Abstract
Object
There is increasing interest in deep brain stimulation (DBS) for the treatment of addiction. Initial testing must be conducted in animals, and the alcohol-preferring (P) rat meets the criteria for an animal model of alcoholism. This study is composed of 2 experiments designed to examine the effects of 1) pharmacological inactivation and 2) DBS of the nucleus accumbens shell (AcbSh) on the consumption of alcohol by P rats.
Methods
In the first experiment, the effects of reversible inactivation of the AcbSh were investigated by administering intracranial injections of γ–aminobutyric acid (GABA) agonists. Bilateral microinjections of drug were administered to the AcbSh in P rats (8–10 rats/group), after which the animals were placed in operant chambers containing 2 levers—one used to administer water and the other to administer 15% EtOH—to examine the acquisition and maintenance of oral EtOH self-administration. In the second experiment, a DBS electrode was placed in each P rat’s left AcbSh. The animals then received 100 or 200 μA (3–4 rats/group) of DBS to examine the effect on daily consumption of oral EtOH in a free-access paradigm.
Results
In the first experiment, pharmacological silencing of the AcbSh with GABA agonists did not decrease the acquisition of EtOH drinking behavior but did reduce EtOH consumption by 55% in chronically drinking rats. Similarly, in the second experiment, 200 μA of DBS consistently reduced EtOH intake by 47% in chronically drinking rats. The amount of EtOH consumption returned to baseline levels following termination of therapy in both experiments.
Conclusions
Pharmacological silencing and DBS of the AcbSh reduced EtOH intake after chronic EtOH use had been established in rodents. The AcbSh is a neuroanatomical substrate for the reinforcing effects of alcohol and may be a target for surgical intervention in cases of alcoholism.
Keywords: nucleus accumbens, alcoholism, deep brain stimulation, functional neurosurgery, alcohol-preferring rat
Alcoholism is a global health issue: an estimated 76.3 million people have received the diagnosis of an alcohol use disorder.58 Alcohol dependence is the third largest contributor to disease burden in developed countries and leads to significant mental and physical consequences.58 Mortality rates in alcoholics are 1.8 to 9.5 times higher than those in the general population,10,12,19,21,28 and death due to unnatural causes, including falls and motor vehicle accidents, is more common in alcoholics.12,22,28 This excess mortality, along with health care expenses, law enforcement costs, property loss, and reduced productivity, contribute to the considerable economic burden of alcoholism,58 which is estimated to be approximately 2% of the United States gross domestic product.52 Traditional treatments for alcoholism suffer from high rates of noncompliance, variable effectiveness, and serious side effects. An estimated 45%–75% of treated alcoholics will relapse within 3 years,1,3,10 indicating that alcoholism can be a chronic and recurring illness, and there is a need for better therapies.
For the surgical treatment of alcoholism, candidate locations need to be identified by investigating the neurocircuitry that regulates alcohol intake in animal models. Ethanol, like many other drugs of abuse, at least partially produces its reinforcing actions through the midbrain dopaminergic system, which includes mesocorticolimbic projections from dopaminergic neurons in the ventral tegmental area to the nucleus accumbens, olfactory tubercle, frontal cortex, and amygdala.25 Dysfunction of this pathway is primarily implicated in addiction, and a subterritory of the nucleus accumbens, specifically the “shell” (AcbSh), has been identified as a critical component for the self-administration of drugs.8,39,48
Pharmacological inactivation, produced with the aid of a neuronal blocking agent, is a fast-acting but temporary method used to investigate the contribution of a particular structure to a skilled behavior.29 Although the mechanism of DBS is clearly more complex than that of a stroke, the ultimate clinical effect of DBS frequently appears similar to that of a lesion,33 and the use of GABA agonists for temporary lesioning of a structure allows us to simulate the potential effects of DBS in the same region. Using this method, one recent study demonstrated that oral alcohol consumption in rodents was decreased by an infusion of a GABAA agonist, specifically muscimol, into the AcbSh just prior to alcohol access.51 In a previous report, the authors also discussed the effect of intraaccumbal muscimol administration on alcohol intake and found a similar decrease in operant responding for alcohol in rodents, although the subregion of the nucleus accumbens was not specified.17 In our study, a mixture of GABA agonists, specifically baclofen and muscimol (Bac-Mus), was chosen as an inactivating agent for use on the AcbSh for 3 reasons: 1) the two compounds do not form an active metabolite, but instead bind to GABAB and GABAA receptors, respectively;29 2) projection cells originating in the AcbSh are potentially influenced by both GABAB and GABAA receptors;36,41 and 3) Bac-Mus infusion produces a rapid reduction in local neuronal activity without inhibiting passing fiber tracts.29,32,44
Preclinical research on the effects of DBS on alcoholism is extremely limited. Deep brain stimulation (150 μA) of the AcbSh reduced EtOH consumption in Long-Evans rats;23 however, these rats did not consume EtOH at a rate that would produce pharmacologically relevant blood alcohol levels (intake of ~ 0.5 g/kg/day). In a study conducted by Henderson et al.,15 DBS was also applied to the AcbSh in alcohol-preferring rats (P rats), a breed that is characterized by unusually intense alcohol-drinking behavior in both free-choice and operant conditions,38 and that meets the standard criteria for an animal model of alcoholism.4 Deep brain stimulation failed to alter free-choice baseline drinking, but did reduce the incidence of EtOH relapse. Similarly, the P rats in the Henderson study consumed unusually low levels of EtOH (0.2 g/kg/day). More work clearly needs to be done to assess the role of the AcbSh in alcohol use and the efficacy of DBS for the treatment of alcoholism.
The current study was designed to assess 2 items: 1) the effects of bilateral pharmacological inactivation of the AcbSh on the self-administration of alcohol in naïve and chronically drinking alcohol-preferring P rats; and 2) the effects of unilateral DBS of the AcbSh on consumption of EtOH in chronically drinking alcohol-preferring P rats.
Methods
Experiment 1
Animals
Experimentally naïve adult female alcohol-preferring (P) rats from the 77th generation, each weighing between 200 and 350 g, were used in the present study. Female rats were used because they maintain body weight and head size better than male rats; this allows more accurate stereotactic placement.48 The rats were double-housed on arrival at the lab and were maintained on a 12-hour reverse light-dark cycle (lights off at 9:00 AM). Food and water were freely available throughout the experiment, except for periods during which the animals were in the test chamber. The rats used in this study were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All research protocols were approved by the institutional animal care and use committee and are in accordance with guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (National Research Council, 1996).
Surgical Procedure
One week before operant training commenced, the animals were given an agent to induce general anesthesia (isoflurane [2%], Webster Veterinary Supply), after which stereotactic implantation of bilateral 22-gauge microinjection guide cannulae (Plastics One, Inc.) into the AcbSh was performed. On average, the coordinates for the AcbSh were 1.6 mm anterior to the bregma, 2.4 mm lateral to the midline, and 7.5 mm ventral from the surface of the skull at a 10° angle to the vertical plane.37 The dorsoventral coordinate was aimed 1 mm above the actual target because the microinjector projected an additional 1 mm beyond the cannula tip. Guide cannulae were fixed into place with cranioplastic material (Ortho-Jet, Lang Dental Manufacturing Co.) layered over 4 stainless steel screws fastened to the skull. After surgery, the rats were housed individually and allowed to recover for 5 to 7 days. The animals were habituated to the microinjection chambers and handled for at least 5 minutes daily after the 2nd postoperative day in preparation for the microinjection procedure.
Pharmacological Silencing
Baclofen and muscimol, GABAB and GABAA agonists, respectively, were used to pharmacologically inactivate the AcbSh. For the infusion, the drugs were dissolved in aCSF at a dose of 0.3 nmol baclofen and 0.03 nmol muscimol in a 0.3-ml injection volume, as described in previous reports that showed efficacy at this dose without significant side effects.32,44 The aCSF consisted of 120.0 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25.0 mM NaHCO3, 2.5-mM CaCl2, and 10.0 mM D-glucose. When necessary, 0.1 M HCl or 0.1 M NaOH was added to the solutions to adjust the pH levels to 7.4 ± 0.1. The rats were randomly assigned for the remainder of the experiment to either the active drug or control group (8 and 10 rats, respectively). Mock (injection-free) microinjection procedures were performed 1 day before the start of the experiment to habituate the rats to the process. Microinjectors were constructed using 30-gauge needles (Plastics One, Inc.); these were connected to PE20 tubing, which was attached to 10-ml glass syringes controlled by an infusion pump (Hamilton Instruments). Before each daily operant session during Weeks 2 and 9, each animal received a bilateral microinjection of either aCSF or active drug. All microinjections (volume 0.3 μl/side) were administered over a 60-second period. The injectors were left in place for an additional 60 seconds to permit diffusion of active drug or aCSF from the injection site, as previously described.32 Injectors were then removed, the cannulae stylets were replaced, and the rats were allowed to recover for approximately 10 minutes prior to entry into the operant chambers.
Operant EtOH Self-Administration
The EtOH self-administration experiments were conducted in standard 2-lever operant chambers (30 × 30 × 26 cm [width × height × depth]) contained within ventilated, sound-attenuated enclosures (64 × 60 × 50 cm; Coulbourn Instruments). Two operant levers—one to dispense water and the other to dispense 15% EtOH—were located on the same wall and were placed 15 cm above a grid floor and 13 cm apart. The levers were raised to this level to avoid accidental brushing against a lever and to reduce responses that would result from general locomotor activation. Directly beneath each lever was a trough through which a dipper cup (0.1 ml) was raised to deliver response-contingent fluid. When a lever was pressed, a small cue light illuminated in the corresponding drinking trough during the 4-second dipper cup access. The assignment of the water and EtOH levers, with respect to the left or right position, was counterbalanced among animals but remained the same for each rat. Throughout the experimental sessions, the operant chambers were illuminated by house lights. Specialized software (Graphic State, Coulbourn Instruments) controlled the operant chambers and recorded the data. All sessions were 60 minutes in duration and were conducted daily during the light period.
We have previously shown that ethanol-naive P rats do not require food/water restrictions or sucrose-fading procedures to acquire EtOH self-administration under operant conditions.46,47 The P rat learns spontaneously to press the bar for oral alcohol in daily 60-minute sessions during the first 2–3 weeks of exposure. This phase of escalating response is termed “acquisition.” Eventually the animals develop a baseline response for alcohol over water, which is termed “maintenance.”45,46 In the present study, the rats were not acclimated to the operant chamber before commencement of acquisition and did not receive any prior operant training. Both the EtOH (15%) and water levers were maintained on a fixed-ratio (FR) 1 schedule of reinforcement for the first 3 weeks, which means that the rats received 1 ethanol reward for each bar press. Subsequently, the reinforcement schedule on the EtOH lever was increased during Weeks 4–6 to FR3 (1 ethanol reward for every 3 bar presses) and again during Weeks 7 to 9 to FR5 (1 ethanol reward for every 5 bar presses) based on a well-established paradigm.27,38,45,46 Even without food or water restriction, P rats show a consummatory drive to obtain alcohol for its pharmacological effects and will press a lever up to 6 or 7 times for a single EtOH reward;27 this is the basis of the fixed-ratio paradigm employed here. Increasing the workload for alcohol increases the sensitivity and specificity of results that can be obtained from operant self-administration experiments. Water was always reinforced on an FR1 schedule (1 water reward for every bar press). The FR1 schedule was maintained for water because increasing the work requirement would further reduce the low level of responding for water. The responses on the water lever were important during the silencing sessions to help evaluate a nonspecific general reduction in motor activity from decreased goal-directed responding on the EtOH lever. The number of responses on both ethanol and water levers was recorded after each session. Presession microinjections were administered daily for 7 consecutive days during acquisition (Week 2) and again for only 5 consecutive days during maintenance (Week 9) due to concerns about the physical integrity and infection rate of 9-week-old surgical sites with further manipulation.
Histology and Data Analysis
At the completion of the experiment, the rats were killed by CO2 inhalation. A 30-gauge microinjector was substituted for the cannula stylet, and 1% bromophenol blue was infused into the site. The rat’s brains were then removed and stored at −70°C. Subsequently, the frozen brains were equilibrated at 15°C in a cryostat microtome and sliced into 40-μm sections. Slices were then examined for verification of the injection site by using the rat brain atlas of Paxinos and Watson.37 Only rats in which accurate placement of cannulae was confirmed were included in the final data analysis.
Analyses for the acquisition and maintenance of operant data consisted of a mixed ANOVA, with a between-subject variable of group and a repeated measure of session performed on the number of EtOH lever responses. Lever discrimination was examined by contrasting EtOH and water lever responses within each group. Data analysis for microinjection days consisted of a mixed ANOVA with between-subject variables of group and treatment and a repeated measure of sessions.
Experiment 2
Animals
Seven adult female alcohol-preferring (P) rats from the 78th generation, each weighing between 200 and 350 g, were used in this experiment.
Surgical Procedure
The surgical procedure was performed in a manner similar to that of Experiment 1, except that, instead of guide cannulae, a twisted electrode pair (Plastics One, Inc.) was implanted into the left AcbSh in each animal. Unilateral, rather than bilateral, implantation was performed, because this was the first time the procedure had been performed in our laboratory, and we simplified the technique as much as possible. Left-sided implantation was chosen based on a previous human study, in which abnormal neural activity was demonstrated in the left AcbSh only (there was no change in the right AcbSh) in response to drug-related cues.14 Average coordinates for the AcbSh were 1.6 mm anterior to the bregma, 2.4 mm lateral to the midline, and 8.5 mm ventral from the surface of the skull at a 10° angle to the vertical plane. Each electrode terminated in 2 female sockets that were connected to a multichannel electrode pedestal. The pedestal was secured firmly using cranioplastic material (Ortho-Jet, Lang Dental Manufacturing Co.), which was layered around the device and over 3 stainless steel screws fastened to the skull. After surgery, the rats were individually housed and allowed to recover for at least 2 days before testing was resumed.
Deep Brain Stimulation Apparatus
The rodent DBS system consisted of an “animal-proof” stainless steel–shielded 6-channel cable, which attached proximally to the implanted electrode pedestal and distally to a multichannel electrical swivel (all from Plastics One, Inc.) to allow free movement within the cage. The electrical swivel was embedded in a custom-made glass and wood panel that fit over the top of the testing cage. A waveform generator (Agilent 33522A, Agilent Technologies) was used to design the stimulus waveform, and a custom-made stimulus isolator was used to convert the voltage waveform to a constant current waveform. The stimulus parameters were as follows: biphasic, anode-leading, rectangular pulses with no interphasic delay; pulse frequency 150 Hz; pulse width 100 μsec; and current intensities of 100 μA (3 rats) or 200 μA (4 rats).
The functionality of the DBS system was tested by periodically measuring the electrochemical impedance spectroscopy (EIS) data of the electrodes and by checking the stimulator output current. The EIS data were measured using a Gamry Reference 600 Potentiostat, and the output current was measured by briefly connecting a digital multimeter (Agilent 34410A, Agilent Technologies) in series with the stimulator and electrodes. All measurements were done outside the 60-minute testing session and did not interfere with animal behavior.
Free-Choice Limited-Access EtOH Administration
We used a free-choice limited-access paradigm in this experiment, in lieu of an operant paradigm, to keep the equipment needs and setup simple since this was the lab’s first experience with DBS therapy. Water and a nonflavored solution of 15% EtOH were made freely available, with no lever pressing required, via two separate 15-ml tubes embedded in the front panel of a Plexiglas training box (30 × 30 × 26 cm [w × h × d]). The rats were placed in the training box with access to the fluids for 1 hr/day (limited access) each day for 4 weeks to establish baseline alcohol intake. Alcohol-preferring (P) rats are natural drinkers and do not typically require food/water restrictions or sucrose-fading procedures23 to acquire stable daily alcohol intake in a free-choice limited-access setting. Once chronic drinking levels were established and remained stable over several days, the rats underwent surgery for DBS lead placement. After a recovery period of 2 days, the rats resumed the daily 1-hour sessions in the training box.
Deep Brain Stimulation
During the 1st postoperative week, the rats were tethered to the DBS system but did not receive any active stimulation, a period termed “mock DBS.” This allowed each rat to acclimate to the new sensation of the DBS cables attached to the head cap electrode pedestal. In the 2nd postoperative week, once the rats reestablished baseline levels of alcohol intake, DBS was delivered to the rats, while they were in the training box, for 5 minutes before and throughout the entire 60-minute drinking session. Deep brain stimulation therapy occurred each day during the 1-hour session for 5 days at 100 μA in 3 rats and for 6 days at 200 μA in 4 rats. A technical issue in the 100-μA group caused DBS not to be delivered on Day 1, but this was not identified until after completion of the experiment, leaving just 5 days of active DBS data for analysis. All 7 rats underwent 3 additional daily sessions after DBS had been administered, during which the animals were tethered but did not receive stimulation, to assess for any posttreatment effects.
Histology and Data Analysis
At the completion of the experiment, the rats were killed by CO2 inhalation. The animal’s brains were removed, and their brain tissues were examined for accurate placement of the DBS electrodes in the manner described above.
The analysis of DBS data was simplified since there were not enough values to fulfill the degrees-of-freedom requirement to perform a mixed-factor ANOVA. Therefore, paired t-tests were performed to compare baseline EtOH consumption to intake during the test session. To adjust for multiple comparisons, the p value of significance was lowered to p < 0.01.
Results
Experiment 1
Acquisition
Both groups had similar baseline low levels of EtOH-lever activity during the 1st week of acquisition and did not display reliable lever discrimination. Pharmacological inactivation of the AcbSh with Bac-Mus did not significantly affect EtOH-lever responding in the active drug group compared with the aCSF group (group F1,11 = 0.008, p = 0.932; group × session interactions term F13,143 = 0.678, p = 0.782) during the 7 injection sessions of Week 2 (Fig. 1). There was a significant effect of the session (F13,143 = 6.357, p < 0.001) on EtOH lever responding. The effect indicated a significant increase in EtOH responding for both groups, and it was a clear indicator that the rats were acquiring the expected EtOH self-administration (t-tests, p < 0.01; Fig. 1). Water lever responses per session were not significantly different between the 2 groups during Weeks 1–3 (p = 0.78; data not shown). Similarly, there was a significant session effect (p < 0.01), but this effect was based on a reduction in the number of water responses across the sessions. Statistically, the reduction (part of the acquisition of the discrimination between EtOH and water) in water responding was indicated by significant differences between the number of water responses during the initial sessions and the number of water responses during the 7-day injection period in Week 2 (t-test, p < 0.02).
Fig. 1.
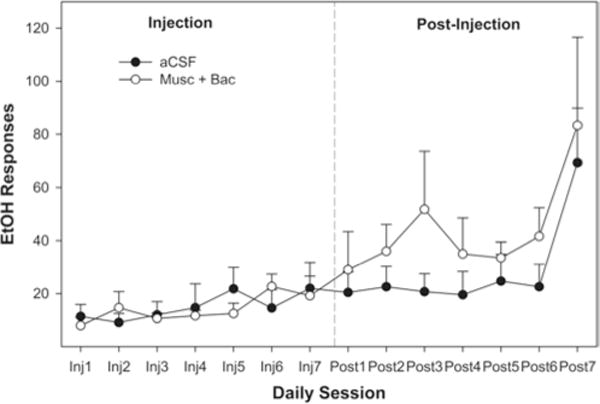
Line graph showing the average number of EtOH-lever presses per 1-hour session during Week 2 (acquisition) over 7 days of microinjections. Each session represents 1 day of testing. The data were collected while the animals were in the operant chambers directly after microinjections during the first 7 days (Inj1–Inj7), and for 7 days after the last day of microinjections (Post1–Post7). No difference between groups was indicated using ANOVA on any day with regard to EtOH self-administration. There were 10 rats in the aCSF group and 8 rats in the Bac-Mus group.
Maintenance
Both groups had similar baseline levels of EtOH-lever activity during the 8th week of maintenance (ANOVA, p = 0.84), with average responding being approximately 150 lever-presses per hour. This level of responding would have produced pharmacological relevant blood ethanol concentrations (predicted to be 50–70 mg%). Fluid intakes were not measured in the present experiment but were measured previously46,47 to ensure there was a relationship between the number of reinforcements presented and the amount of 15% EtOH consumed. The overall analysis indicated that there was a significant session × group interaction (F7, 186 = 1.65, p < 0.01). Decomposing the interaction term by holding the session constant (examining the effects of group on each individual injection session) indicated that a microinjection of Bac-Mus into the AcbSh reduced EtOH self-administration during the microinjection sessions held on Days 4 and 5 of Week 9 (p < 0.05 for both days; Fig. 2). There was a trend for the Bac-Mus group to respond less frequently on the EtOH lever than the control group during posttreatment Sessions 1 and 2, but this finding did not reach statistical significance. Pharmacological silencing of the AcbSh did not alter water responding during any of the daily sessions of Week 9 (Fig. 3). Statistically, there were no effects of session (p = 0.45), group (p = 0.61), or a group × session interaction term (p = 0.35; Fig. 3).
Fig. 2.
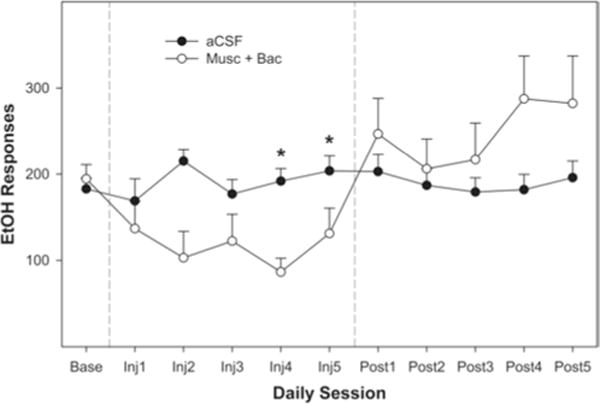
Line graph depicting the average number of EtOH-lever presses per 1-hour session during Week 9 (maintenance) over 5 days of microinjections. Each session represents 1 day of testing. Significant effects (noted by asterisks) were identified by ANOVA on Day 4 (p < 0.004) and Day 5 (p < 0.019). There were 10 rats in the aCSF group and 8 rats in the Bac-Mus group.
Fig. 3.
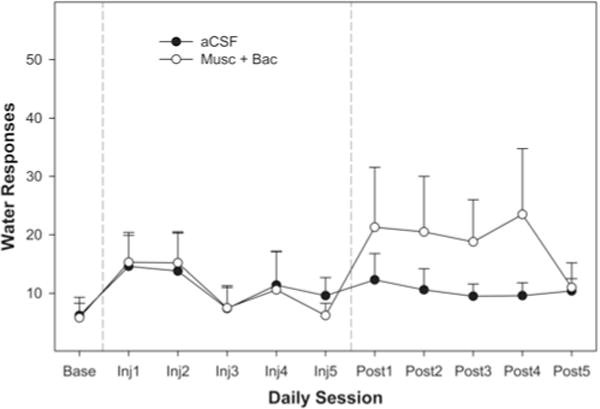
Line graph showing the average number of water-lever presses per 1-hour session during Week 9 (maintenance) over 5 days of microinjections. Each session represents 1 day of testing. No difference between groups was indicated using ANOVA on any day with regard to water self-administration. There were 10 rats in the aCSF group and 8 rats in the Bac-Mus group.
Experiment 2
Deep brain stimulation delivered at 100 μA temporarily reduced EtOH consumption (Fig. 4) with a 35% decrease on Day 2 and a 43% decrease on Day 3 (p < 0.002 for both days) but no effect on Days 1, 4, and 5. Deep brain stimulation delivered at 200 μA consistently reduced EtOH consumption, with a 35% decrease on Day 3, a 42% decrease on Day 4, a 53% decrease on Day 5, and a 59% decrease on Day 6 (p < 0.0018 for all days; Fig. 5). There was no effect of DBS on water consumption (data not shown), and EtOH consumption returned to baseline levels following the termination of DBS treatment (Figs. 4 and 5). After a 4-week implantation period, the DBS system displayed impedance within the functional range, which confirms that the DBS system was still operational. Baseline EtOH intake levels in both groups would have resulted in blood ethanol concentrations ranging from 75–125 mg%, based on our previous work.
Fig. 4.
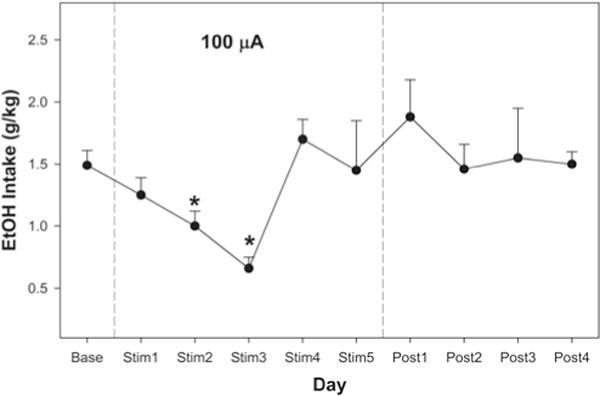
Line graph showing average EtOH intake per 1-hour session over 5 days of 100-μA DBS. Data were collected each day during DBS delivery (Stim1–Stim5) and for 4 days after the last day of DBS delivery (Post1–Post4). There was a 35% decrease on Day 2 and a 43% decrease on Day 3 (both p < 0.002). The EtOH consumption returned to baseline levels following termination of stimulation. Data obtained using 3 rats.
Fig. 5.
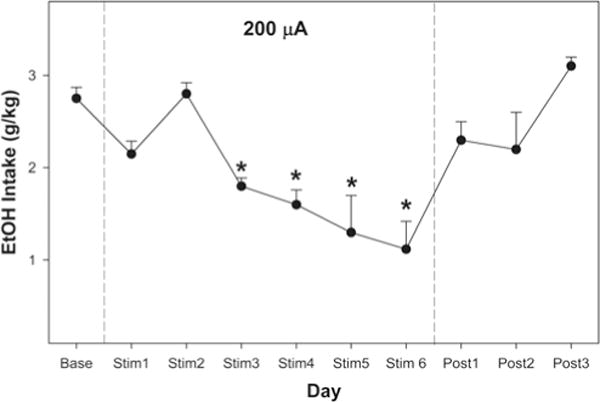
Line graph demonstrating average EtOH intake per 1-hour session over 6 days of 200-μA DBS. Deep brain stimulation consistently reduced EtOH consumption, with decreases of 35%, 42%, 53%, and 59% on Days 3, 4, 5, and 6, respectively (all p < 0.0018). The EtOH consumption returned to baseline levels following termination of stimulation. Data obtained using 4 rats.
Discussion
The major findings of the current project are 1) bilateral pharmacological inactivation of the AcbSh is effective at reducing operant EtOH self-administration; and 2) unilateral DBS of the AcbSh is capable of reducing alcohol consumption in chronically drinking alcohol-preferring (P) rats. These suppressive effects were selective for EtOH—there was no alteration in water responding or intake—and indicate that the AcbSh is not nonspecifically involved in the regulation of fluid intake or the performance of goal-directed activities. We can infer from the data that the AcbSh could be a viable target for the neurosurgical treatment of alcoholism.
Pathophysiology of Addiction
The clinical characteristics of the P rat may reflect a dysregulation of the mesocorticolimbic system, including the dopaminergic neurons in the ventral tegmental area, which project to the nucleus accumbens, the frontal cortex, and the amygdala to facilitate reward.25 Dysfunction of this pathway may also underlie addiction in humans.26,50 An increase in the activity of the mesocorticolimbic system has been well documented in response to the acute administration of multiple drugs of abuse, including alcohol.26 However, chronic drug or alcohol use decreases functioning within this pathway,26,30,31,55 leading to long-standing deficits, particularly in dopamine transmission.35
Chronic addiction is often a damaging, cyclical process composed of 3 repeated stages: 1) binge/intoxication; 2) withdrawal/negative affect; and 3) preoccupation/anticipation (that is, chronic craving and compulsive seeking).26 Each stage is characterized by the involvement of key anatomical structures, and the AcbSh may play a role in all 3 phases. Preclinical research has demonstrated enhanced dopaminergic transmission in the AbcSh with both the administration of drugs39,53,56 and drug-related cues.2 A genetic predisposition to alcoholism in rodents is associated with a “hypersensitive” dopamine system within the AcbSh,7,8 possibly due to a reduction in its baseline dopaminergic innervation.59 Additionally, pharmacological depletion of dopamine in the AcbSh is associated with increased alcohol consumption in normal Sprague-Dawley rats.40
Taken together, these findings suggest that end-stage addiction is a disease of decreased dopaminergic tone, which negatively affects the function of the AbcSh, similar to the effect of Parkinson disease on the striatum. Deep brain stimulation in Parkinson disease may partially normalize the deficient dopaminergic system through alterations in dopamine and dopamine-related enzyme levels, which may be one biological basis for the effects of DBS on parkinsonian symptoms.16 Deep brain stimulation also alters neurophysiological interactions, including abnormal neuronal discharge and excessive synchrony, which occur in and between critical cortical and subcortical sites in diseases due to dopamine depletion.6,57 A cautious but logical conclusion is that DBS mechanistically may also be able to restore function to patients with severe, medically refractory alcoholism.
Preclinical Data on the Nucleus Accumbens Shell
While many neurotransmitters, receptors, and neuroanatomical regions have been implicated in the mechanism of alcoholism, the AcbSh has repeatedly emerged as a key structure in the acquisition,20 maintenance,5,18 and relapse15 of high-alcohol drinking behavior. We demonstrated here that both pharmacological silencing and DBS of the AcbSh were able to decrease alcohol consumption in operant and free-access settings, respectively, in rats with established alcoholism. This is in agreement with findings of other studies that have examined the role of the AcbSh in addiction. Fuchs et al.11 demonstrated a significant decrease in context-induced reinstatement of cocaine seeking in Sprague-Dawley rats that received bilateral AcbSh injections of Bac-Mus. In an electrical equivalent, Vassoler and coauthors54 demonstrated that bilateral high-frequency DBS (160 Hz, 150 μA) of the AcbSh blocked cocaine priming–induced reinstatement of drug seeking. This effect was not seen with DBS of the dorsal striatum and did not occur with the reinstatement of food seeking, suggesting that the positive results were specific to the drug and the location in the AcbSh.
Regarding alcoholism, Hodge and colleagues17 silenced the bilateral nucleus accumbens (subterritory not specified) in Long-Evans rats with muscimol alone and found an approximately 56% decrease in the operant self-administration of ethanol (10% v/v). Similarly another group demonstrated that bilateral AcbSh muscimol injections dramatically decreased consumption of ethanol (10% v/v) during a daily 1-hour limited-access session, while conversely increasing sucrose solution intake—again confirming a drug-specific effect.51 In 2 studies of the role of DBS in rodent alcohol consumption, researchers found a reduction in EtOH consumption. In the first study, Long-Evans rats were daily given 30 minutes of limited access to oral ethanol (10% v/v) until stable drinking levels were established. Bilateral DBS (160 Hz) of the AcbSh was then delivered 5 minutes before and during the daily sessions and reduced EtOH intake by approximately 60% compared with baseline levels.23 Findings of this study also defined 150 μA as the minimum effective dose for a DBS-induced treatment effect, which is in agreement with our results, which show that 100 μA variably and transiently reduces EtOH intake. In the second study, the researchers examined bilateral high-frequency DBS (160 Hz, 200 μA) of the AcbSh in P rats.15 Deep brain stimulation was effective at decreasing alcohol preference (alcohol consumed/total fluid consumed) during 1-hour EtOH-drinking sessions conducted daily for 2 consecutive days after chronic intake had been established. Notably, DBS also significantly reduced total EtOH intake to approximately 50% of baseline levels during a 24-hour EtOH-drinking session after a 4- to 6-week period of forced abstinence. The P rat typically demonstrates a prominent alcohol deprivation effect, which is a temporary, marked increase in EtOH consumption during reexposure after a period of abstinence.34 The alcohol deprivation effect is observed in primates as well,49 and potentially reflects drug craving and likelihood of relapse.34,45 Attenuation of the alcohol deprivation effect in the P rat by DBS, shown in the study by Henderson et al.,15 indicates that neuromodulation may be one approach to relapse prevention. To our knowledge, the current report discusses the only study in which unilateral DBS of the AcbSh on alcohol consumption in rodents has been examined. It is interesting to note that we achieved roughly the same magnitude of treatment effect in our experiments—not only with bilateral pharmacological silencing but with unilateral DBS as well—suggesting that unilateral therapy may be sufficient to alter reward-related behavior.
Neuroadaptation to Reward
Neither pharmacological silencing nor DBS of the AcbSh resulted in an immediate decrease in alcohol consumption. The delayed effect of both therapies indicates that lesioning or stimulating the AcbSh does not simply impair gross motor function. Rather, these therapies may reduce the reinforcing properties of EtOH by possibly devaluing the hedonic, or positive, aspects of alcohol intoxication. Interestingly, in both experiments, a transient increase in daily consumption directly preceded a significant delayed decrease in alcohol intake over the following days. One possibility is that rats are increasing intake temporarily in an attempt to recapture the reward of alcoholic intoxication, but then abruptly decrease intake when the positive aspects fail to occur. Essentially the rats may be “learning” that alcohol is no longer rewarding. In support of this idea, in the study by Hodge and colleagues,17 a detailed analysis of the session time line revealed that the muscimol injection did not alter the onset of alcohol responding or the response rate (lever hits/minute) but did result in premature termination of responding on the active lever compared with responses by controls. This finding suggests that inactivation of the nucleus accumbens resulted in an attenuated internal reward response to alcohol and, as such, these animals stopped responding earlier than sham-injected rats.
Additionally, pharmacological inactivation of the AcbSh with GABA agonists had no effect on the development, or acquisition, of alcohol-drinking behavior. One explanation for these findings might be that widespread neuroadaptation must occur in response to chronic drug use before an intervention can “normalize” functioning. This is the basis of the incentive sensitization theory,43 which states that a bias of attentional processing toward drug-associated stimuli occurs in response to repeated drug use, culminating in a pathological motivation for drugs, or a compulsive “wanting” for the wrong rewards (drugs) over time. While indirectly our data support the idea that chronic drug use causes pathological changes in brain circuitry that were not present initially, the clinical relevance of acquisition is questionable, because surgical interventions for alcoholism would only be directed at long-standing severe users. It is also important to note that the rats did not begin to increase lever-pressing for alcohol exponentially until Week 3, after the microinjections were completed in Week 2, possibly leading to a false negative result due to the inherent lack of sensitivity in the operant paradigm at low levels of responding.
The lack of a simple “on-off” response to GABAergic inactivation of the AcbSh and to DBS of the AcbSh in 2 different experimental paradigms is not surprising. Unlike psychostimulants,26 the mechanisms underlying alcohol abuse are not well defined. Alcoholism is not simply explained by changes in dopaminergic activity in the AcbSh. Multiple studies in which 6-hydroxydopamine injections in the nucleus accumbens were used to dramatically reduce local dopamine levels have failed to attenuate EtOH intake in operant42 and free-access paradigms.9,20 Addiction, including alcoholism, is characterized by a network of specific regions that show progressive, detrimental neuroplasticity in response to chronic drug abuse,26 and the AcbSh probably represents a complex intersection of neural circuits that control the motivational, attentional, impulsive, and compulsive aspects of alcohol abuse, thus predicting a multidimensional response to any regional therapy.
Weaknesses of this study include the use of an animal model that does not fully reflect the complexities of human alcoholism, the lack of measurement of blood alcohol concentrations, and the use of a genetically selective organism that may not reflect the mainstream population. As detailed previously, the alcohol-preferring (P) rat is the best translational model of alcoholism currently available. Blood ethanol concentrations have been previously measured in similar experiments in our lab13 and, due to practical considerations, are not reproduced in every subsequent project. While the P rat may not represent the mainstream population, we believe that it does represent the genetically predisposed, family history–positive sub-population of severe alcoholics who could be candidates for surgical intervention.
Conclusions
Admittedly, there is a substantial treatment gap between people with alcohol dependence and currently available therapies.24 Nevertheless, this situation is not likely to improve, regardless of public health measures, until more effective, longer-lasting therapies become available, particularly for patients with the most refractory alcoholism. We have demonstrated two points: 1) pharmacological inactivation of the bilateral AcbSh reduces EtOH operant responding in the P rat after chronic EtOH use has been established; and 2) unilateral DBS of the AcbSh is effective at reducing daily EtOH consumption in alcoholic animals. The AcbSh is a neuroanatomical substrate for the reinforcing effects of alcohol and may be a target for the neuromodulation of refractory alcoholism. Future work should focus on the following: 1) translational experiments using multiple therapeutic modalities including DBS, pharmacological manipulation, and optogenetic activation in animal models of addiction; 2) the development of a rodent DBS apparatus that minimizes behavioral artifacts; 3) the continuing investigation of the roles of the subthalamic nucleus, the medial prefrontal cortex, and the core of the nucleus accumbens with regard to addiction; and 4) the exploration of DBS treatment on combined nicotine and alcohol abuse, given the impact that nicotine has on mortality in alcoholic persons.19
Acknowledgments
Support for this study was provided to Dr. Zachary Rodd by the National Institute on Alcohol Abuse and Alcoholism (Grants AA12262, AA07642, AA20908, and AA022167). The authors report no conflict of interest regarding the materials and methods used in this study or the findings specified in this paper.
Abbreviations in this paper
- AcbSh
nucleus accumbens shell
- aCSF
artificial cerebrospinal fluid
- Bac-Mus
mixture of baclofen and muscimol
- DBS
deep brain stimulation
- EIS
electrochemical impedance spectroscopy
- EtOH
ethanol alcohol
- FR
fixed-ratio
- GABA
γ–aminobutyric acid
- P rat
alcohol-preferring rat
Footnotes
Disclosure
Conception and design: Wilden, Rodd. Acquisition of data: Wilden, Qing, Hauser. Analysis and interpretation of data: Wilden, Qing, Hauser, Rodd. Drafting the article: Wilden. Critically revising the article: all authors. Reviewed submitted version of manuscript: all authors. Approved the final version of the manuscript on behalf of all authors: Wilden. Statistical analysis: Wilden, Hauser, Rodd. Administrative/technical/material support: Qing, McBride, Irazoqui, Rodd. Study supervision: McBride, Irazoqui, Rodd.
Portions of this study were presented at the AANS Annual Scientific Meeting, April 9–13, 2011, Denver, Colorado; and at the AANS Annual Scientific Meeting, April 27–May 1, 2013, New Orleans, Louisiana.
References
- 1.Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, et al. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 2.Bassareo V, De Luca MA, Di Chiara G. Differential impact of pavlovian drug conditioned stimuli on in vivo dopamine transmission in the rat accumbens shell and core and in the prefrontal cortex. Psychopharmacology (Berl) 2007;191:689–703. doi: 10.1007/s00213-006-0560-7. [DOI] [PubMed] [Google Scholar]
- 3.Bottlender M, Soyka M. Outpatient alcoholism treatment: predictors of outcome after 3 years. Drug Alcohol Depend. 2005;80:83–89. doi: 10.1016/j.drugalcdep.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 4.Cicero TJ, Snider SR, Perez VJ, Swanson LW. Physical dependence on and tolerance to alcohol in the rat. Physiol Behav. 1971;6:191–198. doi: 10.1016/0031-9384(71)90088-6. [DOI] [PubMed] [Google Scholar]
- 5.Cozzoli DK, Courson J, Caruana AL, Miller BW, Greentree DI, Thompson AB, et al. Nucleus accumbens mGluR5-associated signaling regulates binge alcohol drinking under drinking-in-the-dark procedures. Alcohol Clin Exp Res. 2012;36:1623–1633. doi: 10.1111/j.1530-0277.2012.01776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Hemptinne C, Ryapolova-Webb ES, Air EL, Garcia PA, Miller KJ, Ojemann JG, et al. Exaggerated phase-amplitude coupling in the primary motor cortex in Parkinson disease. Proc Natl Acad Sci U S A. 2013;110:4780–4785. doi: 10.1073/pnas.1214546110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ding ZM, Rodd ZA, Engleman EA, McBride WJ. Sensitization of ventral tegmental area dopamine neurons to the stimulating effects of ethanol. Alcohol Clin Exp Res. 2009;33:1571–1581. doi: 10.1111/j.1530-0277.2009.00985.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, et al. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33:2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fahlke CC, Hansen SS, Engel JAJ, Hård EE. Effects of ventral striatal 6-OHDA lesions or amphetamine sensitization on ethanol consumption in the rat. Pharmacol Biochem Behav. 1994;47:345–349. doi: 10.1016/0091-3057(94)90020-5. [DOI] [PubMed] [Google Scholar]
- 10.Finney JW, Moos RH. The long-term course of treated alcoholism: I. Mortality, relapse and remission rates and comparisons with community controls. J Stud Alcohol. 1991;52:44–54. doi: 10.15288/jsa.1991.52.44. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs RA, Ramirez DR, Bell GH. Nucleus accumbens shell and core involvement in drug context-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2008;200:545–556. doi: 10.1007/s00213-008-1234-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris EC, Barraclough B. Excess mortality of mental disorder. Br J Psychiatry. 1998;173:11–53. doi: 10.1192/bjp.173.1.11. [DOI] [PubMed] [Google Scholar]
- 13.Hauser SR, Katner SN, Deehan GA, Jr, Ding ZM, Toalston JE, Scott BJ, et al. Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (p) rats. Alcohol Clin Exp Res. 2012;36:1963–1972. doi: 10.1111/j.1530-0277.2012.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heinze HJ, Heldmann M, Voges J, Hinrichs H, Marco-Pallares J, Hopf JM, et al. Counteracting incentive sensitization in severe alcohol dependence using deep brain stimulation of the nucleus accumbens: clinical and basic science aspects. Front Hum Neurosci. 2009;3:22. doi: 10.3389/neuro.09.022.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henderson MB, Green AI, Bradford PS, Chau DT, Roberts DW, Leiter JC. Deep brain stimulation of the nucleus accumbens reduces alcohol intake in alcohol-preferring rats. Neurosurg Focus. 2010;29(2):E12. doi: 10.3171/2010.4.FOCUS10105. [DOI] [PubMed] [Google Scholar]
- 16.Henning J, Koczan D, Glass Ä, Karopka T, Pahnke J, Rolfs A, et al. Deep brain stimulation in a rat model modulates TH, CaMKIIa and Homer1 gene expression. Eur J Neurosci. 2007;25:239–250. doi: 10.1111/j.1460-9568.2006.05264.x. [DOI] [PubMed] [Google Scholar]
- 17.Hodge CW, Chappelle AM, Samson HH. GABAergic transmission in the nucleus accumbens is involved in the termination of ethanol self-administration in rats. Alcohol Clin Exp Res. 1995;19:1486–1493. doi: 10.1111/j.1530-0277.1995.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoplight BJ, Sandygren NA, Neumaier JF. Increased expression of 5-HT1B receptors in rat nucleus accumbens via virally mediated gene transfer increases voluntary alcohol consumption. Alcohol. 2006;38:73–79. doi: 10.1016/j.alcohol.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 19.Hurt RD, Offord KP, Croghan IT, Gomez-Dahl L, Kottke TE, Morse RM, et al. Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA. 1996;275:1097–1103. doi: 10.1001/jama.275.14.1097. [DOI] [PubMed] [Google Scholar]
- 20.Ikemoto SS, Kohl RRR, McBride WJW. GABA(A) receptor blockade in the anterior ventral tegmental area increases extracellular levels of dopamine in the nucleus accumbens of rats. J Neurochem. 1997;69:137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- 21.John U, Rumpf HJ, Bischof G, Hapke U, Hanke M, Meyer C. Excess mortality of alcohol-dependent individuals after 14 years and mortality predictors based on treatment participation and severity of alcohol dependence. Alcohol Clin Exp Res. 2012;37:156–163. doi: 10.1111/j.1530-0277.2012.01863.x. [DOI] [PubMed] [Google Scholar]
- 22.Kennedy BP, Isaac NE, Graham JD. The role of heavy drinking in the risk of traffic fatalities. Risk Anal. 1996;16:565–569. doi: 10.1111/j.1539-6924.1996.tb01101.x. [DOI] [PubMed] [Google Scholar]
- 23.Knapp CM, Tozier L, Pak A, Ciraulo DA, Kornetsky C. Deep brain stimulation of the nucleus accumbens reduces ethanol consumption in rats. Pharmacol Biochem Behav. 2009;92:474–479. doi: 10.1016/j.pbb.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohn R, Saxena S, Levav I, Saraceno B. The treatment gap in mental health care. Bull World Health Organ. 2004;82:858–866. [PMC free article] [PubMed] [Google Scholar]
- 25.Koob GF, Sanna PP, Bloom FE. Neuroscience of addiction. Neuron. 1998;21:467–476. doi: 10.1016/s0896-6273(00)80557-7. [DOI] [PubMed] [Google Scholar]
- 26.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li TK, Lumeng L, McBride WJ, Waller MB. Progress toward a voluntary oral consumption model of alcoholism. Drug Alcohol Depend. 1979;4:45–60. doi: 10.1016/0376-8716(79)90040-1. [DOI] [PubMed] [Google Scholar]
- 28.Markkula N, Härkänen T, Perälä J, Partti K, Peña S, Koskinen S, et al. Mortality in people with depressive, anxiety and alcohol use disorders in Finland. Br J Psychiatry. 2012;200:143–149. doi: 10.1192/bjp.bp.111.094904. [DOI] [PubMed] [Google Scholar]
- 29.Martin JH, Ghez C. Pharmacological inactivation in the analysis of the central control of movement. J Neurosci Methods. 1999;86:145–159. doi: 10.1016/s0165-0270(98)00163-0. [DOI] [PubMed] [Google Scholar]
- 30.Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, et al. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. (Erratum in Neuropsychopharmacology 29:1763, 2004) [DOI] [PubMed] [Google Scholar]
- 31.Martinez D, Kim JH, Krystal J, Abi-Dargham A. Imaging the neurochemistry of alcohol and substance abuse. Neuroimaging Clin N Am. 2007;17:539–555. x. doi: 10.1016/j.nic.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 32.McFarland K, Kalivas PW. The circuitry mediating cocaine-induced reinstatement of drug-seeking behavior. J Neurosci. 2001;21:8655–8663. doi: 10.1523/JNEUROSCI.21-21-08655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McIntyre CC, Savasta M, Kerkerian-Le Goff L, Vitek JL. Uncovering the mechanism(s) of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- 34.McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, et al. The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcohol Clin Exp Res. 1998;22:1170–1176. [PubMed] [Google Scholar]
- 35.Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noori HR, Spanagel R, Hansson AC. Neurocircuitry for modeling drug effects. Addict Biol. 2012;17:827–864. doi: 10.1111/j.1369-1600.2012.00485.x. [DOI] [PubMed] [Google Scholar]
- 37.Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- 38.Penn PE, McBride WJ, Lumeng L, Gaff TM, Li TK. Neurochemical and operant behavioral studies of a strain of alcohol-preferring rats. Pharmacol Biochem Behav. 1978;8:475–481. doi: 10.1016/0091-3057(78)90087-4. [DOI] [PubMed] [Google Scholar]
- 39.Pontieri FE, Tanda G, Di Chiara G. Intravenous cocaine, morphine, and amphetamine preferentially increase extracellular dopamine in the “shell” as compared with the “core” of the rat nucleus accumbens. Proc Natl Acad Sci U S A. 1995;92:12304–12308. doi: 10.1073/pnas.92.26.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quarfordt SD, Kalmus GW, Myers RD. Ethanol drinking following 6-OHDA lesions of nucleus accumbens and tuberculum olfactorium of the rat. Alcohol. 1991;8:211–217. doi: 10.1016/0741-8329(91)90854-p. [DOI] [PubMed] [Google Scholar]
- 41.Rahman SS, McBride WJ. Involvement of GABA and cholinergic receptors in the nucleus accumbens on feedback control of somatodendritic dopamine release in the ventral tegmental area. J Neurochem. 2002;80:646–654. doi: 10.1046/j.0022-3042.2001.00739.x. [DOI] [PubMed] [Google Scholar]
- 42.Rassnick S, Stinus L, Koob GF. The effects of 6-hydroxydopamine lesions of the nucleus accumbens and the mesolimbic dopamine system on oral self-administration of ethanol in the rat. Brain Res. 1993;623:16–24. doi: 10.1016/0006-8993(93)90004-7. [DOI] [PubMed] [Google Scholar]
- 43.Robinson TE, Berridge KC. Review. The incentive sensitization theory of addiction: some current issues. Philos Trans R Soc Lond B Biol Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rocha A, Kalivas PW. Role of the prefrontal cortex and nucleus accumbens in reinstating methamphetamine seeking. Eur J Neurosci. 2010;31:903–909. doi: 10.1111/j.1460-9568.2010.07134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, et al. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- 46.Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, et al. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- 47.Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, et al. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res. 2002;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- 48.Rodd-Henricks ZA, McKinzie DL, Li TK, Murphy JM, Mc-Bride WJ. Cocaine is self-administered into the shell but not the core of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303:1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- 49.Sinclair JD. The alcohol-deprivation effect in monkeys. Psychon Sci. 1971;25:21–22. [Google Scholar]
- 50.Söderpalm B, Ericson M. Neurocircuitry involved in the development of alcohol addiction: the dopamine system and its access points. Curr Top Behav Neurosci. 2011;13:127–161. doi: 10.1007/7854_2011_170. [DOI] [PubMed] [Google Scholar]
- 51.Stratford TR, Wirtshafter D. Opposite effects on the ingestion of ethanol and sucrose solutions after injections of muscimol into the nucleus accumbens shell. Behav Brain Res. 2011;216:514–518. doi: 10.1016/j.bbr.2010.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thavorncharoensap M, Teerawattananon Y, Yothasamut J, Lertpitakpong C, Chaikledkaew U. The economic impact of alcohol consumption: a systematic review. Subst Abuse Treat Prev Policy. 2009;4:20. doi: 10.1186/1747-597X-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thielen RJ, Engleman EA, Rodd ZA, Murphy JM, Lumeng L, Li TK, et al. Ethanol drinking and deprivation alter dopaminergic and serotonergic function in the nucleus accumbens of alcohol-preferring rats. J Pharmacol Exp Ther. 2004;309:216–225. doi: 10.1124/jpet.103.059790. [DOI] [PubMed] [Google Scholar]
- 54.Vassoler FM, Schmidt HD, Gerard ME, Famous KR, Ciraulo DA, Kornetsky C, et al. Deep brain stimulation of the nucleus accumbens shell attenuates cocaine priming-induced reinstatement of drug seeking in rats. J Neurosci. 2008;28:8735–8739. doi: 10.1523/JNEUROSCI.5277-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- 56.Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- 57.Whitmer DD, de Solages CC, Hill BB, Yu HH, Henderson JM, Bronte-Stewart HH. High frequency deep brain stimulation attenuates subthalamic and cortical rhythms in Parkinson’s disease. Front Hum Neurosci. 2012;6:155. doi: 10.3389/fnhum.2012.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.World Health Organization. WHO Global Status Report on Alcohol 2004. Geneva: WHO; 2004. ( http://www.who.int/substance_abuse/publications/global_status_report_2004_overview.pdf) [Accessed December 15, 2013] [Google Scholar]
- 59.Zhou FC, Zhang JK, Lumeng L, Li TK. Mesolimbic dopamine system in alcohol-preferring rats. Alcohol. 1995;12:403–412. doi: 10.1016/0741-8329(95)00010-o. [DOI] [PubMed] [Google Scholar]


