SUMMARY
The ten-eleven-translocation 5-methylcytosine dioxygenase (TET) family of enzymes catalyzes the conversion of 5-methylcytosine (5-mC) to 5-hydroxyme-thylcytosine (5-hmC), a modified cytosine base that facilitates gene expression. Cells respond to hypoxia by inducing a transcriptional program regulated in part by oxygen-dependent dioxygenases that require Fe(II) and α-ketoglutarate. Given that the TET enzymes also require these cofactors, we hypothesized that the TETs regulate the hypoxia-induced transcriptional program. Here, we demonstrate that hypoxia increases global 5-hmC levels, with accumulation of 5-hmC density at canonical hypoxia response genes. A subset of 5-hmC gains colocalize with hypoxia response elements facilitating DNA demethylation and HIF binding. Hypoxia results in transcriptional activation of TET1, and full induction of hypoxia-responsive genes and global 5-hmC increases require TET1. Finally, we show that 5-hmC increases and TET1 upregulation in hypoxia are HIF-1 dependent. These findings establish TET1-mediated 5-hmC changes as an important epigenetic component of the hypoxic response.
INTRODUCTION
The TET proteins are dioxygenases that convert 5-methylcytosine (5-mC) to 5-hydroxymethylcytosine (5-hmC) (Kriaucionis and Heintz, 2009; Tahiliani et al., 2009). Whereas 5-mC represses transcription (Jones, 2012), elevated 5-hmC levels are associated with increased gene expression (Gan et al., 2013; Madzo et al., 2014; Szulwach et al., 2011). Colocalization of 5-hmC with regulatory regions such as transcription factor binding sites, promoters, and enhancers suggests that 5-hmC has important regulatory functions (Madzo et al., 2014; Pastor et al., 2011; Stroud et al., 2011; Williams et al., 2011; Wu et al., 2011). 5-hmC also serves as an intermediate in demethylation pathways (Branco et al., 2012). Three TET enzymes have been identified: TET1, TET2, and TET3. TET1 is highly expressed in embryonic stem cells and is upregulated in the generation of induced pluripotent stem cells (Ito et al., 2010; Koh et al., 2011; Piccolo et al., 2013). TET2 and TET3 are required for normal hematopoiesis and early reprogramming of the mammalian zygote, respectively (Ficz et al., 2011; Gu et al., 2011; Iqbal et al., 2011; Madzo et al., 2013; Wossidlo et al., 2011). These studies demonstrate that TET-mediated conversion of 5-mC to 5-hmC is an important epigenetic component of transcriptional regulation.
Hypoxia is a pervasive stimulus that affects a wide variety of biological processes. In tumor biology, rapid cellular proliferation and abnormal tumor vasculature result in highly hypoxic regions that confer an aggressive phenotype by upregulating angiogenic, metabolic, survival, proliferative, and metastatic pathways (Majmundar et al., 2010). Study of the transcriptional response to hypoxia has largely focused on the hypoxia inducible factors (HIFs) HIF-1 and HIF-2, which are α-β heterodimer transcription factors. HIFα subunits are targeted for degradation by O2, Fe(II), and α-ketoglutarate-dependent prolyl hydroxylases. These hydroxylases have reduced activity in hypoxia, resulting in HIFα accumulation (Kaelin and Ratcliffe, 2008; Prabhakar and Semenza, 2012).
Epigenetic regulation plays an important role in regulating transcriptional changes in hypoxia. Genes encoding for the O2, Fe(II), and α-ketoglutarate-dependent jumonji domain (JmJ) histone demethylases are transcriptionally upregulated in hypoxia (Beyer et al., 2008; Pollard et al., 2008; Schödel et al., 2011), and global changes in many histone modifications, such as H3K4me3, have also been reported (Johnson et al., 2008). Site-specific changes in histone modifications have been observed at hypoxia-induced genes including CA9, LDHA, and PDK1 (Luo et al., 2012; van den Beucken et al., 2009). In addition, hypoxia affects DNA methylation (Liu et al., 2011; Shahrzad et al., 2007; Skowronski et al., 2010) and regulates noncoding RNAs providing additional layers of epigenetic regulation (Choudhry et al., 2014). Despite the evidence that hypoxia induces epigenetic alterations, whether hypoxia also affects global or site-specific 5-hmC levels is not known. We hypothesized that the TETs play a role in regulating the transcriptional response to hypoxia, because, like the JmJ histone demethylases and the HIFα prolyl hydroxylases, the TETs require O2, Fe(II), and α-ketoglutarate for their activity.
RESULTS
Hypoxia Increases Global 5-hmC Levels
We explored the role of TET-mediated 5-hmC changes in regulating the hypoxia-induced transcriptional program by measuring changes in global 5-hmC levels by high performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) in a variety of cancer cells after treatment with 1% O2 for 48 hr: SK-N-BE(2), NBL-WN, La1-55n, SHEP, and SK-N-AS (neuroblastoma); Farage (non-Hodgkin B cell lymphoma), Karpas 422 (diffuse large B cell lymphoma with t[11;14]), HeLa (cervical cancer), HN5 (head and neck cancer), MDA-MB-231 (triple-negative breast cancer), and T47D (ER+ PR+ breast cancer). Although many cancer cell lines had low levels of 5-hmC in both hypoxia and normoxia, we found that 5-hmC increased after hypoxia in tumorigenic N-type neuroblastoma cells, but not in nontumorigenic S-type cells, despite the fact the TET enzymes depend on oxygen (Figures 1A and S1A). We further pursued the functional significance of 5-hmC increases in neuroblastoma cells for several reasons. First, neuroblastomas are derived from neural crest cells and neuronal cell types have high levels of 5-hmC (Globisch et al., 2010; Szulwach et al., 2011). Second, neuroblastoma cells dedifferentiate in hypoxia (Bhaskara et al., 2012; Jögi et al., 2002, 2004), suggesting that altered differentiation could be driven by 5-hmC changes because the TET enzymes regulate transitions between pluripotent and differentiated states (Ito et al., 2010; Koh et al., 2011; Madzo et al., 2014).
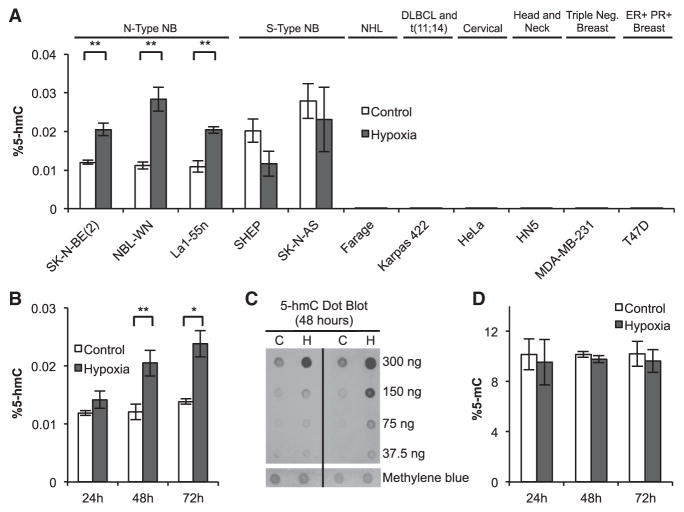
Figure 1. Global 5-hmC Levels Increase in Hypoxia.
(A) Quantitation of 5-hmC by HPLC-MS/MS in various cancer cell lines exposed to 48 hr of hypoxia (NB, neuroblastoma; NHL, non-Hodgkin lymphoma; DLBCL, diffuse large B cell lymphoma; ER, estrogen receptor; PR, progesterone receptor). Percentage 5-hmC is calculated relative to the sum of all cytosine species (n ≥ 3).
(B) Quantitation of 5-hmC by HPLC-MS/MS in SK-N-BE(2) cells exposed to 24, 48, or 72 hr of hypoxia (n ≥ 3).
(C) Dot blot quantification of 5-hmC in SK-N-BE(2) cells exposed to 48 hr of hypoxia.
(D) Quantification of 5-mC by HPLC-MS/MS in SK-N-BE(2) cells. Percentage 5-mC is calculated relative to the sum of all cytosine species (n ≥ 3). Data represent means ± SEM. p values calculated by Student’s t test, *p < 0.05, **p < 0.01. See also Figure S1.
To further characterize the timeline of 5-hmC increases, SK-N-BE(2) cells were exposed to 24, 48, and 72 hr of hypoxia, and 5-hmC levels were analyzed by HPLC-MS/MS. HIF-1α protein levels were elevated in hypoxia exposed cells at all time points, demonstrating that cells were oxygen deprived (Figure S1B). 5-hmC levels were elevated in cells exposed to 48 and 72 hr of hypoxia (Figures 1B and S1C). Increased 5-hmC levels in hypoxic SK-N-BE(2) cells were also observed by dot blot assay after labeling 5-hmC with biotin (Figure 1C) (Song et al., 2011). In contrast to the observed changes in 5-hmC, 5-mC levels remained unchanged at all time points (Figures 1D and S1D). Hypoxia-induced 5-hmC gains were largely reversed following reoxygenation (Figure S1E).
5-hmC Gains Occur at Hypoxia-Regulated Genes
To determine the genomic positions gaining 5-hmC in hypoxia, we purified and sequenced 5-hmC-enriched DNA fragments from SK-N-BE(2) cells exposed to 48 hr of hypoxia using the hMe-Seal method (Song et al., 2011) in two independent biological replicates (Figures S2A and S2B). RNA sequencing (RNA-seq) was performed to quantify transcriptional changes. To determine the distribution of 5-hmC gains across the genome, we summed gained hMe-Seal sequencing tags over various genomic elements (exons, introns, untranslated regions [UTRs], CpG islands/shores, and intergenic regions). To test for potential regulatory function of 5-hmC, the positions of previously published HIF binding sites (Schödel et al., 2011), and the locations of DNase-hypersensitive sites (DHSs) (Bernstein et al., 2012) were included. In absolute terms, 5-hmC gains occurred to the greatest extent over introns (Figure 2A). Enrichment of 5-hmC gains at each element was then calculated by normalizing for element length. 5-hmC gains were enriched at or near known HIF-1 binding sites, at DHSs, and at CpG shores (Figures 2B, S2C, and S2D). Enrichment of 5-hmC gains was greater at or near unique HIF-1 binding sites (sites that bind HIF-1, but not HIF-2) than at unique HIF-2 binding sites (sites that bind HIF-2, but not HIF-1) (Schödel et al., 2011).
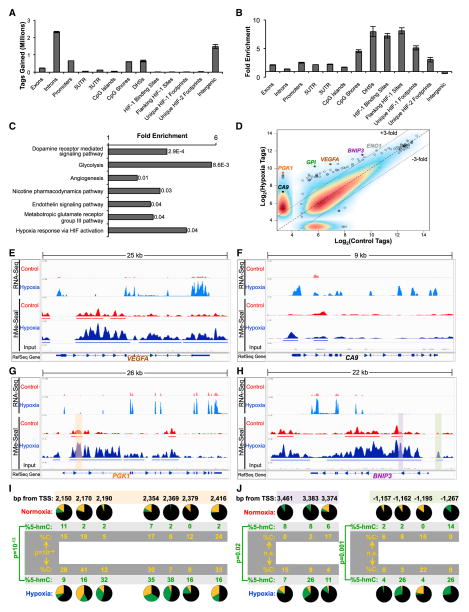
Figure 2. 5-hmC Gains Occur at Hypoxia Regulated Regions in SK-N-BE(2) Cells.
(A and B) Absolute 5-hmC gains (A) and enrichment for 5-hmC gains (B) were calculated at genomic elements. Regions flanking HIF binding sites are regions ±1 kb from the ChIP-seq peak. Footprints include the ChIP-seq peak and flanking region (Schödel et al., 2011). Data represent means ± SD for two biological replicates.
(C) PANTHER pathway analysis of genes with regions gaining at least 3-fold 5-hmC. p values are Bonferroni corrected results of binomial tests (Mi et al., 2013).
(D) Regions gaining the most 5-hmC map to the HIF-1 target genes CA9, PGK1, GPI, VEGFA, BNIP3, and ENO1. Plotted points represent combined data from two experiments.
(E–H) RNA-seq and hMe-Seal sequencing data are plotted in the Integrated Genomics Viewer (IGV) genome browser at VEGFA, CA9, PGK1, and BNIP3 genes. RefSeq gene tracks represent the position of known genes in the hg19 build of the human genome. Arrows represent the direction of transcription.
(I and J) TAB-seq data for the corresponding shaded regions of PGK1 (G) and BNIP3 (H). For each CpG, black, green, and gold represent the percentage of 5-mC, 5-hmC, and unmodified cytosine, respectively. Results represent pooled data over three independent experiments. p values calculated by chi-square tests. See also Figure S2 and Table S2.
We performed PANTHER pathway analysis on the genes with a 3-fold increase of 5-hmC density in hypoxia and identified pathways such as dopamine receptor mediated signaling, glycolysis, angiogenesis, and hypoxia response via HIF activation (Figure 2C). For each genomic position, we plotted the number of sequencing tags mapping to the region for the control and hypoxia samples. This analysis demonstrated that regions gaining the most 5-hmC map to canonical hypoxia response genes, including CA9, PGK1, GPI, VEGFA, BNIP3, and ENO1 (Figure 2D). 5-hmC gains occurred over gene bodies and/or promoter regions of these genes (Figures 2E–2H and S2E–S2H). To resolve 5-hmC, 5-mC, and unmodified cytosine at single-base resolution at loci that gain 5-hmC in hypoxia, we performed Tet-assisted bisulfite sequencing (TAB-seq) (Yu et al., 2012) at the PGK1 first intron, and the BNIP3 promoter and first intron (Figures 2I and 2J). Given previous reports that 5-hmC is associated with transcription factor binding sites (Gan et al., 2013; Madzo et al., 2014), we used Homer motif analysis to calculate enrichment for putative transcription factor binding sites at positions gaining 5-hmC in hypoxia. We identified these regions as enriched for binding sites of ZFX, Eomes, ZNF711, NeuroD1, N-Myc, Foxo1, Max, c-Myc, Stat3, and the androgen receptor (Table S1). Finally, we repeated hMe-Seal and RNA-seq experiments in a second neuroblastoma cell line, NBL-WN. NBL-WN cells gained 5-hmC at similar genomic annotations and hypoxia-responsive genes (Figures S2I–S2L). These results implicate 5-hmC as an important regulator of the hypoxia-induced transcriptional program.
TET1 Expression Increases in Hypoxia and Is Necessary for Hypoxic Gene Induction
Given the dependence of TET enzymes on oxygen, we were surprised to find global increases of 5-hmC in hypoxia. Therefore, we hypothesized that transcriptional increases in one or more TET genes could compensate for potentially lower enzymatic activity in hypoxia, as has been shown for the HIFα prolyl hydroxylases (Stiehl et al., 2006). We found that TET1 transcription increased in SK-N-BE(2) cells in hypoxia as measured by quantitative PCR (qPCR) (Figure 3A), whereas no changes in TET2 or TET3 mRNA levels were observed (Figure 3B). These TET expression results were also observed by RNA-seq (Figure 3C). Like 5-hmC levels, the increase in TET1 mRNA was reversed after reoxygenation (Figure S3A) and was observed in other N-type, but not S-type, cell lines (Figure 3D). To determine whether the TETs are induced in hypoxia in other cancer cell lines, we quantified TET1, TET2, and TET3 expression after 48 hr of hypoxia in Farage, Karpas 422, HeLa, HN5, MDA-MB-231, and T47D cells. We only observed induction of TET2 by hypoxia in Karpas 422 cells (Figures S3B–S3D).
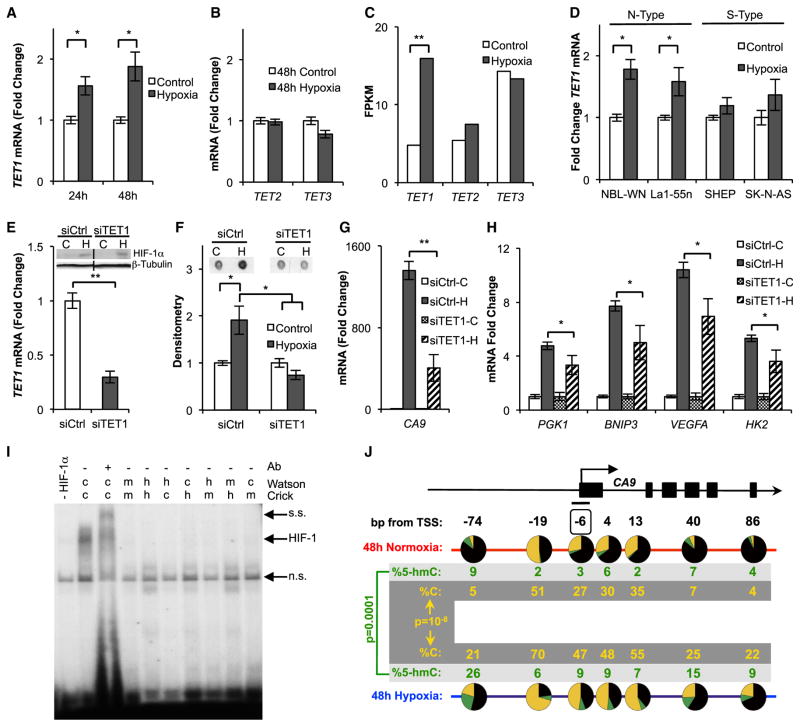
Figure 3. TET1 Is Required for Full Hypoxic Gene Induction.
(A) TET1 mRNA quantified by qPCR from SK-N-BE(2) cells (n ≥ 4).
(B) TET2 and TET3 mRNA quantified by qPCR from SK-N-BE(2) cells exposed to 48 hr of hypoxia (n = 3).
(C) TET mRNA levels measured in fragments per kilobase of transcript per million mapped reads (FPKM) by RNA-seq. Data represent Cuffdiff output from two biological replicates of SK-N-BE(2) cells exposed to 48 hr of hypoxia. p values were false discovery rate corrected.
(D) TET1 mRNA measured by qPCR in neuroblastoma cell lines exposed to 48 hr of hypoxia (n ≥ 4).
(E) TET1 mRNA determined by qPCR, and HIF-1α expression determined by immunoblot, in SK-N-BE(2) cells transfected with siCtrl or siTET1 (n = 3).
(F) 5-hmC quantified by dot blot in SK-N-BE(2) cells transfected with siCtrl or siTET1 and exposed to 48 hr of hypoxia. Quantification represents means ± SEM (n = 3).
(G and H) qPCR quantification of hypoxia-induced gene expression in TET1 depleted SK-N-BE(2) cells after 48 hr of hypoxia (n = 3).
(I) EMSA binding studies of HIF-1 to the CA9 HRE. c, cytosine; h, 5-hmC; m, 5-mC.
(J) TAB-seq of the CA9 TSS. The boxed CpG 6 bp upstream of the TSS resides within the HRE. Results are pooled over three replicates. p values calculated by chi-square tests.
All graphs represent means ± SEM. p values for expression analysis calculated by Student’s t test, *p < 0.05, **p < 0.01. See also Figure S3.
Given the increase of TET1 in hypoxia, we hypothesized that TET1 was responsible for global 5-hmC gains. We tested this by knocking down TET1 with small interfering RNA (siRNA) and noted that this treatment did not interfere with HIF-1α accumulation (Figure 3E). We found that TET1 depletion inhibited global 5-hmC gains (Figure 3F). We then tested if TET1 activity is necessary for full induction of genes gaining 5-hmC in hypoxia using cells transiently depleted of TET1 with siRNA or cells stably expressing a TET1-targeting short hairpin RNA (shTET1) (Figure S3E). In addition to genes identified as gaining large amounts of 5-hmC in Figure 2, we included HK2 in our analysis, because it gained narrow 5-hmC peaks over its promoter and first intron in our hMe-Seal experiments (Figure S3F). TET1 depletion blunted hypoxia-induced induction of CA9, BNIP3, and VEGFA at 16 hr of hypoxia (Figures S3G and S3H). After 48 hr of hypoxia, TET1 depletion attenuated PGK1 and HK2 expression, in addition to CA9, BNIP3, and VEGFA (Figures 3G and 3H). These experiments show that TET1 is required for full induction of the hypoxic transcriptional program.
Interestingly, CA9 expression is delayed in hypoxic neuroblastoma cells relative to other hypoxia-induced genes (Figures S3I and S3J) and closely mirrors the time course of global 5-hmC gains in hypoxia (Figures 1B and S1C), suggesting that CA9 expression could be particularly dependent on epigenetic remodeling. Therefore, we further investigated the role of 5-hmC gains in inducing CA9 expression. CA9 gains 5-hmC over its transcriptional start site (TSS) where there is a well-characterized hypoxia response element (HRE) (Wykoff et al., 2000). Because HIF-1 binding to HREs is methylation sensitive (Wenger et al., 1998), we tested the effect of hydroxymethylation on HIF-1 binding within the HRE using an electrophoretic mobility shift assay (EMSA). Like 5-mC, we found that 5-hmC inhibited HIF-1 binding (Figure 3I). We therefore hypothesized that 5-hmC may accumulate near the HRE to facilitate an open chromatin state, and that 5-hmC within the HRE would be a transient intermediate facilitating demethylation and HIF binding. To test this hypothesis, we performed TAB-seq (Yu et al., 2012) at the CA9 TSS. Consistent with our hypothesis and the observed slow induction kinetics of CA9, we observed small but insignificant accumulation of 5-hmC at the CA9 TSS by 16 hr of hypoxia, accompanied by an increase of unmodified cytosine at the HRE (Figure S3K). By 48 hr of hypoxia, significant accumulation of 5-hmC at a CpG 74 bp upstream of the TSS was observed and further demethylation of multiple CpGs in this region, including at the HRE, was observed (Figure 3J). To determine if 5-hmC gains at HIF-1 chromatin immunoprecipitation sequencing (ChIP-seq) peaks are associated with HRE demethylation at other sites, we performed TAB-seq at two additional HIF-1 ChIP-seq peaks associated with 5-hmC gains in hypoxia (Figures S2C and S2D). Similar to our findings at CA9, we found that these hMe-Seal peaks mark HREs that gained unmodified cytosine over 48 hr of hypoxia (Figures S3L and S3M).
TET1 and Global 5-hmC Increases Are HIF-1 Dependent
We then determined the mechanisms underlying TET1 induction and 5-hmC increases in hypoxia. We hypothesized that these changes may be HIF-1 regulated, because (1) pathways and genes identified in our hMe-Seal analysis showed a strong HIF-1 signature (Figure 2); and (2) SK-N-BE(2) and NBL-WN cells selectively expressed HIF-1α over HIF-2α (Figure S4A), consistent with a previous report (Qing et al., 2010). We generated SK-N-BE(2) cells expressing a previously validated short hairpin RNA targeting either HIF-1α (shHIF-1α) (Stiehl et al., 2012) or a nontargeting control (shCtrl). shHIF-1α efficiently reduced HIF-1α protein levels in hypoxia and inhibited transcriptional upregulation of HIF-1 target genes (Figure 4A). HIF-1α knockdown also prevented the upregulation of TET1 and global 5-hmC increases in hypoxia (Figures 4B, 4C, and S4B). These results demonstrate that HIF-1 is required for hypoxic induction of TET1 and global increases in 5-hmC.
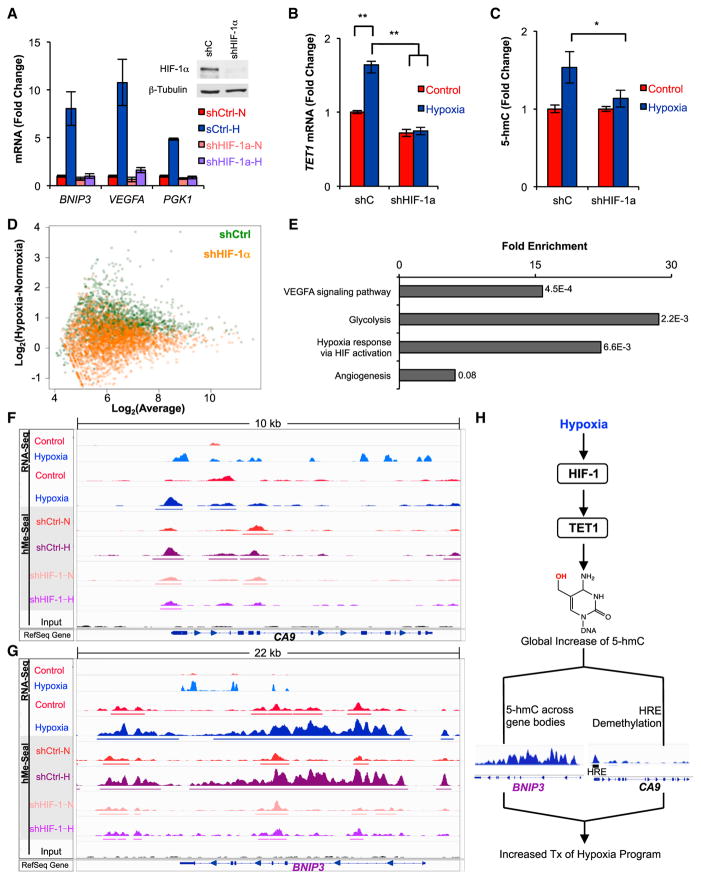
Figure 4. HIF-1 Regulates TET1 and Is Necessary for Genome-wide and Locus-Specific 5-hmC Gains in SK-N-BE(2) Cells.
(A) qPCR quantification of HIF-1 target gene expression in shHIF-1α or shCtrl-transduced cells after 48 hr of hypoxia (n = 3).
(B and C) TET1 mRNA quantification by qPCR (B) and 5-hmC quantification by HPLC-MS/MS (C) in shCtrl or shHIF-1α-transduced SK-N-BE(2) cells exposed to 48 hr of hypoxia (n = 3).
(D) Regions gaining 5-hmC from Figure 2C are plotted in shCtrl or shHIF-1α-expressing cells. Gains of 5-hmC in hypoxia are represented as an upward shift on the y axis.
(E) PANTHER pathway analysis of regions losing 5-hmC in hypoxic shHIF-1α cells relative to hypoxic shCtrl cells. p values are Bonferroni corrected results of binomial tests (Mi et al., 2013).
(F and G) hMe-Seal data for parental and transduced SK-N-BE(2) cells exposed to 48 hr of hypoxia or normoxia.
(H) Hypoxia stimulates HIF-1-dependent upregulation of TET1, which is necessary for genome-wide 5-hmC increases. Site-specific 5-hmC gains are clustered at hypoxia response genes that require TET1 for induction.
All graphs represent means ± SEM. See also Figure S4.
To investigate whether site-specific 5-hmC gains are HIF-1 regulated, we repeated hMe-Seal with cells expressing shCtrl and shHIF-1α. We analyzed genomic positions identified as gaining 5-hmC in our initial hMe-Seal experiment and found that shCtrl-transduced cells gained 5-hmC at these positions, but shHIF-1α-transduced cells did not (Figure 4D). PANTHER pathway analysis of genes losing 5-hmC in hypoxic shHIF-1α cells relative to hypoxic shCtrl cells demonstrated that 5-hmC depletion in hypoxic shHIF-1α cells is specific to genes regulated by HIF-1 (Figure 4E). CA9, BNIP3, VEGFA, PGK1, GPI, and ENO1 gained large amounts of 5-hmC in shCtrl-transduced cells but showed little to no 5-hmC gains in shHIF-1α-transduced cells (Figures 4F, 4G, and S4C–S4F). In contrast, HIF-1α knockdown had no effect on 5-hmC levels at genes that are not regulated by hypoxia (Figures S4G and S4H). These findings indicate that induction of TET1 and gain of 5-hmC-density at hypoxia-induced genes are HIF-1 dependent.
DISCUSSION
Our results demonstrate a hitherto uncharacterized role for the TET1 dioxygenase and 5-hmC in regulating the hypoxia-induced transcriptional program in neuroblastoma cells. We propose a model (Figure 4H) in which TET1 is upregulated in hypoxia by HIF-1. This is associated with TET1 and HIF-1-dependent 5-hmC gains clustered at hypoxia-induced genes. At these genes, TET1 facilitates expression. We find 5-hmC gains in hypoxia at promoters, exons, introns, UTRs, and other regulatory regions, where 5-hmC may have distinct functions. These findings raise numerous questions that will be the subject of future work, including the following: determining whether TET1 is a direct or indirect transcriptional target of HIF-1; how TET1 is directed to specific genes induced in hypoxia; and the function of 5-hmC at different genomic elements.
We observe induction of TET1 and increase in 5-hmC within tumorigenic, N-type neuroblastoma cells. The low levels of 5-hmC in other cancer cells are consistent with previous reports that (1) 5-hmC levels are reduced in numerous cancers (Mariani et al., 2013) and (2) neuronal cells have high levels of 5-hmC (Globisch et al., 2010; Szulwach et al., 2011). It will be important for us and others to test an even wider range of cancer and other cell types in the future to understand if our observations are broadly applicable.
Our work is consistent with previous observations that hypoxia induces global and site-specific epigenetic changes (Beyer et al., 2008; Johnson et al., 2008; Liu et al., 2011; Pollard et al., 2008; Shahrzad et al., 2007; Skowronski et al., 2010). Our finding that TET1 is transcriptionally upregulated in hypoxia and subsequently facilitates hypoxia-induced gene expression is analogous to previous findings regarding the JmJ histone demethylases: genes encoding for JmJ histone demethylases are HIF targets (Beyer et al., 2008; Pollard et al., 2008; Schödel et al., 2011), and the protein products of these genes facilitate hypoxic gene induction by remodeling chromatin at other HIF targets (Luo et al., 2012). Previous work has also demonstrated that treatment of cells with 5-azacytidine augments induction of the hypoxia transcriptional program, particularly by demethylation of HREs (Koslowski et al., 2011). This is consistent with our observation that HIF-1 binding sites gaining 5-hmC in our hMe-Seal experiments exhibit an increase in the amount of unmodified cytosine at the HRE in TAB-seq experiments, which facilitates gene induction. The fact that very little 5-hmC accumulates at the CpG within these HREs suggests that demethylation at these CpGs occurs quickly. Our results further highlight the importance of chromatin remodeling in the hypoxic response and identify 5-hmC changes as a previously unknown component of this process.
Two recent studies have demonstrated that most HIF-target genes have promoter-proximally paused RNA PolII in normoxia, and that HIF binding to these genes releases PolII from the paused state (Choudhry et al., 2014; Galbraith et al., 2013). Nonetheless, a small subset of hypoxia regulated genes recruit PolII de novo on hypoxic exposure (Choudhry et al., 2014). Broad 5-hmC gains across the bodies of genes, such as BNIP3, suggest a potential role for 5-hmC in PolII elongation, whereas narrow 5-hmC peaks gained at or near the TSS of genes like CA9 may facilitate PolII recruitment. Determining if 5-hmC serves to regulate either de novo PolII recruitment or release from the promoter-proximal paused state may further our molecular understanding of how hypoxia-induced genes are regulated.
Finally, our work emphasizes the importance of employing techniques that can distinguish 5-mC from 5-hmC. A previous study examined the effect of hypoxia on covalent cytosine modifications using Illumina 450K methylation arrays (Yuen et al., 2013). These arrays use sodium bisulfite treatment of DNA to distinguish modified and unmodified cytosines but cannot distinguish between 5-mC and 5-hmC (Mariani et al., 2013). Using these arrays, cytosine modification levels in 147 CpGs were found to differ between cytotrophoblastic cells treated with 24 hr of normoxia or hypoxia (Yuen et al., 2013). Only one of the CpGs identified in this study correlates with a location gaining 5-hmC in our data (Illumina cg 23097499, Chr20: 62330618, associated with TNFRSF6B) (Yuen et al., 2013). This is most likely because this previous study would be blind to any transitions between 5-mC and 5-hmC due to the limitations of sodium bisulfite based techniques. Combined with recent work that used techniques specific for 5-hmC to examine lineage commitment of hematopoietic stem cells (Madzo et al., 2014), our work suggests that 5-hmC may play a broad role in regulating cellular responses that require dynamic and widespread transcriptional changes. Future work should continue to test this hypothesis using techniques specific for 5-hmC.
EXPERIMENTAL PROCEDURES
Detailed procedures are provided in the Supplemental Experimental Procedures.
Hypoxia Exposures
Hypoxic cells were incubated under 1% O2 and 10% CO2 in modular incubator chambers (Billups-Rothenberg). Normoxic cells were incubated at 10% CO2 and 20% O2.
Detection of 5-hmC and 5-mC by HPLC-MS/MS
Genomic DNA was hydrolyzed to nucleosides and run on a Zorbax XDB-C18 2.1 3 50 mm column (1.8 mm particle size) attached to an Agilent 1200 Series liquid chromatography machine coupled to an Agilent 6410 Triple Quad Mass Spectrometer (Madzo et al., 2014; Vasanthakumar et al., 2013).
5-hydroxymethylcytosine Selective Chemical Labeling
5-hydroxymethylcytosine selective chemical labeling (hMe-Seal) was performed as previously described (Song et al., 2011). Briefly, 20 μg of sonicated genomic DNA was labeled with UDP-6-N3-glucose and biotinylated using DMCO-S-S-PEG3-Biotin Conjugate (Click Chemistry Tools). Biotinylated DNA was affinity purified and sequenced.
Sequence Mapping and Analysis
DNA reads were mapped to hg19 with the Burrows-Wheeler Aligner. The BWA-mem algorithm was used for 100 bp reads and BWA-backtrack for 50 bp reads. Peak calling was performed using MACS1.4. RNA reads were aligned using TopHat. Cufflinks and Cuffdiff were used for expression analysis.
Enrichment Calculations for 5-hmC Gains at Genomic Elements
Regions gaining 5-hmC in hypoxia were intersected with the genomic element being analyzed. Intersections were weighted by the length of the intersection and the amount of 5-hmC being gained over the intersection and then summed genome-wide to calculate an observed value of 5-hmC gained at each element. Expected values were calculated by scrambling 5-hmC gains over the genome and performing the same calculation. Mean values from 1,000 scrambling experiments were used as the expected value in enrichment calculations.
Generation of Lentivirally Transduced Cells
Production of lentivirus and subsequent infection of neuroblastoma cells followed the protocol found at http://www.addgene.org/tools/protocols/plko/. pLKO.1 plasmids coding for HIF-1α and TET1 targeting hairpins were previously described (Stiehl et al., 2012; Sun et al., 2013).
Transfection with TET1 siRNA
TET1 targeting siRNA (Thermo ON-TARGETplus SMARTpool D-014635) and nontargeting control siRNA (Thermo ON-TARGETplus Non-targeting pool L-001810) were transfected into neuroblastoma cells using Mirus TransIT-siQUEST reagent (MIR 2114).
HIF-1 EMSA Assay
HIF-1α and ARNT were produced by IVTT using the TNT T7 Coupled Coupled Reticulocyte Lysate System (Promega). Nucleotide probes were designed based off of the previously characterized CA9 HIF1 binding site (Wykoff et al., 2000): 5′-CCAATGCACGTACAGCCC-3′ and 5′-GGGCTGTACGTGCATTGG-3′ with the underlined cytosine being unmodified, methylated, or hydroxymethylated.
TAB-Seq
TAB-seq was performed using the Wisegene 5-hmC Tab-seq kit. Ten micrograms of DNA was sonicated to 500 bp and conjugated to UDP-glucose with β-glycosyltransferase. After reaction cleanup, conjugated DNA was oxidized using mTet1, and bisulfite treated. Glycosylated DNA without mTet1 oxidation was used for quantification of modified and unmodified cytosines. After PCR amplification, products were TOPO TA cloned (Life Technologies K4575-02) and sequenced.
Supplementary Material
Acknowledgments
We would like to thank Marsha Rosner for the TET1 shRNA plasmid and Chuan He for UDP-6-N3-glucose. We thank Jeremy Marks and Angela Stoddart for comments on the manuscript and insightful discussions. This work was supported in part by NIH grants T32-HD007009-38 (to C.J.M.) and P01-HL090554-06 (to N.R.P.).
Footnotes
ACCESSION NUMBERS
Next generation sequencing data sets are available at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE55391 (GEO accession number: GSE55391).
Supplemental Information includes Supplemental Experimental Procedures, four figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.04.040.
AUTHOR CONTRIBUTIONS
C.J.M. designed and performed experiments, analyzed data, and wrote the manuscript. A.V. and A.Y. performed experiments. J.M. analyzed data and helped perform experiments. T.B., Y.Y., and S.B. performed library building and next generation sequencing. R.H.W., S.L.C., and J.N. helped design experiments. A.V. supervised next-generation sequencing. N.R.P. and L.A.G. conceived the study, analyzed the data, and edited the manuscript.
References
- Bernstein BE, Birney E, Dunham I, Green ED, Gunter C, Snyder M ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer S, Kristensen MM, Jensen KS, Johansen JV, Staller P. The histone demethylases JMJD1A and JMJD2B are transcriptional targets of hypoxia-inducible factor HIF. J Biol Chem. 2008;283:36542–36552. doi: 10.1074/jbc.M804578200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskara VK, Mohanam I, Rao JS, Mohanam S. Intermittent hypoxia regulates stem-like characteristics and differentiation of neuroblastoma cells. PLoS ONE. 2012;7:e30905. doi: 10.1371/journal.pone.0030905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branco MR, Ficz G, Reik W. Uncovering the role of 5-hydroxymethylcytosine in the epigenome. Nat Rev Genet. 2012;13:7–13. doi: 10.1038/nrg3080. [DOI] [PubMed] [Google Scholar]
- Choudhry H, Schödel J, Oikonomopoulos S, Camps C, Grampp S, Harris AL, Ratcliffe PJ, Ragoussis J, Mole DR. Extensive regulation of the non-coding transcriptome by hypoxia: role of HIF in releasing paused RNApol2. EMBO Rep. 2014;15:70–76. doi: 10.1002/embr.201337642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficz G, Branco MR, Seisenberger S, Santos F, Krueger F, Hore TA, Marques CJ, Andrews S, Reik W. Dynamic regulation of 5-hydroxymethylcytosine in mouse ES cells and during differentiation. Nature. 2011;473:398–402. doi: 10.1038/nature10008. [DOI] [PubMed] [Google Scholar]
- Galbraith MD, Allen MA, Bensard CL, Wang X, Schwinn MK, Qin B, Long HW, Daniels DL, Hahn WC, Dowell RD, Espinosa JM. HIF1A employs CDK8-mediator to stimulate RNAPII elongation in response to hypoxia. Cell. 2013;153:1327–1339. doi: 10.1016/j.cell.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan H, Wen L, Liao S, Lin X, Ma T, Liu J, Song C-X, Wang M, He C, Han C, Tang F. Dynamics of 5-hydroxymethylcytosine during mouse spermatogenesis. Nat Commun. 2013;4:1995. doi: 10.1038/ncomms2995. [DOI] [PubMed] [Google Scholar]
- Globisch D, Münzel M, Müller M, Michalakis S, Wagner M, Koch S, Brückl T, Biel M, Carell T. Tissue distribution of 5-hydroxymethylcytosine and search for active demethylation intermediates. PLoS ONE. 2010;5:e15367. doi: 10.1371/journal.pone.0015367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu TP, Guo F, Yang H, Wu HP, Xu GF, Liu W, Xie ZG, Shi L, He X, Jin SG, et al. The role of Tet3 DNA dioxygenase in epigenetic reprogramming by oocytes. Nature. 2011;477:606–610. doi: 10.1038/nature10443. [DOI] [PubMed] [Google Scholar]
- Iqbal K, Jin SG, Pfeifer GP, Szabó PE. Reprogramming of the paternal genome upon fertilization involves genome-wide oxidation of 5-methylcytosine. Proc Natl Acad Sci USA. 2011;108:3642–3647. doi: 10.1073/pnas.1014033108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito S, D’Alessio AC, Taranova OV, Hong K, Sowers LC, Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jögi A, Øra I, Nilsson H, Lindeheim Å, Makino Y, Poellinger L, Axelson H, Påhlman S. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci USA. 2002;99:7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jögi A, Vallon-Christersson J, Holmquist L, Axelson H, Borg Å, Påhlman S. Human neuroblastoma cells exposed to hypoxia: induction of genes associated with growth, survival, and aggressive behavior. Exp Cell Res. 2004;295:469–487. doi: 10.1016/j.yexcr.2004.01.013. [DOI] [PubMed] [Google Scholar]
- Johnson AB, Denko N, Barton MC. Hypoxia induces a novel signature of chromatin modifications and global repression of transcription. Mutat Res. 2008;640:174–179. doi: 10.1016/j.mrfmmm.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PA. Functions of DNA methylation: islands, start sites, gene bodies and beyond. Nat Rev Genet. 2012;13:484–492. doi: 10.1038/nrg3230. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Koh KP, Yabuuchi A, Rao S, Huang Y, Cunniff K, Nardone J, Laiho A, Tahiliani M, Sommer CA, Mostoslavsky G, et al. Tet1 and Tet2 regulate 5-hydroxymethylcytosine production and cell lineage specification in mouse embryonic stem cells. Cell Stem Cell. 2011;8:200–213. doi: 10.1016/j.stem.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koslowski M, Luxemburger U, Türeci O, Sahin U. Tumor-associated CpG demethylation augments hypoxia-induced effects by positive autoregulation of HIF-1α. Oncogene. 2011;30:876–882. doi: 10.1038/onc.2010.481. [DOI] [PubMed] [Google Scholar]
- Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–930. doi: 10.1126/science.1169786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liu L, Zhao Y, Zhang J, Wang D, Chen J, He Y, Wu J, Zhang Z, Liu Z. Hypoxia induces genomic DNA demethylation through the activation of HIF-1α and transcriptional upregulation of MAT2A in hepatoma cells. Mol Cancer Ther. 2011;10:1113–1123. doi: 10.1158/1535-7163.MCT-10-1010. [DOI] [PubMed] [Google Scholar]
- Luo W, Chang R, Zhong J, Pandey A, Semenza GL. Histone demethylase JMJD2C is a coactivator for hypoxia-inducible factor 1 that is required for breast cancer progression. Proc Natl Acad Sci USA. 2012;109:E3367–E3376. doi: 10.1073/pnas.1217394109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madzo J, Vasanthakumar A, Godley LA. Perturbations of 5-hydroxymethylcytosine patterning in hematologic malignancies. seminars in hematology. Semin Hematol. 2013;50:61–69. doi: 10.1053/j.seminhematol.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Madzo J, Liu H, Rodriguez A, Vasanthakumar A, Sundaravel S, Caces DBD, Looney TJ, Zhang L, Lepore JB, Macrae T, et al. Hydroxymethylation at gene regulatory regions directs stem/early progenitor cell commitment during erythropoiesis. Cell Rep. 2014;6:231–244. doi: 10.1016/j.celrep.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294–309. doi: 10.1016/j.molcel.2010.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariani CJ, Madzo J, Moen EL, Yesilkanal A. Alterations of 5-hydroxymethylcytosine in human cancers. Cancers. 2013;5:786–814. doi: 10.3390/cancers5030786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Muruganujan A, Casagrande JT, Thomas PD. Large-scale gene function analysis with the PANTHER classification system. Nat Protoc. 2013;8:1551–1566. doi: 10.1038/nprot.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor WA, Pape UJ, Huang Y, Henderson HR, Lister R, Ko M, McLoughlin EM, Brudno Y, Mahapatra S, Kapranov P, et al. Genome-wide mapping of 5-hydroxymethylcytosine in embryonic stem cells. Nature. 2011;473:394–397. doi: 10.1038/nature10102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolo FM, Bagci H, Brown KE, Landeira D, Soza-Ried J, Feytout A, Mooijman D, Hajkova P, Leitch HG, Tada T, et al. Different roles for Tet1 and Tet2 proteins in reprogramming-mediated erasure of imprints induced by EGC fusion. Mol Cell. 2013;49:1023–1033. doi: 10.1016/j.molcel.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard PJ, Loenarz C, Mole DR, McDonough MA, Gleadle JM, Schofield CJ, Ratcliffe PJ. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1α. Biochem J. 2008;416:387–394. doi: 10.1042/BJ20081238. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Semenza GL. Adaptive and maladaptive cardio-respiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing G, Skuli N, Mayes PA, Pawel B, Martinez D, Maris JM, Simon MC. Combinatorial regulation of neuroblastoma tumor progression by N-Myc and hypoxia inducible factor HIF-1alpha. Cancer Res. 2010;70:10351–10361. doi: 10.1158/0008-5472.CAN-10-0740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schödel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood. 2011;117:e207–e217. doi: 10.1182/blood-2010-10-314427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrzad S, Bertrand K, Minhas K, Coomber BL. Induction of DNA hypomethylation by tumor hypoxia. Epigenetics. 2007;2:119–125. doi: 10.4161/epi.2.2.4613. [DOI] [PubMed] [Google Scholar]
- Skowronski K, Dubey S, Rodenhiser D, Coomber B. Ischemia dysregulates DNA methyltransferases and p16INK4a methylation in human colorectal cancer cells. Epigenetics. 2010;5:547–556. doi: 10.4161/epi.5.6.12400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C-X, Szulwach KE, Fu Y, Dai Q, Yi C, Li X, Li Y, Chen C-H, Zhang W, Jian X, et al. Selective chemical labeling reveals the genome-wide distribution of 5-hydroxymethylcytosine. Nat Biotechnol. 2011;29:68–72. doi: 10.1038/nbt.1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiehl DP, Wirthner R, Köditz J, Spielmann P, Camenisch G, Wenger RH. Increased prolyl 4-hydroxylase domain proteins compensate for decreased oxygen levels. Evidence for an autoregulatory oxygen-sensing system. J Biol Chem. 2006;281:23482–23491. doi: 10.1074/jbc.M601719200. [DOI] [PubMed] [Google Scholar]
- Stiehl DP, Bordoli MR, Abreu-Rodríguez I, Wollenick K, Schraml P, Gradin K, Poellinger L, Kristiansen G, Wenger RH. Non-canonical HIF-2α function drives autonomous breast cancer cell growth via an AREG-EGFR/ErbB4 autocrine loop. Oncogene. 2012;31:2283–2297. doi: 10.1038/onc.2011.417. [DOI] [PubMed] [Google Scholar]
- Stroud H, Feng S, Morey Kinney S, Pradhan S, Jacobsen SE. 5-Hydroxymethylcytosine is associated with enhancers and gene bodies in human embryonic stem cells. Genome Biol. 2011;12:R54. doi: 10.1186/gb-2011-12-6-r54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Song CX, Huang H, Frankenberger CA, Sankarasharma D, Gomes S, Chen P, Chen J, Chada KK, He C, Rosner MR. HMGA2/TET1/HOXA9 signaling pathway regulates breast cancer growth and metastasis. Proc Natl Acad Sci USA. 2013;110:9920–9925. doi: 10.1073/pnas.1305172110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szulwach KE, Li X, Li Y, Song C-X, Wu H, Dai Q, Irier H, Upadhyay AK, Gearing M, Levey AI, et al. 5-hmC-mediated epigenetic dynamics during postnatal neurodevelopment and aging. Nat Neurosci. 2011;14:1607–1616. doi: 10.1038/nn.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, Rao A. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beucken T, Koritzinsky M, Niessen H, Dubois L, Savelkouls K, Mujcic H, Jutten B, Kopacek J, Pastorekova S, van der Kogel AJ, et al. Hypoxia-induced expression of carbonic anhydrase 9 is dependent on the unfolded protein response. J Biol Chem. 2009;284:24204–24212. doi: 10.1074/jbc.M109.006510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasanthakumar A, Lepore JB, Zegarek MH, Kocherginsky M, Singh M, Davis EM, Link PA, Anastasi J, Le Beau MM, Karpf AR, Godley LA. Dnmt3b is a haploinsufficient tumor suppressor gene in Myc-induced lymphomagenesis. Blood. 2013;121:2059–2063. doi: 10.1182/blood-2012-04-421065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger RH, Kvietikova I, Rolfs A, Camenisch G, Gassmann M. Oxygen-regulated erythropoietin gene expression is dependent on a CpG methylation-free hypoxia-inducible factor-1 DNA-binding site. Eur J Biochem. 1998;253:771–777. doi: 10.1046/j.1432-1327.1998.2530771.x. [DOI] [PubMed] [Google Scholar]
- Williams K, Christensen J, Pedersen MT, Johansen JV, Cloos PAC, Rappsilber J, Helin K. TET1 and hydroxymethylcytosine in transcription and DNA methylation fidelity. Nature. 2011;473:343–348. doi: 10.1038/nature10066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wossidlo M, Nakamura T, Lepikhov K, Marques CJ, Zakhartchenko V, Boiani M, Arand J, Nakano T, Reik W, Walter JOR. 5-Hydroxymethylcytosine in the mammalian zygote is linked with epigenetic reprogramming. Nat Commun. 2011;2:241–248. doi: 10.1038/ncomms1240. [DOI] [PubMed] [Google Scholar]
- Wu H, D’Alessio AC, Ito S, Xia K, Wang Z, Cui K, Zhao K, Sun YE, Zhang Y. Dual functions of Tet1 in transcriptional regulation in mouse embryonic stem cells. Nature. 2011;473:389–393. doi: 10.1038/nature09934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wykoff CC, Beasley NJ, Watson PH, Turner KJ, Pastorek J, Sibtain A, Wilson GD, Turley H, Talks KL, Maxwell PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–7083. [PubMed] [Google Scholar]
- Yu M, Hon GC, Szulwach KE, Song CX, Zhang L, Kim A, Li X, Dai Q, Shen Y, Park B, et al. Base-resolution analysis of 5-hydroxymethylcytosine in the mammalian genome. Cell. 2012;149:1368–1380. doi: 10.1016/j.cell.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen RKC, Chen B, Blair JD, Robinson WP, Nelson DM. Hypoxia alters the epigenetic profile in cultured human placental trophoblasts. Epigenetics. 2013;8:192–202. doi: 10.4161/epi.23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.