Abstract
Anatomy and physiology of the eye makes it a highly protected organ. Designing an effective therapy for ocular diseases, especially for the posterior segment, has been considered as a formidable task. Limitations of topical and intravitreal route of administration have challenged scientists to find alternative mode of administration like periocular routes. Transporter targeted drug delivery has generated a great deal of interest in the field because of its potential to overcome many barriers associated with current therapy. Application of nanotechnology has been very promising in the treatment of a gamut of diseases. In this review, we have briefly discussed several ocular drug delivery systems such as microemulsions, nanosuspensions, nanoparticles, liposomes, niosomes, dendrimers, implants, and hydrogels. Potential for ocular gene therapy has also been described in this article. In near future, a great deal of attention will be paid to develop non-invasive sustained drug release for both anterior and posterior segment eye disorders. A better understanding of nature of ocular diseases, barriers and factors affecting in vivo performance, would greatly drive the development of new delivery systems. Current momentum in the invention of new drug delivery systems hold a promise towards much improved therapies for the treatment of vision threatening disorders.
Keywords: nanotechnology, ocular drug delivery, transporter
Introduction
Ocular drug delivery has remained as one of the most challenging task for pharmaceutical scientists. The unique structure of the eye restricts the entry of drug molecules at the required site of action. Drug delivery to the eye can be broadly classified into anterior and posterior segments (Fig. 1). Conventional systems like eye drops, suspensions and ointments cannot be considered optimal in the treatment of vision threatening ocular diseases (1). However, more than 90% of the marketed ophthalmic formulations are in the form of eye drops. These formulations mainly target the anterior segment eye diseases (2). Most of the topically applied drugs are washed off from the eye by various mechanisms (lacrimation, tear dilution and tear turnover) resulting in low ocular bioavailability of drugs. Moreover, human cornea comprising of epithelium, substantia propria and endothelium also restricts the ocular entry of drug molecules (3). As a result of these factors less than 5% of administered drug enters the eye. Alternative approaches like incorporation of permeation enhancers/cyclodextrins and increasing the viscosity of solutions did not provide any significant improvement. Recently many drug efflux pumps have been identified and significant enhancement in ocular drug absorption was achieved following their inhibition or evasion. But prolonged use of such inhibitors may result in undesirable effects (4).
Fig. 1.
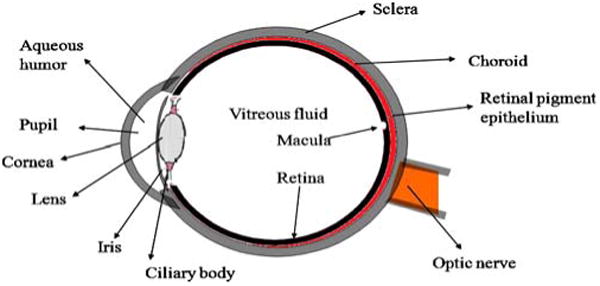
Structure of eye.
Treatment of posterior segment diseases still remains a herculean task for the formulation scientists. The tight junctions of blood retinal barrier (BRB) restrict the entry of systemically administered drugs into the retina (5). High vitreal drug concentrations are required in the treatment of posterior segment diseases. This can be made possible only with the local administration (intravitreal injections/implants and periocular injections). Periocular injections are associated with fairly high patient compliance as compared to intravitreal injections (6).
Dramatic changes have been observed in the field of ocular drug delivery over a decade. Insight into various membrane transporters/receptors present on the eye opened a new window of opportunities. Especially polar drug molecules, which fail to permeate ocular barriers, can be conveniently delivered via transporter/receptor targeted drug delivery systems (7). This review article briefly covers pathology of various ocular diseases and their current therapies. We have also given emphasis on the role of various ocular transporters and recent developments in drug delivery strategies including gene therapy. We have briefly discussed ocular deliver systems, gene therapy, and recent developments like microneedle and iontophoresis
Overview on Anatomy and Diseases Affecting Eye
Broadly we discuss the structure of eye under two subheadings (a) anterior segment and (b) posterior segment. Anterior segment consists of front one-third of eye that mainly includes pupil, cornea, iris, ciliary body, aqueous humor, and lens while the posterior segment consists of the back two-thirds of the eye that includes vitreous humor, retina, choroid, macula, and optic nerve (Fig. 1) (8, 9). In this article we made an attempt to briefly discuss the major diseases affecting the eye. These include age-related macular degeneration (AMD), diabetic macular edema (DME), cataract, proliferative vitreoretinopathy (PVR), uveitis, cytomegalovirus (CMV), and glaucoma. Table I highlights the classification, signs and symptoms and current treatment options for these diseases.
Table I. Summary of Major Ocular Diseases, Symptoms and Treatment.
| Disease | Classification | Signs and symptoms | Treatment | Ref |
|---|---|---|---|---|
| AMD | Dry AMD (non-exudative) | Break down of photoreceptors, retinal pigment epithelium (RPE) and choriocapillaris | Specific high-dose formulations containing antioxidants, zinc and vitamin supplements | (170–178) |
| Wet AMD (exudative) | Growth of abnormal blood vessels behind the retina, macula, disruption of Bruch's membrane and degeneration of RPE leading to complete loss of vision | Intravitreal injection of anti-VEGF agents like ranibizumab, pegaptanib sodium and bevacizumab | ||
| DME | Focal or non-cystoid DME | Small aberrations in retinal blood vessels followed by intra-retinal leakage | Focal or grid lasers and steroids | (179–181) |
| Diffuse or cystoid DME | Formation of microcysts and dilation of retinal capillaries | |||
| PVR | Based on the inflammation of retina: focal, diffuse, subretinal, circumferential, anterior displacement based on the location of scar tissue: anterior, posterior | Simple scar formation and proliferation of cells in vitreous and retina | Surgery and adjunctive treatment after surgery so as to avoid relapses (5-fluorouracil and low molecular weight heparin) | (182–184) |
| Uveitis | Anterior uveitis, intermediate uveitis posterior uveitis, pan-uveitic uveitis | Inflammation occurs in the middle layer of eye (uvea) | Corticosteroids and immunosuppressive agents | (185–187) |
| CMV | Inflammation of the retina, retinal detachment and complete blindness | Cidofovir, ganciclovir (GCV) and foscarnet | (187–189) | |
| Glaucoma | Primary open angle glaucoma (POAG) angle closure glaucoma (ACG) | Obstruction to the outflow of aqueous humor from the anterior segment | Prostaglandin analogs, beta-adrenergic receptor antagonists, alpha2-adrenergic agonists parasympathomimetics, carbonic anhydrase inhibitors | (190–194) |
| Conjunctivitis(or) red eye | Based on the structures: blepharoconjunctivitis (inflammation of eyelids), keratoconjunctivitis inflammation of the cornea) and episcleritis (inflammation between conjunctiva and sclera) | Inflammation of the conjunctiva, redness, irritation, grittiness and watering of eyes | Allergic infections are treated using anti-histaminics and non-steroidal anti-inflammatory agents and bacterial conjunctivitis is treated using antibiotics and corticosteroids | (195–198) |
| Cataract | Basis of etiology: age related, congenital, secondary, traumatic; basis of location of opacity: anterior cortical, anterior polar, anterior subcapsular, nuclear, posterior cortical, posterior subcapsular | Lens opacities that obstruct the passage of light | Surgical treatment may either involve extracapsular surgery and photoemulsification | (199–203) |
Delivery Routes
Conventionally, many ocular diseases are treated with either topical or systemic medications. Topical application of drug has remained the most preferred method due to ease of administration and low cost. Topical application is useful in the treatment of disorders affecting the anterior segment of the eye (10). Anatomical and physiological barriers hinder drugs from reaching posterior segment of eye mainly at choroid and retina. A major fraction of drug following topical administration is lost by lacrimation, tear dilution, nasolacrimal drainage and tear turnover. Such precorneal losses result in very low ocular bioavailability. Typically, less than 5% of the total administered dose reaches aqueous humor (11). So, in order to maintain minimum inhibitory concentrations, the agents need to be frequently dosed resulting in poor patient compliance. Upon topical instillation drugs are absorbed either by corneal route (cornea → aqueous humor → intraocular tissues) or non-corneal route (conjunctiva → sclera → choroid/RPE). The preferred route depends mainly on the corneal permeability of drug molecules (12). Unlike topical administration, systemic dosing helps in the treatment of diseases affecting posterior segment of the eye. A major drawback associated with systemic administration is only 1– 2% of administered drug reaches to vitreous cavity. Blood retinal barrier which is selectively permeable to more lipophilic molecules mainly governs the entry of drug molecules into posterior segment of the eye. This results in frequent administration of high amounts of drugs leading to systemic side effects (13). Though topical and systemic routes are convenient, lack of adequate bioavailability and failure to deliver therapeutic amounts of drugs to the retina prompted the vision scientists to search for alternative routes of administration.
Intravitreal injections have gained considerable momentum during the past two decades. This method involves injection of drug solution directly into vitreous via pars plana using a 30 G needle. Unlike other routes, intravitreal injection offers higher drug concentrations in vitreous and retina. Elimination of drugs following intravitreal administration depends on their molecular weight. Linear and globular shaped molecules (especially protein and peptide drugs) with molecular weight greater than 40 and 70 kDa respectively tend to cause longer retention in vitreous humor (14).
Though intravitreal administration offers high concentrations of drugs in retina, it is associated with various short term complications such as retinal detachment, endophthalmitis and intravitreal hemorrhages (15). Moreover, patients need to be carefully monitored following intravitreal injections.
Periocular route has been considered as the most promising and efficient route for administering drugs to posterior eye segment. Periocular refers to the region surrounding the eye. It is a broad term which includes peribulbar, posterior juxtascleral, retrobulbar, subtenon and subconjunctival routes (Fig. 2). Drug solutions are placed in close proximity to the sclera which results in high retinal and vitreal concentrations. Sclera which is made up of fibrous tissue offers less resistance to permeability of drugs (16). Ghate et al., studied the pharmacokinetics of sodium fluorescein following periocular administration in rabbits. The study concluded that administration of drug via subtenon injection resulted in the highest and sustained vitreous concentration of sodium fluorescein compared to retrobulbar and subconjunctival routes (17). Anterior segment complications have been observed in some patients following periocular injections. These include rise in intraocular pressure, cataract, hyphema, strabismus and corneal decompensation (18).
Fig. 2.
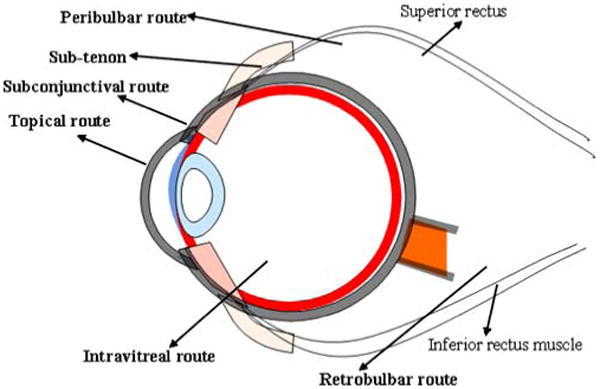
Routes of ocular drug delivery.
Nutrient Transporters and Efflux Pumps in the Eye
Epithelial cells express various nutrient transporters and receptors on their membrane surface. These nutrient transporters aid in the movement of various vitamins and amino acids across the cell membrane. Our laboratory has extensively studied various transporters and receptors which are present on ocular structures. We have also shown that attachment of various transporters/receptors targeted ligands enhances ocular bioavailability significantly. A detailed discussion of all influx transporters is beyond the scope of this review. So, we have mentioned some the important ones.
Peptide Transporter
It is a fairly robust transporter which has gained considerable attention in recent years for targeted drug delivery (19). These proton coupled transporters help in the translocation of di and tripeptides across the epithelium (20). These proteins are mainly classified into PepT1, PepT2 and peptide/histidine transporters (PHT 1 and PHT 2). Many drug molecules are known to be substrates for these transporters. Our laboratory has shown the presence of an oligopeptide transporter on rabbit cornea (21). Other drugs including β-lactam antibiotics, renin inhibitors and ACE-inhibitors are known to be substrates for PepT1 and PepT2. Ocheltree et al., demonstrated the expression of PHT1 in BRPE, HRPE (human RPE cells), ARPE-19 (human RPE cell line), and bovine and human neural retina. However, they could find the expression PEPT2 and PHT2 only in bovine and human retina; and PEPT1was not detected. They also concluded that glycylsarcosine uptake studies did not demonstrate any significant functional activity of PHT1 on plasma membranes of RPE (22). We have also investigated the mechanism of model dipeptide (glycylsarcosine) transport across the blood-ocular barriers following systemic administration in our laboratory. A time and concentration dependent, carrier mediated uptake of glycylsarcosine across the blood-ocular barrier was reported (23). Prodrugs (valine-ACVand valine-valine-ACV) exhibited higher concentrations of ACV in aqueous humor following systemic administration as compared to parent drug. This study indicates that peptide prodrugs are taken up via carrier mediated transport mechanism (24). Hence, drugs with poor ocular bioavailability can be suitably modified so that they can be recognized by peptide transporters.
Glucose Transporter
Glucose is utilized by the retina to meet the energy need of oxidative metabolism. Neural retina is considered to have the highest metabolic rate per unit weight (25). So, an alteration in the metabolic rate can lead to potential ocular complications. Glucose is transported across the blood-retinal and blood aqueous barriers by a stereo-specific, saturable process of facilitated diffusion. Glucose transporters are expressed in various isoforms GLUT1, GLUT2, GLUT3, GLUT4, GLUT5, GLUT6 and GLUT7. Out of these GLU1, GLU3, and GLU4 are high-affinity glucose transporters where as GLUT5 is a high-affinity fructose transporter. GLUT2 is considered to be low affinity glucose transporter. GLUT6 is a pseudogene which does not encode for a functional transporter. GLUT7 is similar to GLUT2 but only expressed in endoplasmic reticulum (26). Mantych et al., studied the presence of various isoforms of glucose transporter (Glut 1, Glut 3, Glut 4, and Glut 5) in human eye using western blot and immunohistochemical techniques. This study reported presence of Glut 1 in RPE, choroid, pars plana, retinal Mueller cells, and lens fiber cells (27). Glucose transporters are more efficient and have more capacity than any other nutrient transporter. But high substrate specificity of glucose transporters renders them inefficient for drug delivery purpose.
Vitamin C Transporter
Vitamin C or ascorbic acid (AA) acts as an antioxidant and thereby protects the cornea and other ocular tissues from UV radiations. Aqueous humor is the main source of AA for cornea and lens. AA levels in aqueous humor are partly responsible for preventing cataract (28). Two specific transporters (SVCT-1 and SVCT-2) of vitamin C were identified in the ocular tissues of human, rabbit and rat. These transporters have identical sequence homology. Transport of AA across the cells is mediated by two different transporter families. One consists of the low affinity and high capacity facilitative hexose transporters (GLUT) which translocate dehydro ascorbic acid (oxidized form of ascorbic acid), and the other consists of high affinity and low capacity sodium dependent vitamin C transporters (SVCT1 and SVCT2) that ferries l-ascorbic acid (reduced form of ascorbic acid) (29).
Amino Acid Transporter
Amino acids are responsible for protein synthesis and maintenance of structural and functional integrity of conjunctiva and retina/RPE. These transporters help in transferring amino acids from circulating blood to various organs. The presence of various amino acid transporters (ASCT1, LAT1 and ATB0+) on the cornea has been confirmed by gene expression. These transporters are actively involved in the transportation of L-alanine, L arginine and L-phenylalanine across the cornea. Amino acid transporters can be classified on the basis of their sodium dependency and substrate specificity (30). System L (large) and system y+ (cationic) amino acid transporters belong to sodium independent transporters while system X- (anionic), system A, B0,+, ASC (anionic, cationic, and neutral amino acid transporters) belong to sodium dependent transporter category (21). Large neutral amino acid transporter is expressed in two isoforms LAT1 and LAT2. LAT1 is mainly involved in the transport of large neutral amino acids, such as Leu, Phe, Ile, Trp, Val, Tyr, His and Met while LAT2 transports both large neutral amino acids and small neutral amino acids (31, 32). Transport systems for amino acids have been characterized on corneal epithelium and endothelium. Gandhi et al., studied the biochemical evidence of sodium independent facilitative transport system, LAT2, with high substrate specificity on ARPE-19 cell line (33). Blisse et al., reported the presence of sodium-dependent, B0,+ amino acid transporter with broad substrate specificity on rabbit corneal epithelium and human cornea. This transporter belongs to neurotransmitter gene family and found to have affinity comparable to PEPT1 in the transport of prodrugs, like valine-ACV and valine-GCV (34, 35). Such transport systems may be efficiently targeted for enhancing ocular delivery of drugs.
Efflux Transporters
Efflux of various substrates across plasma membrane and cytoplasm into extracellular fluid is mainly governed by ABC (ATP-binding cassette) superfamily of proteins which are encoded by MDR1 gene (36). ABC transporters are broadly classified into two types (a) complete transporter, contains four units (two nucleotide-binding domains and two membrane bound domains) and (b) half transporter, which possesses only two units (one nucleotide-binding domain and one membrane-bound domain). Half transporter must attach with another half transporter to perform its action (37). These ABC proteins are actively involved in detoxification process by regulating the transport of various sterols, lipids, endogenous metabolic products and xenobiotics (38). Literature search reveals that mainly two multidrug efflux pumps are responsible for the development of chemoresistance (a) P-glycoprotein (ABCB1) and (b) multidrug resistant protein (MRP) (ABCC1) (39).
P-gp is a 170 kDa membrane bound efflux protein generally expressed on the apical surface of polarized cells (40). P-glycoprotein is actively involved in the efflux of drug molecules thereby reducing drug accumulation inside cells (41). The presence of P-gp in the eye has been confirmed on conjunctival epithelial cells (42), ciliary non-pigmented epithelium (43), human and rabbit cornea (44), retinal capillary endothelial cells, iris and ciliary muscle cells (45). Constable et al., studied the presence of P-gp on three human RPE cell lines, ARPE19, D407 and h1RPE. They concluded that only D407 cell functionally express P-gp and hence can be used for in vitro drug transport studies without any modifications (46).
MRP is a 190 kDa membrane bound efflux protein encoded by MRP1 gene. It is generally expressed on the basolateral surface of intestine, hepatocytes and kidney cells (47, 48). Till now 12 different members of MRP family have been studied (49). MRP's are actively involved in the effluxing glucuronide, sulfate conjugated compounds and organic anions. Aukunuru et al., studied the presence of functionally and biochemically active MRP on human RPE cell line (50). Further studies by Steuer et al., confirmed that MRP is present on the choroidal side of the outer blood retinal barrier (51). Recently, studies from our laboratory revealed the presence of ABCC2 (a MRP homologue) on human corneal epithelium and rabbit cornea (52). Recently Zhang et al., have performed an extensive research on implications of drug transporter and CYP P450 mRNA Expression in ocular drug disposition. They concluded that both BCRP and MRP2 have very low expression levels in the human cornea while there were moderate MRP1 and low MRP3 expression levels in the human cornea. Moreover, designing drugs that can efficiently evade MRP1 efflux may play an important role in enhancement of ocular penetration (53).
Efflux pumps constitute significant barriers to the entry of drug molecules. It is indeed necessary to develop alternative strategies which can improve ocular drug absorption.
Prodrug Approach to Enhance Ocular Bioavailability
Prodrug strategy has been attempted for improving therapeutic efficacy of many drug molecules. Significant advantages in various properties like solubility, stability, permeability and evasion of efflux pump have been gained. Shirasaki has recently reviewed various molecular approaches to enhance drug permeation across the cornea (54). Our laboratory has also aggressively pursued the exploitation of many of these transporters for ocular drug absorption. Amino acid and peptide prodrugs of acyclovir (ACV) and ganciclovir (GCV), targeting various amino acid and peptide transporters have been developed and evaluated in our laboratory. Dipeptide (glycine–valine and tyrosine–valine) monoester prodrugs of GCV were found to show superior corneal absorption and bioavailability compared to parent drug (55). These attempts are highly effective in treating herpes simplex virus-1 (HSV-1) induced corneal epithelial and stromal keratitis (56). Permeability values of valine–GCV, tyrosine– valine–GCV, and glycine–valine–GCV were found to be higher than parent GCV which was attributed to their interaction with oligopeptide transporter present on the retina (57). Sustained release microsphere formulations of GCV and its lipophilic prodrug GCV–monobutyrate, with higher entrapment efficiency, were developed using PLGA in our laboratory (58). By using a similar strategy, l-valyl ester of acyclovir (ACV) was also developed and found to be more permeable across rabbit cornea due to its interaction with the oligopeptide transporter (24). Ocular penetration of peptide prodrug of ACV (valine–valine–ACV), was higher than the parent drug following systemic administration in rabbits. The prodrugs appear to be less cytotoxic, highly water-soluble with excellent in vivo activity against HSV-1 in rabbit epithelial and stromal keratitis (59). Our laboratory has also demonstrated that prodrug derivatization can effectively circumvent P-gp mediated efflux of quinidine across rabbit cornea (60).
TG100801, which converts to TG100572 by de-esterification, was found to be effective in the treatment of ocular neo-vascularization and retinal edema following topical administration (61). Due to this prodrug approach, incidence of systemic toxicity was found to be greatly diminished. Role of phase I and phase II ocular metabolic activities was also reviewed recently to rationalize design of prodrug and co-drug for ocular delivery by Crooks et al., (62). UNIL088, a water-soluble prodrug of cyclosporine A was developed via an ester linkage which gets converted into parent drug by chemical or enzymatic hydrolysis of the terminal ester group (63). Cannabinoids usually lowers IOP by their interaction with the ocular cannabinoid receptors. The solubility related problem of cannabinoids was solved by making arachidony-lethanolamide, R-methanandamide, noladin ether and their water-soluble phosphate ester prodrugs (64). This modification has resulted in higher corneal permeation which could result in more efficient reduction in IOP. In another study, combretastatin A-4-phosphate (CA-4-P), a water soluble prodrug of combretastatin A-4 (CA-4), was found to suppress the development of VEGF-induced neovascularization in the retina and also block development of CNV (65). Topical formulation of nepafenac was found to effectively penetrate into the corneal tissue which also reaches into the posterior segment. In animal models it was found to inhibit CNV and ischemia-induced retinal neovascularization. Nepafenac is converted to amfenac which has significant higher permeability across the cornea. This property is primarily responsible for appearance of the nepafenac to the posterior segment and causing inhibition of VEGF (66).
Novel Ocular Drug Delivery Systems
Colloidal carriers have been widely exploited in the field of drug delivery science. It provides a more selective targeting along with sustained release of molecules at the desired site. Applications of nanotechnology can be very exciting in the treatment of a gamut of diseases affecting the anterior as well as the posterior segment of the eye. One has to understand the physiological and biochemical factors involved in normal and pathological conditions for designing a successful ocular drug delivery system.
An ideal therapy requires selectively targeting of active agent to various diseases like CNV, diabetic retinopathy and solid tumors in the eye. Retina does not possess lymphatic system moreover angiogenesis in this part of eye has similar features to the solid tumor with enhanced permeability and retention (EPR) effects (67, 251). Delivery of a drug via nanotechnology based product fulfills mainly three objectives as follows (1) enhances drug permeation (2) controls the release of drug (3) targets drug (251). Main focus was given to colloidal systems consisting of micro/nanoparticles, micro/nanoemulsions, nanosuspensions and liposomes. In the last decade, dendrimers, niosomes, and cyclodextrins have also been exploited to achieve optimal drug delivery. Ocular implants have also been emerged as an alternative to the conventional delivery systems. The role of iontophoresis and ultrasound has also gained momentum in ocular delivery. Encapsulation of drugs in these colloidal carriers can also significantly enhance permeation across the membrane and prevent degradation from the ocular enzymes (68, 69). Such biodegradable carriers can be developed as an alternative to the implant prepared from nonbiodegradable polymers, which has to be removed surgically after a certain period. Sometimes the surgical process can cause complications like astigmatism, vitreous hemorrhage and patient incompliance.
Microemulsions
Microemulsions are dispersions of water and oil facilitated by a combination of surfactant and co-surfactant in a manner to reduce interfacial tension. These systems are usually characterized by higher thermodynamic stability, small droplet size (∼100 nm) and clear appearance (70). A review on application of microemulsions in ocular drug delivery by Vandamme et al., deals systematically with the various developments and challenges happening in the field. Selection of aqueous phase, organic phase and surfactant/co-surfactant systems are critical parameters which can affect stability of the system. Optimization of these components results in significant improvement in solubility of the drug molecule e.g. indomethacin, chloramphenicol (71). Apart from solubility, microemulsion systems have also been exploited to improve permeation across the cornea. An oil-in-water system consisting of pilocarpine using lecithin, propylene glycol, PEG 200 as surfactant/co surfactants, and isopropyl myristate as the oil phase has been designed, which is nonirritating to the rabbit animal model (72). Such formulations often provide sustained drug release thereby reducing frequency of the drug administration. In case of pilocarpine, microemulsion based system lowers the frequency of administration to two times as compared to four times with conventional eye drops in a day. This was due to enhancement of the permeation by surfactant-co-surfactant combination. Another microemulsion system consisting of pilocarpine hydrochloride (73) was shown to convert in different forms like (liquid crystalline and coarse emulsion) with a change in rheological parameters which changed depending upon change in water content. This results in higher viscosity which will retain the formulation on the cornea resulting in its enhanced effect. Timolol in micro-emulsion system was laden in a 2-hydroxyethyl methacrylate (HEMA) gels which was studied to modulate its transport across the gel (74). The authors have achieved higher drug loading but could not control its release. In another attempt to deliver timolol, a stable o/w and w/o emulsion was formulated which met all the requirements of an eye drop according to Polish Pharmacopeia V. Sirolimus, a highly lipophilic drug with aqueous solubility of 2.6 μg/mL was formulated in microemulsion system which could hold 1 mg of drug in the system with excellent stability and tolerability (75). An alcohol free, microemulsion based formulation consisting chloramphenicol was developed. This formulation exhibited excellent stability when compared to commercially available formulation. Though microemulsions have excellent advantages limitations in selection of surfactant/co-surfactant system and potential toxicity associated with higher concentrations of surfactant/co-surfactant often restricts its use (76).
Nanosuspensions
This can be defined as sub-micron colloidal system which consists of poorly water-soluble drug, suspended in an appropriate dispersion medium stabilized by surfactants. Nanosuspenisons usually consist of colloidal carriers like polymeric resins which are inert in nature. They help in enhancement of drug solubility and thus bioavailability. Unlike microemulsions, they are also popular because of their non irritant nature. Flurbiprofen encapsulated in eudragit RS 100® and RL 100® polymer resins prevents myosis, which might be induced during extracapsular cataract surgery (77). Charge on the surface of nanoparticles facilitates its adhesion to the cornea. Methylprednisolone acetate (MPA) was encapsulated in a copolymer of poly (ethyl-acrylate, methyl-methacrylate and chlorotrimethyl-ammo-nioethyl methacrylate) and examined for its effect on the anti-inflammatory symptoms in rabbits with endotoxin-induced uveitis (EIU) (78). Animal studies have revealed that anti-inflammatory effect of nanosuspensions was more than microsuspensions. Similar studies were carried out using piroxicam in eudragit RS 100. In vivo studies in rabbits have shown significant anti-inflammatory effects compared to microsuspensions (79). In another approach, three different types of glucocorticoids; hydrocortisone, prednisolone and dexamethasone were formulated as nanosuspensions. In vivo study in rabbits suggested that the nanosuspensions significantly enhanced the ocular absorption of glucocorticoids (80). These nanosuspensions also produce sustained drug release and were more effective over a longer duration. Nano-suspensions also impart stability to the drug in the formulation. Cloricromene (AD6) was formulated in nanosuspensions by using eudragit RS100 and RL100. AD6-loaded eudragit retarded nanoparticle suspension offered a significant edge in enhancing the shelf life and bioavailability of the drug following ophthalmic application (81).
Nanoparticles
According to Sahoo et al., “Nanoparticles are defined as particles with a diameter of less than 1 μm, comprising of various biodegradable or non biodegradable polymers, lipids, phospholipids or metals” (251). They can be classified as nanospheres or nanocapsules depending upon whether the drug has been uniformly dispersed or coated within polymeric material. Recent studies have revealed the migration of intact nanoparticles to the RPE cells following intravitreal injection of nanoparticles suspension (82, 251). This migration had been attributed to the rupture of the internal limiting membrane (ILM) and activation of non-specific retinal microglial cells. Such mild transient mechanism in the inflammatory process also modifies permeability and the anchoring mechanism of the ILM. These findings are crucial for the diseases affecting the posterior segment of the eye. Uptake and distribution of nanoparticles depend on the size of the nanoparticles. Kinetics of fluorescein nanospheres (2000, 200 and 50 nm) was studied following intravitreal injection in rabbits. Nanospheres smaller than 200 nm were also observed in the retinal cells other than the vitreous cavity and trabecular meshwork where only larger diameter particles were distributed. This study has shown the importance of particle size in ocular tissue distribution (83). Many formulation parameters have to be considered in designing an ideal formulation. Surface charge interaction of the drug and polymer has played an important role in drug release from the polymer. Incomplete release of progesterone from the polybutyl cyanoacrylate nanospheres was attributed to the surface charge interaction of the drug and polymer (84, 251). A solid lipid nanoparticulate system of tobramycin was developed for topical drug delivery. Such a particulate system can be retained for longer duration on the corneal surface and also on the conjunctival sac compared to an aqueous solution of the drug. In vivo testing has shown sustained drug release over a period of 6 h compared to short duration from equal dose of eye drops (85). Recently Kompella et al., have evaluated how the ocular disposition and distribution of different size of nanoparticles (20 and 200 nm) vary due to blood and lymph circulations following periocular administration in Sprague Dawley rats (86). They found that particles with 20 nm size were transported across the sclera to a small extent and no significant transport was noted across the sclera-choroid-RPE. Such low permeation was attributed to periocular circulation (blood and lymphatic) which play a crucial role in clearing the 20 nm particles. A higher concentration of particles in the ocular tissues was observed in dead animals by the post-mortem studies which they concluded because of the lack of physiological barrier following periocular administration. An ideal nanoparticulate formulation should have low clearance by blood and lymphatic circulation in order for it to be effective in the treatment of posterior segment diseases following periocular administration.
Various naturally occurring polymers were studied as carriers for the nanoparticulate system. Albumin nanoparticles can effectively encapsulate both positively (GCV) and negatively charged (oligonucleotides (ODNs)) molecules (87). Chitosan coated poly (epsilon-caprolactone) nanoparticles of indomethacin resulted in twofold enhancement in ocular bioavailability (88). Enhanced permeation across the cornea was also observed when poly (epsilon-caprolactone) nanoparticles were coated with polyethylene glycol (89). In another study, a formulation comprising dexamethasone acetate encapsulated in biodegradable PLGA nanoparticles was administered by the intravitreal route and the effect on CNV was studied. This study demonstrated a triphasic pattern of drug release. The drug level was found to be more than the level required to treat CMV (90). In another study, mucoadhesive chitosan-sodium alginate nanoparticles were prepared and evaluated for topical delivery of gatifloxacin. This system resulted in burst release during the first hour followed by sustained release for 24 h. This approach helps in reducing the dosing frequency of the antibiotic because of the sustained action observed after single administration (91).
Moshfeghi et al., have extensively reviewed the perspectives of particulate delivery system (micro and nanoparticles) in ophthalmology (92). Intravitreal administration of red nuclear fluorescent protein entrapped in PLGA has been shown to localize in RPE cells where red nuclear fluorescent protein plasmid expression was also observed (93). Phosphorothioate ODNs were either adsorbed or incorporated in albumin nanoparticles and antiviral efficacy was examined. Nanoparticles having entrapped ODNs were found to exhibit higher antiviral activity than nanoparticles with the adsorbed drug on the surface. This may be due to the protection of ODNs from enzymatic degradation (94). Particulate delivery system of ODNs has resulted in their higher stability, efficacy and reduction in non specific toxicity (95, 96). Currently, we are working on the delivery of various steroidal nanoparticles of mean particle diameter ≈200 nm via subconjunctival administration. In our laboratory, we are also working to optimize various in vitro properties of steroidal nanoparticles. These nanoparticles can be suspended in thermosensitive gels for subconjunctival administration. Further attachment of various ligands (folate and biotin) to the surface of these nanoparticles helps in increasing the uptake by RPE cells. This approach can be applied for the treatment of various posterior segment diseases such as wet AMD, PVR and DME.
Microspheres can sustain the release of the ODNs after intravitreal injection for longer time period. As microspheres do not enter the cells, a new approach called “Trojan” delivery system was invented. In this type of delivery system, polymeric microspheres were designed to control release of the nanosized anti-TGFβ2 phosphorothioate ODNs complexes. Such “Trojan” delivery was much more efficient for bleb survival following trabeculectomy than the microsphere or control alone (97). Macugen® was also entrapped in microspheres and studied following transscleral delivery. In vitro release study has shown release of macugen (2 μg/day) up to 20 days across the sclera. Inhibition of endothelial cell proliferation confirmed that biological activity was not affected by encapsulation (98).
Liposomes
Liposomes are lipid vesicles containing aqueous core which have been widely exploited in ocular delivery for various drug molecules. Liposomes containing GCV were formulated by a reversed phase evaporation method and in vivo pharmacokinetic evaluation was performed in a rabbit model. Permeability of GCV solution was compared with the liposomal formulation containing GCV. Transcorneal permeability and area under the curve (AUC) were was 3.9 and 1.7 fold higher than the solution. Ocular tissue distribution was higher in the sclera, cornea and vitreous humor from liposomal formulation (99). Vasoactive intestinal peptide (VIP) was formulated in liposomal formulation (VIP-Lip). Concentration of VIP following intravitreal injection was about 15 times higher in ocular fluid than the concentration achieved by administration of VIP solution alone. The peptide was effective only when formulated in liposomal form (100). Ciprofloxacin (CPFX) was also formulated in liposomal environmental which lowered tear-driven dilution in the conjunctival sac. Multilamellar vesicles from lecithin and alpha-l-dipalmithoyl-phosphatidylcholine were used to prepare liposome containing CPFX. This approach produced sustained release of the drug depending on the nature of the lipid composition selected (101). Acetazolamide was encapsulated in liposomal formulation for delivery via topical route. Liposomes were prepared using reversed-phase evaporation (REVs) and multilamellar vesicles (MLVs). Different molar ratios of phosphatidylcholine and cholesterol were added in the preparation of MLVs which resulted in higher entrapment efficiency as compare to REV. Entrapment efficiency ascended with higher amounts of lipid and charge inducers such as cholesterol and stearylamine. In-vivo intraocular pressure was examined for selective formulation and liposomes. MLVs exhibited prolong efficacy than liposomes prepared by REV (102). In another study cationic liposomes containing herpes simplex virus type 1 (HSV-1) glycoprotein B (gB1s) or, DTK1 and DTK2 (two related polylysine rich peptides) were prepared and examined in the rabbit ocular model of HSV-1 keratitis. These peptides (antigen) in liposomal formulation have the potential to act as very effective anti-HSV vaccine (103). In a similar manner, liposomes of tilisolol were also prepared and studied following topical and intravitreal administrations. Positively charged liposomal formulation of tropicamide and oligolamellar liposomes of ACV were generated. Both formulations resulted in significantly higher absorption across the cornea (104).
Antisense ODNs encapsulated in liposomes have been studied for their potential to treat ocular disorders. Various factors need to be carefully considered before such a system is adopted clinically. In topical delivery, liposome may not be able to release the entire payload of high molecular weight ODNs relative to a free solution form (105). Liposomal formulation can release ODNs in a sustained manner following intravitreal injection (106). PEGylated liposome containing ODNs, results in higher percentage of intact ODNs (30% of the total dose) after 2 weeks. Some researchers have formulated liposomes coated with an envelope of inactivated hemagglutinating virus of Japan (HVJ) to treat CNV in rats. They successfully delivered phosphorothioate ODNs to inhibit VEGF (107).
Niosomes
Niosomes are bilayered structural vesicles made up of non-ionic surfactant which are capable of encapsulating both lipophilic and hydrophilic compounds. In a recent approach to deliver cyclopentolate, niosomal formulation was developed. It released the drug independent of pH resulting in significant enhancement of ocular bioavailability (108, 251). Niosomal formulation of coated (chitosan or carbopol) timolol maleate exhibited significant IOP lowering effect in rabbits as compared to timolol solution. Another novel delivery system known as discomes has been developed. These are large structures with a size range of 12–16 μm derived by incorporating nonionic surfactant Solutan C24 in niosomes. A major advantage of this system was less systemic drainage because of the large size and large residence time in the cul-de-sac due to their disc shape. Timolol maleate was successfully entrapped in discomes and niosomes. In vivo bioavailability of the discomes was better than the niosomes (109, 251).
Dendrimers
According to Sahoo et al., “Dendrimers are macro-molecular compounds made up of a series of branches around a central core. Their nanosize, ease of preparation, functionalization and possibility to attach multiple surface groups render them suitable alternative vehicle for ophthalmic drug delivery” (110, 111, 251). Previously, poly (acrylic) acids were prepared as bioadhesive polymers to achieve better residence time and decrease the frequency of administration. Major drawbacks associated with these systems are blurred vision and formation of a veil leading to vision loss (112). Dendrimers are liquid or semi-solid polymers and contain amine, carboxylic and hydroxyl surface groups, which keep on increasing as the generation number increases (G0, G1, G2, and so on). Dendrimers consisting of poly (amidoamine) (PAMAM) have been widely employed in drug delivery. This system of branched polymers represent unique architecture, and can entrap both hydrophilic and lipophilic drugs into their structure (113–115, 251). Selection of functional group on the surface (amine, carboxylate and hydroxyl), size and molecular weight of the dendrimer are important parameters to be considered in designing a delivery system.
Dendrimer based approach has shown better bioavailability of pilocarpine and tropicamide, when DG1.5 and DG4.0 (OH) dendrimers with carboxylate and hydroxyl surface groups were instilled (116). Some of the researchers have synthesized water soluble conjugates of D(+)-glucosamine and D(+)-glucosamine 6-sulfate to obtain synergistic immunomodulatory and antiangiogenic effect by using anionic PANAM (G3.5) dendrimers (117). The investigators have also shown successful increase in long term surgery after testing the formulation in a rabbit model of scar tissue formation after glaucoma filtration surgery. Cell-adhesion peptides were also attached to dendrimers which were later used to link with collagen scaffolds (118). Stratification of corneal epithelial cell was studied using the above mentioned collagen scaffolds. All peptides have shown promoted effect on stratification. The same investigators have actively developed biocompatible conjugates of dendrimers with collagen scaffolds to obtain better mechanical property and adhesion ability. In another approach, dendrimers based approach was used to deliver anti-VEGF ODN and successfully tested in rats to treat CNV (119). Effect of size, molecular weight and number of surface groups (amine, carboxylate and hydroxyl) of PANAM was studied to understand feasibility for its controlled release of pilocarpine nitrate and some tropicamide (116).
Cyclodextrins
As per Sahoo et al., “Cyclodextrins (CDs) belong to a group of cyclic oligosaccharides capable of forming inclusion complexes with many guest molecules (120). Through CD complexation, aqueous solubility of hydrophobic drugs can be enhanced without changing their molecular structure and their intrinsic ability to permeate biological membranes (251)”. Molecules having low aqueous solubility have been delivered as CD complexes. These complexes have been proven to increase corneal permeation of drugs like dexamethasone, cyclosporine and so on. CD complexation approach can significantly increase ocular bioavailability of poorly water soluble drugs. In some cases such inclusion complexes can impart aqueous stability to drugs within the formulation as well (121). Recently a study was carried out to determine relative concentration of dexamethasone in CDs following topical and systemic absorption in the rabbit eye. Investigators observed a significant amount of drug reaching to the posterior segment tissue like retina and vitreous humor following topical application of eye drops. Such an approach might be very valuable in the treatment of vitreoretinal diseases which require long term delivery of drug molecules (122). In another study, the researchers have prepared inclusion complex of HP-β-CD with zinc diethyldithiocarbamate and disulfiram which resulted in higher corneal permeability and also lower side effects like development of cataract relative to drug alone (123, 124). In a similar approach, inclusion complexes were prepared with rhEGF/HP-β-CD which were suspended in poloxamer gel. This formulation has resulted in significant increase in AUC of the drug. This approach has an additional advantage of imparting stability to the drug (125). Inclusion complexes of drugs like dexamethasone, dexamethasone acetate and pilocarpine with HP-β-CD resulted in higher bioavailability than the conventional eye drops (126, 127). This complexation approach does not disrupt the biological membrane compared to conventional permeation enhancer like benzalkonium chloride. Apart from that, due to inclusion, the free drug is not available, so drugs with inherent irritant properties can be successfully delivered by this approach. CD molecules are inert in nature and HP-β-CD (up to 45%) was found to be non irritant to the human and animal eye (120).
Contact Lenses
Liewen et al., reported an extensive study on polymeric hydrogels for contact lens-based ocular drug delivery (128). Traditionally used soaked contact lenses provide a drug release for a few hours. Current challenges in this mode of drug delivery are to sustain release for longer period and also to incorporate sufficient drug amounts in the lens matrix. Recently, surfactant loaded (Brij 98) poly-hydroxy ethyl methacrylate (p-HEMA) gel has shown sustained release of cyclosporine-A (129). In a similar approach, dexamethasone, dexamethasone 21-disodium phosphate and dexamethasone 21-acetate were suspended in p-HEMA based gel to achieve controlled release (130). The bioavailability of the formulation was found to be better than the topical eye drops. In a different kind of approach, two different kind of silicone and hydrogel lens were compared for 1 month. The silicon based lens was shown to be more beneficial to the patient with hypoxia but also found to form more lipid deposit compared to the other. The same investigators have prepared silicon based hydrogel using different ratios to sustain the release of the timolol, dexamethasone, and dexamethasone 21-acetate over the time of several weeks to 3 months (131). The different ratios of monomers could be changed depending upon the delivery required. The prepared lenses were also studied for its mechanical and physical properties. The residence time of a system could be lengthened by suspending nanoparticulate based drugs in thermogelling polymers. The contact lens, containing a nanoparticulate based drug, is an attractive alternative to the topical drug delivery, especially eye drops (3, 132). A major benefit of this strategy is to prolong residence time in post tear film which in case of topical drops is just a few minutes. Due to increased residence time in eye, drug permeation through the cornea is higher with specific delivery of the drug to targeted tissue. In another study, drug molecule entrapped in a nanoparticulate system was later dispersed in lens made up of poly-2-hydroxyethyl methacrylate (p-HEMA) hydrogels. This approach provide controlled drug release over a few days and delivery rate could be further modulated by changing loading dose of the particles in the gel (133).
Intraocular Implants
Implants have been widely employed to extend the release in ocular fluids and tissues particularly in the posterior segment. Implants can be broadly classified into two categories based on their degradation property (1) biodegradable and (2) non-biodegradable. With implants, the delivery rate could be modulated by varying polymer composition. Implants can be in the form of solid, semi-solid or particulate based delivery systems (134). These implants have been applied in the treatment of diseases affecting both anterior and posterior segments of the eye. The diseases include anterior segment disorders like glaucoma filtering surgery and posterior segment disorders like proliferative vitroretinopathy, CMV retinitis, endophthalmitis, and posterior capsule opacification. Table II summarizes the recent strategies of formulating an implant for various ocular pathologies.
Table II. Implants for the Treatment of Ocular Pathologies.
| Drug | Polymer | Characteristic/treatment | Ref |
|---|---|---|---|
| Episcleral implant of betamethasone | PVA- EVA | Zero order release up to 4 weeks | (204) |
| Triamcinolone acetonide (TA) | PVA | Controls CNV in a rat model | (205) |
| Dexamethasone scleral implant (Surodex®) | PLGA | After phacotrabeculectomy surgery | (206) |
| Intracameral implant of cyclosporine | PLGA | Prevents corneal graft rejection or post-operative inflammation in rats | (207) |
| 5-Fluorouracil (5-FU) | POE | Less toxicity and persistence bleb for longer time | (208) |
| Combination of drug 5-fluorouridine, TA and recombinant tissue plasminogen activator | PLGA | Treatment of PVR after intravitreal injection | (209) |
| Cis-4-hydroxyproline | PLGA 65/35 + PLGA 50/50 | Treatment of PVR | (210) |
| Subretinal implant of sirolimus and TA | Poly butylmethacrylate, poly ethylene-co-vinyl acetate | Treatment of ocular inflammation | (211) |
| Intravitreal implant of fluocinolone acetonide | PVA | Controlled release for more than 1 year to treat severe uveitis | (212) |
| FK506 | PGLC | Prolongation of corneal allograft survival | (139) |
| Cyclosporine A | PGLC | Treatment of uveitis | (138) |
| Intracameral implant of dexamethasone | Corneal graft rejection | (213) | |
| Scleral implants of GCV | PLA | CMV retinitis | (137) |
| Scleral implant of Ethacrynic acid (ECA) | PLGA films | To reduce IOP for 10 days | (214) |
| Cyclosporin A (Oculex Drug Delivery System) | PLGA | Prophylaxis and treatment of corneal transplant rejection in humans | (215) |
| Dexamethasone | PLGA | Anterior segment drug delivery system in patients after phacoemulsification | (216) |
| Intracapsular ring releasing 5-FU | – | Preventing posterior capsule opacification (PCO) in rabbit eyes | (217) |
| Heparin | PLGA | Posterior capsular opacification (PCO) in rabbits | (218) |
PVA–EVA Polyvinyl alcohol–ethylene vinyl acetate, PVA polyvinyl alcohol, PGLC ploy glycolide-co-clatide-co-caprolactone polymer, PLGA poly latide-co-glycolide-acid, PLA poly lactic acid, POE poly ortho esters
Drug release from Poly lactic acid (PLA), poly glycolic acid (PGA) and poly lactic-co-glycolic acid (PLGA) usually follows three phases of drug release which constitute an initial burst, a middle diffusive phase and a final burst of the drug. In recent years, much attention has been paid to manipulate release profiles to achieve longer duration of release from the implants (135, 136). In one study, two PLA monomers of different molecular weight were used in different ratios and, a pseudo-zero order release was obtained with minimum burst effect (137). Recently, co-polymerization of caprolactone with poly-glycolide-co-lactide lead to the development of glycolide-co-lactide-co-caprolactone copolymer (PGLC) which was successfully applied to treat corneal allograft and uveitis (138, 139). Polyanhydrides have also been utilized for these indications (140). Polyorthoesters (POE) are hydrophobic in nature and usually degrade by surface erosion thus providing zero order release kinetics. Among the four generations, third generation POE forms gel like matrix at room temperature. Fourth generation POEs have gained much attention currently because of their slow erosion property due to the presence of latent acid in the polymer backbone (141).
Recently, researchers have also focused on intravitreal injection of implant using cell encapsulation technology. FGF-2 implant embedded in AN69 copolymer delayed photoreceptor degeneration (142). Various synthetic polymers like polyether sulfone, caprolactone and polyvinylpyrrolidone were developed and then positioned with a titanium anchor in the vitreous cavity (143). In a similar study, ciliary neurotrophic factor (CNTF) was released from cells implanted without any side effects for several weeks. A dose dependent protective effect on photoreceptors was noticed with CNTF in rabbits (144). A novel device ECT NT-501 was developed to support the implanted cells (145). After loading CNTF secreting cells in the device, a controlled release of protein was observed for 1 year in the rabbit eye. In a clinical trial, this device appeared to protect photoreceptors in several patients (146).
Hydrogel Systems
These are three-dimensional, hydrophilic, polymeric networks capable of imbibing large amounts of water or biological fluids. Nanjawade et al., have extensively reviewed hydrogels in ocular delivery (147). Residence time can be significantly enhanced with a hydrogel formulation. The gelation can be obtained by changing temperature and pH. Poloxamers, the most widely used polymer, contain the hydrophobic part in the centre surrounded by a hydrophilic part. Though they are widely employed to enhance the residence time, they suffer from a major drawback of having weak mechanical strength, rapid erosion and non-biodegradability (148). Many attempts have been made to address this problem. Oligomerization was carried out to change the critical gelling temperature. In case of cellulose derivative like HPMC, gelation is a result of interaction of hydrophobic components at higher temperature (149). Pilocarpine formulation has shown enhanced miotic response at lower concentration of xyloglucan than poloxamer 407 (150). Pseudolatexes are artificial latexes obtained by dispersing pre-existing polymers in aqueous medium. They offer physical stability of gel along with the drug molecule which are sensitive to aqueous media (151).
In a different sort of gel approach also known as ATRIGEL, a polymer is dissolved in a suitable carrier. The polymer and carrier are both biodegradable and biocompatible. Once injected into the subcutaneous space, water in surrounding tissue causes the precipitation of polymer which immediately entraps the drug and releases it in a controlled manner (www.qltinc.com). The inventors of the product have claimed to use this system for a wide variety of molecules i.e. proteins and peptides. Targeted delivery of drugs, compatibility with a wide variety of molecules, potential to achieve controlled release and biocompatibility were the key features of the system (www.qltinc.com). Currently marketed product, Eligard®, represents a similar technology which is used for the treatment of prostate cancer. Table III summarizes recent development in hydrogel based formulations.
Table III. Recent Developments on the Hydrogel Based Formulations.
| Drug | Polymer | Mechanism of gelation | Ref |
|---|---|---|---|
| Pefloxacin mesylate | Gelrite gellan gum | Ion | (219) |
| Gatifloxacin | Alginate and HPMC | Ion | (220) |
| Indomethacin | Gelrite (gellan gum) | Ion | (221) |
| Ciprofloxacin HCL | Gelrite gellan gum and sodium alginate | Ion | (222) |
| Gatifloxacin | Ion | (223) | |
| Timolol maleate | Gellan gum | Ion | (224) |
| Ofloxacin | Carbopol 940 and HPMC | pH | (225) |
| Puerarin | Carbopol/HPMC-based gel | pH | (226) |
| Puerarin | Poloxamer analogs/carbopol | pH | (227) |
| Fluconazole | In-situ gelling eye drops | – | (228) |
| Ganciclovir | In-situ gelling eye drops | – | (229) |
| Timolol maleate | Pluronic F127 | Temperature | (148) |
| Timolol maleate | poly(Nisopropylacrylamide)-chitosan | Temperature | (230) |
| Pilocarpine | Alginate and pluronic-based in situ gelling | Ion and temperature | (231) |
| Timolol maleate | Pluronic F-127 and chitosan | pH and temperature | (232) |
| Gatifloxacin | Pluronic F127-g-poly(acrylic acid) copolymers | pH and temperature | (233) |
| Pilocarpine | Xyloglucan | Temperature | (150) |
Iontophoresis
Ocular iontophoresis has gained significant interest recently due to its non-invasive nature of delivery to both anterior and posterior segment. It requires a mild electric current which is applied to enhance ionized drug penetration into tissue. This mode of delivery can overcome the potential side effects associated with intraocular injections and implants mentioned earlier. OcuPhor™ system has been designed with an applicator, dispersive electrode and a dose controller for transscleral iontophoresis (DDT) (152, 153). This device releases the active drug into retina-choroid as well. A similar device has been designed called Visulex™ to allow selective transport of ionized molecules through sclera. Transcorneal and transscleral iontophoresis have been extensively employed to deliver a wide variety of drug molecules like gentamicin and dexamethasone. Delivery of antibiotics by iontophoresis has resulted in significant fewer bacterial colonies in cornea compared to the eye drops. Examples of antibiotics successfully employed are gentamicin, tobramycin and ciprofloxacin but not vancomycin because of its high molecular weight (154–156). Successful delivery was obtained with dexamethasone and with antisense ODNs (157, 158). A number of antibiotics, including gentamicin, cephazolin, ticarcilin, amikacin and vancomycin have been successfully delivered into the vitreous of rabbit eyes. Transscleral iontophoresis of steroids (dexamethasone and methyl prednisolone), amikacin, gentamicin and other drugs was also reported (159–164).
Microneedle
Recently, researchers have attempted microneedle to deliver drug to posterior segment as an alternative to topical route. The magnitude of lateral and transverse diffusion of sulforhodamine was found to be similar across human cadaver sclera. An evaluation of coated solid metal microneedle to deliver the drug was carried out both in-vitro and in-vivo. Microneedle had shown excellent in vitro penetration into sclera and rapid dissolution of coating solution after insertion. In-vivo drug level was found to be significantly higher than the level observed following topical drug administration. This mode of drug delivery was also successful in the delivery of pilocarpine (165). The inventors have claimed to deliver drug successfully across both sclera and cornea by coated microneedle with minimum invasion.
Gene Delivery
Recently, various strategies have been adopted to deliver nucleic acids to a specific site within the eye. Designing delivery system for antisense ODNs, aptamers or siRNAs is a challenging task for researchers in ocular delivery field because of high molecular weight, size, surface charge, solubility of the active drug and intrinsic complexities associated with the structure of ocular tissues like retina and cornea (105).
Table IV summarizes the recent developments happened in the field targeting various growth factors. Recently, FDA has approved Vitravene®, an ODNs for the treatment of CMV in AIDS patients and Macugen® (pegaptanib sodium injection) which is an aptamer for the treatment of “wet” AMD (209, 210). Non viral vectors have gained wide acceptance in gene delivery because of their ability to overcome disadvantages of viral vectors such as immunogenicity, safety and potential persistence in the brain (166–168). A major challenge associated with current system is its inability to achieve higher transfection efficiency. “Naked” DNA, physical vectorisation of the genetic material and chemical methods are three main modes of delivery among nonviral gene therapy. An extensive effort to deliver naked DNA by topical, intracameral, intracorneal, subconjunctival, intravitreal and by subretinal routes has been reviewed by Bloquel et al., (169). The same authors have described physical methods such as gene gun, electron transfer, iontophoresis and ultrasound in this review. The authors have also focused on chemical methods such as liposomes, polyplexes, and PEGylated RNA aptamers.
Table IV. Ocular Gene Delivery Targeting Growth Factors.
| Type | Mechanism of action | Effect/treatment | Ref |
|---|---|---|---|
| AS-ODN | Targets stop codon of 5′ untranslated region of the mRNA | Decreases protein expression to treat retinal neovascularization | (234) |
| Phosphorothioate AS-ODN | Decreases VEGF levels by 65% | Tested in vitro (hypoxic condition) and iv vivo (inhibit CNV) in a rat models | (235) |
| AS-ODN | inhibits raf kinases | decreases RPE cell proliferation | (236) |
| Modified pegaptanib Sodium | Better pharmacokinetic parameters and less degradation by exonucleases | Shows extremely higher affinity towards to VEGF165 | (237) |
| siRNA | Down regulation of VEGF | Tested in vivo (subretinal administration in mice) | (238) |
| siRNA | Inhibits the expression of VEGF | Tested in vitro (ARPE-19 cells) and in vivo on (corneal angiogenesis) | (239) |
| AS-ODN | Decreases the conjunctival scarring | Useful in glaucoma filtration surgery | (240) |
| Anti-TGFβ32 aptamer (ARC81) | Inhibits fibroblast migration and decreases matrix deposition | Tested in vitro (mink lung epithelial cell proliferation) and in vivo (inhibits endogenously derived TGFβ2 in rabbit ocular fluid matrix) | (241) |
| Phosphorothioate AS-ODN | Inhibits bFGF-induced angiogenesis | Studied to treat rabbit corneal neovascularization | (242) |
| Phosphorothioate AS-ODN | Inhibits fibronectin | Studied to treat diabetic retinopathy in a rat model | (243) |
| AS-ODN | Targets immediate-early (IE) transcriptional unit | Anti-HSV | (244, 245) |
| Modified 12-mer methylphosphonate | Targeted intron/exon junction of the splice acceptor | Greater affinity and selectivity for HSV- 1 | (246) |
| Phosphodiester AS-ODN | Targets translation start site of IE 110 mRNA | Minimizes non specific effects | (247) |
| AS-ODN | Targeting the tumor necrosis factor alpha (TNFα) | Treatment of HSK | (247) |
| AS-ODN (21-mer, ISIS 2922 Vitravene®) | Targeted to coding region of IE 2 | Inhibits CMV | (248) |
| ISIS 13312 (A modified Vitravene®) | Improves the ocular retention of the AS-ODN | With similar bioactivity to Vitravene®, Its half life was in monkey was 2 months greater | (249, 250) |
AS-ODN Anti sense oligonucleotide, HSK herpetic stromal keratitis, HSV herpes simplex virus, CNV choroidal neovascularization, CMV cytomegalovirus
Conclusions
Effective treatment of ocular diseases is a formidable challenge for scientists in the field especially because of the nature of diseases and presence of the ocular barriers especially in posterior ocular segments. An ideal therapy should maintain effective levels of drug for the longer duration following a single application. Drug delivery by topical and intravitreal routes cannot be considered safe, effective and patient friendly. Drug delivery by periocular route can potentially overcome many of these limitations and also can provide sustained drug levels in ocular pathologies affecting both segments. Transporter targeted delivery can be a promising strategy for many drug molecules. Colloidal carriers can substantially improve the current therapy and may emerge as an alternative following their periocular administration. In recent years, scientists have focused on designing a strategy with a multidisciplinary approach e.g. microneedle, iontophoresis and MRI. Continued innovation in gene delivery appears to be very exciting for a gamut of diseases. Designing these innovative techniques has given an unprecedented momentum for their protection by intellectual rights. In future, much of the emphasis will be given to achieve non-invasive sustained drug release for eye disorders in both segments. A clear understanding of the complexities associated with tissues in normal and pathological conditions, physiological barriers and multicompartmental pharmacokinetics would greatly hasten further development in the field.
Acknowledgments
We would like acknowledge NIH R01EY010659-12 and R01EY09171-15 for financial support.
References
- 1.Hughes PM, Mitra AK. Overview of ocular drug delivery and iatrogenic ocular cytopathologies. In: Mitra AK, editor. Ophthalmic Drug Delivery Systems. 2nd. New York: M Dekker, Inc; 1993. pp. 1–27. [Google Scholar]
- 2.Lang JC. Ocular drug delivery: conventional ocular formulations. Adv Drug Delivery Rev. 1995;16:39–43. [Google Scholar]
- 3.Bourlais CL, Acar L, Zia H, Sado PA, Needham T, Leverge R. Ophthalmic drug delivery systems—recent advances. Prog Retin Eye Res. 1998;17:33–58. doi: 10.1016/S1350-9462(97)00002-5. [DOI] [PubMed] [Google Scholar]
- 4.Jain R, Majumdar S, Nashed Y, Pal D, Mitra AK. Circumventing Pglycoprotein-mediated cellular efflux of quinidine by prodrug derivatization. Mol Pharm. 2004;1:290–299. doi: 10.1021/mp049952s. [DOI] [PubMed] [Google Scholar]
- 5.Janoria KG, Gunda S, Boddu SHS, Mitra AK. Novel approaches to retinal drug delivery. Expert Opin Drug Deliv. 2007;4:371–388. doi: 10.1517/17425247.4.4.371. [DOI] [PubMed] [Google Scholar]
- 6.Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004;1:99–114. doi: 10.1517/17425247.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dias CS, Anand BS, Mitra AK. Effect of mono- and di-acylation on the ocular disposition of ganciclovir: physicochemical properties, ocular bioreversion, and antiviral activity of short chain ester prodrugs. J Pharm Sci. 2002;91:660–668. doi: 10.1002/jps.10072. [DOI] [PubMed] [Google Scholar]
- 8.Idrees F, Vaideanu D, Fraser S, Sowden J, Khaw P. A review of anterior segment dysgeneses. Survey of Ophthal. 2006;51:213–231. doi: 10.1016/j.survophthal.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Geroski H Dayle, Edelhauser HF. Drug Delivery for Posterior Segment Eye Disease. Invest Ophthamol Vis Sci. 2000;4:961–964. [PubMed] [Google Scholar]
- 10.Lee VH, Robinson JR. Topical ocular drug delivery: recent developments and future challenges. J Ocul Pharmacol. 1986;2:67–108. doi: 10.1089/jop.1986.2.67. [DOI] [PubMed] [Google Scholar]
- 11.Hughes PM, Olejnik O, Chang-Lin JE, Wilson CG. Topical and systemic drug delivery to the posterior segments. Adv Drug Deliv Rev. 2005;57:2010–2032. doi: 10.1016/j.addr.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Ahamed I, Patton TF. Importance of the noncorneal absorption route in topical ophthalmic drug delivery. Invest Ophthalmol Vis Sci. 1985;26:584–587. [PubMed] [Google Scholar]
- 13.Duvvuri S, Majumdar S, Mitra AK. Drug delivery to the retina: challenges and opportunities. Expert Opin Biol Ther. 2003;3:45–56. doi: 10.1517/14712598.3.1.45. [DOI] [PubMed] [Google Scholar]
- 14.Marmor MF, Negi A, Maurice DM. Kinetics of macromolecules injected into the subretinal space. Exp Eye Res. 1985;40:687–696. doi: 10.1016/0014-48358590138-1. [DOI] [PubMed] [Google Scholar]
- 15.Ausayakhun S, Yuvaves P, Ngamtiphakom S, Prasitsilp J. Treatment of cytomegalovirus retinitis in AIDS patients with intravitreal ganciclovir. J Med Assoc Thai. 2005;88(Suppl 9):S15–S20. [PubMed] [Google Scholar]
- 16.Raghava S, Hammond M, Kompella UB. Periocular routes for retinal drug delivery. Expert Opin Drug Deliv. 2004;1:99–114. doi: 10.1517/17425247.1.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghate D, Brooks W, McCarey BE, Edelhauser HF. Pharmacokinetics of intraocular drug delivery by periocular injections using ocular fluorophotometry. Invest Ophthalmol Vis Sci. 2007;48:2230–2237. doi: 10.1167/iovs.06-0954. [DOI] [PubMed] [Google Scholar]
- 18.Castellarin A, Pieramici DJ. Anterior segment complications following periocular and intraocular injections. Ophthalmol Clin North Am. 2004;17:583–590. doi: 10.1016/j.ohc.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Ganapathy V, Leibach FH. Peptide transport in intestinal and renal brush border membrane vesicles. Life Sci. 1982;30:2137–2146. doi: 10.1016/0024-32058290287-9. [DOI] [PubMed] [Google Scholar]
- 20.Adibi SA. Renal assimilation of oligopeptides: physiological mechanisms and metabolic importance. Am J Physiol. 1997;272:E723–736. doi: 10.1152/ajpendo.1997.272.5.E723. [DOI] [PubMed] [Google Scholar]
- 21.Anand BS, Mitra AK. Mechanism of corneal permeation of L-valyl ester of acyclovir: targeting the oligopeptide transporter on the rabbit cornea. Pharm Res. 2002;19:1194–1202. doi: 10.1023/A:1019806411610. [DOI] [PubMed] [Google Scholar]
- 22.Ocheltree SM, Keep RF, Shen H, Yang D, Hughes BA, Smith DE. Preliminary investigation into the expression of proton-coupled oligopeptide transporters in neural retina and retinal pigment epithelium (RPE): lack of functional activity in RPE plasma membranes. Pharm Res. 2003;20:1364–1372. doi: 10.1023/A:1025741723724. [DOI] [PubMed] [Google Scholar]
- 23.Atluri H, Anand BS, Patel J, Mitra AK. Mechanism of a model dipeptide transport across blood-ocular barriers following systemic administration. Exp Eye Res. 2004;78:815–822. doi: 10.1016/j.exer.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 24.Dias C, Nashed Y, Atluri H, Mitra A. Ocular penetration of acyclovir and its peptide prodrugs valacyclovir and valvalacyclovir following systemic administration in rabbits: An evaluation using ocular microdialysis and LC–MS. Curr Eye Res. 2002;25:243–252. doi: 10.1076/ceyr.25.4.243.13488. [DOI] [PubMed] [Google Scholar]
- 25.Winkler BS. Glycolytic and oxidative metabolism in relation to retinal function. J Gen Physiol. 1981;77:667–692. doi: 10.1085/jgp.77.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Merriman-Smith R, Donaldson P, Kistler J. Differential expression of facilitative glucose transporters GLUT1 and GLUT3 in the lens. Invest Ophthalmol Vis Sci. 1999;40:3224–3230. [PubMed] [Google Scholar]
- 27.Mantych GJ, Hageman GS, Devaskar SU. Characterization of glucose transporter isoforms in the adult and developing human eye. Endocrinology. 1993;133:600–607. doi: 10.1210/en.133.2.600. [DOI] [PubMed] [Google Scholar]
- 28.Brubaker RF, Bourne WM, Bachman LA, McLaren JW. Ascorbic acid content of human corneal epithelium. Invest Ophthalmol Vis Sci. 2000;41:1681–1683. [PubMed] [Google Scholar]
- 29.Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol Membr Biol. 2001;18:87–95. doi: 10.1080/09687680110033774. [DOI] [PubMed] [Google Scholar]
- 30.Kanai Y, Hediger MA. The glutamate/neutral amino acid transporter family SLC1: molecular, physiological and pharmacological aspects. Pflugers Arch. 2004;447:469–479. doi: 10.1007/s00424-003-1146-4. [DOI] [PubMed] [Google Scholar]
- 31.Fukasawa Y, Segawa H, Kim JY, Chairoungdua A, Kim DK, Matsuo H, Cha SH, Endou H, Kanai Y. Identification and characterization of a Na(+)-independent neutral amino acid transporter that associates with the 4F2 heavy chain and exhibits substrate selectivity for small neutral D- and L-amino acids. J Biol Chem. 2000;275:9690–9698. doi: 10.1074/jbc.275.13.9690. [DOI] [PubMed] [Google Scholar]
- 32.Verrey F, Meier C, Rossier G, Kuhn LC. Glycoprotein-associated amino acid exchangers: broadening the range of transport specificity. Pflugers Arch. 2000;440:503–512. doi: 10.1007/s004240000274. [DOI] [PubMed] [Google Scholar]
- 33.Gandhi MD, Pal D, Mitra AK. Identification and functional characterization of a Na(+)-independent large neutral amino acid transporter (LAT2) on ARPE-19 cells. Int J Pharm. 2004;275:189–200. doi: 10.1016/j.ijpharm.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 34.Jain-Vakkalagadda B, Pal D, Gunda S, Nashed Y, Ganapathy V, Mitra AK. Identification of a Na+-dependent cationic and neutral amino acid transporter, B(0,+), in human and rabbit cornea. Mol Pharm. 2004;1:338–346. doi: 10.1021/mp0499499. [DOI] [PubMed] [Google Scholar]
- 35.Ganapathy ME, Ganapathy V. Amino acid transporter ATB0,+as a delivery system for drugs and prodrugs. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:357–364. doi: 10.2174/156800805774912953. [DOI] [PubMed] [Google Scholar]
- 36.Eytan GD, Kuchel PW. Mechanism of action of P-glycoprotein in relation to passive membrane permeation. Int Rev Cytol. 1999;190:175–250. doi: 10.1016/S0074-7696(08)62148-8. [DOI] [PubMed] [Google Scholar]
- 37.Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.GR-1649R. [DOI] [PubMed] [Google Scholar]
- 38.Sarkadi B, Homolya L, Szakacs G, Varadi A. Human multidrug resistance ABCB and ABCG transporters: participation in a chemoimmunity defense system. Physiol Rev. 2006;86:1179–1236. doi: 10.1152/physrev.00037.2005. [DOI] [PubMed] [Google Scholar]
- 39.Sharom FJ. ABC multidrug transporters: structure, function and role in chemoresistance. Pharmacogenomics. 2008;9:105–127. doi: 10.2217/14622416.9.1.105. [DOI] [PubMed] [Google Scholar]
- 40.Bellamy WT. P-glycoproteins and multidrug resistance. Annu Rev Pharmacol Toxicol. 1996;36:161–183. doi: 10.1146/annurev.pa.36.040196.001113. [DOI] [PubMed] [Google Scholar]
- 41.Gottesman MM, Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- 42.Saha P, Yang JJ, Lee VH. Existence of a p-glycoprotein drug efflux pump in cultured rabbit conjunctival epithelial cells. Invest Ophthalmol Vis Sci. 1998;39:1221–1226. [PubMed] [Google Scholar]
- 43.Wu J, Zhang JJ, Koppel H, Jacob TJ. P-glycoprotein regulates a volume-activated chloride current in bovine non-pigmented ciliary epithelial cells. J Physiol. 1996;491(Pt 3):743–755. doi: 10.1113/jphysiol.1996.sp021254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, Kaliki P, Pal D, Ganapathy V, Mitra AK. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2909–2918. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 45.Holash JA, Stewart PA. The relationship of astrocyte-like cells to the vessels that contribute to the blood-ocular barriers. Brain Res. 1993;629:218–224. doi: 10.1016/0006-89939391323-K. [DOI] [PubMed] [Google Scholar]
- 46.Constable PA, Lawrenson JG, Dolman DE, Arden GB, Abbott NJ. P-Glycoprotein expression in human retinal pigment epithelium cell lines. Exp Eye Res. 2006;83:24–30. doi: 10.1016/j.exer.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 47.Roelofsen H, Hooiveld GJ, Koning H, Havinga R, Jansen PL, Muller M. Glutathione S-conjugate transport in hepatocytes entering the cell cycle is preserved by a switch in expression from the apical MRP2 to the basolateral MRP1 transporting protein. J Cell Sci. 1999;112(Pt 9):1395–1404. doi: 10.1242/jcs.112.9.1395. [DOI] [PubMed] [Google Scholar]
- 48.Yang JJ, Ann DK, Kannan R, Lee VH. Multidrug resistance protein 1 (MRP1) in rabbit conjunctival epithelial cells: its effect on drug efflux and its regulation by adenoviral infection. Pharm Res. 2007;24:1490–1500. doi: 10.1007/s11095-007-9267-7. [DOI] [PubMed] [Google Scholar]
- 49.Kruh GD, Guo Y, Hopper-Borge E, Belinsky MG, Chen ZS. ABCC10, ABCC11, and ABCC12. Pflugers Arch. 2007;453:675–684. doi: 10.1007/s00424-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 50.Aukunuru JV, Sunkara G, Bandi N, Thoreson WB, Kompella UB. Expression of multidrug resistance-associated protein (MRP) in human retinal pigment epithelial cells and its interaction with BAPSG, a novel aldose reductase inhibitor. Pharm Res. 2001;18:565–572. doi: 10.1023/A:1011060705599. [DOI] [PubMed] [Google Scholar]
- 51.Steuer H, Jaworski A, Elger B, Kaussmann M, Keldenich J, Schneider H, Stoll D, Schlosshauer B. Functional characterization and comparison of the outer blood–retina barrier and the blood–brain barrier. Invest Ophthalmol Vis Sci. 2005;46:1047–1053. doi: 10.1167/iovs.04-0925. [DOI] [PubMed] [Google Scholar]
- 52.Karla PK, Pal D, Quinn T, Mitra AK. Molecular evidence and functional expression of a novel drug efflux pump (ABCC2) in human corneal epithelium and rabbit cornea and its role in ocular drug efflux. Int J Pharm. 2007;336:12–21. doi: 10.1016/j.ijpharm.2006.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang T, Xiang CD, Gale D, Carreiro S, Wu EY, Zhang EY. Drug transporter and cytochrome P450 mRNA expression in human ocular barriers: implications for ocular drug disposition. Drug Metab Dispos. 2008;36:1300–1307. doi: 10.1124/dmd.108.021121. [DOI] [PubMed] [Google Scholar]
- 54.Shirasaki Y. Molecular design for enhancement of ocular penetration. J Pharm Sci. 2008;97:2462–2496. doi: 10.1002/jps.21200. [DOI] [PubMed] [Google Scholar]
- 55.Gunda S, Hariharan S, Mitra AK. Corneal absorption and anterior chamber pharmacokinetics of dipeptide monoester prodrugs of ganciclovir (GCV): in vivo comparative evaluation of these prodrugs with Val-GCV and GCV in rabbits. J Ocul Pharmacol Ther. 2006;22:465–476. doi: 10.1089/jop.2006.22.465. [DOI] [PubMed] [Google Scholar]
- 56.Majumdar S, Nashed YE, Patel K, Jain R, Itahashi M, Neumann DM, Hill JM, Mitra AK. Dipeptide monoester ganciclovir prodrugs for treating HSV-1-induced corneal epithelial and stromal keratitis: in vitro and in vivo evaluations. J Ocul Pharmacol Ther. 2005;21:463–474. doi: 10.1089/jop.2005.21.463. [DOI] [PubMed] [Google Scholar]
- 57.Kansara V, Hao Y, Mitra AK. Dipeptide monoester ganciclovir prodrugs for transscleral drug delivery: targeting the oligopeptide transporter on rabbit retina. J Ocul Pharmacol Ther. 2007;23:321–334. doi: 10.1089/jop.2006.0150. [DOI] [PubMed] [Google Scholar]
- 58.Janoria KG, Mitra AK. Effect of lactide/glycolide ratio on the in vitro release of ganciclovir and its lipophilic prodrug (GCV-monobutyrate) from PLGA microspheres. Int J Pharm. 2007;338:133–141. doi: 10.1016/j.ijpharm.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 59.Anand BS, Hill JM, Dey S, Maruyama K, Bhattacharjee PS, Myles ME, Nashed YE, Mitra AK. In vivo antiviral efficacy of a dipeptide acyclovir prodrug, val-val-acyclovir, against HSV-1 epithelial and stromal keratitis in the rabbit eye model. Invest Ophthalmol Vis Sci. 2003;44:2529–2534. doi: 10.1167/iovs.02-1251. [DOI] [PubMed] [Google Scholar]
- 60.Katragadda S, Talluri RS, Mitra AK. Modulation of P-glycoprotein-mediated efflux by prodrug derivatization: an approach involving peptide transporter-mediated influx across rabbit cornea. J Ocul Pharmacol Ther. 2006;22:110–120. doi: 10.1089/jop.2006.22.110. [DOI] [PubMed] [Google Scholar]
- 61.Doukas J, Mahesh S, Umeda N, Kachi S, Akiyama H, Yokoi K, Cao J, Chen Z, Dellamary L, Tam B, Racanelli-Layton A, Hood J, Martin M, Noronha G, Soll R, Campochiaro PA. Topical administration of a multi-targeted kinase inhibitor suppresses choroidal neovascularization and retinal edema. J Cell Physiol. 2008;216:29–37. doi: 10.1002/jcp.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Al-Ghananeem AM, Crooks PA. Phase I and phase II ocular metabolic activities and the role of metabolism in ophthalmic prodrug and codrug design and delivery. Molecules. 2007;12:373–388. doi: 10.3390/12030373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lallem F, Varesio E, Felt-Baeyens O, Bossy L, Hopfgartner G, Gurny R. Biological conversion of a water-soluble prodrug of cyclosporine. A Eur J Pharm Biopharm. 2007;67:555–561. doi: 10.1016/j.ejpb.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 64.Juntunen J, Jarvinen T, Niemi R. In-vitro corneal permeation of cannabinoids and their water-soluble phosphate ester prodrugs. J Pharm Pharmacol. 2005;57:1153–1157. doi: 10.1211/jpp.57.9.0009. [DOI] [PubMed] [Google Scholar]
- 65.Nambu H, Nambu R, Melia M, Campochiaro PA. Combretastatin A-4 phosphate suppresses development and induces regression of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44:3650–3655. doi: 10.1167/iovs.02-0985. [DOI] [PubMed] [Google Scholar]
- 66.Takahashi K, Saishin Y, Saishin Y, Mori K, Ando A, Yamamoto S, Oshima Y, Nambu H, Melia MB, Bingaman DP, Campochiaro PA. Topical nepafenac inhibits ocular neovascularization. Invest Ophthalmol Vis Sci. 2003;44:409–415. doi: 10.1167/iovs.02-0346. [DOI] [PubMed] [Google Scholar]
- 67.Yasukawa T, Ogura Y, Tabata Y, Kimura H, Wiedemann P, Honda Y. Drug delivery systems for vitreoretinal diseases. Prog Retin Eye Res. 2004;23:253–281. doi: 10.1016/j.preteyeres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Kayser O, Lemke A, Hernandez-Trejo N. The impact of nanobiotechnology on the development of new drug delivery systems. Curr Pharm Biotechnol. 2005;6:3–5. doi: 10.2174/1389201053167158. [DOI] [PubMed] [Google Scholar]
- 69.Vandervoort J, Ludwig A. Ocular drug delivery: nanomedicine applications. Nanomed. 2007;2:11–21. doi: 10.2217/17435889.2.1.11. [DOI] [PubMed] [Google Scholar]
- 70.Ansari MJ, Kohli K, Dixit N. Microemulsions as potential drug delivery systems: a review. PDA J Pharm Sci Technol. 2008;62:66–79. [PubMed] [Google Scholar]
- 71.Vandamme TF. Microemulsions as ocular drug delivery systems: recent developments and future challenges. Prog Retin Eye Res. 2002;21:15–34. doi: 10.1016/s1350-9462(01)00017-9. doi:10.1016/S1350-946201 00017-9. [DOI] [PubMed] [Google Scholar]
- 72.Hasse A, Keipert S. Development and characterization of microemulsions for ocular application. Eur J Pharm Biopharm. 1997;43:179–183. [Google Scholar]
- 73.Chan J, Maghraby GM, Craig JP, Alany RG. Phase transition water-in-oil microemulsions as ocular drug delivery systems: in vitro and in vivo evaluation. Int J Pharm. 2007;328:65–71. doi: 10.1016/j.ijpharm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 74.Li CC, Abrahamson M, Kapoor Y, Chauhan A. Timolol transport from microemulsions trapped in HEMA gels. J Colloid Interface Sci. 2007;315:297–306. doi: 10.1016/j.jcis.2007.06.054. [DOI] [PubMed] [Google Scholar]
- 75.Buech G, Bertelmann E, Pleyer U, Siebenbrodt I, Borchert HH. Formulation of sirolimus eye drops and corneal permeation studies. J Ocul Pharmacol Ther. 2007;23:292–303. doi: 10.1089/jop.2006.130. [DOI] [PubMed] [Google Scholar]
- 76.Kaur IP, Kanwar M. Ocular preparations: the formulation approach. Drug Dev Ind Pharm. 2002;28:473–493. doi: 10.1081/DDC-120003445. [DOI] [PubMed] [Google Scholar]
- 77.Pignatello R, Bucolo C, Spedalieri G, Maltese A, Puglisi G. Flurbiprofen-loaded acrylate polymer nanosuspensions for ophthalmic application. Biomaterials. 2002;23:3247–3255. doi: 10.1016/S0142-96120200080-7. [DOI] [PubMed] [Google Scholar]
- 78.Adibkia K, Omidi Y, Siahi MR, Javadzadeh AR, Barzegar-Jalali M, Barar J, Maleki N, Mohammadi G, Nokhodchi A. Inhibition of endotoxin-induced uveitis by methyl-prednisolone acetate nanosuspension in rabbits. J Ocul Pharmacol Ther. 2007;23:421–432. doi: 10.1089/jop.2007.0039. [DOI] [PubMed] [Google Scholar]
- 79.Adibkia K, Siahi Shadbad MR, Nokhodchi A, Javadzedeh A, Barzegar-Jalali M, Barar J, Mohammadi G, Omidi Y. Piroxicam nanoparticles for ocular delivery: physico-chemical characterization and implementation in endotoxin-induced uveitis. J Drug Target. 2007;15:407–416. doi: 10.1080/10611860701453125. [DOI] [PubMed] [Google Scholar]
- 80.Kassem MA, Abdel Rahman AA, Ghorab MM, Ahmed MB, Khalil RM. Nanosuspension as an ophthalmic delivery system for certain glucocorticoid drugs. Int J Pharm. 2007;340:126–133. doi: 10.1016/j.ijpharm.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 81.Pignatello R, Ricupero N, Bucolo C, Maugeri F, Maltese A, Puglisi G. Preparation and characterization of eudragit retard nanosuspensions for the ocular delivery of cloricromene. AAPS PharmSciTech. 2006;7:E27. doi: 10.1208/pt070127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bourges JL, Gautier SE, Delie F, Bejjani RA, Jeanny JC, Gurny R, BenEzra D, Behar-Cohen FF. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44:3562–3569. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- 83.Sakurai E, Ozeki H, Kunou N, Ogura Y. Effect of particle size of polymeric nanospheres on intravitreal kinetics. Ophthalmic Res. 2001;33:31–36. doi: 10.1159/000055638. [DOI] [PubMed] [Google Scholar]
- 84.Li VH, Wood RW, Kreuter J, Harmia T, Robinson JR. Ocular drug delivery of progesterone using nanoparticles. J Microencapsul. 1986;3:213–218. doi: 10.3109/02652048609031575. [DOI] [PubMed] [Google Scholar]
- 85.Cavalli R, Gasco MR, Chetoni P, Burgalassi S, Saettone MF. Solid lipid nanoparticles (SLN) as ocular delivery system for tobramycin. Int J Pharm. 2002;238:241–245. doi: 10.1016/S0378-51730200080-7. [DOI] [PubMed] [Google Scholar]
- 86.Amrite AC, Edelhauser HF, Singh SR, Kompella UB. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis. 2008;14:150–160. [PMC free article] [PubMed] [Google Scholar]
- 87.Irache JM, Merodio M, Arnedo A, Camapanero MA, Mirshahi M, Espuelas S. Albumin nanoparticles for the intravitreal delivery of anticytomegaloviral drugs. Mini Rev Med Chem. 2005;5:293–305. doi: 10.2174/1389557053175335. [DOI] [PubMed] [Google Scholar]
- 88.Calvo P, Vila-Jato JL, Alonso MJ. Comparative in vitro evaluation of several colloidal systems, nanoparticles, nanocapsules, and nanoemulsions, as ocular drug carriers. J Pharm Sci. 1996;85:530–536. doi: 10.1021/js950474+. [DOI] [PubMed] [Google Scholar]
- 89.De Campos AM, Sanchez A, Gref R, Calvo P, Alonso MJ. The effect of a PEG versus a chitosan coating on the interaction of drug colloidal carriers with the ocular mucosa. Eur J Pharm Sci. 2003;20:73–81. doi: 10.1016/S0928-09870300178-7. [DOI] [PubMed] [Google Scholar]
- 90.Xu J, Wang Y, Li Y, Yang X, Zhang P, Hou H, Shi Y, Song C. Inhibitory efficacy of intravitreal dexamethasone acetate-loaded PLGA nanoparticles on choroidal neovascularization in a laser-induced rat model. J Ocul Pharmacol Ther. 2007;23:527–540. doi: 10.1089/jop.2007.0002. [DOI] [PubMed] [Google Scholar]
- 91.Motwani SK, Chopra S, Talegaonkar S, Kohli K, Ahmad FJ, Khar RK. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: formulation, optimisation and in vitro characterisation. Eur J Pharm Biopharm. 2008;68:513–525. doi: 10.1016/j.ejpb.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 92.Moshfeghi AA, Peyman GA. Micro- and nanoparticulates. Adv Drug Deliv Rev. 2005;57:2047–2052. doi: 10.1016/j.addr.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 93.Bejjani RA, BenEzra D, Cohen H, Rieger J, Andrieu C, Jeanny JC, Gollomb G, Behar-Cohen FF. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol Vis. 2005;11:124–132. [PubMed] [Google Scholar]
- 94.Arnedo A, Irache JM, Merodio M, Espuelas Millan MS. Albumin nanoparticles improved the stability, nuclear accumulation and anticytomegaloviral activity of a phosphodiester oligonucleotide. J Control Release. 2004;94:217–227. doi: 10.1016/j.jconrel.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 95.Khan A, Sommer W, Fuxe K, Akhtar S. Site-specific administration of antisense oligonucleotides using biodegradable polymer microspheres provides sustained delivery and improved subcellular biodistribution in the neostriatum of the rat brain. J Drug Target. 2000;8:319–334. doi: 10.3109/10611860008997909. [DOI] [PubMed] [Google Scholar]
- 96.De Rosa G, Quaglia F, Bochot A, Ungaro F, Fattal E. Long-term release and improved intracellular penetration of oligonucleotide-polyethylenimine complexes entrapped in biodegradable microspheres. Biomacromolecules. 2003;4:529–536. doi: 10.1021/bm025684c. [DOI] [PubMed] [Google Scholar]
- 97.Gomes dos Santos AL, Bochot A, Doyle A, Tsapis N, Siepmann J, Siepmann F, Schmaler J, Besnard M, Behar-Cohen F, Fattal E. Sustained release of nanosized complexes of polyethylenimine and anti-TGF-beta 2 oligonucleotide improves the outcome of glaucoma surgery. J Control Release. 2006;112:369–381. doi: 10.1016/j.jconrel.2006.02.010. [DOI] [PubMed] [Google Scholar]
- 98.Carrasquillo KG, Ricker JA, Rigas IK, Miller JW, Gragoudas ES, Adamis AP. Controlled delivery of the anti-VEGF aptamer EYE001 with poly(lactic-co-glycolic)acid microspheres. Invest Ophthalmol Vis Sci. 2003;44:290–299. doi: 10.1167/iovs.01-1156. [DOI] [PubMed] [Google Scholar]
- 99.Shen Y, Tu J. Preparation and ocular pharmacokinetics of ganciclovir liposomes. Aaps J. 2007;9:E371–377. doi: 10.1208/aapsj0903044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lajavardi L, Bochot A, Camelo S, Goldenberg B, Naud MC, Behar-Cohen F, Fattal E, de Kozak Y. Downregulation of endotoxin-induced uveitis by intravitreal injection of vasoactive intestinal peptide encapsulated in liposomes. Invest Ophthalmol Vis Sci. 2007;48:3230–3238. doi: 10.1167/iovs.06-1305. [DOI] [PubMed] [Google Scholar]
- 101.Budai L, Hajdu M, Budai M, Grof P, Beni S, Noszal B, Klebovich I, Antal I. Gels and liposomes in optimized ocular drug delivery: studies on ciprofloxacin formulations. Int J Pharm. 2007;343:34–40. doi: 10.1016/j.ijpharm.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 102.Hathout RM, Mansour S, Mortada ND, Guinedi AS. Liposomes as an ocular delivery system for acetazolamide: in vitro and in vivo studies. AAPS PharmSciTech. 2007;8:1. doi: 10.1208/pt0801001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cortesi R, Argnani R, Esposito E, Dalpiaz A, Scatturin A, Bortolotti F, Lufino M, Guerrini R, Cavicchioni G, Incorvaia C, Menegatti E, Manservigi R. Cationic liposomes as potential carriers for ocular administration of peptides with anti-herpetic activity. Int J Pharm. 2006;317:90–100. doi: 10.1016/j.ijpharm.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 104.Nagarsenker MS, Londhe VY, Nadkarni GD. Preparation and evaluation of liposomal formulations of tropicamide for ocular delivery. Int J Pharm. 1999;190:63–71. doi: 10.1016/S0378-51739900265-3. [DOI] [PubMed] [Google Scholar]
- 105.Bochot A, Mashhour B, Puisieux F, Couvreur P, Fattal E. Comparison of the ocular distribution of a model oligonucleotide after topical instillation in rabbits of conventional and new dosage forms. J Drug Target. 1998;6:309–313. doi: 10.3109/10611869808996838. [DOI] [PubMed] [Google Scholar]
- 106.Bochot A, Fattal E, Boutet V, Deverre JR, Jeanny JC, Chacun H, Couvreur P. Intravitreal delivery of oligonucleotides by sterically stabilized liposomes. Invest Ophthalmol Vis Sci. 2002;43:253–259. [PubMed] [Google Scholar]
- 107.Ogata N, Otsuji T, Matsushima M, Kimoto T, Yamanaka R, Takahashi K, Wada M, Uyama M, Kaneda Y. Phosphorothioate oligonucleotides induction into experimental choroidal neovascularization by HVJ-liposome system. Curr Eye Res. 1999;18:261–269. doi: 10.1076/ceyr.18.4.261.5358. [DOI] [PubMed] [Google Scholar]
- 108.Kaur IP, Garg A, Singla AK, Aggarwal D. Vesicular systems in ocular drug delivery: an overview. Int J Pharm. 2004;269:1–14. doi: 10.1016/j.ijpharm.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 109.Vyas SP, Mysore N, Jaitely V, Venkatesan N. Discoidal niosome based controlled ocular delivery of timolol maleate. Pharmazie. 1998;53:466–469. [PubMed] [Google Scholar]
- 110.Quintana A, Raczka E, Piehler L, Lee I, Myc A, Majoros I, Patri AK, Thomas T, Mule J, Baker JR., Jr Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm Res. 2002;19:1310–1316. doi: 10.1023/A:1020398624602. [DOI] [PubMed] [Google Scholar]
- 111.Ihre HR, Padilla De Jesus OL, Szoka FC, Jr, Frechet JM. Polyester dendritic systems for drug delivery applications: design, synthesis, and characterization. Bioconjug Chem. 2002;13:443–452. doi: 10.1021/bc010102u. [DOI] [PubMed] [Google Scholar]
- 112.Patton TF, Robinson JR. Ocular evaluation of polyvinyl alcohol vehicle in rabbits. J Pharm Sci. 1975;64:1312–1316. doi: 10.1002/jps.2600640811. [DOI] [PubMed] [Google Scholar]
- 113.Milhem OM, Myles C, McKeown NB, Attwood D, D'Emanuele A. Polyamidoamine starburst dendrimers as solubility enhancers. Int J Pharm. 2000;197:239–241. doi: 10.1016/S0378-51739900463-9. [DOI] [PubMed] [Google Scholar]
- 114.Bhadra D, Bhadra S, Jain S, Jain NK. A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int J Pharm. 2003;257:111–124. doi: 10.1016/S0378-51730300132-7. [DOI] [PubMed] [Google Scholar]
- 115.Ooya T, Lee J, Park K. Effects of ethylene glycol-based graft, star-shaped, and dendritic polymers on solubilization and controlled release of paclitaxel. J Control Release. 2003;93:121–127. doi: 10.1016/j.jconrel.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 116.Vandamme TF, Brobeck L. Poly(amidoamine) dendrimers as ophthalmic vehicles for ocular delivery of pilocarpine nitrate and tropicamide. J Control Release. 2005;102:23–38. doi: 10.1016/j.jconrel.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 117.Shaunak S, Thomas S, Gianasi E, Godwin A, Jones E, Teo I, Mireskandari K, Luthert P, Duncan R, Patterson S, Khaw P, Brocchini S. Polyvalent dendrimer glucosamine conjugates prevent scar tissue formation. Nat Biotechnol. 2004;22:977–984. doi: 10.1038/nbt995. [DOI] [PubMed] [Google Scholar]
- 118.Duan X, Sheardown H. Dendrimer crosslinked collagen as a corneal tissue engineering scaffold: mechanical properties and corneal epithelial cell interactions. Biomaterials. 2006;27:4608–4617. doi: 10.1016/j.biomaterials.2006.04.022. [DOI] [PubMed] [Google Scholar]
- 119.Marano RJ, Toth I, Wimmer N, Brankov M, Rakoczy PE. Dendrimer delivery of an anti-VEGF oligonucleotide into the eye: a long-term study into inhibition of laser-induced CNV, distribution, uptake and toxicity. Gene therapy. 2005;12:1544–1550. doi: 10.1038/sj.gt.3302579. [DOI] [PubMed] [Google Scholar]
- 120.Loftssona T, Jarvinen T. Cyclodextrins in ophthalmic drug delivery. Adv Drug Deliv Rev. 1999;36:59–79. doi: 10.1016/S0169-409X9800055-6. [DOI] [PubMed] [Google Scholar]
- 121.Aktas Y, Unlu N, Orhan M, Irkec M, Hincal AA. Influence of hydroxypropyl beta-cyclodextrin on the corneal permeation of pilocarpine. Drug Dev Ind Pharm. 2003;29:223–230. doi: 10.1081/DDC-120016730. [DOI] [PubMed] [Google Scholar]
- 122.Sigurdsson HH, Konraethsdottir F, Loftsson T, Stefansson E. Topical and systemic absorption in delivery of dexamethasone to the anterior and posterior segments of the eye. Acta Ophthalmol Scand. 2007;85:598–602. doi: 10.1111/j.1600-0420.2007.00885.x. [DOI] [PubMed] [Google Scholar]
- 123.Wang S, Li D, Ito Y, Liu X, Zhang J, Wu C. An ocular drug delivery system containing zinc diethyldithiocarbamate and HPbetaCD inclusion complex—corneal permeability, anti-cataract effects and mechanism studies. J Pharm Pharmacol. 2004;56:1251–1257. doi: 10.1211/0022357044526. [DOI] [PubMed] [Google Scholar]
- 124.Wang S, Li D, Ito Y, Nabekura T, Wang S, Zhang J, Wu C. Bioavailability and anticataract effects of a topical ocular drug delivery system containing disulfiram and hydroxypropyl-beta-cyclodextrin on selenite-treated rats. Curr Eye Res. 2004;29:51–58. doi: 10.1080/02713680490513209. [DOI] [PubMed] [Google Scholar]
- 125.Kim EY, Gao ZG, Park JS, Li H, Han K. rhEGF/HP-beta-CD complex in poloxamer gel for ophthalmic delivery. Int J Pharm. 2002;233:159–167. doi: 10.1016/S0378-51730100933-4. [DOI] [PubMed] [Google Scholar]
- 126.Freedman KA, Klein JW, Crosson CE. Beta-cyclodextrins enhance bioavailability of pilocarpine. Curr Eye Res. 1993;12:641–647. doi: 10.3109/02713689309001843. [DOI] [PubMed] [Google Scholar]
- 127.Usayapant A, Karara AH, Narurkar MM. Effect of 2-hydroxypropyl-beta-cyclodextrin on the ocular absorption of dexamethasone and dexamethasone acetate. Pharm Res. 1991;8:1495–1499. doi: 10.1023/A:1015838215268. [DOI] [PubMed] [Google Scholar]
- 128.Xinming L, Yingde C, Lloyd AW, Mikhalovsky SV, Sandeman SR, Howel CA, Liewen L. Polymeric hydrogels for novel contact lens-based ophthalmic drug delivery systems: A review. Cont Lens Anterior Eye. 2008;31:57–64. doi: 10.1016/j.clae.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 129.Kapoor Y, Chauhan A. Drug and surfactant transport In Cyclosporine A and Brij 98 laden p-HEMA hydrogels. J Colloid Interface Sci. 2008;322:624–633. doi: 10.1016/j.jcis.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 130.Kim J, Chauhan A. Dexamethasone transport and ocular delivery from poly(hydroxyethyl methacrylate) gels. Int J Pharm. 2008;353:205–222. doi: 10.1016/j.ijpharm.2007.11.049. [DOI] [PubMed] [Google Scholar]
- 131.Kim J, Conway A, Chauhan A. Extended delivery of ophthalmic drugs by silicone hydrogel contact lenses. Biomaterials. 2008;29:2259–2269. doi: 10.1016/j.biomaterials.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 132.McNamara NA, Polse KA, Brand RJ, Graham AD, Chan JS, McKenney CD. Tear mixing under a soft contact lens: effects of lens diameter. Am J Ophthalmol. 1999;127:659–665. doi: 10.1016/S0002-93949900051-3. [DOI] [PubMed] [Google Scholar]
- 133.Gulsen D, Chauhan A. Ophthalmic drug delivery through contact lenses. Invest Ophthalmol Vis Sci. 2004;45:2342–2347. doi: 10.1167/iovs.03-0959. [DOI] [PubMed] [Google Scholar]
- 134.Bourges JL, Bloquel C, Thomas A, Froussart F, Bochot A, Azan F, Gurny R, BenEzra D, Behar-Cohen F. Intraocular implants for extended drug delivery: therapeutic applications. Adv Drug Deliv Rev. 2006;58:1182–1202. doi: 10.1016/j.addr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 135.Yasukawa T, Kimura H, Tabata Y, Ogura Y. Biodegradable scleral plugs for vitreoretinal drug delivery. Adv Drug Deliv Rev. 2001;52:25–36. doi: 10.1016/S0169-409X0100192-2. [DOI] [PubMed] [Google Scholar]
- 136.Yasukawa T, Ogura Y, Sakurai E, Tabata Y, Kimura H. Intraocular sustained drug delivery using implantable polymeric devices. Adv Drug Deliv Rev. 2005;57:2033–2046. doi: 10.1016/j.addr.2005.09.005. doi:10.1016/ j.addr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 137.Kunou N, Ogura Y, Yasukawa T, Kimura H, Miyamoto H, Honda Y, Ikada Y. Long-term sustained release of ganciclovir from biodegradable scleral implant for the treatment of cytomegalovirus retinitis. J Control Release. 2000;68:263–271. doi: 10.1016/S0168-36590000267-4. [DOI] [PubMed] [Google Scholar]
- 138.Dong X, Shi W, Yuan G, Xie L, Wang S, Lin P. Intravitreal implantation of the biodegradable cyclosporin A drug delivery system for experimental chronic uveitis. Graefes Arch Clin Exp Ophthalmol. 2006;244:492–497. doi: 10.1007/s00417-005-0109-1. [DOI] [PubMed] [Google Scholar]
- 139.Shi W, Liu T, Xie L, Wang S. FK506 in a biodegradable glycolide-co-clatide-co-caprolactone polymer for prolongation of corneal allograft survival. Curr Eye Res. 2005;30:969–976. doi: 10.1080/02713680500320752. [DOI] [PubMed] [Google Scholar]
- 140.Leong KW, D'Amore PD, Marletta M, Langer R. Bioerodible polyanhydrides as drug-carrier matrices. II. Bio-compatibility and chemical reactivity. J Biomed Mater Res. 1986;20:51–64. doi: 10.1002/jbm.820200106. [DOI] [PubMed] [Google Scholar]
- 141.Ng SY, Shen HR, Lopez E, Zherebin Y, Barr J, Schacht E, Heller J. Development of a poly(ortho ester) prototype with a latent acid in the polymer backbone for 5-fluorouracil delivery. J Control Release. 2000;65:367–374. doi: 10.1016/S0168-36599900218-7. [DOI] [PubMed] [Google Scholar]
- 142.Uteza Y, Rouillot JS, Kobetz A, Marchant D, Pecqueur S, Arnaud E, Prats H, Honiger J, Dufier JL, Abitbol M, Neuner-Jehle M. Intravitreous transplantation of encapsulated fibroblasts secreting the human fibroblast growth factor 2 delays photoreceptor cell degeneration in Royal College of Surgeons rats. Proc Natl Acad Sci U S A. 1999;96:3126–3131. doi: 10.1073/pnas.96.6.3126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Tao W, Wen R, Goddard MB, Sherman SD, O'Rourke PJ, Stabila PF, Bell WJ, Dean BJ, Kauper KA, Budz VA, Tsiaras WG, Acland GM, Pearce-Kelling S, Laties AM, Aguirre GD. Encapsulated cell-based delivery of CNTF reduces photoreceptor degeneration in animal models of retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2002;43:3292–3298. [PubMed] [Google Scholar]
- 144.Bush RA, Lei B, Tao W, Raz D, Chan CC, Cox TA, Santos-Muffley M, Sieving PA. Encapsulated cell-based intraocular delivery of ciliary neurotrophic factor in normal rabbit: dose-dependent effects on ERG and retinal histology. Invest Ophthalmol Vis Sci. 2004;45:2420–2430. doi: 10.1167/iovs.03-1342. [DOI] [PubMed] [Google Scholar]
- 145.Thanos CG, Bell WJ, O'Rourke P, Kauper K, Sherman S, Stabila P, Tao W. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng. 2004;10:1617–1622. doi: 10.1089/ten.2004.10.1617. [DOI] [PubMed] [Google Scholar]
- 146.Sieving PA, Caruso RC, Tao W, Coleman HR, Thompson DJ, Fullmer KR, Bush RA. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci U S A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Nanjawade BK, Manvi FV, Manjappa AS. In situ-forming hydrogels for sustained ophthalmic drug delivery. J Control Release. 2007;122:119–134. doi: 10.1016/j.jconrel.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 148.El-Kamel AH. In vitro and in vivo evaluation of Pluronic F127-based ocular delivery system for timolol maleate. Int J Pharm. 2002;241:47–55. doi: 10.1016/S0378-51730200234-X. [DOI] [PubMed] [Google Scholar]
- 149.Ruel-Gariepy E, Leroux JC. In situ-forming hydrogels—review of temperature-sensitive systems. Eur J Pharm Biopharm. 2004;58:409–426. doi: 10.1016/j.ejpb.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 150.Miyazaki S, Suzuki S, Kawasaki N, Endo K, Takahashi A, Attwood D. In situ gelling xyloglucan formulations for sustained release ocular delivery of pilocarpine hydrochloride. Int J Pharm. 2001;229:29–36. doi: 10.1016/S0378-51730100825-0. [DOI] [PubMed] [Google Scholar]
- 151.Vyas SP, Ramchandraiah S, Jain CP, Jain SK. Polymeric pseudolatices bearing pilocarpine for controlled ocular delivery. J Microencapsul. 1992;9:347–355. doi: 10.3109/02652049209021249. [DOI] [PubMed] [Google Scholar]
- 152.Parkinson TM, Ferguson E, Febbraro S, Bakhtyari A, King M, Mundasad M. Tolerance of ocular iontophoresis in healthy volunteers. J Ocul Pharmacol Ther. 2003;19:145–151. doi: 10.1089/108076803321637672. [DOI] [PubMed] [Google Scholar]
- 153.Vollmer DL, Szlek MA, Kolb K, Lloyd LB, Parkinson TM. In vivo transscleral iontophoresis of amikacin to rabbit eyes. J Ocul Pharmacol Ther. 2002;18:549–558. doi: 10.1089/108076802321021090. [DOI] [PubMed] [Google Scholar]
- 154.Frucht-Pery J, Raiskup F, Mechoulam H, Shapiro M, Eljarrat-Binstock E, Domb A. Iontophoretic treatment of experimental pseudomonas keratitis in rabbit eyes using gentamicin-loaded hydrogels. Cornea. 2006;25:1182–1186. doi: 10.1097/01.ico.0000243959.14651.18. [DOI] [PubMed] [Google Scholar]
- 155.Hobden JA, O'Callaghan RJ, Hill JM, Reidy JJ, Rootman DS, Thompson HW. Tobramycin iontophoresis into corneas infected with drug-resistant Pseudomonas aeruginosa. Curr Eye Res. 1989;8:1163–1169. doi: 10.3109/02713688909000041. [DOI] [PubMed] [Google Scholar]
- 156.Hobden JA, Reidy JJ, O'Callaghan RJ, Insler MS, Hill JM. Ciprofloxacin iontophoresis for aminoglycoside-resistant pseudomonal keratitis. Invest Ophthalmol Vis Sci. 1990;31:1940–1944. [PubMed] [Google Scholar]
- 157.Eljarrat-Binstock E, Raiskup F, Frucht-Pery J, Domb AJ. Transcorneal and transscleral iontophoresis of dexamethasone phosphate using drug loaded hydrogel. J Control Release. 2005;106:386–390. doi: 10.1016/j.jconrel.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 158.Berdugo M, Valamanesh F, Andrieu C, Klein C, Benezra D, Courtois Y, Behar-Cohen F. Delivery of antisense oligonucleotide to the cornea by iontophoresis. Antisense Nucleic Acid Drug Dev. 2003;13:107–114. doi: 10.1089/108729003321629647. [DOI] [PubMed] [Google Scholar]
- 159.Barza M, Peckman C, Baum J. Transscleral iontophoresis of cefazolin, ticarcillin, and gentamicin in the rabbit. Ophthalmology. 1986;93:133–139. doi: 10.1016/s0161-6420(86)33780-1. [DOI] [PubMed] [Google Scholar]
- 160.Behar-Cohen FF, El Aouni A, Gautier S, David G, Davis J, Chapon P, Parel JM. Transscleral Coulomb-controlled iontophoresis of methylprednisolone into the rabbit eye: influence of duration of treatment, current intensity and drug concentration on ocular tissue and fluid levels. Exp Eye Res. 2002;74:51–59. doi: 10.1006/exer.2001.1098. [DOI] [PubMed] [Google Scholar]
- 161.Hayden BC, Jockovich ME, Murray TG, Voigt M, Milne P, Kralinger M, Feuer WJ, Hernandez E, Parel JM. Pharmacokinetics of systemic versus focal carboplatin chemotherapy in the rabbit eye: possible implication in the treatment of retinoblastoma. Invest Ophthalmol Vis Sci. 2004;45:3644–3649. doi: 10.1167/iovs.04-0228. [DOI] [PubMed] [Google Scholar]
- 162.Grossman RE, Chu DF, Lee DA. Regional ocular gentamicin levels after transcorneal and transscleral iontophoresis. Invest Ophthalmol Vis Sci. 1990;31:909–916. [PubMed] [Google Scholar]
- 163.Sarraf D, Equi RA, Holland GN, Yoshizumi MO, Lee DA. Transscleral iontophoresis of foscarnet. Am J ophthalmol. 1993;115:748–754. doi: 10.1016/s0002-9394(14)73642-6. [DOI] [PubMed] [Google Scholar]
- 164.Lam TT, Fu J, Chu R, Stojack K, Siew E, Tso MO. Intravitreal delivery of ganciclovir in rabbits by transscleral iontophoresis. J Ocul Pharmacol. 1994;10:571–575. doi: 10.1089/jop.1994.10.571. [DOI] [PubMed] [Google Scholar]
- 165.Jiang J, Gill HS, Ghate D, McCarey BE, Patel SR, Edelhauser HF, Prausnitz MR. Coated microneedles for drug delivery to the eye. Invest Ophthalmol Vis Sci. 2007;48:4038–4043. doi: 10.1167/iovs.07-0066. [DOI] [PubMed] [Google Scholar]
- 166.Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Therapy. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 167.Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Therapy. 2004;11(Suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 168.Provost N, Le Meur G, Weber M, Mendes-Madeira A, Podevin G, Cherel Y, Colle MA, Deschamps JY, Moullier P, Rolling F. Biodistribution of rAAV vectors following intraocular administration: evidence for the presence and persistence of vector DNA in the optic nerve and in the brain. Mol Ther. 2005;11:275–283. doi: 10.1016/j.ymthe.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 169.Bloquel C, Bourges JL, Touchard E, Berdugo M, BenEzra D, Behar-Cohen F. Non-viral ocular gene therapy: potential ocular therapeutic avenues. Adv Drug Deliv Rev. 2006;58:1224–1242. doi: 10.1016/j.addr.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 170.Klein R, Peto T, Bird A, Vannewkirk MR. The epidemiology of age-related macular degeneration. Am J Ophthalmol. 2004;137:486–495. doi: 10.1016/j.ajo.2003.11.069/. [DOI] [PubMed] [Google Scholar]
- 171.Leibowitz HM, Krueger DE, Maunder LR, Milton RC, Kini MM, Kahn HA, Nickerson RJ, Pool J, Colton TL, Ganley JP, Loewenstein JI, Dawber TR. The Framingham Eye Study monograph: an ophthalmological and epidemiological study of cataract, glaucoma, diabetic retinopathy, macular degeneration, and visual acuity in a general population of 2631 adults, 1973–1975. Surv Ophthalmol. 1980;24:335–610. doi: 10.1016/0039-62578090015-6. [DOI] [PubMed] [Google Scholar]
- 172.Iu LP, Kwok AK. An update of treatment options for neovascular age-related macular degeneration. Hong Kong Med J. 2007;13:460–470. [PubMed] [Google Scholar]
- 173.Sunness JS, Rubin GS, Applegate CA, Bressler NM, Marsh MJ, Hawkins BS, Haselwood D. Visual function abnormalities and prognosis in eyes with age-related geographic atrophy of the macula and good visual acuity. Ophthalmology. 1997;104:1677–1691. doi: 10.1016/s0161-6420(97)30079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Spilsbury K, Garrett KL, Shen WY, Constable IJ, Rakoczy PE. Overexpression of vascular endothelial growth factor (VEGF) in the retinal pigment epithelium leads to the development of choroidal neovascularization. Am J Pathol. 2000;157:135–144. doi: 10.1016/S0002-9440(10)64525-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schmidt-Erfurth U, Laqua H, Schlotzer-Schrehard U, Viestenz A, Naumann GO. Histopathological changes following photodynamic therapy in human eyes. Arch Ophthalmol. 2002;120:835–844. [PubMed] [Google Scholar]
- 176.Nobrega M, Bortolotto C, Farah M. Combined photodynamic therapy and intravitreal triamcinolone injection for choroidal neovascularization in Best disease. Can J Ophthalmol. 2007;42:761–762. doi: 10.3129/I07-138. [DOI] [PubMed] [Google Scholar]
- 177.Virgili G, Bini A. Laser photocoagulation for neo-vascular age-related macular degeneration. Cochrane Database Syst Rev. 2007:CD004763. doi: 10.1002/14651858.CD004763.pub2. [DOI] [PubMed] [Google Scholar]
- 178.Costa RA, Jorge R, Calucci D, Cardillo JA, Melo LA, Jr, Scott IU. Intravitreal bevacizumab for choroidal neovascularization caused by AMD (IBeNA Study): results of a phase 1 dose-escalation study. Investig Ophthalmol Vis Sci. 2006;47:4569–4578. doi: 10.1167/iovs.06-0433. [DOI] [PubMed] [Google Scholar]
- 179.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology. 1984;91:1464–1474. doi: 10.1016/s0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 180.Meyer CH. Current treatment approaches in diabetic macular edema. Ophthalmologica. 2007;221:118–131. doi: 10.1159/000098257. [DOI] [PubMed] [Google Scholar]
- 181.Bandello F, Pognuz R, Polito A, Pirracchio A, Menchini F, Ambesi M. Diabetic macular edema: classification, medical and laser therapy. Semin Ophthalmol. 2003;18:251–258. doi: 10.1080/08820530390895262. [DOI] [PubMed] [Google Scholar]
- 182.Charteris DG. Proliferative vitreoretinopathy: pathobiology, surgical management, and adjunctive treatment. Br J Ophthalmol. 1995;79:953–960. doi: 10.1136/bjo.79.10.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Scheer S, Morel C, Touzeau O, Sahel JA, Laroche L. Pharmacological adjuvants for surgical treatment of proliferative vitreoretinopathy. J Fr Ophtalmol. 2004;2:1051–1059. doi: 10.1016/s0181-5512(04)96264-x. [DOI] [PubMed] [Google Scholar]
- 184.Asaria RH, Kon CH, Bunce C, Charteris DG, Wong D, Khaw PT, Aylward GW. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy: results from a randomized, double-blind, controlled clinical trial. Ophthalmology. 2001;108:1179–1183. doi: 10.1016/S0161-64200100589-9. [DOI] [PubMed] [Google Scholar]
- 185.Forrester JV. Endogenous posterior uveitis. Br J Ophthalmol. 1990;74:620–623. doi: 10.1136/bjo.74.10.620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Forrester JV. Intermediate and posterior uveitis. Chem Immunol Allergy. 2007;92:228–243. doi: 10.1159/000099274. [DOI] [PubMed] [Google Scholar]
- 187.Lightman S. Use of steroids and immunosuppressive drugs in the management of posterior uveitis. Eye. 1991;5(Pt 3):294–298. doi: 10.1038/eye.1991.46. [DOI] [PubMed] [Google Scholar]
- 188.Holbrook JT, Jabs DA, Weinberg DV, Lewis RA, Davis MD, Friedberg D. Visual loss in patients with cytomegalovirus retinitis and acquired immunodeficiency syndrome before widespread availability of highly active antiretroviral therapy. Arch Ophthalmol. 2003;121:99–107. doi: 10.1001/archopht.121.1.99. [DOI] [PubMed] [Google Scholar]
- 189.Jacobson MA, Stanley H, Holtzer C, Margolis TP, Cunningham ET. Natural history and outcome of new AIDS-related cytomegalovirus retinitis diagnosed in the era of highly active antiretroviral therapy. Clin Infect Dis. 2000;30:231–233. doi: 10.1086/313612. [DOI] [PubMed] [Google Scholar]
- 190.Hoyng PF, van Beek LM. Pharmacological therapy for glaucoma: a review. Drugs. 2000;59:411–434. doi: 10.2165/00003495-200059030-00003. [DOI] [PubMed] [Google Scholar]
- 191.Camras CB, Bito LZ, Eakins KE. Reduction of intraocular pressure by prostaglandins applied topically to the eyes of conscious rabbits. Investig Ophthalmol Vis Sci. 1977;16:1125–1134. [PubMed] [Google Scholar]
- 192.Bill A, Heilmann K. Ocular effects of clonidine in cats and monkeys (Macaca irus) Exp Eye Res. 1975;21:481–488. doi: 10.1016/0014-48357590129-3. [DOI] [PubMed] [Google Scholar]
- 193.Kaufman PL, Barany EH. Residual pilocarpine effects on outflow facility after ciliary muscle disinsertion in the synomolgus monkey. Invest Ophthalmol. 1976;15:558–561. [PubMed] [Google Scholar]
- 194.Friedl BR, Mallonee J, Anderson DR. Short-term dose response characteristics of acetazolamide in man. Arch Ophthalmol. 1977;95:1809–1812. doi: 10.1001/archopht.1977.04450100111014. [DOI] [PubMed] [Google Scholar]
- 195.Wickstrom K. Acute bacterial conjunctivitis—benefits versus risks with antibiotic treatment. Acta Ophthalmol (Oxf) 2008;86:2–4. doi: 10.1111/j.1600-0420.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- 196.Riva C, Perlino P, Valpreda A, Ricotti E, Castagneri G, Balbo L, Musso A. Long lasting conjunctivitis: research of etiological factors. Minerva Pediatr. 1992;44:595–600. [PubMed] [Google Scholar]
- 197.McCulley JP. Blepharoconjunctivitis. Int Ophthalmol Clin. 1984;24:65–77. doi: 10.1097/00004397-198402420-00008. [DOI] [PubMed] [Google Scholar]
- 198.Warren D, Nelson KE, Farrar JA, Hurwitz E, Hierholzer J, Ford E, Anderson LJ. A large outbreak of epidemic keratoconjunctivitis: problems in controlling nosocomial spread. J Infect Dis. 1989;160:938–943. doi: 10.1093/infdis/160.6.938. [DOI] [PubMed] [Google Scholar]
- 199.Balasubramanian D, Bansal AK, Basti S, Bhatt KS, Murthy JS, Rao CM. The biology of cataract. The Hyderabad Cataract Research Group. Indian J Ophthalmol. 1993;41:153–171. [PubMed] [Google Scholar]
- 200.Basti S, Greenwald MJ. Principles and paradigms of pediatric cataract management. Indian J Ophthalmol. 1995;43:159–176. [PubMed] [Google Scholar]
- 201.Kahn HA, Leibowitz HM, Ganley JP, Kini MM, Colton T, Nickerson RS, Dawber TR. The Framingham Eye Study. I. Outline and major prevalence findings. Am J Epidemiol. 1977;106:17–32. doi: 10.1093/oxfordjournals.aje.a112428. [DOI] [PubMed] [Google Scholar]
- 202.Quillen DA. Common causes of vision loss in elderly patients. Am Fam Phys. 1999;60:99–108. [PubMed] [Google Scholar]
- 203.Sharma YR, Vajpayee RB, Bhatnagar R, Mohan M, Azad RV, Kumar M, Nath R. A simple accurate method of cataract classification. Cataract-I. Indian J Ophthalmol. 1989;37:112–117. [PubMed] [Google Scholar]
- 204.Kato A, Kimura H, Okabe K, Okabe J, Kunou N, Ogura Y. Feasibility of drug delivery to the posterior pole of the rabbit eye with an episcleral implant. Investig Ophthalmol Vis Sci. 2004;45:238–244. doi: 10.1167/iovs.02-1258. [DOI] [PubMed] [Google Scholar]
- 205.Ciulla TA, Criswell MH, Danis RP, Fronheiser M, Yuan P, Cox TA, Csaky KG, Robinson MR. Choroidal neovascular membrane inhibition in a laser treated rat model with intraocular sustained release triamcinolone acetonide microimplants. Br J Ophthalmol. 2003;87:1032–1037. doi: 10.1136/bjo.87.8.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Seah SK, Husain R, Gazzard G, Lim MC, Hoh ST, Oen FT, Aung T. Use of surodex in phacotrabeculectomy surgery. Am J Ophthalmol. 2005;139:927–928. doi: 10.1016/j.ajo.2004.10.052. [DOI] [PubMed] [Google Scholar]
- 207.Xie L, Shi W, Wang Z, Bei J, Wang S. Prolongation of corneal allograft survival using cyclosporine in a polylactide-co-glycolide polymer. Cornea. 2001;20:748–752. doi: 10.1097/00003226-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 208.Einmahl S, Behar-Cohen F, D'Hermies F, Rudaz S, Tabatabay C, Renard G, Gurny R. A new poly(ortho ester)-based drug delivery system as an adjunct treatment in filtering surgery. Investig Ophthalmol Vis Sci. 2001;42:695–700. [PubMed] [Google Scholar]
- 209.Zhou T, Lewis H, Foster RE, Schwendeman SP. Development of a multiple-drug delivery implant for intraocular management of proliferative vitreoretinopathy. J Control Release. 1998;55:281–295. doi: 10.1016/S0168-36599800061-3. [DOI] [PubMed] [Google Scholar]
- 210.Yasukawa T, Kimura H, Tabata Y, Miyamoto H, Honda Y, Ogura Y. Sustained release of cis-hydroxyproline in the treatment of experimental proliferative vitreoretinopathy in rabbits. Graefe Arch Clin Exp Ophthalmol (Albrecht von Graefes Arch Klin Exp Ophthalmol) 2002;240:672–678. doi: 10.1007/s00417-002-0484-9. [DOI] [PubMed] [Google Scholar]
- 211.Beeley NR, Stewart JM, Tano R, Lawin LR, Chappa RA, Qiu G, Anderson AB, de Juan E, Varner SE. Development, implantation, in vivo elution, and retrieval of a biocompatible, sustained release subretinal drug delivery system. J Biomed Materi Res. 2006;76:690–698. doi: 10.1002/jbm.a.30567. [DOI] [PubMed] [Google Scholar]
- 212.Jaffe GJ, Yang CH, Guo H, Denny JP, Lima C, Ashton P. Safety and pharmacokinetics of an intraocular fluocinolone acetonide sustained delivery device. Investig Ophthalmol Vis Sci. 2000;41:3569–3575. [PubMed] [Google Scholar]
- 213.Kagaya F, Usui T, Kamiya K, Ishii Y, Tanaka S, Amano S, Oshika T. Intraocular dexamethasone delivery system for corneal transplantation in an animal model. Cornea. 2002;21:200–202. doi: 10.1097/00003226-200203000-00015. [DOI] [PubMed] [Google Scholar]
- 214.Wang Y, Challa P, Epstein DL, Yuan F. Controlled release of ethacrynic acid from poly(lactide-co-glycolide) films for glaucoma treatment. Biomaterials. 2004;25:4279–4285. doi: 10.1016/j.biomaterials.2003.10.075. [DOI] [PubMed] [Google Scholar]
- 215.Theng JT, Ti SE, Zhou L, Lam KW, Chee SP, Tan D. Pharmacokinetic and toxicity study of an intraocular cyclosporine DDS in the anterior segment of rabbit eyes. Investig Ophthalmol Vis Sci. 2003;44:4895–4899. doi: 10.1167/iovs.02-1112. [DOI] [PubMed] [Google Scholar]
- 216.Wadood AC, Armbrecht AM, Aspinall PA, Dhillon B. Safety and efficacy of a dexamethasone anterior segment drug delivery system in patients after phacoemulsification. J Cataract Refract Surg. 2004;30:761–768. doi: 10.1016/j.jcrs.2003.08.028. [DOI] [PubMed] [Google Scholar]
- 217.Pandey SK, Cochener B, Apple DJ, Colin J, Werner L, Bougaran R, Trivedi RH, Macky TA, Izak AM. Intracapsular ring sustained 5-fluorouracil delivery system for the prevention of posterior capsule opacification in rabbits: a histological study. J Cataract Refract Surg. 2002;28:139–148. doi: 10.1016/S0886-33500101069-0. [DOI] [PubMed] [Google Scholar]
- 218.Xie L, Sun J, Yao Z. Heparin drug delivery system for prevention of posterior capsular opacification in rabbit eyes. Graefe Arch Clin Exp Ophthalmol (Albrecht von Graefes Arch Klin Exp Ophthalmol) 2003;241:309–313. doi: 10.1007/s00417-003-0645-5. [DOI] [PubMed] [Google Scholar]
- 219.Sultana Y, Aqil M, Ali A. Ion-activated, gelrite-based in situ ophthalmic gels of pefloxacin mesylate: comparison with conventional eye drops. Drug Deliv. 2006;13:215–219. doi: 10.1080/10717540500309164. [DOI] [PubMed] [Google Scholar]
- 220.Liu Z, Li J, Nie S, Liu H, Ding P, Pan W. Study of an alginate/HPMC-based in situ gelling ophthalmic delivery system for gatifloxacin. Int J Pharm. 2006;315:12–17. doi: 10.1016/j.ijpharm.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 221.Balasubramaniam J, Kant S, Pandit JK. In vitro and in vivo evaluation of the Gelrite gellan gum-based ocular delivery system for indomethacin. Acta pharmaceutica (Zagreb, Croatia) 2003;53:251–261. [PubMed] [Google Scholar]
- 222.Balasubramaniam J, Pandit JK. Ion-activated in situ gelling systems for sustained ophthalmic delivery of ciprofloxacin hydrochloride. Drug Deliv. 2003;10:185–191. doi: 10.1080/713840402. [DOI] [PubMed] [Google Scholar]
- 223.Liu Z, Yang XG, Li X, Pan W, Li J. Study on the ocular pharmacokinetics of ion-activated in situ gelling ophthalmic delivery system for gatifloxacin by microdialysis. Drug Dev Ind Pharm. 2007;33:1327–1331. doi: 10.1080/03639040701397241. [DOI] [PubMed] [Google Scholar]
- 224.Nelson MD, Bartlett JD, Corliss D, Karkkainen T, Voce M. Ocular tolerability of timolol in gelrite in young glaucoma patients. J Am Optom Assoc. 1996;67:659–663. [PubMed] [Google Scholar]
- 225.Srividya B, Cardoza RM, Amin PD. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J Control Release. 2001;73:205–211. doi: 10.1016/S0168-36590100279-6. [DOI] [PubMed] [Google Scholar]
- 226.Wu C, Qi H, Chen W, Huang C, Su C, Li W, Hou S. Preparation and evaluation of a carbopol/HPMC-based in situ gelling ophthalmic system for puerarin. Yakugaku Zasshi. 2007;127:183–191. doi: 10.1248/yakushi.127.183. [DOI] [PubMed] [Google Scholar]
- 227.Qi H, Chen W, Huang C, Li L, Chen C, Li W, Wu C. Development of a poloxamer analogs/carbopol-based in situ gelling and mucoadhesive ophthalmic delivery system for puerarin. Int J Pharm. 2007;337:178–187. doi: 10.1016/j.ijpharm.2006.12.038. [DOI] [PubMed] [Google Scholar]
- 228.Zhang JJ, Xie LK, Zhao NM, Chen ZJ, Xu Y. Pharmacokinetics of topically applied in-situ-forming gels of fluconazole in rabbit eyes. Yao xue xue bao (Acta Pharmaceutica Sinica) 2000;35:835–838. [PubMed] [Google Scholar]
- 229.Zhang JJ, Gao CF, Wang LY. Ocular pharmacokinetics and bioavailability of 0.2% ganciclovir in-situ gelling eye drops. Zhonghua Yan Ke Za Zhi. 2006;42:637–641. [PubMed] [Google Scholar]
- 230.Cao Y, Zhang C, Shen W, Cheng Z, Yu LL, Ping Q. Poly(N-isopropylacrylamide)-chitosan as thermosensitive in situ gel-forming system for ocular drug delivery. J Control Release. 2007;120:186–194. doi: 10.1016/j.jconrel.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 231.Lin HR, Sung KC, Vong WJ. In situ gelling of alginate/pluronic solutions for ophthalmic delivery of pilocarpine. Bio-macromolecules. 2004;5:2358–2365. doi: 10.1021/bm0496965. [DOI] [PubMed] [Google Scholar]
- 232.Gupta H, Jain S, Mathur R, Mishra P, Mishra AK, Velpandian T. Sustained ocular drug delivery from a temperature and pH triggered novel in situ gel system. Drug Deliv. 2007;14:507–515. doi: 10.1080/10717540701606426. [DOI] [PubMed] [Google Scholar]
- 233.Ma WD, Xu H, Wang C, Nie SF, Pan WS. Pluronic F127-g-poly(acrylic acid) copolymers as in situ gelling vehicle for ophthalmic drug delivery system. Int J Pharm. 2008;350:247–256. doi: 10.1016/j.ijpharm.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 234.Robinson GS, Pierce EA, Rook SL, Foley E, Webb R, Smith LE. Oligodeoxynucleotides inhibit retinal neovascularization in a murine model of proliferative retinopathy. Proc Natl Acad Sci U S A. 1996;93:4851–4856. doi: 10.1073/pnas.93.10.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Garrett KL, Shen WY, Rakoczy PE. In vivo use of oligonucleotides to inhibit choroidal neovascularisation in the eye. J Gene Med. 2001;3:373–383. doi: 10.1002/jgm.197. [DOI] [PubMed] [Google Scholar]
- 236.Hecquet C, Lefevre G, Valtink M, Engelmann K, Mascarelli F. Activation and role of MAP kinase-dependent pathways in retinal pigment epithelial cells: ERK and RPE cell proliferation. Investig Ophthalmol Vis Sci. 2002;43:3091–3098. [PubMed] [Google Scholar]
- 237.Ruckman J, Green LS, Beeson J, Waugh S, Gillette WL, Henninger DD, Claesson-Welsh L, Janjic N. 2′-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J Biol Chem. 1998;273:20556–20567. doi: 10.1074/jbc.273.32.20556. [DOI] [PubMed] [Google Scholar]
- 238.Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, Bennett J, Tolentino MJ. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210–216. [PubMed] [Google Scholar]
- 239.Murata M, Takanami T, Shimizu S, Kubota Y, Horiuchi S, Habano W, Ma JX, Sato S. Inhibition of ocular angiogenesis by diced small interfering RNAs (siRNAs) specific to vascular endothelial growth factor (VEGF) Curr Eye Res. 2006;31:171–180. doi: 10.1080/02713680500514636. [DOI] [PubMed] [Google Scholar]
- 240.Cordeiro MF, Mead A, Ali RR, Alexander RA, Murray S, Chen C, York-Defalco C, Dean NM, Schultz GS, Khaw PT. Novel antisense oligonucleotides targeting TGF-beta inhibit in vivo scarring and improve surgical outcome. Gene Therapy. 2003;10:59–71. doi: 10.1038/sj.gt.3301865. [DOI] [PubMed] [Google Scholar]
- 241.McCauley TG, Kurz JC, Merlino PG, Lewis SD, Gilbert M, Epstein DM, Marsh HN. Pharmacologic and pharmacokinetic assessment of anti-TGFbeta2 aptamers in rabbit plasma and aqueous humor. Pharm Res. 2006;23:303–311. doi: 10.1007/s11095-005-9305-2. [DOI] [PubMed] [Google Scholar]
- 242.Kitajima I, Unoki K, Maruyama I. Phosphorothioate oligodeoxynucleotides inhibit basic fibroblast growth factor-induced angiogenesis in vitro and in vivo. Antisense Nucleic Acid Drug Dev. 1999;9:233–239. doi: 10.1089/oli.1.1999.9.233. [DOI] [PubMed] [Google Scholar]
- 243.Roy S, Zhang K, Roth T, Vinogradov S, Kao RS, Kabanov A. Reduction of fibronectin expression by intravitreal administration of antisense oligonucleotides. Nat Biotechnol. 1999;17:476–479. doi: 10.1038/8654. [DOI] [PubMed] [Google Scholar]
- 244.Kulka M, Smith CC, Aurelian L, Fishelevich R, Meade K, Miller P, Ts'o PO. Site specificity of the inhibitory effects of oligo(nucleoside methylphosphonate)s complementary to the acceptor splice junction of herpes simplex virus type 1 immediate early mRNA 4. Proc Natl Acad Sci U S A. 1989;86:6868–6872. doi: 10.1073/pnas.86.18.6868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kulka M, Smith CC, Levis J, Fishelevich R, Hunter JC, Cushman CD, Miller PS, Ts'o PO, Aurelian L. Synergistic antiviral activities of oligonucleoside methylphosphonates complementary to herpes simplex virus type 1 immediate-early mRNAs 4, 5, and 1. Antimicrob Agents Chemother. 1994;38:675–680. doi: 10.1128/aac.38.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kean JM, Kipp SA, Miller PS, Kulka M, Aurelian L. Inhibition of herpes simplex virus replication by antisense oligo-2′-O-methylribonucleoside methylphosphonates. Biochemistry. 1995;34:14617–14620. doi: 10.1021/bi00045a001. [DOI] [PubMed] [Google Scholar]
- 247.Peyman A, Helsberg M, Kretzschmar G, Mag M, Grabley S, Uhlmann E. Inhibition of viral growth by antisense oligonucleotides directed against the IE110 and the UL30 mRNA of herpes simplex virus type-1. Biol Chem Hoppe-Seyler. 1995;376:195–198. doi: 10.1515/bchm3.1995.376.3.195. [DOI] [PubMed] [Google Scholar]
- 248.Anderson KP, Fox MC, Brown-Driver V, Martin MJ, Azad RF. Inhibition of human cytomegalovirus immediate-early gene expression by an antisense oligonucleotide complementary to immediate-early RNA. Antimicrob Agents Chemother. 1996;40:2004–2011. doi: 10.1128/aac.40.9.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Henry SP, Miner RC, Drew WL, Fitchett J, York-Defalco C, Rapp LM, Levin AA. Antiviral activity and ocular kinetics of antisense oligonucleotides designed to inhibit CMV replication. Invest Ophthalmol Vis Sci. 2001;42:2646–2651. [PubMed] [Google Scholar]
- 250.Detrick B, Nagineni CN, Grillone LR, Anderson KP, Henry SP, Hooks JJ. Inhibition of human cytomegalovirus replication in a human retinal epithelial cell model by antisense oligonucleotides. Invest Ophthalmol Vis Sci. 2001;42:163–169. [PubMed] [Google Scholar]
- 251.Sahoo SK, Dilnawaz F, Krishnakumar S. Nanotechnology in ocular drug delivery. Drug Discov Today. 2008;13:144–151. doi: 10.1016/j.drudis.2007.10.021. [DOI] [PubMed] [Google Scholar]


