Abstract
A new cartilage-specific degradable hydrogel based on photoclickable thiol-ene PEG hydrogels is presented. The hydrogel crosslinks are composed of the peptide, CRDTEGE-ARGSVIDRC, derived from the aggrecanase-cleavable site in aggrecan. This new hydrogel is evaluated for use in cartilage tissue engineering by encapsulating bovine chondrocytes from different cell sources (skeletally immature (juvenile) and mature (adult) donors and adult cells stimulated with pro-inflammatory lipopolysaccharide (LPS)) and culturing for 12 weeks. Regardless of cell source, a two-fold decrease in compressive modulus is observed by 12 weeks, but without significant hydrogel swelling indicating limited bulk degradation. For juvenile cells, a connected matrix rich in aggrecan and collagen II, but minimal collagens I and X is observed. For adult cells, less matrix, but similar quality, is deposited. Aggrecanase activity is elevated, although without accelerating bulk hydrogel degradation. LPS further decreased matrix production, but did not affect aggrecanase activity. In contrast, matrix deposition in the non-degradable hydrogels consisted of aggrecan and collagens I, II and X, indicative of hypertrophic cartilage. Lastly, no inflammatory response in chondrocytes is observed by the aggrecanase-sensitive hydrogels. Overall, we demonstrate that this new aggrecanase-sensitive hydrogel, which is degradable by chondrocytes and promotes a hyaline-like engineered cartilage, is promising for cartilage regeneration.
Keywords: Biodegradation, peptide, hydrogel, poly(ethylene glycol), cartilage tissue engineering
1. Introduction
Autologous chondrocyte implantation (ACI)[1–3] has shown some success in younger (< 35 years) patients,[4] but has poor clinical outcomes in older patients.[1,4] Matrix-assisted ACI (MACI)[5] utilizes a matrix (i.e., scaffold) to deliver chondrocytes and provide a 3D environment for the cells in vivo. The advent of MACI brings the opportunity to expand ACI therapies, via tailoring the scaffold, to patients from a broad age range. The poor success of ACI with older patients is in part attributed to slow tissue synthesis concomitant with elevated tissue degrading activity that leads to an imbalance between anabolism and catabolism.[6–8] Therefore scaffold development must consider and adapt to a wide range of cellular activity in order to advance MACI and expand its indication to older patients.
Synthetic hydrogels derived from poly(ethylene glycol) (PEG) offer a platform for tuning a wide range of scaffold properties while simultaneously maintaining the chondrocyte phenotype and supporting cartilage-specific matrix production.[9–14] Hydrogel degradation is a critical design parameter for matrix elaboration, where non-degrading hydrogels restrict matrix deposition and evolution to the immediate vicinity around cells.[9] Hydrolytically degradable PEG hydrogels that incorporate oligo(lactic acid) into the crosslinks are able to promote macroscopic tissue deposition by chondrocytes,[12,13,15–17] but this mode of bulk degradation is characterized by hydrogel swelling, decreased scaffold mechanics and a significant loss of synthesized matrix from the constructs.[18,19] One of the challenges with using bulk degrading hydrogels is that many of the cartilage-specific extracellular matrix (ECM) molecules are large, and much larger than the mesh size of a hydrogel. As a result, hydrogels must come close to their reverse gelation point before many of the ECM molecules can diffuse and form macroscopic tissue.[15] This requirement means that there is a narrow window for tuning degradation rate with tissue synthesis and elaboration. Identifying this window for a wide range of donors presents a significant design challenge.
Hydrogels that degrade locally through cell-mediated degradation, however, may offer a mechanism to improve matrix elaboration by entrapped chondrocytes and overcome the shortcomings of bulk degrading hydrogels.[20,21] PEG hydrogels with enzyme-sensitive peptides incorporated into the crosslinks have been developed[22–27] and a few studies have investigated them for cartilage regeneration. The first report by Park et al.[28] demonstrated that PEG hydrogels with a matrix metalloproteinase (MMP)-sensitive peptide derived from collagen increased collagen II and aggrecan gene expression in bovine chondrocytes, but matrix deposition remained pericellularly restricted after four weeks in vitro. Bahney et al.[29] incorporated a peptide sensitive to MMP-7 into PEG hydrogels, which is upregulated during chondrogenesis of human mesenchymal stem cells, and showed an elaborated macroscopic tissue rich in collagen II after twelve weeks in vitro. While promising, there remains a need to further develop enzyme sensitive hydrogels for primary chondrocytes, which can be used in conjunction with MACI.
In this paper, we describe a new cartilage-specific enzymatically sensitive PEG hydrogel, which was designed based on chondrocyte activity observed in vivo and in vitro. Cartilage is composed mainly of elastic collagen II fibrils and proteoglycans (primarily aggrecan), and is sparsely populated with chondrocytes that interact with and process the extracellular matrix.[30] In vivo, aggrecan is turned over much more rapidly than collagen II;[31] and therefore we chose to target aggrecan catabolism for the design of a new enzymatically degradable peptide. The aggrecan proteoglycan is composed of a linear core protein with three globular domains, where negatively charged sulfated glycosaminoglycan (sGAG) sidechains are bound to the core protein between globular domains 2 and 3 (G2 and G3).[31,32] The region between G1 and G2 is termed the ‘interglobular domain’ (IGD), and contains the two main sites of aggrecan proteolysis by either MMPs or aggrecanases (A Disintegrin And Metalloproteinase domain with ThromboSpondin motifs, namely ADAMTS-4 and -5, also known as aggrecanase-1 and -2, respectively).[33] Aggrecanases cleave the IGD more efficiently than MMPs.[34–36] Therefore, we designed an aggrecanase-sensitive peptide based on its specific cleavage site within the IGD. The amino acid sequence TEGE-ARGSVI surrounding the E373-A374 cleavage site is conserved between human and bovine aggrecan,[37] and we flanked this sequence with ‘RD’ moieties to improve solubility[38] and thiol-containing cysteines to permit crosslinking, resulting in the final sequence CRDTEGE-ARGSVIDRC. This crosslinker was incorporated into a photoclickable thiol-ene PEG hydrogel, where the network structure has been well described previously,[23] to create a new aggrecanase-sensitive hydrogel. We investigated this new hydrogel for cartilage tissue engineering using several bovine chondrocyte sources, which have been shown to exhibit different anabolic and catabolic characteristics in prior reports.[7,18,39,40] We evaluated scaffold modulus and matrix production, elaboration and catabolism over the course of twelve weeks in the aggrecanase-sensitive hydrogels and compared them to non-degradable hydrogels crosslinked with PEG-dithiol.
2. Results
2.1. Cell-mediated Degradation of Acellular Hydrogels
A photoclickable PEG-based hydrogel was formed by reacting 8-arm PEG-amide-norbornene (8-armPEG-NB) macromer (Figure 1A) with either non-degrading PEG-dithiol (PEGdSH) to form a stable hydrogel or the aggrecanase sensitive peptide (Figure 1B, C) to form a degradable hydrogel (Figure 1D). Degradability of the aggrecanase sensitive crosslinker by bovine chondrocytes was investigated by incubating acellular degradable hydrogels in medium conditioned by adult chondrocytes stimulated with the inflammatory mediator, LPS (Figure 1E). Hydrogel wet weight (n = 3 – 4) was measured as an indicator of hydrogel degradation. Compared to fresh chondrocyte medium, cell-conditioned medium led to an 8% increase (p = 0.02) in hydrogel wet weight after 7 hours, from 100 ± 1 to 108 ± 2 mg, confirming cell-mediated degradation.
Figure 1.
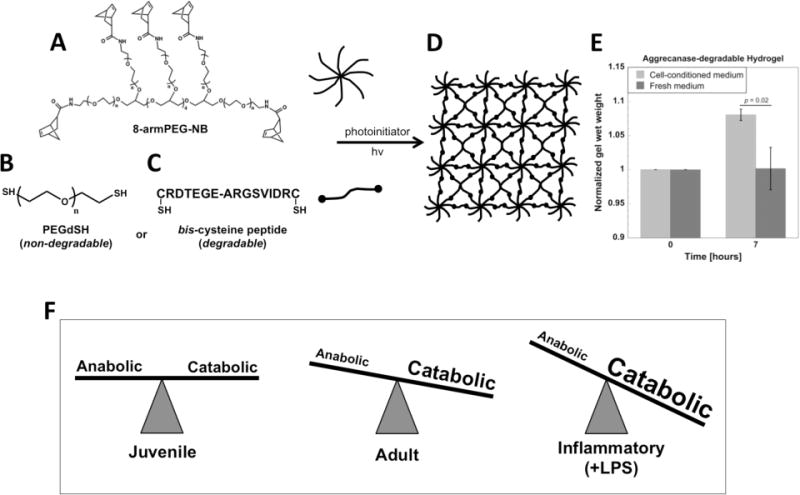
Schematic of hydrogel formation. (A) 8-arm PEG-amide-norbornene (8-armPEG-NB, 20 kDa) is crosslinked with (B) non-degradable PEG-dithiol (PEGdSH, 1000 Da) or (C) aggrecanase-sensitive peptide (CRDTEGE-ARGSVIDRC, 1767 Da) in the presence of photoinitiator and 365 nm light to create (D) a 3D crosslinked hydrogel network within which cells can be encapsulated. (E) Hydrogel degradability was confirmed after 7 hours by incubating acellular hydrogels in adult chondrocyte-conditioned medium (n = 3 – 4). (F) Bovine chondrocytes from three distinct cell sources were studied and included chondrocytes isolated from juvenile and adult donors and adult chondrocytes stimulated with pro-inflammatory lipopolysaccharide (LPS). Adult chondrocytes have been previously reported to exhibit higher catabolic activity compared to juvenile chondrocytes[18] and LPS stimulation enhances catabolic activity in adult chondrocytes.[40]
2.2. Characterization of Cell-laden Hydrogels: Viability, Cellularity, and Bulk Properties
To investigate the applicability of aggrecanase-sensitive hydrogels for cartilage tissue engineering, bovine chondrocytes from three distinct cell sources (Figure 1F) were encapsulated into aggrecanase-degradable and non-degrading PEG hydrogels with the same initial compressive moduli and cultured for 12 weeks. Viable chondrocytes were present in all hydrogel formulations throughout the 12 week culture period and was similar for each cell source and hydrogel formulation. The addition of LPS to the culture medium did not appear to adversely affect cell viability throughout the study. A representative confocal microscopy image from each hydrogel is shown at week 3 (Figure 2A), which were similar throughout the study. Cell number per construct (Figure 2B), however, was affected (p < 0.0001) by time and condition. Most notably the number of juvenile cells per construct for the degrading hydrogel was maintained throughout the culture period. On the contrary, all other conditions led to an overall increase in cell number by 50 – 85% by week 12.
Figure 2.
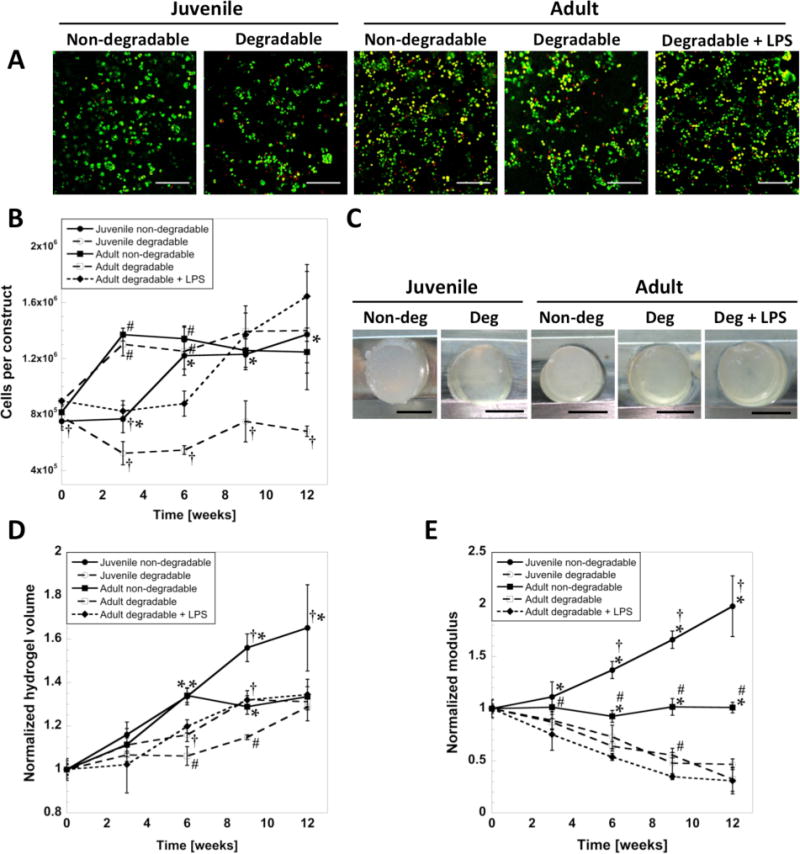
(A) Viability of encapsulated cells after 3 weeks of culture in non-degradable or enzymatically degradable hydrogels. Live cells are green, dead cells are red. Scale bars are 200 μm. (B) Cell number per construct. (C) Representative photographs of hydrogels taken at 12 weeks. Scale bars are 5 mm. (D) Hydrogel volume and (E) compressive modulus normalized to measurements from one day after encapsulation. The initial compressive modulus was similar for all conditions, 13 ± 3 kPa at day one. * indicates significant difference from degradable hydrogels (at same cell age). † indicates significant difference from adult cells (same hydrogel condition). # indicates significant difference from LPS condition (adult cells only) (p < 0.05). Error bars are standard deviation (n = 3).
Hydrogel bulk properties were measured over 12 weeks in the non-degrading and degrading hydrogels in order to assess the overall contributions of matrix deposition and hydrogel degradation. Representative images of hydrogels are shown at 12 weeks (Figure 2C). Hydrogel volume normalized to day 1 (Figure 2D) increased (p < 0.0001) with time for all conditions. At week 12, hydrogel volume was greatest for the juvenile chondrocytes in the non-degrading hydrogel, while all other conditions were similar. Hydrogel volume was higher (p < 0.001) in the LPS condition at weeks 6 and 9 when compared to the untreated hydrogel for the adult cell source. The tangent compressive modulus of the constructs (Figure 2E), which measured 13 ± 3 kPa at day 1 for all conditions, decreased (p < 0.0002) with time by ~50% for enzymatically degradable hydrogels regardless of cell source and treatment, but doubled (p = 0.0001) for juvenile cells in non-degradable hydrogels over 12 weeks.
2.3. sGAG and Aggrecan Production and Deposition
The ability of encapsulated chondrocytes to produce and deposit the cartilaginous ECM molecule aggrecan and its associated sGAGs was assessed quantitatively by measuring cumulative sGAG production over the course of 12 weeks, and spatially by immunohistochemistry. sGAG production per cell (Figure 3A) is shown as the amount in the hydrogels normalized to cell number at 12 weeks and as the cumulative amount released to the medium throughout 12 weeks of culture, with the latter normalized to the corresponding cell number at each time point. The sum of the two amounts represents the total cumulative sGAG produced on average per cell over 12 weeks. The amount in the constructs on a cellular basis was lower (p < 0.0001 for juvenile cells, p < 0.006 for adult cells) in the enzymatically degradable hydrogels, which is supported by the Safranin-O staining for sGAG (Figure 3B). However, the sGAG released to the medium was elevated (p = 0.004) in the enzymatically degradable hydrogels for juvenile cells, but was similar for adult chondrocytes. For adult cells, LPS treatment led to a similar amount of sGAG in the constructs per cell, but the amount released was lower (p < 0.0001) compared to no treatment. Spatially sGAG deposition was present throughout the constructs in the non-degradable hydrogels for both cell sources. In the degradable hydrogels, sGAG was more localized to the regions adjacent to the cells (i.e., pericelluarly) for the juvenile cell source and even more restricted pericellularly for the adult cell source. Aggrecan deposition was present for all cell sources (Figure 3C). For juvenile cells, there was evidence of aggrecan matrix connectivity in the enzymatically degradable hydrogels at 12 weeks, which was not observed in the non-degrading hydrogels. For adult cells, aggrecan deposition was restricted to pericellular regions for both hydrogels and did not appear to be affected by LPS.
Figure 3.
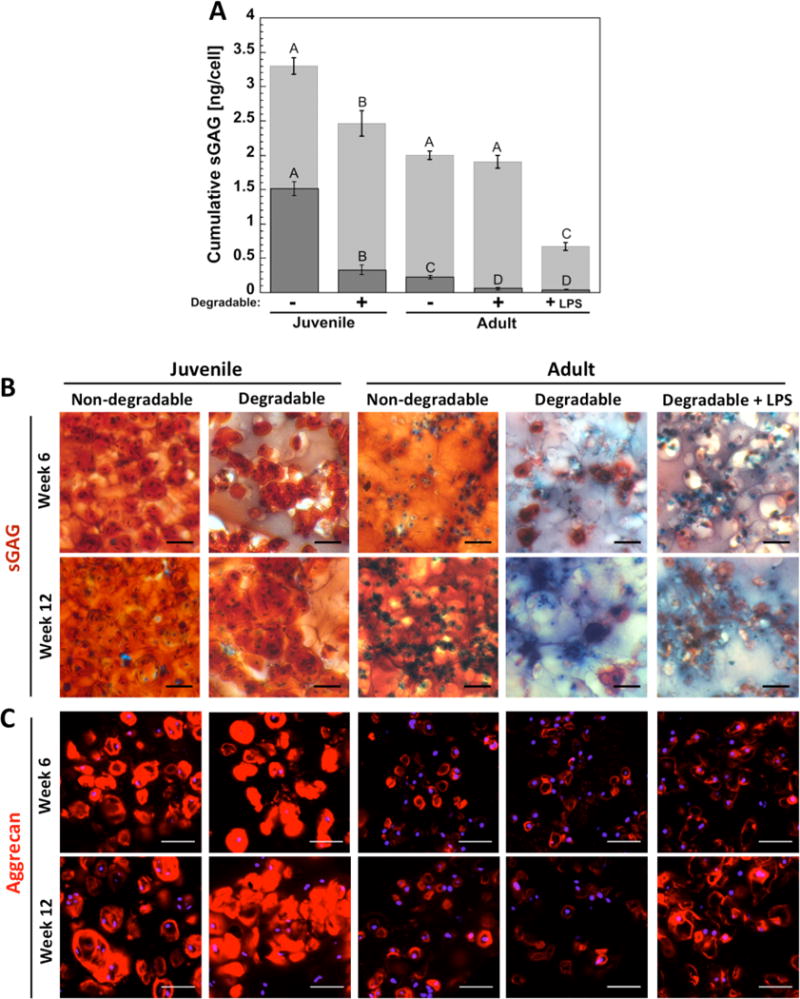
(A) sGAG production per cell in the constructs at 12 weeks is shown by the (Image) bar and that which was released to the medium throughout 12 weeks is shown by the (Image) bar. The sum of the two represents on average the total sGAG production per cell over the entire 12 weeks. Letter groupings show statistical similarities (same letter) and differences (different letters) (p < 0.05). Top letters are for cumulative sGAG from medium and lower letters are for cumulative sGAG in constructs. Error bars are standard deviation (n = 3). (B) Representative images of histological staining for sulfated GAG (sGAG, orange-red) at weeks 6 and 12 with Safranin-O/Fast Green. Background proteins are blue, nuclei are dark blue-purple, scale bars are 50 μm. (C) Representative confocal microscopy images from immunohistochemistry staining for aggrecan (red) in hydrogels at weeks 6 and 12. Nuclei are blue, scale bars are 50 μm.
2.4. Collagen Production, Deposition and Degradation
Collagen production was assessed quantitatively by the production of the total collagens over the course of 12 weeks and qualitatively for the specific type of collagen by immunohistochemistry. Collagen production per cell (Figure 4A) is shown as the amount in the hydrogels normalized to cell number at 12 weeks and as the cumulative amount released to the medium throughout 12 weeks of culture normalized to corresponding cell number at each time point. The sum of the two amounts represents the total cumulative collagen produced on average per cell over 12 weeks. The amount in the constructs on a per cell basis was highest (p < 0.0003) for juvenile cells in non-degrading hydrogels, but enzymatically degradable hydrogels showed greater (p < 0.0001) collagen release to the medium by juvenile cells. Deposition of collagen II, the most abundant collagen type found in articular cartilage (Figure 4B), was present for all cell sources. For juvenile chondrocytes, there was evidence of collagen II matrix connectivity at 12 weeks in the enzymatically degradable hydrogel, which was not observed in the non-degradable hydrogels. For adult cells, collagen II deposition was restricted pericellularly for both hydrogels and did not appear to be affected by LPS. Collagen degradation was assessed by probing for the C1,2C degraded collagen neoepitope, which was present in all conditions. There was evidence of greater C1,2C staining in the degradable hydrogels compared to the non-degradable hydrogels for the juvenile cells, but no large differences among the adult cells.
Figure 4.
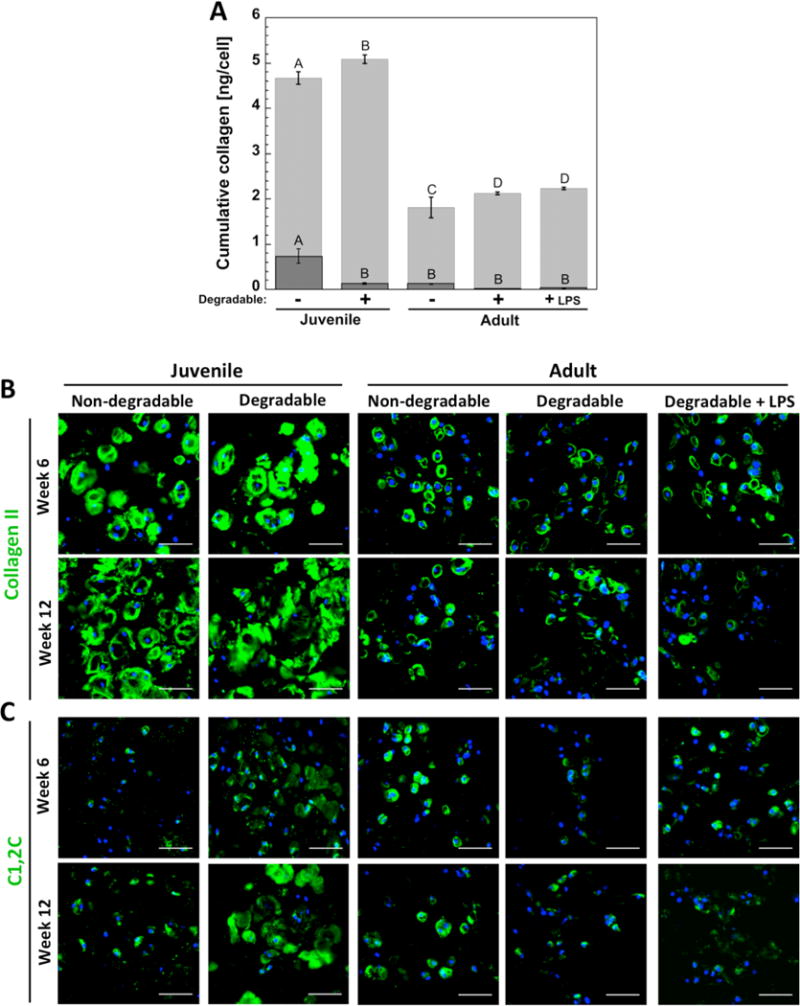
(A) Total collagen production per cell in the constructs at 12 weeks is shown by the (Image) bar and that which was released to the medium throughout 12 weeks is shown by the (Image) bar. The sum of the two represents on average the total collagen production per cell over the entire 12 weeks. Letter groupings show statistical similarities (same letter) and differences (different letters) (p < 0.05). Top letters are for total collagen from medium and lower letters are for total collagen in constructs. Error bars are standard deviation (n = 3). (B) Representative confocal microscopy images of immunohistochemical staining for collagen II (green) and (C) collagenase-generated collagen neoepitope C1,2C (green) in hydrogels at weeks 6 and 12. Nuclei are blue, scale bars are 50 μm.
Collagens I and X were examined in the constructs at weeks 6 and 12 (Figure 5A, B) in order to assess deposition of collagens found in fibrocartilage and hypertrophic cartilage, respectively, which would indicate a loss of the articular chondrocyte phenotype. Deposition of collagens I and X was present in all cell sources and hydrogels. Staining was most intense in non-degradable hydrogels for both cell sources.
Figure 5.
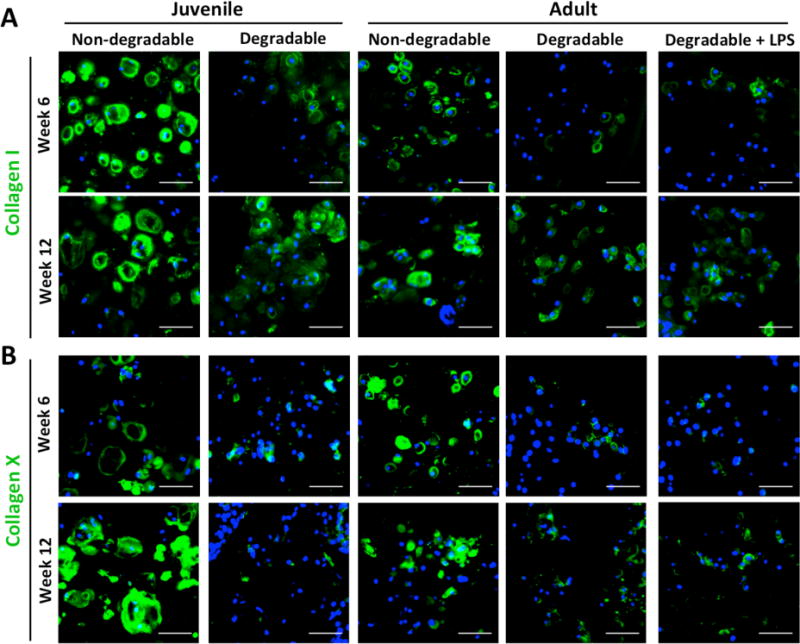
(A) Representative confocal microscopy images of immunohistochemical staining for collagen I (green) and (B) collagen X (green) in hydrogels at weeks 6 and 12. Nuclei are blue, scale bars are 50 μm.
2.5. Matrix Degrading Enzyme Activity and IL-6 Secretion
To determine whether incorporating aggrecanase-degradable peptides into the hydrogel crosslinks could affect catabolic activity or promote chondrocyte inflammation, enzyme activity and interleukin-6 (IL-6) secretion were measured. Aggrecanase-1 and generic MMP activities per cell are shown as the active amount of enzyme measured over all time points within the constructs and in the culture medium (Figure 6A & B, respectively). The sum of the amount in the constructs and in the medium represents the total amount of active enzyme produced on average per cell over 12 weeks. The amount of each active enzyme at each time interval is shown in Figures S1 and S2, respectively. For juvenile cells, degradable hydrogels led to lower (p < 0.0001) aggrecanase-1 and total MMP activity in the constructs, but greater (p < 0.0001) activity in the culture medium when compared to non-degradable hydrogels. Aggrecanase-1 and total MMP activities measured in the constructs were higher (p < 0.0001) for adult cells compared to juvenile cells. For adult cells, aggrecanase-1 activity was not affected by hydrogel degradation, but MMP activity was lower (p = 0.02) in the constructs of the degradable hydrogels. LPS led to the highest (p < 0.004 and p < 0.0001, respectively) aggrecanase-1 and MMP activities measured in the medium. However in the constructs, aggrecanase-1 and MMP activities were lower (p < 0.0001) with LPS treatment. Inflammatory IL-6 secretion is shown as the cumulative quantity measured in the culture medium over 12 weeks (Figure 6C) and the quantities measured at each time interval are shown in figure S3, demonstrating increased IL-6 secretion from adult chondrocytes at 1 day compared to juvenile chondrocytes. There was more (p < 0.0001) IL-6 produced by adult cells than juvenile cells, but hydrogel degradation did not affect IL-6 secretion for either cell source. LPS stimulated the highest (p < 0.0001) amount of IL-6 secretion.
Figure 6.
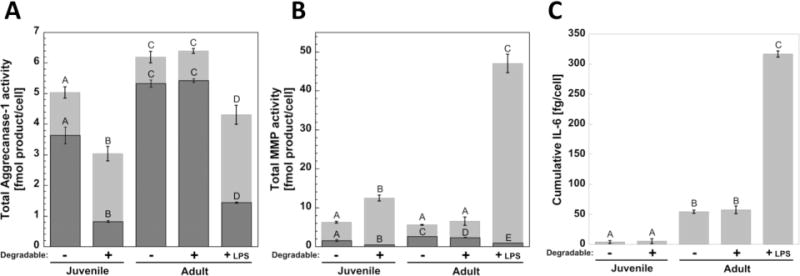
Activity of (A) aggrecanase-1 and (B) generic MMPs measured per cell within the constructs is shown by the (Image) bar and released to the culture medium is shown by the (Image) bar. The sum of the active enzyme in the constructs and released to the medium represents on average the total amount of active enzyme that was produced per cell over the entire 12 weeks. (C) Cumulative IL-6, shown as the additive quantity measured in the medium throughout 12 weeks. Letter groupings show statistical similarities (same letter) and differences (different letters) (p < 0.05). Top letters are for total activity in medium and lower letters are for total activity in constructs. Error bars are standard deviation (n = 3).
3. Discussion
A new aggrecanase-degradable hydrogel was developed and tested with three bovine chondrocyte sources, which exhibited differences in metabolic activity as confirmed in this study. We demonstrated that hydrogel degradation occurs in the presence of cell-conditioned medium, indicating that cells are able to degrade the hydrogel. Regardless of chondrocyte source, enzymatically degradable hydrogels promoted cartilage-specific matrix deposition rich in aggrecan and collagen II with reduced collagen I and minimal collagen X. We confirmed that degradation of the aggrecanase-sensitive hydrogel, which leads to exposed peptide fragments, did not elevate aggrecanase activity or elevate chondrocyte secretion of pro-inflammatory cytokines (specifically IL-6). Overall, we present a new and promising enzyme-degradable hydrogel for cartilage tissue engineering.
Juvenile chondrocytes, representing a cell source that in native tissue exhibits a homeostatic balance between anabolic and catabolic activity,[7] abundantly produced and deposited sGAGs and collagens when encapsulated in both aggrecanase-sensitive hydrogels and non-degradable hydrogels. In non-degrading hydrogels, matrix accumulated pericellularly resulting in a two-fold increase in modulus by twelve weeks. Hydrogel degradation, however, led to higher matrix release concomitant with lower matrix retention within hydrogels, which is consistent with the decrease in modulus over time. This finding suggests that enzymes were able to diffuse through the hydrogel leading to bulk degradation, which is supported by the presence of active MMPs and aggrecanases in the culture medium. Although matrix deposition within the hydrogel was lower with degradation, the quality of the engineered tissue was markedly improved. Most notably, the degradable hydrogels promoted an engineered tissue that was more consistent with hyaline cartilage, while the engineered tissue in the non-degradable hydrogels was composed of collagens I, II and X, characteristic of hypertrophic cartilage. We attribute this improved cartilaginous phenotype in the degradable hydrogels to several possibilities. The structure and mechanics of the pericellular matrix will evolve as the hydrogel degrades locally around the cells and therefore may impact cellular signaling.[41,42] The presence of peptide crosslinks, which better mimics native tissue environments, may improve the chondrocyte phenotype, which has been observed in self-assembling peptide hydrogels.[43,44] In addition, connectivity of deposited aggrecan and collagen II was evident by 12 weeks in the degradable hydrogel, but not in the non-degradable hydrogel. Because aggrecan and collagen molecules are very large, connectivity can only occur after reverse gelation happens when a sufficient number of crosslinks have been broken and the polymer dissolves. These observations therefore suggest that juvenile chondrocytes are able to degrade the aggrecanase-sensitive hydrogels both locally around the cells and in the bulk and that this degradation is critical to producing a hyaline-like engineered cartilage.
Adult chondrocytes represent a cell source with lower anabolic, but higher catabolic activity compared to juvenile chondrocytes[7,18] which was confirmed here with lower sGAG and collagen contents, higher aggercanase activity and higher IL-6 secretion compared to the juvenile chondrocytes. In the non-degrading hydrogels, modulus was maintained over 12 weeks for adult chondrocytes, but was lower when compared to the juvenile chondrocytes, which is consistent with less matrix deposition in the hydrogel. While the modulus decreased in the degradable hydrogels, the quality of the engineered tissue was improved and similar to that for the juvenile chondrocytes. Specifically, a hyaline-like engineered cartilage was formed in the degradable hydrogels while a hypertrophic-like engineered cartilage was formed in the non-degradable hydrogels. Interestingly, degradation impacted the spatial distribution of sGAGs resulting in few sGAG detected in the extracellular space of the hydrogel. While most sGAG molecules in cartilage are attached to the aggrecan core protein, the sGAG-rich domain in aggrecan is readily degraded,[33] releasing smaller sGAG-laden aggrecan fragments into the extracellular space[45] where they can either remain within or diffuse out of the hydrogel. In the degrading hydrogels, there was more aggrecanase detected in the construct with adult cells than juvenile cells, which is consistent with chondrocytes isolated from older donors.[18,46,47] It is possible that more of the aggrecan secreted by the adult cells was processed and degraded into smaller sGAG fragments. We therefore attribute the lack of sGAG staining in the extracellular space to the presence of smaller sGAG fragments, which rapidly diffuse out of the hydrogel as the mesh size increases from hydrogel degradation. This observation is further confirmed by the large of amount of sGAG released to the culture medium. Tissue connectivity was not observed with the adult cells, which is attributed to their lower matrix synthesis rates, and therefore hydrogels with adult cells may require longer culture times.
As crosslinks in the aggrecanase-sensitive hydrogels are cleaved, cells will be exposed to peptide fragments for some period of time until a sufficient number of crosslinks are broken to release the polymer chains. ECM fragments have been shown to elicit catabolic responses in chondrocytes. For example, degraded fibronectin fragments have been shown to stimulate aggrecanase catabolism of aggrecan[48] and aggrecanase activity is elevated in osteoarthritis.[49,50] Therefore, it was important to determine whether hydrogel degradation and exposure to peptide fragments negatively affect the encapsulated cells by eliciting elevated catabolism and/or an inflammatory response.
Aggrecanase-degradable hydrogels did not increase aggrecanase activity for either adult or juvenile chondrocytes. Generic MMP activity was assessed to probe for more general cartilage catabolism, which is necessary for tissue homeostasis, but can be increased in disease and inflammatory states.[30] Hydrogel degradability increased MMP activity in juvenile chondrocytes, but not adult chondrocytes. This finding is supported by the intense staining for the C1,2C epitope associated with MMP-cleaved collagen in the degradable hydrogels with juvenile chondrocytes, especially at week 12. As deposited ECM diffuses further into the extracellular space, it is possible that the cells begin to remodel the tissue by elevating MMP activity. This observation is supported by the fact that MMP levels were higher compared to non-degradable hydrogels after 6 weeks when ECM transitioned from primarily pericellular at 6 weeks to interconnected by 12 weeks. In addition, others have reported that MMPs contribute to the mechanical properties of engineered cartilage.[51] The pro-inflammatory cytokine, IL-6, was also assessed as an indicator for an inflammatory response, which has been shown to cause proteoglycan release from human articular cartilage,[52] to be upregulated with osteoarthritis,[53] and to lead to increased aggrecanase-mediated degradation of aggrecan.[54] While IL-6 secretion was higher for adult compared to juvenile chondrocytes, it was unaffected by hydrogel degradation.
These findings suggest that the aggrecanase-degradable hydrogel does not have adverse effects on the encapsulated cells. However, it is important to note that aggrecanase-1 (ADAMTS-4) activity was measured in this study, but aggrecanase-2 (ADAMTS-5) is also secreted by chondrocytes and elevated in osteoarthritis.[52] Aggrecanase-2 is capable of cleaving the E373-A374 site on aggrecan[55] similar to aggrecanase-1, but at a much slower rate.[56] Therefore, it is likely that the enzyme activity assay, which uses a peptide that spans this sequence, would have detected aggrecanase-2 activity. We recognize that the generic MMP assay does not differentiate between different MMPs and therefore changes in the type of MMP activity may have occurred in the degradable hydrogel, but which were not detected. Although hydrogel degradation was attributed mainly to aggrecanases, it was surprising that cells with different aggrecanase activities led to similar changes in the hydrogel modulus over time (an indication of hydrogel degradation). While adult chondrocytes produced more active aggrecanase than juvenile chondrocytes, the majority of the aggrecanase was detected within the hydrogels and therefore could be more cell-associated and unable to diffuse. Additionally, aggrecanase released from cells can degrade either peptide crosslinks or aggrecan produced by the chondrocytes. Studies have shown that the glycosylation of native aggrecan can enhance degradation rates,[57,58] and as a result aggrecan produced by adult chondrocytes may be even more susceptible to degradation due to increased keratan sulfate content, which increases degradation rates.[58] Therefore even if overall aggrecanase activity was elevated, the adult chondrocyte-produced aggrecan may be more reactive than the peptide crosslinks.
Adult chondrocytes were exposed to LPS to represent a cell source with even higher catabolic activity relative to anabolic activity,[39,40] which was confirmed in this study. Specifically, LPS led to a significant up-regulation of MMP activity and interleukin-6 secretion, confirming its inflammatory effect on chondrocytes. In addition, LPS led to decreased sGAGs synthesis, which is consistent with previous observations.[59] Interestingly though, LPS did not affect the amount of sGAGs retained in the hydrogel nor the spatial distribution of sGAGs or aggrecan. LPS also did not affect total collagen production or the quality of the engineered tissue with respect to collagen type. The compressive modulus, however, was consistently lower than the modulus of constructs with unstimulated adult chondrocytes, but this difference was only significant at 9 weeks. It is interesting to note that C1,2C staining appeared more intense at the six week time point, which is consistent with the high MMP activity and IL-6 secretion. IL-6 secretion was the highest at weeks 6 and 9 and began to drop after week 9, suggesting that the inflammatory effect of LPS subsided, although MMP activity remained high throughout the study. Interestingly, aggrecanase was down-regulated by LPS, which may in part be related to the lower sGAG production. Previous work showed that LPS stimulation upregulated aggrecanase-1 gene expression by chondrocytes[39] with 1000 ng ml−1 LPS, albeit at non-physiologically relevant levels, which is 100-fold higher than the concentration used in this study. Nonetheless, these findings suggest the aggrecanase-sensitive hydrogel constructs consistently exposed to an inflammatory stimulant do not experience accelerated degradation and are capable of supporting neotissue deposition.
Overall, the aggrecanase-sensitive hydrogels exhibit improvements over more traditional hydrolytic and bulk degrading hydrogels. Although hydrogel degradation led to a 2-fold decrease in compressive modulus over twelve weeks, the decrease was much less dramatic when compared to hydrolytically degrading PEG hydrogels where an 8-fold decrease in modulus was reported after only four weeks.[60] In addition, hydrogel degradation did not appear to increase hydrogel volume. These findings suggest a degradation behavior that is a combination of both bulk and localized around the cell, which is distinctly different from traditional hydrolytically degrading hydrogels. However, one shortcoming of degrading hydrogels regardless of the type of degradation mechanisms (enzymatic or hydrolysis) is the loss of matrix molecules with hydrogel degradation. One of the promising attributes of this type of hydrogel system with enzyme sensitive crosslinks is that degradation behavior and rate can be further optimized to minimize bulk degradation, which is a current focus of our research. For example, increasing the initial hydrogel crosslinking density (and thereby increasing compressive modulus) or making slight changes to the peptide sequence to alter the degradation kinetics[25] can shift the degradation behavior even more towards localized degradation to minimize matrix loss due to bulk degradation.[20,21] However, one of the design challenges is that many of the enzyme sensitive peptide sequences can be cleaved by multiple enzymes.[25] For example the hydrogel in this study was designed as an aggrecanase-sensitive hydrogel, but the aggrecanase targeted sequence E373-A374 on aggrecan can be cleaved by MMPs including MMP-8, but with decreased kinetics compared to aggrecanases.[61]
4. Conclusion
The presented work details a new aggrecanase-sensitive hydrogel showing promise for cartilage tissue engineering by preserving the chondrocyte phenotype and promoting a hyaline-like engineered cartilage tissue produced by both juvenile and adult chondrocytes with minimal collagens I and X. Even though the half-lives of aggrecan and collagen molecules are on the order of 15–100 days and 100 years, respectively,[31,62,63] encapsulated cells were able to degrade the aggrecanase-sensitive hydrogel. The efficacy of the degradable hydrogel was dependent on the cell source, where juvenile chondrocytes produced an engineered tissue exhibiting matrix connectivity, which was not evident in adult cells with or without inflammatory stimulation, which showed increased catabolic activity and decreased anabolic activity. Nonetheless, we demonstrate the promise for aggrecanase-sensitive hydrogels in cartilage tissue engineering and future efforts will focus on further tailoring the degradation rates and localization, as well as combining with mechanical stimulation, which is important in cartilage homeostasis[17,64–66] and may influence expression and production of catabolic enzymes.[67,68] These strategies may further improve matrix elaboration, matrix deposition, and overall modulus of hydrogels with encapsulated cells from patients spanning a wide range of ages.
5. Experimental Section
Materials
8-arm PEG amine (MW 20,000) was from JenKem Technology USA (Allen, TX). O-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate (HATU) and N,N′-diisopropylethylamine (DIEA) were from Chem-Impex International, Inc. (Wood Dale, IL). Hoechst 33258 was from Polysciences, Inc. (Warrington, PA). Collagenase type II, pepsin A, and papain were from Worthington Biochemical (Lakewood, NJ). Ethyl ether, N,N-dimethylformamide (DMF), bovine IL-6 ELISA kit, and Triton X-100 were from Fisher Scientific (Fair Lawn, NJ). SpectraPor 7 1000 MWCO dialysis tubing was from Spectrum Labs (Rancho Dominguez, CA). The custom aggrecanase-degradable peptide (CRDTEGE-ARGSVIDRC) was synthesized by GenScript (Piscataway, NJ). Irgacure 2959 was from Ciba Specialty Chemicals (Tarrytown, NY). Fetal bovine serum (FBS) was from Atlanta Biologicals (Lawrenceville, GA). The LIVE/DEAD® assay, phosphate-buffered saline (PBS), DMEM, penicillin-streptomycin (P/S), fungizone, gentamicin, HEPES buffer, GlutaGro (L-glutamine), minimal essential medium non-essential amino acids (MEM-NEAA), trypsin-EDTA, trypan blue, DAPI, AlexaFluor 488-conjugated goat anti-rabbit IgG, and AlexaFluor 546-conjugated goat anti-mouse IgG were from Invitrogen (Carlsbad, CA). 5-norbornene-2-carboxylic acid, PEG-dithiol, L-proline, L-ascorbic acid, bovine serum albumin (BSA), dimethyl methylene blue (DMMB), lipopolysaccharides (LPS) from S. enterica, chondroitinase ABC, hyaluronidase, and protease from Streptomyces griseus were from Sigma-Aldrich (St. Louis, MO). Keratanase I was from MP Biomedical (Solon, OH). Mouse anti-aggrecan antibody (A1059-53E) and rabbit anti-collagen II antibody (C5710-20F) were from US Biologicals (Swampscott, MA). Rabbit anti-collagen I (ab34710), and X (ab58632) antibodies were from Abcam (Cambridge, MA). Rabbit anti-C1,2C (collagenase-generated collagen neoepitope) antibody (50-1035) was from IBEX Pharmaceuticals (Quebec, Canada). Generic MMP and aggrecanase-1 SensoLyte™ assay kits were from Anaspec (Fremont, CA). Human ADAMTS-4 was from Millipore (Billerica, MA). Retrievagen A antigen retrieval solution was from BD Biosciences (San Jose, CA).
Macromer Synthesis
8-armPEG-NB was synthesized by reacting 8-arm PEG amine (MW 20,000) with norbornene acid.[23,69,70] Briefly, norbornene acid (8x molar excess per amine-terminated PEG arms) in DMF was activated by reacting for 5 minutes under argon with HATU (4x excess) and DIEA (4x excess) at room temperature. The activated norbornene mixture was combined with 8-arm PEG amine in DMF, and the reaction proceeded overnight under argon at room temperature. 8-armPEG-NB was recovered by precipitation in ethyl ether, purified by dialyzing against DI H2O for 2–3 days, filtered (0.2 μm) and finally lyophilized. Using 1H-NMR spectroscopy, norbornene conjugation (δ = 5.9 – 6.3 ppm) per arm of the 8-arm PEG molecule (δ = 3.4 – 3.9 ppm) was determined to be on average 100%.
Chondrocyte Isolation
Bovine articular chondrocytes were isolated from the metacarpal-phalangeal joints of four skeletally mature (1–2 year old) steers (Arapahoe Meat Co., Lafayette, CO), which are referred to as adult chondrocytes. Bovine articular chondrocytes were also isolated from the femoral-patellar groove and articular condyles of a skeletally immature (1–3 week old) calf (Research 87, Marlborough, MA), which are referred to as juvenile chondrocytes. In quadrupeds, both joints are load bearing and characterized by hyaline cartilage.[71] Briefly, cartilage slices were washed in PBS supplemented with 1% P/S, 0.5 μg ml−1 fungizone and 20 μg ml−1 gentamicin (PBS-antis), and digested 16 hours at 37 °C in 0.2% collagenase II in DMEM with 5% FBS. Isolated chondrocytes were washed in PBS-antis + 0.02% EDTA, pelleted followed by PBS-antis and then passed through a 100 μm cell strainer. Cells were maintained in chondrocyte medium (DMEM supplemented with 10% FBS, 1% P/S, 10 mM HEPES, 0.1 M MEM-NEAA, 0.4 μM L-proline, 50 μg ml−1 L-ascorbic acid, 4 mM L-glutamine, 0.5 μg ml−1 fungizone and 20 μg ml−1 gentamicin). Using the trypan blue exclusion assay, initial cell viabilities were 92% for adult chondrocytes and 87% for juvenile chondrocytes.
Hydrogel Formation
A 10% w/w macromer solution of 8-armPEG-NB and crosslinker (non-degradable PEGdSH, MW 1000; or degradable bis-cysteine peptide, CRDTEGE-ARGSVIDRC; at 0.45:1 or 0.65:1 thiol:norbornene molar ratio, respectively) was prepared in chondrocyte medium. The thiol:norbornene ratios were chosen to yield hydrogels with similar initial compressive moduli, and hence similar initial crosslink density, after swelling overnight in the absence of cells. The thiol:norbornene ratios are different because of the slower reactivity of the thiol in the cysteine compared to the thiol on PEG.[72] Chondrocytes were mixed with macromer solution at 50 million cells ml−1 and photopolymerized with 0.05% w/w Irgacure 2959 into cylindrical constructs (5 mm diameter × 2 mm height) for 7 min with 365 nm light (6 mW cm−2). Each construct was cultured in 4 ml chondrocyte medium (replaced twice per week) for up to 12 weeks at 37 °C in 5% CO2. Conditioned medium was collected, snap frozen in liquid nitrogen, stored at −80 °C, and pooled together in three week increments. For the lipopolysaccharides (LPS) condition, culture medium was supplemented with 10 ng ml−1 LPS starting one day after encapsulation. Cell viability in constructs (n = 2) was assessed using a LIVE/DEAD® membrane integrity assay at 3 weeks. Images were acquired using a confocal laser-scanning microscope (CLSM, Zeiss LSM 510, Thornwood, NY) at 100x magnification. To assess hydrogel degradability, acellular hydrogels were formed in fresh chondrocyte medium and swelled overnight to equilibrium. The hydrogels were placed in cell-conditioned medium and wet weight monitored for 7 hours. Cell-conditioned medium was prepared from chondrocytes that were placed in suspension culture at 1.5 million cells ml−1 and activated with 1 μg ml−1 LPS overnight. After activation, cells suspension was centrifuged and the supernatant collected as the cell-conditioned medium.
Hydrogel Construct Characterization
Hydrogels were assessed for wet weight and compressive modulus after 1 day, and week 3, 6, 9, and 12 (n = 3). Hydrogels were compressed to 15% strain at a strain rate of 0.5 mm min−1, to obtain stress-strain curves (MTS Synergie 100, 10N). The modulus was estimated as the slope of the linear region of stress-strain curves. Hydrogels were lyophilized to measure dry weight. Hydrogel volume was determined from height and diameter measurements.
Biochemical Analysis
On day 1 and weeks 3, 6, 9, and 12, hydrogel constructs were removed (n = 3), weighed, snap frozen in liquid nitrogen and stored at −80 °C. Hydrogels were lyophilized, then homogenized and digested with papain for 16 hours at 60 °C. DNA content was measured using Hoechst 33258, assuming 7.7 pg of DNA per chondrocyte.[73] The constructs and conditioned medium were assayed for collagen using the hydroxyproline assay, where hydroxyproline is assumed to make up 10% of collagen,[74] and for sGAGs using the dimethyl methylene blue (DMMB) dye assay.[75] sGAG and collagen content were normalized to cell number.
Histological and Immunohistochemical Analysis
At weeks 6 and 12, constructs (n = 2) were fixed in 4% paraformaldehyde, dehydrated, paraffin embedded and sectioned to 10 μm. Sections were stained with Safranin-O/Fast Green to visualize sGAGs and imaged at 200x magnification with a bright field microscope (Axiovert 40 C, Zeiss, Thornwood, NY). Sections were treated with primary antibodies against aggrecan (1:5), collagen type II (1:50), collagen type X (1:50), collagen type I (1:50), and C1,2C (1:100). Before primary antibody treatment, sections underwent antigen retrieval, then were treated with appropriate enzymes for 1 h at 37°C: hyaluronidase (200 U) for aggrecan, collagen II, and C1,2C; chondroitinase ABC (10 mU) and keratanase I (4 mU) for aggrecan; pepsin A (280 kU) for collagens I and X; and protease (400 U) and 0.25% trypsin for collagen X. Sections were treated with AlexaFluor 488 or 546-conjugated secondary antibodies and counterstained with DAPI. Images were acquired by laser scanning confocal microscopy at 400x magnification using the same settings and post-processing for all images. Sections that received no primary antibody treatment were used as negative controls. Sections of juvenile and adult hyaline cartilage were used as positive controls.
Enzyme Activity Assays and IL-6 ELISA
On day 1 and at weeks 3, 6, 9, and 12, constructs (n = 3) were snap-frozen in liquid nitrogen and stored at −80°C. The constructs were homogenized in Sensolyte kit assay buffer with 0.1% Triton X-100. Construct lysate and conditioned medium were assayed using Sensolyte 520 assay kits for substrates specific for ADAMTS-4 (aggrecanase-1) and generic MMPs (probes for MMP-1-3, 7-10, and 12–14 simultaneously). Activity was measured as a molar amount of cleaved substrate generated after incubating 1 hour at 37°C. Human ADAMTS-4 and collagenase II were used as positive controls for active enzymes. Conditioned culture medium (n = 3) from all time points was assayed for bovine interleukin-6 (IL-6) using a sandwich ELISA kit.
Statistical Analysis
Data are presented as mean (standard deviation). Measures of wet weight, modulus, biochemical content, enzyme activity and IL-6 were analyzed by two-way ANOVA where the factors were culture time and condition, followed by one-way ANOVA with Fisher’s LSD post-hoc test, α = 0.05, to determine significant difference between conditions at specific time points. Normal probability plots of the residuals were generated and were found to support the normal distribution assumption (plots not shown).
Supplementary Material
Acknowledgments
This research was supported by NIH grant # 1R01AR065441 and # R21AR061011, a fellowship from the National Science Foundation Graduate Research Fellowship Program (NSF GRFP), a National Institute of Health (NIH) Pharmaceutical Biotechnology Training Grant, and a Chancellor’s Fellowship from the University of Colorado.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr Stacey C. Skaalure, Department of Chemical and Biological Engineering, University of Colorado, Boulder, CO 80309; BioFrontiers Institute, University of Colorado, Boulder, CO 80309.
Stanley Chu, Department of Chemical and Biological Engineering, University of Colorado, Boulder, CO 80309; BioFrontiers Institute, University of Colorado, Boulder, CO 80309.
Prof Stephanie J. Bryant, Email: stephanie.bryant@colorado.edu, Department of Chemical and Biological Engineering, University of Colorado, Boulder, CO 80309; BioFrontiers Institute, University of Colorado, Boulder, CO 80309; Materials Science and Engineering Program, University of Colorado, Boulder, CO 80309.
References
- 1.Gillogly SD, Myers TH. Orthop Clin North Am. 2005;36:433. doi: 10.1016/j.ocl.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 2.Richardson JB, Caterson B, Evans EH, Ashton BA, Roberts S. J Bone Jt Surgery-British Vol. 1999;81B:1064. doi: 10.1302/0301-620x.81b6.9343. [DOI] [PubMed] [Google Scholar]
- 3.Vanlauwe J, Almqvist F, Bellemans J, Huskin JP, Verdonk R, Victor J. Acta Orthop Belg. 2007;73:145. [PubMed] [Google Scholar]
- 4.Bartlett W, a Skinner J, Gooding CR, Carrington RWJ, Flanagan aM, Briggs TWR, Bentley G. J Bone Joint Surg Br. 2005;87:640. doi: 10.1302/0301-620X.87B5.15905. [DOI] [PubMed] [Google Scholar]
- 5.Kon E, Verdonk P, Condello V, Delcogliano M, Dhollander A, Filardo G, Pignotti E, Marcacci M. Am J Sports Med. 2009;37:156S. doi: 10.1177/0363546509351649. [DOI] [PubMed] [Google Scholar]
- 6.Loeser RF. Arthritis Rheum. 2006;54:1357. doi: 10.1002/art.21813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin JA, Buckwalter JA. Biogerontology. 2002;3:257. doi: 10.1023/a:1020185404126. [DOI] [PubMed] [Google Scholar]
- 8.Buckwalter JA, Woo SL, Goldberg VM, Hadley EC, Booth F, Oegema TR, Eyre DR. J Bone Jt Surg Am. 1993;75:1533. doi: 10.2106/00004623-199310000-00015. [DOI] [PubMed] [Google Scholar]
- 9.Nicodemus GD, Skaalure SC, Bryant SJ. Acta Biomater. 2011;7:492. doi: 10.1016/j.actbio.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elisseeff J, Anseth K, Sims D, McIntosh W, Randolph M, Yaremchuk M, Langer R. Plast Reconstr Surg. 1999;104:1014. doi: 10.1097/00006534-199909040-00017. [DOI] [PubMed] [Google Scholar]
- 11.Sontjens SHM, Nettles DL, Carnahan MA, Setton LA, Grinstaff MW. Biomacromolecules. 2006;7:310. doi: 10.1021/bm050663e. [DOI] [PubMed] [Google Scholar]
- 12.Bryant SJ, Durand KL, Anseth KS. J Biomed Mater Res Part A. 2003;67A:1430. doi: 10.1002/jbm.a.20003. [DOI] [PubMed] [Google Scholar]
- 13.Bryant SJ, Anseth KS. J Biomed Mater Res. 2002;59:63. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 14.Bryant SJ, Chowdhury TT, Lee DA, Bader DL, Anseth KS. Ann Biomed Eng. 2004;32:407. doi: 10.1023/b:abme.0000017535.00602.ca. [DOI] [PubMed] [Google Scholar]
- 15.Bryant SJ, Bender RJ, Durand KL, Anseth KS. Biotechnol Bioeng. 2004;86:747. doi: 10.1002/bit.20160. [DOI] [PubMed] [Google Scholar]
- 16.Anseth KS, Bryant SJ. J Biomed Mater Res Part A. 2003;64A:70. doi: 10.1002/jbm.a.10319. [DOI] [PubMed] [Google Scholar]
- 17.Roberts JJ, Nicodemus GD, Greenwald EC, Bryant SJ. Clin Orthop Relat Res. 2011 doi: 10.1007/s11999-011-1823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skaalure SC, Milligan IL, Bryant SJ. Biomed Mater. 2012:7. doi: 10.1088/1748-6041/7/2/024111. [DOI] [PubMed] [Google Scholar]
- 19.Skaalure SC, Dimson SO, Pennington AM, Bryant SJ. Acta Biomater. 2014 doi: 10.1016/j.actbio.2014.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Vernerey FJ, Greenwald EC, Bryant SJ. Comput Methods Biomech Biomed Engin. 2012;15:1197. doi: 10.1080/10255842.2011.585973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dhote V, Vernerey FJ. Biomech Model Mechanobiol. 2013 doi: 10.1007/s10237-013-0493-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.West JL, Hubbell JA. Macromolecules. 1999;32:241. [Google Scholar]
- 23.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. Adv Mater. 2009;21:5005. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters aT, Weber FE, Fields GB, Hubbell Ja. Proc Natl Acad Sci U S A. 2003;100:5413. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patterson J, Hubbell Ja. Biomaterials. 2010;31:7836. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 26.West JL, Lee SH, Miller JS, Moon JJ. Biotechnol Prog. 2005;21:1736. doi: 10.1021/bp0502429. [DOI] [PubMed] [Google Scholar]
- 27.Lévesque SG, Shoichet MS. Bioconjug Chem. 2007;18:874. doi: 10.1021/bc0602127. [DOI] [PubMed] [Google Scholar]
- 28.Park Y, Lutolf MP, Hubbell JA, Hunziker EB, Wong M. Tissue Eng. 2004;10:515. doi: 10.1089/107632704323061870. [DOI] [PubMed] [Google Scholar]
- 29.Bahney CS, Hsu CW, Yoo JU, West JL, Johnstone B. FASEB J. 2011;25:1486. doi: 10.1096/fj.10-165514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Manicourt DH, Devogelaer JP, Thonar EJMA. In: Dynamics of Bone and Cartilage Metabolism. Seibel MJ, Robins SP, Bilezikian JP, editors. Vol. 1. Elsevier; Burlington: 2006. pp. 421–449. [Google Scholar]
- 31.Knudson CB, Knudson W. Semin Cell Dev Biol. 2001;12:69. doi: 10.1006/scdb.2000.0243. [DOI] [PubMed] [Google Scholar]
- 32.Buckwalter JA, Rosenberg LC. J Biol Chem. 1982;257:9830. [PubMed] [Google Scholar]
- 33.Caterson B, Flannery CR, Hughes GE, Little CB. Matrix Biol. 2000;19:333. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 34.Durigova M, Nagase H, Mort JS, Roughley PJ. Matrix Biol. 2011;30:145. doi: 10.1016/j.matbio.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lark MW, Gordy JT, Weidner JR, Ayala J, Kimura JH, Williams HR, Mumford RA, Flannery CR, Carlson SS, Iwata M, Sandy JD. J Biol Chem. 1995;270:2550. doi: 10.1074/jbc.270.6.2550. [DOI] [PubMed] [Google Scholar]
- 36.Ilic MZ, Handley CJ, Robinson HC, Mok MT. Arch Biochem Biophys. 1992;294:115. doi: 10.1016/0003-9861(92)90144-l. [DOI] [PubMed] [Google Scholar]
- 37.Flannery CR, Little CB, Caterson B. Matrix Biol. 1998;16:507. doi: 10.1016/s0945-053x(98)90021-x. [DOI] [PubMed] [Google Scholar]
- 38.Lutolf MP, Raeber GP, Zisch AH, Tirelli N, Hubbell JA. Adv Mater. 2003;15:888. [Google Scholar]
- 39.Patel L, Sun W, Glasson SS, Morris EA, Flannery CR, Chockalingam PS. BMC Musculoskelet Disord. 2011:12. doi: 10.1186/1471-2474-12-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee MS, Ikenoue T, Trindade MCD, Wong N, Goodman SB, Schurman DJ, Smith RL. J Orthop Res. 2003;21:117. doi: 10.1016/S0736-0266(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 41.Adams JC, Watt FM. Development. 1993;117:1183. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- 42.Guilak F, Alexopoulos LG, Upton ML, Youn I, Choi JB, Cao L, Setton LA, Haider MA. Ann New York Acad Sci Skelet Dev Remodel Heal Dis Aging. 2006;1068:498. doi: 10.1196/annals.1346.011. [DOI] [PubMed] [Google Scholar]
- 43.Kisiday J, Jin M, Kurz B, Hung H, Semino C, Zhang S, Grodzinsky AJ. Proc Natl Acad Sci U S A. 2002;99:9996. doi: 10.1073/pnas.142309999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu J, Song H, Zhang L, Xu H, Zhao X. Macromol Biosci. 2010;10:1164. doi: 10.1002/mabi.200900450. [DOI] [PubMed] [Google Scholar]
- 45.Mok SS, Masuda K, Hauselmann HJ, Aydelotte MB, Thonar EJMA. J Biol Chem. 1994;269:33021. [PubMed] [Google Scholar]
- 46.Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR, Mumford RA, Lohmander LS. J Clin Invest. 1997;100:93. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sztrolovics R, Alini M, Roughley PJ, Mort JS. Biochem J. 1997;326:235. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Homandberg GA, Davis G, Maniglia C, Shrikhande A. Osteoarthr Cartil. 1997;5:450. doi: 10.1016/s1063-4584(97)80049-0. [DOI] [PubMed] [Google Scholar]
- 49.Struglics A, Larsson S, Hansson M, Lohmander LS. Osteoarthr Cartil. 2009;17:497. doi: 10.1016/j.joca.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 50.Nagase H, Kashiwagi M. Arthritis Res Ther. 2003;5:94. doi: 10.1186/ar630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Connelly JT, Wilson CG, Levenston ME. Osteoarthr Cartil. 2008;16:1092. doi: 10.1016/j.joca.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Best Pract Res Clin Rheumatol. 2008;22:351. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Middleton J, Manthey A, Tyler J. J Histochem Cytochem. 1996;44:133. doi: 10.1177/44.2.8609369. [DOI] [PubMed] [Google Scholar]
- 54.Flannery CR, Little CB, Hughes CE, Curtis CL, Caterson B, Jones SA. Matrix Biol. 2000;19:549. doi: 10.1016/s0945-053x(00)00111-6. [DOI] [PubMed] [Google Scholar]
- 55.Edwards DR, Porter S, Clark IM, Kevorkian L. Biochem J. 2005;386:15. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E. Matrix Biol. 2002;21:499. doi: 10.1016/s0945-053x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 57.Campbell MA, Handley CJ, D’Souza SE. Biochem J. 1989;259:21. doi: 10.1042/bj2590021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pratta MA, Tortorella MD, Arner EC. J Biol Chem. 2000;275:39096. doi: 10.1074/jbc.M006201200. [DOI] [PubMed] [Google Scholar]
- 59.Morales TI, Wahl LM, Hascall VC. J Biol Chem. 1984;259:6720. [PubMed] [Google Scholar]
- 60.Roberts JJ, Nicodemus GD, Greenwald EC, Bryant SJ. Clin Orthop Relat Res. 2011 doi: 10.1007/s11999-011-1823-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buttner FH, Hughes CE, Margerie D, Lichte A, Tschesche H, Caterson B, Bartnik E. Biochem J. 1998;333:159. doi: 10.1042/bj3330159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mok SS, Masuda K, Hauselmann HJ, Aydelotte MB, Thonar EJMA. J Biol Chem. 1994;269:33021. [PubMed] [Google Scholar]
- 63.Hauselmann HJ, Masuda K, Hunziker EB, Neidhart M, Mok SS, Michel BA, Thonar EJMA. Am J Physiol Physiol. 1996;271:C742. doi: 10.1152/ajpcell.1996.271.3.C742. [DOI] [PubMed] [Google Scholar]
- 64.Roberts JJ, Nicodemus GD, Giunta S, Bryant SJ. J Biomed Mater Res Part A. 2011;97A:281. doi: 10.1002/jbm.a.33057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guilak F, Butler DL, Goldstein SA. Clin Orthop Relat Res. 2001:S295. [PubMed] [Google Scholar]
- 66.Darling EM, Athanasiou KA. Ann Biomed Eng. 2003;31:1114. doi: 10.1114/1.1603752. [DOI] [PubMed] [Google Scholar]
- 67.Nicodemus GD, Bryant SJ. Osteoarthr Cartil. 2010;18:126. doi: 10.1016/j.joca.2009.08.005. [DOI] [PubMed] [Google Scholar]
- 68.De Croos JNA, Dhaliwal SS, Grynpas MD, Pilliar RM, Kandel RA. Matrix Biol. 2006;25:323. doi: 10.1016/j.matbio.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Roberts JJ, Bryant SJ. Biomaterials. 2013;34:9969. doi: 10.1016/j.biomaterials.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shih H, Lin CC. Macromol Rapid Commun. 2013;34:269. doi: 10.1002/marc.201200605. [DOI] [PubMed] [Google Scholar]
- 71.Muir P, Peterson AL, Sample SJ, Scollay MC, Markel MD, Kalscheur VL. J Anat. 2008;213:706. doi: 10.1111/j.1469-7580.2008.00996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoyle CE, Bowman CN. Angew Chem Int Ed Engl. 2010;49:1540. doi: 10.1002/anie.200903924. [DOI] [PubMed] [Google Scholar]
- 73.Kim YJ, Sah RLY, Doong JYH, Grodzinsky AJ. Anal Biochem. 1988;174:168. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 74.Woessner JF. Arch Biochem Biophys. 1961;93:440. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 75.Templeton DM. Connect Tissue Res. 1988;17:23. doi: 10.3109/03008208808992791. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


