Abstract
Cidofovir (CDF) and its cyclic analogue (cCDF) have shown potential in vitro and in vivo antiviral activity against cytomegalovirus (CMV) retinitis. However, hydrophilic nature of CDF may affect cell permeation across lipophilic epithelium and thus limit its effectiveness in the treatment of CMV retinitis. In the present study, we have tested a novel hypothesis, which involves chemical derivatization of cCDF into lipophilic transporter-targeted prodrug [via conjugation with different carbon chain length of lipid raft and targeting moiety (biotin) for sodium-dependent multivitamin transporter (SMVT)]. We have synthesized and characterized three derivatives of cCDF including biotin B-C2–cCDF, B-C6–cCDF, and B-C12–cCDF. Physicochemical properties such as solubility, partition coefficient (n-octanol/water and ocular tissue), bioreversion kinetics, and interaction with SMVT transporter have been determined. Among these novel conjugates, B-C12–cCDF has shown higher interaction to SMVT transporter with lowest half maximal inhibitory concentration value, higher cellular accumulation, and high tissue partitioning. Improvement in physicochemical properties, lipophilicity, and interaction with transporter was observed in the trend of increasing the lipid chain length, that is, B-C12–cCDF > B-C6–cCDF > B-C2–cCDF. These results indicate that transporter-targeted lipid analogue of cCDF exhibits improved cellular accumulation along with higher transporter affinity and hence could be a viable strategy for the treatment of CMV retinitis.
Keywords: transporters, bioavailability, drug transport, HIV/AIDS, lipids, MDCK cells, membrane transport, ophthalmic drug delivery, prodrugs, targeted drug delivery
INTRODUCTION
Cidofovir (CDF), an acyclic phosphonate nucleotide analogue of cytosine, exhibits potential antiviral activity against herpes simplex viruses (HSVs), varicella zoster viruses, adenoviruses, papillomaviruses, polyomaviruses, and poxviruses.1 CDF and its cyclic analogue (cCDF) are highly potent and clinically indicated in the treatment of human cytomegalovirus (hCMV) infections.2 In addition to retinal diseases including diabetic retinopathy, glaucoma, and age-related macular degeneration,3 CMV retinitis is another potential cause of vision impairment.4 hCMV is a major cause of an opportunistic infection in patients with AIDS, congenital immunodeficiency, or organ transplant recipients.4 Although the incidence and prevalence of CMV retinitis in AIDS patients have declined primarily due to the highly active antiretroviral therapy (HAART), it remains a major cause of the vision loss in 20%–40% AIDS patients.5 Moreover, a longitudinal study of the ocular complications of AIDS revealed that ocular findings in patients with newly diagnosed CMV retinitis in the post-HAART era resembled those prior to the introduction of HAART.6 In addition, when CDF was directly compared for its efficacy with other antiviral drugs [acyclovir (ACV) or ganciclovir (GCV)], it proved clearly superior to ACV in the treatment of mice infected intracerebrally with HSV7,8 and to GCV in the treatment of both immune-competent and immune-deficient mice infected with murine CMV.9–11
Although CDF has shown potential activity against CMV retinitis, major treatment failure is due to the lower oral bioavailability and emergence of resistance.12 Emergence of drug resistance by various multidrug resistance (MDR)-associated efflux proteins have been reported as a potential barrier to the success of ocular therapies.13 Therefore, there is an urgency to develop potential analogues of CDF, which shows high permeability, low toxicity, and simultaneously overcomes an incidence of drug resistance. In order to resolve such problems, researchers have synthesized lipid conjugates of cCDF (octadecyloxyethyl–cCDF) for intraocular injection to improve its vitreal half-life, bioavailability, and antiviral efficacy.14 However, the major limitation of lipid conjugates is the higher retention in lipid bilayer of plasma membrane and inability to translocate into the cytoplasm due to which it is not effective in treating deeper tissue infection such as CMV retinitis. Moreover, the low aqueous solubility of lipid prodrugs can further restrict the application of this approach.15 Therefore, it is important to balance the prodrug lipophilicity necessary for transcellular absorption with adequate aqueous solubility. Researchers have opted to synthesize peptide prodrug of cCDF to target peptide transporter in order to improve its antiviral efficacy and bioavailability following oral administration.16 However, the major limitations for transporter-targeted prodrugs are substrate specificity and transport capacity.17 Therefore, lipid conjugates and transporter-targeted prodrugs exhibit partial response since individually these strategies hold both advantages as well as limitations. Hence, a balanced approach is needed to produce better permeability via transporter-targeted moiety along with suitable lipophilicity via lipid raft. In this study, we hypothesized that combination of both transporter-targeted moiety and lipid raft may provide synergistic effect.
In our laboratory, various transporters have been investigated on the retina, which can be utilized for the delivery of therapeutic agents. Transporters such as peptide/histidine,18 organic cationic transporter,19 amino acid transporter,20,21 folic acid,22 and sodium-dependent multivitamin transporter (SMVT) have been reported on the human retinal pigment epithelium cells (ARPE-19).23 SMVT is an important transport system carrying vitamins such as biotin and co-factors essential for the ocular tissues. It was observed in our laboratory that SMVT is a saturable transporter with Km of 138.25 µM and Vmax of 38.85 pmol/ (min mg) protein on ARPE-19 cells.23 Biotin prodrugs were able to utilize this low capacity and high affinity SMVT transporter to enhance the permeability of therapeutic drug conjugates. We have previously reported that biotin-conjugated GCV (biotin–GCV) was recognized by SMVT transporter on ARPE-19 cells and rabbit retina. Moreover, biotin–GCV has shown therapeutically desirable pharmacokinetics profile as compared with parent GCV.23
Therefore, in the present study to delineate our hypothesis of attaining synergetic effect by simultaneously conjugating both transporter-targeted moiety (biotin) and lipid raft, we have synthesized and characterized several biotin-conjugated lipid prodrugs of cCDF to target SMVT transporter on the retina for the treatment of hCMV retinitis. We hypothesized that the lipid raft of synthesized targeted lipid prodrug will facilitate enhanced interaction with the cell membrane and thereby will assist docking of targeted moiety (i.e., biotin) into the binding domain of transporter (SMVT). The net effect will be rapid translocation of targeted lipid prodrug across the plasma membrane, improving cellular absorption. These modifications will not only help to improve the permeability (via SMVT transporter and lipid raft) but will also attain desired antiviral efficacy of parent CDF. Therefore, transporter-targeted lipid prodrug design may be a promising approach to treat hCMV retinitis.
MATERIALS
Cidofovir was a generous gift from the Gilead Science (Foster city, California) for this project. Biotin–N-hydroxysuccinimide (biotin–NHS) and Benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate (PyBOP) were obtained from Chem-Impex International (Wood Dale, Illinois). 1-Dodecanol, 1-hexanol, 6-amino 1-hexanol, and 12-amino 1-dodecanol were obtained from TCI America (Portland, Oregon). [3H]-Biotin (35.6 Ci/mmol, radiochemical purity >98.6%) was obtained from Perkin Elmer (Waltham, Massachusetts). N,N-Dimethylformamide (DMF), N,N-diisopropylethylamine (DIPEA), methanol, and dichloromethane were purchased from Sigma–Aldrich (St. Louis, Missouri). All other reagents of analytical grade were purchased from local supplier and used as such without any purification. Distilled–deionized water (DDW) was used to prepare buffer and mobile phase. Madin–Darby canine kidney (MDCK)–multidrug resistance protein 1 (MDR1) cells were donated by P. Borst (Netherlands Cancer Institute, Amsterdam, the Netherlands). Dulbecco’s modified Eagle’s medium–Nutrient Mixture F-12 (1:1) (DMEM/F12), DMEM, fetal bovine serum (FBS), and Non-essential aminoa cids (NEAA) were purchased from Gibco (Invitrogen Corporation, Grand Island, New York). Antibiotics and sodium bicarbonate were purchased from Sigma–Aldrich. Transwell® inserts were procured from Fisher Scientific (Pittsburgh, Pennsylvania), and tenofovir and tenofovir disoproxil fumarate were purchased from Santa Cruz Biotechnology (Santa Cruz, California). Nonradioactive cytotoxicity kit (CellTiter 96® AQueous Non-Radioactive Cell Proliferation Assay) was purchased from Promega (Madison, Wisconsin) and scintillation cocktail and Bio-Rad Protein Assay kit was purchased from MP bioscience (Solon, Ohio) and Bio-Rad (Hercules, California), respectively. Culture flasks (75 cm2 growth area) and 12-well plates (3.8 cm2 growth area per well) were procured from MidSci (St. Louis, Missouri). Nuclear magnetic resonance (NMR) spectra were collected by Bruker 400 MHz NMR with sample dissolved in deuterated dimethyl sulfoxide (DMSO-d6).
ANIMALS
Adult male New Zealand albino rabbits weighing between 2 and 2.5 kg were obtained from Myrtle’s Rabbitry (Thompson Station, Tennessee). Ketamine HCl and Rompun (Xylazine®) were purchased from Fort Dodge Animal Health (Fort Dodge, Iowa) and Bayer Animal Health (Shawnee Mission, Kansas), respectively. Protocol for performing all the surgical procedure was approved by Institutional Animal Care and Use Committee of the University of Missouri-Kansas City (Kansas City, Missouri).
METHODS
Synthesis of Biotin-Conjugated Lipid Prodrugs of cCDF
Biotin-Lipid Conjugates
To biotin–NHS (1 equiv), anhydrous DMF and lipid moieties i.e. 2-amino 1-ethanol/6-amino 1-hexanol/ 12-amino 1-dodecanol (1.2 equiv) were added and the reaction mixture was stirred overnight under N2 at room temperature. Solvent was removed under vacuum, and the white residue was purified by the silica gel column chromatography with 20% dichloromethane in methanol as the eluent to yield products (biotin-conjugated lipid moieties, biotin-C2/biotin-C6/biotin-C12) as white solid.
Biotin–Lipid Conjugates with cCDF
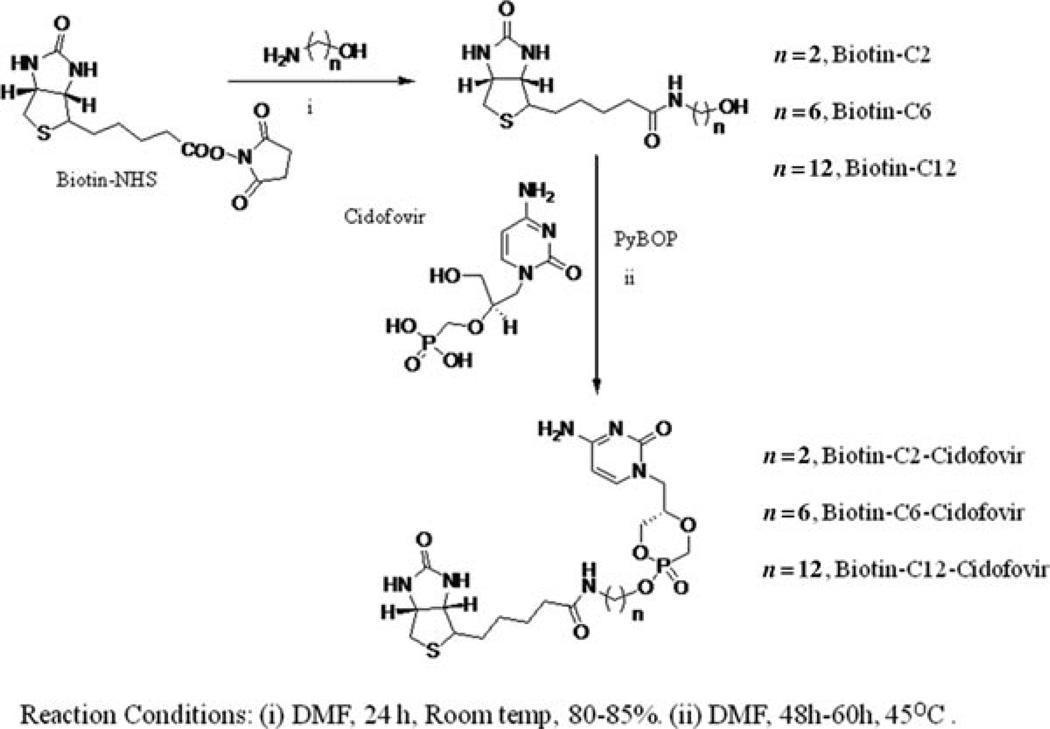
In a separate flask, CDF(1 equiv), 20mL of anhydrous DMF, and 2.1 mL of dry DIPEA were added. The reaction vessel was warmed by a heat gun to facilitate the dissolution of the CDF–DIPEA salt. The solvent was then removed under vacuum. To that residue, 20mL of anhydrous DMF, DIPEA (5 equiv), the relevant biotin-conjugated lipid (1.5 equiv) in anhydrous DMF, and PyBOP (2.1 equiv) were added, and the reaction mixture was stirred under N2 at 45°C for 48– 60 h. The reaction was monitored by 31P NMR, and additional portions of PyBOP were added as necessary. After completion of reaction, solvent was removed under vacuum, and the brownish-red residue was purified by repetitive silica gel column chromatography using gradient solvent combination of dichloromethane and methanol as the eluent, to yield products as brownish sticky solid (Figure 1).
Figure 1.
Schematic representation of the synthesis of biotin-conjugated lipid prodrugs of cyclic cidofovir.
Biotin-C2–cyclic Cidofovir (B-C2–cCDF) was obtained as brownish sticky solid in 45% yield. 31P NMR (400 MHz, DMSO-d6) δ: 13.92 (s), 12.84 (s), electrospray ionization–mass spectrometry (ESI–MS): m/z calculated 530.5, found 531.4 (M + H)+.
Biotin-C6–cyclic Cidofovir (B-C6–cCDF) was obtained as brownish sticky solid in 56% yield. 31P NMR (400 MHz, DMSO-d6) δ: 13.12 (s), 11.55 (s), ESI–MS: m/z calculated 586.6, found 587.2 (M + H)+.
Biotin-C12–cyclic Cidofovir (B-C12–cCDF) was obtained as brownish sticky solid in 61% yield. 31P NMR (400 MHz, DMSO-d6) δ: 13.84 (s), 12.6 (s), ESI–MS: m/z calculated 670.7, found 671.6 (M + H)+.
Synthesis of Lipid Prodrugs of cCDF
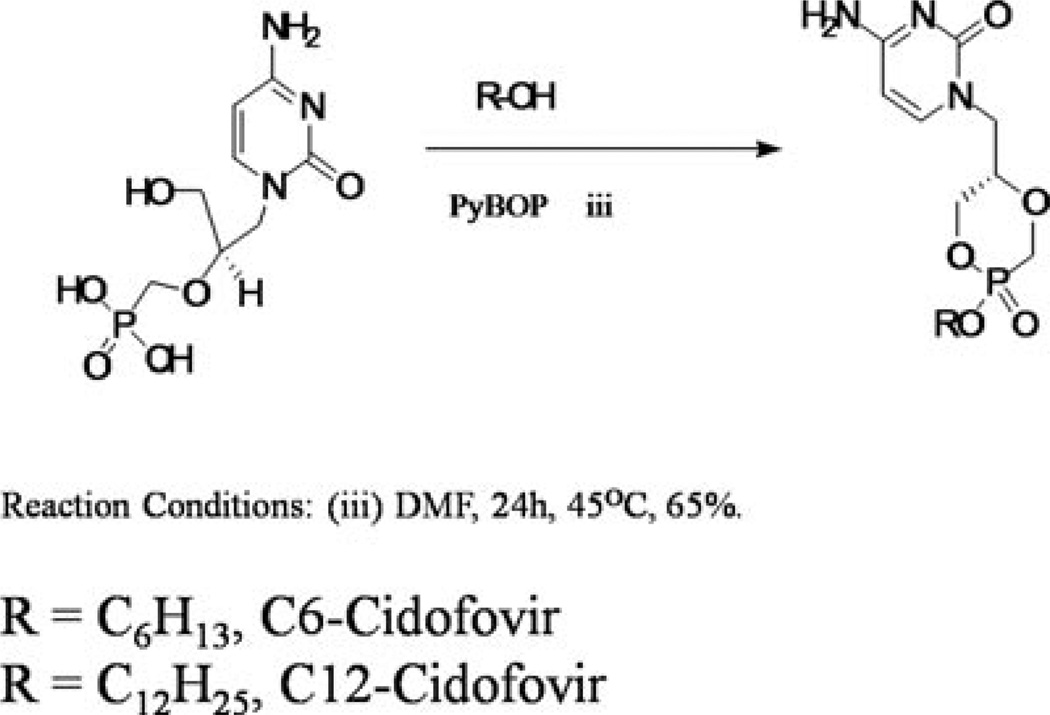
To CDF (1 equiv), 20mL of anhydrous DMF and 2.1mL of dry DIPEA were added. The reaction flask was warmed by a heat gun to facilitate the dissolution of the CDF–DIPEA salt. The solvent was then removed under vacuum. To that residue, 20mL of anhydrous DMF, DIPEA (5 equiv), the relevant lipid moieties (1.5 equiv) in dry DMF, and PyBOP (2.1 equiv) were added, and the reaction mixture was stirred under N2 at 45°C for 24 h. The reaction was monitored by 31P NMR, and additional portions of PyBOP were added as necessary. After completion of the reaction, solvent was removed under vacuum, and the brownish-red residue was purified by repetitive silica gel column chromatography using gradient solvent combination of dichloromethane and methanol to generate white solid product (Figure 2). The purity of the prodrugs were confirmed via High-performance liquid chromatography (HPLC).
Figure 2.
Schematic representation of the synthesis of lipid prodrugs of cyclic cidofovir.
C6-cyclic Cidofovir (C6-cCDF) was obtained as white solid in 65% yield. 31P NMR (400 MHz, DMSO-d6) δ: 12.92 (s), 11.66 (s), ESI–MS: m/z calculated 345.3, found 346.2 (M + H)+.
C12-cyclic Cidofovir (C12-cCDF) was obtained as white solid in 62% yield. 31P NMR (400 MHz, DMSO-d6) δ: 13.47 (s), 12.20 (s), ESI–MS: m/z calculated 429.5, found 430.3 (M + H)+.
Cell Culture
MDCK-MDR1 cells (passages 5–15) were cultured in DMEM supplemented with 10% FBS (heat inactivated), 1% nonessential amino acids, penicillin (100 U/mL), streptomycin (100µg/mL), 20mM HEPES, and 29mM sodium bicarbonate adjusting pH 7.4. Cells were grown and incubated at 37°C in a culture incubator with 5% CO2. The medium was changed every alternate day. Cells were passaged every 4–6 days till it reached 80%–90% confluency. Cells were seeded at a density of 25,000 per well in 12-well tissue culture-treated plates and collagen-coated Transwell® permeable inserts (Costar®) at a density of 10,000 per well in 96-well plates. These cells were then allowed to grow for 4–6 days and used for further studies.
High-Performance Liquid Chromatography Analysis
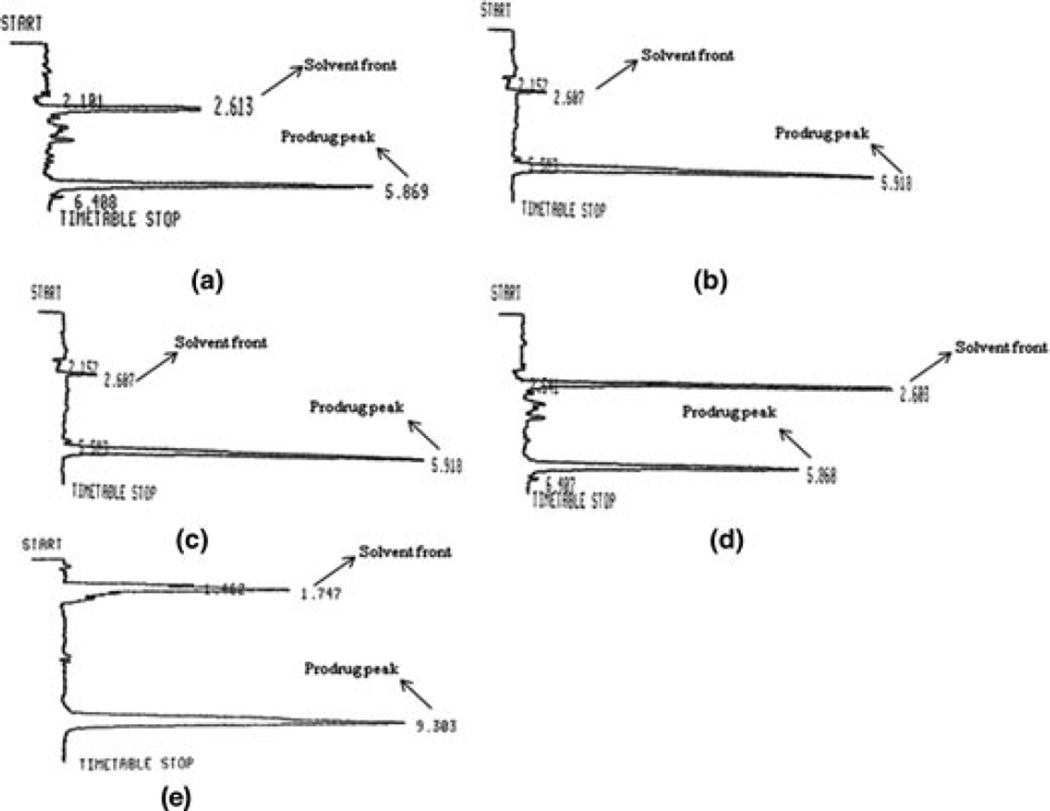
High-performance liquid chromatography (HPLC) methods for simultaneous analysis of CDF, its biotin conjugate (B-C2–cCDF, B-C6–cCDF, and B-C12– cCDF) and lipid prodrugs (C6-cCDF and C12-cCDF) were developed (Figure 3). Stability, solubility, n-octanol/water partition coefficient, and tissue partition coefficient samples were analyzed by an HPLC system comprising Waters 515 pump (Waters, Milford, Massachusetts), Alcott 718AL refrigerated autosampler, ultraviolet detector (RAININ, Dynamax, Absorbance Detector Model UV-C), HP 3395 integrator, Phenomenex C18 (4.6 × 250) column with C18 guard column. Mobile phases comprised 20mM phosphate buffer (pH 6.8) and methanol (90:10, v/v) for CDF and biotin-conjugated lipid prodrugs of cCDF, and 20mM phosphate buffer (pH 6.8) and acetonitrile (50:50, v/v) for lipid conjugates of cCDF. All the compounds were detected at λmax 274nm and flow rate of 0.5mL/min. The analytical method was validated with respect to precision (% RSD < 1), linearity (r2 > 0.998), reproducibility (% Relative standard deviation < 2), and selectivity (no interfering peaks were observed).
Figure 3.
Representative chromatogram of cyclic cidofovir prodrugs (a) B-C2-cCDF, (b) B-C6–cCDF, (c) B-C12–cCDF, (d) C6-cCDF, and (e) C12-cCDF.
Liquid Chromatography-Tandem Mass Spectrometry Analysis
MDS Sciex API 3200 Triple Quadrupole linear QTrap mass spectrometry (Applied Biosystems/MDS Sciex, Foster City, California) system interfaced by turbo ion spray with positive ion source in Multiple Reaction Monitoring (MRM) mode was used for detection. Mass-dependent parameters were tuned and optimized for CDF, B-C2–cCDF, B-C6–cCDF, B-C12–cCDF, C6–cCDF, C12–cCDF, tenofovir and tenofovir diisoproxil fumarate. Tenofovir was used as an internal standard (IS) for analysis of biotin-conjugated lipid prodrugs. Initially, full scan mass spectra were acquired in positive ion mode for all the compounds. During a direct infusion experiment, the mass spectra for B-C2–cCDF, B-C6–cCDF, B-C12–cCDF, and tenofovir peaks at mass to charge ratio (m/z) of 280.09, 531.18, 587.24, 671.37, and 288.13, respectively, as a protonated molecular ions [M + H]+. The most stable abundant fragment ions observed in each product tandem mass spectrometry (MS/MS) spectrum were at m/z 262.30 for CDF, 262.16 for B-C2–cCDF, 262.30 for B-C6–cCDF, 262.13 for B-C12–cCDF, and 270.03 for tenefovir. Quantitative determination was performed in MRM scan positive ion mode using the following mass transitions: 280.09 → 262.30 for CDF, 531.18 → 262.16 for B-C2–cCDF, 587.24 → 262.30 for B-C6–cCDF, 671.37 → 262.13 for B-C12–cCDF, and 288.13 → 270.00 for tenofovir (IS). The method was developed over the linear (r2 > 0.99) concentration range 60–1000 ng/mL. Lower limit of quantification (LLOQ) of all the compounds was 30.0 ng/mL.
Tenofovir disoproxil fumarate was used as an IS for the analysis of lipid prodrugs of cCDF. Similarly, a full scan mass spectra were acquired in positive ion mode for C6-cCDF, C12-cCDF, and tenofovir disoproxil fumarate peaks at m/z of 346.20, 430.33, and 674.10, respectively, as a protonated molecular ion [M + H]+. The most stable abundant fragment ions observed in each product MS/MS spectrum were at m/z 262.20 for C6-cCDF, C12-cCDF, and 326.10 for tenefovir disoproxil fumarate. Quantitative determination was performed in MRM scan positive ion mode using the following mass transitions: 346.20 → 262.20 for C6-cCDF, 430.33 → 262.20 for C12-cCDF, and 674.10 → 326.10 for tenofovir disoproxil fumarate (IS). The method was developed over the linear (r2 > 0.99) concentration range 30–1000 ng/mL. LLOQ of all the compounds was 15.0 ng/mL.
Sample Preparation and Extraction
Frozen samples were thawed at room temperature and thoroughly mixed using vortex for 1 min. For biotin-conjugated lipid prodrugs of cCDF: Using calibrated pipettes, 100 µL of samples was aliquoted into a 1.5mL polypropylene microcentrifuge tubes. To these samples, 20µL of 10.0µg/mL freshly prepared IS working solution (tenofovir) (except for blank sample) was added. The mixture was vortexed for 30 s. The samples were diluted with DDW upto 1mL and then loaded on to the Bond EluteSCX cartridge, which is preequilibrated with 2mL of methanol and 2 mL of DDW. Following loading of CDF and its biotin-conjugated lipid prodrugs, the samples were eluted from Bond EluteSCX cartridge with 2mL of 3% Ammonium hydroxide-methanol. The eluent phase was evaporated in Speed Vac® at 40°C for 2 h, following reconstitution in 100 µ L of mobile phase.
For lipid conjugates of cCDF: To 100 µL of samples aliquoted into a 1.5mL polypropylene microcentrifuge, 20 µL of 10.0 µg/mL freshly prepared tenofovir disoproxil fumarate (IS) working solution (except for blank sample) was added. The mixture was vortexed for 30 s. A 900 µ L ice-cold ethyl acetate was added and vortexed for 1 min, centrifuged, and 800 µ L supernatant was aliquoted in microfuge tube. The eluent phase was evaporated in Speed Vac® at 40°C for 4 h, following reconstitution in 100 µL of mobile phase. A 10 µL of the resulting solution was injected onto liquid chromatography (LC)–MS/MS for analysis using XTerrs® MS C18 5 µ m column.
Aqueous Solubility and n-Octonal/Buffer Partition Coefficient
Aqueous solubility study was performed by following previously published protocol from our laboratory.24 Briefly, excess amount of CDF and its biotin-conjugated prodrugs (B-C2–cCDF, B-C6–cCDF, and B-C12–cCDF) were added to 1 mL of DDW in glass vials. These vials were kept in shaker bath for 24 h at 37°C. After 24 h, the content of the vial was carefully transferred in an Eppendorf tube and centrifuged for 10 min at 10,000X g. The supernatant was collected and filtered through 0.45 µm Nalgene syringe filter membrane. The supernatant was immediately stored in −20°C refrigerator until analysis was performed using HPLC. All the experiments were conducted in triplicate.
A partition coefficient study was performed with n-octanol and Dulbecco’s Phosphate-Buffered Saline (DPBS) (pH 7.4) as per previously published protocol from our laboratory.25 Briefly, n-octanol and DPBS were presaturated for 48 h with each other. After presaturation, 1mL aliquots of presaturated DPBS was placed in glass vial and stock solution of CDF and its biotin-conjugated lipid prodrugs (100 µM) were added. The mixture was vortexed and an initial sample was collected (Caqu). After equilibration, 1mL aliquot of presaturated n-octanol was added. The glass vial was kept at constant temperature shaker bath (37°C) for 24 h. Samples were collected from aqueous and organic phase and analyzed for the determination of partition coefficient and log P values.
Stability Studies
Buffer Stability
Prodrugs need to display a good stability and must be absorbed intact across cell membrane. Simultaneously, it should be converted to parent molecule following its active/passive transport in target tissue to exhibit therapeutic activity. Chemical stability studies will help to screen an ideal prodrug candidate for in vivo studies. The chemical stability of prodrugs was studied in various buffers at different pH (1.4–7.4). For pH 1.4 hydrochlorate buffer (8.5 mL of 0.2M hydrochloric acid and 5.0mL of 0.2 M potassium chloride), pH 4.0 acetate buffer (0.3 g sodium acetate tri-hydrate and 3.9mL of 2N acetic acid), pH 6.0 phosphate buffer (50mL of 0.2M monobasic potassium phosphate and 5.6mL of 0.2M sodium hydroxide), and pH 7.4 phosphate buffer (50mL of 0.2 M monobasic potassium phosphate and 39.1mL of 0.2M sodium hydroxide) were prepared within the range of ± 0.05 units.26 The buffer strength was kept constant at 100 mM. To initialize the study, 4.8mL of buffer was taken in a glass vial and 200 µ L of freshly prepared prodrug solution (10 mg/mL) in methanol was added. The glass vials were kept in the water bath at 37°C and 60 rpm. Aliquots (100 µ L) were taken at predetermined time interval and immediately freeze at −80°C until further analysis by HPLC. Experiments were performed in triplicate.
Ocular Tissues Hydrolysis
Previously published protocol was followed to separate the ocular tissues from the animals.27,28 Briefly, New Zealand white male albino rabbits were euthanized with sodium pentobarbital injection through the marginal ear vein. Both the eyeballs were enucleated immediately and rinsed with DPBS pH 7.4. Both ocular segments were carefully separated by making an incision at the scleral–limbus junction. The posterior segment ocular tissues such as conjunctiva, sclera, choroid–retina, and vitreous humor were separated. These tissues were stored at −80°C prior to use. Target tissue was retina for bioreversion and the therapeutic action of prodrugs. So, the retina was homogenized in ice bath and the homogenate was collected. Protein concentration of supernatant was measured with a Bio-Rad protein estimation kit (Bio-Rad). In all the stability studies, protein concentration of 1.0mg/ mL was employed. To initiate the study, 2.8mL of supernatant was taken in a glass vial and 200 µL of prodrugs solution (1mg/mL of stock solution of prodrugs) was added. At predetermined time interval, 100 µ L sample was taken and immediately diluted with 100 µL of ice-cold methanol containing 0.1% trifluroacetic acid to terminate enzyme activity. Samples were immediately centrifuged and supernatant was stored at −80° C until HPLC analysis. Finally, apparent first-order rate constants (k) from Eq. 1 and half-life (t1/2) from Eq. 2 were calculated and corrected for any chemical hydrolysis observed with the control. This experiment was performed in triplicate.
| (1) |
| (2) |
Cytotoxicity Studies
Cytotoxicity of CDF and its prodrugs was evaluated in MDCK –MDR1 (passage no 5–15) using an aqueous nonradioactive cytotoxicity kit. This kit is based on the principle of MTS assay and has been successfully used to study cytotoxicity of various pro-drugs in our laboratory29 MDCK–MDR1 cells were cultured following procedure mentioned in the Methods section. Prior to the experiment, a fixed number of MDCK–MDR1 cells (10,000 cells per well) were plated in 96-well plate for 24 h. After 24 h, culture medium was replaced with 100 µ L of freshly prepared solutions of CDF and its biotin-conjugated lipid prodrugs (B-C2–cCDF, B-C6–cCDF, B-C12–cCDF) in culture medium at different concentrations and cells were incubated for 48 h (in a humidified, 5% CO2 atmosphere). CDF and its conjugates were tested in the concentration range of 50, 100, and 250 µM. After 48 h, the solution was removed and the above-mentioned dye was added and incubated for 2h. At the end of 2 h, cell viability was calculated using color determination at 485 nm with a 96-well microtiter plate reader (SpectraFluor Plus, Tecan, Switzerland). Five percent Triton-X 100 and culture medium were used as positive and negative control, respectively.
Interaction with SMVT Transporter
Inhibition of Uptake of [3H]-Biotin
This experiment was performed to investigate if biotin-conjugated lipid prodrugs can be recognized by the SMVT transporter. For this study, a model cell line MDCK–MDR1 was selected to delineate in vitro interaction with SMVT.30 MDCK–MDR1 cells were cultured on 12-well plates according to an earlier stated protocol from the Methods section. Biotin, a well-known substrate of SMVT transporter, was selected for this study. After the confluency, cells were washed with DPBS pH 7.4 two times for 10 min at 37°C. The uptake study was initiated by adding 1 mL of [3H]-Biotin (0.25µCi/mL in DPBS pH 5.5) in the presence and absence of biotin-conjugated lipid prodrug of cCDF solution (all at 50 µM concentration) as a competitive inhibitor. Uptake study was carried out for 10min at 37°C. At the end of an incubation period, drug solutions were removed and the cells were washed with ice-cold stop solution (200mM KCl and 2mM HEPES) three times. Furthermore, cells were lysed with 1mL of lysis solution (0.1%, w/v Triton X-100 in 0.3N sodium hydroxide) and kept at room temperature overnight. Next day, 500 µL of aliquots was transferred to scintillation vials containing 3mL scintillation cocktail. Finally, these samples were analyzed with a Beckman scintillation counter (Model LS-6500, Beckman Instruments, Inc.). Uptake was normalized to the protein content of each well. Protein content of cell lysate was quantified using Bio-Rad assay.
Inhibition of Transport of [3H]-Biotin
Transwell diffusion chamber system was utilized for this study. Prior to a transport experiment, cell monolayers grown on the Transwell® inserts were rinsed with DPBS pH 7.4 and incubated at 37°C for 10min for two times for both apical (AP) and basolateral (BL) sides. Transport was initiated by adding 500 µ L of [3H]-biotin (in DPBS pH 5.5) in presence and absence of biotin-conjugated lipid prodrugs of cCDF (all at 50 µ M concentration) toward AP side of cells (donor chamber). The receiver chamber (BL side) contains DPBS pH 7.4. The cell monolayer integrity (around 250Ωcm2) was determined by transepithelial electrical resistance measurement. Transport experiment was conducted for a total time period of 3 h. Sampling (100 µL) from the receiver chamber was carried out at predetermined time intervals of 15, 30, 45, 60, 90, 120, 150, and 180 min and fresh DPBS pH 7.4 was replaced to maintain sink conditions in receiver chamber. The samples were then analyzed in a Beckman scintillation counter (Model LS-6500, Beckman Instruments, Inc.). All experiments are performed in triplicate. The permeability (cm/s) (P) of [3H]-biotin was determined according to Eq. 3.
| (3) |
where dM/dt (mol/min) represents the rate of transport across the cell monolayer, A (cm2) is the cross-sectional area available for transport, and Cd (µ M) is the donor concentration.
Affinity Measurements Using [3H]-Biotin on MDCK-MDR1 Cells
For dose–response studies in which [3H]-biotin uptake was inhibited in the presence of biotin-conjugated lipid prodrugs, the inhibitory effect was calculated by Eq. 4.
| (4) |
Here, x denotes the logarithm of the concentration of the biotin-conjugated lipid prodrugs, whereas Y is the intracellular uptake of [3H]-biotin. IC50 represents the inhibitor concentration where the uptake of [3H]-biotin was inhibited by 50%, and H is the Hill constant. Y starts at a minimum (min) value (at low inhibitor concentration) and then plateaus at a maximum (max) value (at high inhibitor concentration), resulting in a sigmoidal profile.
Nonradioactive Cellular Accumulation
Cellular accumulation study was performed on MDCK–MDR1. Cells were grown in monolayer on 12-well plates for 4 –6 days (with confluency of 90%–95%), the cellular accumulation of CDF, biotin-conjugated lipid prodrugs, and lipid prodrugs of cCDF were initiated. Briefly, cells were washed with DPBS pH 7.4 and 1mL of CDF, biotin-conjugated lipid prodrugs, and lipid conjugates of cCDF (all at 50 µM in DPBS pH 5.5) was added in the presence and absence of competitive inhibitor biotin at 37°C. At the end of an experiment, the drug/prodrugs solution was removed and cell monolayer was washed with ice-cold stop solution. Cells were lysed by rapid freeze–thaw cycle (three times). The samples were analyzed by LC–MS/MS after Bond Elute SCX solid-phase extraction with 3% Ammonium hydroxide–methanol and cold ethyl acetate extraction. All the experiments were performed in quadruplicate and cellular accumulation was normalized using protein count.
Tissue Partition Coefficient
Previously published protocol was followed to separate the posterior segment ocular tissues from New Zealand rabbits.27,28 The retina–choroid and sclera were removed carefully and placed in preweighed vials on ice. A 990 µ L of isotonic phosphate buffer saline pH 7.4 was added to the vials containing retina–choroid and incubated at 37°C for 15 min for tissue equilibration. Ten microliters of drug and pro-drugs solution was spiked, gently vortexed, and incubated for 6h. At the end of incubation, the vials were centrifuged at 5000 rpm for 2 min and the supernatant and tissue pellet were collected in different microfuge tubes and stored at −80?C for further analysis. One hundred microliters of supernatant was mixed with ice-cold methanol for protein precipitation (CPBS pH 7.4). Tissue pellet was homogenized with DPBS pH 7.4 and immediately mixed with the ice-cold methanol for protein precipitation and terminating enzymatic degradation before analysis (Ctis). Tissue partition calculation was calculated with Eq. 5. All the analysis was performed by HPLC or LC–MS/MS.
| (5) |
Statistical Analysis
All experiments were conducted at least in triplicate and cellular accumulation studies were conducted in quadruplicate. Results are expressed as mean ± SD. Student’s t-test was applied to detect statistical significance between the parameters of the biotin-conjugated lipid prodrugs, lipid prodrugs, and CDF. p < 0.05 and p < 0.01 were considered to be statistically significant.
RESULTS
Aqueous Solubility and n-Octanol-Water Partition Coefficient
Aqueous solubility of biotin-conjugated lipid prodrugs was determined in DDW at 37°C. The aqueous solubility and partition coefficient values are shown in Table 1. As expected, the solubility of biotin-conjugated lipid prodrugs of cCDF was found to be lower with increasing carbon chain length of lipid raft. Drastic reduction in the solubility of lipid prodrugs of cCDF has been observed compared with biotin-conjugated lipid prodrugs. The reduction in solubility was about 1.5-, 4.5-, and 5.5-fold for B-C2–cCDF, B-C6–cCDF, and B-C12–cCDF, respectively. However, reduction in solubility was about 56- and 74-fold for C6-cCDF and C12-cCDF, respectively (Table 1). Octanol–buffer partition studies reveal log P value of CDF to be −1.86. These results clearly indicate an improvement in the lipophilicity of prodrugs due to the presence of lipid raft conjugation.
Table 1.
Physicochemical Properties (Aqueous Solubility and log P) for Biotin-Conjugated Lipid Prodrugs and Lipid Prodrugs of Cyclic-Cidofovir
| Prodrugs | Solubility (mg/mL) in DDWa |
Log P |
|
|---|---|---|---|
| Predictedb | Calculatedc | ||
| CDF | 72.44 ± 3.33 | −2.89 | −1.86 ± 0.39 |
| B-C2–cCDF | 56.08 ± 1.63 | 0.23 | −0.64 ± 0.24 |
| B-C6–cCDF | 17.31 ± 1.02 | 1.04 | −0.47 ± 0.18 |
| B-C12–cCDF | 13.21 ± 0.98 | 2.61 | −0.16 ± 0.04 |
| C6-cCDF | 1.27 ± 0.48 | 3.64 | 2.84 ± 0.56 |
| C12-cCDF | 0.95 ± 0.37 | 4.23 | 3.89 ± 1.23 |
Distilled–deionized water.
Log P from Chem draw.
Log P = Log [(ConcentrationOctanol)/ConcentrationWater)].
Buffer and Tissue Homogenate Stability
Buffer (at various pH) and ocular tissue homogenate stability studies aid in the screening of the ideal prodrug candidates for further studies.
Buffer Stability
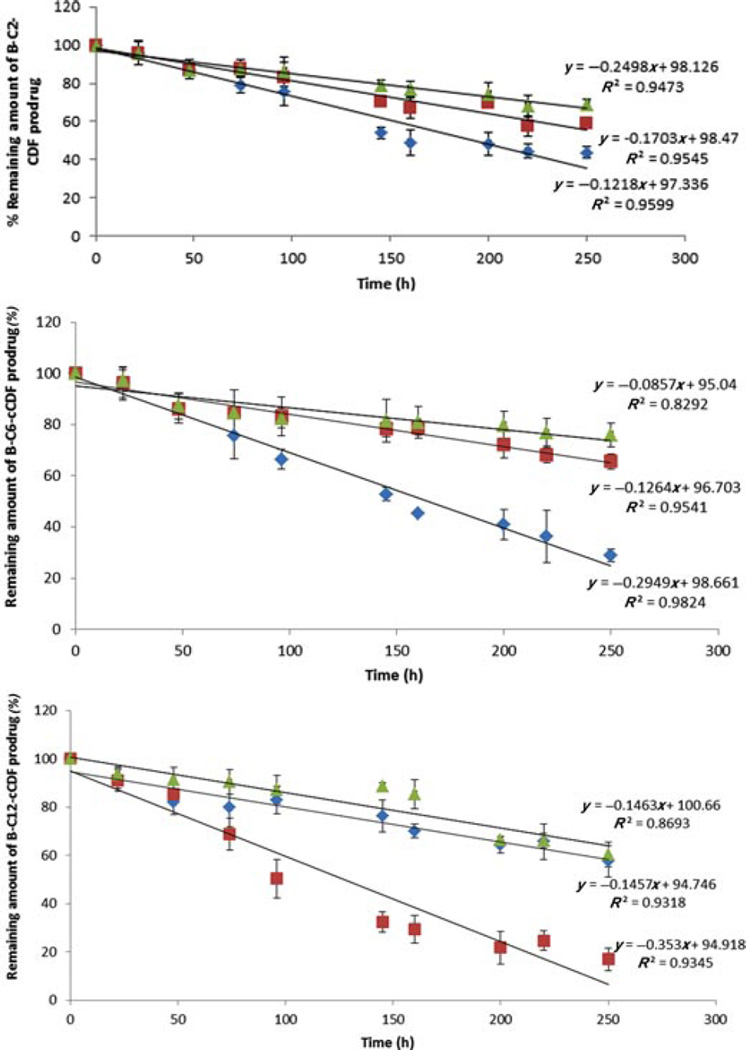
Chemical stability was determined for all the biotin-conjugated lipid prodrugs in different pH buffers (pH 1.4 to 7.4). We did not observe any appreciable degradation of prodrugs at pH 1.4 (data not shown). Upon raising pH toward alkaline range, an accelerated degradation has been observed. Figure 4 and Table 2 summarize the apparent first-order degradation rate constants at various pH. B-C2–cCDF was found to be more stable than B-C6–cCDF and B-C12–cCDF.
Figure 4.
pH-dependent degradation of (a) biotin-C2-cCidofovir, (b) biotin-C6-cCidofovir, and (c) biotin-C12-cCidofovir. δ,pH 4.0; ■, pH 6.0; ◊, pH 7.4. Each data point represents mean ± SD (n= 3).
Table 2.
First-Order Rate Constant (k) and Half-Life (t1/2) of Biotin-Conjugated Lipid Prodrugs at Various pH at 37°C
| pH 4.0 |
pH 6.0 |
pH7.4 |
||||
|---|---|---|---|---|---|---|
| k (h−1) | t1/2 (h) | k (h−1) | t1/2 (h) | k (h−1) | t1/2 (h) | |
| B-C2-cCidofovir | 1.4 ± 0.20 | 504.80 ± 66.74 | 2.2 ± 0.23 | 318.28 ± 32.06 | 3.6 ± 0.32 | 192.97 ± 16.30 |
| B-C6-cCidofovir | 6.1 ± 1.1 | 115.93 ± 22.71 | 1.8 ± 0.051 | 390.77 ± 17.30 | 6.1 ± 0.20 | 486.35 ± 17.86 |
| B-C12-cCidofovir | 1.7 ± 0.03 | 406.08 ± 8.24 | 1.8 ± 0.042 | 387.88 ± 9.27 | 7.6 ± 0.036 | 89.60 ± 12.40 |
Hydrolysis rate constant values are k × 10−3.
Each data point represents mean ± SD (n= 3).
Ocular Tissue Homogenate Stability
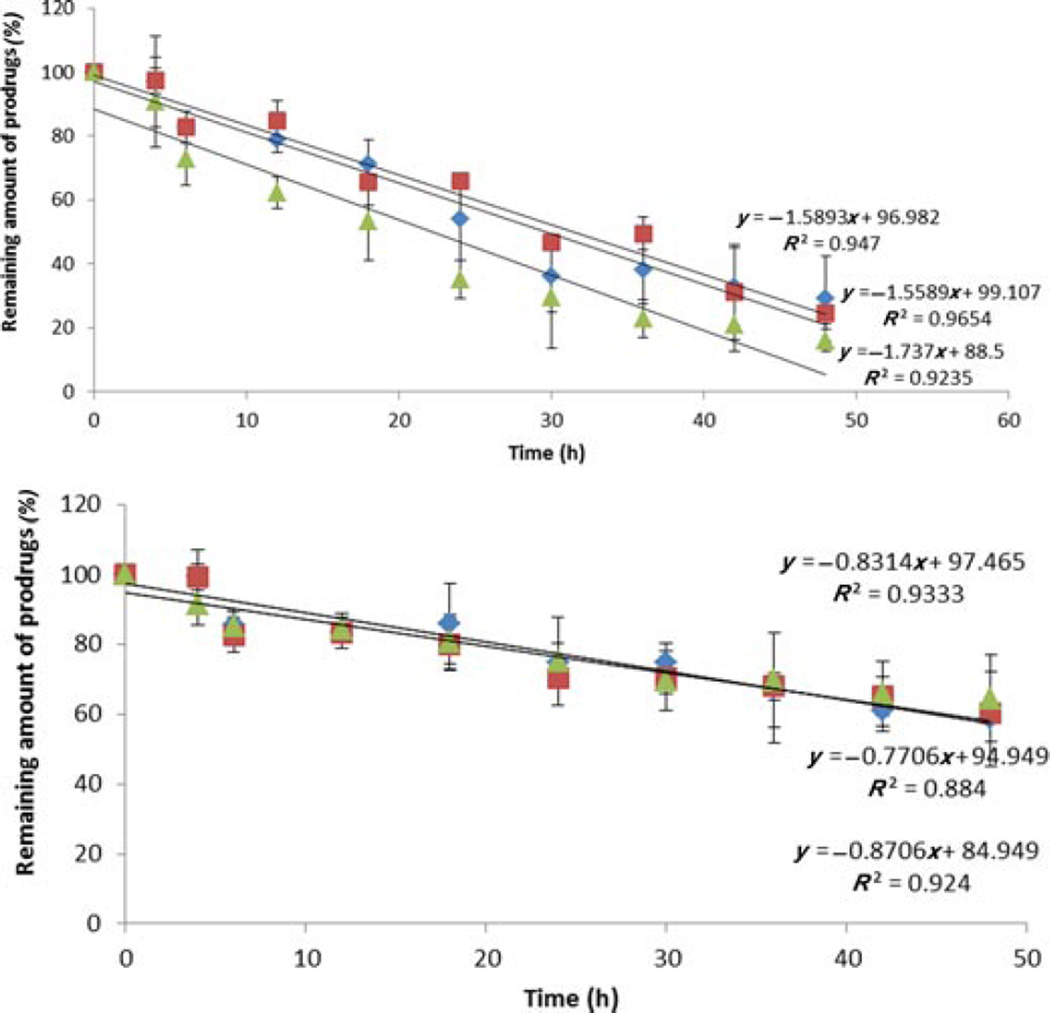
A prodrug molecule is pharmacologically inactive and hence would not exhibit any therapeutic effect unless it converts into the parent drug. Therefore, we also performed the enzymatic stability studies of prodrugs in presence of retina–choroid and vitreous humor homogenate (posterior segment tissue). Figure 5 and Table 3 summarize the apparent first-order degradation rate constant, indicating the loss of the prodrugs in the retina–choroid and vitreous.
Figure 5.
Enzymatic degradation biotin-conjugated lipid prodrugs in (a) retina–choroid tissue homogenate and (b) vitreous humor homogenate. ■, B-C2-cCidofovir; ◊, B-C6-cCidofovir; and Δ,B-C12-cCidofovir. Each data point represents mean ± SD (n= 3).
Table 3.
First-Order Rate Constant (k) and Half-Life (t1/2) of Biotin-Conjugated Lipid Prodrugs in Ocular Tissue Homogenate at 37°C
| Retina–Choroid |
Vitreous Humor |
|||
|---|---|---|---|---|
| k (h−1) | t1/2 (h) | k (h−1) | t1/2 (h) | |
| B-C2-cCidofovir | 2.82 ± 0.0024 | 24.57 ± 2.02 | 1.07 ± 0.0012 | 65.05 ± 6.87 |
| B-C6-cCidofovir | 2.71 ± 0.0026 | 26.09 ± 2.35 | 1.22 ± 0.00076 | 58.85 ± 3.90 |
| B-C12-cCidofovir | 3.94 ± 0.0025 | 17.85 ± 2.18 | 1.02 ± 0.00027 | 68.23 ± 8.10 |
Hydrolysis rate constant values are k× 10−2.
Each data point represents mean ± SD (n= 4).
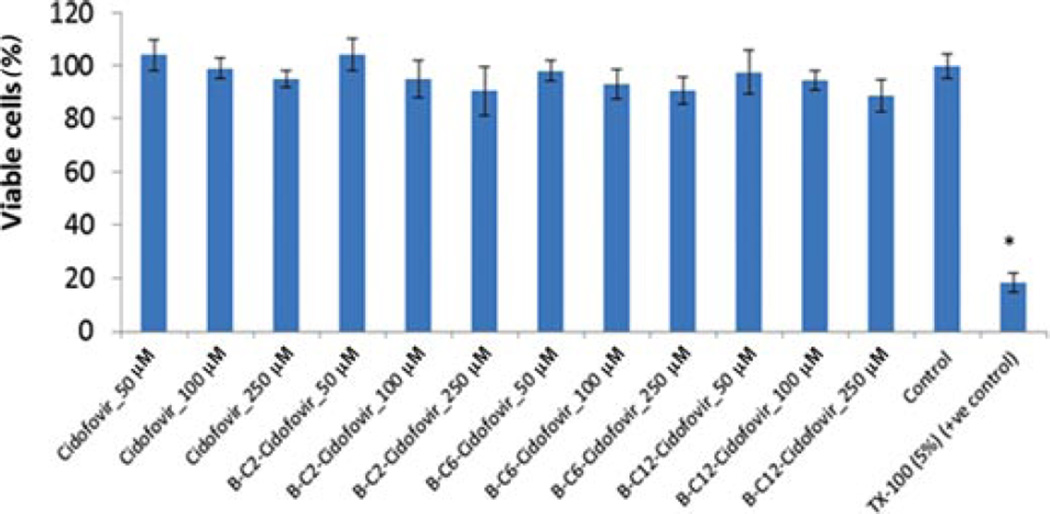
Cytotoxicity Studies
Cidofovir and all the prodrugs of CDF were studied for cytotoxicity studies in MDCK–MDR1 cell line. Prodrugs were found to be nontoxic up to 250 µM concentration for 48 h. Triton X-100 and culture medium were used as a positive and negative control, respectively. Figure 6 summarizes the percentage of viable cells remaining after exposure of all the compounds studied.
Figure 6.
Cytotoxicity study (MTS) for the biotin-conjugated lipid prodrugs on MDCK–MDR1 for 48 h. Each data point represents mean ± SD (n= 3). *p< 0.05.
Interaction with SMVT Transporter
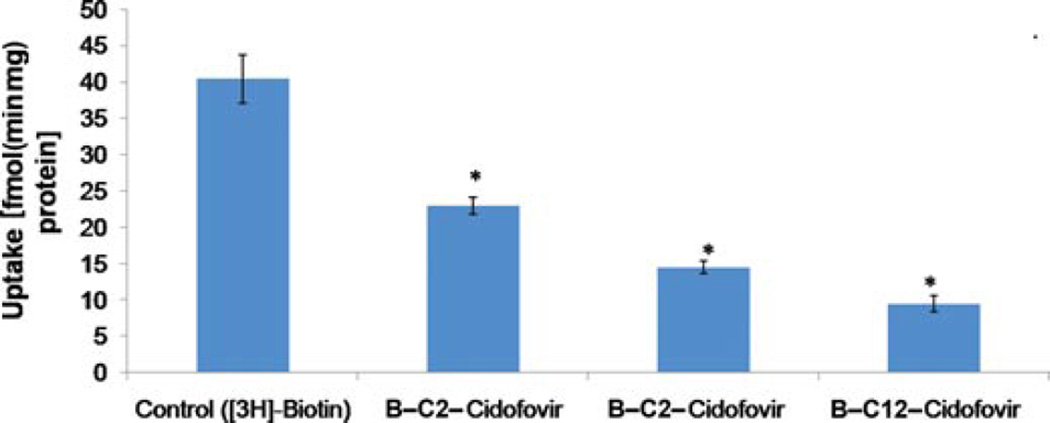
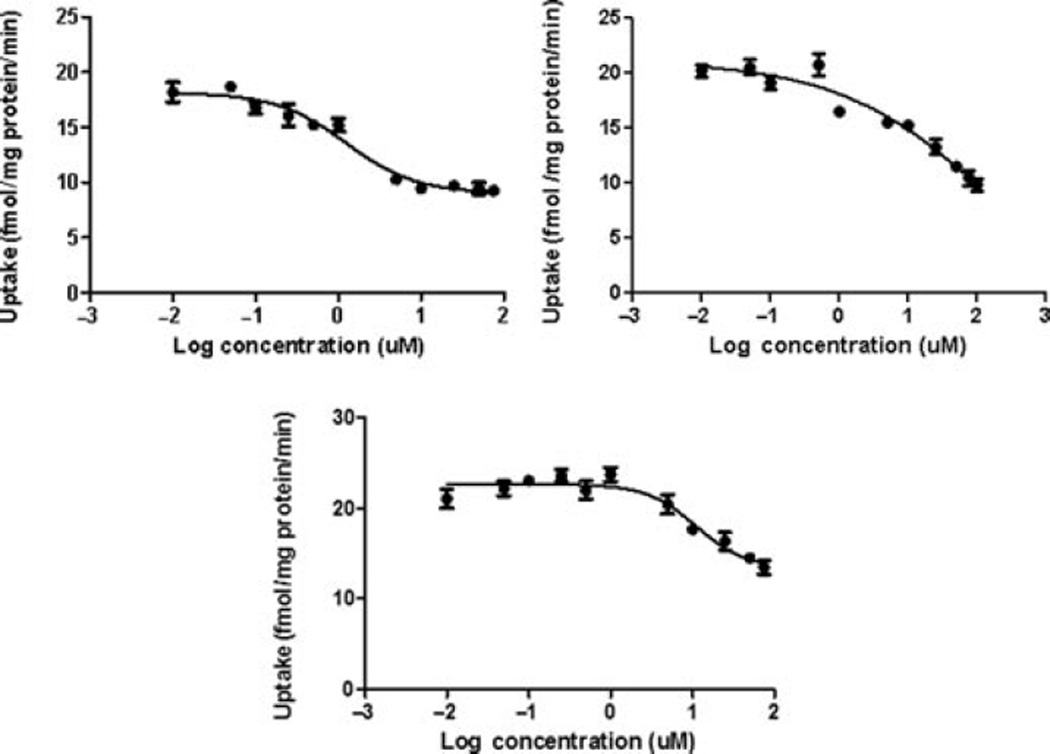
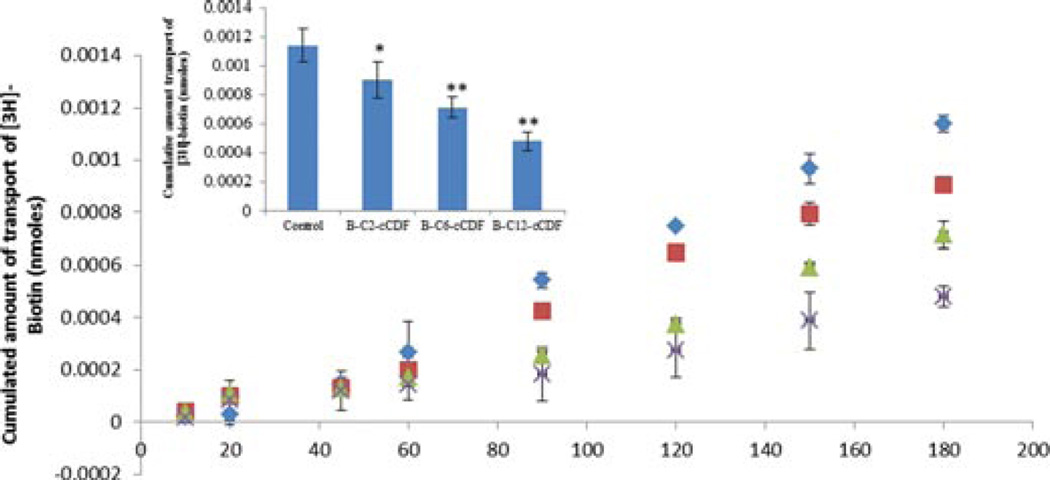
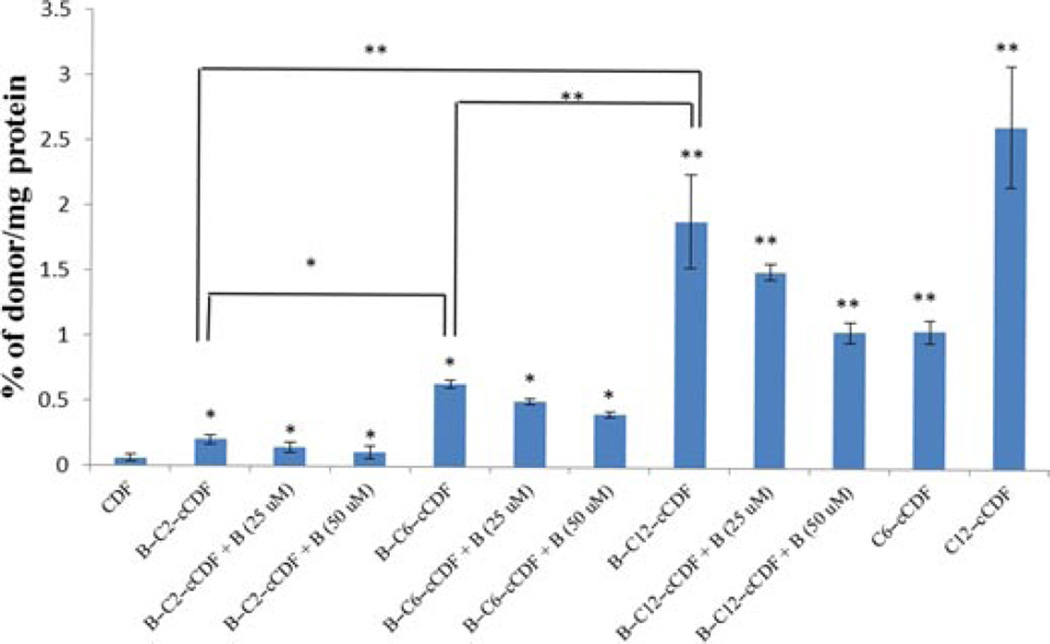
The presence of SMVT transporter on the MDCK– MDR1 cell line has been characterized previously from our laboratory.30 This cell line is widely used because it possesses tight junctions and it is also easy to culture.31 Results of this study are shown in Figure 7. All the biotin-conjugated prodrugs (50 µM) have shown significant reduction in uptake of [3H]-biotin (0.25 Ci/mL in DPBS pH 5.5). Uptake of [3H]-biotin was reduced by 50%, 58%, and 73% for B-C2–cCDF, B-C6–cCDF, and B-C12–cCDF, respectively on MDCK–MDR1 cells. These results indicate the effect of lipid chain length of biotin-conjugated lipid prodrugs on the inhibition of [3H]-biotin uptake. Moreover, the affinity measurement of these prodrugs for SMVT transporter shows that all the prodrugs possess high affinity for SMVT transporters (Fig. 8). IC50 values are listed in Table 4. These values are calculated with GraphPad Prism software 5.04. In addition, we also performed the transport of [3H]-biotin (0.25 Ci/mL) across MDCK–MDR1 monolayer using transwell. The result clearly indicates significant reduction in the transport of [3H]-biotin in presence of all the biotin-conjugated prodrugs (at 50µM concentration). However, the reduction in cumulative transport of [3H]-biotin was higher in presence of B-C12–cCDF, followed by B-C6–cCDF and B-C2–cCDF. Cumulative transport of [3H]-biotin across MDCK– MDR1 at the end of 180 min was reduced by 44%, 58%, and 70% in presence of B-C2–cCDF, B-C6–cCDF, and B-C12–cCDF, respectively (Fig. 9). We also performed the cellular accumulation of all the prodrugs on MDCK–MDR1 cells. Cellular accumulation of B-C2–cCDF, B-C6–cCDF, B-C12–cCDF, C6-cCDF, and C12-cCDF were 3.5-, 10.5-, 32-, 17.5-, and 42-fold higher than the parent CDF (Fig. 10).
Figure 7.
Cellular accumulation of [3H]-biotin (0.25 µCi/mL) in presence of biotin-conjugated lipid prodrugs (50 µM) on MDCK–MDR1 cells. Each data point represents mean ± SD (n= 3). *p< 0.05.
Figure 8.
Dose-dependent inhibition of [3H]-biotin (0.25 µCi/mL) uptake by (a) biotin-C2-cCidofovir, (b) biotin-C6-cCidofovir, and (c) biotin-C12-cCidofovir in MDCK–MDR1. Each data point represents mean ± SD (n = 3).
Table 4.
IC50 Values from the Graphpad Prism 5.0 for the Biotin-Conjugated Lipid Prodrugs of Cyclic Cidofovir Toward SMVT Transporter
| Drug | IC50 Value (µM) |
|---|---|
| B-C2–cCDF | 31.42 ± 3.05 |
| B-C6–cCDF | 11.25 ± 1.48 |
| B-C12–cCDF | 2.90 ± 0.07 |
Figure 9.
Transport of [3H]-biotin (0.25 µCi/mL) across MDCK–MDR1 cells in absence and presence of biotin-conjugated lipid prodrugs (50 µM). ◊, Control; ■, B-C2-cCidofovir; Δ,B-C6-cCidofovir; X, B-C12-cCidofovir. Each data point represents mean ± SD (n= 3). Insert: Cumulative amount transport at the end of 180 min. *p< 0.05 and **p< 0.01.
Figure 10.
Cellular accumulation of biotin-conjugated lipid prodrugs [alone and in presence of biotin (25µ and 50 µM)] and lipid prodrugs on MDCK–MDR1 cells for 60 min. Each data point represents mean ± SD (n= 4). *p< 0.05 and **p< 0.01.
Tissue Partition Coefficient
Following intravitreal injection into vitreous body, the prodrugs needs to partition into retina–choroid in order to exert their antiviral activity. Therefore, we have performed the tissue partitioning experiments with biotin-conjugated lipid prodrugs for retina–choroid and the results for all the biotin-conjugated prodrugs are listed in Table 5. Results indicate about 3, 5, and 10-fold improvement in the partition of these prodrugs into retina–choroid, relative to parent CDF. This improvement is mainly due to an increase lipophilicity of these conjugates.
Table 5.
Tissue (Retina–Choroid) Partition Coefficient for the Biotin-Conjugated Lipid Prodrug of Cyclic-Cidofovir
| Drug | Partition Coefficient (Retina–Choroid) |
Fold Increase |
|---|---|---|
| CDF | 0.29 ± 0.06 | 1 |
| B-C2–cCDF | 0.89 ± 0.27 | 3.07 |
| B-C6–cCDF | 1.42 ± 0.45 | 4.90 |
| B-C12–cCDF | 2.84 ± 0.96 | 9.80 |
DISCUSSION
Cidofovir is one of the most potent drug molecule indicated in the treatment of HIV-induced opportunistic infections such as CMV retinitis. Posterior segment delivery of CDF is challenging because of its high hydrophilicity and poor permeability across the retina (target tissue for the CMV retinitis). Retinal pigment epithelium (RPE) cells display high membrane lipophilicity and tight junctions. So far, different approaches have been employed to enhance permeability of CDF such as lipid prodrugs14 or transporter-targeted prodrugs of cCDF1. However, individually, these approaches have exhibited limited advantages. In the present study, we have synthesized various transporter-targeted lipid prodrugs of cCDF. We hypothesized that the presence of lipid raft will improve the interaction of these prodrugs with the cell membrane and transporter-targeting moiety will further improve the translocation of these prodrugs across the plasma membrane. This may exhibit synergistic effect due to the simultaneous conjugation of lipid raft and transporter-targeted moiety, which may ultimately improve the uptake of these prodrugs across the cell layers. Our laboratory has previously confirmed the presence of SMVT transporter on the AP side of RPE.23 Therefore, we sought to utilize SMVT transporter for our approach. We have successfully synthesized and characterized the biotin-conjugated lipid prodrugs and lipid prodrugs of cCDF (B-C2–cCDF, B-C6–cCDF, B-C12–cCDF, C6-cCDF, and C12-cCDF) using 31P NMR, LC–MS/MS, and HPLC. The general synthesis scheme for the prodrugs has been shown in Figures 1 and 2. All the prodrugs have exhibited 95% or more purity along with brownish sticky (biotin-conjugated prodrugs) or white solid crystalline (lipid prodrugs) appearance.
It is well known that the rate-limiting barrier for the uptake and transport of highly hydrophilic drugs such as CDF is the lipophilic bilayer of the cell membrane. Hence, we performed the solubility and n-octanol/buffer partition coefficient studies of these prodrugs. The results have indicated that the solubility of biotin-conjugated lipid prodrugs was significantly lowered, relative to the parent CDF. However, higher water solubility of biotin-conjugated lipid prodrugs have been attributed due to the addition of water-soluble vitamin such as biotin as the transporter-targeting moiety to the lipid conjugates of cCDF as compare to lipid prodrug of cyclic cidofovir. Moreover, partition coefficient study has shown an increment in log P value for biotin-conjugated lipid prodrugs. The lipophilic RPE prevents the entry of relatively hydrophilic antiviral drugs following systemic or intravitreal administration. The improved lipophilicity of synthesized biotin-conjugated lipid prodrugs will enhance their ocular bioavailability by overcoming this limitation. Moreover, an enhanced lipophilicity of these prodrugs may enhance the melanin binding in the retina, which will form the depot in the retina–choroid for longer residence and, therefore, may prolong the antiviral activity.32
Furthermore, we have determined the chemical stability of all the prodrugs in different pH buffers (pH 1.4 to 7.4). We did not observe any appreciable degradation of all the prodrugs at pH 1.4. However, upon rising pH toward alkaline range, the degradation appears to be faster. Table 2 summarizes the apparent first-order degradation rate constant indicating the loss of the prodrugs. The B-C12–cCDF showed faster degradation relative to B-C6–cCDF and B-C2–cCDF. We hypothesized that this could be due to steric hindrance caused by the presence of CDF and biotin at the terminals. This may lead to the low accessibility of phosphoester group to nucleophilic attack by hydroxyl ion. Therefore, it is likely that due to the steric hindrance, nucleophilic attack on B-C2–cCDF is slow as compared with B-C6–cCDF and B-C12–cCDF.
We have also performed enzymatic stability studies of all the prodrugs in the presence of retina–choroid and vitreous tissue homogenates. A rapid conversion of these prodrugs into parent drug in the retina— choroid relative to vitreous homogenates has been observed (Fig. 5 and Table 3). These results further indicate that all the prodrugs remain stable for longer duration upon intravitreal administration. Following active transport across deeper tissue in the retina, prodrugs will convert into its parent moiety to exert its antiviral activity.
Another important objective of the present study was to target the SMVT transporter. Recognition of prodrugs by SMVT transporters may enhance their translocation within inner retinal tissues and eventually a higher concentration of drug can be achieved at the target site. Therefore, we performed in vitro uptake study using a model MDCK–MDR1 cell line. The results indicate that all the prodrugs show strong interaction with the SMVT transporter. An inhibition trend (of [3H]-biotin) was observed in the following order: B-C12–cCDF >B-C6–cCDF >B-C2–cCDF. These results clearly indicate that elongation of lipid chain causes stronger interaction of biotin-conjugated prodrugs with cell membrane. Moreover, high percentage of transport inhibition of [3H]-biotin in the presence of B-C12–cCDF in comparison to B-C6–cCDF and B-C2–cCDF suggested the significant role of lipid raft. In addition, results of IC50 value clearly indicate the higher affinity of B-C12–cCDF toward SMVT transporter as compared with other prodrugs. Non-radioactive cellular accumulation study shows that there is 3.5-, 10.5-, 32-, 17.5-, and 42-fold increase in the uptake of B-C2–cCDF, B-C6–cCDF, B-C12–cCDF, C6-cCDF, and C12-cCDF, respectively, as compared with the parent CDF (Fig. 10). The observed cellular uptake of B-C6–cCDF and B-C12–cCDF was not more than their lipid prodrugs (i.e., C6-cCDF and C12-cCDF). Biotin-conjugated lipid prodrugs have exhibited higher water solubility, which may have lead to the reduced cell bilayer partitioning relative to the lipid prodrugs. Cellular uptake of B-C12–cCDF was found to be comparable to C12-cCDF, wherein the lipid raft (C12) facilitates the enhanced interaction of B-C12–cCDF with membrane protein and, thereby, assisting docking of biotin into the binding domain of SMVT transporter. The net effect will be rapid translocation across RPE cell membrane, which will not be the case with lipid prodrugs. Thus, our novel B-C12–cCDF prodrug may significantly enhance the delivery of CDF across lipophilic RPE. In addition, the uptake of biotin-conjugated lipid prodrugs across MDCK–MDR1 was significantly inhibited by biotin (due to competitive inhibition). These results clearly indicate that improvement in uptake of biotin-conjugated prodrugs was via SMVT interaction (Fig. 10). The overall results from the interaction of all biotin-conjugated lipid prodrugs with SMVT transporters suggest that these compounds are strongly recognized by SMVT transporter. Moreover, results further clarify that upon increasing the lipid chain length of the biotin-conjugated lipid prodrugs, an interaction with the SMVT transporter can be enhanced.
The major limiting factor for in vivo absorption of hydrophilic molecules is their low tissue partitioning. The parent drug CDF is highly hydrophilic in nature and following intravitreal administration, a large fraction may remain in the vitreous body and may not partition enough into lipophilic retina– choroid. However, the tissue–water partition study of biotin-conjugated lipid prodrugs clearly indicates an improvement in the uptake into retina–choroid tissue. As all the prodrugs have high lipophilicity; these compounds may partition into retina–choroid as compared with the parent CDF. Moreover, because of the strong interaction with SMVT transporter, these prodrugs may accumulate into the deeper tissue of retina/ choroid, thereby improving the therapy.
Overall, these studies have shown that B-C12– cCDF is the most promising prodrug among all the synthesized compounds, owing to its higher lipophilicity among all biotin-conjugated prodrugs, strong interaction with SMVT transporters, lowest IC50 value (high affinity for SMVT), higher tissue– water partition coefficient, and rapid conversion into parent molecule (in retina–choroid). On the basis of above findings, we conclude that novel transporter-targeted lipid prodrugs can be utilized as a viable strategy for the treatment of hCMV retinitis.
ACKNOWLEDGMENTS
This study was supported by National Institutes of Health grants R01 EY 09171-14 and R01 EY 10659-12. We would like to thank Gilead Science for the generous gift of Cidofovir. We would also like to thank Dr. Ravinder Earla for his assistance in LC–MS/MS analysis.
REFERENCES
- 1.Eriksson U, Peterson LW, Kashemirov BA, Hilfinger JM, Drach JC, Borysko KZ, Breitenbach JM, Kim JS, Mitchell S, Kijek P, McKenna CE. Serine peptide phosphoester prodrugs of cyclic cidofovir: Synthesis, transport, and antiviral activity. Mol Pharm. 2008;5(4):598–609. doi: 10.1021/mp8000099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Clercq E, Holy A. Acyclic nucleoside phosphonates: A key class of antiviral drugs. Nat Rev Drug Discov. 2005;4(11):928–940. doi: 10.1038/nrd1877. [DOI] [PubMed] [Google Scholar]
- 3.Barot M, Gokulgandhi MR, Mitra AK. Mitochondrial dysfunction in retinal diseases. Curr Eye Res. 2011;36(12):1069–1077. doi: 10.3109/02713683.2011.607536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merodio M, Arnedo A, Renedo MJ, Irache JM. Ganciclovir-loaded albumin nanoparticles: Characterization and in vitro release properties. Eur J Pharm Sci. 2001;12(3):251–259. doi: 10.1016/s0928-0987(00)00169-x. [DOI] [PubMed] [Google Scholar]
- 5.Skiest DJ. Cytomegalovirus retinitis in the era of highly active antiretroviral therapy (HAART) Am J Med Sci. 1999;317(5):318–335. doi: 10.1097/00000441-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Jabs DA, Van Natta ML, Holbrook JT, Kempen JH, Meinert CL, Davis MD. Longitudinal study of the ocular complications of AIDS: 2. Ocular examination results at enrollment. Ophthalmology. 2007;114(4):787–793. doi: 10.1016/j.ophtha.2006.07.065. [DOI] [PubMed] [Google Scholar]
- 7.Yang H, Datema R. Prolonged and potent therapeutic and prophylactic effects of (S)-1-[(3-hydroxy-2-phosphonylmethoxy)propyl]cytosine against herpes simplex virus type 2 infections in mice. Antimicrob Agents Chemother. 1991;35(8):1596–1600. doi: 10.1128/aac.35.8.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Clercq E, Holy A. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine in various models of herpes simplex virus infection in mice. Antimicrob Agents Chemother. 1991;35(4):701–706. doi: 10.1128/aac.35.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neyts J, Sobis H, Snoeck R, Vandeputte M, De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)-cytosine and 9-(1,3-dihydroxy-2-propoxymethyl)-guanine in the treatment of intracerebral murine cytomegalovirus infections in immunocompetent and immunodeficient mice. Eur J Clin Microbiol Infect Dis. 1993;12(4):269–279. doi: 10.1007/BF01967257. [DOI] [PubMed] [Google Scholar]
- 10.Smee DF, Morris JL, Leonhardt JA, Mead JR, Holy A, Sidwell RW. Treatment of murine cytomegalovirus infections in severe combined immunodeficient mice with ganciclovir, (S)-1-[3-hydroxy-2-(phosphonylmethoxy)propyl]cytosine, interferon, and bropirimine. Antimicrob Agents Chemother. 1992;36(9):1837–1842. doi: 10.1128/aac.36.9.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neyts J, Balzarini J, Naesens L, De Clercq E. Efficacy of (S)-1-(3-hydroxy-2-phosphonylmethoxypropyl)cytosine and 9-(1,3-dihydroxy-2-propoxymethyl)guanine for the treatment of murine cytomegalovirus infection in severe combined immunodeficiency mice. J Med Virol. 1992;37(1):67–71. doi: 10.1002/jmv.1890370112. [DOI] [PubMed] [Google Scholar]
- 12.Smith IL, Taskintuna I, Rahhal FM, Powell HC, Ai E, Mueller AJ, Spector SA, Freeman WR. Clinical failure of CMV retinitis with intravitreal cidofovir is associated with antiviral resistance. Arch Ophthalmol. 1998;116(2):178–185. doi: 10.1001/archopht.116.2.178. [DOI] [PubMed] [Google Scholar]
- 13.Barot M, Gokulgandhi MR, Haghnegahdar M, Dalvi P, Mitra AK. Effect of emergence of fluoroquinolone resistance on intrinsic expression of P-glycoprotein phenotype in corneal epithelial cells. J Ocul Pharmacol Ther. 2011;27(6):553–559. doi: 10.1089/jop.2011.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng L, Beadle JR, Tammewar A, Hostetler KY, Hoh C, Freeman WR. Intraocular pharmacokinetics of a crystalline lipid prodrug, octadecyloxyethyl-cyclic-cidofovir, for cytomegalovirus retinitis. J Ocul Pharmacol Ther. 2011;27(2):157–162. doi: 10.1089/jop.2010.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beaumont K, Webster R, Gardner I, Dack K. Design of ester prodrugs to enhance oral absorption of poorly permeable compounds: Challenges to the discovery scientist. Curr Drug Metab. 2003;4(6):461–485. doi: 10.2174/1389200033489253. [DOI] [PubMed] [Google Scholar]
- 16.Peterson LW, Sala-Rabanal M, Krylov IS, Serpi M, Kashemirov BA, McKenna CE. Serine side chain-linked peptidomimetic conjugates of cyclic HPMPC and HPMPA: Synthesis and interaction with hPEPT1. Mol Pharm. 2010;7(6):2349–2361. doi: 10.1021/mp100186b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li F, Maag H, Alfredson T. Prodrugs of nucleoside analogues for improved oral absorption and tissue targeting. J Pharm Sci. 2008;97(3):1109–1134. doi: 10.1002/jps.21047. [DOI] [PubMed] [Google Scholar]
- 18.Ocheltree SM, Keep RF, Shen H, Yang D, Hughes BA, Smith DE. Preliminary investigation into the expression of proton-coupled oligopeptide transporters in neural retina and retinal pigment epithelium (RPE): Lack of functional activity in RPE plasma membranes. Pharm Res. 2003;20(9):1364–1372. doi: 10.1023/a:1025741723724. [DOI] [PubMed] [Google Scholar]
- 19.Han YH, Sweet DH, Hu DN, Pritchard JB. Characterization of a novel cationic drug transporter in human retinal pigment epithelial cells. J Pharmacol Exp Ther. 2001;296(2):450–457. [PubMed] [Google Scholar]
- 20.Yamamoto A, Akanuma S, Tachikawa M, Hosoya K. Involvement of LAT1 and LAT2 in the high- and low-affinity transport of L-leucine in human retinal pigment epithelial cells (ARPE-19 cells) J Pharm Sci. 2010;99(5):2475–2482. doi: 10.1002/jps.21991. [DOI] [PubMed] [Google Scholar]
- 21.Gu S, Roderick HL, Camacho P, Jiang JX. Characterization of an N-system amino acid transporter expressed in retina and its involvement in glutamine transport. J Biol Chem. 2001;276(26):24137–24144. doi: 10.1074/jbc.M009003200. [DOI] [PubMed] [Google Scholar]
- 22.Smith SB, Kekuda R, Gu X, Chancy C, Conway SJ, Ganapathy V. Expression of folate receptor alpha in the mammalian retinol pigmented epithelium and retina. Invest Ophthalmol Vis Sci. 1999;40(5):840–848. [PubMed] [Google Scholar]
- 23.Janoria KG, Boddu SH, Wang Z, Paturi DK, Samanta S, Pal D, Mitra AK. Vitreal pharmacokinetics of biotinylated ganciclovir: Role of sodium-dependent multivitamin transporter expressed on retina. J Ocul Pharmacol Ther. 2009;25(1):39–49. doi: 10.1089/jop.2008.0040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel K, Trivedi S, Luo S, Zhu X, Pal D, Kern ER, Mitra AK. Synthesis, physicochemical properties and antiviral activities of ester prodrugs of ganciclovir. Int J Pharm. 2005;305(1–2):75–89. doi: 10.1016/j.ijpharm.2005.08.024. [DOI] [PubMed] [Google Scholar]
- 25.Dias CS, Anand BS, Mitra AK. Effect of mono- and diacylation on the ocular disposition of ganciclovir: Physicochemical properties, ocular bioreversion, and antiviral activity of short chain ester prodrugs. J Pharm Sci. 2002;91(3):660–668. doi: 10.1002/jps.10072. [DOI] [PubMed] [Google Scholar]
- 26.Gupta D, Gupta SV, Lee KD, Amidon GL. Chemical and enzymatic stability of amino acid prodrugs containing methoxy, ethoxy and propylene glycol linkers. Mol Pharm. 2009;6(5):1604–1611. doi: 10.1021/mp900084v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Majumdar S, Srirangam R. Solubility, stability, physicochemical characteristics and in vitro ocular tissue permeability of hesperidin: A natural bioflavonoid. Pharm Res. 2009;26(5):1217–1225. doi: 10.1007/s11095-008-9729-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katragadda S, Gunda S, Hariharan S, Mitra AK. Ocular pharmacokinetics of acyclovir amino acid ester prodrugs in the anterior chamber: Evaluation of their utility in treating ocular HSV infections. Int J Pharm. 2008;359(1–2):15–24. doi: 10.1016/j.ijpharm.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agarwal S, Boddu SH, Jain R, Samanta S, Pal D, Mitra AK. Peptide prodrugs: Improved oral absorption of lopinavir, a HIV protease inhibitor. Int J Pharm. 2008;359(1–2):7–14. doi: 10.1016/j.ijpharm.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luo S, Kansara VS, Zhu X, Mandava NK, Pal D, Mitra AK. Functional characterization of sodium-dependent multi-vitamin transporter in MDCK-MDR1 cells and its utilization as a target for drug delivery. Mol Pharm. 2006;3(3):329–339. doi: 10.1021/mp0500768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal S, Jain R, Pal D, Mitra AK. Functional characterization of peptide transporters in MDCKII-MDR1 cell line as a model for oral absorption studies. Int J Pharm. 2007;332(1–2):147–152. doi: 10.1016/j.ijpharm.2006.09.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheruvu NP, Amrite AC, Kompella UB. Effect of eye pigmentation on transscleral drug delivery. Invest Ophthalmol Vis Sci. 2008;49(1):333–341. doi: 10.1167/iovs.07-0214. [DOI] [PMC free article] [PubMed] [Google Scholar]