Abstract
Cytomegalovirus (CMV) retinitis is the most common ocular opportunistic complication and a serious cause of vision loss in immunocompromised patients. Even though, a rise in human immunodeficiency virus (HIV) infected individuals seems to be a major factor responsible for the prevalence of CMV retinitis, the introduction of highly active antiretroviral therapy (HAART) significantly reduced the incidence and severity of CMV retinitis. Thorough evaluation of the patient’s immune status and an exact classification of the retinal lesions may provide better understanding of the disease etiology, which would be necessary for optimizing the treatment conditions. Current drugs such as ganciclovir, valganciclovir, cidofovir and foscarnet have been highly active against CMV, but prolonged therapy with these approved drugs is associated with dose-limiting toxicities thus limiting their utility. Moreover development of drug-resistant mutants has been observed particularly in patients with acquired immunodeficiency syndrome (AIDS). Continuous efforts by researchers in the industry and academia have led to the development of newer candidates with enhanced antiviral efficacy and apparently minimal side effects. These novel compounds can suppress viral replication and prevent reactivation in the target population. Though some of the novel therapeutics possess potent viral inhibitory activity, these compounds are still in stages of clinical development and yet to be approved. This review provides an overview of disease etiology, existing anti-CMV drugs, advances in emerging therapeutics in clinical development and related recent patents for the treatment of CMV retinitis.
Keywords: Antivirals, cytomegalovirus, patents, retinitis, therapeutics, treatment
INTRODUCTION
Cytomegalovirus (CMV), a member of the beta class of herpes virus family, is the most common cause of blindness and death in patients with acquired immunodeficiency syndrome (AIDS). It generally occurs in HIV infected individuals whose CD4+ T cells count is less than 50 cells/μL. CMV is a life-threatening, opportunistic pathogen mainly associated with significant morbidity and mortality in immature or immunocompromised individuals [1, 2]. More than fifty percent of kidney, heart and liver transplant recipients showed evidence of CMV infections in the first year post-transplantation [3]. This virus has a long life cycle and can remain in the host system for life time [4].
CMV is the largest human herpes virus with doublestranded DNA of about 235 kbp [5, 6]. The genome is enclosed by an icosahedral protein capsid. A lipid bilayer envelope, consisting of two glycoprotein complexes encompasses the capsid. These complexes play a vital role in cellular adsorption into the host system, virion assembly, replication and infection. The tegument lies in between the protein capsid and the lipid envelope. The tegument has several proteins which help in viral replication and are highly immunogenic. The function of many tegument proteins still remains unclear [7-12]. Similar to many other viral infections, CMV primary infection is followed by a period of latentiation. The virus reactivates when the host immune system is severely compromised [13].
CMV translocates to the retina through endothelial cells and peripheral blood leukocytes in AIDS and immunocompromised transplant patients, respectively [14]. Viral particles cross the retinal vascular endothelial cells via endocytosis. Further replication of virions in these cells, disrupts the integrity of blood-retinal barrier allowing their access to Muller cells, other glial cells and finally the retinal pigment epithelium [15, 16]. CMV retinitis is characterized by progressive, necrotizing retinitis that can lead to retinal detachment, optic atrophy and finally loss of vision [17, 18].
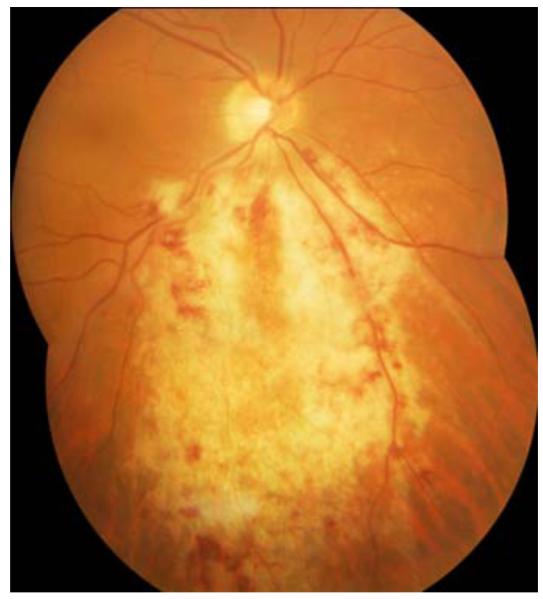
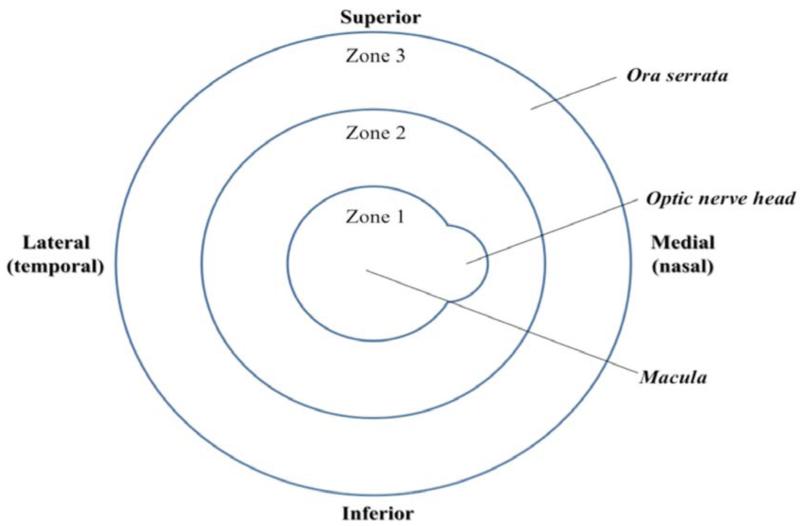
CMV retinitis is the most common and severe intraocular complication in patients with HIV. Although primary infection is asymptomatic and subclinical in immunocompetent hosts, reactivated CMV infection is responsible for vision and life-threatening complications, in the setting of immunosuppression. Clinical manifestation of CMV disease in these individuals involves retinitis, pneumonitis and central nervous system infections [19]. CMV retinitis may be present in three main clinical forms. The granular form is indolent, and is characterized by whitish granular lesions often starting at the retinal periphery and slowing destroying the retina. The hemorrhagic/edematous form is more aggressive; rapidly leading to extensive yellowish foci of retinal necrosis intermingled with retinal hemorrhages (ketchup and cheese pattern). The last form is called frosted branch angiitis and is manifested by an occlusive retinal vasculitis with exuberant whitening of the vessel wall, as if it were frozen. As depicted in Fig. (1), retinitis spreads centrifugally along vessels in a brush-fire fashion, clearing the central portion and destroying the retina. The extent of vision loss is determined by the location of CMV retinal lesions. The ocular fundus is categorized into three different zones. Zone 1 encircles the area within 1500 μm of the nerve or 3000 μm of the center of the fovea. Zone 2 includes the area outside of zone 1 but posterior to the equator and zone 3 comprises the peripheral retina between the equator and the ora serrata [20]. The lesions are described according to zones affected; zone-1 representing posterior part of retina and zone-3 representing anterior (more peripheral) part of retina Fig. (2). Zone-1 lesions cause immediate vision loss due to damage to optic nerve and macula whereas zone-3 lesions cause retinal detachment [21].
Fig. (1).
Characteristics of CMV retinitis (Reproduced with permission from (1)).
Fig. (2).
Depiction of the three anatomical zones for classification of CMV retinitis.
With the introduction of highly active antiretroviral therapy (HAART) for HIV treatment, the incidence and severity of CMV retinitis went down by 55-95% [22]. However, with a rise in AIDS among patients, an augmented prevalence of CMV retinitis has been observed [23, 24]. In spite of significant advances in the treatment and development of vaccines for specific viral diseases (i.e. polio and measles), and the eradication of particular viruses from the human population (e.g. smallpox), viral diseases remain an imperative medical and public health problem and encompass a devastating effect on the economic output of society. In many developing countries, CMV retinitis seems to be ignored, with modest data unfolding the scope of the problem, and minimal/no strategic approach for management of this disease.
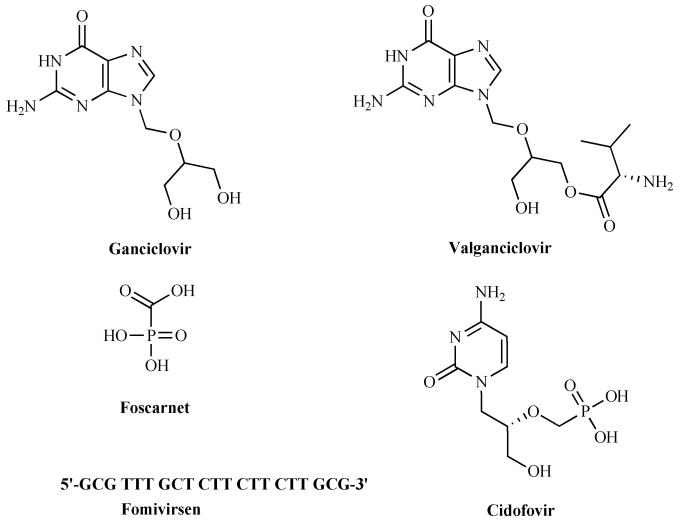
The advent of antiviral drugs, in particular nucleoside analogs has raised potential for chemical diversity within the class based on the differentiation of target viral DNA polymerases or reverse transcriptases from host enzymes. These nucleoside analogs are specifically activated by the viral nucleoside kinases, selectively phosphorylated and are subsequently converted to triphosphate forms which serve as substrates or inhibitors for the viral DNA polymerases in infected cells. Current drugs for the treatment of CMV infections are ganciclovir, valganciclovir, cidofovir and foscarnet Fig. (3). Though acyclovir (ACV) is approved in European countries for CMV prophylaxis, it is devoid of adequate potency for treating the active disease. Advances in biotechnology led to the development of an anti-sense RNA drug (fomivirsen) which is also approved for the treatment of CMV retinitis.
Fig. (3).
Structures of currently marketed anti-CMV drugs.
EXISTING ANTIVIRAL AGENTS
Ganciclovir (GCV) and its Prodrug
GCV was the first antiviral drug approved to treat CMV infections. It is a synthetic compound and a derivative of acyclic nucleoside analogue of 2′-deoxyguano-sine. In CMV infected human cells, it gets monophosphorylated by enzyme UL97 and then converted to GCV triphosphate. The triphosphate then inhibits viral DNA synthesis catalyzed by viral DNA polymerase (encoded by UL54 gene) [25]. An intravenous formulation (Cytovene-IV®) was approved for CMV retinitis in 1989. It was administered twice daily at a dose of 5mg/kg in order to achieve high concentrations. Such high doses caused hematologic abnormalities (neutropenia, anemia, and thrombocytopenia) and probably long term reproductive toxicity. The cumulative percentage of patients with mutations in either UL97 or UL54 genes has increased from 2.2% at three months to 15.3% at 18 months during therapy [26]. To avoid the risks of catheter related sepsis and inconvenience associated with intravenous GCV administration, an oral formulation (Cytovene®) was developed. However, low solubility, poor bioavailability (5%) and insufficient viral suppression resulting from the lower systemic exposure from oral GCV lead to the development of Valganciclovir (Valcyte®), the L-valyl ester prodrug of GCV. Upon oral administration, valganciclovir was enzymatically metabolized to active GCV, resulting in higher oral bioavailability (60%). It was approved for the treatment of CMV retinitis in HIV infected immunocompromised individuals in 2000 [27] and became the prodrug of choice for clinical usage of oral GCV. Intravitreal administration of GCV resulted in achieving high retinal concentrations with no systemic bone marrow suppression. Interestingly, repeated administration of doses ranging from 200μg/0.1 mL to 2000 μg/0.1 mL showed no significant retinal toxicity [28, 29]. But the disadvantages such as retinal detachment and vitreous hemorrhages owing to repeated intravitreal injections limit its usage. A sustained-release GCV intraocular implant (Vitrasert®) approved in 1996 provided long term treatment (3-6 months) for CMV retinitis in the infected eye. But a significant risk of infection in the contralateral eye in individuals treated with implants alone was noted [30]. Also, implantation is associated with high cost as well as retinal detachment needing surgery.
Foscarnet (FOS)
Foscavir®, a pyrophosphonate analogue, the trisodium salt of phosphormophonic acid, is the second drug approved in 1991 for the treatment of CMV retinitis in AIDS patients. The compound binds to the pyrophosphate site and blocks the cleavage of pyrophosphate from the terminal nucleoside triphosphate, supplementary to the growing DNA chain. Resistance to FOS alone and with GCV was sometimes observed in patients primarily due to point mutations in UL54 gene. Renal impairment is considered to be a major dose-limiting toxicity and necessitates regular monitoring of serum creatinine levels. For patients with GCV resistant viral strains and dose-limiting neutropenia or leucopenia, FOS is considered to be a second line therapy [31, 32].
Cidofovir (CDV)
Cidofovir (Vistide®) is a broad spectrum antiviral drug with potent activity against CMV and herpes virus infections both in vitro and in vivo [33-36]. It is an acyclic nucleoside phosphonate, approved in 1996 for the treatment of CMV retinitis in AIDS patients. CDV upon intracellular accumulation is phosphorylated to diphosphate by host kinases and acts as a DNA chain terminator by competitively inhibiting viral DNA polymerase. The oral bioavailability of CDV is less than 5% but its notable intracellular half life (> 24 hrs) makes it suitable for infrequent dosing. Similar mutations of UL54 gene leading to viral resistance have been observed. Long term therapy of CDV is known to cause severe renal toxicity, neutropenia and ocular hypotony. For these reasons it is considered as a second line therapy. Renal toxicity is due to high affinity of CDV towards the anion transporter localized in the convoluted proximal tubules [37].
Fomivirsen (FMV)
Fomivirsen (Vitravene®) is an antisense compound with a 21-nucleotide sequence (5′-GCG TTT GCT CTT CTT CTT GCG-3′) complementary to the immediate early transcriptional unit region 2 of CMV messenger ribonucleic acid (RNA). It is administered by intraocular injection. This drug was approved in 1998 as a second line therapy for local treatment of CMV retinitis. Treatment protocol involves a 4-week induction phase with a single injection every alternate week, followed by maintenance dose in which a single injection is administered every 4 weeks. Though intravitreal FMV is well tolerated with an acceptable safety profile, its frequent usage is associated with adverse local side effects such as intraocular inflammation [38]. However, these side effects can be managed with corticosteroids.
All the above existing antiviral agents, their mechanism of action, mode of delivery and side effects have been summarized in Table 1.
Table 1. Summary of the Existing Drugs for the Treatment of CMV Retinitis.
| Existing Antiviral Agents | Mechanism of Action | Mode of Delivery | Side Effects |
|---|---|---|---|
| Ganciclovir | Inhibits viral DNA synthesis | Intravenous formulation-Cytovene- IV® (Roche) Oral formulation-Cytovene® (Roche) Intraocular implant-Vitrasert® (Bausch & Lomb) |
Hematological abnormalities Poor oral bioavailability (5%) leading to insufficient viral suppression Infection in the contralateral eye and risk of retinal detachment |
| Valganciclovir | Inhibits viral DNA synthesis | Oral formulation-Valcyte® (Roche) | Myelosuppression |
| Foscarnet | Blocks the cleavage of pyrophosphate from the terminal nucleoside triphosphate |
Intravenous formulation-Foscavir® AstraZeneca LP) |
Renal impairment |
| Cidofovir | Inhibits viral DNA synthesis | Intravenous formulation-Vistide® (Gilead Sciences) |
Severe renal toxicity |
| Fomivirsen | Inhibits translation of early CMV proteins | Intravitreal injection-Vitravene® (Isis Pharmaceuticals) |
Adverse ocular side effects such as uveitis |
NEW ANTI-CMV DRUGS
Severe acute and long-term toxicities associated with the use of currently approved antiviral drugs mandate further development of safer and newer drugs for the treatment of CMV diseases [39]. Moreover, incidence of viral resistance observed so often with current drugs may be lowered with the newly developed antiviral agents Fig. (4).
Fig. (4).
Structures of anti-CMV drugs currently in clinical development.
Maribavir
Maribavir is a novel benzimidazole riboside analog, chemically termed 1-(β-L-ribofuranosyl)-2-isopropylamino-5,6-dichlorobenzimidazole, and also known as 1263W94, Benzimidavir, GW 1263, VP41263, GW1263W94. It is selectively potent against CMV and EBV (Epistein Barr Virus). This compound does not inhibit viral DNA polymerase in CMV infected cells, as noticed with other drugs [40, 41]. It has been reported to interfere with viral nucleocapsid egress from the infected cell nucleus, thus reducing infectious virions. This drug can block phosphorylation of the polymerase accessory protein, pUL44, by the viral-encoded protein kinase, pUL97, which in turn blocks viral DNA synthesis [42-44]. Preclinical data demonstrated excellent in vitro potency, bioavailability, and safety profile in acute, chronic and genetic toxicology studies. A different mechanism of action reduces the cross resistance associated with earlier and currently available agents. Phase I studies in HIV-infected individuals during a 28-day study exhibited a significant reduction in viral shedding in the semen and urine [45]. Inherent viral inhibitory potential makes this drug a suitable candidate for the treatment of CMV retinitis and other systemic CMV infections [46, 47].
BAY 38-4766
BAY 38-4766 belongs to a class of non-nucleoside antiviral agents and is chemically termed 3-hydroxy-2,2-dimethyl-N-[4([[5-(dimethylamino)-1-naphthyl]sulfonyl]amino)-phenyl]propanamide. It is found to be highly active against CMV in vitro [48]. This molecule exhibited anti-CMV activity similar to GCV in an immunodeficient mouse model [49]. The compound is active against strains resistant to currently approved anti-CMV drugs. Moreover cross-resis-tance to these agents in virus selected for resistance to BAY 38-4766 was not observed [48, 50]. BAY 38-4766 acts by inhibiting DNA maturation and gene mutations responsible for drug resistance, of which UL89 and UL56 genes encode subunits of the viral terminase [51]. Treatment with BAY 38-4766 reduced both viremia and DNAemia in CMV infected guinea pigs. It also reduced mortality from 83% (placebo-treated guinea pigs) to 17% (BAY 38-4766-treated animals) [52]. Though this compound demonstrated encouraging safety profile in healthy male volunteers at single oral doses up to 2000mg, no recent reports of the compound itself or any other related molecules have been found in the literature regarding its contemporary status.
Cidofovir Esters
CDV, a highly active phosphonate drug against CMV suffers from low bioavailability and nephrotoxicity. Alkoxyalkyl esters of CDV such as hexadecyloxypropyl CDV (HDP-CDV, now known as CMX001) and octadecyloxyethyl CDV (ODE-CDV) have been developed to overcome toxicities without losing any efficacy of the parent compound. These derivatives exhibited enhanced in vitro anti-CMV efficacy. In particular CMX001 was found to be 1000 fold more potent than CDV against human and murine CMV [53, 54]. These compounds showed a significant improvement in cellular absorption and oral bioavailability (88-97%) without causing any renal toxicity [55]. The data acquired from pharmacokinetic, metabolic, and toxicologic studies has lead CMX001 to be selected for clinical evaluation. Phase I clinical trials have been successfully completed [56]. It was well tolerated confirming the previously reported studies in mice which showed significantly reduced accumulation of the compound in the kidney, thereby avoiding the frequently observed nephrotoxicity associated with the parent compound, CDV [55, 57]. Currently, CMX001 is being evaluated in Phase II clinical studies for the treatment of polyomavirus BK virus and CMV infections (ClinicalTrials.gov identifier: NCT00793598 and NCT00942305) as well as adenovirus infections [58].
Benzimidazole Ribosides
Halogenated benzimidazole analogs were identified for its activity to block the cleavage/packaging of viral genomes and to eliminate the formation of monomer viral genomes in infected cells [59]. GW275175X, chemically 2-bromo-5,6-dichloro-1-β-d-ribopyranosyl-1H-benzimidazole is a β-D-pyranosyl sugar analog of 1H-β-D-ribofuranosyl-2-bromo-5,6-dichlorobenzimidazole (BDCRB), an excellent anti-CMV inhibitor both in vitro and in vivo [60, 61]. GW275175X is more stable and retains the mechanism of action of BDCRB. Though this compound advanced to Phase I single-escalating dose trial of safety, tolerability and pharmacokinetics, no Phase II/III clinical studies are currently in progress to evaluate its clinical potential.
Cyclopropavir and Methylenecyclopropane Analogs
The methylenecyclopropane derivatives are quite new therapeutic agents which have been found to possess antiviral activity. Later, the first generation derivatives represent bioisosteric analogs of ACV and are followed by the second generation which was closely related to GCV. Both Z- and E-isomeric analogs were synthesized for each series of compounds. Reports show that Z-isomers of the first generation of methylenecyclopropane analogs demonstrated high antiviral activity against CMV, murine CMV, rat CMV, rhesus monkey CMV, guinea pig CMV, human herpes virus 6 (HHV-6) and human herpes virus 8 (HHV-8). Recently, cyclopropavir (CPV), also known as ZSM-I-62 has been reported to be very active in vitro against CMV, HHV-6A, HHV-6B and HHV-8. It is a 2, 2-bis-hydroxymethyl derivative and is classified as a second generation of methylenecyclopropane analogs. In CMV infected cells, this compound requires UL97 kinase for its antiviral activity and is also reported to retain its activity against most GCV-resistant and clinical isolates. UL97 kinase is stereoselective and phosphorylates CPV to its (+)-monophosphate. Furthermore, phosphonate modifications of CPV also showed excellent antiviral activity against CMV similar to monophosphate analogs. Valcyclopropavir, an L-valine ester of CPV, inhibited replication of hCMV to a similar extent as the parent drug CPV. Improved pharmacokinetic parameters of this L-valine prodrug has been observed over CPV. Halogenated methylenecyclopropane analogs ((Z)- and (E)-[2- fluoro-2-(hydroxyl methyl) cyclopropylidene] methyl-purines and – pyrimidines) demonstrated antiviral activity against CMV, HSV, VZV, and EBV. This highly active lead molecule will be soon evaluated in Phase 1 studies.
AIC246
More recently, a new inhibitor, AIC246 has been identified with excellent antiviral activity [62]. It appears to act by interfering at a very late stage of viral replication and possibly by inhibiting cleavage/packaging of viral DNA. The compound retained its activity against clinical isolates of CMV, including GCV-resistant isolates. The drug is well tolerated and is currently in Phase II clinical trial.
All the above new anti-CMVdrugs, their mechanism of action and advantages over the existing drugs have been summarized in Table 2.
Table 2. Summary of the Novel Drugs for the Treatment of CMV Retinitis.
| New Anti-CMV Drugs | Mechanism of Action | Advantages |
|---|---|---|
| Maribavir | Interferes with viral nucleocapsid egress from infected cell nucleus |
Different mechanism of action reduces the cross resistance associated with earlier drugs |
| BAY 38-4766 | Inhibits DNA maturation | Decreases mutation in UL89 and UL56 genes responsible for development of drug resistance |
| Cidofovir esters | Inhibits viral DNA synthesis | Significant improvement in oral bioavailability (88-97%) without any renal toxicity |
| Benzimidazole ribosides | Block the cleavage/packaging of viral genomes | Novel mechanism of action confers efficacy to drug resistant strains |
|
Cyclopropavir and methyle-necyclopropane
analogs |
Inhibits viral DNA synthesis and the normal activity of the UL97 kinase |
Retains its activity against most GCV-resistant and clinical isolates |
| AIC246 | Inhibits the cleavage/packaging of viral DNA | Excellent antiviral activity against clinical isolates of CMV |
RECENT PATENTS FOR TREATMENT OF CYTOMEGALOVIRUS RETINITIS
A recent patent by Mitra discloses peptide prodrugs of ACV and GCV to treat herpes virus and CMV ocular infections [63]. Significant attention towards transporter targeted prodrug approach has led to the identification of numerous membrane transporters on ocular tissues such as cornea, retina and conjunctiva [64, 65]. This patent provides di-and tripeptide mono- and di-esters of GCV which could be formulated into eye drops for topical instillation. The disclosed compounds have been designed to target oligopeptide transporters for delivery into corneal and retinal tissues. These conjugates exhibit excellent antiviral activity against herpes and cytomegaloviruses. Effective absorption by the oligopeptide transporter at blood ocular barrier (BOB) requires the prolonged presence of intact prodrug at the site of absorption. However, dipeptide prodrugs were found to be rapidly metabolized to parent drug resulting in limited bioavailability. In order to provide enhanced residence time of intact prodrug in the systemic circulation, besides facilitating transporter targeting on BOB after oral or systemic administration, fairly stable prodrugs have been disclosed by Mitra et al. in another patent. This patent is directed to specific stereoisomers of di-peptidyl esters of ACV and mono- and di-esters of GCV with enhanced enzymatic stability and efficient transporter translocation across ocular barriers for the treatment of herpes keratitis and CMV retinitis. These pro-drugs demonstrated excellent solution stability and solubility facilitating formulation of stable eye drops [66].
Recent advancements in the field of genetics and molecular biology has revealed the significance of short interfering RNA (siRNA) or RNA interference (RNAi) as a physiological mechanism in regulating gene expression. A patent application filed by Kowalik [67] discloses utilization of RNAi to block mRNA translation into proteins which are vital for replication of CMV. Introduction of siRNA induces the RNAi, which then inhibits viral proliferation by interfering with post-translational modifications. siRNA disclosed is usually about 18-29 nucleotides in length unmodified, with modified 3′ terminal (3′PO4 and 3′dTdT or 3′TT) or enclosed in a vector. An RNAi agent may consist of 1E1, 1E2, DNA polymerase, a scaffold protease or glycoproteins (gB and gH). IE1 and IE2 are viral regulatory proteins of human CMV, expressed immediately after infection of a host cell. The siRNA degrades or inhibits viral RNA transcripts, or blocks the translation of viral CMV RNA transcripts or inhibits the expression of two or more proteins. siRNA can mediate RNAi during an early or late viral replication stage. This composition has been described to be very effective in the treatment of CMV retinitis when administered as an intravitreal injection.
A US patent [68] reports the design and synthesis of antisense oligonucleotides to inhibit CMV replication and treat CMV infections. An antisense phosphorothioate deoxyoligonucleotide drug, ISIS 2922 demonstrated clinical efficacy in treating CMV retinitis in AIDS patients. Though this compound is well tolerated with less ocular complications and no systemic side effects, it is found to be relatively less stable than an analog of FMV. The analog possess atleast one 2′-methoxyethoxy modification and may be chimeric oligonucleotide. The analog exhibits a prolonged increased residence time in the vitreous and retina compared to FMV. These compounds are targeted to IE1, IE2, or DNA polymerase genes of CMV. These oligonucleotides can be targeted to a portion of mRNA cap site, AUG region, the conserved amino acid region, or the CMV insertion regions between bases 608-697 or 1109-1159 of the DNA polymerase gene. Also, GEM132, a second antisense drug targeted against CMV has been discussed in this patent. It is a phosphorothioate oligonucleotide with modified nucleotides at both ends and is targeted to the intron-exon boundary of human CMV (hCMV) genes UL36 and UL37. These drugs have been intended for administration by an intravitreal injection for the treatment of CMV retinitis.
A recent US patent application discusses novel antibody sequences which can bind to hCMV and neutralize the infection. These antibody sequences were isolated from human B cells having biological and therapeutic activities specifically for hCMV. These antibodies have the ability to recognize a segment in the hCMV envelope glycoprotein H (gH), which is known to be bound by antibodies that neutralize CMV infections. The identified sequences further can be employed to produce recombinant proteins and can be used for therapeutic management of all CMV related disorders [69].
Novel sirtuin modulating compounds have been patented by Sirtris Pharmaceuticals, Inc. [70, 71]. These compounds have been reported to modulate (raise or lower) the level or activity of a sirtuin protein that ultimately results in increasing the lifespan of an infected cell by preventing or treating various ocular disorders. This patent discloses sirtuin modulating compounds which could either upregulate (activate or stimulate), downregulate (inhibit or suppress) or otherwise alter a functional property or biological activity of a sirtuin protein. The biological activity of a sirtuin protein is dependent on its deacetylation ability. Silent Information Regulator (SIR) family of genes signifies a greatly conserved group of genes in the genome of organisms ranging from prokaryotes to eukaryotes. The encoded SIR proteins are involved in diverse processes from regulation of gene silencing to DNA repair. The present invention also mentions the utility of these compounds for treating and/or preventing a wide variety of ocular diseases and disorders including CMV retinitis. These novel compounds have been proposed to treat vision impairment when administered to a patient at a therapeutic amount of a sirtuin modulator or a pharmaceutically acceptable salt, prodrug or its metabolic derivative. A sirtuinmodulating compound may be delivered in a pharmaceutically acceptable ophthalmic vehicle. The delivery platform may provide ocular surface contact for adequate time period to allow the compound to permeate corneal and other anterior segment tissues. Also, the inventors mentioned that these compounds may be injected directly into vitreous and aqueous humor to prevent/treat various ocular complications.
Tokars et al. provided methods for synthesizing 9-(2-phosphonylmethoxyethyl) adenine (PMEA) lipid conjugates and utilizing these compounds to treat viral diseases caused by herpes and CMV [72]. PMEA is an acyclic nucleoside phosphonate with excellent antiviral activity, particularly against HIV. The present invention discloses lipid conjugates of PMEA including PMEA-oleyl amide and PMEA-oleyl carbamate. The former was found to be active against herpes simplex virus type 1 (HSV-1) and herpes simplex virus type 2 (HSV-2), while the latter was active against hCMV. In cells, PMEA converts to the mono- and di-phosphorylated metabolites (PMEAp and PMEApp) which are dose-dependent and rise proportionally to initial extracellular PMEA concentrations. PMEApp acts as a potent DNA chain terminator. The compounds of the present invention showed excellent antiviral activity against CMV infected cells, thus indicating their utility in the treatment of CMV retinitis in subjects with a weakened immune system.
A recent US patent by Zimmermann et al. [73] discloses the methods of preparation of heterocyclylamide-substituted imidazoles which may be useful as antiviral agents in the treatment and/or prophylaxis of CMV infections, especially CMV retinitis in AIDS patients. The compounds of the present invention have shown an unforeseeable, surprising spectrum of antiviral activity against family of Herpes viridae (herpes viruses), especially on CMV, in particular on the hCMV. Owing to their better pharmacological properties and excellent antiviral activities, these imidazole compounds may be used alone and also in combination with other active antiviral drugs such as GCV or ACV, for the treatment and/or prevention of viral infections, in particular hCMV infections. Another patent by Zimmermann et al. [74] discloses novel 4-aminocarbonylamino-substituted imidazole compounds with excellent antiviral activity against hCMV. The compounds of this invention may, depending on their structure, exist in stereoisomeric forms (enantiomers, diastereomers). The invention for that reason relates to the enantiomers or diastereomers and their respective mixtures. The stereoisomerically pure constituents can be extracted or separated in a known method from such mixtures of enantiomers and/or diastereomers.
Another recent patent application by Fryszman et al. [75] reports the utility of 5-membered heterocycle-based p38 kinase inhibitors for treating p38 kinase mediated diseases or disorders, or diseases or disorders in which p38 kinase activity, including p38α and p38β kinase activity, is involved. This application discusses compositions comprising pyrazole- or imidazole-based compounds which are employed as kinase inhibitors, including p38α and p38β kinases. Four isoforms of p38 (p38α, p38β, p38γ and p38δ) are known so far. The α and β isoforms are expressed in inflammatory cells and are key modulators of TNF-α production. As a result of inhibition of the p38α and β kinases, the expression of TNF-α level is reduced. This process prevents or ameliorates one or more symptoms of p38 kinase-mediated diseases or disorders. Furthermore, administration of p38α and β inhibitors in animal models of inflammatory disease including CMV retinitis has confirmed that such inhibitors are effective in treating those diseases.
Another patent related to the methods of inhibiting (preventing and/or treating) viral infection using prothymosinalpha (ProTα) molecules is disclosed [76]. ProTα is a polypeptide comprising the amino acid sequence of SEQ ID NO: 2. When administered in therapeutically effective amounts, this compound may reduce the symptoms associated with CMV infections. ProTα could be a purified naturally occurring polypeptide or a purified recombinant polypeptide or a purified synthetic polypeptide or a non-phosphorylated polypeptide. The present invention is intended for identification of a viral inhibitor which can prevent or eliminate latent viruses in the body. The inventors reported that viral inhibitors of the present invention may also be combined with drugs which can treat secondary complications of HIV infection, e.g., GCV for the treatment of CMV retinitis. The inventors anticipated that combination therapy may hinder the development of drug-resistant mutants by entailing multiple mutation events for the emergence of a fully drug-resistant isolate.
A recent US patent application by Exley et al. [77] discloses 2-aminopurine derivatives, i.e., aminocyclopropylcarboxylate derivatives of ACV, penciclovir (PCV), and GCV to prevent or treat autoimmune diseases. The inventors mention that certain 2-amino purine derivatives are useful for treating a wide variety of diseases or conditions formerly not adequately treated by any known medications. This patent application discusses a method of treating patients possessing a herpes simplex, herpes zoster, CMV or herpes simplex keratitis infection which comprises administering an effective amount of any 2-aminopurine derivative or its pharmaceutically acceptable salt. The 2-amino purine derivative of GCV discussed in the application may be particularly useful for treating CMV retinitis or herpes simplex keratitis. The inventor reveals that these compounds when administered in relatively smaller amounts would yield similar results of antiviral activities to that of antiviral agents previously employed. These compounds would have enhanced stability to hydrolytic conditions at both acidic and neutral pH, permitting longer retention in the stomach and blood, prior to being converted to a biologically active form for necessitating treatment.
A recent US patent application by Ware et al. [78] describes the utility of a novel polypeptide ligand, p30, or LIGHT for herpes virus entry mediator (HVEM). LIGHT is responsible for modulating immune responses and inhibiting viral infection and subsequent proliferation by CMV. This endogenous polypeptide functions as a ligand for HVEM, which earlier was known only to bind D glycoprotein (gD) of HSV. gD is a transmembrane protein located in the virion envelope which initiates infection by binding to cellular receptors. The inventors stated in the application that the homotrimeric soluble forms of this polypeptide (apparent molecular weight of about 30kDa) may be employed to obstruct cellular entry of herpesvirus/CMV. This is due to the ability of polypeptide ligand (LIGHT) to compete with HSV gD for HVEM. Also, this application provides methods for modulating lymphotoxin beta receptor (LTβR)-mediated cellular responses by administering an amount of a LTβR or TNFR1 agonist adequate to treat viral disorders such as CMV retinitis.
Another recent US patent by Melnik et al. reports the design and development of peptides which exclusively inhibits the fusion of hCMV to the host cell membrane. These molecules inhibit viral infection and/or replication including CMV infection by a novel mechanism [79]. This patent discloses an isolated peptide possessing a sequence with high degree of similarity to a segment of hCMV glycoprotein B (gB), which is involved in fusion of the virion to cell membranes. The peptides inhibit virionxell membrane fusion which is essential for infecting a non-infected cell. Such peptides are capable of inhibiting infection at the cellular level, despite its origin from an exogenous source or from an aggravation of a preexisting dormant infection. The peptides of the present invention could be either cyclic homodetic or cyclic heterodetic and possibly include at least one D- amino acid residue. The peptides discussed in this patent include portions of residues 146 to 315 of SEQ ID NO: 1 or a sequence variant of residues 146 to 315 of SEQ ID NO: 1 possessing at least 70% sequence identity. These compounds may particularly be useful for treating infections including CMV retinitis in immuno-compromised patients. The peptides can be administered either alone or in combination with one or more antiviral agents for better efficacy.
CURRENT & FUTURE DEVELOPMENTS
Treatment of CMV infections still remains a challenging task, despite the introduction of numerous therapeutics agents with excellent intrinsic antiviral activity. With the introduction of highly active antiretroviral therapies, the frequency and severity of CMV infections have significantly diminished along with a decline in AIDS-related mortality. However due to an increase in the number of immunocompromised individuals all over the world, CMV retinitis still remains the major cause of vision loss, particularly in HIV infected populations. Currently available drugs for the treatment of CMV infections are relatively safe and essentially effective, but the toxicity associated with these molecules upon long term therapy restricts their utility. Moreover, long term therapy may lead to emergence of resistance and development of drug-resistant variants due to the partial suppression/inhibition of viral replication. Therefore, it is clear that the development of newer therapeutics with a novel mechanism of action, possessing superior efficacy and diminished potential for adverse side effects appears to be a necessary requisite for treating CMV related disorders. Several new compounds with distinct molecular targets which could reduce the development of resistance are currently in different phases of clinical trials. Nevertheless, combination therapy would possibly improve the contemporary treatment standards in immunocompromised populations. Development of newer therapeutics with intrinsic ability to both target DNA polymerase and the viral proteins would be advantageous and are expected to significantly improve the status of CMV therapy. Hopefully, newer and highly active antiviral drugs would rapidly progress to various stages of formulation development and enter the pharmaceutical market, further contributing to a reduction in the incidence of CMV infections.
ACKNOWLEDGEMENTS
We would like to acknowledge NIH grants R01EY09171-16 and R01EY010659-14 for financial support.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- [1].Heiden D, Ford N, Wilson D, Rodriguez WR, Margolis T, Janssens B, et al. Cytomegalovirus Retinitis: The Neglected Disease of the AIDS Pandemic. PLoS Med. 2007;4(12):e334. doi: 10.1371/journal.pmed.0040334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Biron KK. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 2006;71(2-3):154–63. doi: 10.1016/j.antiviral.2006.05.002. [DOI] [PubMed] [Google Scholar]
- [3].Rubin RH, Kemmerly SA, Conti D, Doran M, Murray BM, Neylan JF, et al. Prevention of primary cytomegalovirus disease in organ transplant recipients with oral ganciclovir or oral acyclovir prophylaxis. Transpl Infect Dis. 2000;2(3):112–7. [PubMed] [Google Scholar]
- [4].Britt WJ, Mach M. Human Cytomegalovirus Glycoproteins. Intervirology. 1996;39(5-6):401–12. doi: 10.1159/000150510. [DOI] [PubMed] [Google Scholar]
- [5].McGavran MH, Smith MG. Ultrastructural, cytochemical, and microchemical observations on cytomegalovirus (salivary gland virus) infection of human cells in tissue culture. Exp Mol Pathol. 1965;76:1–10. doi: 10.1016/0014-4800(65)90019-5. [DOI] [PubMed] [Google Scholar]
- [6].Wright HT, Jr., Goodheart CR, Lielausis A. Human Cytomegalovirus. Morphology by negative staining. Virology. 1964;23:419–24. doi: 10.1016/0042-6822(64)90265-x. [DOI] [PubMed] [Google Scholar]
- [7].Rasmussen L. Molecular pathogenesis of human cytomegalovirus infection. Transpl Infect Dis. 1999;1(2):127–34. doi: 10.1034/j.1399-3062.1999.010206.x. [DOI] [PubMed] [Google Scholar]
- [8].Baldick CJ, Jr., Shenk T. Proteins associated with purified human cytomegalovirus particles. J Virol. 1996;70(9):6097–105. doi: 10.1128/jvi.70.9.6097-6105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Roby C, Gibson W. Characterization of phosphoproteins and protein kinase activity of virions, noninfectious enveloped particles, and dense bodies of human cytomegalovirus. J Virol. 1986;59(3):714–27. doi: 10.1128/jvi.59.3.714-727.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gibson W. Protein counterparts of human and simian cytomegaloviruses. Virology. 1983;128(2):391–406. doi: 10.1016/0042-6822(83)90265-9. [DOI] [PubMed] [Google Scholar]
- [11].Gibson W. Structure and assembly of the virion. Intervirology. 1996;39(5-6):389–400. doi: 10.1159/000150509. [DOI] [PubMed] [Google Scholar]
- [12].Spaete RR, Gehrz RC, Landini MP. Human cytomegalovirus structural proteins. J Gen Virol. 1994;75(12):3287–308. doi: 10.1099/0022-1317-75-12-3287. [DOI] [PubMed] [Google Scholar]
- [13].Kedhar SR, Jabs DA. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Herpes. 2007;14(3):66–71. [PubMed] [Google Scholar]
- [14].Gerna G, Baldanti F, Revello MG. Pathogenesis of human cytomegalovirus infection and cellular targets. Hum Immunol. 2004;65(5):381–6. doi: 10.1016/j.humimm.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [15].Bodaghi B, Slobbe-van Drunen ME, Topilko A, Perret E, Vossen RC, van Dam-Mieras MC, et al. Entry of human cytomegalovirus into retinal pigment epithelial and endothelial cells by endocytosis. Invest Ophthalmol Vis Sci. 1999;40(11):2598–607. [PubMed] [Google Scholar]
- [16].Rao NA, Zhang J, Ishimoto S. Role of retinal vascular endothelial cells in development of CMV retinitis. Trans Am Ophthalmol Soc. 1998;96:111–23. discussion 24-6. [PMC free article] [PubMed] [Google Scholar]
- [17].Thorne JE, Holbrook JT, Jabs DA, Kempen JH, Nichols C, Meinert CL. Effect of cytomegalovirus retinitis on the risk of visual acuity loss among patients with AIDS. Ophthalmology. 2007;114(3):591–8. doi: 10.1016/j.ophtha.2006.08.008. [DOI] [PubMed] [Google Scholar]
- [18].Deayton JR, Wilson P, Sabin CA, Davey CC, Johnson MA, Emery VC, et al. Changes in the natural history of cytomegalovirus retinitis following the introduction of highly active antiretroviral therapy. AIDS. 2000;14(9):1163–70. doi: 10.1097/00002030-200006160-00013. [DOI] [PubMed] [Google Scholar]
- [19].Gariano RF, Rickman LS, Freeman WR. Ocular examination and diagnosis in patients with the acquired immunodeficiency syndrome. West J Med. 1993;158(3):254–62. [PMC free article] [PubMed] [Google Scholar]
- [20].Stewart MW. Optimal management of cytomegalovirus retinitis in patients with AIDS. Clin Ophthalmol. 2010;4:285–99. doi: 10.2147/opth.s6700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Holland GN, Buhles WC, Jr., Mastre B, Kaplan HJ. A controlled retrospective study of ganciclovir treatment for cytomegalovirus retinopathy. Use of a standardized system for the assessment of disease outcome. UCLA CMV Retinopathy. Study Group. Arch Ophthalmol. 1989;107(12):1759–66. doi: 10.1001/archopht.1989.01070020841024. [DOI] [PubMed] [Google Scholar]
- [22].Skiest DJ, Chiller T, Chiller K, Park A, Keiser P. Protease inhibitor therapy is associated with markedly prolonged time to relapse and improved survival in AIDS patients with cytomegalovirus retinitis. Int J STD AIDS. 2001;12(10):659–64. doi: 10.1258/0956462011923886. [DOI] [PubMed] [Google Scholar]
- [23].Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114(4):787–93. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- [24].Thorne JE, Jabs DA, Kempen JH, Holbrook JT, Nichols C, Meinert CL. Causes of visual acuity loss among patients with AIDS and cytomegalovirus retinitis in the era of highly active antiretroviral therapy. Ophthalmology. 2006;113(8):1441–5. doi: 10.1016/j.ophtha.2006.03.022. [DOI] [PubMed] [Google Scholar]
- [25].Sullivan V, Talarico CL, Stanat SC, Davis M, Coen DM, Biron KK. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature. 1992;358(6382):162–4. doi: 10.1038/358162a0. [DOI] [PubMed] [Google Scholar]
- [26].Boivin G, Gilbert C, Gaudreau A, Greenfield I, Sudlow R, Roberts NA. Rate of emergence of cytomegalovirus (CMV) mutations in leukocytes of patients with acquired immunodeficiency syndrome who are receiving valganciclovir as induction and maintenance therapy for CMV retinitis. J Infect Dis. 2001;184(12):1598–602. doi: 10.1086/324672. [DOI] [PubMed] [Google Scholar]
- [27].Cvetkovic RS, Wellington K. Valganciclovir: a review of its use in the management of CMV infection and disease in immunocompromised patients. Drugs. 2005;65(6):859–78. doi: 10.2165/00003495-200565060-00012. [DOI] [PubMed] [Google Scholar]
- [28].Young SH, Morlet N, Heery S, Hollows FC, Coroneo MT. High dose intravitreal ganciclovir in the treatment of cytomegalovirus retinitis. Med J Aust. 1992;157(6):370–3. doi: 10.5694/j.1326-5377.1992.tb137242.x. [DOI] [PubMed] [Google Scholar]
- [29].Henry K, Cantrill H, Fletcher C, Chinnock BJ, Balfour HH., Jr. Use of intravitreal ganciclovir (dihydroxy propoxymethyl guanine) for cytomegalovirus retinitis in a patient with AIDS. Am J Ophthalmol. 1987;103(1):17–23. doi: 10.1016/s0002-9394(14)74163-7. [DOI] [PubMed] [Google Scholar]
- [30].Musch DC, Martin DF, Gordon JF, Davis MD, Kuppermann BD. Treatment of cytomegalovirus retinitis with a sustained-release ganciclovir implant. The Ganciclovir Implant Study Group. N Engl J Med. 1997;337(2):83–90. doi: 10.1056/NEJM199707103370203. [DOI] [PubMed] [Google Scholar]
- [31].Razonable RR, Emery VC. Management of CMV infection and disease in transplant patients. 27-29 February 2004. Herpes. 2004;11(3):77–86. [PubMed] [Google Scholar]
- [32].Jacobson MA, O’Donnell JJ, Mills J. Foscarnet treatment of cytomegalovirus retinitis in patients with the acquired immunodeficiency syndrome. Antimicrob Agents Chemother. 1989;33(5):736–41. doi: 10.1128/aac.33.5.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].De Clercq E, Holy A. Acyclic nucleoside phosphonates: a key class of antiviral drugs. Nat Rev Drug Discov. 2005;4(11):928–40. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- [34].De Clercq E. Antiviral drugs in current clinical use. J Clin Virol. 2004;30(2):115–33. doi: 10.1016/j.jcv.2004.02.009. [DOI] [PubMed] [Google Scholar]
- [35].Kern ER, Rybak RJ, Hartline CB, Bidanset DJ. Predictive efficacy of SCID-hu mouse models for treatment of human cytomegalovirus infections. Antivir Chem Chemother. 2001;12(Suppl 1):149–56. [PubMed] [Google Scholar]
- [36].Kern ER. Value of animal models to evaluate agents with potential activity against human cytomegalovirus. Transplant Proc. 1991;23(3 Suppl 3):152–5. discussion 5. [PubMed] [Google Scholar]
- [37].Ho ES, Lin DC, Mendel DB, Cihlar T. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000;11(3):383–93. doi: 10.1681/ASN.V113383. [DOI] [PubMed] [Google Scholar]
- [38].Safety of intravitreous fomivirsen for treatment of cytomegalovirus retinitis in patients with AIDS. Am J Ophthalmol. 2002;133(4):484–98. doi: 10.1016/s0002-9394(02)01332-6. [DOI] [PubMed] [Google Scholar]
- [39].Wang WW, Ye JJ. Present status and advances in pharmacotherapy for cytomegalovirus retinitis associated with acquired immunodeficiency syndrome. Zhonghua Yan Ke Za Zhi. 2010;46(12):1148–52. [PubMed] [Google Scholar]
- [40].Biron KK, Harvey RJ, Chamberlain SC, Good SS, Smith AA, 3rd, Davis MG, et al. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob Agents Chemother. 2002;46(8):2365–72. doi: 10.1128/AAC.46.8.2365-2372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zacny VL, Gershburg E, Davis MG, Biron KK, Pagano JS. Inhibition of Epstein-Barr virus replication by a benzimidazole L-riboside: novel antiviral mechanism of 5, 6-dichloro-2-(isopropylamino)-1-beta-L-ribofuranosyl-1H-benzimidazole. J Virol. 1999;73(9):7271–7. doi: 10.1128/jvi.73.9.7271-7277.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Marschall M, Freitag M, Suchy P, Romaker D, Kupfer R, Hanke M, et al. The protein kinase pUL97 of human cytomegalovirus interacts with and phosphorylates the DNA polymerase processivity factor pUL44. Virology. 2003;311(1):60–71. doi: 10.1016/s0042-6822(03)00147-8. [DOI] [PubMed] [Google Scholar]
- [43].Krosky PM, Baek MC, Jahng WJ, Barrera I, Harvey RJ, Biron KK, et al. The human cytomegalovirus UL44 protein is a substrate for the UL97 protein kinase. J Virol. 2003;77(14):7720–7. doi: 10.1128/JVI.77.14.7720-7727.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Krosky PM, Baek MC, Coen DM. The human cytomegalovirus UL97 protein kinase, an antiviral drug target, is required at the stage of nuclear egress. J Virol. 2003;77(2):905–14. doi: 10.1128/JVI.77.2.905-914.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lalezari JP, Aberg JA, Wang LH, Wire MB, Miner R, Snowden W, et al. Phase I dose escalation trial evaluating the pharmacokinetics, anti-human cytomegalovirus (HCMV) activity, and safety of 1263W94 in human immunodeficiency virus-infected men with asymptomatic HCMV shedding. Antimicrob Agents Chemother. 2002;46(9):2969–76. doi: 10.1128/AAC.46.9.2969-2976.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Maribavir: 1263W94, Benzimidavir, GW 1263, GW 1263W94, VP41263. Drugs R D. 2007;8(3):188–92. doi: 10.2165/00126839-200708030-00006. [DOI] [PubMed] [Google Scholar]
- [47].Trofe J, Pote L, Wade E, Blumberg E, Bloom RD. Maribavir: A Novel Antiviral Agent with Activity Against Cytomegalovirus. Annals Pharmacotherap. 2008;42(10):1447–57. doi: 10.1345/aph.1L065. [DOI] [PubMed] [Google Scholar]
- [48].Reefschlaeger J, Bender W, Hallenberger S, Weber O, Eckenberg P, Goldmann S, et al. Novel non-nucleoside inhibitors of cytomegaloviruses (BAY 38-4766): In vitro and in vivo antiviral activity and mechanism of action. J Antimicrob Chemother. 2001;48(6):757–67. doi: 10.1093/jac/48.6.757. [DOI] [PubMed] [Google Scholar]
- [49].Weber O, Bender W, Eckenberg P, Goldmann S, Haerter M, Hallenberger S, et al. Inhibition of murine cytomegalovirus and human cytomegalovirus by a novel non-nucleosidic compound in vivo. Antiviral Res. 2001;49(3):179–89. doi: 10.1016/s0166-3542(01)00127-9. [DOI] [PubMed] [Google Scholar]
- [50].McSharry JJ, McDonough A, Olson B, Hallenberger S, Reefschlaeger J, Bender W, et al. Susceptibilities of human cytomegalovirus clinical isolates to BAY38-4766, BAY43-9695, and ganciclovir. Antimicrob Agents Chemother. 2001;45(10):2925–7. doi: 10.1128/AAC.45.10.2925-2927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Buerger I, Reefschlaeger J, Bender W, Eckenberg P, Popp A, Weber O, et al. A novel nonnucleoside inhibitor specifically targets cytomegalovirus DNA maturation via the UL89 and UL56 gene products. J Virol. 2001;75(19):9077–86. doi: 10.1128/JVI.75.19.9077-9086.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schleiss MR, Bernstein DI, McVoy MA, Stroup G, Bravo F, Creasy B, et al. The non-nucleoside antiviral, BAY 38-4766, protects against cytomegalovirus (CMV) disease and mortality in immunocompromised guinea pigs. Antiviral Res. 2005;65(1):35–43. doi: 10.1016/j.antiviral.2004.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Williams-Aziz SL, Hartline CB, Harden EA, Daily SL, Prichard MN, Kushner NL, et al. Comparative activities of lipid esters of cidofovir and cyclic cidofovir against replication of herpesviruses in vitro. Antimicrob Agents Chemother. 2005;49(9):3724–33. doi: 10.1128/AAC.49.9.3724-3733.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Wan WB, Beadle JR, Hartline C, Kern ER, Ciesla SL, Valiaeva N, et al. Comparison of the antiviral activities of alkoxyalkyl and alkyl esters of cidofovir against human and murine cytomegalovirus replication in vitro. Antimicrob Agents Chemother. 2005;49(2):656–62. doi: 10.1128/AAC.49.2.656-662.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ciesla SL, Trahan J, Wan WB, Beadle JR, Aldern KA, Painter GR, et al. Esterification of cidofovir with alkoxyalkanols increases oral bioavailability and diminishes drug accumulation in kidney. Antiviral Res. 2003;59(3):163–71. doi: 10.1016/s0166-3542(03)00110-4. [DOI] [PubMed] [Google Scholar]
- [56].Painter GR, Hostetler KY. Design and development of oral drugs for the prophylaxis and treatment of smallpox infection. Trends Biotechnol. 2004;22(8):423–7. doi: 10.1016/j.tibtech.2004.06.008. [DOI] [PubMed] [Google Scholar]
- [57].Quenelle DC, Lampert B, Collins DJ, Rice TL, Painter GR, Kern ER. Efficacy of CMX001 against herpes simplex virus infections in mice and correlations with drug distribution studies. J Infect Dis. 2010;202(10):1492–9. doi: 10.1086/656717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Prichard MN, Kern ER. The search for new therapies for human cytomegalovirus infections. Virus Res. 2011;157(2):212–21. doi: 10.1016/j.virusres.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Underwood MR, Harvey RJ, Stanat SC, Hemphill ML, Miller T, Drach JC, et al. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J Virol. 1998;72(1):717–25. doi: 10.1128/jvi.72.1.717-725.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kern ER, Hartline CB, Rybak RJ, Drach JC, Townsend LB, Biron KK, et al. Activities of benzimidazole D- and L-ribonucleosides in animal models of cytomegalovirus infections. Antimicrob Agents Chemother. 2004;48(5):1749–55. doi: 10.1128/AAC.48.5.1749-1755.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Townsend LB, Devivar RV, Turk SR, Nassiri MR, Drach JC. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-D-ribofuranosyl)benzimidazoles. J Med Chem. 1995;38(20):4098–105. doi: 10.1021/jm00020a025. [DOI] [PubMed] [Google Scholar]
- [62].Lischka P, Hewlett G, Wunberg T, Baumeister J, Paulsen D, Goldner T, et al. In vitro and in vivo activities of the novel anticytomegalovirus compound AIC246. Antimicrob Agents Chemother. 2010;54(3):1290–7. doi: 10.1128/AAC.01596-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Mitra AK. Acyclovir-peptide analogs. 2010 US7825086. [Google Scholar]
- [64].Majumdar S, Duvvuri S, Mitra AK. Membrane transporter/receptor-targeted prodrug design: Strategies for human and veterinary drug development. Adv Drug Deliv Rev. 2004;56(10):1437–52. doi: 10.1016/j.addr.2004.02.006. [DOI] [PubMed] [Google Scholar]
- [65].Yang C, Tirucherai GS, Mitra AK. Prodrug based optimal drug delivery via membrane transporter/receptor. Expert Opin Biol Ther. 2001;1(2):159–75. doi: 10.1517/14712598.1.2.159. [DOI] [PubMed] [Google Scholar]
- [66].Mitra AK, Samanta SK, Talluri RS. Stereochemically defined dipeptide esters of antiviral agents for enhanced ocular treatment. 2011 US7951774. [Google Scholar]
- [67].Kowalik TF. RNAi targeting of viruses. 2004 US20040248839. [Google Scholar]
- [68].Draper KG, Kisner DL, Anderson KP, Chapman S. Composition and method for treatment of CMV infections. 2000 US6153595. [Google Scholar]
- [69].Funaro A, Gribaudo G, Landolfo S. Antibodies against human cytomegalovirus (HCMV) 2011 US20110171233. [Google Scholar]
- [70].Nunes JJ, Milne J, Bemis J, Xie R, Vu CB, Ng PY, Disch J, Salzmann T, Armistead D. Sirtuin modulating compounds. 2011 US20110130387. [Google Scholar]
- [71].Bemis J, Disch JS, Jirousek M, Lunsmann WJ, Ng PY, Vu CB. Sirtuin modulating compounds. 2010 US7829556. [Google Scholar]
- [72].Tokars M, Lawrence R, Bradley M. PMEA lipid conjugates. 2009 US20090111774. [Google Scholar]
- [73].Zimmermann H, Brueckner D, Henninger K, Rosentreter U, Hendrix M, Keldenich J, Lang D, Radtke M, Paulsen D, Kern A. Heterocyclyamide-substituted imidazoles. 2011 US7919489. [Google Scholar]
- [74].Zimmermann H, Brueckner D, Heimbach D, Hendrix M, Henninger K, Hewlett G, Rosentreter U, Keldenich J, Lang D, Radtke M. Antiviral 4-aminocarbonylamino-substituted imidazole compounds. 2010 US7767704. [Google Scholar]
- [75].Fryszman OM, Lang H, Lan J, Chang E, Fang Y. 5-Membered heterocycle-based p38 kinase inhibitors. 2011 US20110071152. [Google Scholar]
- [76].Mosoian A, Klotman ME, Teixeira A. Methods of inhibiting viral infection. 2010 US7754687. [Google Scholar]
- [77].Exley RW. Treatment of herpes virus related diseases. 2011 US20110112117. [Google Scholar]
- [78].Ware C. Ligand for herpes simplex virus entry mediator and methods of use. 2011 US20110171242. [Google Scholar]
- [79].Melnik LI, Garry RF, Morris CA. Peptide compositions and methods for inhibiting herpesvirus infection. 2011 WO2011053798. [Google Scholar]