Abstract
Purpose of review
Glucocorticoids (GCs) have been universally regarded as anti-inflammatory, however a considerable number of studies now demonstrate that under some conditions, GCs are capable of potentiating neuroinflammatory processes (i.e. priming), a permissive function of GCs. The present review addresses recent evidence that provides insight into the mechanism(s) of GC-induced neuroinflammatory priming.
Recent findings
GCs have been found to prime inflammasomes (i.e. NLRP3), which are intra-cellular multiprotein complexes that mediate pro-inflammatory processes. Inflammasomes are activated by products of stressed or damaged cells. Interestingly, these products (damage-associated molecular patterns) are induced by stress and mediate stress-induced neuroinflammatory priming.
Summary
In light of these findings, we propose a model of GC-induced neuroinflammatory priming whereby stress and GCs induce cellular damage/stress in the brain, the products of which prime the NLRP3 inflammasome. Thus, GC-induced priming of the NLRP3 inflammasome may mediate the potentiated neuroinflammatory response to a subsequent pro-inflammatory immune challenge. We propose that during a fight/flight response available energy stores should be diverted to defensive behaviors, and it might be after the emergency is over that resources should be shifted to recuperation and host defense against infection. This is the scenario that would be promoted by elevated GCs reducing ongoing inflammation while simultaneously priming the NLRP3 inflammasome.
Keywords: glucocorticoid, stress, microglia, neuroinflammation, priming
Introduction
Glucocorticoids (GCs) are considered, with few exceptions, to be anti-inflammatory [1]. Indeed, exogenous GCs are highly effective at suppressing inflammatory responses and play an important physiological role in the HPA response to pro-inflammatory stimuli (e.g. infection, sterile injury)[2]. However, mounting evidence now suggests that GCs, in some contexts, may promote or potentiate inflammatory immune responses [3], findings that have prompted a re-evaluation of the dogma that GCs are universally anti-inflammatory. The suggestion is that even though elevated GCs suppress ongoing inflammation through a set of well-known mechanisms, they may at the same time potentiate inflammation to subsequent challenges. This evidence comes primarily from studies focused on understanding the mechanisms involved in stress- and GC-induced priming of neuroinflammatory responses to a subsequent pro-inflammatory stimulus. Here we will provide an overview of the conditions under which these phenomena occur, a discussion that will serve as a basis for exploring the mechanistic underpinnings of GC potentiation of neuroinflammation.
Central nervous system (CNS) innate immunity and its primary immune effector cell, microglia, are key immunologic substrates for understanding how stress and GCs potentiate neuroinflammatory responses to pro-inflammatory challenges. Thus, a brief orientation on neuroinflammation and its main effector cell, microglia, will provide a springboard for examining the role of GCs in neuroinflammatory priming.
Microglia and neuroinflammation
Innate immunity is the first line of defense against infection. Within the CNS, microglia, as part of the myeloid lineage, constitute the predominant innate immune cell in the brain parenchyma and serve many functions including immunosurveillance for pathogens, cellular debris, apoptotic cells, and alterations in neuronal phenotype [4]. Other macrophage subtypes also serve a critical role in the brain’s innate immune response [5] and may contribute to the processes under discussion here. In the healthy CNS, microglia send out processes that sample the local environment at a rate of several times per second [6] and have been termed “surveillant” [4]. If microglia encounter an infectious agent or cellular debris, the cell undergoes rapid morphological and functional changes that include the synthesis and secretion of inflammatory mediators including pro-inflammatory cytokines (e.g., interleukin-1beta, IL-1β), chemokines, nitric oxide, prostaglandins, and reactive oxygen species. This response induces neuroinflammation. Of course, most infectious agents do not cross the blood brain barrier (BBB) and enter the brain. Rather, pathogenic stimuli such as bacteria typically stimulate an innate immune response in the periphery (outside the BBB) resulting in an inflammatory profile quite similar to that observed after microglia directly encounter an infectious agent. However, peripheral inflammatory mediators such as pro-inflammatory cytokines can induce the de novo production of pro-inflammatory cytokines in the brain through several routes of communication connecting peripheral and central innate immune responses. These include both humoral and neural routes of communication (See review by Maier and Watkins, [7]). Notably, pro-inflammatory cytokines induced in the brain orchestrate a constellation of physiological and behavioral modifications known as the sickness response. This response manifests as cognitive (memory alterations), affective (mood changes), vegetative (sleep and eating disturbances) and physiological (fever) endophenotypes, which play an adaptive role in an organism’s host defense against infection, trauma, and injury [8].
Microglia are complex, and it is common to consider whether these cells are activated classically or alternatively, each of which produces cells with different properties. However, recent views suggest that microglia can enter a spectrum of activation states, producing varying blends of pro- and anti-inflammatory products [9]. Of particular relevance here, these cells can enter a state characterized as primed [10]. Primed microglia undergo immunophenotypic changes such as cell surface up-regulation of myeloid markers (e.g. major histocompatibility complex II). Primed microglia do not produce inflammatory or anti-inflammatory products but, if further stimulated, produce exaggerated levels of inflammatory products. Interestingly, a primed microglia immunophenotype can also be induced by exposure to stress and GCs.
Stress- and GC-induced priming of neuroinflammation
The basic phenomenon of stress- and GC-induced neuroinflammatory priming involves the following general schema. Initially, an organism is exposed to an acute or chronic stressor, or for that matter exogenous GCs. After exposure to the stressor, the organism is given a peripheral immune challenge by administering a pro-inflammatory agent, which induces inflammatory mediators. Typically, the agent consists of lipopolysaccharide (LPS), which is a non-infectious component of gram-negative bacteria (i.e. E. coli) and highly effective at eliciting a pro-inflammatory response (e.g. IL-1β) in the brain via immune-to-brain communication. Peripheral LPS signals through Toll-like receptor-4 (TLR4) on peripheral innate immune cells such as macrophages and microglia in the brain [11]. Signaling through TLR4 induces activation of NF-κB, a transcription factor that is critical for pro-inflammatory cytokine transcription to occur [12]. Usually LPS is administered at least 24h after termination of the stressor. Inflammatory mediators are then measured in brain within hours (2–12) or sometimes days of LPS exposure. The end result is that prior exposure to a stressor potentiates the neuroinflammatory response to the immune challenge, thus indicating that stress induces a primed immunophenotype in the CNS.
Indeed, a considerable number of studies have demonstrated that exposure to acute and chronic stressors shifts the neuroimmune microenvironment towards a microglial activation state that predisposes the CNS to a heightened pro-inflammatory response (primed) if exposure to a subsequent pro-inflammatory challenge should occur (reviewed in Frank et al., 2013, [13*]). Moreover, a subset of these studies found that pharmacological blockade of GC signaling (GR antagonist RU486) prior to or during stress exposure resulted in an attenuation of the stress-induced potentiation of the neuroinflammatory response to an immune challenge. These findings suggest that stress-induced GCs were necessary for stress-induced priming of the neuroinflammatory response.
These findings raise the key question of which CNS immune substrate is primed by stress-induced GCs? Because the above studies administered the immune challenge (LPS) in vivo, it is not possible to determine which cell type(s) was primed by GCs as many different types of innate immune cells may contribute to the neuroinflammatory response. To address this question, we conducted a set of studies in which animals were either adrenalectomized (ADX) or administered a GC receptor antagonist (RU486) prior to acute stress exposure, with the purpose of suppressing the GC response or GC signaling due to stress [14**]. 24h after the stress session ended, hippocampal microglia were isolated and directly challenged with LPS. As noted above, microglia express the receptor for LPS (i.e. TLR4). Here, LPS was used to directly stimulate the microglia pro-inflammatory response. Importantly prior stress exposure potentiated the pro-inflammatory response of microglia to LPS, indicating that stress primes microglia. Furthermore, surgical (ADX) and pharmacological suppression (RU486) of stress-induced GCs blocked the potentiated pro-inflammatory response of microglia to LPS. These findings suggest that stress-induced GCs prime the pro-inflammatory response of microglia. Similarly, neuroinflammatory priming effects have also been observed by simply administering exogenous GCs.
GC-induced priming of neuroinflammation
Earlier studies demonstrated that prior exposure to acute or chronic exogenous GCs also potentiates the neuroinflammatory response to a subsequent immune challenge. In other words, exogenous GC exposure was sufficient to replicate the priming effects of stress on microglial and neuroinflammatory responses to an immune challenge. These studies are covered in a recent review by Bellavance and Rivest [2*] and will not be addressed here. Here, we will discuss several recent investigations that provide insight into potential mechanisms responsible for GC-induced neuroinflammatory priming. An investigation by Kelly and colleagues [15**] assessed the effects of chronic GC treatment on the neuroinflammatory effects of methamphetamine (METH). Animals were treated with a high concentration of GCs administered in their drinking water, which induces many of the immunosuppressive features of high dose GCs such as thymic involution. On day 7 of GC treatment, a pro-inflammatory dose of METH was administered subcutaneously and neuroinflammatory endpoints measured 12 and 72h later. Kelly and colleagues found that prior chronic GC exposure potentiated the neuroinflammatory response to METH in a number of brain regions. Moreover, GC pretreatment enhanced dopaminergic cell death due to METH treatment in striatum, as measured by tyrosine hydroxylase immuno-staining. Interestingly, similar findings have been reported using a chronic stressor regimen [16**]. De Pablos and colleagues exposed subjects to chronic variable stress for 9 days and were given an intra-cerebral immune challenge with LPS, rather than METH as above, which was delivered into the substantia nigra. Consistent with prior findings from their laboratory [17], chronic stress potentiated the neuroinflammatory response to LPS. Interestingly, exposure to chronic stress increased cell death as well as potentiated the LPS-induced cell death of dopaminergic neurons. These effects of chronic stress were blocked by treatment with the GC receptor antagonist RU486 suggesting that stress-induced GCs mediated the neuroinflammatory priming effects as well as the effects of stress on cell death.
It is notable that both Kelly et al. and De Pablos et al. found that stress and/or GCs potentiated dopaminergic cell death or damage. It is well established that products of dead or damaged cells are capable of inducing inflammation [18], and so it is possible that GC-induced cell damage may play a pivotal role in the priming effects of stress on neuroinflammation. The notion that cell death or damage can induce an inflammatory response was originally proposed by Matzinger (1994), who developed the danger model of immunogenicity [19]. This model proposes that the immune system will respond to a stimulus only if that stimulus results in the release of endogenous danger-associated molecular patterns (DAMPs), which signal cellular damage and activate the innate immune system [20]. The danger model is particularly relevant to pathophysiological conditions involving sterile injury or trauma, wherein an inflammatory event is induced in the absence of infection. The damaged tissue and cells then release DAMPs such as high mobility group box-1 (HMGB1)[20]. These DAMPs bind their cognate receptors on innate immune cells such as microglia, thereby inducing neuroinflammatory processes.
The findings above raise the possibility that stress-induced GCs may damage neurons and other cell types, which then release DAMPs. Subsequently, these molecules may prime microglia and other innate immune cells to pro-inflammatory agents. Indeed, we found that HMGB1 administered into the cerebrospinal fluid (i.e. intra-cisterna magna, ICM injection) primed the pro-inflammatory response of microglia [21**]. Further, we found that exposure to an acute stressor increased HMGB1 protein levels in the hippocampus immediately after the stress session, as well as 24hr later. In addition, we found that hippocampal microglia, isolated immediately after stress exposure, exhibited increased secretion of HMGB1 compared to control animals.
In light of these effects of stress on HMGB1, it was of interest to determine whether HMGB1 plays a role in stress-induced priming of the microglia pro-inflammatory response. Towards this aim, an HMGB1 antagonist was injected ICM immediately prior to stress exposure. Microglia were then isolated from hippocampus 24h after stress exposure and were challenged with LPS to probe for stress-induced priming. Indeed, blockade of HMGB1 signaling blocked the effect of stress on microglia priming, suggesting that HMGB1 mediates the priming effects of stress on microglia. However, in this study, stress-induced cell death or damage was not assessed, and so it is unclear whether these processes played a role in neuroinflammatory priming. Nonetheless, taken together, these findings implicate DAMPs as potential mediators of stress and GC-induced priming of neuroinflammatory processes. A key question that remains is the mechanism whereby GCs prime neuroinflammatory processes and the possible role of DAMPs in these processes.
Mechanisms of GC priming: the NLRP3 (Nucleotide-binding domain, Leucine-Rich Repeat, Pyrin domain containing proteins-3) inflammasome
Interestingly, GCs have been implicated in NLRP3 inflammasome priming [22*], which plays a pivotal role in the processing and maturation of the pro-inflammatory cytokine IL-1β [23]. Inflammasomes are intracellular multi-protein complexes involved in the activation of inflammatory caspases, which mediate the processing and maturation of pro-inflammatory cytokines. Importantly, the NLRP3 inflammasome is a sensor for a diverse array of DAMPs, including HMGB1, that have been implicated in the pathophysiology of sterile inflammatory diseases [24]. Formation and activation of the NLRP3 inflammasome requires both a priming step and a second activation step [25]. Priming of the NLRP3 inflammasome is induced by a stimulus that signals, in part, through TLR4 to increase NLRP3 expression to a critical level necessary for inflammasome formation. Of note, the DAMP HMGB1 exerts its pro-inflammatory effects through TLR4 [26]. If the inflammasome is then exposed to a subsequent activating signal, the primed increase in NLRP3 permits formation of a molecular complex with the adaptor apoptosis-associated speck-like protein (ASC), which recruits and cleaves pro-caspase-1 to mature caspase-1. Finally, caspase-1 converts pro-IL-1β to the mature and active form of IL-1β, which is released into the extra-cellular space.
Intriguingly, Busillo and colleagues found that GCs increase NLRP3 mRNA and protein in macrophages without inducing a pro-inflammatory response, an effect mediated by the GC receptor [27**]. If GC-exposed macrophages were subsequently challenged with the NLRP3 inflammasome-activating stimulus adenosine triphosphate (ATP), the pro-inflammatory response to ATP was amplified suggesting that GCs primed macrophages by increasing expression of NLRP3. We have also found that GC treatment of isolated microglia increases NLRP3 expression (unpublished observations). Moreover, we found similar results with chronic GC exposure [28**]. Animals were treated with varying concentrations of GCs in drinking water for 10 days. The high GC treatment condition potentiated the microglia pro-inflammatory response to LPS. Further, high GC treatment increased expression of NLRP3 in hippocampus and potentiated the LPS induction of NLRP3 in microglia.
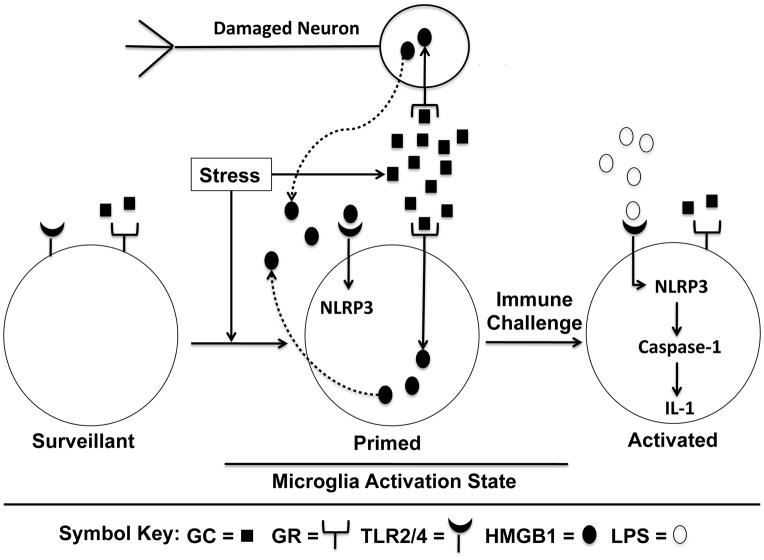
In light of these findings that GCs prime the NLRP3 inflammasome, we propose that GC priming of neuroinflammatory processes is mediated, in part, through priming of the NLRP3 inflammasome. Thus, if an organism is subsequently challenged with a pro-inflammatory stimulus (i.e. LPS), the NLRP3 inflammasome is activated resulting in a potentiated pro-inflammatory cytokine response. Finally, two lines of evidence converge to suggest that DAMPs such as HMGB1 may mediate GC-induced priming of the NLRP3 inflammasome. First, the pro-inflammatory effects of HMGB1 are mediated, in part, through TLR4 and second, the NLRP3 inflammasome is also primed through TLR4. Therefore, the following scenario of GC-induced neuroinflammatory priming is suggested (Fig. 1). During exposure to a stressor or exogenous GCs, GCs via the GC receptor induce the release of HMGB1 within the brain. HMGB1 may be actively released from innate immune cells (microglia) or passively released from damaged or stressed neurons. HMGB1 then signals through TLR4 on microglia to prime the NLRP3 inflammasome by increasing NLRP3 protein levels to a critical threshold. This results in a primed neuroinflammatory state. If a subsequent immune challenge occurs, the NLRP3 inflammasome is activated resulting in a potentiated pro-inflammatory cytokine response. This mechanism of priming may help explain the paradoxical effects of GCs in some clinical conditions, where GCs potentiate, rather than suppress pro-inflammatory processes.
Fig. 1. A proposed model of GC-induced neuroinflammatory priming.
Microglia are depicted in 3 activation states. Surveillant: Microglia serve a housekeeping function in the brain (e.g. removal of cellular debris) to maintain homeostasis. Primed: If an organism is exposed to stress or exogenous GCs, GC levels in the brain are increased resulting in a cascade of cellular events. 1) GCs ligate the low affinity GR located on multiple cell types including neurons and microglia, 2) ligation of the GR on neurons results in cellular stress or damage and the passive release of the DAMP HMGB1, while ligation of the GR on microglia leads to the active secretion of HMGB1, and 3) extra-cellular HMGB1 binds TLR4 leading to increased NLRP3 mRNA and protein. Activated: If an organism is subsequently exposed to a pro-inflammatory immune challenge (LPS), the NLRP3 inflammasome is activated resulting in activation of caspase-1, which cleaves pro-IL-1 to mature IL-1. IL-1 is then released to induce a cascade of pro-inflammatory events resulting in neuroinflammation. Abbreviations: GC, glucocorticoid; GR, glucocorticoid receptor; TLR, Toll-like receptor; DAMP, damage-associated molecular pattern; HMGB1, high mobility group box-1; LPS, lipopolysaccharide; NLRP3, Nucleotide-binding domain, Leucine-Rich Repeat, Pyrin domain containing proteins-3; IL-1, interleukin-1
Conclusion
From an adaptive point of view it has always seemed mysterious as to why GCs should inhibit innate immune responses since the likelihood of infection and injury should increase during a fight/flight emergency. Perhaps during such an emergency, available energy stores should be diverted to defensive behaviors, and it might be after the emergency is over that resources should be shifted to recuperation and host defense against infection. This is the scenario that would be promoted by elevated GCs reducing ongoing inflammation while simultaneously priming the NLRP3 inflammasome.
Key Points.
Stress-induced GCs prime neuroinflammatory responses to a subsequent immune challenge.
Exogenous GCs are also sufficient to prime neuroinflammatory responses to a subsequent immune challenge.
The NLRP3 inflammasome may mediate GC-induced priming of neuroinflammation.
GCs may serve an adaptive role by priming neuroinflammatory response to injury, trauma or infection, which are more likely to occur during a fight/flight response.
Acknowledgments
Financial support and sponsorship
The present work was supported by an NIH grant (R21MH096224) to MGF and SFM.
Footnotes
Conflicts of interest
None.
References
- 1.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factor-kappaB or activator protein-1: molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- *2.Bellavance MA, Rivest S. The HPA - Immune Axis and the Immunomodulatory Actions of Glucocorticoids in the Brain. Frontiers in immunology. 2014;5:136. doi: 10.3389/fimmu.2014.00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sorrells SF, Caso JR, Munhoz CD, Sapolsky RM. The stressed CNS: when glucocorticoids aggravate inflammation. Neuron. 2009;64:33–39. doi: 10.1016/j.neuron.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468:253–262. doi: 10.1038/nature09615. [DOI] [PubMed] [Google Scholar]
- 5.Schiltz JC, Sawchenko PE. Signaling the brain in systemic inflammation: the role of perivascular cells. Front Biosci. 2003;8:s1321–1329. doi: 10.2741/1211. [DOI] [PubMed] [Google Scholar]
- 6.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 7.Maier SF, Watkins LR. Immune-to-central nervous system communication and its role in modulating pain and cognition: Implications for cancer and cancer treatment. Brain Behav Immun. 2003;17 (Suppl 1):S125–131. doi: 10.1016/s0889-1591(02)00079-x. [DOI] [PubMed] [Google Scholar]
- 8.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perry VH. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 12.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- *13.Frank MG, Watkins LR, Maier SF. Stress-induced glucocorticoids as a neuroendocrine alarm signal of danger. Brain, behavior, and immunity. 2013;33:1–6. doi: 10.1016/j.bbi.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **14.Frank MG, Thompson BM, Watkins LR, Maier SF. Glucocorticoids mediate stress-induced priming of microglial pro-inflammatory responses. Brain, behavior, and immunity. 2012;26:337–345. doi: 10.1016/j.bbi.2011.10.005. This study demonstrated that stress-induced GCs play a mediating role in the priming effects of GCs on microglia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **15.Kelly KA, Miller DB, Bowyer JF, O’Callaghan JP. Chronic exposure to corticosterone enhances the neuroinflammatory and neurotoxic responses to methamphetamine. Journal of neurochemistry. 2012;122:995–1009. doi: 10.1111/j.1471-4159.2012.07864.x. This study demonstrated that chronic exposure to exogenous GCs was sufficient to prime the neuroinflammatory response as well as the neuronal damage to methamphetamine. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.de Pablos RM, Herrera AJ, Espinosa-Oliva AM, Sarmiento M, Munoz MF, Machado A, et al. Chronic stress enhances microglia activation and exacerbates death of nigral dopaminergic neurons under conditions of inflammation. J Neuroinflammation. 2014;11:34. doi: 10.1186/1742-2094-11-34. This study demonstrated that GCs mediate the priming effects of chronic stress on neuroinflammation and neuronal damage to LPS exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Pablos RM, Villaran RF, Arguelles S, Herrera AJ, Venero JL, Ayala A, et al. Stress increases vulnerability to inflammation in the rat prefrontal cortex. J Neurosci. 2006;26:5709–5719. doi: 10.1523/JNEUROSCI.0802-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kono H, Rock KL. How dying cells alert the immune system to danger. Nat Rev Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matzinger P. Tolerance, danger, and the extended family. Annual review of immunology. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leukoc Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- **21.Weber MD, Frank MG, Tracey KJ, Watkins LR, Maier SF. Stress Induces the Danger-Associated Molecular Pattern HMGB-1 in the Hippocampus of Male Sprague Dawley Rats: A Priming Stimulus of Microglia and the NLRP3 Inflammasome. J Neurosci. 2015;35:316–324. doi: 10.1523/JNEUROSCI.3561-14.2015. This study found that the DAMP HMGB1 is induced by stress and mediates the neuroinflammatory priming effects of stress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *22.Busillo JM, Cidlowski JA. The five Rs of glucocorticoid action during inflammation: ready, reinforce, repress, resolve, and restore. Trends Endocrinol Metab. 2013;24:109–119. doi: 10.1016/j.tem.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martinon F, Mayor A, Tschopp J. The inflammasomes: guardians of the body. Annual review of immunology. 2009;27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 24.Leemans JC, Cassel SL, Sutterwala FS. Sensing damage by the NLRP3 inflammasome. Immunological reviews. 2011;243:152–162. doi: 10.1111/j.1600-065X.2011.01043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hornung V, Latz E. Critical functions of priming and lysosomal damage for NLRP3 activation. Eur J Immunol. 2010;40:620–623. doi: 10.1002/eji.200940185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, et al. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **27.Busillo JM, Azzam KM, Cidlowski JA. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. The Journal of biological chemistry. 2011;286:38703–38713. doi: 10.1074/jbc.M111.275370. This study found that GC-induced priming of the NLRP3 inflammasome mediated the GC-induced potentiation of inflammatory processes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **28.Frank MG, Hershman SA, Weber MD, Watkins LR, Maier SF. Chronic exposure to exogenous glucocorticoids primes microglia to pro-inflammatory stimuli and induces NLRP3 mRNA in the hippocampus. Psychoneuroendocrinology. 2014;40:191–200. doi: 10.1016/j.psyneuen.2013.11.006. This study demonstrated that chronic exposure to exogenous GCs induces expression of NLRP3 while priming the microglial pro-inflammatory response to a subsequent immune challenge. [DOI] [PMC free article] [PubMed] [Google Scholar]