Abstract
Objective
While patients suffering from fibromyalgia (FM) are known to exhibit hyperalgesia, the central mechanisms contributing to this altered pain processing are not fully understood. In this study we investigate potential dysregulation of the neural circuitry underlying cognitive and hedonic aspects of the subjective experience of pain such as anticipation of pain and of pain relief.
Methods
FMRI was performed on 31 FM patients and 14 controls while they received cuff pressure pain stimuli on their leg, calibrated to elicit a pain rating of ∼50/100. During the scan, subjects also received visual cues informing them of impending pain onset (pain anticipation) and pain offset (relief anticipation).
Results
Patients exhibited less robust activations during both anticipation of pain and anticipation of relief within regions commonly thought to be involved in sensory, affective, cognitive and pain-modulatory processes. In healthy controls, direct searches and region-of-interest analyses in the ventral tegmental area (VTA) revealed a pattern of activity compatible with the encoding of punishment: activation during pain anticipation and pain stimulation, but deactivation during relief anticipation. In FM patients, however, VTA activity during pain and anticipation (of both pain and relief) periods was dramatically reduced or abolished.
Conclusion
FM patients exhibit disrupted brain responses to reward/punishment. The VTA is a source for reward-linked dopaminergic/GABAergic neurotransmission in the brain and our observations are compatible with reports of altered dopaminergic/GABAergic neurotransmission in FM. Reduced reward/punishment signaling in FM may relate to the augmented central processing of pain and reduced efficacy of opioid treatments in these patients.
Fibromyalgia (FM) is a chronic, relatively common pain disorder characterized by persistent, widespread body pain and myofascial tenderness, and is considered the quintessential “functional” pain disorder. The prevalence of FM in the general population is estimated in the United States to be 3.4% in women and 0.5% in men, and increases with age (reaching more than 7% in women between 60 and 79) (1). Some of the hallmarks of FM include alterations in central nervous system pain-modulatory processes, a prominent role of negative affective factors in maintaining pain and disability and a poor enduring response to “peripheral” treatments such as topical agents or trigger point injections, as well as opioids (2). These characteristics highlight the ‘central’ nature of FM pathophysiology, and have made this disorder a subject for several brain imaging studies. Collectively, evidence derived from psychophysical and functional neuroimaging studies supports the notion of “augmented sensitivity to painful stimulation in FM”, which is thought to be due predominantly to aberrant brain processing of pain-related information (2, 3).
However, while the neural correlates of experimental (4-6) and clinical (7) pain in FM have been the object of several investigations, potential dysregulation of the neural mechanisms underlying anticipation of pain and anticipation of pain relief in this chronic pain population has received little attention. This is an important distinction, as cognitive, motivational and affective processes are intimately involved in the perception and reporting of pain, including in FM (3, 8, 9). Importantly, the brain state preceding a painful stimulation has been shown to predict responses to experimental (10), as well as clinical (11) pain. Expectancy and pain-relevant anxiety, in particular, have been shown to shape subsequent perceptual states (12). Relief from pain, on the other hand, is a positive hedonic experience intrinsically linked to pain (13). It has been suggested that the experience of relief may be altered in patients with chronic pain (14). As both pain, and the anticipation of pain and relief have strong hedonic value linked to their punishment/reward properties, it is reasonable to suspect that these states may be processed differently in FM patients, particularly in structures involved in the encoding of appetitive or aversive stimuli. In the present study we used functional magnetic resonance imaging (fMRI) and cuff pain algometry (CPA) to investigate the brain responses to deep tissue noxious stimulation as well as to the anticipation of pain and relief in FM patients and healthy controls. We adopted both a whole-brain approach and a region-of-interest (ROI) approach focused on the nucleus accumbens (NAc) and the ventral tegmental area (VTA), two mesolimbic structures known to be involved in the processing of reward/punishment (15), and that were implicated in FM pathophysiology in positron emission tomography (PET) studies (16, 17).
Materials and methods
Subjects
31 FM patients and 14 healthy controls were recruited to participate in this experiment. Enrolled patients were diagnosed with fibromyalgia (as confirmed by physician and medical records) and met the recently-proposed Wolfe et al criteria, which require the presence of widespread pain as well as the endorsement of a number of somatic and cognitive symptoms (18). Healthy controls were free from chronic pain and rheumatic disease. Exclusion criteria for both groups included age below 18 years, current or past history of significant psychiatric, neurological or cardiovascular disorders, history of significant head injury, current use of opioids, implanted medical or metallic objects and pregnancy. All participants in the study provided written informed consent in accordance with the Partners Human Research Committee.
Study overview
Subjects participated in two separate sessions, on different days: one training (behavioral-only) session and one imaging session. The training session was used to familiarize subjects with the stimuli and rating procedures and determine appropriate stimulus intensities to be used subsequently in the imaging session (see below).
Painful stimulation was achieved via cuff pain algometry. We chose CPA over other more commonly used methods of pain stimulation (e.g., contact heat) because CPA stimuli appear to have a preferential effect on deep tissue nociceptors (19). As most clinical pain originates in deep tissue rather than cutaneous receptors, the investigation of brain responses to deep tissue pain might prove to be more clinically relevant than brain responses to evoked cutaneous pain. As in our previous studies (20, 21), mechanical stimuli were delivered on the right calf using a 13.5cm-wide velcro-adjusted pressure cuff, connected to a rapid cuff inflator (Hokanson E20 AG101, Hokanson Inc, Bellevue, WA, USA). The cuff inflator was adapted to ramp up more gradually to target pressure over ∼2 seconds, to minimize abrupt subject motion.
After providing informed consent and completing questionnaires (Beck Depression Inventory, Fatigue Visual Analog Scale (VAS), Widespread Pain Index, Short Form 36 Health Survey, Brief Pain Inventory), subjects were familiarized with the CPA procedures. Subjects sat comfortably on a chair with the left foot resting on a support at a slightly elevated position. The vascular cuff was then secured around the left gastrocnemius muscle. The quantitative sensory testing began by inflating the cuff to 60 mmHg of pressure and making adjustments in 10 mmHg increments until a pain intensity rating of ∼50/100 was first obtained.
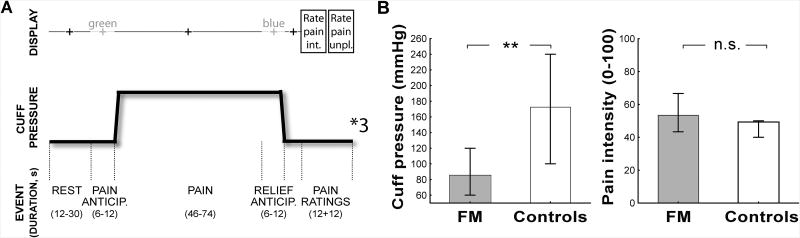
On the day of the imaging session, ratings of intensity and unpleasantness of clinical pain (VAS, 0-100) were obtained from patients. The stimulus pressure was briefly recalibrated prior to scanning, using procedures similar to those adopted during the training session. During a functional imaging scan run, brain activity was investigated using Blood Oxygen Level Dependent (BOLD) fMRI, while undergoing 3 separate tonic (i.e., 46 – 74 sec) cuff pain stimuli set to the same intensity level (∼50/100) (Figure 1A). Prior to each cuff inflation, a cross projected to the subjects' visual field changed from black to green in order to signal the period of pain anticipation, and then turned black again at stimulus onset. Prior to cuff deflation, the cross switched in color from black to blue to signal the period of pain anticipation relief, and then turned black again at cuff stimulus offset. These visual cues had a duration of six to twelve seconds (i.e., jittered in time). The use of relatively long pain stimuli was chosen to maximize the emotional responses associated with expectancy of pain and relief, and to ensure temporal separation between regressors in the design matrix (see below). For each of the 3 pain blocks, eight seconds after stimulus offset, subjects used an MR-compatible button box to rate the intensity and unpleasantness of the cuff pain stimuli (0-100), on electronic scales presented via ePrime (Psychology Software Tools, Sharpsburg, PA).
Figure 1. Experimental design (A) and psychophysical results (B).
Bars represent median and 25-75% interquartile range.
FMRI data were acquired using a 3T Siemens TIM Trio MRI System (Siemens Medical, Erlangen, Germany) equipped for echo planar imaging with a 32-channel head coil. A whole brain T2*-weighted gradient echo BOLD EPI pulse sequence was used (TR/TE=2sec/30ms, f.a.=90°, 32 AC-PC aligned axial slices, voxel size=3.1×3.1×4mm). We also collected anatomical data, using a multi-echo MPRAGE pulse sequence (TR/TE1/TE2/TE3/T4=2530/1.64/3.5/5.36/7.22 ms, flip angle=7°, voxel size=1mm isotropic).
Data Analysis
All statistical analyses for behavioral data were performed with Statistica 10.0 (StatSoft Inc., USA), using an alpha level of 0.05. Differences in gender distribution across groups were assessed using the Fisher exact test. Deviation from normality was assessed using the Kolmogorov-Smirnov test for all variables of interest: cuff pressure values (i.e., pressure values, in mmHg, eliciting the target pain intensity rating of ∼50/100 in the recalibration performed at the beginning of the imaging visit) and mean intensity and unpleasantness ratings (averaged over three trials). As distribution of cuff pressure values in both patients and controls, and of pain intensity ratings in the controls significantly deviated from normality (p's < 0.05), all group comparisons were performed using the nonparametric Mann-Whitney U (M-W U) test. Group analyses were performed to compare cuff pressure values (in order to determine differences in pain sensitivity between FM and controls), and pain ratings (to assess successful calibration of cuff pressure, and possible differences in the affective responses associated with the stimulus) for both pain intensity and unpleasantness separately, averaged across the 3 trials.
FMRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB's Software Library, www.fmrib.ox.ac.uk/fsl; (22)). The following preprocessing was applied: motion correction, fieldmap-based EPI unwarping, non-brain removal, spatial smoothing (FWHM=5mm), grand-mean intensity normalization by a single multiplicative factor, and high-pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma=72s). Time-series statistical analysis was carried out using FILM with local autocorrelation correction. Cortical surface reconstruction was performed using FreeSurfer (http://surfer.nmr.mgh.harvard.edu/; (23)) for improved structural-functional co-registration purposes. Co-registration used a recently developed automated boundary-based registration algorithm (FreeSurfer's bbregister tool). Registration to the MNI152 standard space was carried out using FSL's FLIRT.
A first level within-subject general linear model (GLM) analysis was performed by modeling the pain expectancy cue, cuff pain stimulus application, and expectancy of pain relief cue as regressors of interest. We also modeled the period between stimulus offset and rating periods, and the rating periods as regressors of no-interest. A canonical double-gamma hemodynamic response function was adopted. Parameter estimates and relative variances for each explanatory variable were then passed up to mixed effects group level analyses, performed using FLAME (FMRIB's Local Analysis of Mixed Effects) 1+2, with automatic outlier detection enabled. Whole-brain statistical parametric maps were computed for the following regressors: pain anticipation, pain stimulus, relief anticipation. All maps were thresholded using clusters determined by a voxel-wise threshold (Z>2.3) and a (corrected) cluster significance threshold of P=0.05.
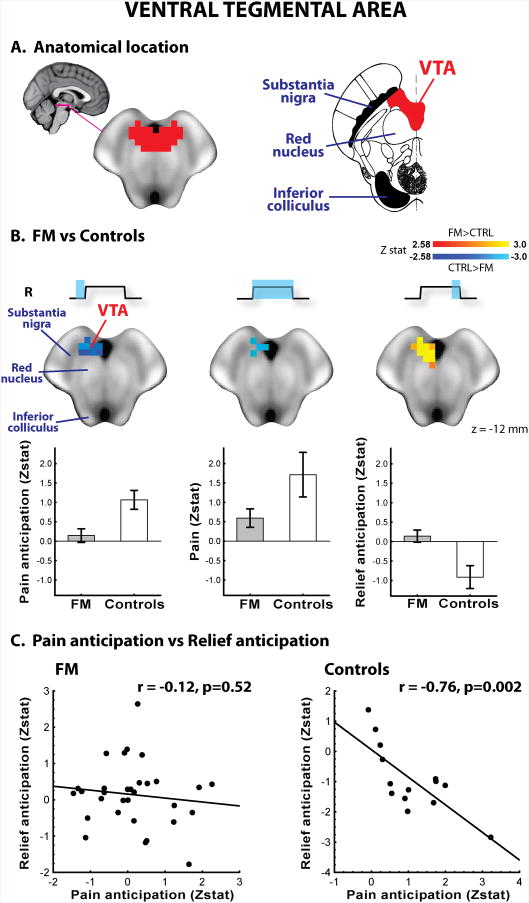
Brain responses to pain anticipation, pain and relief anticipation were also compared across groups with a direct search restricted to the NAc and the VTA. The NAc direct search was performed within the NAc labels from the Harvard-Oxford Subcortical Structural Atlas (http://www.cma.mgh.harvard.edu/fsl_atlas.html), thresholded at a (arbitrary) value of 80 (sizes: NAcRIGHT = 11 voxels; NAcLEFT = 14 voxels; Supplementary Figure 1). The VTA direct search was performed within an anatomically defined mask manually drawn on the MNI152 brain at 0.5mm resolution, based on its location medial to the substantia nigra and the red nuclei (sizes: VTARIGHT = 77 voxels; VTALEFT = 81 voxels; Figure 5, bottom right) (24). The correct coregistration between each of these masks and each subject's spatially normalized fMRI maps was confirmed by visual inspection. These direct searches were performed with an uncorrected threshold of z=2.58 and a minimum cluster size of 5 voxels. From these regions mean z-statistic values were extracted to create correlational plots, as well as to display group differences (for illustrative purposes). In order to further corroborate the significant results obtained from the VTA direct searches (see Results), a region-of-interest analysis was performed by averaging the z-score from all the voxels within the VTA mask (split into left and right VTA). An unpaired t-test was performed to compare average VTA z-scores across groups using Statistica 10.0 (StatSoft Inc., USA), using an alpha level of 0.05.
Figure 5. Direct searches in the ventral tegmental area.
The VTA mask for the ROI analyses (left) was drawn in the midbrain, medial to the substantia nigra and ventral to the red nucleus (right; adapted from the Duvernoy's atlas (24)) (A). In FM, VTA responses to anticipation of pain, pain and anticipation of relief were statistically reduced, compared to controls. Bars represent mean ± SEM (B). VTA responses to pain anticipation and relief anticipation were negatively correlated in healthy controls, but not in FM patients (C).
Results
Psychophysical results
Demographic and clinical data are included in Table 1. Gender distribution was not statistically different across groups (p = 0.23). Prior to scanning, FM patients reported their current clinical pain at an intensity of 34.3 ± 25.19 (range: 0 to 78) and unpleasantness of 32.3 ± 26.7 (0 to 90) out of 100, respectively. Ratings of intensity and unpleasantness of clinical pain were highly correlated (r = 0.88, p<0.0001). In patients, baseline clinical pain ratings tended to be negatively correlated with cuff pressure values subsequently selected to elicit target rating of 50/100 (clinical pain intensity: r = -.33, p = 0.071; clinical pain unpleasantness: r = -.35, p = 0.051). As Figure 1B shows, the pain ratings elicited by the cuff pressure were not statistically significantly different between FM and controls (p's ≥ 0.30), as expected due to percept-matched calibration. However, the pressure needed to induce target pain rating was significantly lower in FM than in controls (p<0.01).
Table 1. Demographic and clinical data.
Values represent means ± SD. PCS = Pain Catastrophizing Scale, BDI = Beck Depression Inventory, WPI = Widespread Pain Index, SF36 = Short Form (36) Health Survey, BPI = Brief Pain Inventory.
| Variable | Mean + SD | |
|---|---|---|
| Controls | FM | |
| N | 14 | 31 |
| Age (years) | 44.2 ± 14.3 | 44.0 ± 11.9 |
| Sex (% Female) | 71.4% | 87.1% |
| Symptom duration (years) | - | 12.5 ± 12.2 |
| Clinical pain intensity (0-100) | - | 34.3 ± 25.19 |
| Clinical pain unpleasantness (0-100) | - | 32.3 ± 26.7 |
| Fatigue (0-100) | 13.0 ± 16.4 | 64.6 ± 22.3** |
| BDI (0-63) | 2.8 ± 3.8 | 17.0 ± 13.6** |
| WPI (number of pain sites; 0-19) | 0.4 ± 0.8 | 11.6 ± 8.1** |
| SF36, General Health (0-100) | 88.6 ± 13.8 | 39.0 ± 23.7** |
| SF36, Physical Function (0-100) | 90.4 ± 26.4 | 47.4 ± 26.0** |
| BPI, Pain Interference (0-10) | 0.0 ± 0.0 | 5.5 ± 2.0** |
| BPI, Pain Severity (0-10) | 0.3 ± 0.6 | 5.3 ± 2.0** |
p<0.001
Imaging results - whole brain analyses
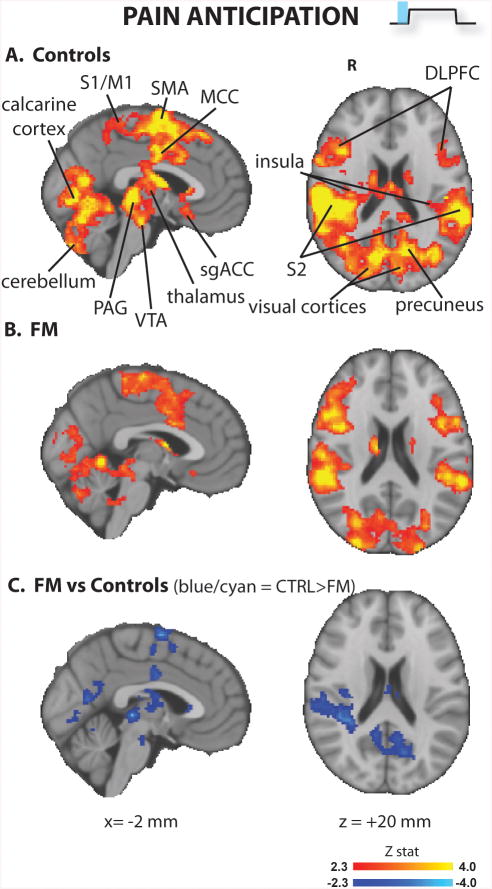
The pain anticipation cue (Figure 2 and Supplementary Table 1) elicited activation in multiple regions in both groups, including primary somatosensory and motor cortices (S1/M1), supplementary motor area (SMA), dorsolateral prefrontal (DLPFC), secondary somatosensory cortex (S2), posterior (PCC), middle (MCC) and subgenual (sgACC) cingulate cortices, the superior parietal lobule (SPL), insula/frontal operculum, the periaqueductal gray (PAG), basal ganglia, medial and lateral visual areas, parahippocampal gyrus, and cerebellum. The brain responses to pain anticipation in the controls were significantly stronger in several of these areas, including SMA, MCC, PCC, PAG, VTA and visual cortices bilaterally, caudate nucleus (head) and globus pallidus on the left, and S2 and posterior insula on the right. In no regions did patients exhibit a stronger BOLD response to pain anticipation than controls.
Figure 2. Brain responses to pain anticipation (whole brain analyses).
FM patients exhibited lower brain responses in several brain regions.
S1/M1 = primary somatosensory / motor cortices; SMA = supplementary motor area; MCC = middle cingulate area; sgACC = subgenual anterior cingulate cortex; VTA = ventral tegmental area; PAG = periaqueductal gray; DLPFC = dorsolateral prefrontal cortex
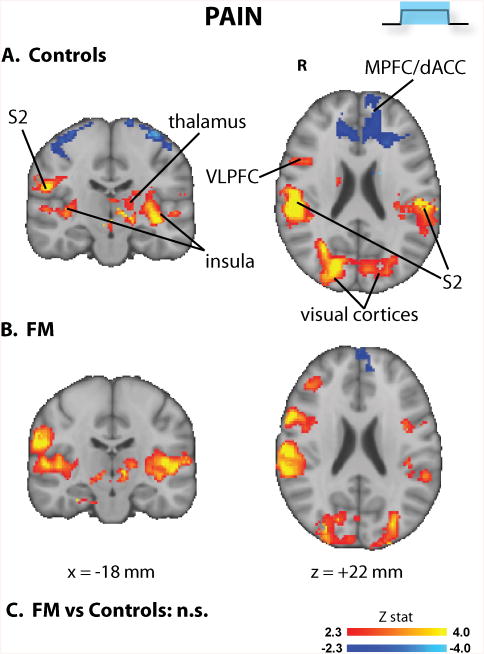
In both groups, cuff pain stimuli evoked brain activity changes in regions frequently observed as activated or deactivated during experimental pain (Figure 3 and Supplementary Table 2). Activated regions included thalamus, insula/frontal operculum, S2, DLPFC, basal ganglia and cerebellum. Medial and lateral visual cortices were also activated. Deactivations were observed in both groups at the level of the medial prefrontal cortex (MPFC). No group differences were observed for pain-induced brain activity, in the whole brain analyses.
Figure 3. Brain responses to pain (whole brain analyses).
In whole-brain searches, the responses to cuff pain were not statistically different across groups.
S2 = secondary somatosensory cortex; VLPFC = ventrolateral prefrontal cortex; MPFC = medial prefrontal cortex.
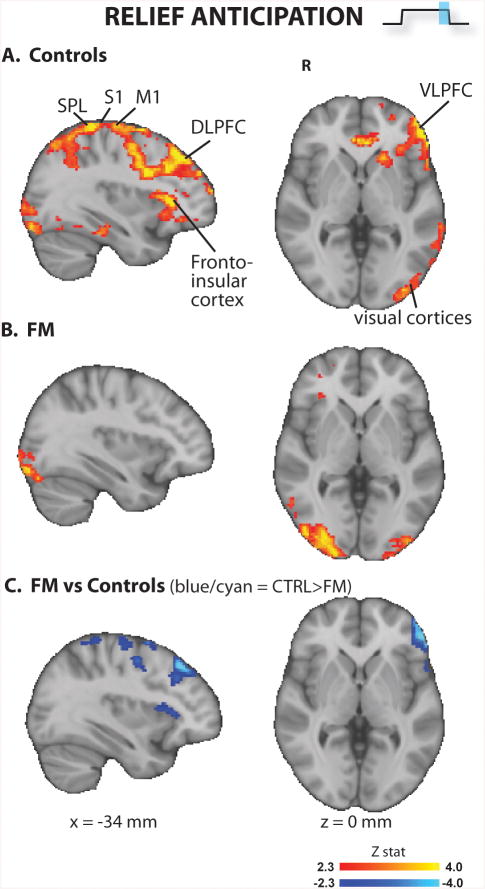
The visual cue for relief anticipation (Figure 4 and Supplementary Table 3) produced significant activations in S1/M1, lateral and medial prefrontal cortices, operculo-insular cortex, precuneus, and visual areas in both groups. The BOLD signal was statistically stronger for the controls in left S1/M1 (sensorimotor representation of the leg), SPL, DLPFC, VLPFC, and operculo-insular cortices. In the whole brain analyses, in no regions did FM patients exhibit a stronger BOLD response than controls to the expectancy of pain relief cue.
Figure 4. Brain responses to relief anticipation (whole brain analyses).
FM patients exhibited lower brain responses in several brain regions. S1/M1 = primary somatosensory / motor cortices; DLPFC = dorsolateral prefrontal cortex; SPL = superior parietal lobule; VLPFC = ventrolateral prefrontal cortex.
Imaging results - VTA and NAc analyses
No group differences reached statistical significance for the nucleus accumbens in the direct searches. In the right VTA, a voxelwise direct search revealed group differences in all three statistical contrasts (Figure 5). Healthy controls exhibited an increase in BOLD signal during pain anticipation and during pain stimulation, but a decrease during relief anticipation. In FM, however, these responses were either significantly reduced (pain), or null (pain anticipation and relief anticipation; Figure 5B). Similar results were observed in ROI analyses computed averaging the values from all voxels in the right VTA mask. Compared to FM patients, controls exhibited stronger activations during pain anticipation (p<0.01) and were trending stronger for pain (p=0.059), while stronger deactivations were found during relief anticipation (p<0.05). Using the left VTA as ROI, no statistically significant group differences were observed during pain stimulation as well as anticipation of relief, similar to the results from the direct search (p's>0.6). However, the left VTA did reveal a statistically significant group difference for pain anticipation (CTRL>FM: p<0.05). In the VTA subregion showing statistically significant group differences in all contrasts, responses to pain anticipation were positively correlated with VTA responses to pain in both groups (controls: r = 0.54, p = 0.048; FM: r = 0.55, p = 0.001). VTA responses to pain anticipation were also negatively correlated with VTA responses to relief anticipation in the controls (r = 0.76, p = 0.002), but not in the FM patients (r = -0.12, p = 0.52; Figure 5C).
Discussion
Our results present evidence for differences in brain processing during pain, as well as anticipation of pain and of pain relief, between FM patients and healthy controls.
During pain anticipation (Figure 2), healthy volunteers activated multiple regions including ACC, PAG, thalamus, premotor cortex and VTA, i.e., areas previously reported as being associated with expectancy of pain (25), as well as other regions thought to be involved in sensory, affective, cognitive and pain-modulatory processes, such as S1/M1, S2, DLPFC, fronto-insular cortex, and basal ganglia (26). Interestingly, brain responses to pain anticipation were significantly reduced in FM patients. Cuff pain stimuli (Figure 3) evoked brain activity changes in regions frequently observed as activated (thalamus, insula/frontal operculum, S2, DLPFC, basal ganglia and cerebellum) or deactivated (medial prefrontal cortex) during experimental pain (21, 26), which were statistically indistinguishable across groups in whole-brain analyses. Of note, we were able to observe these brain responses even if the stimuli used had a longer duration (i.e., 46 – 74 sec) than most published fMRI pain studies. Still, the lack of activation within the primary somatosensory cortex (which we have previously observed with cuff pain stimuli of shorter duration (21)) could be due to the length of stimulation. During the relief anticipation period (Figure 4), FM patients exhibited decreased brain activation compared to controls, as they activated visual areas (likely in response to the processing of the visual cue) but, unlike healthy volunteers, failed to activate S1/M1, SPL, ventro- and dorso-lateral prefrontal and fronto-insular cortices. Overall, these results add to a growing literature supporting the reduced responsiveness of FM patients to a variety of experimental manipulations (4, 27, 28).
Analyses focused on mesolimbic regions (direct search, as well as ROI analyses) revealed group differences in responses to pain anticipation, pain, and relief anticipation in the right VTA (Figure 5). The VTA is a dopamine-rich region that occupies the ventromedial portion of the midbrain. While dopaminergic neurons in the VTA and other regions have been traditionally linked with processing of signals for reward, it has become increasingly clear that a portion of these cells also encode aversive/punishment signals (29). Indeed, in our healthy controls the VTA responses to all three experimental periods were compatible with the encoding of signals of punishment and reward: this region was activated during pain anticipation and pain receipt, but deactivated during relief anticipation. Furthermore, VTA responses during pain anticipation were positively correlated with those during pain stimulation, and negatively correlated with VTA responses during relief anticipation – i.e. subjects with greater VTA activation during pain anticipation had greater VTA deactivation during relief anticipation. In FM patients, however, the VTA responses to all experimental periods were dramatically reduced or abolished, and the activity during pain anticipation and relief anticipation were not related.
Our observation that a region rich in dopaminergic neurons such as the VTA exhibits less reactivity to all experimental periods is compatible with the results of other studies showing altered dopaminergic neurotransmission in FM patients. For instance, recent PET studies have revealed that FM patients exhibit reduced activity levels of DOPA decarboxylase, an enzyme involved in dopamine metabolism, in several regions including the VTA (17), as well as reduced dopaminergic brain responses to evoked pain (4), compared to healthy volunteers. Interestingly, human PET studies reveal that higher binding potential for D2/D3 ligands, potentially indicative of lower levels of endogenous dopamine release, is associated with higher pain sensitivity in healthy adults, as well as FM patients (4, 30). Thus, altered dopaminergic neurotransmission may, at least in part, underlie the noted hyperalgesia in FM patients (31-33), as was also observed in the present study (Figure 1). Interestingly, lower responsiveness of VTA and other ‘reward regions’ to noxious stimuli predicts lower opioid-induced analgesia in healthy subjects (34). Thus, reduced VTA response to pain (as well as pain anticipation/relief) in FM suggests that altered neurotransmission in this and similar reward/punishment processing brain regions might support the lack of therapeutic efficacy of opioids in treating FM pain (opioid use for management of pain in FM is in fact not recommended by any current guideline (35-37)). Furthermore, recent evidence suggests a strong link between corticostriatal circuitry and chronic pain (38). This circuitry is under the modulatory control of dopaminergic midbrain nuclei including the VTA, and therefore our study provides further support for a role of dopaminergic neurotrasmission in the etiopathology underlying pain disorders.
While up to 65% of neurons in the VTA are dopaminergic, a large portion of the remaining neurons are mostly GABAergic (39). Recent studies have shown that most VTA GABAergic neurons are excited by aversive stimuli, including noxious stimuli, suggesting a role for these cells in processing signals for punishment (15, 40). Notably, in the study by Cohen et al., these neurons exhibited a small increase in firing rate during the exposure to a conditional cue immediately preceding an aversive stimulus, and a larger increase during receipt of the aversive stimulus itself (15), an activity profile which was very similar to the VTA responses we observed in our healthy controls. As GABA levels are diminished in some brain regions FM patients (41), it is possible that reduced GABAergic neurotransmission might also contribute to the group differences observed in brain activity in this study. However, as no direct measure of GABA or dopamine was obtained in this study, the neurochemical correlates of our results are only speculative and will need to be directly investigated.
One possible explanation for the brain activity differences observed in other brain regions across groups during the pain anticipation/relief periods involves the concept of salience, i.e., the ability of a given stimulus to ‘stand out’ from its background. As most patients reported experiencing some amount of ongoing pain (i.e., their clinical pain) even in the absence of cuff stimulation, the cues may have only signaled the transition from a lower level of pain to a higher level of pain (or vice versa), rather than the transition from a pain-free state to a moderately strong pain state (or vice versa), as was the case for the healthy controls. It is therefore possible that the observed group differences might partly reflect a lower salience attributed by the patients to the impending onset or offset of cuff pain stimulation. Since several of the regions observed activated during the pain anticipation/relief periods, including somatosensory, insular, cingulate, frontal and parietal areas, have been implicated in the detection of salient changes in the sensory environment (42-44), our data at least in part support this interpretation. Moreover, stimuli with high emotional salience induce stronger activations of visual areas compared to less salient stimuli (45). Therefore, the group differences in visual cortex activation during pain anticipation also provide corroboration to potential differences in processing of salient events.
Furthermore, reduced brain responses to the pain anticipation relief were observed in regions, including VLPFC, DLPFC and insula, often implicated in placebo analgesia (46-48). It is therefore possible that such results could be partly explained by the fact that patients might expect a lower degree of relief from pain, as for them the end of the stimulus does not mean the end of pain perception – i.e. their clinical pain continues.
Other factors might also contribute to explain the observed brain activity differences across groups, such as a reduced ability to engage pain coping mechanisms in FM patients. Among the regions that FM patients activated to a lesser degree during pain anticipation was the periaqueductal gray. The PAG is a midbrain structure that a large number of studies have implicated in descending pain modulation. For instance, electrical stimulation of subregions of the PAG in animal models has been shown to reduce behavioral responses to noxious stimulation by inhibiting nociceptive dorsal horn neurons, indirectly through its projections to the rostral ventromedial medulla (49). Therefore, activation of the PAG during expectancy of pain in healthy volunteers may reflect the engagement of the descending pain inhibitory mechanisms in preparation to the upcoming pain stimulus. According to this view, the reduced PAG activation in FM patients would be indicative of a reduced ability to engage such coping mechanisms, a notion also supported by the results from other studies (6).
Yet another mechanism potentially contributing to the reduced of responsiveness exhibited by the FM patients to the various experimental conditions may be related to levels of perceived helplessness. A recent study in a different chronic pain population (temporomandibular disorder) has shown a relationship between reported helplessness and cortical thickness in the supplementary motor area and midcingulate cortex (50). As these were among the regions exhibiting lower responsiveness to pain anticipation in FM patients in our study, future studies should investigate whether catastrophizing-related factors such as helplessness and structural brain changes may contribute to explain our observations.
Several caveats should be taken into consideration. First, we have not collected behavioral data directly measuring perceived reward or punishment. Thus, linking altered VTA responses in FM patients to alterations in the processing of punishment and reward is only based on the well-accepted role of the VTA in the processing of aversive/rewarding stimuli, as well as on the assumption that anticipating or perceiving a painful stimulus should be a punishing experience, while anticipating relief from pain should be a rewarding experience. Similarly, we have not collected behavioral data allowing us to test the hypothesis that the experimental pain stimuli may be less salient for patients, due to the presence of more highly salient ongoing clinical pain. It is also important to note that as the patients were more sensitive to pain stimuli, they required less pressure to achieve the target pain sensation, compared to the healthy volunteers. Thus, we cannot exclude that the differences in the physical intensity of the stimulation might explain at least part of the brain effects observed in this study.
In summary, we demonstrate the existence in FM patients of altered brain processing within circuitry that other studies have related to reward/punishment and salience. Our results further support a reduced ability to engage the descending pain modulatory system in these patients. While we did not directly investigate neurotransmitter release, our observations are also compatible with previous reports of altered dopaminergic/GABAergic neurotransmission in FM patients, and could contribute to our understanding of some hallmarks of FM, including augmented central processing of pain and the lack of therapeutic efficacy of opioid treatments.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Karin Jensen for helpful comments on the manuscript. This research was supported by NCCAM, NIH (R01-AT004714, P01-AT002048, P01-AT006663, R01-AT005280; R01-AG034982, R21-AR057920)
Grants: Supported by NCCAM, NIH (R01-AT004714, P01-AT002048, P01-AT006663, R01-AT005280; R01-AG034982, R21-AR057920).
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Wolfe F, Ross K, Anderson J, Russell IJ, Hebert L. The prevalence and characteristics of fibromyalgia in the general population. Arthritis Rheum. 1995;38(1):19–28. doi: 10.1002/art.1780380104. [DOI] [PubMed] [Google Scholar]
- 2.Clauw DJ, Williams D. Fibromyalgia. In: Mayer EA, Bushnell MC, editors. Functional Pain Syndromes. Seattle: IASP Press; 2009. p. 580. [Google Scholar]
- 3.Gracely RH, Ambrose KR. Neuroimaging of fibromyalgia. Best Pract Res Clin Rheumatol. 2011;25(2):271–84. doi: 10.1016/j.berh.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Wood PB, Schweinhardt P, Jaeger E, Dagher A, Hakyemez H, Rabiner EA, et al. Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci. 2007;25(12):3576–82. doi: 10.1111/j.1460-9568.2007.05623.x. [DOI] [PubMed] [Google Scholar]
- 5.Gracely RH, Petzke F, Wolf M, Clauw D. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46(5):1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 6.Jensen KB, Kosek E, Petzke F, Carville S, Fransson P, Marcus H, et al. Evidence of dysfunctional pain inhibition in Fibromyalgia reflected in rACC during provoked pain. Pain. 2009;144(1-2):95–100. doi: 10.1016/j.pain.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ambrose KR, Gracely RH, Glass JM. Fibromyalgia dyscognition: concepts and issues. Reumatismo. 2012;64(4):206–15. doi: 10.4081/reumatismo.2012.206. [DOI] [PubMed] [Google Scholar]
- 9.Fields HL. Proceedings of the 11th world congress on pain. Seattle: IASP press; 2006. A motivation-decision model of pain: the role of opioids; pp. 449–59. [Google Scholar]
- 10.Ploner M, Lee MC, Wiech K, Bingel U, Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proc Natl Acad Sci U S A. 2010;107(1):355–60. doi: 10.1073/pnas.0906186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, et al. Default mode network connectivity encodes clinical pain: An arterial spin labeling study. Pain. 2012 doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ploghaus A, Narain C, Beckmann CF, Clare S, Bantick S, Wise R, et al. Exacerbation of pain by anxiety is associated with activity in a hippocampal network. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21(24):9896–903. doi: 10.1523/JNEUROSCI.21-24-09896.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leknes S, Lee M, Berna C, Andersson J, Tracey I. Relief as a reward: hedonic and neural responses to safety from pain. PLoS One. 2011;6(4):e17870. doi: 10.1371/journal.pone.0017870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–60. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen JY, Haesler S, Vong L, Lowell BB, Uchida N. Neuron-type-specific signals for reward and punishment in the ventral tegmental area. Nature. 2012;482(7383):85–8. doi: 10.1038/nature10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RE, Clauw DJ, Scott DJ, McLean SA, Gracely RH, Zubieta JK. Decreased central mu-opioid receptor availability in fibromyalgia. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(37):10000–6. doi: 10.1523/JNEUROSCI.2849-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wood PB, Patterson JC, 2nd, Sunderland JJ, Tainter KH, Glabus MF, Lilien DL. Reduced presynaptic dopamine activity in fibromyalgia syndrome demonstrated with positron emission tomography: a pilot study. J Pain. 2007;8(1):51–8. doi: 10.1016/j.jpain.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res. 2010;62(5):600–10. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 19.Polianskis R, Graven-Nielsen T, Arendt-Nielsen L. Pressure-pain function in desensitized and hypersensitized muscle and skin assessed by cuff algometry. J Pain. 2002;3(1):28–37. doi: 10.1054/jpai.2002.27140. [DOI] [PubMed] [Google Scholar]
- 20.Edwards RR, Mensing G, Cahalan C, Greenbaum S, Narang S, Belfer I, et al. Alteration in Pain Modulation in Women With Persistent Pain After Lumpectomy: Influence of Catastrophizing. J Pain Symptom Manage. 2012 doi: 10.1016/j.jpainsymman.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Loggia ML, Edwards RR, Kim J, Vangel MG, Wasan AD, Gollub RL, et al. Disentangling linear and nonlinear brain responses to evoked deep tissue pain. Pain. 2012;153(10):2140–51. doi: 10.1016/j.pain.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–19. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- 23.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 24.Naidich TP, Duvernoy HM, Delman BN, Sorensen AG, Kollias SS, Haacke EM. Duvernoy's Atlas of the Human Brain Stem and Cerebellum. Wien: Springer-Verlag; 2009. [Google Scholar]
- 25.Fairhurst M, Wiech K, Dunckley P, Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 2007;128(1-2):101–10. doi: 10.1016/j.pain.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–84. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 27.Lee SJ, Song HJ, Decety J, Seo J, Kim SH, Nam EJ, et al. Do patients with fibromyalgia show abnormal neural responses to the observation of pain in others? Neurosci Res. 2013 doi: 10.1016/j.neures.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 28.Thieme K, Rose U, Pinkpank T, Spies C, Turk DC, Flor H. Psychophysiological responses in patients with fibromyalgia syndrome. J Psychosom Res. 2006;61(5):671–9. doi: 10.1016/j.jpsychores.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 29.Bromberg-Martin ES, Matsumoto M, Hikosaka O. Dopamine in motivational control: rewarding, aversive, and alerting. Neuron. 2010;68(5):815–34. doi: 10.1016/j.neuron.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Scott DJ, Heitzeg MM, Koeppe RA, Stohler CS, Zubieta JK. Variations in the human pain stress experience mediated by ventral and dorsal basal ganglia dopamine activity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(42):10789–95. doi: 10.1523/JNEUROSCI.2577-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desmeules JA, Cedraschi C, Rapiti E, Baumgartner E, Finckh A, Cohen P, et al. Neurophysiologic evidence for a central sensitization in patients with fibromyalgia. Arthritis Rheum. 2003;48(5):1420–9. doi: 10.1002/art.10893. [DOI] [PubMed] [Google Scholar]
- 32.Granges G, Littlejohn G. Pressure pain threshold in pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis Rheum. 1993;36(5):642–6. doi: 10.1002/art.1780360510. [DOI] [PubMed] [Google Scholar]
- 33.Petzke F, Clauw DJ, Ambrose K, Khine A, Gracely RH. Increased pain sensitivity in fibromyalgia: effects of stimulus type and mode of presentation. Pain. 2003;105(3):403–13. doi: 10.1016/S0304-3959(03)00204-5. [DOI] [PubMed] [Google Scholar]
- 34.Wanigasekera V, Lee MC, Rogers R, Kong Y, Leknes S, Andersson J, et al. Baseline reward circuitry activity and trait reward responsiveness predict expression of opioid analgesia in healthy subjects. Proc Natl Acad Sci U S A. 2012;109(43):17705–10. doi: 10.1073/pnas.1120201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carville SF, Arendt-Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, et al. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67(4):536–41. doi: 10.1136/ard.2007.071522. [DOI] [PubMed] [Google Scholar]
- 36.Hauser W, Thieme K, Turk DC. Guidelines on the management of fibromyalgia syndrome - a systematic review. Eur J Pain. 2010;14(1):5–10. doi: 10.1016/j.ejpain.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Fitzcharles MA, Ste-Marie PA, Gamsa A, Ware MA, Shir Y. Opioid use, misuse, and abuse in patients labeled as fibromyalgia. The American journal of medicine. 2011;124(10):955–60. doi: 10.1016/j.amjmed.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 38.Baliki MN, Petre B, Torbey S, Herrmann KM, Huang L, Schnitzer TJ, et al. Corticostriatal functional connectivity predicts transition to chronic back pain. Nat Neurosci. 2012;15(8):1117–9. doi: 10.1038/nn.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nair-Roberts RG, Chatelain-Badie SD, Benson E, White-Cooper H, Bolam JP, Ungless MA. Stereological estimates of dopaminergic, GABAergic and glutamatergic neurons in the ventral tegmental area, substantia nigra and retrorubral field in the rat. Neuroscience. 2008;152(4):1024–31. doi: 10.1016/j.neuroscience.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan KR, Yvon C, Turiault M, Mirzabekov JJ, Doehner J, Labouebe G, et al. GABA neurons of the VTA drive conditioned place aversion. Neuron. 2012;73(6):1173–83. doi: 10.1016/j.neuron.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foerster BR, Petrou M, Edden RA, Sundgren PC, Schmidt-Wilcke T, Lowe SE, et al. Reduced insular gamma-aminobutyric acid in fibromyalgia. Arthritis Rheum. 2012;64(2):579–83. doi: 10.1002/art.33339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–83. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- 43.Iannetti GD, Mouraux A. From the neuromatrix to the pain matrix (and back) Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 2010;205(1):1–12. doi: 10.1007/s00221-010-2340-1. [DOI] [PubMed] [Google Scholar]
- 44.Mouraux A, Diukova A, Lee MC, Wise RG, Iannetti GD. A multisensory investigation of the functional significance of the “pain matrix”. Neuroimage. 2011;54(3):2237–49. doi: 10.1016/j.neuroimage.2010.09.084. [DOI] [PubMed] [Google Scholar]
- 45.Lang PJ, Bradley MM, Fitzsimmons JR, Cuthbert BN, Scott JD, Moulder B, et al. Emotional arousal and activation of the visual cortex: an fMRI analysis. Psychophysiology. 1998;35(2):199–210. [PubMed] [Google Scholar]
- 46.Petrovic P, Kalso E, Petersson KM, Andersson J, Fransson P, Ingvar M. A prefrontal non-opioid mechanism in placebo analgesia. Pain. 2010;150(1):59–65. doi: 10.1016/j.pain.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 47.Zubieta JK, Stohler CS. Neurobiological mechanisms of placebo responses. Ann N Y Acad Sci. 2009;1156:198–210. doi: 10.1111/j.1749-6632.2009.04424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kong J, Gollub RL, Rosman IS, Webb JM, Vangel MG, Kirsch I, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. J Neurosci. 2006;26(2):381–8. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basbaum AI, Clanton CH, Fields HL. Opiate and stimulus-produced analgesia: functional anatomy of a medullospinal pathway. Proc Natl Acad Sci U S A. 1976;73(12):4685–8. doi: 10.1073/pnas.73.12.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Salomons TV, Moayedi M, Weissman-Fogel I, Goldberg MB, Freeman BV, Tenenbaum HC, et al. Perceived helplessness is associated with individual differences in the central motor output system. The European journal of neuroscience. 2012;35(9):1481–7. doi: 10.1111/j.1460-9568.2012.08048.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.