Dear editor
Lung cancer is the most commonly diagnosed cancer and the leading cause of cancer death worldwide,1 with the majority of patients presenting with advanced disease.2 Treg cells diminish the activation and function of lymphocytes via cell-cell contact and secretion of soluble mediators.3 PD-1 is expressed on the surface of activated T and B cells and regulates their activation and proliferation.4 PD-L1 binds to the PD-1 receptor, leading to, among other responses, negative regulation of immune activity. Both Tregs and the PD-1/PD-L1 pathway play important roles in lung cancer pathogenesis;5,6 however, the association between these two factors remains poorly understood. Here, we examined PD-1 expression on Tregs, and compared these results with established clinical indicators of lung cancer. These analyses revealed significant expression of PD-1 on Tregs in lung cancer, which may be used to inform clinical diagnoses.
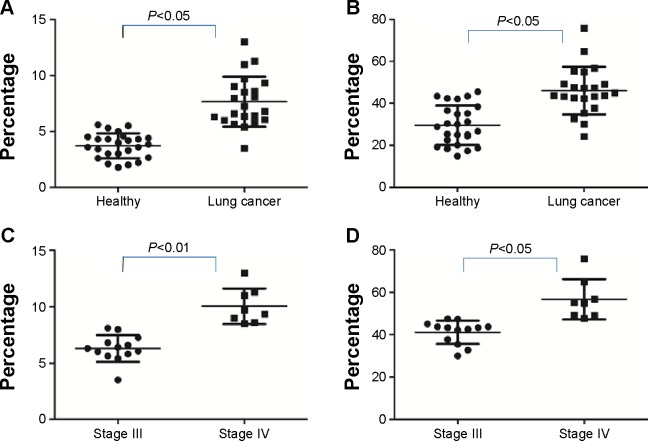
PD-1 expression was studied on CD4+CD25+CD127low Tregs isolated from peripheral blood mononuclear cells by flow cytometry. Twenty-two primary lung cancer patients and 25 healthy volunteers were recruited from the Second Affiliated Hospital of Soochow University (Suzhou, People’s Republic of China). Lung cancer patients had a nearly 2-fold increase in the number of circulating Tregs relative to healthy controls (7.66%±2.25% versus [vs] 3.76%±1.06%, respectively; P<0.05). The levels of PD-1 expression by CD4+CD25+CD127low Tregs were also higher in lung cancer samples compared to controls (46.01%±11.33% vs 33.34%±13.54%, respectively). Significant differences in both CD4+CD25+CD127low Treg abundance (6.29%±1.18% vs 10.06%±1.58%; P<0.01) and Treg PD-1 expression (41.85%±6.1% vs 56.57%±12.52%; P<0.05) were observed between clinical stages III and IV, respectively (Figure 1); no differences were seen among other pathologic subtypes, or in terms of lymphatic metastasis.
Figure 1.
Expression of Tregs PD-1 on Tregs in peripheral blood.
Notes: (A) Expression of Tregs in peripheral blood in lung cancer patients and healthy volunteers. (B) Expression of PD-1 on Tregs in lung cancer patients and healthy volunteers. (C) Expression Tregs in clinical stages III and IV lung cancer patients. (D) Expression of PD-1 on Tregs in clinical stages III and IV lung cancer patients.
Tregs play a critical role in a variety of immunologic processes, including self-tolerance, anti-tumor immune responses, and transplantation.7,8 Our study revealed a significant increase in the number of CD4+CD25+CD127low Tregs in patients with lung cancer, relative to healthy controls. While the mechanisms underlying the increase in lung cancer-associated Tregs are not known, the overall increase in the number of immunosuppressive immune cells present in these patients may play an important role in both the development and progression of lung cancer.9,10
Costimulatory molecule receptors interacting with their corresponding ligand mediate both positive and negative costimulatory signals, which regulate immune cell activation, including the activation and proliferation of T cells, cytokine production, apoptosis, cell survival, and cytotoxicity.11–13 PD-1 belongs to the CD28 family of receptors, and is expressed on activated T, B, and myeloid cells.4 PD-1 and its ligand PD-L1 deliver inhibitory signals that regulate the balance between effector T cell activation and immune-mediated tissue damage.11,12 In addition, the proliferation and immune inhibitory activity of Tregs is directly related to the expression of costimulatory molecules on the cell surface.14 Conflicting reports on the role of PD-1 on Treg function have suggested that PD-1–PD-L1 ligation on Tregs promotes Treg stability and expansion,15–18 while others have suggested that this pathway inhibits Treg expansion and function.19,20 Our study revealed a distinct increase in Treg number and Treg PD-1 expression in patients with lung cancer, suggesting that the PD-1/PD-L1 pathway may play a role in Treg induction and is associated with impaired adaptive immunity. From these results, we hypothesize that the over-expression of PD-1 on Tregs and/or the increase in Treg number may participate in the immune inhibitory state of lung cancer patients. The cross-talk between Treg cells and PD-1/PD-L1-induced inhibition in lung cancer warrants further exploration for lung cancer associated immune pathogenesis.
Acknowledgments
Our studies were supported by grants from the Natural Science Foundation of China (no 81272610).
Footnotes
Authors’ contributions
Both Anyuan Zhong and Xue Pan contributed equally to this letter. This letter has been approved by all authors for publication. All authors contributed toward data analysis, drafting, and revising the letter.
Disclosure
The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S. Naturally arising Foxp3-expressing CD25+CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat Immunol. 2005;6(4):345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- 4.Agata Y, Kawasaki A, Nishimura H, et al. Expression of the PD-1 antigen on the surface of stimulated mouse T and B lymphocytes. Int Immunol. 1996;8(5):765–772. doi: 10.1093/intimm/8.5.765. [DOI] [PubMed] [Google Scholar]
- 5.Pircher A, Gamerith G, Amann A, et al. Neoadjuvant chemo-immunotherapy modifies CD4(+)CD25(+) regulatory T cells (Treg) in non-small cell lung cancer (NSCLC) patients. Lung Cancer. 2014;85(1):81–87. doi: 10.1016/j.lungcan.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Reck M, Paz-Ares L. Immunologic Checkpoint Blockade in Lung Cancer. Semin Oncol. 2015;42(3):402–417. doi: 10.1053/j.seminoncol.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 7.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101(5):455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 8.Morse M, Clay TM, Mosca P, Lyerly HK. Immunoregulatory T cells in cancer immunotherapy. Expert Opin Biol Ther. 2002;2(8):827–834. doi: 10.1517/14712598.2.8.827. [DOI] [PubMed] [Google Scholar]
- 9.Zhang D, Chen Z, Wang DC, Wang X. Regulatory T cells and potential inmmunotherapeutic targets in lung cancer. Cancer Metastasis Rev. 2015 May 12; doi: 10.1007/s10555-015-9566-0. Epub. [DOI] [PubMed] [Google Scholar]
- 10.Domagala-Kulawik J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl Lung Cancer Res. 2015;4(2):177–190. doi: 10.3978/j.issn.2218-6751.2015.01.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8(3):239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 12.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19(3):309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 14.Kitazawa Y, Fujino M, Wang Q, et al. Involvement of the programmed death-1/programmed death-1 ligand pathway in CD4+CD25+ regulatory T-cell activity to suppress alloimmune responses. Transplantation. 2007;83(6):774–782. doi: 10.1097/01.tp.0000256293.90270.e8. [DOI] [PubMed] [Google Scholar]
- 15.Francisco LM, Salinas VH, Brown KE, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Periasamy S, Dhiman R, Barnes PF, et al. Programmed death 1 and cytokine inducible SH2-containing protein dependent expansion of regulatory T cells upon stimulation With Mycobacterium tuberculosis. J Infect Dis. 2011;203(9):1256–1263. doi: 10.1093/infdis/jir011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Q, Munger ME, Highfill SL, et al. Program death-1 signaling and regulatory T cells collaborate to resist the function of adoptively transferred cytotoxic T lymphocytes in advanced acute myeloid leukemia. Blood. 2010;116(14):2484–2493. doi: 10.1182/blood-2010-03-275446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20(1):107–118. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 19.Franceschini D, Paroli M, Francavilla V, et al. PD-L1 negatively regulates CD4+CD25+Foxp3+ Tregs by limiting STAT-5 phospho-rylation in patients chronically infected with HCV. J Clin Invest. 2009;119(3):551–564. doi: 10.1172/JCI36604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karim R, Jordanova ES, Piersma SJ, et al. Tumor-expressed B7-H1 and B7-DC in relation to PD-1+ T-cell infiltration and survival of patients with cervical carcinoma. Clin Cancer Res. 2009;15(20):6341–6347. doi: 10.1158/1078-0432.CCR-09-1652. [DOI] [PubMed] [Google Scholar]