Abstract
Acute kidney injury (AKI) is associated with high morbidity and mortality. Urinary tract infection (UTI) may be associated with sepsis or septic shock, and cause sudden deterioration of renal function. This study investigated the clinical characteristics and change of renal function to identify the risk factors for development of AKI in UTI patients. This retrospective study was conducted in a tertiary referral center. From January 2006 to January 2013, a total of 790 UTI patients necessitating hospital admission were included for final analysis. Their demographic and clinical characteristics and comorbidities were collected and compared. Multivariate logistic regression analysis was performed to evaluate the risk factors for AKI in UTI patients. There were 97 (12.3%) patients developing AKI during hospitalization. Multivariate logistic regression analysis showed that patients with older age (OR 1.02, 95% CI 1.00–1.04, P = 0.04), diabetes mellitus (DM) (OR 2.23, 95% CI 1.35–3.68, P = 0002), upper UTI (OR 2.63, 95% CI 1.53–4.56, P = 0001), afebrile during hospitalization (OR 1.71, 95% CI 1.04–2.83, P = 0036) and lower baseline eGFR [baseline eGFR 45–59 mL/min/1.73 m2 (OR 2.12, 95% CI 1.12–4.04, P = 0.022), baseline eGFR 30-44 mL/min/1.73 m2 (OR 4.44, 95% CI 2.30–8.60 P < 0.001) baseline eGFR < 30 mL/min/1.73 m2 (OR 4.72, 95% CI 2.13–10.45, P <0.001), respectively] were associated with increased risk for development of AKI. were associated with increased risk for development of AKI. Physicians should pay attention to UTI patients at risk of AKI (advancing age, DM, upper UTI, afebrile, and impaired baseline renal function).
Introduction
Urinary tract infection (UTI) is one of the most common bacterial infections [1]. The overall annual incidence of UTI was 1.75% among residents of the Calgary Health Region, Canada during 2004–2005 [2]. The 2007 National Ambulatory Medical Care Surveys estimated UTIs were responsible for nearly two million visits to emergency departments annually in the USA [3].
UTI can be either asymptomatic or symptomatic, characterized by a wide spectrum of symptoms ranging from mild irritative voiding to bacteremia, sepsis, shock or even death. In specific patient groups, urosepsis may show high mortality rates of 25% to 60% [4].
Sepsis is one of the most common triggers of acute kidney injury (AKI) [5], and about 60% patients with septic shock developed AKI [6]. Acute UTI may cause sudden deterioration of renal function, especially for urinary tract obstruction [7]. AKI is associated with high morbidity and mortality during acute care [8–10]. Patients with severe AKI were under increased risk of end stage renal disease and even death after hospital discharge [11–13].
Since sepsis-related AKI leads to poor outcomes and increased healthcare costs, identification of the risk factors for development of AKI in patients with UTI is critical. However, there were few studies focusing on this important issue. In this retrospective study, we investigated the clinical characteristics and change of renal function to identify the risk factors for development of AKI in UTI patients.
Materials and Methods
Ethics statement
This retrospective observational study complied with the guidelines of the Declaration of Helsinki and was approved by the Medical Ethics Committee of Chia-Yi Christian Hospital, a tertiary referral center located in the southwestern part of Taiwan. Since this study involved retrospective review of existing data, approval from the Institutional Review Board of Chia-Yi Christian Hospital was obtained (Approval # CYCH-IRB-100015), but without specific informed consent from patients. Furthermore, not only were all data securely protected (by delinking identifying information from the main data sets) and made available only to investigators, but they were also analyzed anonymously. The Institutional Review Board of Chia-Yi Christian Hospital specifically waived the need for consent for these studies. Finally, all primary data were collected according to procedures outlined in epidemiology guidelines to strengthen the reporting of observational studies.
Study Conduct
This retrospective study was conducted in a tertiary referral center located in a city of southern Taiwan with a population of 547,000 people. The hospital has 1,000 acute care beds, and serves approximately 3,800 outpatients and 260 emergency patients daily. The authors had full access to the results and vouch for the completeness and accuracy of the data and analysis.
Study population
From January 2006 to January 2013, clinical data of 938 consecutive hospitalized patients with baseline creatinine values diagnosed with UTI in the Chia-Yi Christian Hospital were enrolled. The criteria for the diagnosis of UTI were symptomatic, including pain on urination, lumbago or fever with bacterial isolation of more than 104 colony forming units (CFU)/mL. Patients with concurrent infections other than UTI or receiving chronic dialysis therapy (i.e., regular dialysis therapy more than 3 months) before UTI episode were excluded from this study (Fig 1). Finally a total of 790 patients with UTI were included for analyses.
Fig 1. Rectangle below: patients included for analyses.
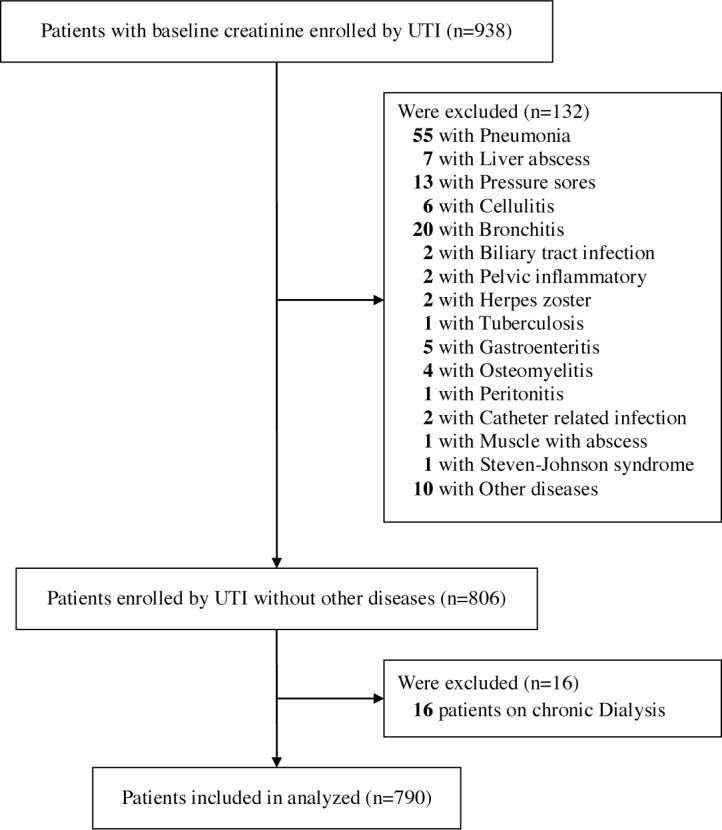
Hospital Course
Inpatients were assessed by standard laboratory and diagnostic procedures. Clinical data including age, sex, diabetes mellitus (DM), hypertension, coronary artery disease (CAD), congestive heart failure (CHF), cerebrovascular disease, malignancy, and medications such as antihypertensive drugs or nephrotoxic agents (i.e., aminoglycosides, nonsteroidal anti-inflammatory drugs (NSIAD), contrast media, and trimethoprim/sulfamethoxazole) were recorded. Patients admitted were treated with antibiotics based on the standard protocol. The initial regimens of empiric antibiotic therapy were parenteral first generation cephalosporin plus aminoglycoside (if no impaired renal function), parenteral second generation cephalosporin or parenteral fluoroquinolones to treat the common UTI pathogens for patients with stable hemodynamic condition. Parenteral empiric antibiotic therapy according to previous culture results and antimicrobial susceptibility was prescribed for patients with recurrent UTI. Specific antibiotic therapy was administered according to the culture results and antimicrobial susceptibility during hospitalization. The four main vital signs including pulse rate, respiration rate, blood pressure, and temperature were routinely monitored.
Major Outcomes and Definitions
AKI was diagnosed by a decrease in glomerular filtration rate (GFR) more than 50% or doubling of serum creatinine compared to that at baseline according to The RIFLE GFR criteria [14]. Estimated GFR (eGFR) was determined according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine equation [15]. The baseline level of serum creatinine was obtained at dates 3 months before admission. The diagnosis and classification of chronic kidney disease (CKD) were established according to the criteria of the National Kidney Foundation K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease [16]. Diagnosis of DM was based on the American Diabetes Association and the World Health Organization criteria. Upper UTI was an infection of the kidney or ureter; lower UTI included cystitis, urethritis, and prostatitis. Bacteremia was an invasion of the bloodstream by bacteria and confirmed by blood culture. Fever was defined as a temperature above 38.3°C (101°F) [17, 18]. Afebrile was defined as UTI patients who did not have a temperature above 38.3°C (101°F). Septic shock was defined as sepsis with hypotension (systolic blood pressure less than 90 mmHg or a fall in systolic blood pressure > 40 mmHg) lasting for at least 1 hour despite adequate fluid resuscitation [19].
Study Design
Patients were divided into two groups based on the presence (group 1) or absence (group 2) of AKI during the hospital stay. Demographic data of gender, age, co-morbidities (diabetes mellitus, hypertension, CHF, CAD, stroke, and malignancy), upper or lower UTI, baseline renal function, indwelling urinary catheter, vital signs at admission, and presence of bacteremia or septic shock during hospitalization were reviewed from the medical charts and data were collected for further analyzed.
Statistical analysis
Data are expressed as means and standard deviations for continuous variables and as frequency and proportions for categorical variables. Continuous data were analyzed using a Mann-Whitney U-test. Categorical data were analyzed by Fisher’s exact test or chi-square test. We performed a conditional logistic regression analysis with AKI as the outcome variable and baseline demography as well as clinical relevant data as the main exposure of interest. A P value under 0.05 was considered significant. Statistical analyses were performed by the software SPSS 17.0 (International Business Machines Corp., Armonk, NY, USA).
Results
There were 790 UTI patients enrolled for final analysis, their demographic and clinical characteristics of UTI patients are shown in Table 1. The mean age was 65 ± 18 years, 543 (68.7%) were female. There were 335 hypertensive patients, among which 180 were treated with angiotensin-converting enzyme inhibitors (ACE inhibitors) or angiotensin receptor blockers (ARBs). A total of 47 patients were found to have urinary tract obstruction. A total of 369 patients were afebrile UTI during hospitalization, and 15 of them had hypothermia. There were 97 patients (12.3%) developing AKI after admission with 4 patients (0.5%) necessitating dialysis therapy. The overall mortality rate was 0.38% (3/790). Patients who developed AKI had older age (72 ± 13 versus 64 ± 19 years, P <0.001) and higher serum creatinine at admission (3.37 ±1.96 versus 1.32 ± 0.94 mg/dL, P <0.001), higher prevalence of DM (56.7% versus 35.6%, P <0.001), hypertension (54.6% versus 40.7%, P = 0.009), upper UTI (46.4% versus 35.5%, P = 0.037), afebrile (59.8% versus 44.9%, P = 0.006), septic shock (22.7% versus 9.8%, P <0.001) and bacteremia (39.2% versus 28.6%, P = 0.033), lower values of systolic and diastolic blood pressure (127 ± 33 versus 137 ± 30 mmHg, and 70 ± 17 versus 77 ± 15 mmHg, P = 0.006 and P <0.001, respectively) and baseline eGFR (53 ± 23 versus 72 ± 27 mL/min/1.73m2, P <0.001), and less exposure to nephrotoxic agents (47.4% versus 69.3%, P <0.001) compared with those without AKI.
Table 1. Characteristics of the 790 patients with urinary tract infection.
| Characteristic | All (n = 790) | Acute kidney injury | P-Value | |
|---|---|---|---|---|
| Yes (n = 97) | No (n = 693) | |||
| Age (year) | 65 ± 18 | 72 ± 13 | 64 ± 19 | <0.001 |
| Gender (female) | 543 (68.7) | 72 (74.2) | 471 (68.0) | 0.213 |
| Systolic blood pressure (mmHg) | 135 ± 30 | 127 ± 33 | 137 ± 30 | 0.006 |
| Diastolic blood pressure (mmHg) | 75.82 ± 15.8 | 70 ± 17 | 77 ± 15 | <0.001 |
| Diabetes mellitus | 302 (38.2) | 55 (56.7) | 247 (35.6) | <0.001 |
| Hypertension | 335 (42.4) | 53 (54.6) | 282 (40.7) | 0.009 |
| Congestive heart failure | 29 (3.7) | 5 (5.2) | 24 (3.5) | 0.387 |
| Coronary artery disease | 55 (7.0) | 10 (10.3) | 45 (6.5) | 0.167 |
| Stroke | 178 (22.5) | 21 (21.6) | 157 (22.7) | 0.824 |
| Malignancy | 92 (11.6) | 16 (16.5) | 76 (11.0) | 0.112 |
| Indwelling foley catheter | 54 (6.8) | 8 (8.2) | 46 (6.6) | 0.556 |
| Afebrile | 369 (46.7) | 58 (59.8) | 311 (44.9) | 0.006 |
| Upper urinary tract infection | 291 (36.8) | 45 (46.4) | 246 (35.5) | 0.037 |
| Bacteremia | 236 (29.9) | 38 (39.2) | 198 (28.6) | 0.033 |
| Septic shock | 90 (11.4) | 22 (22.7) | 68 (9.8) | <0.001 |
| Hospitalized serum creatinine (mg/dL) | 1.57 ± 1.30 | 3.37 ± 1.96 | 1.32 ± 0.94 | <0.001 |
| Baseline eGFR (mL/min/1.73 m2) | 70 ± 28 | 53 ± 23 | 72 ± 27 | <0.001 |
| Baseline renal function (eGFR) | <0.001 | |||
| ≥ 60 mL/min/1.73m2 | 497 (62.9) | 32 (33.0) | 465 (67.1) | |
| 45–59 mL/min/1.73m2 | 132 (16.7) | 23 (23.7) | 109 (15.7) | |
| 30–44 mL/min/1.73m2 | 103 (13.0) | 27 (27.8) | 76 (11.0) | |
| < 30 mL/min/1.73m2 | 58 (7.3) | 15 (15.5) | 43 (6.2) | |
| Nephrotoxic agents | 526 (66.6) | 46 (47.4) | 480 (69.3) | <0.001 |
| Aminoglycosides | 290 (36.8) | 16 (16.3) | 274 (39.7) | <0.001 |
| Nonsteroidal anti-inflammatory drugs | 392 (49.6) | 36 (36.7) | 356 (51.4) | 0.006 |
| Contrast media | 99 (12.5) | 16 (16.3) | 83 (12.0) | 0.225 |
| Trimethoprim/sulfamethoxazole | 21 (2.7) | 4 (4.1) | 17 (2.5) | 0.317 |
| Antihypertensive agents | 429 (54.3) | 66 (68.0) | 363 (52.4) | 0.004 |
Data are expressed as mean ± SD or number (percentage)
In multivariate logistic regression analysis, older age (OR 1.02, 95% CI 1.00–1.04, P = 0.04), DM (OR 2.23, 95% CI 1.35–3.68, P = 0002), upper UTI (OR 2.63, 95% CI 1.53–4.56, P = 0001), afebrile during hospitalization (OR 1.71, 95% CI 1.04–2.83, P = 0036) and lower baseline eGFR [baseline eGFR 45–59 mL/min/1.73 m2 (OR 2.12, 95% CI 1.12–4.04, P = 0.022), baseline eGFR 30–44 mL/min/1.73 m2 (OR 4.44, 95% CI 2.30–8.60 P < 0.001) baseline eGFR < 30 mL/min/1.73 m2 (OR 4.72, 95% CI 2.13–10.45, P <0.001), respectively] were independently associated with increased risk for development of AKI in patients admitted with UTI (Table 2) (Fig 2).
Table 2. Multivairate logistic regression model for factors related to acute kidney injury.
| Covariate | β | OR (95% CI) | P-Value |
|---|---|---|---|
| Age (year) | 0021 | 1.02 (1.00–1.04) | 0040 |
| Gender (female) | 0280 | 1.32 (0.77–2.27) | 0307 |
| Systolic blood pressure (mmHg) | -0010 | 0.99 (0.98–1.00) | 0079 |
| Diastolic blood pressure (mmHg) | -0015 | 0.99 (0.96–1.01) | 0165 |
| Diabetes mellitus | 0801 | 2.23 (1.35–3.68) | 0002 |
| Hypertension | 0172 | 1.19 (0.71–1.99) | 0515 |
| Congestive heart failure | -0397 | 0.67 (0.20–2.21) | 0513 |
| Coronary artery disease | -0033 | 0.97 (0.42–2.21) | 0937 |
| Stroke | -0216 | 0.81 (0.44–1.49) | 0490 |
| Malignancy | 0325 | 1.38 (0.71–2.68) | 0337 |
| Indwelling foley catheter | 0294 | 1.34 (0.56–3.23) | 0513 |
| Afebrile | 0538 | 1.71 (1.04–2.83) | 0036 |
| Upper urinary tract infection | 0970 | 2.63 (1.53–4.56) | 0001 |
| Septic shock | 0552 | 1.74 (0.89–3.38) | 0104 |
| Baseline eGFR group | |||
| 45–59 versus 60 ml/min/1.73 m2 | 0.753 | 2.12 (1.12–4.04) | 0.022 |
| 30–44 versus 60 ml/min/1.73 m2 | 1.491 | 4.44 (2.30–8.60) | <0.001 |
| < 30 versus 60 ml/min/1.73 m2 | 1.552 | 4.72 (2.13–10.45) | <0.001 |
Fig 2. Multivariate analysis of associations between eGFR and acute kidney injury.
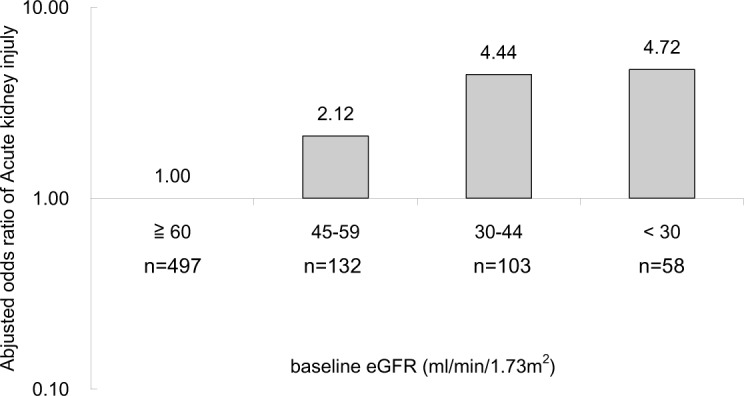
aN = 790. bMultivariate model adjusted for gender, diabetes mellitus, hypertension, congestive heart failure, coronary artery disease, stroke, malignancy, indwelling foley catheter, afebrile, upper UTI, septic shock, baseline eGFR group.
Discussion
AKI is a common complication of sepsis and septic shock. UTI is one of the common causes of sepsis and may cause sudden deterioration in renal function. Several studies suggested that AKI was not a common complication among patients with acute pyelonephritis [20–22]. In this study, the incidence of AKI in UTI patient necessitating admission was 12.3%, with a bacteremia rate of 29.9%, septic shock 11.4%, and mortality 0.38%. Only a few studies investigated the risk factors for AKI in UTI patients. Previous studies indicated that hypovolemia, hypotension, sepsis, the use of nephrotoxic drugs, contrast media and urinary obstruction were AKI risk factors in UTI patients [23, 24]. Our study showed that UTI patients with DM, upper UTI, afebrile or septic shock during hospitalization and impaired baseline renal function were at higher risk for development of AKI.
The risk of UTI in CKD patients might be increased by disease and host factors (e.g., papillary necrosis, nephrolithiasis, neurogenic bladder, immunodeficiency, malnutrition, low urinary flow rate or urinary concentration defect) and management of comorbidity (foley catheters and intravenous lines) [25]. In addition, CKD has been recognized as a risk factor for development of AKI. A lot of comorbidities are associated with CKD, including high incidence of cardiovascular disease in CKD with increased exposure to contrast agents, use of ACE inhibitors or ARBs in the existence of undiagnosed renal artery stenosis, and impaired autoregulation of renal blood flow in diabetic patients permitting low renal perfusion during systemic hypotension. These comorbidities per se may lead to more frequent exposure to nephrotoxic agents and/or alter the response to an acute insult, which cause increased susceptibility to AKI [26]. Previous studies reported that CKD was a potent predictor of acute decline in kidney function following exposure to radio-contrast and major surgery [27, 28]. Hsu et al. compared CKD patients with hospital-acquired AKI treated with and without dialysis, and identified a significantly increased AKI risk if eGFR <60 mL/min/1.73 m2 [29]. Moreover, subjects with eGFR of 45–59 mL/min/1.73 m2 had on average a twofold increase in adjusted odds ratio of AKI compared with subjects with eGFR of 60 mL/min/1.73 m2 or above. In this study, there was a higher incidence of eGFR of <60 mL/min/1.73 m2 in the AKI group compared with that in the non-AKI group (67.0% versus 32.9%, respectively). In addition, compared with subjects with baseline eGFR of 60 mL/min/1.73 m2 or above, the odds ratio for AKI in subjects with baseline eGFR 45–59 mL/min/1.73 m2, 30–44 mL/min/1.73 m2, and < 30 mL/min/1.73 m2 were 2.12, 4.44, and 4.72, respectively. Our results suggested that impaired baseline renal function played a predictive role for development of AKI in UTI patient.
Our study showed that the UTI patients with AKI were older than those without (72 ± 13 versus 64 ± 19 years, P < 0.001), with an odds ratio of 1.02 increment each year. UTI is a type of infection which is common among older people. The prevalence of UTI in the elderly is much higher than younger individuals. At least 20% women and 10% of men aged 65 years or older have bacteriuria [30]. In our study, 59.2% (468/790) of the cases are over 65 years old. Multiple age-related changes including cell-mediated immunity recession, bladder defenses alteration due to obstructive uropathy, neurogenic dysfunction, bacterial receptivity intensification of uroepithelial cells [31], contamination due to fecal and urinary incontinence, uretheral instrumentation and catheterization, and antibacterial factors reduction in prostate and vagina associated with changes in zinc levels, PH and hormones [32] contribute to the risk associated with UTI in elderly. Additionally, age-related decline of GFR may cause older patients at risk for acute kidney injury. [33] In our study, patients who were over 65 years old had higher prevalence of eGFR <60 compared with those under 65 years old (53.2% versus 13.7%, respectively). Since age and impaired baseline renal function are risks for development of AKI in UTI patients, physicians should pay attention to elderly UTI patients with CKD.
DM has been reported as a risk factor for the development of both upper and lower UTIs [34–36]. In addition, DM is a potent predictor of AKI following exposure to contrast media [27], cardiac surgery [37, 38], oral sodium phosphate bowel preparation [39], and sepsis [40]. In our study, UTI patients, compared with the general population, had a higher incidence of DM (38.2%). In the AKI group, 56.7% had DM. Patients with diabetes had a higher risk of AKI (OR 2.23, 95% CI 1.35–3.68, P = 0.002). UTI is one of the most common sources of bacteremia in diabetic patients [41], and patients with DM are at a greater risk of developing various complications of UTI including sepsis [42]. Severe sepsis may induce vital organ dysfunction, including AKI [43]. Robbins et al. showed that UTI induced acute kidney injury in approximately 40% of diabetic patients with bacteremia [44]. Our results also suggested that DM was an independent risk factor for AKI in UTI patients.
Fever is known as an important feature of sepsis, and is considered to be an adaptive response to strengthen the immune system in order fighting against the invading organisms [45]. Lack of fever may contribute to lower resistance to infection, delayed recovery [17], higher mortality rate, and poor prognosis [46]. A number of factors, including acute alcoholism, hypothyroidism and elderliness have been identified in patients who had experienced afebrile bacteremia [47–49]. In this study, there was a higher incidence of being afebrile in patients with AKI (59.8%) than those without AKI (44.9%). Being afebrile played a predictive role for AKI in UTI patient (OR 1.71, 95% CI 1.04–2.83, P = 0.036). Wolk PJ et al. found that patients with renal impairment exhibited a lower febrile response to bacteremia [50]. Our study showed that 50.4% (137/272) of patients with eGFR <60 mL/min/1.73m2 experienced afebrile UTI. Among the UTI patients with eGFR <60 mL/min/1.73m2 developing AKI, 64.5% (40/62) presented with being afebrile. Several reasons might account for afebrile UTI patients being prone to developing AKI. First, patients with afebrile UTI have higher chance to have CKD, whereas CKD per se is a risk of AKI in UTI patients. Second, afebrile UTI with sepsis is more likely to be unrecognized, and more serious complications including AKI may result from the delayed treatment.
Upper UTI is not a well-recognized cause of AKI. Acute pyelonephritis can involve entire lobules of the medulla and cortex [51]. Interstitial infiltration of neutrophils and phagocytes and extensive destruction of the parenchymal by the acute inflammatory process were found in AKI patients with acute pyelonephritis [52. 53]. These reports demonstrated that severe upper UTI might cause serious damage to the kidney and resulted in AKI. Our study found that patients with upper UTI had higher risk of AKI than those with lower UTI (OR 2.63, 95% CI 1.53–4.56, P = 0.001). Early institution of appropriate antibiotic therapy for patients with upper UTI, especially those with septic shock, is important to prevent the development of AKI.
It is well known that nephrotoxic medications contribute to the development of AKI [54, 55]. In this study, there was a lower incidence of AKI in patients treated with nephrotoxic agents compared with those not treated with nephrotoxic agents (8.7% versus 19.7%, respectively). Nephrotoxic agent was usually avoided in patients with impaired baseline renal function, unstable hemodynamics, or at risk of AKI. This would result in a lower incidence of use of nephrotoxic agents in patients developing AKI in this study.
There are several limitations in this study. First, the retrospective data collection may lead to missing data and bias. However, we comprehensively collected the data using a standard form to reduce bias. Second, although the treatments were prescribed by qualified attending physicians according to the standard treatment for UTI, medications potentially contribute to AKI (e.g., aminoglycosides, nonsteroid anti-inflammatory drugs, contrast media), which may result in a potential bias. Nephrotoxic agents were usually avoided in patients with impaired baseline renal function or at risk of AKI. Contrast media may be required for emergency imaging survey of complicated UTI, urosepsis, or septic shock. Because of the selection bias, putting the use of nephrotoxic agent into the multivariate analysis will yield inaccurate results or conclusions. Further prospective studies will be needed to confirm the relationship between nephrotoxic agents and AKI in UTI patients. Third, this is a single center study, and the results may not be generalized to the entire community. A prospective, randomized study with larger sample size will be needed to confirm our results. Fourth, UTI patients’ urine output was not routinely recorded in this retrospective study. Therefore, we could not use the definition of AKI based on the urine output of Kidney Disease: Improving Global Outcomes (KDIGO) criteria to define the development of AKI. Of course, this underestimated the true incidence of AKI in our UTI patients. A prospective study designed to record the serial serum creatinine and urine output after admission will be needed to provide the true incidence of AKI after UTI using the KDIGO criteria based on both serum creatinine and urine output criteria. In conclusion, we found that impaired baseline renal function, advancing age, DM, upper UTI, and afebrile during hospitalization are all important and independent risk factors for developing AKI in UTI patients. Physicians should pay attention to UTI patients at risk of AKI.
Supporting Information
(XLS)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Nicolle LE. Epidemiology of urinary tract infections. Infect Med. 2001; 18: 153–162. [Google Scholar]
- 2. Laupland KB, Ross T, Pitout JD, Church DL, Gregson DB. Community-onset urinary tract infections: a population-based assessment. Infection. 2007; 35: 150–153. [DOI] [PubMed] [Google Scholar]
- 3. Schappert SM, Rechtsteiner EA. Ambulatory medical care utilization estimates for 2007. Vital Health Stat. 2011; 13: 1–38. [PubMed] [Google Scholar]
- 4. Rosser CJ, Bare RL, Meredith JW. Urinary tract infections in the critically ill patient with a urinary catheter. Am J Surg. 1999; 177: 287–290. [DOI] [PubMed] [Google Scholar]
- 5. Mori T, Shimizu T, Tani T. Septic acute renal failure. Contrib Nephrol. 2010; 166: 40–46. 10.1159/000314849 [DOI] [PubMed] [Google Scholar]
- 6. Plataki M, Kashani K, Cabello-Garza J, Maldonado F, Kashyap R, Kor DJ, et al. Predictors of acute kidney injury in septic shock patients: an observational cohort study. Clin J Am Soc Nephrol. 2011; 6: 1744–1751. 10.2215/CJN.05480610 [DOI] [PubMed] [Google Scholar]
- 7. Baker LR, Cattell WR, Fry IK, Mallinson WJ. Acute renal failure due to bacterial pyelonephritis. Q J Med. 1979; 48: 603–612. [PubMed] [Google Scholar]
- 8. Hou SH, Bushinsky DA, Wish JB, Cohen JJ, Harrington JT. Hospital-acquired renal insufficiency: A prospective study. Am J Med. 1983; 74: 243–248. [DOI] [PubMed] [Google Scholar]
- 9. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005; 16: 3365–3370. [DOI] [PubMed] [Google Scholar]
- 10. Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: A Veterans Administration study. Crit Care Med. 2009; 37: 2552–2558. 10.1097/CCM.0b013e3181a5906f [DOI] [PubMed] [Google Scholar]
- 11. Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, et al. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009; 76: 893–899. 10.1038/ki.2009.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hsu CY, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Go AS, et al. Nonrecovery of kidney function and death after acute or chronic renal failure. Clin J Am Soc Nephrol. 2009; 4: 891–898. 10.2215/CJN.05571008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA. 2009; 302: 1179–1185. 10.1001/jama.2009.1322 [DOI] [PubMed] [Google Scholar]
- 14. Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure–definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of Acute Dialysis Quality Initiative (ADQI) group. Crit Care. 2004; 8: R204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 150: 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002; 39: S1–266. [PubMed] [Google Scholar]
- 17. Dalal S, Zhukovsky DS. Pathophysiology and Management of Fever. J Support Oncol. 2006; 4: 9–16. [PubMed] [Google Scholar]
- 18. Kushimoto S, Yamanouchi S, Endo T, Sato T, Nomura R, Fujita M, et al. Body temperature abnormalities in non-neurological critically ill patients: a review of the literature. J Intensive Care. 2014; 2: 14 10.1186/2052-0492-2-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992; 101: 1644–1655. [DOI] [PubMed] [Google Scholar]
- 20. Jones SR. Acute renal failure in adults with uncomplicated acute pyelonephritis reports and review. Clin Infect Dis. 1992; 14: 243–246. [DOI] [PubMed] [Google Scholar]
- 21. Smith JW. Prognosis in pyelonephritis: promise or progress? Am J Med Sci. 1989; 297: 53–62. [DOI] [PubMed] [Google Scholar]
- 22. Fünfstück R, Ott U, Naber KG. The interaction of urinary tract infection and renal insufficiency. Int J Antimicrob Agents. 2006; 28 Suppl 1: S72–77. [DOI] [PubMed] [Google Scholar]
- 23. Kooman JP, Barendregt JN, van der Sande FM, van Suylen RJ. Acute pyelonephritis: a cause of acute renal failure? Neth J Med. 2000; 57: 185–189. [DOI] [PubMed] [Google Scholar]
- 24. Nahar A, Akom M, Hanes D, Briglia A, Drachenberg CB, Weinman EJ. Pyelonephritis and acute renal failure. Am J Med Sci. 2004; 328: 121–123. [DOI] [PubMed] [Google Scholar]
- 25. Gilbert DN. Urinary tract infections in patients with chronic renal insufficiency. Clin J Am Soc Nephrol. 2006; 1: 327–331. [DOI] [PubMed] [Google Scholar]
- 26. Singh P, Rifkin DE, Blantz RC. Chronic kidney disease: an inherent risk factor for acute kidney injury? Clin J Am Soc Nephrol. 2010; 5: 1690–1695. 10.2215/CJN.00830110 [DOI] [PubMed] [Google Scholar]
- 27. Parfrey PS, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, et al. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989; 320: 143–149. [DOI] [PubMed] [Google Scholar]
- 28. Browner WS, Li J, Mangano DT. In-hospital and long-term mortality in male veterans following noncardiac surgery. JAMA. 1992; 268: 228–232. [PubMed] [Google Scholar]
- 29. Hsu CY, Ordonez JD, Chertow GM, Fan D, McCulloch CE, Go AS. The risk of acute renal failure in patients with chronic kidney disease. Kidney Int. 2008; 74: 101–107. 10.1038/ki.2008.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boscia JA, Kaye D. Asymptomatic bacteriuria in the elderly. Infect Dis Clin North Am. 1987; 1: 893–905. [PubMed] [Google Scholar]
- 31. Schaeffer AJ. Urinary tract infections in the elderly. Eur Urol. 1991; 19 Suppl 1: 2–6. [DOI] [PubMed] [Google Scholar]
- 32. Sant GR. Urinary tract infection in the elderly. Semin Urol. 1987; 5: 126–133. [PubMed] [Google Scholar]
- 33. Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014; 371: 58–6 10.1056/NEJMra1214243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Patterson JE, Andriole VT. Bacterial urinary tract infections in diabetes. Infect Dis Clin North Am. 1997; 11: 735–750. [DOI] [PubMed] [Google Scholar]
- 35. Boyko EJ, Fihn SD, Scholes D, Chen CL, Normand EH, Yarbro P. Diabetes and the risk of acute urinary tract infection among postmenopausal women. Diabetes Care. 2002; 25: 1778–1783. [DOI] [PubMed] [Google Scholar]
- 36. Scholes D, Hooton TM, Roberts PL, Gupta K, Stapleton AE, Stamm WE. Risk factors associated with acute pyelonephritis in healthy women. Ann Intern Med. 2005; 142: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, et al. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007; 297: 1801–1809. [DOI] [PubMed] [Google Scholar]
- 38. Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005; 16: 162–168. [DOI] [PubMed] [Google Scholar]
- 39. Ma RC, Chow CC, Yeung VT, So WY, Kong AP, Tong PC, et al. Acute renal failure following oral sodium phosphate bowel preparation in diabetes Diabetes Care. 2007; 30: 182–183. [DOI] [PubMed] [Google Scholar]
- 40. Medeiros P, Nga HS, Menezes P, Bridi R, Balbi A, Ponce D. Acute kidney injury in septic patients admitted to emergency clinical room: risk factors and outcome. Clin Exp Nephrol. 2014. December 27. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 41. Qari FA. Bacteremia and septicemia in diabetic patients in Western Saudi Arabia. Saudi Med J. 2003; 24: 1064–1067. [PubMed] [Google Scholar]
- 42. Kalra OP, Raizada A. Approach to a patient with urosepsis. J Glob Infect Dis. 2009; 1: 57–63 10.4103/0974-777X.52984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nduka OO, Parrillo JE. The pathophysiology of septic shock. Crit Care Clin. 2009; 25: 677–702. 10.1016/j.ccc.2009.08.002 [DOI] [PubMed] [Google Scholar]
- 44. Robbins SL, Tucker AW. The cause of death in diabetes. A report of 307 autopsied cases. N Engl J Med. 1944; 231: 865–868.http://www.nejm.org/toc/nejm/231/26/ [Google Scholar]
- 45. Tiruvoipati R, Ong K, Gangopadhyay H, Arora S, Carney I, Botha J. Hypothermia predicts mortality in critically ill elderly patients with sepsis. BMC Geriatr. 2010; 10: 70 10.1186/1471-2318-10-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Roberts NJ Jr. Impact of temperature elevation on immunologic defenses. Rev Infect Dis. 1991; 13: 462–472. [DOI] [PubMed] [Google Scholar]
- 47. Gleckman R, Hibert D. Afebrile bacteremia. A phenomenon in geriatric patients. JAMA. 1982; 248: 1478–1481. [DOI] [PubMed] [Google Scholar]
- 48. Holloway WA, Reinhardt J. Septic shock in the elderly. Geriatrics. 1984; 39: 48–54. [PubMed] [Google Scholar]
- 49. Lee CC, Chen SY, Chang IJ, Chen SC, Wu SC. Comparison of Clinical manifestations and outcome of community-acquired bloodstream infections among the oldest old, elderly, and adult patients. Medicine (Baltimore). 2007; 86: 138–144. [DOI] [PubMed] [Google Scholar]
- 50. Wolk PJ, Apicella MA. The effect of renal function on the febrile response to bacteremia. Arch Intern Med. 1978; 138: 1084 [PubMed] [Google Scholar]
- 51. Cotran RS, Pennington JE. Urinary tract infection, pyelonephritis, and reflux nephropathy Philadelphia: W.B. Saunders; 1981: 1571–1632. [Google Scholar]
- 52. Lorentz WB, Iskandar S, Browning MC, Reynolds GD. Acute renal failure due to pyelonephritis. Nephron. 1990; 54: 256–258. [DOI] [PubMed] [Google Scholar]
- 53. Söylemezoğlu O, Kale G, Saatçi U, Akçaören Z. Acute renal failure due to acute pyelonephritis. Int Urol Nephrol. 1995; 27: 137–139. [DOI] [PubMed] [Google Scholar]
- 54. Liangos O. Drugs and AKI. Minerva Urol Nefrol. 2012; 64: 51–62. [PubMed] [Google Scholar]
- 55. Thatte L, Vaamonde CA. Drug-induced nephrotoxicity: the crucial role of risk factors. Postgrad Med. 1996; 100: 83–84, 87–88, 91 passim. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


