Abstract
Objective
To evaluate the relationship between alcohol consumption and the risk of acute exacerbation of COPD (AECOPD).
Methods and measurements
We conducted a secondary analysis of data previously collected in a large, multicenter trial of daily azithromycin in COPD. To analyze the relationship between amount of baseline self-reported alcohol consumption in the past 12 months and subsequent AECOPD, we categorized the subjects as minimal (<1 drink/month), light-to-moderate (1–60 drinks/month), or heavy alcohol users (>60 drinks/month). The primary outcome was time to first AECOPD and the secondary outcome was AECOPD rate during the 1-year study period.
Results
Of the 1,142 enrolled participants, 1,082 completed baseline alcohol questionnaires and were included in this analysis. Six hundred and forty-five participants reported minimal alcohol intake, 363 reported light-to-moderate intake, and 74 reported heavy intake. There were no statistically significant differences in median time to first AECOPD among minimal (195 days), light-to-moderate (241 days), and heavy drinkers (288 days) (P=0.11). The mean crude rate of AECOPD did not significantly differ between minimal (1.62 events per year) and light-to-moderate (1.44 events per year) (P=0.095), or heavy drinkers (1.68 events per year) (P=0.796). There were no significant differences in hazard ratios for AECOPD after adjustment for multiple covariates.
Conclusion
Among persons with COPD at high risk of exacerbation, we found no significant relationship between self-reported baseline alcohol intake and subsequent exacerbations. The number of patients reporting heavy alcohol intake was small and further study is needed to determine the effect of heavy alcohol intake on AECOPD risk.
Keywords: pulmonary disease, chronic obstructive, ethanol, alcohol, alcoholism
Introduction
COPD is a leading cause of death and disability both in the US and worldwide.1,2 Recent studies indicate that in the US alone, COPD is responsible for over 3.5 million hospital days, 15 million physician office visits, and $38.8 billion in direct and indirect costs, annually.3,4 Acute exacerbation of COPD (AECOPD) not only accounts for the majority of COPD-related costs,5 but has also been associated both with disease progression and increased mortality among those with COPD.6–8 Greater understanding of factors that affect AECOPD risk could have a significant public health impact.
While heavy alcohol intake has long been associated with adverse health effects including increased risk of respiratory infection,9–12 observational studies have shown that moderate alcohol intake may confer health benefits ranging from decreased risk of diabetes and cardiovascular events to a reduction in all-cause mortality.13–15 These benefits have been, in part, attributed to an alcohol-related reduction in inflammatory mediators.
Little is known about the effects of alcohol consumption on susceptibility to AECOPD. Using previously collected data from a large randomized controlled trial of patients with COPD, we performed a secondary analysis to evaluate the relationship between alcohol consumption and the risk of AECOPD.
Methods
We conducted a nested prospective cohort study using data collected in a randomized trial that tested the effect of daily azithromycin on reducing AECOPD risk (ClinicalTrials.gov NCT00325897). The detailed protocol methods and main results of the trial have been previously published.16
Study participants
Inclusion criteria were as follows: age 40 years or older, a ratio of forced expiratory volume in 1 second (FEV1)/forced vital capacity (FVC) <70%, FEV1 <80% of predicted, ≥10 pack-year smoking history, and an increased risk of AECOPD (defined as requiring oxygen, having received systemic corticosteroids or having an emergency department visit or hospitalization for COPD within 1 year of study entry). Exclusion criteria included a diagnosis of asthma or a disease resulting in the patient being either medically unstable or having a predicted life expectancy <3 years, macrolide hypersensitivity, taking medications with azithromycin interactions, electrocardiographic QTc interval >450 ms, resting heart rate >120 beats/minute, hepatic or renal insufficiency (creatinine >1.5 mg/dL and estimated creatinine clearance <20 mL/min), bronchiectasis, or hearing impairment. Participants were required to be free of AECOPD for ≥4 weeks prior to enrollment. Patients were not excluded on the basis of their alcohol consumption.
Participants were randomly assigned in a 1:1 ratio to either daily azithromycin or placebo for 1 year given in addition to their usual treatment. Participants were seen in clinic or contacted by phone on alternate months and detailed exacerbation information was collected over 1 year. AECOPDs were defined as a complex of respiratory symptoms (increased or new onset) of more than one of the following: cough, sputum, wheezing, dyspnea, or chest tightness with a duration of at least 3 days and requiring treatment with an antibiotic or systemic corticosteroid.
All participants provided written informed consent to participate in the study. Each participating institution’s institutional review board approved the protocol.
At the time of enrollment, study participants were administered an alcohol intake questionnaire quantifying self-reported alcohol consumption in the preceding 12-month period (Figure 1).17 Mean monthly alcohol consumption was calculated based on responses to the following questions: “In the past 12 months, how many days of the year did you drink any alcoholic beverage?” and “On the average, on the days that you drank alcohol, how many drinks did you have a day? (By a drink, I mean a 12 oz [355 mL] beer, a 4 oz [128 mL] glass of wine, or an oz [30 mL] of liquor)”.
Figure 1.
Alcohol use questionnaire.
Categories of alcohol intake were determined using cut points based on previously published population data demonstrating decreased COPD mortality in those with alcohol intake equaling 1–60 14 g drinks per month.18,19 We categorized subjects’ alcohol use as “minimal to none”, “light-to-moderate”, and “heavy” according to the following criteria: “minimal” if reported <12 drinks in a lifetime or <12 drinks in the past 12 months; “light-to-moderate” if reported 1–60 drinks per month; “heavy” if reported >60 drinks per month.
Participants also completed a St George’s Respiratory Questionnaire (SGRQ), which is a self-completed, validated questionnaire to assess respiratory health status.20 SGRQ scores range from 0 to 100, with lower scores indicating better respiratory health status; the minimal clinically important difference in SGRQ scores is 4 points.21 Good reliability has been demonstrated with SGRQ.22 Participants also completed a Hospital Anxiety and Depression Scale (HADS) questionnaire, performed post-bronchodilator spirometry, and had their medication use recorded. HADS has been validated in general populations of medical patients as well as COPD populations, and good reliability has been established.23,24
We hypothesized that light-to-moderate alcohol use would be associated with a lower risk of AECOPD compared to minimal or no use; secondarily, we hypothesized that heavy alcohol use would be associated with a higher risk of AECOPD. The primary outcome was time to first AECOPD and the secondary outcome was exacerbation rate during the 1-year study period.
Statistical methods
Univariate analysis of continuous and categorical data utilized Student’s t-tests and chi-square tests, respectively.
Time to first AECOPD was analyzed by comparing alcohol-use groups using log-rank testing, as well as by use of a multivariate Cox proportional hazard model to adjust for factors that might influence exacerbations. Covariates forced into the regression model included study treatment group (azithromycin or placebo control) and known predictors of AECOPD, age, and sex. Candidate variables for stepwise regression covariates included those with potential effect on subsequent AECOPD including smoking status, chronic bronchitis, oxygen use, gastroesophageal reflux, FEV1/FVC ratio, COPD medications, SGRQ score, HADS anxiety score, and HADS depression score. Previous AECOPD is another established AECOPD risk factor, and was assessed by two ways in this trial: 1) use of antibiotics or steroids for COPD in the previous year, or 2) hospitalization or unscheduled emergency visit for COPD in the previous year. These were combined into one variable (exacerbation in past year at baseline) and also included as a candidate covariate in stepwise Cox regression. Stepwise covariates were included in the final model if they were significant at P<0.05.
Rate of AECOPD was determined by dividing the number of AECOPDs by person-years of follow-up, allowing use of data from patients with multiple exacerbations during follow-up. The relationship between alcohol consumption and rate of AECOPD was analyzed using zero-inflated negative binomial regression.
Statistical analyses were conducted using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Among the 1,142 participants enrolled in the trial, 1,082 completed alcohol questionnaires and were included in this analysis. Follow-up AECOPD data were available for all the 1,082 included patients.
Baseline characteristics of the study population are presented in Table 1. Of the 1,082 subjects, 645 participants (59.6%) reported minimal to no alcohol intake, 363 (33.5%) reported light-to-moderate intake, and 74 (6.8%) reported heavy intake.
Table 1.
Baseline characteristics*
| All (n=1,082) |
Alcohol intake category
|
P-value** | |||
|---|---|---|---|---|---|
| Minimal to none (n=645) |
Light-to-moderate (n=363) |
Heavy (n=74) |
|||
| Age, years | 65.2±8.7 | 64.9 ±8.7 | 65.4±8.5 | 66.8±9.0 | 0.189 |
| Female sex, % | 41 | 44 | 40 | 16 | <0.0001 |
| Race or ethnicity, % | |||||
| White | 81 | 79 | 84 | 89 | 0.086 |
| African-American | 14 | 17 | 12 | 4 | |
| Asian | 0.9 | 1 | 0.2 | 1 | |
| Native American | 0.4 | 0.5 | 0.2 | 0 | |
| More than one | 3 | 3 | 3 | 5 | |
| Hispanic | 2 | 2 | 4 | 1 | 0.139 |
| Post-bronchodilator FEV1, L | 1.11±0.51 | 1.08±0.49 | 1.14±0.52 | 1.27±0.55 | 0.003 |
| Post-bronchodilator FEV1 % | 39.6 ±15.7 | 39.1 ±15.5 | 40.2±16.1 | 41.3±15.4 | 0.357 |
| predicted FEV1/FVC, % | 42.6±12.8 | 42.9±12.9 | 41.7±12.7 | 44.4±12.4 | 0.163 |
| Smoking history, pack-years | 58.6±32.3 | 58.3±32.3 | 56.9±29.7 | 70.4±41.0 | 0.004 |
| Current smokers, % | 22 | 21 | 22 | 31 | 0.111 |
| Medication use, % | |||||
| ICS only | 5 | 6 | 4 | 3 | 0.256 |
| LAMA only | 7 | 8 | 7 | 1 | 0.116 |
| LABA only | 2 | 2 | 2 | 0 | 0.416 |
| ICS + LABA | 20 | 20 | 20 | 27 | 0.322 |
| ICS + LAMA | 5 | 5 | 5 | 4 | 0.939 |
| LABA + LAMA | 5 | 4 | 6 | 4 | 0.612 |
| ICS + LABA + LAMA | 48 | 46 | 49 | 50 | 0.648 |
| None | 9 | 10 | 8 | 10 | 0.518 |
| Oxygen use, % | 60 | 62 | 59 | 43 | 0.007 |
| Chronic bronchitis, % | 55 | 56 | 53 | 64 | 0.223 |
| SGRQ total (mean ± SD) | 50.6±16.5 | 52.0±16.5 | 48.6±16.7 | 48.1±14.2 | 0.003 |
| GERD, % | 43 | 44 | 43 | 38 | 0.612 |
| HAD anxiety scale | 5.4±3.7 | 5.6±3.9 | 5.2±3.6 | 4.5±3.3 | 0.019 |
| HAD depression scale | 4.9±0.2 | 5.1±3.3 | 4.6±3.0 | 4.5±3.0 | 0.030 |
| AECOPD previous year | 88 | 90 | 83 | 91 | 0.007 |
| Randomized to azithromycin/placebo | 50%/50% | 60%/59% | 33%/34% | 7%/7% | 0.660 |
Notes:
Data are presented as mean ± standard deviation or frequency (%).
P-values calculated by chi-square (categorical) and t-test (continuous).
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GERD, gastroesophageal reflux disease; HAD, hospital anxiety and depression; LABA, long-acting β2 agonist; LAMA, long-acting muscarinic antagonist; SGRQ, St George’s Respiratory Questionnaire; ICS, inhaled corticosteroid; SD, standard deviation.
There were small but statistically significant differences in FEV1 among the alcohol intake categories. Minimal users had worse respiratory health status as assessed by SGRQ scores and more anxiety as assessed by HADS scores. Heavy users of alcohol had a higher mean smoking pack-year history. There were no differences in proportions of current smokers or in use of inhaled COPD medications. Randomized allocation (azithromycin vs placebo) was similar in each alcohol intake category.
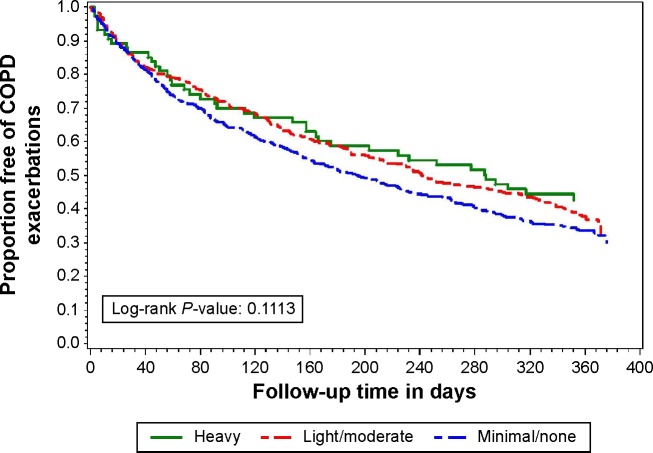
A total of 1,593 exacerbations were observed during the study period. There were no statistically significant differences in crude median time to first AECOPD among minimal (195 days; 95% confidence interval [CI], 163–225), light-to-moderate (241 days; 95% CI, 205–302), and heavy users of alcohol (288 days; 95% CI, 164–372) (P=0.111, log-rank test). Compared to minimal alcohol users, the crude hazard ratio for time to first AECOPD in light-to-moderate alcohol users was 0.87 (95% CI, 0.74–1.03) and in heavy alcohol users was 0.78 (95% CI, 0.56–1.07; P=0.123). Adjustment for treatment group (azithromycin vs control) and multiple known risk factors for AECOPD had little effect on these results (Table 2). At the completion of the 1-year follow-up period, 44% of heavy drinkers, 38% of light-to-moderate drinkers, and 32% of minimal drinkers remained exacerbation free. These differences did not reach statistical significance (log-rank P=0.111) (Figure 2).
Table 2.
Time to first AECOPD by alcohol consumption pattern
| Total (n=1,082) |
Alcohol intake
|
P-value | |||
|---|---|---|---|---|---|
| Minimal (n=645) |
Light-to moderate (n=363) |
Heavy (n=74) |
|||
| Median time to first exacerbation, days (95% CI) | 216 (190–241) | 195 (163–225) | 241 (205–302) | 288 (164–372) | 0.1113 |
| Crude HR (95% CI) | NA | (Referent) | 0.87 (0.74–1.03) P=0.097 |
0.78 (0.56–1.07) P=0.123 |
– |
| Adjusted HR (95% CI)* | NA | (Referent) | 0.89 (0.75–1.05) P=0.155 |
0.93 (0.70–1.29) P=0.656 |
– |
Notes:
Covariates included in final stepwise model: treatment group, age, sex, smoking status, bronchitis, oxygen, FEV1/FVC ratio, exacerbation in past year, SGRQ.
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CI, confidence interval; HR, hazard ratio; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; NA, not applicable; SGRQ, St George’s Respiratory Questionnaire.
Figure 2.
Proportion free from AECOPD for 1 year, according to alcohol consumption pattern.
Abbreviation: AECOPD, acute exacerbation of chronic obstructive pulmonary disease.
The overall mean rate of AECOPD was 1.66 events per year (95% CI, 1.51–1.80). The mean AECOPD rate was lower among light-to-moderate drinkers (1.44 exacerbations per year; 95% CI, 1.31–1.71) than minimal drinkers (1.62 exacerbations per year; 95% CI, 1.53–1.94), but the difference was not statistically significant (P=0.095) (Table 3).
Table 3.
AECOPD event rates by alcohol consumption pattern
| Alcohol intake
|
||||
|---|---|---|---|---|
| Total (n=1,082) |
Minimal (n=645) |
Light-to-moderate (n=363) |
Heavy (n=74) |
|
| Mean exacerbation rate, exacerbations per year (95% CI) | 1.66 (1.51–1.80) | 1.62 (1.53–1.94) | 1.44 (1.31–1.71) P=0.095 |
1.68 (1.17–2.19) P=0.796 |
Note: P-values comparing to referent group of minimal alcohol intake.
Abbreviations: AECOPD, acute exacerbation of chronic obstructive pulmonary disease; CI, confidence interval.
Alternative alcohol intake categorizations were formed using cut points based on tertiles, deciles, and intake strata previously described by Sisson et al17 and the results were likewise statistically nonsignificant.
Discussion
We identified no statistically significant association between self-reported alcohol consumption at baseline and AECOPD risk over the ensuing year.
Existing evidence regarding the effect of alcohol on respiratory health has shown both harm and benefit, depending on the intensity, duration, and frequency of alcohol exposure.25,26 At a high level of consumption, alcohol impairs airway clearance and both innate and adaptive immunity,10 and observational studies have demonstrated increased risk of pneumonia.9,12,27
The data available in this analysis do not suggest that a self-reported heavy alcohol consumption pattern was associated with increased frequency of AECOPD compared to minimal intake. There was even some suggestion that heavy alcohol users might have had longer time to first exacerbation, although they also had better FEV1, less chronic oxygen use, and there was ultimately no statistically significant difference in time to first exacerbation or exacerbation frequency. We note that the subjects reporting heavy alcohol intake were few in number and further study is needed to more closely characterize the relationship between heavy alcohol consumption and AECOPD.
Among the therapies that have been shown to reduce AECOPD risk, inhaled corticosteroids, macrolide antibiotics, and selective phosphodiesterase-4 inhibitors are thought to exert their beneficial effect by reducing or modifying airway inflammation. It is plausible that low-level alcohol consumption could also provide some degree of protective reduction in inflammation.28
While we observed no significant trend of risk reduction for low-level alcohol consumption in this study, multiple population studies analyzing alcohol intake and COPD mortality have reported a U-shaped curve, with the lowest risk of death among men with light-to-moderate alcohol intake, equaling 1–60 drinks per month.18,19 A previous prospective cohort study in a US Veteran population identified no association between Alcohol Use Disorders Identification Test – Consumption (AUDIT-C) scores and risk of AECOPD.29 Although the AUDIT-C tool is a validated screening tool for alcohol misuse, it does not specifically quantify alcohol consumption patterns, particularly in those without a pattern of abuse such as in light or moderate drinkers. The study was further limited by an inability to capture AECOPD events treated at non-VA facilities, a lack of spirometry data confirming COPD diagnosis, and an overwhelmingly male population.
The strengths of our current study include its large size, use of spirometry to confirm COPD, and its careful collection of prospective AECOPD data, which was the primary outcome of the main trial.
There remain notable limitations. Capturing accurate clinical information about alcohol behavior, particularly for those with large intake, is challenging and study participants may have misreported alcohol intake. Self-reporting of alcohol intake, as in the National Health and Nutrition Examination Survey (NHANES) alcohol questionnaire, has been validated by surrogate reporting and by biomarker studies. Therefore, self-report is generally accepted as a valid and reasonably accurate way to quantify alcohol intake for population research studies.30 The retrospective nature of the alcohol intake questionnaire is also subject to recall bias. We only administered the alcohol questionnaire at baseline, so although subsequent changes in alcohol consumption behavior seem unlikely, we cannot exclude the possibility that baseline data may not reflect prospective alcohol intake during the follow-up period. We also note that we had a very limited number of heavy alcohol users, so we have limited ability to draw conclusions about that particular group. Recruitment of such subjects is challenging, particularly in the context of a longitudinal trial such as the trial within which the present study was nested.
Conclusion
Among persons with COPD at high risk of exacerbation, we found no significant relationship between self-reported baseline alcohol intake and subsequent exacerbations. The number of patients reporting heavy alcohol intake was small and further study is needed to determine the effect of heavy alcohol intake on AECOPD risk.
Acknowledgments
The COPD Clinical Research Network is supported by a Cooperative Agreement from the Division of Lung Diseases of the National Heart, Lung, and Blood Institute. Members of this network are: Brigham and Women’s Hospital (affiliated sites: Fallon Clinic, West Roxbury Veterans Hospital) – JJ Reilly Jr (principal investigator [PI]), GR Washko (co-PI), R Rosiello, ML Moy (investigators), Grant HL074428; Den-ver Health Medical Center (affiliated sites: National Jewish Medical and Research Center, University of Colorado) – RK Albert (PI), B Make (co-PI), M Schwarz, C Welch (investigators), Grant HL074409; Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center – R Casaburi (PI), J Porszasz (investigator), Grant HL074407; Minnesota Veterans Research Institute, Minneapolis (affiliated sites: HealthPartners Research Foundation, Mayo Clinic) – DE Niewoehner (PI), C McEvoy, KR Rice, PD Scanlon (co-PIs), Grant HL074416; Temple University – GJ Criner (PI), W Chatila, N Marchetti, V Kim, G D’Alonzo, S Krachman, F Cordova, K Brennan, N Patel, J Mamary (investigators), Grant HL074408; University of Alabama at Birmingham – WC Bailey, JAD Cooper (co-PIs), MT Dransfield, LB Gerald, P O’Reilly (investigators), Grant HL074418; University of California, San Francisco – SC Lazarus (PI), HA Boushey, PG Woodruff (investigators), Grant HL074431; University of Maryland, Baltimore – SM Scharf (PI), M Alattar, P Amelung, M Cowan, J Hanson, J Hasday, A Iacono, C Shanholtz, N Todd, A Verceles (investigators), Grant HL074441; University of Michigan, Ann Arbor – FJ Martinez (PI), JL Curtis, MK Han, KR Flaherty, SE Gay, TE Sisson (investigators), Grant HL074422; University of Pittsburgh, Pittsburgh – F Sciurba (PI), J Bon (investigator), Grant HL074439; University of Minnesota (Data Coordinating Center), Minneapolis – JE Connett (PI), NR Anthonisen (Steering Committee Chair), C Wendt (co-PI), Grant HL074424.
This study was also supported by the National Institute on Alcohol Abuse and Alcoholism, University of Nebraska Medical Center, Omaha – JH Sisson (PI), Grant 5R01AA008769.
Footnotes
Author contributions
EEW conceived and designed the study, directed the statistical analyses, interpreted the data, drafted the manuscript, and approved the final version. DEN and KMK assisted in conception and design of the study, directed the statistical analyses, interpreted the data, revised the manuscript critically for important intellectual content, and approved the final version. JHS contributed to interpretation of the data, revised the manuscript critically for important intellectual content, and approved the final version. SL and JEC performed the statistical analyses, contributed to interpretation of the data, revised the manuscript critically for important intellectual content, and approved the final version. EEW and KMK had full access to the data and take responsibility for the integrity of the data and the accuracy of the data analyses.
Disclosure
The views expressed in this article are those of the authors and do not necessarily represent the views of the Minneapolis VA Health Care System, the US Department of Veterans Affairs, the National Institutes of Health, the US Government, or the authors’ affiliated academic institutions. The authors report no conflicts of interest in this work.
References
- 1.Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Burden of Disease Collaborators The state of US health, 1990–2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. doi: 10.1001/jama.2013.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster TS, Miller JD, Marton JP, Caloyeras JP, Russell MW, Menzin J. Assessment of the economic burden of COPD in the U.S.: a review and synthesis of the literature. COPD J Chronic Obstr Pulm Dis. 2006;3(4):211–218. doi: 10.1080/15412550601009396. [DOI] [PubMed] [Google Scholar]
- 4.National Heart Lung and Blood Institute . Morbidity and Mortality: 2012 Chart Book on Cardiovascular, Lung, and Blood Diseases. Bethesda, MD: National Heart Lung and Blood Institute; 2012. [Google Scholar]
- 5.Celli BR, MacNee W, Agusti A, et al. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 6.Kanner RE, Anthonisen NR, Connett JE. Lower respiratory illnesses promote FEV(1) decline in current smokers but not ex-smokers with mild chronic obstructive pulmonary disease: results from the lung health study. Am J Respir Crit Care Med. 2001;164(3):358–364. doi: 10.1164/ajrccm.164.3.2010017. [DOI] [PubMed] [Google Scholar]
- 7.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138. doi: 10.1056/NEJMoa0909883. [DOI] [PubMed] [Google Scholar]
- 9.Szabo G, Mandrekar P. A recent perspective on alcohol, immunity, and host defense. Alcohol Clin Exp Res. 2009;33(2):220–232. doi: 10.1111/j.1530-0277.2008.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Happel KI, Nelson S. Alcohol, immunosuppression, and the lung. Proc Am Thorac Soc. 2005;2(5):428–432. doi: 10.1513/pats.200507-065JS. [DOI] [PubMed] [Google Scholar]
- 11.Samokhvalov AV, Irving HM, Rehm J. Alcohol consumption as a risk factor for pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2010;138(12):1789–1795. doi: 10.1017/S0950268810000774. [DOI] [PubMed] [Google Scholar]
- 12.Fernández-Solá J, Junqué A, Estruch R, Monforte R, Torres A, Urbano-Márquez A. High alcohol intake as a risk and prognostic factor for community-acquired pneumonia. Arch Intern Med. 1995;155(15):1649–1654. doi: 10.1001/archinte.1995.00430150137014. [DOI] [PubMed] [Google Scholar]
- 13.Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption on diabetes mellitus: a systematic review. Ann Intern Med. 2004;140(3):211–219. doi: 10.7326/0003-4819-140-6-200403160-00011. [DOI] [PubMed] [Google Scholar]
- 14.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Castelnuovo A, Costanzo S, Bagnardi V, Donati MB, Iacoviello L, de Gaetano G. Alcohol dosing and total mortality in men and women: an updated meta-analysis of 34 prospective studies. Arch Intern Med. 2013;166(22):2437–2445. doi: 10.1001/archinte.166.22.2437. [DOI] [PubMed] [Google Scholar]
- 16.Albert RK, Connett J, Bailey WC, et al. Azithromycin for prevention of exacerbations of COPD. N Engl J Med. 2011;365(8):689–698. doi: 10.1056/NEJMoa1104623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sisson JH, Stoner JA, Romberger DJ, et al. Alcohol intake is associated with altered pulmonary function. Alcohol. 2005;36(1):19–30. doi: 10.1016/j.alcohol.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 18.Tabak C, Smit HA, Räsänen L, et al. Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology. 2001;12(2):239–245. doi: 10.1097/00001648-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 19.Reilly KH, Gu D, Duan X, et al. Risk factors for chronic obstructive pulmonary disease mortality in Chinese adults. Am J Epidemiol. 2008;167(8):998–1004. doi: 10.1093/aje/kwm393. [DOI] [PubMed] [Google Scholar]
- 20.Jones PW. Quality of life measurement for patients with diseases of the airways. Thorax. 1991;46(9):676–682. doi: 10.1136/thx.46.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Make B, Casaburi R, Leidy NK. Interpreting Results from clinical trials: understanding minimal clinically important differences in COPD outcomes. COPD J Chronic Obstr Pulm Dis. 2005;2(1):1–5. doi: 10.1081/copd-200051363. [DOI] [PubMed] [Google Scholar]
- 22.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. Am Rev Respir Dis. 1992;145:1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 23.Nowak C, Sievi NA, Clarenbach CF, et al. Accuracy of the hospital anxiety and depression scale for identifying depression in chronic obstructive pulmonary disease patients. Pulm Med. 2014;2014:1–7. doi: 10.1155/2014/973858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. an updated literature review. J Psychosom Res. 2002;52(2):69–77. doi: 10.1016/s0022-3999(01)00296-3. [DOI] [PubMed] [Google Scholar]
- 25.Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41(5):293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kornum JB, Due KM, Nørgaard M, et al. Alcohol drinking and risk of subsequent hospitalisation with pneumonia. Eur Respir J Off J Eur Soc Clin Respir Physiol. 2012;39(1):149–155. doi: 10.1183/09031936.00000611. [DOI] [PubMed] [Google Scholar]
- 27.Loeb M, Neupane B, Walter SD, et al. Environmental risk factors for community-acquired pneumonia hospitalization in older adults. J Am Geriatr Soc. 2009;57(6):1036–1040. doi: 10.1111/j.1532-5415.2009.02259.x. [DOI] [PubMed] [Google Scholar]
- 28.Pai JK, Hankinson SE, Thadhani R, Rifai N, Pischon T, Rimm EB. Moderate alcohol consumption and lower levels of inflammatory markers in US men and women. Atherosclerosis. 2006;186(1):113–120. doi: 10.1016/j.atherosclerosis.2005.06.037. [DOI] [PubMed] [Google Scholar]
- 29.Greene CC, Bradley KA, Bryson CL, et al. The association between alcohol consumption and risk of COPD exacerbation in a veteran population. Chest. 2008;134(4):761–767. doi: 10.1378/chest.07-3081. [DOI] [PubMed] [Google Scholar]
- 30.Del Boca FK, Darkes J. The validity of self-reports of alcohol consumption: state of the science and challenges for research. Addiction. 2003;98(Suppl 2):1–12. doi: 10.1046/j.1359-6357.2003.00586.x. [DOI] [PubMed] [Google Scholar]