactive) P-Rh. (d) Additional changes induced by light activation of P-Rh to P-Rh*. Upon binding to dark P-Rh (c), finger loop residues (I72, V74, M75) become less mobile, while the mobility of the C-tail residues increases. Light activation further decreases the mobility of the finger loop residues (d), while mobility of V139 increases [this loop was later shown to move out of the way of incoming receptors (Kim et al. 2012; Vishnivetskiy et al. 2013)]. Ribbon and surface cartoons rendered with UCSF Chimera 1.8
Existing data indicate that the receptor-binding arrestin elements likely include noncontiguous residues distributed through this surface of the protein. Each individual interaction between arrestins and receptors is relatively low-affinity, but simultaneous engagement of several elements yields a high-affinity complex (Gurevich and Benovic 1993; Krupnick et al. 1994). As a result, not all potential interaction sites on both partners need to be engaged to allow arrestin to perform its functions. The complexes held together by fewer elementary interactions would have reduced affinity and stability. This is the probable mechanistic basis of the functional differences between class B GPCRs that hold arrestins tightly and travel with them all the way to late endosomes (Oakley et al. 2000) and class A receptors that readily release bound arrestins upon internalization.
3 Few Arrestin Elements Determine Receptor Preference
Discrete interactions of individual arrestin residues distributed over an extensive receptor-binding surface were shown to account for receptor selectivity that determines arr-1 preference for rhodopsin, as well as preferential binding of nonvisual arrestins to other GPCRs. This was elegantly demonstrated in a study where multiple elements were swapped between arr-1 and arr-2 in an attempt to identify those that determine this specificity (Vishnivetskiy et al. 2004). In this study, the parts of arr-1 that increased arr-2 binding to P-Rh* and the parts of arr-2 that improved arr-1 binding to M2 muscarinic acetylcholine receptor were identified. It turned out that two elements encompassing residues 49–90 (β-strands V and VI with adjacent loops) in the N-domain and residues 237–268 (β-strands XV and XVI) in the C-domain of visual arr-1 and homologous elements in arr-2 are the key players in receptor preference (Vishnivetskiy et al. 2004). The exchange of these two elements between arr-1 and arr-2 completely reversed receptor specificity of these two subtypes (Vishnivetskiy et al. 2004).
Individual residues that determine receptor preference of arrestins were identified in a subsequent study (Vishnivetskiy et al. 2011). Due to high homology between arr-1 and -2, as few as 35 residues in the two elements that engage receptors are different, and only 22 of these differences represent nonconservative substitutions (Vishnivetskiy et al. 2004). An attempt to construct arr-2 with arr-1-like preference for P-Rh* demonstrated that only five arr-2 residues (Leu-68, Ser-86, Asp-240, Asp-259, and Thr-261) are the key in determining its receptor specificity, whereas nine additional residues (Leu-48, Glu-50, Arg-51, Tyr-238, Cys-242, Lys-250, Cys-251, Pro-252, and Met-255) play a supporting role (Vishnivetskiy et al. 2011). Moreover, alanine substitution of ten of these residues (four in the N-domain and six in the C-domain) completely blocked the binding of arr-1, arr-2, and arr-3 to all GPCRs tested, including P-Rh* (Vishnivetskiy et al. 2011; Gimenez et al. 2012a).
An interesting feature that distinguishes nonvisual arrestins from arr-1 is revealed by the comparison of the crystal structures of arr-2 and arr-1 (Hirsch et al. 1999; Han et al. 2001). Each arrestin domain is a β-strand “sandwich,” in which the two β-sheets are “glued” together via hydrophobic interactions between the side chains pointing inside the sandwich (Hirsch et al. 1999; Han et al. 2001; Sutton et al. 2005; Zhan et al. 2011). In visual arr-1, Val90 is one of these residues, participating in multiple interactions with hydrophobic side chains of Val45, Val57, Val59, and Phe118 (Hirsch et al. 1999). In nonvisual arrestins, this valine is absent, being replaced with serine (arr-2) or alanine (arr-3) (Han et al. 2001; Zhan et al. 2011). Even though all its potential partners are conserved in arr-2 (Val41, Val53, Val55, and Phe115), the absence of this valine apparently makes the N-domain more flexible. In contrast to arr-2, arr-1 demonstrates relatively low binding to active phosphorylated M2 muscarinic receptors (Han et al. 2001; Vishnivetskiy et al. 2004). The Val90Ser mutation in arr-1, which apparently “loosens up” the N-domain, dramatically reduces its preference for P-Rh*, enhancing the binding to M2 receptors (Han et al. 2001). The magnitude of the effect of the mutation of this one residue (the side chain of which is not even exposed) strongly suggests that a relatively rigid N-domain stabilized by the interactions of Val90 with its partners is an important contributor to the high specificity of arr-1 for P-Rh* (Han et al. 2001; Vishnivetskiy et al. 2004). In fact, the Val90Ser substitution increases arr-1 binding to active phosphorylated M2 muscarinic receptors more than any other point mutation reported (Han et al. 2001; Vishnivetskiy et al. 2011).
This proof-of-concept protein engineering highlights the importance of the insight provided by the availability of high-resolution structural data. It also suggested that any mutants of nonvisual arrestins designed for increased receptor specificity must have Val (found in the two visual subtypes, arr-1 and arr-4) (Hirsch et al. 1999; Sutton et al. 2005) in the equivalent position. It seemed reasonable to expect that on this rigid background predisposed to be receptor selective, substitutions of relatively few residues that determine receptor preference (Vishnivetskiy et al. 2011) would yield nonvisual arrestins with enhanced receptor specificity.
4 Construction of Nonvisual Arrestins with Increased Receptor Specificity
This approach was used to create a set of mutants on arr-3-Ala87Val background specifically intended for the generation of variants with high receptor specificity (Gimenez et al. 2012b). Arr-3 was used in this study because it was reported to be even more promiscuous than arr-2, capable of binding numerous GPCRs (Barak et al. 1997; Kohout et al. 2001; Zhan et al. 2011).
The Val87Ala mutation per se had negligible impact on arr-3 binding to M2 muscarinic and D1 and D2 dopamine receptors and slightly increased the binding to β2 adrenergic receptor (β2AR) (Gimenez et al. 2012b). The next study focused on ten exposed residues, four in the N-domain and six in the C-domain, that were previously identified as critical for the receptor–arrestin interaction (Vishnivetskiy et al. 2011; Gimenez et al. 2012a). However, if one considers all possible permutations, where each position can be occupied by 20 different amino acids, the number of possible combinations is 2010 (i.e., more than 10 trillion), which is too large for experimental testing. However, the analysis of known arrestin sequences (Gurevich and Gurevich 2006a) shows that only two to three different residues were found in equivalent positions in arrestins from Caenorhabditis elegans to mammals. The logical assumption that amino acids that are never found in a particular position should not be there narrows the number of possible combinations down to manageable. Evolutionary sequence analysis (Gurevich and Gurevich 2006a) shows that the residues affecting receptor preference (Vishnivetskiy et al. 2011; Gimenez et al. 2012a) are actually islands of variability within highly conserved elements. Replacement of arr-3 residues only with those that naturally occur in equivalent positions in arrestins from other species virtually eliminates the possibility of misfolding.
The substitutions following this logic were introduced into eight out of these ten positions (Gimenez et al. 2012b). The recruitment of the generated arr-3 mutants to agonist-activated M2, D1, D2, and β2AR (Gimenez et al. 2012b) was measured using bioluminescence resonance energy transfer (BRET) between GPCRs tagged with Renilla luciferase on the C terminus and arrestins N-terminally tagged with Venus, a version of GFP (Namkung et al. 2009b; Walther et al. 2010). Interestingly, none of the mutations appreciably increased arr-3 binding to any of the receptors tested. However, seven out of ten significantly reduced the interaction with some of the receptors, but not with others, changing the selectivity up to fourfold (Gimenez et al. 2012b). This unexpectedly high ~70% success rate clearly shows that the key players in receptor specificity were correctly identified (Vishnivetskiy et al. 2011). This notion was further supported by the finding, with the use of direct in vitro binding assay with P-Rh* (Gurevich and Benovic 1992, 1993), that most substitutions significantly affected the ability of arr-3 mutants to interact with this model receptor (Gimenez et al. 2012b). Importantly, the combination of two mutations that significantly reduced β2AR binding without affecting the interactions with M2 and D2 receptors (Asp260Lys + Gln262Pro) yielded an arrestin with ~50-fold preference for these receptors over the β2AR (Gimenez et al. 2012b). Similarly, the combination of two substitutions that reduced the binding to D2, but not D1 receptors (Tyr239Thr + Gln256Tyr), generated an arrestin with more than fivefold preference for the D1 over D2 receptor (Gimenez et al. 2012b). Thus, the effects of individual mutations appear to be additive, which demonstrates the feasibility of the construction of nonvisual arrestins with high specificity for particular GPCRs (Fig. 2).
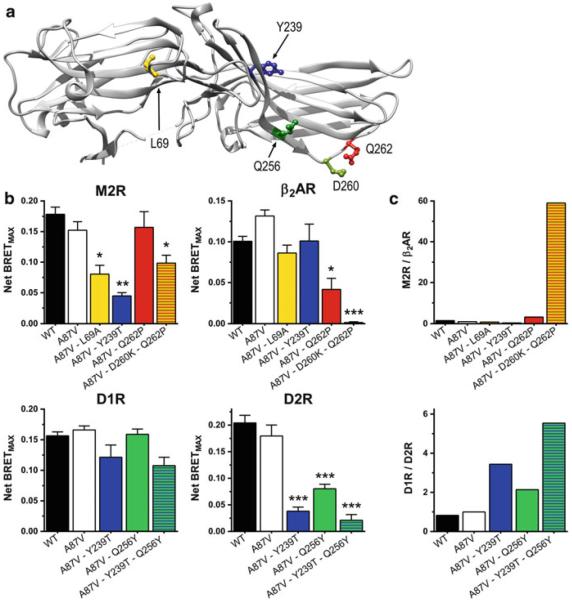
Fig. 2.
Mutations of few residues increase the selectivity of arr-3 for certain GPCRs. (a) The residues on the receptor-binding surface of bovine arr-3 that affected receptor selectivity the most (Gimenez et al. 2012b) are shown as ball-and-stick models. (b) The effect of these mutations and their combinations (on the Ala87Val background) on agonist-induced arr-3 recruitment (Net BRETmax) to M2 muscarinic (M2R), D1 (D1R) and D2 (D2R) dopamine, and β2-adrenergic (β2AR) receptors. (c) Ratios of net BRETMAX [shown in panel (b)] for the indicated mutants and receptor pairs are shown. For normalization, the binding ratio of the Ala87Val base mutant was set at 1. Asp260Lys + Gln256Tyr increased arr-3 preference for M2R over β2AR to >50-fold, whereas Tyr239Tre + Gln256Tyr increased arr-3 preference for D1R over D2R to approximately fivefold
In-cell analysis of the binding of these arr-3 mutants to different GPCRs yielded yet another interesting finding. The arrestin–receptor interactions were found to have two distinct components: a basal, agonist-independent and an agonist-induced, each accounting for about half of the maximum observed binding (Gimenez et al. 2012a, b). Interestingly, the manipulation of the receptor-binding surface changed these two components in the same direction to a similar extent, which was reflected in a very good correlation between mutation-induced changes in both basal banding and its agonist-induced increase (Gimenez et al. 2012b). Thus, a limited set of exposed residues mediates both the basal and agonist-induced arrestin binding to GPCRs, and targeted mutagenesis of these elements is a feasible approach for the generation of inherently selective nonvisual arrestins specifically targeting individual receptor subtypes.
Arrestin mutants that combine narrow receptor specificity with increased ability to desensitize GPCRs that cannot be phosphorylated or have excessive activity for other reasons are likely to be effective tools for normalizing GPCR signaling in conditions where excessive signaling underlies the pathology. This promising research direction is still in its infancy, and a lot of additional work needs to be done to generate receptor-specific arrestins with high therapeutic potential.
5 Differential Role of Receptor-Attached Phosphates in the Binding of Different Arrestins
As a rule, arrestins preferentially bind active phosphorylated forms of their cognate receptors. The main phosphorylation sensor in all arrestins is structurally similar: the polar core, localized between the two arrestin domains, includes two positively charged arginines and three negatively charged aspartates (Hirsch et al. 1999; Han et al. 2001; Sutton et al. 2005; Zhan et al. 2011) (see Chap. 7, Fig. 3). Usually, in soluble proteins, charged residues are exposed on the surface, whereas the polar core in arrestins is buried. An arginine in β-strand X (Arg175, Arg169, or Arg170 in arr-1, arr-2, or arr-3, respectively) directly binds the phosphates attached to the intracellular loops and/or C terminus of GPCRs by GRKs (Gurevich and Benovic 1993, 1995, 1997; Granzin et al. 1998; Vishnivetskiy et al. 1999; Celver et al. 2002; Gurevich and Gurevich 2006b; Hanson and Gurevich 2006). Neutralization or reversal of the charge of this arginine by appropriate mutations artificially turns the phosphate sensor “on,” greatly increasing arrestin binding to unphosphorylated active forms of their cognate receptors: Rh* in case of arr-1 (Gurevich and Benovic 1995, 1997; Gray-Keller et al. 1997; Gurevich 1998; Vishnivetskiy et al. 1999) or various nonvisual receptors in case of arr-2 and arr-3 (Gurevich and Benovic 1993; Gurevich et al. 1997; Kovoor et al. 1999; Celver et al. 2001, 2002; Pan et al. 2003; Schattauer et al. 2012). Each arrestin has numerous lysines and arginines that bind receptor-attached phosphates: several in β-strand X and preceding loop (Gurevich and Benovic 1995) and two lysines in β-strand I (Vishnivetskiy et al. 2000; Shukla et al. 2013) (see Chap. 7, Fig. 2). Arr-1 has an additional phosphate-binding residues, Arg19 in the loop between β-strands I and II (Sutton et al. 2005), which explains why arr-1 is more dependent on receptor-attached phosphates than nonvisual subtypes (Vishnivetskiy et al. 2000; Gimenez et al. 2012a; Kim et al. 2012; Zhuang et al. 2013). Interestingly, this remains true even in case of arr-1 binding to non-cognate receptors (Gimenez et al. 2012a, b). As far as nonvisual GPCRs are concerned, the role of receptor-attached phosphates varies widely, depending on a particular arrestin–GPCR combination (Mukherjee et al. 2002; Kim et al. 2004; Namkung et al. 2009a; Gimenez et al. 2012a) [see also Chap. 2 and Gurevich and Gurevich (2006b) for review]. Using BRET between receptor-RLuc and Venus-arrestin it was recently shown that in case of the β2AR, phosphates play an important, although not as decisive role as in arr-1 binding (Gimenez et al. 2012a). As for the M2 muscarinic and D2 dopamine receptors, the role of phosphorylation in arrestin recruitment (Gimenez et al. 2012a) and signaling regulation (Namkung et al. 2009b) appears to be minimal, even though the phosphorylation of a particular cluster of serines and threonines in the third intracellular loop of M2 was shown to enable arrestin binding (Pals-Rylaarsdam et al. 1997; Lee et al. 2000). In all arrestin subtypes mutations that destabilize the polar core or delete or forcibly detach the C-tail displaced by receptor binding yielded “pre-activated” enhanced nonvisual arrestins that readily interact with cognate GPCRs in a phosphorylation-independent manner (Gurevich et al. 1997; Kovoor et al. 1999; Celver et al. 2001, 2002; Pan et al. 2003).
An enhanced phosphorylation-independent mutant of arr-1 was shown to compensate for the lack of rhodopsin phosphorylation in vivo, prolonging the survival and improving functional performance of rod photoreceptors (Song et al. 2009) (see Chap. 7). Enhanced nonvisual arrestins were shown to effectively shut off the signaling by several unphosphorylated GPCRs in cells (Kovoor et al. 1999; Celver et al. 2001, 2002) and in vivo (Bruchas et al. 2006). However, nonvisual arrestins are inherently promiscuous (Gurevich et al. 1995; Barak et al. 1997; Kohout et al. 2001; Gimenez et al. 2012b), and activating mutations make them even more flexible (Carter et al. 2005), so that the expression of phosphorylation-independent nonvisual arrestins in any cell, in addition to the desired suppression of the signaling by overactive receptors, would likely also dampen the signaling by other GPCRs present in the same cell, causing serious side effects. Thus, therapeutic use of enhanced nonvisual arrestins will be feasible when activating mutations are combined with those that narrow down their receptor specificity, preferably to small groups of receptors or individual GPCRs.
6 Usefulness of Arrestins with Greater Specificity for Individual Receptors
Overactive GPCRs cause signaling imbalances leading to disease via different mechanisms: excessive stimulation of a normal receptor by a ligand (Hernandez et al. 2003, 2008; Ribeiro et al. 2007; Stavrakis et al. 2009, 2011; Ahmed et al. 2010), activating mutations (Schipani et al. 1995; Paschke 1996; Schöneberg et al. 2004; Vassart and Costagliola 2011), or aberrant desensitization (Apfelstedt-Sylla et al. 1993; Kim et al. 1993; Restagno et al. 1993; Chen et al. 1995; Rim and Oprian 1995; Barak et al. 2001; Moaven et al. 2013). The development of enhanced nonvisual arrestins targeting a specific malfunctioning receptor holds promise of compensation with a potential of bringing the signaling closer to normal. Recent advances in the development of gene delivery methods suitable for therapy (Ishikawa et al. 2011; Bartel et al. 2012; Nguyen and Szoka 2012; Dalkara et al. 2013) make the introduction of protein-based tools feasible (see chapter “Therapeutic potential of small molecules and engineered proteins”).
Controlling runaway GPCRs is not the only potential therapeutic use of reengineered arrestins with narrow receptor specificity. In addition to shutting of G protein-mediated signaling (Carman and Benovic 1998), arrestins recruit GPCRs to coated pits for internalization via direct binding to clathrin (Goodman et al. 1996) and AP2 (Laporte et al. 1999) and initiate the second round of signaling by recruiting various non-receptor partners (Gurevich and Gurevich 2006a; DeWire et al. 2007). New generations of GPCR agonists biased towards G proteins or arrestins are becoming increasingly available (see chapter “Arrestin-biased GPCR agonists”) with some currently tested in clinical trials for the treatment of pain and control of elevated blood pressure and even food intake (Reiter et al. 2012; Kenakin and Christopoulos 2013). Signaling-biased arrestin mutants with disabled individual functions, such as the ability to bind clathrin/AP2 (Kim and Benovic 2002) or MEK1 (Meng et al. 2009) and activate ERK1/2 (Coffa et al. 2011) or JNK3 (Seo et al. 2011; Breitman et al. 2012), are also becoming available. These designer arrestins equipped with additional mutations that make them specific for particular GPCRs can be used for selective channeling of arrestin-mediated signaling to desired pathways, while excluding unwanted ones. In combination with conventional or biased agonists, these arrestins can also be used to enhance traditional pharmacological therapy and make it more targeted. Phosphorylation-independent arrestin mutants were shown to support rapid internalization and recycling of GPCRs, preventing receptor downregulation (Pan et al. 2003). In several pathological conditions, such as congestive heart failure, excessive desensitization and downregulation of β-adrenergic receptors is at the root of the disease (Rockman et al. 1998). Arrestin mutants that can selectively prevent downregulation of β-adrenergic receptors have a potential to improve the performance of the failing heart.
Arrestins modulate an amazing variety of physiological processes, from GPCR trafficking (chapters “Arrestin interactions with G protein-coupled receptors” and “Arrestin binding to clathrin, AP2, and role in GPCR trafficking”), MAP activity (chapters “Arrestin-dependent activation of ERK and Src Family kinases”, “Arrestin-dependent activation of JNK family kinases”, and “Arrestin-mediated activation of p38 MAPK: molecular mechanisms and behavioral consequences”), cell motility (chapter “Molecular Mechanisms underlying beta-arrestin-dependent chemotaxis and actin-cytoskeletal reorganization”) and heart function (Rockman et al. 1998) to aging (Gimenez et al. 2013). In most cases, arrestin interactions with particular GPCRs are responsible for these effects, both normal and pathological. Thus, nonvisual arrestins combining strict receptor specificity with different types of signaling bias have many potential therapeutic uses.
Footnotes
Different systems of arrestin names are used in the field and in this book. We use systematic names of arrestin proteins: arrestin-1 (historic names S-antigen, 48 kDa protein, visual or rod arrestin), arrestin-2 (β-arrestin or β-arrestin1), arrestin-3 (β-arrestin2 or hTHY-ARRX), and arrestin-4 (cone or X-arrestin; for unclear reasons its gene is called “arrestin 3” in the HUGO database).
References
- Ahmed MR, Berthet A, Bychkov E, Porras G, Li Q, Bioulac BH, Carl YT, Bloch B, Kook S, Aubert I, Dovero S, Doudnikoff E, Gurevich VV, Gurevich EV, Bezard E. Lentiviral overexpression of GRK6 alleviates L-dopa-induced dyskinesia in experimental Parkinson's disease. Sci Transl Med. 2010;2:28ra28. doi: 10.1126/scitranslmed.3000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almen MS, Nordstrom KJ, Fredriksson R, Schioth HB. Mapping the human membrane proteome: a majority of the human membrane proteins can be classified according to function and evolutionary origin. BMC Biol. 2009;7:50. doi: 10.1186/1741-7007-7-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfelstedt-Sylla E, Kunisch M, Horn M, Ruther K, Gerding H, Gal A, Zrenner E. Ocular findings in a family with autosomal dominant retinitis pigmentosa and a frameshift mutation altering the carboxyl terminal sequence of rhodopsin. Br J Ophthalmol. 1993;77:495–501. doi: 10.1136/bjo.77.8.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo AR, Reis M, Rocha H, Aguiar B, Morales-Hojas R, Macedo-Ribeiro S, Fonseca NA, Reboiro-Jato D, Reboiro-Jato M, Fdez-Riverola F, Vieira CP, Vieira J. The Drosophila melanogaster methuselah gene: a novel gene with ancient functions. PloS One. 2013;8:e63747. doi: 10.1371/journal.pone.0063747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attramadal H, Arriza JL, Aoki C, Dawson TM, Codina J, Kwatra MM, Snyder SH, Caron MG, Lefkowitz RJ. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992;267:17882–17890. [PubMed] [Google Scholar]
- Barak LS, Ferguson SS, Zhang J, Caron MG. A beta-arrestin/green fluorescent protein biosensor for detecting G protein-coupled receptor activation. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- Barak LS, Oakley RH, Laporte SA, Caron MG. Constitutive arrestin-mediated desensitization of a human vasopressin receptor mutant associated with nephrogenic diabetes insipidus. Proc Natl Acad Sci USA. 2001;98:93–98. doi: 10.1073/pnas.011303698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel MA, Weinstein JR, Schaffer DV. Directed evolution of novel adeno-associated viruses for therapeutic gene delivery. Gene Ther. 2012;19:694–700. doi: 10.1038/gt.2012.20. [DOI] [PubMed] [Google Scholar]
- Breitman M, Kook S, Gimenez LE, Lizama BN, Palazzo MC, Gurevich EV, Gurevich VV. Silent scaffolds: inhibition of c-Jun N-terminal kinase 3 activity in cell by dominant-negative arrestin-3 mutant. J Biol Chem. 2012;287:19653–19664. doi: 10.1074/jbc.M112.358192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchas MR, Macey TA, Lowe JD, Chavkin C. Kappa opioid receptor activation of p38 MAPK is GRK3- and arrestin-dependent in neurons and astrocytes. J Biol Chem. 2006;281:18081–18089. doi: 10.1074/jbc.M513640200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman CV, Benovic JL. G-protein-coupled receptors: turn-ons and turn-offs. Curr Opin Neurobiol. 1998;8:335–344. doi: 10.1016/s0959-4388(98)80058-5. [DOI] [PubMed] [Google Scholar]
- Carter JM, Gurevich VV, Prossnitz ER, Engen JR. Conformational differences between arrestin2 and pre-activated mutants as revealed by hydrogen exchange mass spectrometry. J Mol Biol. 2005;351:865–878. doi: 10.1016/j.jmb.2005.06.048. [DOI] [PubMed] [Google Scholar]
- Celver J, Lowe J, Kovoor A, Gurevich VV, Chavkin C. Threonine 180 is requred for G protein-coupled receptor kinase 3 and b-arrestin mediated desensitization of the m-opioid receptor in Xenopus oocytes. J Biol Chem. 2001;276:4894–4900. doi: 10.1074/jbc.M007437200. [DOI] [PubMed] [Google Scholar]
- Celver J, Vishnivetskiy SA, Chavkin C, Gurevich VV. Conservation of the phosphate-sensitive elements in the arrestin family of proteins. J Biol Chem. 2002;277:9043–9048. doi: 10.1074/jbc.M107400200. [DOI] [PubMed] [Google Scholar]
- Chan S, Rubin WW, Mendez A, Liu X, Song X, Hanson SM, Craft CM, Gurevich VV, Burns ME, Chen J. Functional comparisons of visual arrestins in rod photoreceptors of transgenic mice. Invest Ophthalmol Vis Sci. 2007;48:1968–1975. doi: 10.1167/iovs.06-1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Makino CL, Peachey NS, Baylor DA, Simon MI. Mechanisms of rhodopsin inactivation in-vivo as revealed by a CooH-terminal truncation mutant. Science. 1995;267:374–377. doi: 10.1126/science.7824934. [DOI] [PubMed] [Google Scholar]
- Claus M, Maier J, Paschke R, Kujat C, Stumvoll M, Fuhrer D. Novel thyrotropin receptor germline mutation (Ile568Val) in a Saxonian family with hereditary nonautoimmune hyper-thyroidism. Thyroid. 2005;15:1089–1094. doi: 10.1089/thy.2005.15.1089. [DOI] [PubMed] [Google Scholar]
- Coffa S, Breitman M, Spiller BW, Gurevich VV. A single mutation in arrestin-2 prevents ERK1/2 activation by reducing c-Raf1 binding. Biochemistry. 2011;50:6951–6958. doi: 10.1021/bi200745k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft CM, Whitmore DH, Wiechmann AF. Cone arrestin identified by targeting expression of a functional family. J Biol Chem. 1994;269:4613–4619. [PubMed] [Google Scholar]
- Dalkara D, Byrne LC, Klimczak RR, Visel M, Yin L, Merigan WH, Flannery JG, Schaffer DV. In vivo-directed evolution of a new adeno-associated virus for therapeutic outer retinal gene delivery from the vitreous. Sci Transl Med. 2013;5:189ra176. doi: 10.1126/scitranslmed.3005708. [DOI] [PubMed] [Google Scholar]
- DeWire SM, Ahn S, Lefkowitz RJ, Shenoy SK. Beta-arrestins and cell signaling. Annu Rev Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- Dinculescu A, McDowell JH, Amici SA, Dugger DR, Richards N, Hargrave PA, Smith WC. Insertional mutagenesis and immunochemical analysis of visual arrestin interaction with rhodopsin. J Biol Chem. 2002;277:11703–11708. doi: 10.1074/jbc.M111833200. [DOI] [PubMed] [Google Scholar]
- Dohlman HG, Thorner J, Caron MG, Lefkowitz RJ. Model systems for the study of seven-transmembrane-segment receptors. Ann Rev Biochem. 1991;60:653–688. doi: 10.1146/annurev.bi.60.070191.003253. [DOI] [PubMed] [Google Scholar]
- Dorn GW, 2nd, Force T. Protein kinase cascades in the regulation of cardiac hypertrophy. J Clin Investig. 2005;115:527–537. doi: 10.1172/JCI24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez LE, Hernandez CC, Mattos EC, Brandao IT, Olivieri B, Campelo RP, Araujo-Jorge T, Silva CL, Campos de Carvalho AC, Kurtenbach E. DNA immunizations with M2 muscarinic and beta1 adrenergic receptor coding plasmids impair cardiac function in mice. J Mol Cell Cardiol. 2005;38:703–714. doi: 10.1016/j.yjmcc.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Gimenez LE, Kook S, Vishnivetskiy SA, Ahmed MR, Gurevich EV, Gurevich VV. Role of receptor-attached phosphates in binding of visual and non-visual arrestins to G protein-coupled receptors. J Biol Chem. 2012a;287:9028–9040. doi: 10.1074/jbc.M111.311803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez LE, Vishnivetskiy SA, Baameur F, Gurevich VV. Manipulation of very few receptor discriminator residues greatly enhances receptor specificity of non-visual arrestins. J Biol Chem. 2012b;287:29495–29505. doi: 10.1074/jbc.M112.366674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimenez LE, Ghildyal P, Fischer KE, Hu H, Ja WW, Eaton BA, Wu Y, Austad SN, Ranjan R. Modulation of methuselah expression targeted to Drosophila insulin-producing cells extends life and enhances oxidative stress resistance. Aging cell. 2013;12:121–129. doi: 10.1111/acel.12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OB, Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature. 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- Granzin J, Wilden U, Choe HW, Labahn J, Krafft B, Buldt G. X-ray crystal structure of arrestin from bovine rod outer segments. Nature. 1998;391:918–921. doi: 10.1038/36147. [DOI] [PubMed] [Google Scholar]
- Gray-Keller MP, Detwiler PB, Benovic JL, Gurevich VV. Arrestin with a single amino acid sustitution quenches light-activated rhodopsin in a phosphorylation-independent fasion. Biochemistry. 1997;36:7058–7063. doi: 10.1021/bi963110k. [DOI] [PubMed] [Google Scholar]
- Gurevich VV. The selectivity of visual arrestin for light-activated phosphorhodopsin is controlled by multiple nonredundant mechanisms. J Biol Chem. 1998;273:15501–15506. doi: 10.1074/jbc.273.25.15501. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL. Cell-free expression of visual arrestin. Truncation mutagenesis identifies multiple domains involved in rhodopsin interaction. J Biol Chem. 1992;267:21919–21923. [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL. Visual arrestin interaction with rhodopsin. Sequential multisite binding ensures strict selectivity toward light-activated phosphorylated rhodopsin. J Biol Chem. 1993;268:11628–11638. [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL. Visual arrestin binding to rhodopsin. Diverse functional roles of positively charged residues within the phosphorylation-recognition region of arrestin. J Biol Chem. 1995;270:6010–6016. doi: 10.1074/jbc.270.11.6010. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Benovic JL. Mechanism of phosphorylation-recognition by visual arrestin and the transition of arrestin into a high affinity binding state. Mol Pharmacol. 1997;51:161–169. doi: 10.1124/mol.51.1.161. [DOI] [PubMed] [Google Scholar]
- Gurevich EV, Gurevich VV. Arrestins: ubiquitous regulators of cellular signaling pathways. Genome Biol. 2006a;7:236. doi: 10.1186/gb-2006-7-9-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Gurevich EV. The structural basis of arrestin-mediated regulation of G-protein-coupled receptors. Pharmacol Ther. 2006b;110:465–502. doi: 10.1016/j.pharmthera.2005.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurevich VV, Richardson RM, Kim CM, Hosey MM, Benovic JL. Binding of wild type and chimeric arrestins to the m2 muscarinic cholinergic receptor. J Biol Chem. 1993;268:16879–16882. [PubMed] [Google Scholar]
- Gurevich VV, Dion SB, Onorato JJ, Ptasienski J, Kim CM, Sterne-Marr R, Hosey MM, Benovic JL. Arrestin interaction with G protein-coupled receptors. Direct binding studies of wild type and mutant arrestins with rhodopsin, b2-adrenergic, and m2 muscarinic cholinergic receptors. J Biol Chem. 1995;270:720–731. doi: 10.1074/jbc.270.2.720. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Pals-Rylaarsdam R, Benovic JL, Hosey MM, Onorato JJ. Agonist-receptor-arrestin, an alternative ternary complex with high agonist affinity. J Biol Chem. 1997;272:28849–28852. doi: 10.1074/jbc.272.46.28849. [DOI] [PubMed] [Google Scholar]
- Gurevich VV, Hanson SM, Song X, Vishnivetskiy SA, Gurevich EV. The functional cycle of visual arrestins in photoreceptor cells. Prog Retin Eye Res. 2011;30:405–430. doi: 10.1016/j.preteyeres.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M, Gurevich VV, Vishnivetskiy SA, Sigler PB, Schubert C. Crystal structure of beta-arrestin at 1.9 A: possible mechanism of receptor binding and membrane translocation. Structure. 2001;9:869–880. doi: 10.1016/s0969-2126(01)00644-x. [DOI] [PubMed] [Google Scholar]
- Hanson SM, Gurevich VV. The differential engagement of arrestin surface charges by the various functional forms of the receptor. J Biol Chem. 2006;281:3458–3462. doi: 10.1074/jbc.M512148200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson SM, Francis DJ, Vishnivetskiy SA, Kolobova EA, Hubbell WL, Klug CS, Gurevich VV. Differential interaction of spin-labeled arrestin with inactive and active phosphor-hodopsin. Proc Natl Acad Sci USA. 2006;103:4900–4905. doi: 10.1073/pnas.0600733103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez CC, Barcellos LC, Gimenez LE, Cabarcas RA, Garcia S, Pedrosa RC, Nascimento JH, Kurtenbach E, Masuda MO, Campos de Carvalho AC. Human chagasic IgGs bind to cardiac muscarinic receptors and impair L-type Ca2+ currents. Cardiovasc Res. 2003;58:55–65. doi: 10.1016/s0008-6363(02)00811-8. [DOI] [PubMed] [Google Scholar]
- Hernandez CC, Nascimento JH, Chaves EA, Costa PC, Masuda MO, Kurtenbach E, Campos DECAC, Gimenez LE. Autoantibodies enhance agonist action and binding to cardiac muscarinic receptors in chronic Chagas' disease. J Recept Signal Transduct Res. 2008;28:375–401. doi: 10.1080/10799890802262319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch JA, Schubert C, Gurevich VV, Sigler PB. The 2.8 A crystal structure of visual arrestin: a model for arrestin's regulation. Cell. 1999;97:257–269. doi: 10.1016/s0092-8674(00)80735-7. [DOI] [PubMed] [Google Scholar]
- Ishikawa K, Tilemann L, Fish K, Hajjar RJ. Gene delivery methods in cardiac gene therapy. J Gene Med. 2011;13:566–572. doi: 10.1002/jgm.1609. [DOI] [PubMed] [Google Scholar]
- Kenakin T, Christopoulos A. Signalling bias in new drug discovery: detection, quantification and therapeutic impact. Nat Rev Drug Discov. 2013;12:205–216. doi: 10.1038/nrd3954. [DOI] [PubMed] [Google Scholar]
- Khoo DH, Parma J, Rajasoorya C, Ho SC, Vassart G. A germline mutation of the thyrotropin receptor gene associated with thyrotoxicosis and mitral valve prolapse in a Chinese family. J Clin Endocrinol Metab. 1999;84:1459–1462. doi: 10.1210/jcem.84.4.5620. [DOI] [PubMed] [Google Scholar]
- Kim YM, Benovic JL. Differential roles of arrestin-2 interaction with clathrin and adaptor protein 2 in G protein-coupled receptor trafficking. J Biol Chem. 2002;277:30760–30768. doi: 10.1074/jbc.M204528200. [DOI] [PubMed] [Google Scholar]
- Kim RY, al-Maghtheh M, Fitzke FW, Arden GB, Jay M, Bhattacharya SS, Bird AC. Dominant retinitis pigmentosa associated with two rhodopsin gene mutations. Leu-40-Arg and an insertion disrupting the 5'-splice junction of exon 5. Arch Ophthalmol. 1993;111:1518–1524. doi: 10.1001/archopht.1993.01090110084030. [DOI] [PubMed] [Google Scholar]
- Kim OJ, Gardner BR, Williams DB, Marinec PS, Cabrera DM, Peters JD, Mak CC, Kim KM, Sibley DR. The role of phosphorylation in D1 dopamine receptor desensitization: evidence for a novel mechanism of arrestin association. J Biol Chem. 2004;279:7999–8010. doi: 10.1074/jbc.M308281200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Vishnivetskiy SA, Van Eps N, Alexander NS, Cleghorn WM, Zhan X, Hanson SM, Morizumi T, Ernst OP, Meiler J, Gurevich VV, Hubbell WL. Conformation of receptor-bound visual arrestin. Proc Natl Acad Sci USA. 2012;109:18407–18412. doi: 10.1073/pnas.1216304109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci USA. 2001;98:1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovoor A, Celver J, Abdryashitov RI, Chavkin C, Gurevich VV. Targeted construction of phosphorylation-independent b-arrestin mutants with constitutive activity in cells. J Biol Chem. 1999;274:6831–6834. doi: 10.1074/jbc.274.11.6831. [DOI] [PubMed] [Google Scholar]
- Kraakman L, Lemaire K, Ma P, Teunissen AW, Donaton MC, Van Dijck P, Winderickx J, de Winde JH, Thevelein JM. A Saccharomyces cerevisiae G-protein coupled receptor, Gpr1, is specifically required for glucose activation of the cAMP pathway during the transition to growth on glucose. Mol Microbiol. 1999;32:1002–1012. doi: 10.1046/j.1365-2958.1999.01413.x. [DOI] [PubMed] [Google Scholar]
- Krupnick JG, Gurevich VV, Schepers T, Hamm HE, Benovic JL. Arrestin-rhodopsin interaction. Multi-site binding delineated by peptide inhibition. J Biol Chem. 1994;269:3226–3232. [PubMed] [Google Scholar]
- Kühn H. Light-regulated binding of rhodopsin kinase and other proteins to cattle photoreceptor membranes. Biochemistry. 1978;17:4389–4395. doi: 10.1021/bi00614a006. [DOI] [PubMed] [Google Scholar]
- Kühn H, Hall SW, Wilden U. Light-induced binding of 48-kDa protein to photoreceptor membranes is highly enhanced by phosphorylation of rhodopsin. FEBS Lett. 1984;176:473–478. doi: 10.1016/0014-5793(84)81221-1. [DOI] [PubMed] [Google Scholar]
- Lagerstrom MC, Schioth HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov. 2008;7:339–357. doi: 10.1038/nrd2518. [DOI] [PubMed] [Google Scholar]
- Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SG, Caron MG, Barak LS. The 2-adrenergic receptor/arrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci USA. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KB, Ptasienski JA, Pals-Rylaarsdam R, Gurevich VV, Hosey MM. Arrestin binding to the M2 muscarinic acetylcholine receptor is precluded by an inhibitory element in the third intracellular loop of the receptor. J Biol Chem. 2000;275:9284–9289. doi: 10.1074/jbc.275.13.9284. [DOI] [PubMed] [Google Scholar]
- Lin YJ, Seroude L, Benzer S. Extended life-span and stress resistance in the Drosophila mutant methuselah. Science. 1998;282:943–946. doi: 10.1126/science.282.5390.943. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990;248:1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- Meng D, Lynch MJ, Huston E, Beyermann M, Eichhorst J, Adams DR, Klusmann E, Houslay MD, Baillie GS. MEK1 binds directly to betaarrestin1, influencing both its phosphorylation by ERK and the timing of its isoprenaline-stimulated internalization. J Biol Chem. 2009;284:11425–11435. doi: 10.1074/jbc.M806395200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moaven H, Koike Y, Jao CC, Gurevich VV, Langen R, Chen J. Visual arrestin interaction with clathrin adaptor AP-2 regulates photoreceptor survival in the vertebrate retina. Proc Natl Acad Sci USA. 2013;110:9463–9468. doi: 10.1073/pnas.1301126110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Gurevich VV, Preninger A, Hamm HE, Bader M-F, Fazleabas AT, Birnbaumer L, Hunzicker-Dunn M. Aspartic acid 564 in the third cytoplasmic loop of luteinizing hormone/choriogonadotropin receptor is crucial for phosphorylation-independent interaction with arrestin2. J Biol Chem. 2002;277:17916–17927. doi: 10.1074/jbc.M110479200. [DOI] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Javitch JA, Sibley DR. G protein-coupled receptor kinase-mediated phosphorylation regulates post-endocytic trafficking of the D2 dopamine receptor. J Biol Chem. 2009a;284:15038–15051. doi: 10.1074/jbc.M900388200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung Y, Dipace C, Urizar E, Javitch JA, Sibley DR. G protein-coupled receptor kinase-2 constitutively regulates D2 dopamine receptor expression and signaling independently of receptor phosphorylation. J Biol Chem. 2009b;284:34103–34115. doi: 10.1074/jbc.M109.055707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen J, Szoka FC. Nucleic acid delivery: the missing pieces of the puzzle? Acc Chem Res. 2012;45:1153–1162. doi: 10.1021/ar3000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikonov SS, Brown BM, Davis JA, Zuniga FI, Bragin A, Pugh ENJ, Craft CM. Mouse cones require an arrestin for normal inactivation of phototransduction. Neuron. 2008;59:462–474. doi: 10.1016/j.neuron.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordstrom KJ, Sallman Almen M, Edstam MM, Fredriksson R, Schioth HB. Independent HHsearch, Needleman–Wunsch-based, and motif analyses reveal the overall hierarchy for most of the G protein-coupled receptor families. Mol Biol Evol. 2011;28:2471–2480. doi: 10.1093/molbev/msr061. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Ohguro H, Palczewski K, Walsh KA, Johnson RS. Topographic study of arrestin using differential chemical modifications and hydrogen-deuterium exchange. Protein Sci. 1994;3:2428–2434. doi: 10.1002/pro.5560031226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pals-Rylaarsdam R, Gurevich VV, Lee KB, Ptasienski J, Benovic JL, Hosey MM. Internalization of the m2 muscarinic acetylcholine receptor: arrestin-independent and -dependent pathways. J Biol Chem. 1997;272:23682–23689. doi: 10.1074/jbc.272.38.23682. [DOI] [PubMed] [Google Scholar]
- Pan L, Gurevich EV, Gurevich VV. The nature of the arrestin x receptor complex determines the ultimate fate of the internalized receptor. J Biol Chem. 2003;278:11623–11632. doi: 10.1074/jbc.M209532200. [DOI] [PubMed] [Google Scholar]
- Paschke R. Constitutively activating TSH receptor mutations as the cause of toxic thyroid adenoma, multinodular toxic goiter and autosomal dominant non autoimmune hyperthyroidism. Exp Clin Endocrinal Diabetes. 1996;104:129–132. doi: 10.1055/s-0029-1211720. [DOI] [PubMed] [Google Scholar]
- Pulvermuller A, Schroder K, Fischer T, Hofmann KP. Interactions of metarhodopsin II. Arrestin peptides compete with arrestin and transducin. J Biol Chem. 2000;275:37679–37685. doi: 10.1074/jbc.M006776200. [DOI] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol. 2012;52:179–197. doi: 10.1146/annurev.pharmtox.010909.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Restagno G, Maghtheh M, Bhattacharya S, Ferrone M, Garnerone S, Samuelly R, Carbonara A. A large deletion at the 3' end of the rhodopsin gene in an Italian family with a diffuse form of autosomal dominant retinitis pigmentosa. Hum Mol Genet. 1993;2:207–208. doi: 10.1093/hmg/2.2.207. [DOI] [PubMed] [Google Scholar]
- Ribeiro AL, Gimenez LE, Hernandez CC, de Carvalho AC, Teixeira MM, Guedes VC, Barros MV, Lombardi F, Rocha MO. Early occurrence of anti-muscarinic autoantibodies and abnormal vagal modulation in Chagas disease. Int J Cardiol. 2007;117:59–63. doi: 10.1016/j.ijcard.2006.04.053. [DOI] [PubMed] [Google Scholar]
- Rim J, Oprian DD. Constitutive activation of opsin: interaction of mutants with rhodopsin kinase and arrestin. Biochemistry. 1995;34:11938–11945. doi: 10.1021/bi00037a035. [DOI] [PubMed] [Google Scholar]
- Rockman HA, Chien KR, Choi DJ, Iaccarino G, Hunter JJ, Ross JJ, Lefkowitz RJ, Koch WJ. Expression of a beta-adrenergic receptor kinase 1 inhibitor prevents the development of myocardial failure in gene-targeted mice. Proc Natl Acad Sci USA. 1998;95:7000–7005. doi: 10.1073/pnas.95.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schattauer SS, Miyatake M, Shankar H, Zietz C, Levin JR, Liu-Chen LY, Gurevich VV, Rieder MJ, Chavkin C. Ligand directed signaling differences between rodent and human κ-opioid receptors. J Biol Chem. 2012;287:41595–41607. doi: 10.1074/jbc.M112.381368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipani E, Kruse K, Juppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- Schöneberg T, Schulz A, Biebermann H, Hermsdorf T, Römpler H, Sangkuhl K. Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther. 2004;104:173–206. doi: 10.1016/j.pharmthera.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Seo J, Tsakem EL, Breitman M, Gurevich VV. Identification of arrestin-3-specific residues necessary for JNK3 activation. J Biol Chem. 2011;286:27894–27901. doi: 10.1074/jbc.M111.260448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Manglik A, Kruse AC, Xiao K, Reis RI, Tseng WC, Staus DP, Hilger D, Uysal S, Huang LY, Paduch M, Tripathi-Shukla P, Koide A, Koide S, Weis WI, Kossiakoff AA, Kobilka BK, Lefkowitz RJ. Structure of active β-arrestin-1 bound to a G-protein-coupled receptor phosphopeptide. Nature. 2013;497:137–141. doi: 10.1038/nature12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Vishnivetskiy SA, Gross OP, Emelianoff K, Mendez A, Chen J, Gurevich EV, Burns ME, Gurevich VV. Enhanced arrestin facilitates recovery and protects rods lacking rhodopsin phosphorylation. Curr Biol. 2009;19:700–705. doi: 10.1016/j.cub.2009.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrakis S, Yu X, Patterson E, Huang S, Hamlett SR, Chalmers L, Pappy R, Cunningham MW, Morshed SA, Davies TF, Lazzara R, Kem DC. Activating autoantibodies to the beta-1 adrenergic and m2 muscarinic receptors facilitate atrial fibrillation in patients with Graves' hyperthyroidism. J Am Coll Cardiol. 2009;54:1309–1316. doi: 10.1016/j.jacc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavrakis S, Kem DC, Patterson E, Lozano P, Huang S, Szabo B, Cunningham MW, Lazzara R, Yu X. Opposing cardiac effects of autoantibody activation of beta-adrenergic and M2 muscarinic receptors in cardiac-related diseases. Int J Cardiol. 2011;148:331–336. doi: 10.1016/j.ijcard.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton RB, Vishnivetskiy SA, Robert J, Hanson SM, Raman D, Knox BE, Kono M, Navarro J, Gurevich VV. Crystal structure of cone arrestin at 2.3A: evolution of receptor specificity. J Mol Biol. 2005;354:1069–1080. doi: 10.1016/j.jmb.2005.10.023. [DOI] [PubMed] [Google Scholar]
- Suwa M, Ono Y. Computational overview of GPCR gene universe to support reverse chemical genomics study. Methods Mol Biol. 2009;577:41–54. doi: 10.1007/978-1-60761-232-2_4. [DOI] [PubMed] [Google Scholar]
- Vassart G, Costagliola S. G protein-coupled receptors: mutations and endocrine diseases. Nat Rev Endocrinol. 2011;7:362–372. doi: 10.1038/nrendo.2011.20. [DOI] [PubMed] [Google Scholar]
- Versele M, Lemaire K, Thevelein JM. Sex and sugar in yeast: two distinct GPCR systems. EMBO Rep. 2001;2:574–579. doi: 10.1093/embo-reports/kve132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Paz CL, Schubert C, Hirsch JA, Sigler PB, Gurevich VV. How does arrestin respond to the phosphorylated state of rhodopsin? J Biol Chem. 1999;274:11451–11454. doi: 10.1074/jbc.274.17.11451. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Schubert C, Climaco GC, Gurevich YV, Velez MG, Gurevich VV. An additional phosphate-binding element in arrestin molecule. Implications for the mechanism of arrestin activation. J Biol Chem. 2000;275:41049–41057. doi: 10.1074/jbc.M007159200. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Hosey MM, Benovic JL, Gurevich VV. Mapping the arrestin-receptor interface: structural elements responsible for receptor specificity of arrestin proteins. J Biol Chem. 2004;279:1262–1268. doi: 10.1074/jbc.M308834200. [DOI] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Francis D, Van Eps N, Kim M, Hanson SM, Klug CS, Hubbell WL, Gurevich VV. The role of arrestin alpha-helix I in receptor binding. J Mol Biol. 2010;395:42–54. doi: 10.1016/j.jmb.2009.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Gimenez LE, Francis DJ, Hanson SM, Hubbell WL, Klug CS, Gurevich VV. Few residues within an extensive binding interface drive receptor interaction and determine the specificity of arrestin proteins. J Biol Chem. 2011;286:24288–24299. doi: 10.1074/jbc.M110.213835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vishnivetskiy SA, Baameur F, Findley KR, Gurevich VV. Critical role of the central 139-loop in stability and binding selectivity of arrestin-1. J Biol Chem. 2013;288:11741–11750. doi: 10.1074/jbc.M113.450031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther C, Nagel S, Gimenez LE, Morl K, Gurevich VV, Beck-Sickinger AG. Ligand-induced internalization and recycling of the human neuropeptide Y2 receptor is regulated by its carboxyl-terminal tail. J Biol Chem. 2010;285:41578–41590. doi: 10.1074/jbc.M110.162156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Gimenez LE, Gurevich VV, Spiller BW. Crystal structure of arrestin-3 reveals the basis of the difference in receptor binding between two non-visual subtypes. J Mol Biol. 2011;406:467–478. doi: 10.1016/j.jmb.2010.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang T, Chen Q, Cho MK, Vishnivetskiy SA, Iverson TM, Gurevich VV, Sanders CR. Involvement of distinct arrestin-1 elements in binding to different functional forms of rhodopsin. Proc Natl Acad Sci USA. 2013;110:942–947. doi: 10.1073/pnas.1215176110. [DOI] [PMC free article] [PubMed] [Google Scholar]