Abstract
During the last 2 years, two new oral anticoagulants, dabigatran and rivaroxaban, have been approved in the United States. Phase II and Phase III clinical trials of dabigatran, rivaroxaban, and apixaban are summarized. Approach to perioperative management depends on the half-life of the medication, risk of surgical bleeding, and the patient's renal function. No reversal agent is available for any of the neworal anticoagulants. Management of bleeding patients is based on local measures and consideration of antifibrinolytic therapy and activated factor VII or prothrombin complex concentrate infusion based on healthy volunteer and animal studies. The new oral anticoagulants provide additional options to prevent venous thromboembolism in patients after orthopedic surgery or stroke in patients with atrial fibrillation but present unique challenges compared to warfarin.
Keywords: Anticoagulation, thromboembolism, dabigatran, rivaroxaban, apixaban
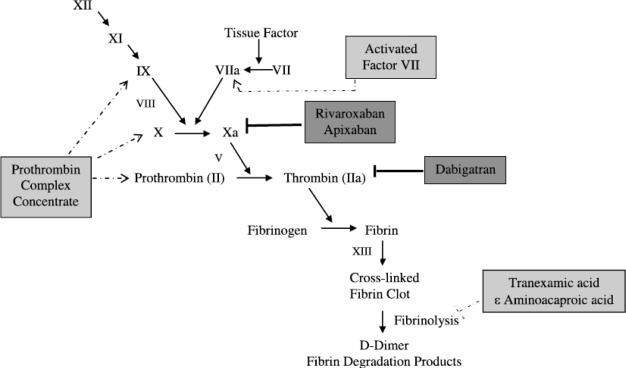
Fifty-seven years since warfarin was approved by the US Food and Drug Administration (FDA), two new oral anticoagulants have entered the US market. These drugs have given patients and providers alternatives to heparin and warfarin for prophylaxis against stroke in patients with atrial fibrillation and venous thromboembolism (VTE) after orthopedic procedures. As more patients have switched to these anticoagulants, issues have arisen such as management of bleeding and perioperative management. This review will focus on dabigatran and rivaroxaban as they are approved for clinical use and apixaban as it has completed Phase III studies. The pharmacokinetic data for these agents are summarized in Table 1. A review of the coagulation cascade and the sites of action of these agents is seen in Figure 1. The new oral drugs are very effective anticoagulants because they inhibit proteins at the end of the coagulation cascade. Reversal of the anticoagulant effect is challenging because antidotes for these anticoagulants do not exist. This review will summarize available clinical trial evidence and a proposed approach to management of bleeding and the perioperative setting.
TABLE 1.
Pharmacokinetic Properties of New Oral Anticoagulants
| Dabigatran | Rivaroxaban | Apixaban | |
|---|---|---|---|
| FDA-approved indications | Prevention of stroke and systemic embolism in nonvalvular atrial fibrillation | VTE prophylaxis after hip and knee replacement, stroke and systemic embolism prophylaxis in nonvalvular atrial fibrillation | Pending |
| Activity | Inhibits free and clot bound thrombin (factor IIa) | Inhibits factor Xa | Inhibits factor Xa |
| Dosing for atrial fibrillation | 150 mg BID, 75 mg BID if CrCl 15Y30 mL/min | 20 mg/d, 15 mg/d if CrCl 15Y50 mL/min | 5 mg BID‡ |
| Dosing for VTE prophylaxis | 10 mg/d | 2.5 mg BID‡ | |
| Onset of action | 1.5-3 h | 2-4 h | 3 h |
| Half-life | 14-17 h | 5-9 h, 11-13 h elderly§ | 8-15 h |
| Metabolism and excretion | 80% renal, 20% fecal | 66% renal, 33% fecal | 25% renal 75% biliary, fecal |
| Drug Interactions | P-glycoprotein inhibitors* | Potent CYP3A4 inhibitors†, P-glycoprotein inhibitors* | Potent CYP3A4 inhibitors†, P-glycoprotein inhibitors* |
| Detection of anticoagulant effect | ECT if available, TT most sensitive | Anti-Xa assay | Anti-Xa assay |
| Unique issues | Must be stored in original bottle | Highly protein bound and not dialyzable, take with evening meal | Highly protein bound and not dialyzable |
Rifampin, amiodarone.
Ketoconazole, itraconazole, voriconazole, ritonavir.
Dosing not approved by FDA.
Despite half-life, daily dosing because of persistence of anti-Xa activity. CrCl, creatinine clearance.
Figure 1.
Clotting cascade and location of activity of new oral anticoagulants and hemostatic agents. Proteins are depicted by their zymogen symbols. The new oral anticoagulants are depicted in dark gray boxes with bold inhibition lines. Hemostatic agents are in light gray boxes with dashed lines in the area of activity. The PCC is depicted as containing nonactivated proteins for simplicity but can contain activated proteins and factor VII also depending on the product.
DABIGATRAN
Clinical Trials
Dabigatran is an oral direct thrombin inhibitor that is FDA approved for stroke and systemic embolism prevention in patients with nonvalvular atrial fibrillation. The RE-LY trial randomized more than 18,000 patients with nonvalvular atrial fibrillation to blinded treatment with dabigatran 150 mg or 110 mg orally twice a day (BID) or open-label warfarin (Table 2). Dabigatran 150 mg was superior to warfarin in prevention of stroke or systemic embolism with the primary end point occurring in 1.11% per year of patients managed with dabigatran compared with 1.69% per year in patients treated with warfarin (p G 0.001 superiority). The 110-mg dose was noninferior to warfarin. The rate of ischemic stroke was significantly less only in patients treated with 150 mg of dabigatran. Life-threatening hemorrhage occurred less often with either dose of dabigatran. Intracranial hemorrhage occurred significantly less in the dabigatran 110-mg and 150-mg groups compared with warfarin with a rate of 0.23% per year, 0.3% per year, and 0.74% per year, respectively.1 The FDA approved dabigatran (Pradaxa; Boehringer Ingelheim, Ingelheim, Germany) 150 mg orally BID in October 2010 for prevention of stroke and systemic embolism in patients with nonvalvular atrial fibrillation. The 110-mg dose was not approved as subset analyses did not find a group in which the risk-benefit profile was superior to the 150-mg dose.2 Based on pharmacokinetic data, 75 mg orally BID was approved for patients with creatinine clearances between 15 mL per minute and 30 mL per minute, but other authors suggest caution with use in this group.3 Subsequent analysis has shown that poorly controlled patients on warfarin with international normalized ratio (INR) measurements in therapeutic range less than 65% of the time benefited the most and may be the best candidates for dabigatran therapy.4
TABLE 2.
Phase II and III Clinical Trials Using Dabigatran
| Trial Name | Indication | Dabigatran Dose | Comparator | Treatment Duration | Thrombotic Outcome | Major Bleeding |
|---|---|---|---|---|---|---|
| PETRO44 Phase II |
Nonvalvular atrial fibrillation | 50 mg, 150 mg or 300 mg BID | Warfarin (INR 2Y3) | 12 wk | Stroke and systemic embolism 50 mg: 1.7% 150 mg: 0% 300 mg: 0% Warfarin: 0% | 50 mg: 0% 150 mg: 0% 300 mg: 0% Warfarin: 0% |
| RE-LY1 Phase III |
Nonvalvular atrial fibrillation | 110 mg or 150 mg BID | Warfarin (INR 2Y3) | Median follow-up 2 y | Stroke and systemic embolism 110 mg: 1.53%/y‡ 150 mg: 1.11%/y* Warfarin: 1.69%/y | 110 mg: 2.71%/y* 150 mg: 3.11%/y Warfarin: 3.36%/y |
| RE-DEEM5 Phase II |
Secondary prevention after ACS | 50 mg, 75 mg, 110 mg, 150 mg BID | Placebo | 6 mo | CV death, MI, or stroke 50 mg BID: 4.6% 75 mg BID: 4.9% 110 mg BID: 3.0% 150 mg BID: 3.5% placebo: 3.8% | 50 mg BID: 0.8% 75 mg BID: 0.3% 110 mg BID: 2.0%† 150 mg BID: 1.2%† placebo: 0.5% |
| BISTRO I45 Phase II |
VTE prevention after THR | Dose escalation 12.5Y300 mg twice daily, 150 mg or 300 mg/d | N/A | 6Y10 d | All VTE 12.5 mg BID: 20.8% 25 mg BID: 9.5% 50 mg BID: 14.8% 100 mg BID: 19.4% 150 mg Daily: 9.1% 150 mg BID: 9.5% 200 mg BID: 19.0% 300 mg Daily: 0% | 0% in all groups |
| BISTRO II46 Phase II |
VTE prevention after THR or TKR | 50 mg, 150 mg, or 225 mg twice daily, 300 mg/d | Enoxaparin 40 mg/d | 6Y10 d | All VTE 50 mg BID: 28.5% 150 mg BID: 17.4%* 300 mg/d: 16.6%* 225 mg BID: 13.1%* Enoxaparin: 24% | 50 mg BID: 0.3%* 150 mg BID: 4.1% 300 mg/d: 4.7% 225 mg BID: 3.8% Enoxaparin: 2% |
| RE-MODEL47 Phase III |
VTE prevention after TKR | 150 mg or 220 mg/d | Enoxaparin 40 mg/d | 6Y10 d | VTE and all-cause mortality 150 mg: 40.5%‡ 220 mg: 36.4%‡ Enoxaparin: 37.7% |
150 mg: 1.3% 220 mg: 1.5% Enoxaparin: 1.3% |
| RE-NOVATE48 Phase III |
VTE prevention after THR | 150 mg or 220 mg/d | Enoxaparin 40 mg/d | 28Y35 d | VTE and all-cause mortality 150 mg: 8.6%‡ 220 mg: 6%‡ Enoxaparin: 6.7% | 150 mg: 1.3% 220 mg: 2% Enoxaparin: 1.6% |
| RE-MOBILIZE49 Phase III |
VTE prevention after TKR | 150 mg or 220 mg/d | Enoxaparin 30 mg BID | 12Y15 d | VTE and all-cause mortality 150 mg: 34%† 220 mg: 31%† Enoxaparin: 25% | 150 mg: 0.6% 220 mg: 0.6% Enoxaparin: 1.4% |
| RE-NOVATE II7 Phase III |
VTE prevention after THR | 220 mg/d | Enoxaparin 40 mg/d | 208Y35 d | VTE and all-cause mortality 220 mg: 7.7%‡ Enoxaparin: 8.8% | 220 mg: 1.4% Enoxaparin: 0.9% |
| RE-COVER8 Phase III |
Acute VTE treatment | 150 mg BID | Warfarin (INR 2Y3) | 6 mo | Recurrent VTE Dabigatran: 2.4%‡ Warfarin: 2.1% | Dabigatran: 1.6% Warfarin: 1.9% |
| RE-MEDY Phase III |
Secondary VTE prophylaxis | 150 mg BID | Warfarin (INR 2Y3) | 18 mo | Not reported | Not reported |
Statistically significant superiority demonstrated over comparator.
Statistically significant inferiority demonstrated over comparator.
Statistically significant noninferiority demonstrated to comparator.
ACS, acute coronary syndrome; TKR, total knee replacement; THR, total hip replacement.
A phase II placebo-controlled dose escalation study (50Y150 mg BID) of dabigatran in patients after myocardial infarction showed equal rates of cardiovascular death, myocardial infarction, and stroke, but a dose-dependent increase in bleeding rates.5 Additional studies of dabigatran for secondary prevention after acute coronary syndromes are not currently available.
Dabigatran has also been studied in prophylaxis of VTE after knee and hip replacement. The RE-MODEL and REMOBILIZE trials compared 150 mg and 220 mg of dabigatran with enoxaparin 40 mg per day subcutaneously or 30 mg BID subcutaneously, respectively. Both dabigatran doses were found to have equal bleeding rates in comparison with enoxaparin. However, enoxaparin 30 mg BID was superior to dabigatran, whereas dabigatran was noninferior to enoxaparin 40 mg per day (Table 2).6 In a study of 2,000 patients treated after total hip replacement, VTE or death occurred in 2.2% of patients treated with dabigatran 220 mg compared with 4.2% of patients treated with enoxaparin 40 mg per day (risk difference, −1.9%; p = 0.03 superiority). Major bleeding was similar between the groups (1.4% dabigatran, 0.9% enoxaparin; p = 0.4).7 Overall, these data suggest similar efficacy to enoxaparin 40 mg per day in VTE prophylaxis after orthopedic surgery with a similar bleeding risk. Dabigatran has been approved in Europe and Canada for prevention of VTE after orthopedic surgery based on these data.
The RE-COVER trial examined the use of dabigatran to treat VTE in 2,500 patients with proximal deep vein thrombosis or pulmonary embolism. All patients were treated with low-molecular-weight heparin and then randomized to dabigatran 150 mg BID or warfarin for 6 months in a double-blind, double-dummy design. Recurrent VTE occurred in 2.4% in the dabigatran arm and 2.1% in the warfarin group (p G 0.001, noninferiority). Major bleeding was equal, but the location of bleeding was more often in a critical organ (nine intracranial hemorrhages with warfarin vs. one intracranial hemorrhage with dabigatran). The incidence of any bleeding was also higher in thewarfarin group (21.9 vs. 16.1%; hazard ratio [HR], 0.71).8 The RE-MEDY trial is an extension of the RE-COVER trial examining the use of dabigatran for secondary prevention of VTE. The study was completed in October 2010, and we anticipate results in the next year.
Laboratory Testing
One of the major benefits of dabigatran over warfarin is that laboratory monitoring is not required during therapy. However, there are many instances in which knowing the degree of anticoagulation is paramount. For patients on dabigatran, the activated partial thromboplastin time (aPTT) increases with larger doses; however, the dose response is not linear and plateaus at higher concentrations of dabigatran.3,9 The prothrombin time (PT/INR) is variably affected but has been shown to rise with therapeutic doses.9 The INR is an insensitive measure of dabigatran activity and should not be used to monitor patients. Elevations in activated clotting time measured by thromboelastography (TEG) have been reported,10 but animal studies showed similar TEG profiles in pigs on dabigatran and without anticoagulation.11 The thrombin time (TT) measures the direct activity of thrombin and is the most sensitive to the effects of dabigatran. If the TT is normal, there is no dabigatran in the sample. At high concentrations of dabigatran, however, the thrombin time may be above a measurable level. The ecarin clotting time (ECT) also directly measures the anticoagulant effect of direct thrombin inhibitors but is less sensitive than the TT, thus, a more accurate measure of the concentration of dabigatran. The ECTis not widely available, thus, most hospitals may be limited to aPTT and TT to interpret the extent of anticoagulation in patients on dabigatran.3
RIVAROXABAN
Clinical Trials
Rivaroxaban is an oral direct inhibitor of activated factor X (Xa) that is FDA approved for stroke and systemic embolization prevention in nonvalvular atrial fibrillation and VTE prevention after knee and hip replacement. The ROCKET-AF trial randomized more than 14,000 patients with atrial fibrillation and two stroke risk factors to rivaroxaban 20 mg per day or warfarin (Table 3). Rivaroxaban was noninferior to warfarin in the prevention of stroke and systemic embolism (2.1% per year rivaroxaban vs. 2.4% per year warfarin, p G 0.001 noninferiority). Major bleeding was equal between the rivaroxaban and warfarin groups at 5.6% and 5.4%, respectively. Fatal bleeding was 50% lower in the rivaroxaban group (0.4% vs. 0.8%, p = 0.003). Intracranial hemorrhage rates were also decreased with rivaroxaban (0.8% vs. 1.2%, p = 0.02).12 Rivaroxaban was approved for prevention of stroke in patients with nonvalvular atrial fibrillation in November 2011 (20 or 15 mg per day orally if creatinine clearance 15Y50 mL per minute).13 After discontinuation of rivaroxaban in the ROCKET-AF trial, an increased risk of stroke was found, leading to a black box warning.13 To maintain blinding in the trial at its completion, patients were not bridged when switching from rivaroxaban to warfarin. Inadequate anticoagulation in high-risk patients likely led to increased stroke risk.
TABLE 3.
Phase II and III Clinical Trials Using Rivaroxaban
| Trial Name | Indication | Rivaroxaban Dose | Comparator | Treatment Duration | Thrombotic Outcome | Major Bleeding |
|---|---|---|---|---|---|---|
| ROCKET-AF12 Phase III |
Nonvalvular atrial fibrillation |
20 mg/d | Warfarin (INR 2Y3) |
Median treatment 19.7 mo |
Stroke and Systemic embolism Rivaroxaban: 2.12%/y‡ Warfarin: 2.42%/y |
Rivaroxaban: 3.6%/y Warfarin: 3.4%/y |
| ATLAS ACS-TIMI 4614 Phase II |
Secondary prevention after ACS |
5Y20 mg/d total dose |
Placebo | 6 mo | CV death, MI, stroke, revascularization 5 mg: 5.8% 10 mg: 3.8% 15 mg: 6.2% 20 mg: 5.5% Placebo: 5.1% |
5 mg: 0.7%† 10 mg: 1.5%† 15 mg: 1.8%† 20 mg: 1.8%† Placebo: 0.1% |
| ATLAS ACS-TIMI 5115 Phase III |
Secondary prevention after ACS |
2.5 mg and 5 mg BID |
Placebo | 31 mo | CV death, MI, stroke 2.5 mg BID: 9.1%* 5 mg BID: 8.8%* Placebo: 10.7% |
2.5 mg BID: 1.8%† 5 mg BID: 2.4%† Placebo: 0.6% |
| RECORD116 Phase III |
VTE prevention after THR |
10 mg/d | Enoxaparin 40 mg/d |
31 Y39 d | VTE and all-cause mortality Rivaroxaban: 1.1%* Enoxaparin: 3.7% |
Rivaroxaban: 0.3% Enoxaparin: 0.1% |
| RECORD217 Phase III |
VTE prevention after THR |
10 mg/d | Enoxaparin 40 mg/d |
31Y39 d, enoxaparin 10Y14 d |
VTE and all-cause mortality Rivaroxaban: 2%* Enoxaparin: 9.3% |
Rivaroxaban: G0.1% Enoxaparin: G0.1% |
| RECORD318 Phase III |
VTE prevention after TKR |
10 mg/d | Enoxaparin 40 mg/d |
10Y14 d | VTE and all-cause mortality Rivaroxaban: 9.6%* Enoxaparin: 18.9% |
Rivaroxaban: 0.6% Enoxaparin: 0.5% |
| RECORD419 Phase III |
VTE prevention after TKR |
10 mg/d | Enoxaparin 30 mg BID |
10Y14 d | VTE and all-cause mortality Rivaroxaban: 6.9%* Enoxaparin: 10.1% |
Rivaroxaban: 0.7% Enoxaparin: 0.3% |
| MAGELLAN50 Phase III |
VTE medical patients |
10 mg/d | Enoxaparin 40 mg daily |
35Y39 d, enoxaparin 10Y14 d |
VTE death and all VTE Rivaroxaban: 4.4%* Enoxaparin: 5.7% |
Rivaroxaban: 1.1%† Enoxaparin: 0.4% |
| ODIXa-DVT51 Phase II |
Acute VTE treatment |
10 mg, 20 mg, 30 mg BID or 40 mg/d |
Enoxaparin/warfarin (INR 2Y3) |
12 mo | Thrombotic burden and VTE death 10 mg BID: 53% 20 mg BID: 59.2% 30 mg BID: 56.9% 40 mg: 43.8% Warfarin: 45.9% |
10 mg BID: 1.7% 20 mg BID: 1.7% 30 mg BID: 3.3% 40 mg: 1.7% Warfarin: 0% |
| EINSTEIN21 Phase III |
Acute VTE treatment |
15 mg BID for 3 wk, 20 mg/d |
Enoxaparin/warfarin (INR 2Y3) |
3Y12 mo | Recurrent VTE Rivaroxaban: 2.1%‡ Warfarin: 3.0% |
Rivaroxaban: 0.8% Warfarin: 1.2% |
Statistically significant superiority demonstrated over comparator.
Statistically significant inferiority demonstrated over comparator.
Statistically significant noninferiority demonstrated to comparator.
ACS, acute coronary syndrome; MI, myocardial infarction; THR, total hip replacement; TKR, total knee replacement.
The ATLAS-TIMI 46 and 51 studies used rivaroxaban in patients after acute coronary syndromes to reduce cardiovascular end points.14,15 Compared with placebo, rivaroxaban decreased the composite end point of cardiovascular death, myocardial infarction, and stroke but caused a significantly higher major bleeding. The use of rivaroxaban after acute coronary syndromes currently is not standard of care.
In the RECORD trials, rivaroxaban was compared with enoxaparin for VTE prophylaxis after total knee and hip replacement.16-19 Rivaroxaban 10 mg per day orally was found to be superior to enoxaparin 40 mg per day and 30 mg BID. In a systematic review of these studies, the relative risk of VTE was 0.38 compared with that of enoxaparin 40 mg per day (p < 0.0001) and 0.77 in comparison with enoxaparin 30 mg BID (p = 0.05). No significant difference in postoperative bleeding was noted. In all of these studies, rivaroxaban was started within 6 to 8 hours of surgery and continued for an average of 12 days after knee replacement and 35 days after hip replacement.20 In July 2011, the FDA approved rivaroxaban (Xarelto; Janssen Pharmaceuticals, Titusville, NJ) for VTE prophylaxis after orthopedic surgery.
Rivaroxaban has also been tested against warfarin in the treatment of VTE. The EINSTEIN trial was an open-label randomized noanferiority study of rivaroxaban 15 mg BID for 3 weeks then 20 mg per day compared with enoxaparin bridged to warfarin. Recurrent VTE occurred in 3% of patients treated with enoxaparin/warfarin and 2.1% patients treated with rivaroxaban (p < 0.001 noninferiority). Major and clinically relevant bleeding was similar between the groups (major bleed 0.8% rivaroxaban vs. 1.2% enoxaparin/warfarin; p = 0.21). The EINSTEIN extension study showed that rivaroxaban was effective for secondary VTE prophylaxis, with recurrent VTE in 7.1% of the placebo group versus 1.3% on rivaroxaban (p < 0.001). Major bleeding occurred in 0.7% of patients on rivaroxaban and zero patients on placebo (p = 0.11).21 This suggests that bleeding risk is low with rivaroxaban, but comparison to bleeding rates for long-term anticoagulation on warfarin would require extrapolation from other studies.
Laboratory Testing
Routine laboratory monitoring of rivaroxaban is not required. As factor is a part of the common coagulation pathway, inhibitors of factor Xa should prolong the PT and aPTT. The degree of prolongation is dependent on the reagent used. No effect was seen on the TT or fibrinogen activity assays.22 Dose dependent prolongation of TEG parameters (R and K times) has been reported.23 Chromogenic anti-Xa assays can be standardized to measure rivaroxaban, but this test may not be routinely available.24
APIXABAN
Clinical Trials
Apixaban is an oral direct factor Xa inhibitor that has completed several phase III trials but has not yet been approved by the FDA (Table 4). Apixaban has been studied in two large trials in patients with atrial fibrillation. The AVERROES trial randomized 5,600 patients unsuitable for warfarin therapy to apixaban 5 mg BID versus aspirin. With a mean follow-up of 1.1 years, the trial was stopped early for benefit as the rate of stroke or systemic embolism was 3.7% per year in the aspirin group versus 1.6% per year with apixaban. Similar rates of bleeding were seen in both treatment groups including rates of intracranial hemorrhage (0.4% per year in both groups).25 This trial has been criticized because of the lack of standardization of the aspirin dose and the use of enteric-coated aspirin. The ARISTOTLE trial compared apixaban 5mg BID with warfarin in more than 18,000 patients with atrial fibrillation and one stroke risk factor. Apixaban was superior to warfarin in prevention of stroke or systemic embolization. The rate of hemorrhagic stroke was reduced by one half with apixaban (0.24% per year apixaban vs. 0.47% per year warfarin; p < 0.001). The rate of death from any cause was also lower in the apixaban group (HR, 0.89; p = 0.047). Intracranial hemorrhage was reduced from 0.8% per year with warfarin to 0.33% per year with apixaban (p < 0.001). Major bleeding occurred significantly less often with apixaban compared with warfarin and a 7.7% absolute risk reduction for all bleeding was noted with apixaban (p < 0.001).26 Overall, apixaban appears to be an effective alternative to warfarin for prevention of stroke and systemic embolism in patients with atrial fibrillation.
TABLE 4.
Phase II and III Clinical Trials of Apixaban
| Trial Name | Indication | Dose | Comparator | Treatment Duration | Thrombotic Outcome | Major Bleeding |
|---|---|---|---|---|---|---|
| AVERROES25 Phase III |
Nonvalvular atrial fibrillation | 5 mg BID | Aspirin 81Y324 mg | Median follow-up 1.1 y | Stroke or systemic embolism Apixaban: 1.6%/y* Aspirin: 3.7%/y | Apixaban: 1.4%/y Aspirin: 1.2%/y |
| ARISTOTLE26 Phase III |
Nonvalvular atrial fibrillation | 5 mg BID | Warfarin (INR 2-3) | Median follow-up 1.8 y | Stroke or systemic embolism Apixaban: 1.3%/y* Warfarin: 1.6%/y | Apixaban: 2.1%/y* Warfarin: 3.1%/y |
| APPRAISE27 Phase II |
Secondary prevention after ACS | 5Y20 mg/d | Placebo | 26 wk | CV Death, MI, revascularization and Stroke 2.5 mg BID: 7.6% 10 mg/d: 6.0% Placebo: 8.7% | 2.5 mg BID: 0.8% 10 mg/d: 0% 10 mg BID: 2.9%† 20 mg/d: 4.1%† Placebo: 0% |
| APPRAISE-228 Phase III |
Secondary prevention after ACS | 5 mg BID | Placebo | Median follow-up 241 d | CV Death, MI, and stroke Apixaban: 7.5% Placebo: 7.9% | Major Bleeding Apixaban: 1.3%† Placebo: 0.5% |
| ADVANCE129 Phase III |
VTE prevention after TKR | 2.5 mg BID | Enoxaparin 30 mg BID | 12 d | VTE and all-cause mortality Apixaban: 9% Enoxaparin: 8.8% | Apixaban: 0.7%* Enoxaparin: 1.4% |
| ADVANCE-230 Phase III |
VTE prevention after TKR | 2.5 mg BID | Enoxaparin 40 mg/d | 12 d | VTE and all-cause mortality Apixaban: 15%* Enoxaparin: 24% | Apixaban: 0.6% Enoxaparin: 0.9% |
| ADVANCE-352 Phase III |
VTE prevention after THR | 2.5 mg BID | Enoxaparin 40 mg/d | 35 d | VTE and all-cause mortality Apixaban: 1.4%* Enoxaparin: 3.9% | Apixaban: 0.8% Enoxaparin: 0.7% |
| Botticelli DVT53 Phase II |
Acute VTE treatment | 5 mg or 10 mg BID, 20 mg/d | Enoxaparin/warfarin | 84Y91 d | VTE and increased thrombotic burden: 5 mg BID: 6% 10 mg BID: 5.6% 20 mg/d: 2.6% Warfarin: 4.2% | 5 mg BID: 0.7% 10 mg BID: 0% 20 mg/d: 1.6% Warfarin: 0% |
| Metastatic cancer54 Phase II |
VTE prevention metastatic cancer | 5Y20 mg | Placebo | 12 wk | Symptomatic VTE Apixaban: 0% Placebo: 10% | 5 mg: 0% 10 mg: 0% 20 mg: 6% Placebo: 3% |
Statistically significant superiority demonstrated over comparator.
Statistically significant inferiority demonstrated over comparator.
ACS, acute coronary syndrome; MI, myocardial infarction; THR, total hip replacement; TKR, total knee replacement.
A Phase II and III study tested the use of apixaban with antiplatelet therapy after acute coronary syndromes.27,28 The APPRAISE-2 studywas discontinued early because of increased major bleeding without a decrease in the composite primary end point of cardiovascular death, recurrent myocardial infarction, and stroke.
Apixaban has been evaluated in prevention but not treatment of VTE. The ADVANCE trials have examined the use of apixaban 2.5 mg BID versus enoxaparin in prophylaxis of VTE after orthopedic surgery. When compared with enoxaparin 30 mg BID, apixaban failed noninferiority to enoxaparin with rates of VTE and all-cause mortality in 9% of the apixaban and 8.9% of the enoxaparin groups.29
However, when compared with the European regimen of enoxaparin 40 mg per day, apixabanwas found to be superior with equal bleeding rates after total knee and hip replacement.30,31
Laboratory Testing
Apixaban has a mechanism of action similar to rivaroxaban with direct inhibition of factor Xa. Apixaban also prolongs the aPTT and PT levels with variability in the PT depending on the reagents used in testing. The linear correlation of the plasma concentration of apixaban and anti-Xa levels standardized to apixaban or to low-molecular-weight heparin are equally strong (r = 0.967). Therefore, recalibration of anti-Xa testing may not be necessary to determine the degree of anticoagulation with apixaban.32
MANAGEMENT OF BLEEDING WITH NEW ORAL ANTICOAGULANTS
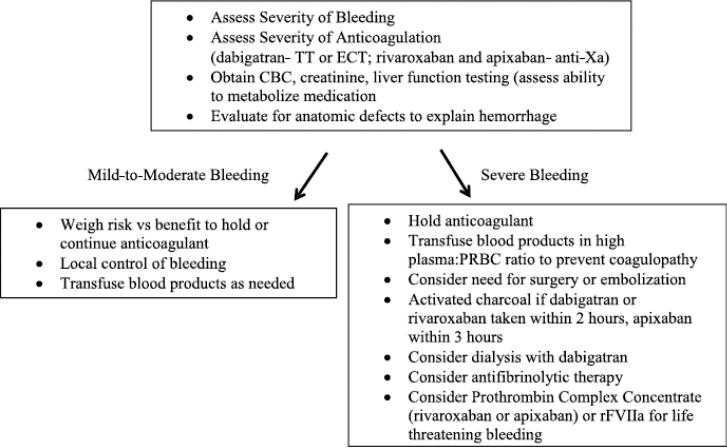
The bleeding rates with the new oral anticoagulants are generally equal to or less than bleeding rates with warfarin, but antidotes are not available. Figure 1 shows the sites of action of the neworal anticoagulants and hemostatic agents that could be used. Algorithms for managing hemorrhage in patients on dabigatran have been developed.33 A proposed management guideline is presented in Figure 2.
Figure 2.
Management guideline for bleeding while taking dabigatran, rivaroxaban, or apixaban.
Initial evaluation for bleeding patients on the new oral anticoagulants includes an assessment of hemodynamic stability, severity of bleeding, and level of anticoagulation. Life threatening bleeding (i.e., intracranial hemorrhage) requires the most aggressive response. Baseline clotting times, fibrinogen activity, complete blood count, creatinine, and liver function tests should be obtained. Alteration in renal function will affect the metabolism of dabigatran the most and apixaban the least. Apixaban and rivaroxaban metabolism is altered by changes in liver function. Assessment for anatomic etiology of the hemorrhage should be sought with use of local control measures if possible. Activated charcoal will decrease absorption of the anticoagulants if administered within 2 to 3 hours of ingestion of the anticoagulant. Dialysis will remove dabigatran because of its low plasma protein binding, whereas rivaroxaban and apixaban are likely not dialyzable.3,34 The volume of distribution of dabigatran is large (60-70 L);3 therefore, multiple sessions of dialysis may be required. Extrapolating from the trauma literature, if massive transfusion is required, we recommend transfusion in 1:1 plasma:packed red blood cell ratio to prevent dilutional and consumptive coagulopathy.35
In cases of significant bleeding, additional hemostatic agents should be considered (Table 5). Antifibrinolytic medication provides clot stabilization if fibrin is able to form. In a large randomized trial of injured patients not taking the new oral anticoagulants, tranexamic acid was shown to decrease the risk of death caused by hemorrhage when given within the first 3 hours of injury.36 Antifibrinolytic agents have been ineffective in reducing bleeding times with direct thrombin inhibitors and may not be useful for patients taking dabigatran.3 A recent prospective case series suggests decreased postoperative blood loss in patients treated with both rivaroxaban prophylaxis and tranexamic acid.37 Reversal agents for the new oral anticoagulants including an inactivated Xa product are in development but are not currently available.38 In healthy subjects, the anticoagulant effect of rivaroxaban can be reversed with administration of 50 units/kg of Cofact (Sanquin; Amsterdam, Netherlands), a nonactivated four-factor prothrombin complex concentrate (PCC) (Table 5). In patients on dabigatran, clotting times remained prolonged after PCC infusion, showing inadequate reversal of anticoagulation effect.39 In a rat tail model of bleeding, recombinant activated factor VII, nonactivated four-factor PCC, and activated PCC were shown to significantly reduce bleeding times in dabigatran-treated animals.3,40 Laboratory coagulation tests did not predict the reversal of bleeding in the mice, however.40 In a mouse model of intracranial hemorrhage with dabigatran use, a nonactivated fourfactor PCC prevented hematoma expansion, but activated factor VII did not have an effect.41 Clinical data on dabigatran and rivaroxaban reversal using PCCs and activated factor VII in humans are not available. In addition, four-factor PCCs are not available in the United States (Table 5). Thrombosis and disseminated intravascular coagulation have occurred with administration of activated factor VII and activated and nonactivated PCCs. Therefore, the risk of hemorrhage needs to be weighed against the risk of using any of these procoagulant agents and patients must be monitored closely.
TABLE 5.
Potential Useful Medications for Bleeding While on New Anticoagulants
| Name | Agent Category | Clotting Factors in Product | Available in United States? |
|---|---|---|---|
| Tranexamic acid | Antifibrinolytic | None | Yes |
| ? Aminoacaproic acid | Antifibrinolytic | None | Yes |
| NovoSeven | Activated factor VII | Activated VII | Yes |
| Cofact | 4-Factor PCC | Nonactivated II, VII, IX, X | No |
| Beriplex, Octaplex | 4-Factor PCC | Nonactivated II, VII, IX, X, Protein C and S | No |
| Profilnine, Bebulin | 3-Factor PCC | Nonactivated II, IX, X, small amounts VII | Yes |
| Feiba | Activated PCC | Activated VII, nonactivated II, IX, X | Yes |
PERIOPERATIVE MANAGEMENT
Timing of anticoagulant discontinuation before surgery depends on the half-life of the anticoagulant, the patient's renal function, and the surgical risk of bleeding. Creatinine clearance plays the largest role in perioperative management of dabigatran. Table 6 summarizes recommendations regarding timing of discontinuation in standard risk procedures. High-risk procedures including cardiac surgery, neurosurgery, abdominal surgery, or procedures requiring spinal anesthesia may require 2 to 4 days off dabigatran in patients with normal renal function and 4 days off therapy with creatinine clearance 30 to 50 mL per minute.3 Checking an ECT or TT in patients with renal impairment on dabigatran is an option to ensure that minimal anticoagulant effect remains before the procedure. Rivaroxaban has a significantly shorter half-life than dabigatran and thus could be discontinued 24 hours before surgery.13 The half life of rivaroxaban in elderly patients increases, so 48 hours may be necessary to allow for proper elimination. An increased risk of stroke has been reported after discontinuation of rivaroxaban, thus, minimizing the duration without anticoagulation in high-risk patients is recommended.13 In elderly patients, higher levels of apixaban have been reported. Providers should consider discontinuing apixaban for 48 hours or checking an anti-Xa level before surgery.42
TABLE 6.
Timing of Discontinuation of New Oral Anticoagulants Before Standard Risk Procedures
| Creatinine Clearance | Dabigatran | Rivaroxaban | Apixaban |
|---|---|---|---|
| >50 mL/min | 24 h | 24 h | 24-36 h |
| 30-50 mL/min | 48 h | 48 h | 48 h |
| <30 mL/min | 5 d |
Timing of resumption of the new anticoagulants after surgery is dependent on bleeding risks and the dose used. It is important to remember that these drugs fully anticoagulate the patient in 2 to 4 hours. In clinical trials for VTE prophylaxis after orthopedic surgery, dabigatran was initiated at one-half dose 1 to 4 hours after surgery and full dose 12 hours later.6 Rivaroxaban was initiated 6 to 8 hours after wound closure and apixaban was started 12 to 24 hours postoperatively.20,43 For procedures with a low bleeding risk, full anticoagulation with apixaban could be restarted after 24 hours, whereas resumption of anticoagulation after major surgery could be considered 48 hours postoperatively. 43 Additional clinical data and experience with the new anticoagulants will influence perioperative and postoperative management in the future.
CONCLUSIONS
Two new oral anticoagulants are available in the United States with additional agents likely to be approved in the near future. Each of these agents has the benefit of oral administration and uniform dosing. A majority of the clinical benefit is likely secondary to consistent anticoagulant effect. Monitoring of anticoagulant activity is not required but may be necessary in specific instances such as bleeding. Determining the anticoagulant effect of dabigatran requires special coagulation testing using the TT or ECT. Rivaroxaban and apixaban can be monitored through standardized anti-Xa assays. Perioperative and postoperative management of anticoagulation should be determined by the surgical risk of bleeding and renal function of the patient, which may be affected by age. In the bleeding patient, reversal of anticoagulant effect depends on the severity of hemorrhage and hepatic and renal function, which will determine the metabolism of the drugs. In healthy volunteers, PCC can reverse the effects of rivaroxaban, but it is unknown if this can be extrapolated to bleeding individuals on any Xa inhibitor if severe hemorrhage occurred. Reversal of dabigatran with activated PCC and factor VII has only been shown in animals. Until additional clinical data become available, physicians will need to rely on a hemorrhage management algorithm and clinical judgment.
Contributor Information
Lisa M. Baumann Kreuziger, University of Minnesota Regions Hospital.
Colleen T Morton, University of Minnesota Regions Hospital.
David J Dries, University of Minnesota Regions Hospital.
REFERENCES
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, et al. RE-LY Steering Committee and Investigators Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- 2.Beasley BN, Unger EF, Temple R. Anticoagulant optionsVwhy the FDA approved a higher but not a lower dose of dabigatran. N Engl J Med. 2011;364:1788–1790. doi: 10.1056/NEJMp1103050. [DOI] [PubMed] [Google Scholar]
- 3.van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilateVa novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–1127. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- 4.Wallentin L, Yusuf S, Ezekowitz MD, Alings M, Flather M, Franzosi MG, Pais P, Dans A, Eikelboom J, Oldgren J, et al. RE-LY Investigators Efficacy and safety of dabigatran compared with warfarin at different levels of international normalized ratio control for stroke prevention in atrial fibrillation: an analysis of the RE-LY trial. Lancet. 2010;376:975–983. doi: 10.1016/S0140-6736(10)61194-4. [DOI] [PubMed] [Google Scholar]
- 5.Oldgren J, Budaj A, Granger CB, Khder Y, Roberts J, Siegbahn A, Tijssen JG, Van de Werf F, Wallentin L, RE-DEEM Investigators Dabigatran vs. placebo in patients with acute coronary syndromes on dual antiplatelet therapy: a randomized, double-blind, phase II trial. Eur Heart J. 2011;32:2781–2789. doi: 10.1093/eurheartj/ehr113. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson BI, Friedman RJ. Dabigatran etexilate: pivotal trials for venous thromboembolism prophylaxis after hip or knee arthroplasty. Clin Appl Thromb Hemost. 2009;15(suppl 1):25S–31S. doi: 10.1177/1076029609340668. [DOI] [PubMed] [Google Scholar]
- 7.Eriksson BI, Dahl OE, Huo MH, Kurth AA, Hantel S, Hermansson K, Schnee JM, Friedman RJ, RENOVATE II Study Group Oral dabigatran versus enoxaparin for thromboprophylaxis after primary total hip arthroplasty (RE-NOVATE II*). A randomized, double-blind, noninferiority trial. Thromb Haemost. 2011;105:721–729. doi: 10.1160/TH10-10-0679. [DOI] [PubMed] [Google Scholar]
- 8.Schulman S, Kearon C, Kakkar AK, Mismetti P, Schellong S, Eriksson H, Baanstra D, Schnee J, Goldhaber SZ, RE-COVER Study Group Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 9.Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cotton BA, McCarthy JJ, Holcomb JB. Acutely injured patients on dabigatran. N Engl J Med. 2011;365:2039–2040. doi: 10.1056/NEJMc1111095. [DOI] [PubMed] [Google Scholar]
- 11.McKellar SH, Abel S, Camp CL, Suri RM, Ereth MH, Schaff HV. Effectiveness of dabigatran etexilate for thromboprophylaxis of mechanical heart valves. J Thorac Cardiovasc Surg. 2011;141:1410–1416. doi: 10.1016/j.jtcvs.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 12.Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, et al. ROCKETAF Investigators Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 13.Janssen Pharmaceuticals Xarelto prescribing information. 2011 [Google Scholar]
- 14.Mega JL, Braunwald E, Mohanavelu S, Burton P, Poulter R, Misselwitz F, Hricak V, Barnathan ES, Bordes P, Witkowski A, et al. Rivaroxaban versus placebo in patients with acute coronary syndromes (ATLAS ACS-TIMI 46): a randomized, double-blind, phase II trial. Lancet. 2009;374:29–38. doi: 10.1016/S0140-6736(09)60738-8. [DOI] [PubMed] [Google Scholar]
- 15.Mega JL, Braunwald E, Wiviott SD, Bassand JP, Bhatt DL, Bode C, Burton P, Cohen M, Cook-Bruns N, Fox KA, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson BI, Borris LC, Friedman RJ, Haas S, Huisman MV, Kakkar AK, Bandel TJ, Beckmann H, Muehlhoffer E, Misselwitz F, et al. Rivaroxaban versus enoxaparin for thromboprophylaxis after hip arthroplasty. N Engl J Med. 2008;358:2765–2775. doi: 10.1056/NEJMoa0800374. [DOI] [PubMed] [Google Scholar]
- 17.Kakkar AK, Brenner B, Dahl OE, Eriksson BI, Mouret P, Muntz J, Soglian AG, Pap AF, Misselwitz F, Haas S, RECORD2 Investigators Extended duration rivaroxaban versus short-term enoxaparin for the prevention of venous thromboembolism after total hip arthroplasty: a double-blind, randomised controlled trial. Lancet.;2008;372:31–39. doi: 10.1016/S0140-6736(08)60880-6. [DOI] [PubMed] [Google Scholar]
- 18.Lassen MR, Ageno W, Borris LC, Lieberman JR, Rosencher N, Bandel TJ, Misselwitz F, Turpie AG, RECORD3 Investigators Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty. N Engl J Med. 2008;358:2776–2786. doi: 10.1056/NEJMoa076016. [DOI] [PubMed] [Google Scholar]
- 19.Turpie AG, Lassen MR, Davidson BL, Bauer KA, Gent M, Kwong LM, Cushner FD, Lotke PA, Berkowitz SD, Bandel TJ, et al. RECORD4 Investigators Rivaroxaban versus enoxaparin for thromboprophylaxis after total knee arthroplasty (RECORD4): a randomized trial. Lancet. 2009;373:1673–1680. doi: 10.1016/S0140-6736(09)60734-0. [DOI] [PubMed] [Google Scholar]
- 20.Turun S, Banghua L, Yuan Y, Zhenhui L, Ying N, Jin C. A systematic review of rivaroxaban versus enoxaparin in the prevention of venous thromboembolism after hip or knee replacement. Thromb Res. 2011;127:525–534. doi: 10.1016/j.thromres.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 21.EINSTEIN Investigators. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 22.Mani H, Hesse C, Stratmann G, Lindhoff-Last E. Rivaroxaban differentially influences ex vivo global coagulation assays based on the administration time. Thromb Haemost. 2011;106:156–164. doi: 10.1160/TH10-10-0667. [DOI] [PubMed] [Google Scholar]
- 23.Samama MM, Martinoli JL, LeFlem L, Guinet C, Plu-Bureau G, Depasse F, Perzborn E. Assessment of laboratory assays to measure rivaroxaban--an oral, direct factor Xa inhibitor. Thromb Haemost. 2010;103:815–825. doi: 10.1160/TH09-03-0176. [DOI] [PubMed] [Google Scholar]
- 24.Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit. 2010;32:673–679. doi: 10.1097/FTD.0b013e3181f2f264. [DOI] [PubMed] [Google Scholar]
- 25.Connolly SJ, Eikelboom J, Joyner C, Diener HC, Hart R, Golitsyn S, Flaker G, Avezum A, Hohnloser SH, Diaz R, et al. AVERROES Steering Committee and Investigators Apixaban in patients with atrial fibrillation. N Engl J Med. 2011;364:806–817. doi: 10.1056/NEJMoa1007432. [DOI] [PubMed] [Google Scholar]
- 26.Granger CB, Alexander JH, McMurray JJ, Lopes RD, Hylek EM, Hanna M, Al-Khalidi HR, Ansell J, Atar D, Avezum A, et al. ARISTOTLE Committees and Investigators Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–992. doi: 10.1056/NEJMoa1107039. [DOI] [PubMed] [Google Scholar]
- 27.Alexander JH, Becker RC, Bhatt DL, Cools F, Crea F, Dellborg M, Fox KA, Goodman SG, Harrington RA, Huber K, et al. APPRAISE Steering Committee and Investigators Apixaban, an oral, direct, selective factor Xa inhibitor, in combination with antiplatelet therapy after acute coronary syndrome: results of the Apixaban for Prevention of Acute Ischemic and Safety Events (APPRAISE) trial. Circulation. 2009;119:2877–2885. doi: 10.1161/CIRCULATIONAHA.108.832139. [DOI] [PubMed] [Google Scholar]
- 28.Alexander JH, Lopes RD, James S, Kilaru R, He Y, Mohan P, Bhatt DL, Goodman S, Verheugt FW, Flather M, et al. APPRAISE-2 Investigators Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- 29.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361:594–604. doi: 10.1056/NEJMoa0810773. [DOI] [PubMed] [Google Scholar]
- 30.Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P, ADVANCE-2 Investigators Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomized double-blind trial. Lancet. 2010;375:807–815. doi: 10.1016/S0140-6736(09)62125-5. [DOI] [PubMed] [Google Scholar]
- 31.Lassen MR, Gallus A, Raskob GE, Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, ADVANCE-3 Investigators Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 32.Becker RC, Yang H, Barrett Y, Mohan P, Wang J, Wallentin L, Alexander JH. Chromogenic laboratory assays to measure the factor Xa-inhibiting properties of apixabanVan oral, direct and selective factor Xa inhibitor. J Thromb Thrombolysis. 2011;32:183–187. doi: 10.1007/s11239-011-0591-8. [DOI] [PubMed] [Google Scholar]
- 33.Morton C, Pruthi R, Zhu D, Bergeron B, Ney A, Baumann Kreuziger L, Burnett B, Zantek ND, Dries D, Eginton M. [October 31, 2011];Dabigatran: consensusbased statement on emergency care of bleeding. 2011 Available at: http://www.icsi.org/guidelines_and_more/protocols_/cardiovascular_protocols/dabigatran__consensus-based_statement_on_emergency_care_of_bleeding_protocol/dabigatran__consensus-based_statement_on_emergency_care_of_bleeding_protocol_63874.html.
- 34.Wong PC, Pinto DJ, Zhang D. Preclinical discovery of apixaban, a direct and orally bioavailable factor Xa inhibitor. J Thromb Thrombolysis. 2011;31:478–492. doi: 10.1007/s11239-011-0551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johansson PI, Stensballe J. Hemostatic resuscitation for massive bleeding: the paradigm of plasma and plateletsVa review of the current literature. Transfusion. 2010;50:701–710. doi: 10.1111/j.1537-2995.2009.02458.x. [DOI] [PubMed] [Google Scholar]
- 36.CRASH-2 Investigators. Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt BJ, et al. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomized controlled trial. Lancet. 2011;377:1096–1101. 1101, e1–e2. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 37.Clav A, Fazilleau F, Dumser D, Lacroix J. Efficacy of tranexamic acid on blood loss after primary cementless total hip replacement with rivaroxaban thromboprophylaxis: a case-control study in 70 patients. Orthop Traumatol Surg Res. 2012 Apr 27; doi: 10.1016/j.otsr.2011.12.005. [ePub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Ageno W, Gallus AS, Wittkowsky A, Crowther M, Hylek EM, Palareti G. American College of Chest Physicians. Oral anticoagulant therapy: antithrombotic therapy and prevention of thrombosis--American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:e44S–e88S. doi: 10.1378/chest.11-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eerenberg ES, Kamphuisen PW, Sijpkens MK, Meijers JC, Buller HR, Levi M. Reversal of rivaroxaban and dabigatran by prothrombin complex concentrate: a randomized, placebo-controlled, crossover study in healthy subjects. Circulation. 2011;124:1573–1579. doi: 10.1161/CIRCULATIONAHA.111.029017. [DOI] [PubMed] [Google Scholar]
- 40.van Ryn J, Schurer J, Kink-Eiband M, Clemens A. The successful reversal of dabigatran-induced bleeding by coagulation factor concentrates in a rat tail bleeding model do not correlate with ex vivo markers of anticoagulation. Blood. 2011;118:2316. [Google Scholar]
- 41.Zhou W, Schwarting S, Illanes S, Liesz A, Middelhoff M, Zorn M, Bendszus M, Heiland S, van Ryn J, Veltkamp R. Hemostatic therapy in experimental intracerebral hemorrhage associated with the direct thrombin inhibitor dabigatran. Stroke. 2011;42:3594–3599. doi: 10.1161/STROKEAHA.111.624650. [DOI] [PubMed] [Google Scholar]
- 42.European Medicines Agency Eliquis product information. 2011 [Google Scholar]
- 43.Douketis JD. Pharmacologic properties of the new oral anticoagulants: a clinician-oriented review with a focus on perioperative management. Curr Pharm Des. 2010;16:3436–3441. doi: 10.2174/138161210793563338. [DOI] [PubMed] [Google Scholar]
- 44.Ezekowitz MD, Reilly PA, Nehmiz G, Simmers TA, Nagarakanti R, Parcham-Azad K, Pedersen KE, Lionetti DA, Stangier J, Wallentin L. Dabigatran with or without concomitant aspirin compared with warfarin alone in patients with nonvalvular atrial fibrillation (PETRO Study). Am J Cardiol. 2007;100:1419–1426. doi: 10.1016/j.amjcard.2007.06.034. [DOI] [PubMed] [Google Scholar]
- 45.Eriksson BI, Dahl OE, Ahnfelt L, Kälebo P, Stangier J, Nehmiz G, Hermansson K, Kohlbrenner V. Dose escalating safety study of a new oral direct thrombin inhibitor, dabigatran etexilate, in patients undergoing total hip replacement: BISTRO I. J Thromb Haemost. 2004;2:1573–1580. doi: 10.1111/j.1538-7836.2004.00890.x. [DOI] [PubMed] [Google Scholar]
- 46.Eriksson BI, Dahl OE, Bller HR, Hettiarachchi R, Rosencher N, Bravo ML, Ahnfelt L, Piovella F, Stangier J, Kälebo P, et al. BISTRO II Study Group A new oral direct thrombin inhibitor, dabigatran etexilate, compared with enoxaparin for prevention of thromboembolic events following total hip or knee replacement: the BISTRO II randomized trial. J Thromb Haemost. 2005;3:103–111. doi: 10.1111/j.1538-7836.2004.01100.x. [DOI] [PubMed] [Google Scholar]
- 47.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Kälebo P, Christiansen AV, Hantel S, Hettiarachchi R, et al. RE-MODEL Study Group. Oral dabigatran etexilate vs. subcutaneous enoxaparin for the prevention of venous thromboembolism after total knee replacement: the REMODEL randomized trial. J Thromb Haemost. 2007;5:2178–2185. doi: 10.1111/j.1538-7836.2007.02748.x. [DOI] [PubMed] [Google Scholar]
- 48.Eriksson BI, Dahl OE, Rosencher N, Kurth AA, van Dijk CN, Frostick SP, Prins MH, Hettiarachchi R, Hantel S, Schnee J, et al. RE-NOVATE Study Group Dabigatran etexilate versus enoxaparin for prevention of venous thromboembolism after total hip replacement: a randomised, doubleblind, noninferiority trial. Lancet. 2007;370:949–956. doi: 10.1016/S0140-6736(07)61445-7. [DOI] [PubMed] [Google Scholar]
- 49.Ginsberg JS, Davidson BL, Comp PC, Francis CW, Friedman RJ, Huo MH, Lieberman JR, Muntz JE, Raskob GE, Clements ML, et al. RE-MOBILIZE Writing Committee Oral thrombin inhibitor dabigatran etexilate vs North American enoxaparin regimen for prevention of venous thromboembolism after knee arthroplasty surgery. J Arthroplasty. 2009;24:1–9. doi: 10.1016/j.arth.2008.01.132. [DOI] [PubMed] [Google Scholar]
- 50.Cohen A. [May 30, 2012];Rivaroxaban compared with enoxaparin for the prevention of venous thromboembolism in acutely ill medical patients. 2011 Available at: http://my.americanheart.org/idc/groups/ahamah-public/@wcm/@sop/@scon/documents/downloadable/ucm_425442.pdf.
- 51.Agnelli G, Gallus A, Goldhaber SZ, Haas S, Huisman MV, Hull RD, Kakkar AK, Misselwitz F, Schellong S, ODIXa-DVT Study Investigators Treatment of proximal deep-vein thrombosis with the oral direct factor Xa inhibitor rivaroxaban (BAY 59-7939): the ODIXa-DVT (Oral Direct Factor Xa Inhibitor BAY 59-7939 in Patients With Acute Symptomatic Deep-Vein Thrombosis) study. Circulation. 2007;116:180–187. doi: 10.1161/CIRCULATIONAHA.106.668020. [DOI] [PubMed] [Google Scholar]
- 52.Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM, ADVANCE-3 Investigators Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363:2487–2498. doi: 10.1056/NEJMoa1006885. [DOI] [PubMed] [Google Scholar]
- 53.Botticelli Investigators. Buller H, Deitchman D, Prins M, Segers A. Efficacy and safety of the oral direct factor Xa inhibitor apixaban for symptomatic deep vein thrombosis. The Botticelli DVT dose-ranging study. J Thromb Haemost. 2008;6:1313–1318. doi: 10.1111/j.1538-7836.2008.03054.x. [DOI] [PubMed] [Google Scholar]
- 54.Levine MN, Gu C, Liebman HA, Escalante CP, Solymoss S, Deitchman D, Ramirez L, Julian J. A randomized phase II trial of apixaban for the prevention of thromboembolism in patients with metastatic cancer. J Thromb Haemost. 2012;10:807–814. doi: 10.1111/j.1538-7836.2012.04693.x. [DOI] [PubMed] [Google Scholar]