Abstract
Introduction
The eye is considered as the most privileged organ because of the blood–ocular barrier that acts as a barrier to systemically administered xenobiotics. However, there has been a significant increase in the number of reports on systemic drug-induced ocular complications. If such complications are left untreated, then it may cause permanent damage to vision. Hence, knowledge of most recent updates on ever-increasing reports of such toxicities has become imperative to develop better therapy while minimizing toxicities.
Areas covered
The article is mainly divided into anterior and posterior segment manifestations caused by systemically administered drugs. The anterior segment is further elaborated on corneal complications where as the posterior segment is focused on optic nerve, retinal and vitreous complications. Furthermore, this article includes recent updates on acute and chronic ocular predicaments, in addition to discussing various associated symptoms caused by drugs.
Expert opinion
Direct correlation of ocular toxicities due to systemic drug therapy is evident from current literature. Therefore, it is necessary to have detailed documentation of these complications to improve understanding and predict toxicities. We made an attempt to ensure that the reader is aware of the characteristic ocular complications, the potential for irreversible drug toxicity and indications for cessation.
Keywords: corneal toxicities, ocular complications, optic neuropathy, retinopathy, xenobiotics
1. Introduction
Patient safety is one of the primary concerns in drug therapy. In current treatment modalities, much emphasis is placed on medication errors, drug-dependent adverse events; information on adverse drug reactions and preventive strategies for drug-related adverse effects [1]. Ample disclosure on adverse reactions would make favorable impact on patient safety to a certain extent. Information on ocular adverse effects from systemically administered xenobiotics has gained recent attention.
Rich vasculature and miniature mass of the eye render it particularly vulnerable to various drug-induced toxicities. Xenobiotics or drug molecules administered systemically may selectively distribute into specific ocular tissues such as the cornea, lens, retina, and optic nerve leading to drug toxicity. Early detection of adverse reactions can prevent such ocular toxicity. If such reactions are ignored or undetected, toxicity may cause irreversible ocular damage, ultimately leading to vision or permanent loss [2,3].
Adverse ocular reaction can be a consequence of many factors. For example, use of a particular medicament for prolonged periods may increase the chances of ocular adverse effects. It may be perplexing to establish, whether a certain ocular pathology is due to current condition which is being treated or by drugs employed to treat such a condition. Also, systemic administration of a similar medication or a combination of various drugs may yield different adverse reactions depending on the age and health condition of the patients. Elderly patients often use multiple medicaments for prolonged periods. In such conditions, both metabolic and renal excretion rates may be affected by altered efficiency of the liver and/or kidney. Sometimes, drug response cannot be expected from drug levels alone. Genetic and environmental factors may cause unpredictable and irregular responses.
Incidence of adverse reactions is often directly associated with drug dosage. Current literature also confirms that most ocular adverse reactions occur when drug doses are typically above the therapeutic concentrations during a particular disease condition. Since the eye is a highly sensory organ, an introduction of newer systemic drugs and alterations in dosing regimens has caused an increase in ocular toxicities [2,4,5]. It is necessary for clinicians to determine whether the observed adverse reactions are due to initiation of drug therapy or caused by a change in dosage. Nevertheless, reactions can occur at any time during or after certain duration and may even persist for many years after drug cessation. However, anticipation of treatment-associated side effects may allow pharmacists or physicians to develop intervention strategies that could reduce or eliminate adverse toxicities. Though there are plethora of prescription and non-prescription drugs that can cause serious ocular drug toxicities, this review article is intended to describe toxicities affecting the cornea, retina, and optic nerve.
2. Corneal manifestations
Drugs administered by systemic route may reach the cornea via the tear film, aqueous humor and limbal vasculature, which may potentially lead to toxicity. The location and extent of corneal involvement often determine the corneal toxicity. All layers of the cornea can be affected by systemic medications. Corneal depositions of systemically administered xenobiotics often include corneal changes comprising epithelial, stromal, and endothelial alterations (Table 1). In most situations, corneal deposition can be resolved by cessation of drug therapy. Several studies suggest that the potential for alteration in corneal morphology is dependent on the chemical properties of the drugs rather than its pharmacologic action [6].
Table 1.
Ocular complications (Anterior segment) associated with systemically administered xenobiotics.
| Drug | Current Indication | Ophthalmic side effects | Remarks/Comments | Ref. |
|---|---|---|---|---|
| Isotretinoin | To treat psoriasis, cystic acne and various skin conditions | Corneal steepening, optic neuritis, dry eye, decrease in night vision and transitory myopia | Annual eye examination is recommended | [116,117] |
| Phenothiazines | To treat depression and anxiety | Corneal deposits, keratoconjunctivitis, abnormal pigmentation of the eyelids, night blindness and anterior subcapsular cataract | Dosage around 800 mg/day increase toxicity risks | [36,118] |
| Amiodarone | To suppress abnormal rhythms of the heart (cardiac arrhythmias) | Whorl keratopathy, optic neuropathy, and photophobia | Generally observed with 400–1,400 mg/day dosage | [119–121] |
| Digoxin | To treat congestive heart failure | Reddish-green color defects, flashing of light, hazy vision and xanthopsia | Visual changes are reversible | [122,123] |
| Hydroxychloroquine | To treat malaria, rheumatoid arthritis and systemic lupus erythematosus | Corneal deposits, bull’s eye maculopathy | Corneal drug deposits clear on stopping or reducing the dose of hydroxy-chloroquine | [124–126] |
| Topiramate | To treat epilepsy and preventing migraine headaches | Angle-closure glaucoma, bilateral anterior uveitis, massive choroidal effusion, ocular inflammatory reactions, visual field defects, probable effects on retina, cornea, and sclera, and neuroophthalmologic complications | Reversible ocular side effects when diagnosed early and discontinued in time | [127–129] |
| Amantadine | To reduce tremors of Parkinson’s disease | Corneal endothelial dysfunction, bilateral severe reversible corneal edema, visual loss, mydriasis, punctuate subepithelial opacities, stromal and epithelial edema and oculogyric crisis | On drug cessation, rapid resolution of corneal edema and recovery of visual acuity is observed | [130–133] |
| Rofecoxib | To treat inflammatory diseases | Corneal neovascularization and scarring, conjunctivitis and blurred vision | Transient inflammation of the conjunctiva is observed which resolves on drug cessation | [134,135] |
| Tamoxifen | Widely used in the management of breast cancer | Lens and corneal deposits | Yearly ophthalmic examinations are mandatory for patients on tamoxifen therapy | [17,136] |
| Cetrizine | Seasonal allergic rhinitis, perennial allergic rhinitis, and chronic urticaria | Pupillary changes, blurred vision, and keratoconjunctivitis sicca | Cuase imbalance between dopaminergic and cholinergic blockades Ocular side effects were cease upon discontinuation of therapy |
[137] |
2.1 Epithelial complications
The most common corneal epithelial complications include vortex keratopathy and formation of epithelial cysts.
2.1.1 Vortex keratopathy
Vortex keratopathy, also known as cornea verticillata, is typically differentiated by a distinctive bilateral corneal subepithelial whorl-like fashion of grayish or golden-brown deposits in corneal epithelium. Ocular manifestations such as cornea verticillata are common in Fabry’s disease and are often a consequence of progressive deposition of glycosphingolipids in various ocular structures. Other specific ocular complications of Fabry’s disease include conjunctival vascular abnormalities, lens opacities, and retinal vascular abnormalities [7]. Francois was the first one to report the corneal verticillata of Fabry’s disease that was observed with amiodarone and chloroquine therapies [8]. Currently, several systemic medications have been reported to cause intralysosomal accumulation of lipids and vortex keratopathy identical to Fabry’s disease.
The cationic, amphiphilic properties of a drug can allow it to penetrate lysosomes even though the molecules inducing lipidoses causes diverse pharmacologic actions. Upon lysosomal entry, active molecule or metabolites bind with cellular lipids resulting in the formation of drug–lipid complexes. Such complexes may lead to accumulation of lysosomal inclusions and corneal depositions due to their inability to evade lysosomes or resist enzymatic degradation. The whorl-like pattern may result from the centripetal migration of deposit-laden limbal epithelial cells [9,10]. However, the corneal deposits of drug-induced lipidoses seem to be resolved shortly after cessation of drug therapy.
A recent report demonstrated vortex keratopathy in both eyes of a patient treated with vandetanib, a dual epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor 2 (VEGFR2) inhibitor indicated in the treatment of non-small cell lung carcinoma (NSCLC) [11]. This report suggests that anti-EGFR properties which affects normal corneal epithelial cell migration and wound healing or drug-associated metabolite deposition may be responsible for the formation of this corneal toxicity. Other ocular complications due to the use of EGFR inhibitors include reversible recurrent corneal erosions, conjunctival hyperemia, telangiectasia of the eyelid margins, meibomitis, and tear film dysfunction. Also, tortuous eyelashes associated with gefitinib, corneal opacities with administration of high doses of EGFR inhibitors, trichomegaly and periorbital rash after erlotinib administration are reported [12–14]. Similarly, symptomatic corneal verticillata was observed after vandetanib therapy for anaplastic astrocytoma [15].
Tamoxifen, an estrogen receptor antagonist, is indicated in the treatment of breast cancer. It caused both keratopathy and retinopathy with both high-dose therapy of 180–200 mg/day and low-dose therapy [16,17]. In another study, corneal verticillata was reported in 10.8% of patients undergoing tamoxifen therapy [17,18]. Reversible and dose-related keratopathy may be attributed to the cationic amphiphilic property of the drug.
Amiodarone is an anti-arrhythmic drug to treat and prevent certain types of serious, life-threatening ventricular arrhythmias. It is often reported to cause vortex keratopathy within 1–4 months of the initiation of therapy [19–21]. Slit lamp examination of both eyes of a patient treated for 6 years showed a bilateral, symmetric, whorl-like pattern of brown deposits in the infero-central corneal epithelium. Also, diffuse and fine deposits with resemblance of keratic precipitation in the central portion of the endothelium are also detected [22]. Electron microscopy analysis of amiodarone-induced keratopathy has shown lipid-bearing intralysosomal inclusions in the corneal epithelium similar to that observed with Fabry’s disease [23,24]. Keratopathy associated with Fabry’s disease cannot be distinguished from amiodarone therapy by conventional slit-lamp microscopy. However, Wasielica-Poslednik et al. tried to compare the microstructure of Fabry-induced cornea verticillata with amiodarone-induced keratopathy by in vivo confocal laser-scanning microscopy (CLSM). This technique allows the differentiation between both etiologies in majority of patients, but does not allow quantitative monitoring of corneal changes in Fabry’s patients under enzyme replacement therapy [25]. Amiodarone was also found in tears and severe keratopathy has been reported in a patient wearing soft contact lenses, potentially secondary to trapping of tears [26]. This drug may also lead to anterior subcapsular lens opacities, retinopathy (choroidal neovascularization), and optic neuropathy [20,27,28].
2.1.2 Epithelial cysts
Cytarabine (cytosine arabinoside or Ara-C) is a powerful antimetabolite indicated in the treatment of acute myeloid leukemia (AML). It is a cell cycle (S phase)-specific drug that inhibits DNA synthesis [29]. Systemic use of high-dose intravenous cytarabine (> 1 g/m2) may cause corneal and conjunctival epithelial toxicity with conjunctival hyperemia, punctuate keratopathy, anterior uveitis, and fine refractile corneal epithelial microcysts [30–32]. Intermittent low-dose intravenous cytarabine therapy resulted in the formation of bilateral corneal epithelial microcysts. The formation of microcysts were more densely noted in the central region than the mid-periphery of cornea (Figure 1) [29]. Formation of epithelial microcysts by intense degeneration of the rapidly dividing basal epithelial cells was observed by histopathologic examination [32]. Upon intravenous administration, cytarabine penetrates the blood–ocular barrier, affecting cornea. The drug is also found in the aqueous and tears. Use of this agent may produce visual symptoms including rapid tear turn over, photophobia, foreign body sensation, pain and diminished visual acuity. Corneal toxicity has been reported to occur after 5–7 days of treatment initiation and can be prevented by the use of topical corticosteroids. The mechanism by which a topical corticosteroid reduces the toxic effects is unclear. Dosing of topical corticosteroids 1 day prior to cytarabine administration is often recommended to prevent or reduce the formation of corneal epithelial microcysts, keratopathy, and conjunctival hyperemia [33–35].
Figure 1.
Corneal epithelial microcysts visible with direct slit-beam illumination.
Reproduced with permission of Nature Publishing Group from Lochhead et al. [29].
2.2 Stromal complications
Potential drug entry to the cornea via aqueous humor, limbal vasculature, and tear film has led to the development of corneal stromal deposition. Drug deposits in the stroma are categorized predominantly as being pigmented, crystalline, or refractile. A variable visual impact of stromal drug deposition is observed even after the drug administration has been stopped.
2.2.1 Pigmented deposition
Pigmentary changes in the cornea and lens are typically due to drug or metabolite deposition through the aqueous humor. With low dosages, typical corneal changes involve brown granular pigments in posterior stroma, Descemet’s membrane, and endothelium. Anterior stroma, subepithelial layer, and epithelium may also be affected at higher dose ranges.
Chlorpromazine is one of the most common psychotropic agents which can potentially induce numerous and diverse unwanted ocular effects. At high dosages, the drug can commonly cause abnormal pigmentation of the eyelids, interpalpebral conjunctiva, lens, and cornea. It can also cause corneal edema [36]. Deposition of many white granules was observed in the superficial subepithelial corneal stroma by HRT II RCM in a patient receiving chlorpromazine therapy [37]. Chronic chlorpromazine therapy may cause corneal opacities, which can be seen with both direct and retro-illumination. Lenticular changes have been reported to occur at lower doses, resulting in anterior subcapsular opacities which can advance to form a stellate pattern.
Isotretinoin, an isomer of retinoic acid, is a well-known agent indicated in the treatment of severe recalcitrant cystic acne. Though this drug has proven to be very effective, it is frequently associated with adverse ocular effects such as conjunctivitis, blepharoconjunctivitis, dry eye, pseudotumor cerebri, eye irritation, decreased tolerance to contact lens, decreased vision, increased tear osmolarity, keratitis, myopia, ocular discomfort, ocular sicca, and several other corneal opacities [38–40]. Recent reports suggest additional adverse events associated with isotretinoin are including permanent sicca, corneal ulcers, diplopia, and eyelid edema [41]. Typically fine, diffuse gray deposits in the superficial stroma have been observed in patients treated with isotretinoin. These opacities do not impede vision and generally do not appear after treatment cessation (within 2–10 months). However, persistent and recurrent stromal opacities were reported 6 years after discontinuation of isotretinoin [38,39,42].
Clofazimine is a phenazine red dye derivative, recommended for the treatment of leprosy, psoriasis, pyoderma gangrenosum, and discoid lupus. Systemic administration of clofazimine has been reported to cause discoloration (reddish-brown) of the conjunctiva and cornea. Effect of clofazimine on eye was studied in 76 patients with multibacillary leprosy as part of multidrug therapy for 6–24 months. Reddish brown conjunctival and corneal pigmentation was evident in 46 and 53% of the patients, respectively. Also, clofazimine crystals in tears were found in 32% of the patients [43,44]. Ohman et al. reported that the use of 100–300 mg drug/day produced subepithelial deposits in 38.5% of the patients undergoing the treatment, while these deposits were lost following treatment termination [45]. Fine, brownish pigmented superficial lines in a whorl-like pattern were seen in 10.5% of psoriatic patients subjected to clofazimine treatment. No other functional disturbance was observed and the changes were reversible [46]. Clofazimine at a dosage of 100 mg twice daily produced polychromatic crystals in the cornea and perilimbal conjunctiva of both eyes. Crystalline deposits in the anterior stroma of cornea and conjunctiva were noted, which disappeared with discontinuation but reappeared upon reinitiation of clofazimine [47]. Similarly, long-term therapy with clofazimine resulted in numerous polychromatic crystalline deposits within the cornea and conjunctiva in a leprosy patient [48]. A severe case associated with clofazimine therapy has been reported to induce bull’s eye retinopathy. Such a treatment for 5 months showed bilateral anterior pigmentary corneal deposits in a whorl pattern and caused infectious retinitis in the left eye as well as bilateral annular macular pigmentary abnormalities [44].
2.2.2 Crystalline deposition
Crystalline keratopathy has been previously observed with the use of immunoglobulins in the management of pyoderma gangrenosum, where a corneal deposition was evident [49,50]. Crystalline deposition was observed after 4 years of intravenous treatment with immunoglobulin G (IgG). This deposition is thought to be originated from the limbal vessels. Also, a similar ring-like deposition was produced by subconjunctival injections of human IgG [51]. A 62-year-old female was diagnosed with monoclonal gammopathy of undetermined significance (MGUS)-associated crystalline keratopathy after corneal biopsy. MGUS is generally difficult to diagnose and may eventually progress to loss of vision [52]. Recent studies have shown atypical corneal immunoglobulin deposition in a patient with dysproteinemia. Amorphous, cloud-like opacities in the midperiphery at the level of deep stroma and Descemet’s membrane is evident by slit lamp examination. Immunotactoid keratopathy, a different form of paraprotein crystalline keratopathy associated with a monoclonal immunoglobulin G kappa light chain (IgGk) protein has been reported [53–55].
2.2.3 Refractile deposition
Systemic administration of colloidal gold salts generally indicated for the treatment of rheumatoid arthritis may allow gold deposition in the cornea. This deposition is termed as chrysiasis. At a dosage of > 1 g, most patients demonstrate this type of deposition in their corneas, devoid of inflammation. Higher deposition was found in the posterior stroma relative to the endothelium and Descemet’s membrane. Prolonged therapy may cause lenticular chrysiasis with anterior subcapsular deposition [56–58].
However, a recent study documented gold deposits with high reflectivity particularly in the anterior stroma in a patient treated with gold sodium thiomalate [59]. Gold deposits started appearing when dosage of sodium thiomalate exceeded 100 mg. Initial appearance was observed after 7 months of therapy and persisted even after 9 months following treatment cessation. Fortunately, no increase in the density of deposit was observed probably due to the effect of epithelial turnover [57,60]
2.3 Endothelial complications
Endothelial complications are very minimal relative to epithelial or stromal complications in terms of drug-related deposition affects. However, rifabutin, a derivative of rifampin, indicated for the treatment and prophylaxis of Mycobacterium Avium Complex (MAC) infections has caused endothelial deposition. MAC infections are commonly found in AIDS patients. The most common side effect associated with rifabutin in both immunocompromised and immunocompetent patients is the hypopyon uveitis [61–64]. A number of patients with fine, diffuse, white, stellate corneal endothelial deposits occurring predominantly in the periphery were noted. These deposits were found to be associated with rifabutin use independent of the presence of CMV retinitis [65,66]. Several studies have demonstrated that the normal laser cell flare photometry readings confirmed the absence of aqueous inflammation. In due course, these deposits appear to assume a golden color even after the cessation of rifabutin. Moreover, deposition in anterior lens and a reversible retinal dysfunction have been noted. Long-term treatment with rifabutin may also allow the drug to accumulate irreversibly on the posterior surface of the cornea and on the anterior surface of the lens [65,67,68]. Despite many observations, the mechanism underlying the endothelial deposition still remains unclear.
3. Optic neuropathy
Optic neuropathy is a physiologic condition which is characterized by a functional disturbance or pathologic changes in the optic nerve. It mainly involves optic nerve dysfunctioning and damages. Optic nerve damage can occur in presence of various toxic substances, shock, radiation, or trauma. Optic neuropathy can be classified as hereditary (leber’s hereditary optic neuropathy, congenital recessive optic atrophy, apparent sex-linked optic atrophy, dominant optic atrophy, and autosomal recessive chiasmal optic neuropathy) or acquired (vascular disturbances due to occlusions of the central retinal vein or artery or arteriosclerotic changes within the optic nerve itself, degenerative retinal disease, e.g., optic neuritis, pressure against the optic nerve, metabolic diseases, e.g., diabetes, glaucoma, and toxicity due to alcohol, tobacco, or other poisons). Such drug-induced optic neuropathy is often reversible upon drug withdrawal and eventually eases the associated symptoms. Therefore, it is important to be aware of the adverse effects and symptoms associated with optic neuropathy at the initial stage in order to avoid any further damage to the optic nerve (Table 2).
Table 2.
List of xenobiotics causing optic nerve toxicity upon systemic administration.
| Drug | Symptoms | Pathogenesis | Ref. |
|---|---|---|---|
| Methotrexate | Optic disc edema, centrocaceal scotoma, increased lacrimation, photophobia, reduce visual acuity and vision loss | Not clear but thought to be demyelinating neuropathy | [138,139] |
| Sildenafil | Decreased vision, change in color perception (typically blue tinge to vision), increase sensitivity to light | Increased blood flow velocity in retrobulbar and choroid circulation | [140–142] |
| Infliximab | Headache, blurred vision, bilateral optic neuritis, optic disc edema, minor hemorrhages at disc margin | Demyelination of optic nerve | [143,144] |
| Bisphosphonate (Alendronate and Pamidronate sodium) | Eyelid edema, optic neuritis, periorbital edema, scleritis and uveitis | Not known | [145–147] |
| Hepatitis B vaccination | Optic disc edema, Parapapillary hemorrhage, acute placoid pigment epitheliopathy and optic inflammation | Not known | [148,149] |
| Isoniazid | Bilateral hyperemic optic discs, blurred vision and single flame-shaped hemorrhage in the right eye | Isoniazid blockade of pyridoxal phosphate synthesis which depletes neurotransmitters | [150] |
Linezolid, an oxazolidinone derivative, is an antibacterial agent used for the treatment of severe infections of gram positive bacteria (streptococci, vancomycin-resistant enterococci (VRE), and methicillin-resistant staphylococcus aureus (MRSA)) that are resistant to other antimicrobial therapies. Following few months of treatment, linezolid may cause reversible optic neuropathy and irreversible peripheral neuropathy. Linezolid-induced optic neuropathy appears to be dependent on the duration of treatment. Optic neuropathy was observed in patients on this drug ranging from 5 to 10 months (mean of 9 months) [69,70]. Linezolid also causes symmetric painless visual obstructions, bilateral visual field defect, swollen or pale optic disc, bilateral central scotomas, and peripheral neuropathy [70–72].
Amiodarone, a potent antiarrhythmic medication, has been recommended in certain types of life-threatening ventricular arrhythmias and atrial fibrillation. Amiodarone, a cationic amphophilic moiety, has a long half-life (21–78 days) and high interaction with polar lipids. These characteristics lead to subsequent accumulation of amiodarone and its metabolites as lysosomal inclusion bodies in multiple tissues, including the optic nerve [73,74]. Moreover, the simultaneous occurrence of optic neuropathy in both eyes upon administration of amiodarone and reversal upon withdrawal of therapy clearly indicate a relation between them. In contrast to other drug-induced optic neuropathies, amiodarone optic neuropathy is not a dose-dependent phenomenon, and has no proven pathogenic mechanism. Amiodarone optic neuropathy is often characterized by gradual onset, slow progression, bilateral vision loss with prolonged disc swelling. On the other hand, non-arteric ischemic optic neuropathy which is associated with amiodarone usage is often characterized by acute, unilateral vision loss, disk edema that resolves over several weeks [73,75,76]. The appearance of optic nerve head in both the conditions is similar and sometimes indistinguishable (Figure 2) [76]. Amiodarone cause the visual acuity loss ranging from 0 to 11.4% in patients. Amiodarone therapy is sometimes not interchangeable with other alternative drugs and therefore baseline ophthalmic examination and frequent monitoring (every 6 months) is highly recommended for such patients [71].
Figure 2.
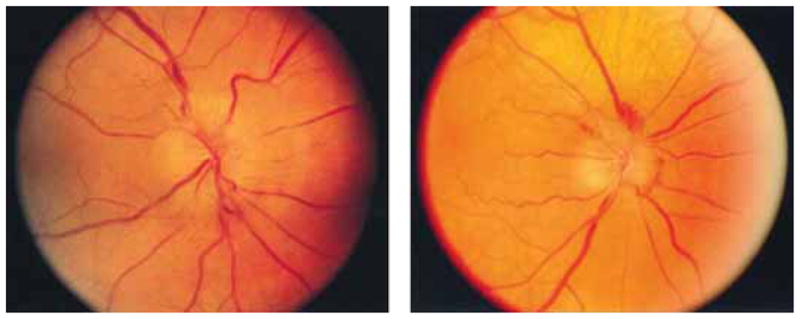
(Left) An optic nerve appearance in non-arteritic ischemic optic neuropathy. (Right) An optic nerve in amiodarone induced optic neuropathy has a similar appearance.
Reproduced with permission of Elsevier from Macaluso et al. [76].
Ethambutol is an antimycobacterial drug commonly indicated as first-line defense against tuberculosis. This treatment can be associated with toxic effect of optic neuropathy, which is generally uncommon and mostly reversible upon withdrawal. Incidence of optic neuropathy in patients on ethambutol is dose-dependent and the average duration of therapy until optic neuropathy onsets is around 7–8 months [71]. Incidence of ethambutol-induced optic neuropathy may occur in up to 6% at a daily dose of 25 mg/kg and rare with daily dose not exceeding 15 mg/kg. However, the incidence of ethambutol-induced ocular toxicity is highly unpredictable. Toxicity includes loss of visual function, visual field defect, decrease nerve fiber thickness, normal or slightly swollen optic discs, central scotomas, impaired red-green color vision, and damage to optic nerve [77–80]. Moreover, ethambutol induced rapid “bleaching” or “decoloration” of tapetal fundus which is completely reversible upon withdrawal even after prolonged dosing in dogs [81,82]. The mechanism of ethambutol toxicity is still not clear, however, it chelates with copper in the retinal ganglionic cells and their axons in the optic nerve. This chelation may affect cytochrome c oxidase activity in the mitochondria, which may lead to optic neuropathy [83].
Carboplatin, a platinum-based drug, is a chemotherapeutic agent applied for the treatment of solid tumors like ovarian carcinoma, lung, head, and neck cancers. Long-term therapy of carboplatin causes various ocular complications including visual obstruction. Ocular complications include severe orbital inflammation that resulted in a loss of visual acuity, proptosis, optic neuropathy, and loss of eye reflexes and movement [84]. However, the patients overcome such ocular toxicities after a few months of treatment cessation.
4. Retinal manifestations
Blood–retinal barrier is the major hurdle for systemically administered drugs to enter into the retina. However, drug molecules permeate into the retina via the circulatory system. In addition, a wide variety of systemically administered drugs can generate retinal toxicity (Table 3). However, in a majority of the cases, toxicity-induced loss of visual function is minimal or reversible following discontinuation of therapy.
Table 3.
List of xenobiotics causing retinal and choroidal toxicity upon systemic administration.
| Drug | Current indication | Ocular side effects | Ref. |
|---|---|---|---|
| Didanosine | Use as antiretroviral agent for the treatment of HIV infection in children | Peripheral retinal degeneration, retinal pigment epithelium atrophy, optic neuritis and night blindness | [151] |
| Rofecoxib (Vioxx) | Rheumatoid arthritis, acute pain condition and dysmenorrhea | Retinal vein occlusion and numerous flecked hemorrhage, painless decrease in vision | [152] |
| Cidofovir and ganciclovir | In patients with acquired immune deficiency (AIDS) and bilateral cytomegalovirus retinitis (CMV) | Hypermetropia with a choroidal edema, bilateral cataract and uveitis | [153] |
| Cisplatin | Use in treatment of small-cell lung cancer, ovarian cancer and lymphomas | Macular pigment changes, peripheral neuropathy | [104] |
| Doxorubicin | Breast cancer, ovarian cancer, and pancreatic cancer | Maculopathy, enhanced desferroxamine-induced blindness, visual field defects and retinal pigment changes | [104] |
4.1 Retinopathy
Retinopathy is an indication of damage to the retina. Drugs involved in retinal disorders can cause visual damage, which is sometimes irreversible. In drug-induced retinopathy, the retina suffers from degeneration, edema, alterations in the pigment, detachment, inflammation, hemorrhages, and crystalline deposits.
Vigabatrin, an analog of gamma amino butyric acid (GABA), irreversibly inhibits GABA transminase and is considered as an antiepileptic drug. Visual dysfunction is a common side effect with vigabatrin. Many recent reports suggest that vigabatrin induces retinopathy. The incidence of retinopathy in patients with vigabatrin is around 30–40% and the loss of visual function is irreversible. Patients on vigabatrin treatment suffer from visual degradation, hazziness, and loss in the field of vision. A previous published report has demonstrated that vigabatrin in rabbit causes changes in retinal immunohistopathology characterized by an enhancement of the glial cells located in the peripheral parts of the retina, which may explain the mechanism of visual field defect found in patients on vigabatrin [85].
Interferon (INF) is a small protein that induces production of cytokine via immune system in response to viral infections. INF therapy has been used to treat several types of infections such as chronic hepatitis B & C, genital warts, leukemia, AIDS-related Kaposi’s sarcoma, and malignant melanoma. A previous report has suggested that increase in retinal blood flow and retinal wall shear rate in patients with INF therapy indicates that retinal vascular endothelial dysfunction may be associated with INF-induced retinopathy [86]. Incidence of retinopathy is reported to be dependent on initial dose of INF-α and patients receiving high dosages are usually at increased risk of retinopathy. However, this condition is reversible upon discontinuation of INF therapy [87]. Ocular manifestations include bilateral cotton wool spot, retinal hemorrhage and edema, retinal microaneurisms, central retinal vein occlusion, and loss of visual acuity.
Tamoxifen, a triphenylethylene nonsteroid estrogen antagonist, is widely indicated for the treatment of breast cancer. Several reports suggested that tamoxifen increases cell membrane fluidity and protein kinase c activity which influences the rod outer segment binding and ingestion by retinal pigment epithelial cells, suggesting membrane-mediated pathways may contribute to the tamoxifen-induced retinopathy [88]. Tamoxifen can cause severe bilateral pigmentary and crystalline retinopathy and such patients require discontinuing therapy [17,89]. Tamoxifen is metabolized by cytochrome P450 and myeloperoxidase. The resulting metabolite is then conjugated with glutathione causing the depletion of glutathione resulting in oxidative stress and tissue damage, a possible mechanism of tamoxifen-induced retinopathy [90]. Tamoxifen-induced ocular toxicities are associated with a cumulative dose of 100 g or more. In contrast with current therapeutic regimens, rare cases of crystalline retinal deposition (characterized by retinal crystalline deposits that can be localized in the macular area or sometime in the whole retina) and pigmentary changes of the macula such as macular edema have been reported with long-term (typically greater than 2 years) use of tamoxifen [91]. Along with retinopathy, tamoxifen therapy may also cause visual field loss and obstruction [92].
Nitrofurantoin, a synthetic nitrofuran, is an antibiotic generally used in the treatment and prophylaxis of urinary tract infections. Long-term use may result in visual impairment associated with superficial and deep intraretinal glistering crystalline deposits, distributed throughout the retina [93]. Apart from the crystalline deposits, nitrofurantoin therapy may cause peripheral neuropathy which is rare but a potentially reversible adverse effect [94].
4.2 Retinal pigment epithelium (RPE) detachment
Retinal detachment is a separation of the neurosensory retina from the retinal pigment epithelium (RPE) layer. It is a vision-degenerating situation that is considered among one of the few ocular emergencies. Detachment of neurosensory retina from the RPE can occur via different mechanism, e. g., rhegmatogenous, traction, exudative or serous, and combined traction-rhegmatogenous. However, the main cause of retinal detachment involves accumulation of subretinal fluids.
Serous or exudative retinal detachment may occur due to buildup of serous or hemorrhage fluid in the subretinal space because of hydrostatic pressure or inflammation. There are reports which indicate that long-term therapy of systemic corticosteroids causes severe, chronic and recurrent central serous chorioretinopathy (CSC) and occasionally exudative retinal detachment which may lead to vision loss [95]. The mechanism of corticosteroid-induced retinal detachment may involve therapy-induced CSC. It is thought to occur in the choroid, followed by the breakdown of the outer blood–retinal barrier in the RPE resulting in development of serous retinal detachment [96].
Sunitinib, a small molecule and multitargeted tyrosine kinase inhibitor, is given orally to treat metastatic renal cell carcinoma. It is administered at intermittent schedule of 4/2 (total 6 weeks, 4 weeks of therapy and 2 weeks off treatment) for the treatment of carcinoma. A recent report suggests that ocular examination shows reduction in visual acuity, bilateral neurosensory retinal detachment, and diffuse edema [97]. The primary mechanism of subretinal exudation detachment was thought to be due to changes in choroidal vascular permeability and perfusion. However, sunitinib-induced retinal detachment and loss of visual acuity seem to be reversible upon discontinuation of therapy [97].
4.3 Macular degeneration
Macula, a miniature and highly sensitive part, is the center of retina mainly responsible for sharp, clear, and straight-ahead vision. The center of the macula is called the fovea, an indentation, which is packed with cone cells and responsible for central and high-resolution vision. Any drug which exerts macular toxicity may cause persistent visual impairment and subsequently vision loss. Along with drugs, some of the oxidative stress can also induce macular degeneration through mitochondrial DNA damages [98].
Topiramate, an analogue of β-D-fructopyranose sulfamate, is prescribed as an anticonvulsant drug to treat epilepsy. Its anticonvulsant activity is attributed mainly due to Na+ channel blockade, activation of GABA receptors, and weak anti-carbonic anhydrase activity. The incidence of maculopathy in patients on topiramate therapy for symptomatic epilepsy has been observed [99]. Topiramate induced maculopathy and ocular complications seem to be irreversible even upon discontinuation. Ophthalmologic examination in patient shows ocular complications such as bilateral paracentral scotomas. Fundus examination indicates bilateral maculopathy. The mechanism behind topiramate-induced retinopathy is unclear; however, an interesting observation of oral administration of topiramate to rabbits is a significant reduction in retinal function demonstrated by changes in the electroretinogram. Large accumulation of gamma-amino butyric acid in the inner retina may explain the retinal accumulation and toxicity of systemic topiramate [100].
4.4 Bull’s eye maculopathy
A variety of different conditions can lead to the formation of bull’s eye maculopathy. This condition is usually referred to an area of center hyperpigmentation of the retina. It is surrounded by an annulus of hypopigmentation and rarely surrounded circumferentially by hyperpigmentation. The main causes of this type of maculopathy include central areolar choroidal-RPE dystrophy, concentric annular macular dystrophy, cone dystrophy, the Bardet-Biedl syndrome, and the Hallervoden-Spatz syndrome.
Chloroquine and hydroxychloroquine-induced bull’s eye maculopathy is very common in patients on long-term therapy. Chloroquine diphosphate has been a drug of choice in the treatment of various rheumatic diseases, including rheumatoid arthritis. A recent report suggests that patients on long-term chloroquine therapy developed chloroquine-induced maculopathy. Ocular manifestation involves initial chloroquine-induced maculopathy lesions, which results in loss of the foveal reflex, parafoveal retinal pigment epithelium irregularities, and light parafoveal hipocromic lesions [101]. Moreover, the report also indicates that the progression of bull’s eye maculopathy even after withdrawal of chloroquine demands systematic and frequent ophthalmologic examination particularly in high-risk patients [101].
5. Choroidal effusion/edema
The suprachoroidal space, approximately 30 μm thick, forms a transition and potential space between the choroid and sclera which is composed of fibrous connective tissue. Since there are virtually no capillaries or lymphatic spaces available to drain fluid that collects in this area, it accumulates and generates choroidal edema (Table 3). Choroidal effusion is a condition where there is an accumulation of fluid in the suprachoroidal space, i.e., between the choroid and sclera. Clinical features of choroidal effusions include ocular hypotony or scleral enfolding, ocular neoplasms, and inflammatory choroidal disorders. The clinical features of a choroidal effusion involves choroidal edema, shallow anterior chamber, low intraocular pressure, visualization of the ora serrata without scleral depression, Verhoeff’s streaks, and Hagen’s sign. Acetazolamide, a carbonic anhydrous inhibitor, is clinically used for the treatment of glaucoma, epilepsy, diuretics, and idiopathic intracranial hypertension. A clinical report suggests that patients unresponsive to intravenous dexamethasone therapy receiving oral acetazolaminde therapy after surgery to control glaucoma complains severe pain in the eye few hours after the therapy. Ophthalmologic examination shows bilateral choroidal effusion along with circumcorneal congestion, corneal edema, and elevated intraocular pressure [102].
Chlorthalidone is a diuretic which is clinically used to treat the hypertension. A clinical report suggests that chlorthalidone therapy may cause loss of vision due to the development of acute myopia during the treatment of systemic hypertension. Ophthalmologic examination of such case reveals ciliary spasm, shallow peripheral choroidal effusion, and retinal irregularities at the macula. Moreover, increase in macular thickness is also observed by optical coherence tomography. However, upon withdrawal of therapy these complications are reversed completely reversal in case of chlorthalidone [103].
6. Vitreous opacification
Carmustine, a β-chloro-nitrosourea compound, is used as an alkylating agent. This drug can penetrate strong barriers like blood–brain barrier, therefore it is widely indicated in the treatment of brain tumor, multiple myeloma, and malignant melanoma [104]. However, due to the excellent penetrability it can also cross blood–retinal barrier which may cause posterior segment ocular toxicities. Carmustine has been used alone or in combination following intravenous and intracarotid administration. Moreover, the occurrence of ocular side effects is dependent on the dose as well as the number of carmustine administration, and the rate of infusion. Most of the patients have shown ocular toxicities within 2–14 weeks of therapy. Ocular complications include vitreous opacification along with nonspecific blurred vision, corneal edema and opacities, internal ophthalmoplegia, optic neuritis, blindness with optic atrophy, and exudative retinopathy with exudates and hemorrhage [105,106].
7. Melanin binding and drug toxicity
Retinal pigment epithelium (RPE) is a monolayer of cuboidal cells, lying between choriocapillaris and light sensitive photoreceptors. The pigment melanin in the RPE plays an important role as photoprotective by absorbing radiation and scavenging free radicals and reactive oxygen species (ROS) [107,108]. Therapeutic agents can bind to ocular melanin. However, the mechanism of drug binding to melanin responsible for retinal toxicity still remains a topic of further research [109]. While some authors consider drug binding to melanin and ocular toxicity as two separate entities [110], the others consider that binding of drugs to melanin produce direct toxicities to retina [111–113].
Thioridazine, a phenothiazine, is an antipsychotic drug of low potency. It is an antipsychotic drug indicated for the treatment of schizophrenia and disorganization. Ocular toxicity of thioridazine is dose-dependent and at higher doses it can cause pigment disruption of the retina. Phenothiazines have shown binding affinity toward pigmented tissue and consequently remains bound to the RPE melanin [114]. It can lead to progressive retinal/macular degeneration and loss of visual acuity if not closely monitored and treated. Moreover, funduscopy revealed granular retinal pigmentary changes that form large plaques of depigmented RPE with loss of choriocapillaries [115].
There are many examples of structurally and pharmacologically unrelated agents that bind to melanin. Anti-muscarinic drug (atropine), β-blocker drug (timolol), CNS acting sympathomimetics (epinephrine) and antibiotics (fluoroquinolones) are considered to be “gentle,” than anti-psychotics (chlorpromazine), and anti-inflammatory (chloroquine and hydroxychloroquine), which binds to melanin for months to a year after single administration. However, melanin binding is not a true predication of retinal toxicity [110]. It is evident that drug-related toxic effects on the retina are dependent on intrinsic toxicity of the drugs rather than its ability to bind to melanin. Moreover, melanin binding has also been found to be protective against ocular toxicity of a few agents [110].
8. Conclusion
The overall purpose of clinical trial and safety management is to assure maximum benefit from the treatment while balancing drug-associated risk factors. Unlike the toxicity to other tissues such as kidney, liver or heart, toxicity associated with the eye is usually not predictive for short-term systemic exposure of xenobiotics or their metabolites. Prediction and evaluation of ocular adverse reactions upon systemic drug administration require careful examination of prevalent symptoms following long-term medication history. This review provides a critical insight about ocular adverse reactions following systemic administration of several important therapeutic agents. This information may be helpful to guide ophthalmologist for screening potential drug-induced toxicities and suggesting possible alternative therapies. The side effects of cornea, lens, retina, and optic nerve are crucial and if left untreated may impart vision loss. Corneal manifestations (corneal epithelium, stroma, and endothelial) and retinal and optic nerve complications associated with systemic medication possess immense threat to vision loss. In addition, the knowledge of microscopic and clinical examination of these complications will further help to avoid vision deterioration.
9. Expert opinion
There have been increasing reports of ocular complications due to systemic drug therapy over the last decade. Knowledge of these complications paved a path to minimize the strenuous efforts of ophthalmologists in identifying the cause of ocular ailments and maximized the treatment options for effective therapy. However, ophthalmologic examinations and frequent clinical monitoring of ocular complications associated with these xenobiotics is an important aspect of long term systemic therapy. It is imperative that the physician should have information about the nature (reversible/irreversible toxicity) and duration of occurrence of complications which will not only help them to adjust the doses but will provide directions to consider an alternative therapy.
Article highlights.
Ocular complications are vision threatening outcomes of chronic systemic drug therapy.
The anterior segment complications include corneal toxicities (epithelium, stroma, and endothelium), uveitis and conjunctivitis.
The location and extent of corneal involvement determines the corneal drug toxicity. All layers of the cornea are affected by systemic medications with differential impact on visual acuity.
Though corneal deposition is not a typical indicator for drug cessation, it should be considered for any lenticular changes as well as reversible or irreversible corneal adverse effects including epithelial, stromal and endothelial manifestations.
Patient discomfort, photophobia and glare are typical features of corneal deposition.
The posterior segment complications include toxicities to sclera, retina, optic nerve, macula, and vitreous fluid.
Some ocular complications are reversible, i.e., the symptoms are relieved upon withdrawal of drug therapy whereas few complications are not.
Vision threatening complications of posterior segment of the eye includes retinal toxicities (especially retinal detachment and maculopathy) and optic nerve complications.
This box summarizes key points contained in the article.
Footnotes
Declaration of interest
This work is supported by NIH grants R01 EY 09171-16 and R01 EY 10659-14.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Brick DC. Medication errors result in costly claims for ophthalmologists. Surv Ophthalmol. 1995;40(3):232–6. doi: 10.1016/s0039-6257(95)80031-x. [DOI] [PubMed] [Google Scholar]
- 2••.Santaella RM, Fraunfelder FW. Ocular adverse effects associated with systemic medications: recognition and management. Drugs. 2007;67(1):75–93. doi: 10.2165/00003495-200767010-00006. An important review highlighting several retrospective cases and reported adverse effects. [DOI] [PubMed] [Google Scholar]
- 3•.Moorthy RS, Valluri S. Ocular toxicity associated with systemic drug therapy. Curr Opin Ophthalmol. 1999;10(6):438–46. doi: 10.1097/00055735-199912000-00012. This article highlights some of the systemic drug induced ocular side effects. [DOI] [PubMed] [Google Scholar]
- 4•.Blomquist PH. Ocular complications of systemic medications. Am J Med Sci. 2011;342(1):62–9. doi: 10.1097/MAJ.0b013e3181f06b21. Focus on common ocular side effects are discussed. [DOI] [PubMed] [Google Scholar]
- 5.Garralda Luquin A. Ocular toxicity induced by medication. An Sist Sanit Navar. 2008;31(Suppl 3):147–53. [PubMed] [Google Scholar]
- 6.Hollander DA, Aldave AJ. Drug-induced corneal complications. Curr Opin Ophthalmol. 2004;15(6):541–8. doi: 10.1097/01.icu.0000143688.45232.15. [DOI] [PubMed] [Google Scholar]
- 7.Sodi A, Ioannidis A, Pitz S. Ophthalmological manifestations of Fabry disease. In: Mehta A, Beck M, Sunder-Plassmann G, editors. Fabry Disease: Perspectives from 5 Years of FOS. Chapter 26 Oxford: Oxford PharmaGenesis; 2006. [Google Scholar]
- 8.Francois J. Cornea verticillata. Bull Soc Belge Ophtalmol. 1968;150:656–70. [PubMed] [Google Scholar]
- 9.Dua HS, Singh A, Gomes JA, et al. Vortex or whorl formation of cultured human corneal epithelial cells induced by magnetic fields. Eye (Lond) 1996;10(Pt 4):447–50. doi: 10.1038/eye.1996.98. [DOI] [PubMed] [Google Scholar]
- 10.D’Amico DJ, Kenyon KR. Drug-induced lipidoses of the cornea and conjunctiva. Int Ophthalmol. 1981;4(1–2):67–76. doi: 10.1007/BF00139581. [DOI] [PubMed] [Google Scholar]
- 11.Ahn J, Wee WR, Lee JH, Hyon JY. Vortex keratopathy in a patient receiving vandetanib for non-small cell lung cancer. Korean J Ophthalmol. 2011;25(5):355–7. doi: 10.3341/kjo.2011.25.5.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang G, Basti S, Jampol LM. Acquired trichomegaly and symptomatic external ocular changes in patients receiving epidermal growth factor receptor inhibitors: case reports and a review of literature. Cornea. 2007;26(7):858–60. doi: 10.1097/ICO.0b013e318064584a. [DOI] [PubMed] [Google Scholar]
- 13.Tullo AB, Esmaeli B, Murray PI, et al. Ocular findings in patients with solid tumours treated with the epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (‘Iressa’, ZD1839) in Phase I and II clinical trials. Eye (Lond) 2005;19(7):729–38. doi: 10.1038/sj.eye.6701630. [DOI] [PubMed] [Google Scholar]
- 14.Yano S, Kondo K, Yamaguchi M, et al. Distribution and function of EGFR in human tissue and the effect of EGFR tyrosine kinase inhibition. Anticancer Res. 2003;23(5A):3639–50. [PubMed] [Google Scholar]
- 15.Yeh S, Fine HA, Smith JA. Corneal verticillata after dual anti-epidermal growth factor receptor and anti-vascular endothelial growth factor receptor 2 therapy (vandetanib) for anaplastic astrocytoma. Cornea. 2009;28(6):699–702. doi: 10.1097/ICO.0b013e3181922146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salomao SR, Watanabe SE, Berezovsky A, Motono M. Multifocal electroretinography, color discrimination and ocular toxicity in tamoxifen use. Curr Eye Res. 2007;32(4):345–52. doi: 10.1080/02713680701229638. [DOI] [PubMed] [Google Scholar]
- 17.Noureddin BN, Seoud M, Bashshur Z, et al. Ocular toxicity in low-dose tamoxifen: a prospective study. Eye (Lond) 1999;13(Pt 6):729–33. doi: 10.1038/eye.1999.217. [DOI] [PubMed] [Google Scholar]
- 18.Pavlidis NA, Petris C, Briassoulis E, et al. Clear evidence that long-term, low-dose tamoxifen treatment can induce ocular toxicity. A prospective study of 63 patients. Cancer. 1992;69(12):2961–4. doi: 10.1002/1097-0142(19920615)69:12<2961::aid-cncr2820691215>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 19.Ingram DV, Jaggarao NS, Chamberlain DA. Ocular changes resulting from therapy with amiodarone. Br J Ophthalmol. 1982;66(10):676–9. doi: 10.1136/bjo.66.10.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dolan BJ, Flach AJ, Peterson JS. Amiodarone keratopathy and lens opacities. J Am Optom Assoc. 1985;56(6):468–70. [PubMed] [Google Scholar]
- 21.Harris L, McKenna WJ, Rowland E, Krikler DM. Side effects and possible contraindications of amiodarone use. Am Heart J. 1983;106(4 Pt 2):916–23. doi: 10.1016/0002-8703(83)90016-9. [DOI] [PubMed] [Google Scholar]
- 22.Erdurmus M, Selcoki Y, Yagci R, Hepsen IF. Amiodarone-induced keratopathy: full-thickness corneal involvement. Eye Contact Lens. 2008;34(2):131–2. doi: 10.1097/ICL.0b013e31814934c0. [DOI] [PubMed] [Google Scholar]
- 23.Haug SJ, Friedman AH. Identification of amiodarone in corneal deposits. Am J Ophthalmol. 1991;111(4):518–20. doi: 10.1016/s0002-9394(14)72398-0. [DOI] [PubMed] [Google Scholar]
- 24.D’Amico DJ, Kenyon KR, Ruskin JN. Amiodarone keratopathy: drug-induced lipid storage disease. Arch Ophthalmol. 1981;99(2):257–61. doi: 10.1001/archopht.1981.03930010259007. [DOI] [PubMed] [Google Scholar]
- 25.Wasielica-Poslednik J, Pfeiffer N, Reinke J, Pitz S. Confocal laser-scanning microscopy allows differentiation between Fabry disease and amiodarone-induced keratopathy. Graefes Arch Clin Exp Ophthalmol. 2011;249(11):1689–96. doi: 10.1007/s00417-011-1726-5. [DOI] [PubMed] [Google Scholar]
- 26.Rivera RP, Younge BR, Dyer JA. Atypical amiodarone-induced keratopathy in a patient wearing soft contact lenses. CLAO J. 1989;15(3):219–21. [PubMed] [Google Scholar]
- 27.Thystrup JD, Fledelius HC. Retinal maculopathy possibly associated with amiodarone medication. Acta Ophthalmol (Copenh) 1994;72(5):639–41. doi: 10.1111/j.1755-3768.1994.tb07194.x. [DOI] [PubMed] [Google Scholar]
- 28••.Feiner LA, Younge BR, Kazmier FJ, et al. Optic neuropathy and amiodarone therapy. Mayo Clin Proc. 1987;62(8):702–17. doi: 10.1016/s0025-6196(12)65224-0. This was the earliest finding suggesting that all patients who receive amiodarone should undergo complete ophthalmologic examinations, including careful evaluation of the ocular funduscopy. [DOI] [PubMed] [Google Scholar]
- 29.Lochhead J, Salmon JF, Bron AJ. Cytarabine-induced corneal toxicity. Eye (Lond) 2003;17(5):677–8. doi: 10.1038/sj.eye.6700451. [DOI] [PubMed] [Google Scholar]
- 30.Fintelmann RE, Qian Y, Skalet A, Jeng BH. Anterior uveitis associated with high-dose cytosine arabinoside. Ocul Immunol Inflamm. 2010;18(6):485–7. doi: 10.3109/09273948.2010.510258. [DOI] [PubMed] [Google Scholar]
- 31.Guthoff T, Tietze B, Meinhardt B, et al. Cytosine-arabinoside-induced keratopathy: a model of corneal proliferation kinetics. Ophthalmologica. 2010;224(5):308–11. doi: 10.1159/000298751. [DOI] [PubMed] [Google Scholar]
- 32.Hopen G, Mondino BJ, Johnson BL, Chervenick PA. Corneal toxicity with systemic cytarabine. Am J Ophthalmol. 1981;91(4):500–4. doi: 10.1016/0002-9394(81)90240-3. [DOI] [PubMed] [Google Scholar]
- 33.Lazarus HM, Hartnett ME, Reed MD, et al. Comparison of the prophylactic effects of 2-deoxycytidine and prednisolone for high-dose intravenous cytarabine-induced keratitis. Am J Ophthalmol. 1987;104(5):476–80. doi: 10.1016/s0002-9394(14)74104-2. [DOI] [PubMed] [Google Scholar]
- 34.Kumar L, Dua H, Agarwal S, et al. Ocular toxicity of low dose cytosar. NZ Med J. 1987;100(825):361. [PubMed] [Google Scholar]
- 35.Lass JH, Lazarus HM, Reed MD, Herzig RH. Topical corticosteroid therapy for corneal toxicity from systemically administered cytarabine. Am J Ophthalmol. 1982;94(5):617–21. doi: 10.1016/0002-9394(82)90006-x. [DOI] [PubMed] [Google Scholar]
- 36••.Richa S, Yazbek JC. Ocular adverse effects of common psychotropic agents: a review. CNS Drugs. 2010;24(6):501–26. doi: 10.2165/11533180-000000000-00000. A comprehensive review highlighting the unwanted ocular effects frequently associated with administration of psychotropic drugs. [DOI] [PubMed] [Google Scholar]
- 37.Toshida H, Uesugi Y, Ebihara N, Murakami A. In vivo observations of a case of chlorpromazine deposits in the cornea using an HRT II Rostock corneal module. Cornea. 2007;26(9):1141–3. doi: 10.1097/ICO.0b013e318124a42b. [DOI] [PubMed] [Google Scholar]
- 38.Fraunfelder FT, Fraunfelder FW, Edwards R. Ocular side effects possibly associated with isotretinoin usage. Am J Ophthalmol. 2001;132(3):299–305. doi: 10.1016/s0002-9394(01)01024-8. [DOI] [PubMed] [Google Scholar]
- 39.Fraunfelder FT, LaBraico JM, Meyer SM. Adverse ocular reactions possibly associated with isotretinoin. Am J Ophthalmol. 1985;100(4):534–7. doi: 10.1016/0002-9394(85)90676-2. [DOI] [PubMed] [Google Scholar]
- 40.Rumsfield JA, West DP, Tse CS, et al. Isotretinoin in severe, recalcitrant cystic acne: a review. Drug Intell Clin Pharm. 1983;17(5):329–33. doi: 10.1177/106002808301700502. [DOI] [PubMed] [Google Scholar]
- 41.Labiris G, Katsanos A, Karapetsa M, et al. Association between isotretinoin use and central retinal vein occlusion in an adolescent with minor predisposition for thrombotic incidents: a case report. J Med Case Reports. 2009;3:58. doi: 10.1186/1752-1947-3-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ellies P, Dighiero P, Legeais JM, et al. Persistent corneal opacity after oral isotretinoin therapy for acne. Cornea. 2000;19(2):238–9. doi: 10.1097/00003226-200003000-00020. [DOI] [PubMed] [Google Scholar]
- 43.Kaur I, Ram J, Kumar B, et al. Effect of clofazimine on eye in multibacillary leprosy. Indian J Lepr. 1990;62(1):87–90. [PubMed] [Google Scholar]
- 44.Craythorn JM, Swartz M, Creel DJ. Clofazimine-induced bull’s-eye retinopathy. Retina. 1986;6(1):50–2. [PubMed] [Google Scholar]
- 45.Ohman L, Wahlberg I. Letter: ocular side-effects of clofazimine. Lancet. 1975;2(7941):933–4. doi: 10.1016/s0140-6736(75)92180-7. [DOI] [PubMed] [Google Scholar]
- 46.Walinder PE, Gip L, Stempa M. Corneal changes in patients treated with clofazimine. Br J Ophthalmol. 1976;60(7):526–8. doi: 10.1136/bjo.60.7.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Font RL, Sobol W, Matoba A. Polychromatic corneal and conjunctival crystals secondary to clofazimine therapy in a leper. Ophthalmology. 1989;96(3):311–15. doi: 10.1016/s0161-6420(89)33071-5. [DOI] [PubMed] [Google Scholar]
- 48.Barot RK, Viswanath V, Pattiwar MS, Torsekar RG. Crystalline deposition in the cornea and conjunctiva secondary to long-term clofazimine therapy in a leprosy patient. Indian J Ophthalmol. 2011;59(4):328–9. doi: 10.4103/0301-4738.82012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Budde M, Gusek-Schneider GC, Mayer U, Seitz B. Annular crystalline keratopathy in association with immunoglobulin therapy for pyoderma gangrenosum. Cornea. 2003;22(1):82–5. doi: 10.1097/00003226-200301000-00021. [DOI] [PubMed] [Google Scholar]
- 50.Ormerod LD, Collin HB, Dohlman CH, et al. Paraproteinemic crystalline keratopathy. Ophthalmology. 1988;95(2):202–12. doi: 10.1016/s0161-6420(88)33200-8. [DOI] [PubMed] [Google Scholar]
- 51.Osusky R, Morell A, Imbach P, Lerch PG. Diffusion of immunoglobulins into rabbit cornea after subconjunctival injection: experimental demonstration and mathematical model. Graefes Arch Clin Exp Ophthalmol. 1993;231(2):122–8. doi: 10.1007/BF00920226. [DOI] [PubMed] [Google Scholar]
- 52.Koo H, Oh DH, Chun YS, Kim JC. A case of crystalline keratopathy in monoclonal gammopathy of undetermined significance (MGUS) Korean J Ophthalmol. 2011;25(3):202–5. doi: 10.3341/kjo.2011.25.3.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lisch W, Saikia P. Immunotactoid microtubular corneal deposits in bilateral paraprotein crystalline keratopathy and atypical corneal immunoglobulin deposition in a patient with dysproteinemia. Cornea. 2011;30(2):247. doi: 10.1097/ICO.0b013e3181d52931. author reply -8. [DOI] [PubMed] [Google Scholar]
- 54.Matoba AY, Chevez-Barrios P, Jones DB. Atypical corneal immunoglobulin deposition in a patient with dysproteinemia. Cornea. 2010;29(1):105–7. doi: 10.1097/ICO.0b013e31819e34ab. [DOI] [PubMed] [Google Scholar]
- 55.Singh K. Immunotactoid microtubular corneal deposits in bilateral paraprotein crystalline keratopathy. Cornea. 2009;28(7):829–31. doi: 10.1097/ICO.0b013e318191449a. [DOI] [PubMed] [Google Scholar]
- 56.McCormick SA, DiBartolomeo AG, Raju VK, Schwab IR. Ocular chrysiasis. Ophthalmology. 1985;92(10):1432–5. doi: 10.1016/s0161-6420(85)33846-0. [DOI] [PubMed] [Google Scholar]
- 57.Bron AJ, McLendon BF, Camp AV. Epithelial deposition of gold in the cornea in patients receiving systemic therapy. Am J Ophthalmol. 1979;88(3 Pt 1):354–60. doi: 10.1016/0002-9394(79)90633-0. [DOI] [PubMed] [Google Scholar]
- 58.Hashimoto A, Maeda Y, Ito H, et al. Corneal chrysiasis. A clinical study in rheumatoid arthritis patients receiving gold therapy. Arthritis Rheum. 1972;15(3):309–15. doi: 10.1002/art.1780150313. [DOI] [PubMed] [Google Scholar]
- 59.Paladini I, Menchini U, Mencucci R. Corneal chrysiasis: in vivo confocal microscopy analysis. Eur J Ophthalmol. 2010;20(4):776–9. doi: 10.1177/112067211002000421. [DOI] [PubMed] [Google Scholar]
- 60.Lopez JD, del Castillo JM, Lopez CD, Sanchez JG. Confocal microscopy in ocular chrysiasis. Cornea. 2003;22(6):573–5. doi: 10.1097/00003226-200308000-00017. [DOI] [PubMed] [Google Scholar]
- 61.Bhagat N, Read RW, Rao NA, et al. Rifabutin-associated hypopyon uveitis in human immunodeficiency virus-negative immunocompetent individuals. Ophthalmology. 2001;108(4):750–2. doi: 10.1016/s0161-6420(00)00586-8. [DOI] [PubMed] [Google Scholar]
- 62.Schaller UC, Michl G, Goebel FD, Klauss V. Acute hypopyon uveitis with rifabutin therapy of systemic Mycobacterium avium complex (MAC) infection in AIDS. Ophthalmologe. 1999;96(4):267–9. doi: 10.1007/s003470050404. [DOI] [PubMed] [Google Scholar]
- 63.Frau E, Gregoire-Cassoux N, Hannouche D, et al. Uveitis with hypopyon in patients with acquired immunodeficiency syndrome, treated with Rifabutin. J Fr Ophtalmol. 1995;18 (6–7):435–8. [PubMed] [Google Scholar]
- 64.Saran BR, Maguire AM, Nichols C, et al. Hypopyon uveitis in patients with acquired immunodeficiency syndrome treated for systemic Mycobacterium avium complex infection with rifabutin. Arch Ophthalmol. 1994;112(9):1159–65. doi: 10.1001/archopht.1994.01090210043015. [DOI] [PubMed] [Google Scholar]
- 65.Arevalo JF, Freeman WR. Corneal endothelial deposits in children positive for human immunodeficiency virus receiving rifabutin prophylaxis for Mycobacterium avium complex bacteremia. Am J Ophthalmol. 2000;129(3):410–11. doi: 10.1016/s0002-9394(00)00343-3. [DOI] [PubMed] [Google Scholar]
- 66.Holland SP, Chang CW, Vagh M, Courtright P. Corneal endothelial deposits in patients with HIV infection or AIDS: epidemiologic evidence of the contribution of rifabutin. Can J Ophthalmol. 1999;34(4):204–9. [PubMed] [Google Scholar]
- 67.Ponjavic V, Granse L, Bengtsson Stigmar E, Andreasson S. Retinal dysfunction and anterior segment deposits in a patient treated with rifabutin. Acta Ophthalmol Scand. 2002;80(5):553–6. doi: 10.1034/j.1600-0420.2002.800519.x. [DOI] [PubMed] [Google Scholar]
- 68.Smith JA, Mueller BU, Nussenblatt RB, Whitcup SM. Corneal endothelial deposits in children positive for human immunodeficiency virus receiving rifabutin prophylaxis for Mycobacterium avium complex bacteremia. Am J Ophthalmol. 1999;127(2):164–9. doi: 10.1016/s0002-9394(98)00310-9. [DOI] [PubMed] [Google Scholar]
- 69.Nambiar S, Rellosa N, Wassel RT, et al. Linezolid-associated peripheral and optic neuropathy in children. Pediatrics. 2011;127(6):e1528–382. doi: 10.1542/peds.2010-2125. [DOI] [PubMed] [Google Scholar]
- 70.Rucker JC, Hamilton SR, Bardenstein D, et al. Linezolid-associated toxic optic neuropathy. Neurology. 2006;66(4):595–8. doi: 10.1212/01.wnl.0000201313.24970.b8. [DOI] [PubMed] [Google Scholar]
- 71••.Li J, Tripathi RC, Tripathi BJ. Drug-induced ocular disorders. Drug Saf. 2008;31(2):127–41. doi: 10.2165/00002018-200831020-00003. This article had broad information on the most commonly recognized drug-induced ocular disorders, their specific clinical features, the medications that can cause the problem, the differential diagnosis and possible mechanisms of action, as well as guidelines for the management of the adverse reactions. [DOI] [PubMed] [Google Scholar]
- 72.Lee E, Burger S, Shah J, et al. Linezolid-associated toxic optic neuropathy: a report of 2 cases. Clin Infect Dis. 2003;37(10):1389–91. doi: 10.1086/379012. [DOI] [PubMed] [Google Scholar]
- 73•.Le-Nguyen XT, Lee TK, Do DV, et al. Diagnostic and therapeutic challenges. Retina. 2011;31(8):1732–9. doi: 10.1097/IAE.0b013e31820f4825. This report is interesting because it discuss the opinion from the several expert in the field of ophthalmology and highlights the need to obtain a complete history, including medical history, of every patient. [DOI] [PubMed] [Google Scholar]
- 74.Arnold AC. Ischemic optic neuropathies. Ophthalmol Clin North Am. 2001;14(1):83–98. [PubMed] [Google Scholar]
- 75.Sharma P, Sharma R. Toxic optic neuropathy. Indian J Ophthalmol. 2011;59(2):137–41. doi: 10.4103/0301-4738.77035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76••.Macaluso DC, Shults WT, Fraunfelder FT. Features of amiodarone-induced optic neuropathy. Am J Ophthalmol. 1999;127(5):610–12. doi: 10.1016/s0002-9394(99)00016-1. An important article dealing with clinical features of amiodarone induced optic neuropathy. [DOI] [PubMed] [Google Scholar]
- 77.Kokkada SB, Barthakur R, Natarajan M, et al. Ocular side effects of antitubercular drugs - a focus on prevention, early detection and management. Kathmandu Univ Med J (KUMJ) 2005;3(4):438–41. [PubMed] [Google Scholar]
- 78.Vistamehr S, Walsh TJ, Adelman RA. Ethambutol neuroretinopathy. Semin Ophthalmol. 2007;22(3):141–6. doi: 10.1080/08820530701457134. [DOI] [PubMed] [Google Scholar]
- 79.Chai SJ, Foroozan R. Decreased retinal nerve fibre layer thickness detected by optical coherence tomography in patients with ethambutol-induced optic neuropathy. Br J Ophthalmol. 2007;91(7):895–7. doi: 10.1136/bjo.2006.113118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan RY, Kwok AK. Ocular toxicity of ethambutol. Hong Kong Med J. 2006;12(1):56–60. [PubMed] [Google Scholar]
- 81.Dillberger JE, Peiffer RL, Dykstra MJ, et al. The experimental antipsychotic agent 1192U90 targets tapetum lucidum in canine eyes. Toxicol Pathol. 1996;24(5):595–601. doi: 10.1177/019262339602400509. [DOI] [PubMed] [Google Scholar]
- 82.Vogel AW, Kaiser JA. Ethambutol-induced transient change and reconstitution (in Vivo) of the tapetum lucidum color in the dog. Exp Mol Pathol. 1963;26:136–49. [PubMed] [Google Scholar]
- 83.Kozak SF, Inderlied CB, Hsu HY, et al. The role of copper on ethambutol’s antimicrobial action and implications for ethambutol-induced optic neuropathy. Diagn Microbiol Infect Dis. 1998;30(2):83–7. doi: 10.1016/s0732-8893(97)00217-4. [DOI] [PubMed] [Google Scholar]
- 84.Lauer AK, Wobig JL, Shults WT, et al. Severe ocular and orbital toxicity after intracarotid etoposide phosphate and carboplatin therapy. Am J Ophthalmol. 1999;127(2):230–3. doi: 10.1016/s0002-9394(98)00346-8. [DOI] [PubMed] [Google Scholar]
- 85.Ponjavic V, Granse L, Kjellstrom S, et al. Alterations in electroretinograms and retinal morphology in rabbits treated with vigabatrin. Doc Ophthalmol. 2004;108(2):125–33. doi: 10.1023/b:doop.0000036780.96560.74. [DOI] [PubMed] [Google Scholar]
- 86.Nagaoka T, Sato E, Takahashi A, et al. Retinal circulatory changes associated with interferon-induced retinopathy in patients with hepatitis C. Invest Ophthalmol Vis Sci. 2007;48(1):368–75. doi: 10.1167/iovs.06-0182. [DOI] [PubMed] [Google Scholar]
- 87.Kadayifcilar S, Boyacioglu S, Kart H, et al. Ocular complications with high-dose interferon alpha in chronic active hepatitis. Eye (Lond) 1999;13(Pt 2):241–6. doi: 10.1038/eye.1999.59. [DOI] [PubMed] [Google Scholar]
- 88•.Engelke M, Tykhonova S, Zorn-Kruppa M, Diehl H. Tamoxifen induces changes in the lipid composition of the retinal pigment epithelium cell line D407. Pharmacol Toxicol. 2002;91(1):13–21. doi: 10.1034/j.1600-0773.2002.910103.x. This was first ever report suggesting that membrane-mediated pathways contribute to the tamoxifen-induced retinopathy. The mechanism involved compensatory decrease in the cholesterol content of the plasma membrane, reduction of phosphatidylcholine content by 50%, formation of a second messenger via phospholipase pathway and sustained activation of protein kinase C. [DOI] [PubMed] [Google Scholar]
- 89.Heier JS, Dragoo RA, Enzenauer RW, Waterhouse WJ. Screening for ocular toxicity in asymptomatic patients treated with tamoxifen. Am J Ophthalmol. 1994;117(6):772–5. doi: 10.1016/s0002-9394(14)70321-6. [DOI] [PubMed] [Google Scholar]
- 90.Toler SM. Oxidative stress plays an important role in the pathogenesis of drug-induced retinopathy. Exp Biol Med (Maywood) 2004;229(7):607–15. doi: 10.1177/153537020422900704. [DOI] [PubMed] [Google Scholar]
- 91.Drenser K, Sarraf D, Jain A, Small KW. Crystalline retinopathies. Surv Ophthalmol. 2006;51(6):535–49. doi: 10.1016/j.survophthal.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 92.Parkkari M, Paakkala AM, Salminen L, Holli K. Ocular side-effects in breast cancer patients treated with tamoxifen and toremifene: a randomized follow-up study. Acta Ophthalmol Scand. 2003;81(5):495–9. doi: 10.1034/j.1600-0420.2003.00116.x. [DOI] [PubMed] [Google Scholar]
- 93.Ibanez HE, Williams DF, Boniuk I. Crystalline retinopathy associated with long-term nitrofurantoin therapy. Arch Ophthalmol. 1994;112(3):304–5. doi: 10.1001/archopht.1994.01090150034012. [DOI] [PubMed] [Google Scholar]
- 94••.Kammire LD, Donofrio PD. Nitrofurantoin neuropathy: a forgotten adverse effect. Obstet Gynecol. 2007;110(2 Pt 2):510–12. doi: 10.1097/01.AOG.0000267134.21517.41. This report indicate nitrofurantoin-induced rare and potentially reversible adverse effect, peripheral neuropathy, unreported in the obstetric and gynecologic literature, and commonly unrecognized by physicians who prescribe it. This report can be useful for the physicians who are unaware of such ocular side effects of nitrofurantoin. [DOI] [PubMed] [Google Scholar]
- 95.Loo JL, Lee SY, Ang CL. Can long-term corticosteriods lead to blindness? A case series of central serous chorioretinopathy induced by corticosteroids. Ann Acad Med Singapore. 2006;35(7):496–9. [PubMed] [Google Scholar]
- 96.Kishi S, Yoshida O, Matsuoka R, Kojima Y. Serous retinal detachment in patients under systemic corticosteroid treatment. Jpn J Ophthalmol. 2001;45(6):640–7. doi: 10.1016/s0021-5155(01)00428-2. [DOI] [PubMed] [Google Scholar]
- 97.Wegner A, Khoramnia R. Neurosensory retinal detachment due to sunitinib treatment. Eye (Lond) 2011;25(11):1517–18. doi: 10.1038/eye.2011.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Barot M, Gokulgandhi MR, Mitra AK. Mitochondrial dysfunction in retinal diseases. Curr Eye Res. 2011;36(12):1069–77. doi: 10.3109/02713683.2011.607536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Beyenburg S, Weyland C, Reuber M. Presumed topiramate-induced maculopathy. Epilepsy Behav. 2009;14(3):556–9. doi: 10.1016/j.yebeh.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 100.Kjellstrom S, Bruun A, Isaksson B, et al. Retinal function and histopathology in rabbits treated with Topiramate. Doc Ophthalmol. 2006;113(3):179–86. doi: 10.1007/s10633-006-9027-8. [DOI] [PubMed] [Google Scholar]
- 101.Shinjo SK, Maia OO, Jr, Tizziani VA, et al. Chloroquine-induced bull’s eye maculopathy in rheumatoid arthritis: related to disease duration? Clin Rheumatol. 2007;26(8):1248–53. doi: 10.1007/s10067-006-0478-9. [DOI] [PubMed] [Google Scholar]
- 102.Parthasarathi S, Myint K, Singh G, et al. Bilateral acetazolamide-induced choroidal effusion following cataract surgery. Eye (Lond) 2007;21(6):870–2. doi: 10.1038/sj.eye.6702741. [DOI] [PubMed] [Google Scholar]
- 103.Mahesh G, Giridhar A, Saikumar SJ, Fegde S. Drug-induced acute myopia following chlorthalidone treatment. Indian J Ophthalmol. 2007;55(5):386–8. doi: 10.4103/0301-4738.33830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104••.Schmid KE, Kornek GV, Scheithauer W, Binder S. Update on ocular complications of systemic cancer chemotherapy. Surv Ophthalmol. 2006;51(1):19–40. doi: 10.1016/j.survophthal.2005.11.001. This review article will be interesting for physicians to observe any sign of ocular side effects in the patients who are under chemotherapy. This article provides update on ophthalmic complications of currently used cytotoxic chemotherapeutics based on their category and anatomical ocular parts. [DOI] [PubMed] [Google Scholar]
- 105.Hilliard LM, Berkow RL, Watterson J, et al. Retinal toxicity associated with cisplatin and etoposide in pediatric patients. Med Pediatr Oncol. 1997;28(4):310–13. doi: 10.1002/(sici)1096-911x(199704)28:4<310::aid-mpo12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 106.Shingleton BJ, Bienfang DC, Albert DM, et al. Ocular toxicity associated with high-dose carmustine. Arch Ophthalmol. 1982;100(11):1766–72. doi: 10.1001/archopht.1982.01030040746007. [DOI] [PubMed] [Google Scholar]
- 107.Boulton M, Rozanowska M, Rozanowski B. Retinal photodamage. J Photochem Photobiol B. 2001;64(2–3):144–61. doi: 10.1016/s1011-1344(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 108.Rozanowska M, Sarna T, Land EJ, Truscott TG. Free radical scavenging properties of melanin interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free Radic Biol Med. 1999;26(5–6):518–25. doi: 10.1016/s0891-5849(98)00234-2. [DOI] [PubMed] [Google Scholar]
- 109.Mecklenburg L, Schraermeyer U. An overview on the toxic morphological changes in the retinal pigment epithelium after systemic compound administration. Toxicol Pathol. 2007;35(2):252–67. doi: 10.1080/01926230601178199. [DOI] [PubMed] [Google Scholar]
- 110.Leblanc B, Jezequel S, Davies T, et al. Binding of drugs to eye melanin is not predictive of ocular toxicity. Regul Toxicol Pharmacol. 1998;28(2):124–32. doi: 10.1006/rtph.1998.1243. [DOI] [PubMed] [Google Scholar]
- 111.Marmor MF, Carr RE, Easterbrook M, et al. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109(7):1377–82. doi: 10.1016/s0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 112.Eves P, Smith-Thomas L, Hedley S, et al. A comparative study of the effect of pigment on drug toxicity in human choroidal melanocytes and retinal pigment epithelial cells. Pigment Cell Res. 1999;12(1):22–35. doi: 10.1111/j.1600-0749.1999.tb00504.x. [DOI] [PubMed] [Google Scholar]
- 113.Larsson BS. Interaction between chemicals and melanin. Pigment Cell Res. 1993;6(3):127–33. doi: 10.1111/j.1600-0749.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 114.Buszman E, Rozanska R. Interaction of thioridazine with ocular melanin in vitro. Acta Pol Pharm. 2003;60(4):257–61. [PubMed] [Google Scholar]
- 115.To TQ, Townsend JC. Ocular toxicity of systemic medications: a case series. Optometry. 2000;71(1):29–39. [PubMed] [Google Scholar]
- 116.Brelsford M, Beute TC. Preventing and managing the side effects of isotretinoin. Semin Cutan Med Surg. 2008;27(3):197–206. doi: 10.1016/j.sder.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Santodomingo-Rubido J, Barrado-Navascues E, Rubido-Crespo MJ. Drug-induced ocular side-effects with isotretinoin. Ophthalmic Physiol Opt. 2008;28(5):497–501. doi: 10.1111/j.1475-1313.2008.00590.x. [DOI] [PubMed] [Google Scholar]
- 118.Choong YY, Lim KS. Phenothiazine deposits in corneo and lens. Med J Malaysia. 2001;56(1):92–4. [PubMed] [Google Scholar]
- 119.Bratulescu M, Zemba M, Gheorghieva V, et al. Ocular manifestation in amiodarone toxicity–case report. Oftalmologia. 2005;49(4):18–23. [PubMed] [Google Scholar]
- 120.Chilov MN, Moshegov CN, Booth F. Unilateral amiodarone keratopathy. Clin Experiment Ophthalmol. 2005;33(6):666–8. doi: 10.1111/j.1442-9071.2005.01121.x. [DOI] [PubMed] [Google Scholar]
- 121.Domingues MF, Barros H, Falcao-Reis FM. Amiodarone and optic neuropathy. Acta Ophthalmol Scand. 2004;82(3 Pt 1):277–82. doi: 10.1111/j.1600-0420.2004.00255.x. [DOI] [PubMed] [Google Scholar]
- 122.Honrubia A, Andres JM, Alcaine F, et al. Visual disorders induced by therapeutic levels of digoxin. Arch Soc Esp Oftalmol. 2000;75(1):55–6. [PubMed] [Google Scholar]
- 123.Hobley A, Lawrenson J. Ocular adverse effects to the therapeutic administration of digoxin. Ophthalmic Physiol Opt. 1991;11(4):391–3. [PubMed] [Google Scholar]
- 124.Grierson DJ. Hydroxychloroquine and visual screening in a rheumatology outpatient clinic. Ann Rheum Dis. 1997;56(3):188–90. doi: 10.1136/ard.56.3.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Easterbrook M. Is corneal deposition of antimalarial any indication of retinal toxicity? Can J Ophthalmol. 1990;25(5):249–51. [PubMed] [Google Scholar]
- 126.Petrohelos MA. Chloroquine-induced ocular toxicity. Ann Ophthalmol. 1974;6(6):615–18. [PubMed] [Google Scholar]
- 127.Abtahi MA, Abtahi SH, Fazel F, et al. Topiramate and the vision: a systematic review. Clin Ophthalmol. 2012;6:117–31. doi: 10.2147/OPTH.S27695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pai K, Rajashekaran P. Glaucoma: adverse event on use of topiramate in alcohol de-addiction. Indian J Psychiatry. 2011;53(2):163–5. doi: 10.4103/0019-5545.82552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jabbarpoor Bonyadi MH, Soheilian R, Soheilian M. Topiramate-induced bilateral anterior uveitis associated with hypopyon formation. Ocul Immunol Inflamm. 2011;19(1):86–8. doi: 10.3109/09273948.2010.523805. [DOI] [PubMed] [Google Scholar]
- 130.Hood CT, Langston RH, Schoenfield LR, Dupps WJ., Jr Amantadine-associated corneal edema treated with descemet’s stripping automated endothelial keratoplasty. Ophthalmic Surg Lasers Imaging. 2010;41:1–4. doi: 10.3928/15428877-20100726-11. [DOI] [PubMed] [Google Scholar]
- 131.Esquenazi S. Bilateral reversible corneal edema associated with amantadine use. J Ocul Pharmacol Ther. 2009;25(6):567–70. doi: 10.1089/jop.2009.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chang KC, Kim MK, Wee WR, Lee JH. Corneal endothelial dysfunction associated with amantadine toxicity. Cornea. 2008;27(10):1182–5. doi: 10.1097/ICO.0b013e318180e526. [DOI] [PubMed] [Google Scholar]
- 133.Hughes B, Feiz V, Flynn SB, Brodsky MC. Reversible amantadine-induced corneal edema in an adolescent. Cornea. 2004;23(8):823–4. doi: 10.1097/01.ico.0000130417.91438.7e. [DOI] [PubMed] [Google Scholar]
- 134.Fraunfelder FW, Solomon J, Mehelas TJ. Ocular adverse effects associated with cyclooxygenase-2 inhibitors. Arch Ophthalmol. 2006;124(2):277–9. doi: 10.1001/archopht.124.2.277. [DOI] [PubMed] [Google Scholar]
- 135.Goldberg D, Panigrahi D, Barazi M, et al. A case of rofecoxib-associated stevens-johnson syndrome with corneal and conjunctival changes. Cornea. 2004;23(7):736–7. doi: 10.1097/01.ico.0000126330.77228.a3. [DOI] [PubMed] [Google Scholar]
- 136.Ah-Song R, Sasco AJ. Tamoxifen and ocular toxicity. Cancer Detect Prev. 1997;21(6):522–31. [PubMed] [Google Scholar]
- 137.Fraunfelder FW, Fraunfelder FT. Oculogyric crisis in patients taking cetirizine. Am J Ophthalmol. 2004;137(2):355–7. doi: 10.1016/S0002-9394(03)00869-9. [DOI] [PubMed] [Google Scholar]
- 138.Sbeity ZH, Baydoun L, Schmidt S, Loeffler KU. Visual field changes in methotrexate therapy. Case report and review of the literature. J Med Liban. 2006;54(3):164–7. [PubMed] [Google Scholar]
- 139.Balachandran C, McCluskey PJ, Champion GD, Halmagyi GM. Methotrexate-induced optic neuropathy. Clin Experiment Ophthalmol. 2002;30(6):440–1. doi: 10.1046/j.1442-9071.2002.00578.x. [DOI] [PubMed] [Google Scholar]
- 140.Harris A, Kagemann L, Ehrlich R, et al. The effect of sildenafil on ocular blood flow. Br J Ophthalmol. 2008;92(4):469–73. doi: 10.1136/bjo.2007.131789. [DOI] [PubMed] [Google Scholar]
- 141.Su DH, Ang PS, Tow SL. Bilateral posterior ischemic optic neuropathy associated with use of sildenafil. J Neuroophthalmol. 2008;28(1):75. doi: 10.1097/WNO.0b013e318167554a. [DOI] [PubMed] [Google Scholar]
- 142.Laties A, Sharlip I. Ocular safety in patients using sildenafil citrate therapy for erectile dysfunction. J Sex Med. 2006;3(1):12–27. doi: 10.1111/j.1743-6109.2005.00194.x. [DOI] [PubMed] [Google Scholar]
- 143.Chan JW, Castellanos A. Infliximab and anterior optic neuropathy: case report and review of the literature. Graefes Arch Clin Exp Ophthalmol. 2010;248(2):283–7. doi: 10.1007/s00417-009-1227-y. [DOI] [PubMed] [Google Scholar]
- 144.Mumoli N, Niccoli G, Scazzeri F, et al. Infliximab-induced retrobulbar optic neuritis. QJM. 2007;100(8):531. doi: 10.1093/qjmed/hcm060. [DOI] [PubMed] [Google Scholar]
- 145.McKague M, Jorgenson D, Buxton KA. Ocular side effects of bisphosphonates: a case report and literature review. Can Fam Physician. 2010;56(10):1015–17. [PMC free article] [PubMed] [Google Scholar]
- 146.Papapetrou PD. Bisphosphonate-associated adverse events. Hormones (Athens) 2009;8(2):96–110. doi: 10.14310/horm.2002.1226. [DOI] [PubMed] [Google Scholar]
- 147.Leung S, Ashar BH, Miller RG. Bisphosphonate-associated scleritis: a case report and review. South Med J. 2005;98(7):733–5. doi: 10.1097/01.SMJ.0000152753.80490.9F. [DOI] [PubMed] [Google Scholar]
- 148.Stewart O, Chang B, Bradbury J. Simultaneous administration of hepatitis B and polio vaccines associated with bilateral optic neuritis. Br J Ophthalmol. 1999;83(10):1200–1. doi: 10.1136/bjo.83.10.1194g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.van de Geijn EJ, Tukkie R, van Philips LA, Punt H. Bilateral optic neuritis with branch retinal artery occlusion associated with vaccination. Doc Ophthalmol. 1994;86(4):403–8. doi: 10.1007/BF01204599. [DOI] [PubMed] [Google Scholar]
- 150.Kulkarni HS, Keskar VS, Bavdekar SB, Gabhale Y. Bilateral optic neuritis due to isoniazid (INH) Indian Pediatr. 2010;47(6):533–5. doi: 10.1007/s13312-010-0083-5. [DOI] [PubMed] [Google Scholar]
- 151.Whitcup SM, Butler KM, Caruso R, et al. Retinal toxicity in human immunodeficiency virus-infected children treated with 2′,3′-dideoxyinosine. Am J Ophthalmol. 1992;113(1):1–7. doi: 10.1016/s0002-9394(14)75744-7. [DOI] [PubMed] [Google Scholar]
- 152.Meyer CH, Schmidt JC, Rodrigues EB, Mennel S. Risk of retinal vein occlusions in patients treated with rofecoxib (vioxx) Ophthalmologica. 2005;219(4):243–7. doi: 10.1159/000085735. [DOI] [PubMed] [Google Scholar]
- 153.Rapp P, Pilon F, Chiambaretta F, et al. Bilateral uveitis with definitive hypotony caused by systemic cidofovir. J Fr Ophtalmol. 2003;26(7):717–19. [PubMed] [Google Scholar]


