Abstract
The Valsalva maneuver is frequently used to test autonomic function. Previous work demonstrated that the blood pressure decrease during the Valsalva maneuver relates to thoracic hypovolemia, which may preclude pressure recovery during phase II, even with normal resting peripheral vasoconstriction. We hypothesized that increased regional blood volume, specifically splanchnic hypervolemia, accounts for the degree of thoracic hypovolemia during the Valsalva maneuver. We studied 17 healthy volunteers aged 15–22 yr. All had normal blood volumes by dye dilution. Subjects also had normal vascular resistance while supine as well as normal vasoconstrictor responses during 35° upright tilt. We assessed changes in estimated splanchnic, pelvic-thigh, and lower leg blood volume, along with thoracic blood volume shifts, by impedance plethysmography before and during the Valsalva maneuver performed in the supine position. Early increases in splanchnic blood volume dominated the regional vascular changes during the Valsalva maneuver. The increase in splanchnic blood volume correlated well (r2 = 0.65, P < 0.00001) with the decrease in thoracic blood volume, there was less correlation of the increase in pelvic blood volume (r2 = 0.21, P < 0.03), and there was no correlation of the increase in leg blood volume (r2 = 0.001, P = 0.9). There was no relation of thoracic hypovolemia with blood volume or peripheral resistance in supine or upright positions. Thoracic hypovolemia during the Valsalva maneuver is closely related to splanchnic hyperemia and weakly related to regional changes in blood volume elsewhere. Changes in baseline splanchnic vascular properties may account for variability in thoracic blood volume changes during the Valsalva maneuver.
Keywords: vasoconstriction, veins, capacitance, mesenteric, autonomic
Forced exhalation during the Valsalva maneuver increases intrapleural pressure and impedes venous return of blood to the heart (10). However, venous return and thoracic blood volume decrease to different degrees in different individuals (27). Thus, although a standardized “quantitative Valsalva maneuver” (3), with open glottis and controlled expiratory pressure, can generate consistent increases in intrapleural and right atrial pressures across subjects (14), it may produce a wide range of thoracic blood volume shifts. Some of these volume shifts may be sufficiently large that they preclude autonomically mediated blood pressure recovery during phase II of the maneuver (27). This can compromise the use of heart rate and blood pressure responses to assess circulatory autonomic function (20). Such findings might suggest sympathetic impairment when none is present.
The splanchnic circulation comprising the liver, spleen, gastrointestinal tract, and pancreas is a major reservoir of blood in humans and animals, accounting for ~30% of venous blood volume (23). Moreover, its contents are highly adjustable, responding well to sympathetic input, producing arterial vasoconstriction and venoconstriction during orthostasis and exercise (21, 25). We investigated the role of variable rapid filling of regional leg, pelvis, and splanchnic circulations in thoracic blood volume findings. We hypothesized that reciprocal changes in splanchnic blood volume (i.e., splanchnic hypervolemia), but not in pelvic or lower extremity blood volume, determine the extent of decrease in thoracic blood volume and, thus, the ability to compensate during phase II of the Valsalva maneuver.
MATERIALS AND METHODS
Subjects and Experimental Outline
To test this hypothesis, we studied 17 healthy (free from all systemic illnesses) volunteers (15–22 yr of age, median 17.5 yr, 7 men and 10 women). They were taking no medications. All subjects had normal ECG and echocardiograms, no other evidence for cardiovascular illness, and normal estimated resting cardiac output by indocyanine dilution. We excluded subjects with a history of syncope or orthostatic intolerance. There were no trained competitive athletes or bedridden subjects. Informed consent was obtained from subjects or from parents and subjects in those <18 yr old. All protocols were approved by the Committee for the Protection of Human Subjects (Institutional Review Board) of New York Medical College.
We assessed changes in estimated thoracic, splanchnic, pelvic, and calf blood segmental volumes (defined below) by impedance plethysmography before, during, and after the Valsalva maneuver, which was performed with the subjects in the supine position. The Valsalva response has been shown to be strongly posture dependent (7, 24). We chose to perform the maneuver in the supine position to separate autonomic stimuli arising from orthostasis from stimuli due to the Valsalva maneuver. Synchronous changes in heart rate and blood pressure were recorded.
Protocol
Tests began in a temperature-controlled room after an overnight fast. After a 30-min acclimatization period, tests were performed in the following order, with ≥15 min allowed for recovery between procedures: supine peripheral blood flow and arterial resistance measurement, supine Valsalva maneuver, tilt to 35°, upright peripheral blood flow, and peripheral arterial resistance measurement. Supine and upright peripheral blood flow and resistance measurements were used to confirm that subjects had normal vasoconstrictor responses.
Details of the Method
Peripheral blood flow, venous pressure, and peripheral arterial resistance
In all subjects, forearm and calf blood flow were measured by venous occlusion strain gauge plethysmography to demonstrate that peripheral blood flow, venous pressure, and peripheral arterial resistance were normal. Supine measurements were made at the beginning of experiments, and measurements were repeated in the upright 35° tilt position. Measurements were made in a standard manner, with exclusion of ankle and hand circulation. We employed these techniques previously to show intact peripheral vasoconstrictive ability (26, 27).
Heart rate, respirations, and blood pressure monitoring
ECG strips were monitored continuously. Relative respiratory volume was measured with a respiratory inductance plethysmograph placed around the maximum thoracic circumference and attached to a Respitrace monitor (NIMS Scientific). Upper extremity blood pressure was continuously monitored with an arterial tonometer (Colin Instruments, San Antonio, TX) placed on the right radial artery and recalibrated automatically every 5 min against oscillometric blood pressure. ECG and tonometric pressure data were interfaced to a personal computer through an analog-to-digital converter (DataQ, Milwaukee, WI). All data were multiplexed with strain gauge and impedance data and were effectively synchronized.
Dye-dilution measurement of blood volume
We used the indocyanine green dye-dilution technique (1). We employed a noninvasive spectrophotometric finger photosensor (DDG, Nihon-Kohden) that was verified during clinical studies (12, 13). The dye decay curve is a monoexponential representing clearance by the liver. Once the hematocrit is measured, extrapolation of the dye decay curve to the time of dye injection (time 0) yields estimated blood volume.
Impedance plethysmography for measurement of changes in segmental blood volume
Impedance plethysmography has been used to detect internal volume shifts (8), including those produced during orthostatic stress (2, 5). We used a tetrapolar high-resolution impedance monitor four-channel digital impedance plethysmograph (UFI) to measure volume shifts in four anatomic segments: the thoracic segment, the splanchnic segment, the pelvic segment (lower pelvis to upper leg), and the leg segment (2, 18, 28, 30). Ag-AgCl ECG electrodes were attached to the left foot and left hand, which served as current injectors. Additional electrodes were placed in pairs representing anatomic segments as follows: ankle-upper calf just below the knee (leg segment), knee-iliac crest (pelvic segment), iliac crest-midline xiphoid process (splanchnic segment), and midline xiphoid process to supraclavicular area (thoracic segment). Impedance plethysmograhy introduces a high-frequency (50 kHz), low-amperage (0.1 mA root mean square) constant-current signal between the foot and hand electrodes that is completely insensible to the subjects. Electrical resistance values were measured using the segmental pairs as sampling electrodes. Anatomic features were selected as the most appropriate locations for comparing changes within and across subjects. This combination of electrodes gives highly repeatable changes in computed volume shifts and has been tested in a wide range of experiments by our group (18, 30). The distance between the sampling electrodes (L) was measured carefully with a tape measure. We estimated the change in blood volume in each segment during the Valsalva maneuver from the following formula (8)
where ρ is electrical conductivity of blood estimated as 53.2 * exp(Hct * 0.022) (where Hct is hematocrit, or packed cell volume, which we measured), given by Geddes and Kidder (9), R0 is the baseline resistance of a specific segment, R1 is the resistance during the Valsalva maneuver, and ΔR is change in resistance (R1 − R0) in a specific segment during the maneuver. ρ was regarded as constant during the maneuver. Impedance plethysmography measurements allow us to follow blood volume changes in the various segments during the Valsalva maneuver.
Quantitative Valsalva Maneuver
The quantitative Valsalva maneuver was performed with the subject supine by exhalation with an open glottis into a mouthpiece connected to the mercury column of a sphygmomanometer with a small air leak. The air leak enabled the glottis to remain open because the subject was very slowly exhaling. Thus 35–40 mmHg of pressure (at the mouthpiece and, presumably, at the pleural level) was maintained for ≥15 s. Pressure was released, with care taken to prevent deep breathing. Two attempts with 10 min of intervening quiet breathing were made to secure an adequate Valsalva maneuver with sustained intraoral pressure. The first adequate exhalation was used for data acquisition. All subjects were able to produce the maneuver. Blood pressure, ECG, heart rate, and thoracic impedance were recorded continuously throughout the maneuver. The blood pressure response was quantified during straining and during the pressure overshoot. We used the maximum decline in blood pressure to indicate the end of early phase II. Typically, this occurred at ~7 s into exhalation. We used the point of subsequent maximum systolic blood pressure preceding release to indicate late phase II. Early blood pressure changes are independent of the sympathetic nervous system, which requires at least some seconds to exert any effect (22, 29).
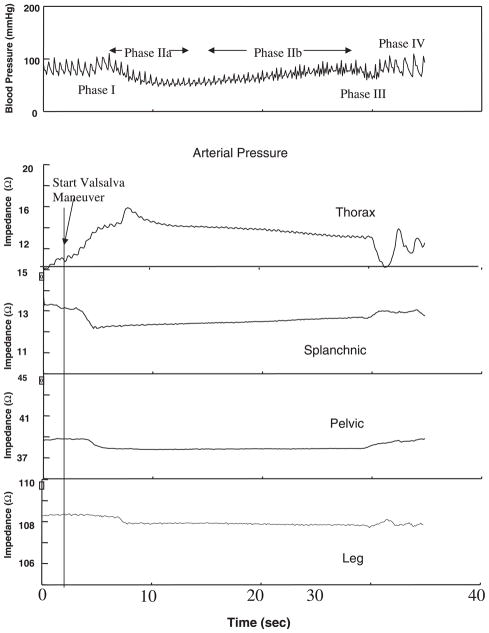
The classic Valsalva maneuver blood pressure response (11) is illustrated Fig. 1. There is a brief increase in blood pressure, denoted phase I, during which there is a mechanical increase in blood pressure due to propulsion of blood from the thorax. This is followed by a decrease in blood pressure and an increase in heart rate produced by decreased venous return and decreased cardiac output during early phase II. Late phase II is usually marked by recovery of blood pressure produced, presumably, by a combination of vasoconstriction, sympathetic cardiac stimulation, and tachycardia. Once exhalation is complete, the release of strain restores normal negative intrathoracic pressure, leading to the blood pressure decrease of phase III followed by the pressure overshoot of phase IV and associated reflex bradycardia.
Fig. 1.
Time course of blood pressure and thoracic, splanchnic, pelvic, and leg impedances during a representative Valsalva maneuver. Increase in thoracic impedance precedes phase I blood pressure change. Decreases in splanchnic, pelvic, and leg impedances occur at successively later times. Splanchnic impedance falls while thoracic impedance rises initially; thereafter, splanchnic impedance rises while thoracic impedance falls. Pelvic and leg impedance changes remain relatively stable throughout phase II.
We chose to examine patients in the supine position, because we wanted to separate contributions from orthostasis from contributions due only to the Valsalva maneuver. Clearly, the Valsalva response has been shown to relate strongly to posture (7, 24).
Time Lag in Onset of Venous Filling During the Valsalva Maneuver
There was a time lag between onset of the Valsalva maneuver, assessed by respiratory plethysmography, and onset of impedance changes in each anatomic segment. We assessed this with the following heuristic. For each segment, the average baseline impedance was computed for 30 s before the maneuver. Inhalation preceding the actual Valsalva maneuver was easily detected by Respitrace and was generally accompanied by a decrease in impedance (increase in blood volume) in the thoracic segment and an increase in impedance (decrease in blood volume) in the splanchnic, pelvic, and leg segments (although less discernible in the leg). The peak of the Respitrace volume indicated onset of the Valsalva maneuver. Onset of the Valsalva-related increase in thoracic impedance and the Valsalva-related decreases in splanchnic, pelvic, and leg impedances was detected when the impedance for each segment decreased below its respective baseline.
Statistics
Supine and upright blood flow data, venous pressure, and peripheral resistance were compared by paired t-tests. Correlations were obtained using Spearman’s rank-order correlation statistic. Results are reported as means ± SE. We used a signed-run test based on binomial statistics to determine whether there is a progression of segmental time lags.
RESULTS
All subjects were able to perform the supine quantitative Valsalva maneuver, maintaining the expiratory pressure at 35–40 mmHg for ≥15 s. Valsalva maneuver results are shown in Figs. 1–4.
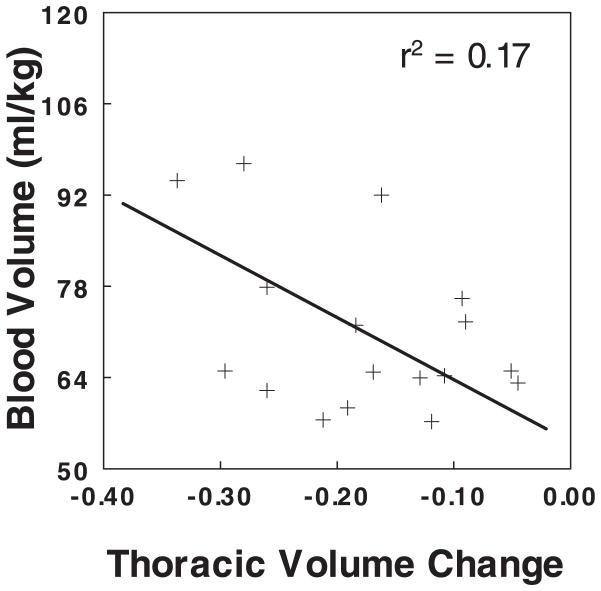
Fig. 4.
Relation between fractional change in segmental thoracic blood volume calculated from impedance plethysmography and blood volume, assessed in the supine position, using indocyanine green dye dilution. There is no significant correlation between blood volume and thoracic blood volume change.
Hemodynamics and Size Measurements
Weight was 68 ± 14 kg, height was 169 ± 10 cm, body surface area was 1.78 ± 0.12 m2, and blood volume was 71 ± 13 ml/kg, which are not different from subject data previously reported (27). Total blood volume, thoracic volume, and body surface area did not correlate with the magnitude of volume changes during the Valsalva maneuver. Blood volume also did not correlate with changes in blood pressure during the Valsalva maneuver.
Supine and corresponding upright hemodynamic data are shown in Table 1. Resting heart rate, mean arterial pressure in the calf, and venous pressure in the calf increased significantly with upright tilt to 35°. Forearm mean arterial pressure and venous pressure were not significantly changed by tilt. Leg venous pressure increased to a similar extent with tilt in all subjects. Forearm and calf peripheral arterial resistance increased in all subjects, indicating postural vasoconstriction.
Table 1.
Size and hemodynamic data
| Posture
|
P | ||
|---|---|---|---|
| Supine | Upright | ||
| HR, beats/min | 69±6 | 80±6* | 0.004 |
| MAP (arm), mmHg | 78±4 | 75±4 | |
| Pv, mmHg | |||
| Arm | 10±2 | 11±1 | |
| Leg | 14±2 | 31±6* | 0.0002 |
| Blood flow, ml·100 ml−1·min−1 | |||
| Arm | 2.6±0.4 | 1.9±0.5* | 0.03 |
| Leg | 2.4±0.4 | 2.0±0.4 | 0.06 |
| Arterial resistance, ml·100 ml−1·min·mmHg−1 | |||
| Arm | 28±6 | 44±12* | 0.0002 |
| Leg | 26±12 | 43±20* | 0.003 |
Values are means ± SE. HR, heart rate; MAP, mean arterial pressure, Pv, venous pressure.
P < 0.05 vs. supine.
Impedance and Segmental Blood Volume Changes During the Valsalva Maneuver
Figure 1 shows the change in blood pressure and associated changes in segmental impedance during the Valsalva maneuver in a typical representative subject. Impedance is expressed as a fractional change, normalized to baseline R0. The relative absolute magnitude of impedance change is greatest in the thorax, next highest in the splanchnic segment, relatively small in the pelvic segment, and smallest in the calf segment. Changes in thoracic impedance are directionally opposite to changes in the other segmental impedances.
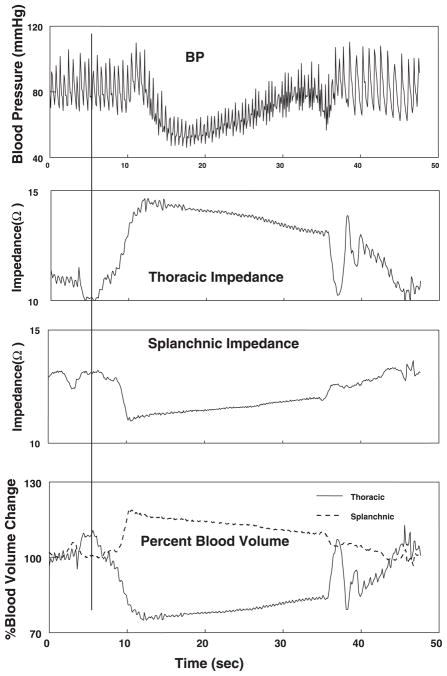
Figure 2 shows data from another representative subject depicting only changes in blood pressure, thoracic and splanchnic segmental impedances, and calculated fractional changes in segmental blood volume. Directionally opposite changes in blood volumes occur with a decrease in thoracic segmental volume and a reciprocal increase in splanchnic segmental blood volume.
Fig. 2.
Time course of blood pressure (BP), thoracic and splanchnic impedance, and calculated segmental blood volume during a typical representative Valsalva maneuver in a representative subject. A reciprocal relation between thoracic and splanchnic impedances is reflected in blood volume changes. Again, initial rise in splanchnic blood volume and fall in thoracic blood volume are followed by opposite changes.
To explore whether a relation exists between changes in segmental thoracic blood volume and changes in other segments, we correlated maximum changes in segmental blood volume for splanchnic, pelvic, and calf segments with maximum changes in thoracic blood volume. Figure 3 demonstrates good correlation of splanchnic blood volume change with thoracic blood volume change (r2 = 0.65, P = 0.0001), relatively poor correlation of pelvic blood volume change with thoracic blood volume change (r2 = 0.21, P = 0.014), and no significant correlation between calf and thoracic blood volume changes (r2 = 0.001, P = 0.9).
Fig. 3.
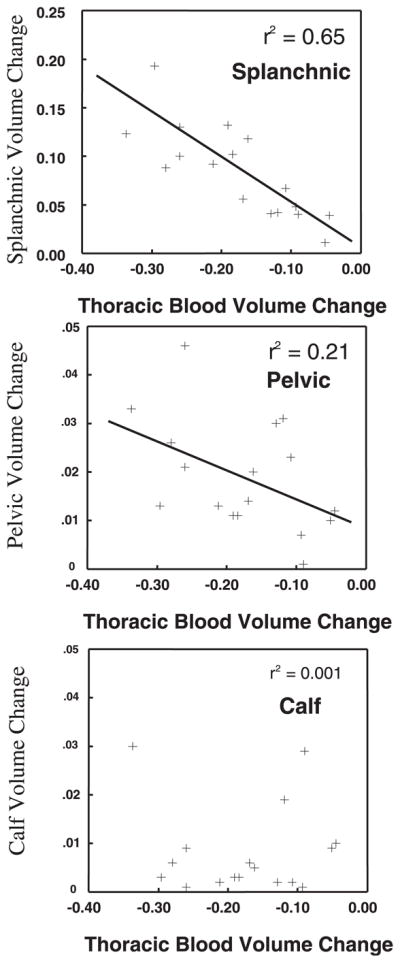
Relation between fractional calculated splanchnic blood volume and fraction of thoracic blood volume, fractional calculated pelvic blood volume and fraction of thoracic blood volume, and fractional calculated leg blood volume and fraction of thoracic blood volume during the Valsalva maneuver. Splanchnic volume changes are highly and inversely correlated to thoracic volume changes. Pelvic blood volume changes correlate less well, and leg volume changes do not correlate with thoracic blood volume decreases.
Relation of Total Blood Volume to Thoracic Blood Volume Change During the Valsalva Maneuver
We similarly correlated total blood volume with thoracic blood volume change during the Valsalva maneuver (Fig. 4). Total blood volume did not significantly correlate (r2 = 0.17, P = 0.10) with thoracic blood volume change in these experiments, although there was a trend toward smaller total blood volume related to larger change in fractional segmental thoracic blood volume change.
Time Lag in Onset of Venous Filling During the Valsalva Maneuver
Onset of the characteristic increase in thoracic impedance precedes reciprocal decreases in splanchnic impedance, which in turn precedes decreased pelvic and then calf impedances. This implies venous wave propagation. Thus time lags were consistently shortest for thoracic volume changes (0.45 ± 0.18 s), longer for the splanchnic segment (1.4 ± 0.5 s), and longest for pelvic and leg segments (2.2 ± 0.6 and 2.8 ± 0.5 s, respectively, P < 0.01, by signed-run test). Thoracic time lag was often close to zero. Pelvic and leg changes were often similar, although sign testing invariably yielded a positive difference between leg and pelvic times.
DISCUSSION
Our results are the first to demonstrate a strong correlation between decrease in thoracic blood volume and increase in splanchnic blood volume during phase II of the Valsalva maneuver. There is also a weak correlation with changes in pelvic blood volume. Previously, we demonstrated that large decreases in thoracic blood volume relate to large decreases in early blood pressure during early phase II of the Valsalva maneuver and may prevent pressure recovery during late phase II (27). We can now say that sequestration of blood within the splanchnic circulation accounts in large measure for the changes in thoracic blood volume and, thus, changes in blood pressure. There is wide variation in venous return from subject to subject, produced by a wide variation in splanchnic pooling from subject to subject. The wide dispersion in splanchnic volume increase and reciprocal thoracic volume decrease unrelated to autonomic status during the Valsalva maneuver indicate that it is not a simple autonomic test. Rather, vascular, as well as autonomic, dependence of the Valsalva maneuver broadens its utility as a tool to study the splanchnic venous system.
Thoracic Blood Volume Decrease During Early Valsalva Maneuver Depends on Splanchnic Filling
We have shown that, without measurement of altered splanchnic venous filling, phase II blood pressure recovery cannot be used as an accurate reflection of sympathetic vasoconstrictive capability. The interpretation problem could be solved by measuring splanchnic filling, as we have done. Although it complicates analysis of the Valsalva maneuver, it adds to its utility as a vascular probe.
Sympathetic activation is not instantaneous, but, rather, using data of Tyden (29), Rowell (22) estimated a lag of ~5–15 s before vasoconstriction. Smith et al. (25) carefully demonstrated a similar, but somewhat shorter, delay in the onset of muscle sympathetic nerve activity after Valsalva straining. Arguably, the decrease in thoracic blood volume during early phase II of the Valsalva maneuver and the reciprocal increase in splanchnic blood volume and other regional blood volumes relate more to baseline venous tone and venous compliance and less to resting arterial tone (21). Baseline sympathetic tone could play a role, but there is no evidence among our subjects of a relation between arm and leg peripheral resistance and thoracic blood volume. A more direct and invasive measure of mesenteric tone would be helpful in this regard. Although there is copious evidence that the Valsalva response relates strongly to blood volume (7, 24), all our subjects had similar normal total blood volumes, and we did not obtain significant correlations between total blood volume and thoracic blood volume during the Valsalva maneuver.
Thoracic-Splanchnic Reciprocity
We propose, along with others (3, 3, 14, 25), that during expiratory strain there is a rapid decrease in venous return, which we detected as an increase in thoracic impedance. From Figs. 1 and 2, we note that the increase in impedance and, hence, the decrease in thoracic volume precede changes in blood pressure by a few seconds, which may relate to pulmonary vascular emptying. Increased splanchnic filling begins shortly after thoracic emptying. The decrease in thoracic filling, which varies from patient to patient, depends on blood volume and on the time-dependent changes in venous resistance and venous pressure in regional circulations and in right atrial pressure. Intrapleural pressure is very similar to intraoral pressure (6). Right atrial pressure appears to change in a deterministic way with increasing intrapleural pressure, although the increase in atrial pressure is only ~70% of the increase in intrapleural pressure (15). Therefore, in subjects of similar total blood volume, thoracic filling depends mostly on venous properties and provides insight into venous mechanisms. From Figs. 1 and 2, we may note that changes in thoracic blood volume and changes in splanchnic blood volume are nearly mirror images (within a scaling factor) of one another, reflected in the x-axis. This not only applies to early phase II, where we propose that splanchnic filling occurs at the expense of thoracic emptying, but also to late phase II, in which we propose that splanchnic emptying promotes thoracic filling and blood pressure restoration. Such splanchnic emptying is caused by sympathetic activation, resulting in splanchnic arterial vasoconstriction, with passive emptying of the gut circulation and also active splanchnic venoconstriction known to occur in humans (4, 22, 23). Similar emptying is not observed in the pelvic or leg segments. Although pelvic volume remains steady throughout the Valsalva maneuver, increases in volume continue in the leg. The data may be displaying splanchnic venoconstriction, which is conspicuously absent in the pelvic and leg regional circulatory beds.
Previous work has supported the ability to generate well-defined venous return curves from graded use of the quantitative Valsalva maneuver (17); other investigators have shown that such graded expiratory pressures produce graded changes in splanchnic venous pooling (16, 19). This supports our observation of a reciprocal relation between thoracic and splanchnic segments.
Time Lag in Onset of Venous Filling During the Valsalva Maneuver
Figure 1 illustrates an interesting and consistent finding. The onset of impedance change precedes blood pressure changes during the Valsalva maneuver and progresses caudally in sequence from thorax to splanchnic, pelvic, and leg segments. This suggests that, in the supine position at least, initial retrograde volume shifts may occur when the venous system is used.
Limitations
Vasoconstrictive capability
We did not directly measure vasoconstriction during the Valsalva maneuver. This is not feasible with occlusion plethysmography because of time scale factors. Segmental impedance flow methods may remediate this difficulty, but tracing artifact obliterates the flow signal. Instead, we used resting and orthostatic peripheral vasoconstriction as surrogates of sympathetic vasoconstrictive adequacy. Alternatively, a direct measure of sympathetic activity, such as muscle sympathetic nerve activity, could enhance our ability to state that volume changes per se, in the presence of intact sympathetic vasoconstriction, account for our findings. Such instrumentation is often problematic in subjects in the adolescent age range and was therefore not pursued. Also, normal vasoconstrictor responses of the limbs do not necessarily indicate normal vasoconstrictor responses of regions such as the splanchnic circulation. Reduced splanchnic vasoconstriction could contribute to splanchnic hypervolemia during the Valsalva maneuver in some of our subjects.
Constancy of Valsalva pressure
The subjects performed a so-called “quantitative” Valsalva maneuver by maintaining as close to constant exhalation pressure in a mouthpiece connected to a mercury manometer. However, although every effort was made to maintain nearly constant pressure of 35–40 mmHg for 15 s, occasionally pressure would fluctuate, which could potentially lead to systematic differences. We were unable to detect such differences in practice, but we did not record exhalation pressure. Respitrace indicated a smooth gradual exhalation for the duration required. A device to control expiratory pressure for a period of time would be ideal and is under development.
Age
Age limitations to generalization may exist. Young adults and adolescents may not perfectly represent findings for mature adults. However, cardiovascular structure and function are essentially mature by puberty; therefore, results can be regarded as at least qualitatively similar to results from older age groups. Moreover, younger patients generally have the advantage of absence of confounding illness, such as heart disease, renal disease, hypertension, and diabetes, which may impact on autonomic or circulatory function.
Acknowledgments
We thank members of the Division of Pediatric Cardiology, especially Michael H. Gewitz, for unflagging support and Drs. Thomas H. Hintze, David Robertson, and Phillip Low for constant inspiration and stimulation.
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants 1-R01-HL-66007 and 1-R01-HL-074873.
References
- 1.Bloomfield DA. Dye Curves: The Theory and Practice of Indicator Dye Dilution. Baltimore, MD: University Park Press; 1974. [Google Scholar]
- 2.Convertino VA, Montgomery LD, Greenleaf JE. Cardiovascular responses during orthostasis: effect of an increase in V̇O2 max. Aviat Space Environ Med. 1984;55:702–708. [PubMed] [Google Scholar]
- 3.De Jong-de Vos van Steenwijk CC, Imholz BP, Wesseling KH, Wieling W. The Valsalva manoeuvre as a cardiovascular reflex test in healthy children and teenagers. Clin Auton Res. 1997;7:167–171. doi: 10.1007/BF02267977. [DOI] [PubMed] [Google Scholar]
- 4.Donegan JF. The physiology of veins. J Physiol. 1921;55:226–245. doi: 10.1113/jphysiol.1921.sp001964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ebert TJ, Smith JJ, Barney JA, Merrill DC, Smith GK. The use of thoracic impedance for determining thoracic blood volume changes in man. Aviat Space Environ Med. 1986;57:49–53. [PubMed] [Google Scholar]
- 6.Elisberg EI, Goldberg H, Snider GL. Value of intraoral pressure as a measure of intrapleural pressure. J Appl Physiol. 1951;4:171–176. doi: 10.1152/jappl.1951.4.3.171. [DOI] [PubMed] [Google Scholar]
- 7.Fritsch-Yelle JM, Convertino VA, Schlegel TT. Acute manipulations of plasma volume alter arterial pressure responses during Valsalva maneuvers. J Appl Physiol. 1999;86:1852–1857. doi: 10.1152/jappl.1999.86.6.1852. [DOI] [PubMed] [Google Scholar]
- 8.Geddes LA, Baker LE. Principles of Applied Biomedical Instrumentation. New York: Wiley; 1989. Detection of physiological events by impedance; pp. 594–600. [Google Scholar]
- 9.Geddes LA, Kidder H. Specific resistance of blood at body temperature. Part II. Med Biol Eng. 1976;14:180–185. doi: 10.1007/BF02478745. [DOI] [PubMed] [Google Scholar]
- 10.Gorlin R, Knowles JH, Storey CF. The Valsalva maneuver as a test of cardiac function: pathologic physiology and clinical significance. Am J Med. 1957;22:197–212. doi: 10.1016/0002-9343(57)90004-9. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton WF, Woodbury RA, Harper HT., Jr Arterial, cerebrospinal and venous pressures in man during cough and strain. Am J Physiol. 1944;141:42–50. [Google Scholar]
- 12.He YL, Tanigami H, Ueyama H, Mashimo T, Yoshiya I. Measurement of blood volume using indocyanine green measured with pulse spectrophotometry: its reproducibility and reliability. Crit Care Med. 1998;26:1446–1451. doi: 10.1097/00003246-199808000-00036. [DOI] [PubMed] [Google Scholar]
- 13.Iijima T, Aoyagi T, Iwao Y, Masuda J, Fuse M, Kobayashi N, Sankawa H. Cardiac output and circulating blood volume analysis by pulse dye densitometry. J Clin Monit. 1997;13:81–89. doi: 10.1023/a:1007339924083. [DOI] [PubMed] [Google Scholar]
- 14.Korner PI, Tonkin AM, Uther JB. Reflex and mechanical circulatory effects of graded Valsalva maneuvers in normal man. J Appl Physiol. 1976;40:434–440. doi: 10.1152/jappl.1976.40.3.434. [DOI] [PubMed] [Google Scholar]
- 15.Luster EA, Baumgartner N, Adams WC, Convertino VA. Effects of hypovolemia and posture on responses to the Valsalva maneuver. Aviat Space Environ Med. 1996;67:308–313. [PubMed] [Google Scholar]
- 16.Manyari DE, Wang Z, Cohen J, Tyberg JV. Assessment of the human splanchnic venous volume-pressure relation using radionuclide plethysmography. Effect of nitroglycerin. Circulation. 1993;87:1142–1151. doi: 10.1161/01.cir.87.4.1142. [DOI] [PubMed] [Google Scholar]
- 17.Mastenbrook SM, Jr, Webster JG, Updike SJ. Venous return curves obtained from graded series of Valsalva maneuvers. Med Res Eng. 1977;12:20–29. [PubMed] [Google Scholar]
- 18.Montgomery LD, Hanish HM, Marker RA. An impedance device for study of multisegment hemodynamic changes during orthostatic stress. Aviat Space Environ Med. 1989;60:1116–1122. [PubMed] [Google Scholar]
- 19.Pang CC. Autonomic control of the venous system in health and disease: effects of drugs. Pharmacol Ther. 2001;90:179–230. doi: 10.1016/s0163-7258(01)00138-3. [DOI] [PubMed] [Google Scholar]
- 20.Porth CJ, Bamrah VS, Tristani FE, Smith JJ. The Valsalva maneuver: mechanisms and clinical implications. Heart Lung. 1984;13:507–518. [PubMed] [Google Scholar]
- 21.Rothe CF. Handbook of Physiology. The Cardiovascular System. Peripheral Circulation and Organ Blood Flow. pt. 1. III. Bethesda, MD: Am. Physiol. Soc; 1983. Venous system: physiology of the capacitance vessels; pp. 397–452. sect. 2. chapt. 13. [Google Scholar]
- 22.Rowell LB. Reflex control of regional circulations in humans. J Auton Nerv Syst. 1984;11:101–114. doi: 10.1016/0165-1838(84)90069-9. [DOI] [PubMed] [Google Scholar]
- 23.Shepherd JT, Vanhoutte PM. Role of the venous system in circulatory control. Mayo Clin Proc. 1978;53:247–255. [PubMed] [Google Scholar]
- 24.Singer W, Opfergehrking TL, McPhee BR, Hilz MJ, Low PA. Influence of posture on the Valsalva manoeuvre. Clin Sci (Colch) 2001;100:433–440. [PubMed] [Google Scholar]
- 25.Smith ML, Beightol LA, Fritsch-Yelle JM, Ellenbogen KA, Porter TR, Eckberg DL. Valsalva’s maneuver revisited: a quantitative method yielding insights into human autonomic control. Am J Physiol Heart Circ Physiol. 1996;271:H1240–H1249. doi: 10.1152/ajpheart.1996.271.3.H1240. [DOI] [PubMed] [Google Scholar]
- 26.Stewart JM, Lavin J, Weldon A. Orthostasis fails to produce active limb venoconstriction in adolescents. J Appl Physiol. 2001;91:1723–1729. doi: 10.1152/jappl.2001.91.4.1723. [DOI] [PubMed] [Google Scholar]
- 27.Stewart JM, Medow MA, Bassett B, Montgomery LD. Effects of thoracic blood volume on Valsalva maneuver. Am J Physiol Heart Circ Physiol. 2004;287:H798–H804. doi: 10.1152/ajpheart.01174.2003. [DOI] [PubMed] [Google Scholar]
- 28.Stewart JM, Montgomery LD. Regional blood volume and peripheral blood flow in the postural tachycardia syndrome. Am J Physiol Heart Circ Physiol. 2004;287:H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tyden G. Aspects of cardiovascular reflex control in man. An experimental study. Acta Physiol Scand Suppl. 1977;448:1–62. [PubMed] [Google Scholar]
- 30.White DD, Montgomery LD. Pelvic blood pooling of men and women during lower body negative pressure. Aviat Space Environ Med. 1996;67:555–559. [PubMed] [Google Scholar]