Abstract
Humans escalate their cigarette smoking over time, and a major obstacle in the field of pre-clinical nicotine addiction research has been the inability to produce escalated nicotine self-administration in rats. In Experiment 1, male Wistar rats were trained to respond for nicotine in 2-hr operant sessions, then exposed to chronic intermittent (12 hrs/day) nicotine vapor and repeatedly tested for nicotine self-administration at 8-12 hrs withdrawal. Rats were tested intermittently on days 1, 3 and 5 of the vapor exposure procedure, then tested on consecutive days 6-15 of nicotine vapor exposure. Rats exhibited transient increases in operant nicotine responding during intermittent testing, regardless of vapor condition, and this responding returned to baseline levels upon resumption of consecutive-days testing (i.e., nicotine deprivation effect). Nicotine vapor-exposed rats then escalated nicotine self-administration relative to both their own baseline (~200% increase) and non-dependent controls (~3x higher). In Experiment 2, rats were exposed or not exposed to chronic intermittent nicotine vapor, then tested for spontaneous and precipitated somatic signs of nicotine withdrawal. Eight hrs following removal from nicotine vapor, rats exhibited robust mecamylamine- precipitated somatic signs of withdrawal. There was a strong correlation between nicotine flow rate and air-nicotine concentration, and the air-nicotine concentrations used in Experiments 1 & 2 resemble concentrations experienced by human smokers. Collectively, these results suggest that chronic intermittent nicotine vapor inhalation produces somatic and motivational signs of nicotine dependence, the latter of which is evidenced by escalation of nicotine self-administration.
Keywords: Nicotine Dependence, Nicotine Self-Administration, Escalation, Withdrawal, Mecamylamine
INTRODUCTION
Nicotine addiction is responsible, either directly or indirectly, for millions of deaths worldwide each year (NIH, WHO). The financial cost of nicotine-related problems to U.S. society alone was recently estimated at $250 billion annually (NIH), a majority of which is due to negative biological outcomes (e.g., cancers and cardiovascular disease). Although nicotine itself is not always the compound responsible for health problems and mortality associated with smoking, nicotine is the psychoactive ingredient that produces addiction to smoking and understanding the neurobiology of this addictive behavior is critical for developing smoking cessation treatments.
Rats have long been used as an animal model of nicotine self-administration that mimics acquisition, maintenance, and relapse-like behaviors in human smokers. Rats exhibit reliable intravenous (i.v.) self-administration of nicotine during limited-access operant sessions in which they are allowed to press a lever for nicotine infusions, and will adjust their lever-pressing behavior to account for increasing or decreasing unit doses of nicotine (O'Dell & Koob, 2007). Rats allowed long periods of access (up to 23 hrs/day) to nicotine self-administration do not typically exhibit escalation, often defined as higher intakes during the first hour of access relative to short-access rats (Paterson & Markou, 2004). That said, increases in long-access self-administration over time are facilitated by (1) intermittence of testing and (2) co-administration of a monoamine oxidase inhibitor (MAOI), which is another ingredient in cigarettes (Cohen et al., 2012). Indeed, rats exhibit a nicotine deprivation effect, defined as a transient increase in nicotine responding following a period without nicotine access, under some (i.e., long-access) conditions (George et al., 2007; O'Dell & Koob, 2007). In sum, achieving escalated voluntary nicotine self-administration in rats has been an obstacle for animal models that aim to mimic patterns of escalated smoking observed in humans (e.g., Kim et al., 2009). Therefore, the aim of the current investigation was to utilize a route (inhalation) of chronic nicotine administration with face validity for the human condition, without co-administration of other drugs, to produce escalation of nicotine self-administration in rats with short access to nicotine self-administration.
Machines have existed for some time to expose rodents to cigarette smoke in quantities representative of first-hand or second-hand smoke, most often to assess the biological consequences of exposure to the carcinogens contained in cigarettes (Griffith & Standafer, 1985). More recently, a nicotine vapor model has been described that exposes rats to pure nicotine in breathing air on a pattern that can be controlled by the experimenter (George et al., 2010), similar to what has been described for alcohol vapor inhalation procedures (Gilpin et al., 2008). This nicotine vapor model is sufficient to produce somatic signs of withdrawal following systemic injection of the non-specific nicotinic acetylcholine receptor (nAchR) antagonist, mecamylamine. In these experiments, we hypothesized that chronic intermittent (12 hrs/day) nicotine vapor inhalation would increase nicotine self-administration during vapor withdrawal, and also produce somatic signs of dependence.
METHODS
Subjects
Twelve adult male Wistar rats were used in Experiment 1 and 11 male Wistar rats were used in Experiment 2, all obtained from Charles River (Kingston, NY). Animals were group-housed in standard plastic cages with wood chip bedding under a 12 hr light/12 hr dark cycle (lights off at 8 AM). Animals were given ad libitum access to food and water throughout except during experimental test sessions. All procedures were conducted in the dark cycle and met the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Experiment 1
Male Wistar rats (352.76 ± 6.61 g body weight at start of self-administration) were handled for 4-5 days then implanted with indwelling jugular catheters. Rats were anesthetized with isoflurane and implanted with a silastic catheter (0.3 mm i.d. x 0.64 mm o.d.; Dow Corning Co. Midland, MI) into the right external jugular vein under sterile conditions. The distal end of the catheter was subcutaneously threaded to the back of the rat where it exited via metal guide cannula (22 gauge; Plastics One, Roanoke, VA) anchored to the back of the rat. After surgery, rats were injected once with an analgesic (Flunixin, 2.5 mg/kg s.c.; Sigma-Aldrich, St. Louis, MO). Starting at 4 days post-surgery, catheters were flushed once daily with cefazolin (20 mg i.v.; Sigma-Aldrich, St. Louis, MO) in heparinized saline (30 U/ml, 0.1 ml total volume). On self-administration days, catheters were flushed once immediately prior to operant sessions with heparinized saline (0.1 ml) and once after sessions with cefazolin in heparinized saline (0.1 ml).
Following 5 days of recovery, rats were allowed 12 consecutive days of 2-hr daily sessions in which they pressed an active lever for i.v. nicotine infusions (0.03mg/kg/100μl/1s, free base, FR-1, timeout 20s) or an inactive lever that had no consequence. Nicotine solution was prepared twice per week (to account for changing body weights) by dissolving nicotine hydrogen tartrate salt in saline. No food or water was available during 2-hr sessions, but rats were never otherwise food-deprived during training. Across the last 3 days of baseline rats exhibited 7.26±1.90 responses on the active lever per 2-hr session (no rats were excluded from the study due to baseline responding), at which point rats were divided into two groups (n=6/group), those that would be exposed to chronic intermittent nicotine vapor (nicotine-dependent group) and those that would be exposed to ambient air (non-dependent group). Rats were tested for nicotine self-administration in 2-hr sessions (as described above) 8-12 hrs into withdrawal (WD) from vapor on days 1, 3, and 5-15 of vapor exposure.
To induce dependence on nicotine, animals were housed in Plexiglass chambers in a vapor delivery system (La Jolla Alcohol Research, Inc., La Jolla, CA) and exposed daily to intermittent (12 hrs ON/12 hrs OFF) nicotine vapor (George et al., 2010). Nicotine vapor was produced by bubbling air at a flow rate of 5 L/min (LPM) through a gas-washing bottle containing a solution of pure nicotine (free base, Sigma-Aldrich). The highly concentrated nicotine vapor was then passed through a drop-catch bottle and further diluted by the addition of 60 LPM of clean air in a 2000 mL Erlenmeyer vacuum flask at room temperature. The final nicotine–air mixture was homogeneously distributed between chambers at a flow rate of 15 LPM. Nicotine vapor concentrations were adjusted by varying the flow rate at which nicotine is bubbled. Air controls were treated in a similar manner except that air entering the cages did not contain nicotine.
Experiment 2
Rats were exposed to chronic intermittent nicotine vapor (as described above; n=6) or ambient air (n=5), and tested for behavioral signs of physical dependence on nicotine (Malin et al., 1992). At 8 hrs WD from nicotine vapor on day 13 of vapor exposure, rats were injected with saline (3 ml/kg, s.c.) and observed (10 min) for spontaneous behavioral signs of nicotine dependence. On day 14 of vapor exposure, rats were injected with mecamylamine (1.5 mg/3 ml/kg, s.c.), a non-specific antagonist of nicotinic acetylcholine receptors (nAchRs), and observed (10 min) for precipitated behavioral signs of nicotine dependence. During each test, each rat was observed by a treatment-blind experimenter for 10 min, during which time the number of blinks, gasps, writhes, head shakes, ptosis (drooping eyelids), teeth chattering, and yawns were recorded. Multiple successive counts of any sign required a distinct pause between episodes. Total occurrences of all somatic signs were summed for a single overall WD score for each rat.
Measurement of Air-Nicotine Concentrations
Measurement of air-nicotine levels was performed using the method developed by the National Institute for Occupational Safety and Health (NIOSH). Briefly, Nicotine air was sampled on sorbent tubes (XAD-2, 80/40 mg) at 1 L/m for 60 min. Samples were analyzed by the Hartford Laboratory using gas chromatography coupled with a nitrogen phosphorous detector (method 2544; NIOSH, 1977a,b).
Statistical Analysis
Data from FR-1 self-administration tests were analyzed using two-way repeated-measures analyses of variance (RM ANOVAs) where day was the within-subjects factor, and nicotine vapor history was the between-subjects factor. Data from somatic WD tests were analyzed using two-way repeated-measures analyses of variance (RM ANOVAs) where mecamylamine dose (0 or 1.5 mg/kg) was the within-subjects factor, and nicotine vapor history was the between-subjects factor. Post-hoc comparisons were conducted using the Student Newman-Keuls test. Statistical significance was set at p<0.05.
RESULTS
Self-administration on days 1, 3, and 5 of vapor exposure were analyzed relative to baseline to assess the effects of intermittent testing (i.e., deprivation effect) and also the early effects of vapor on nicotine self-administration. Two-way RM ANOVA indicated a marginally significant main effect of day on nicotine self-administration, F(3,30)=2.80, p=0.057, suggestive of a nicotine deprivation effect across all rats (Figure 1A). There was no effect of vapor on nicotine self-administration at this early stage of testing (p>0.05), nor was there a day x dependence interaction effect. Rats were then tested on consecutive days to assess the effect of chronic nicotine vapor on nicotine self- administration (Figure 1A). A two-way RM ANOVA for data from vapor days 6-15 indicated a significant day x dependence interaction effect F(10,100)=2.12, p=0.029, on nicotine responding. There was a tendency toward a main effect of nicotine vapor (p=0.07) and no main effect of day (p>0.05) on operant nicotine responding. Post-hoc analyses revealed that nicotine-dependent rats responded more for nicotine than non- dependent controls on days 13, 14, and 15 (p<0.02 in all cases). When active lever presses were expressed as function of baseline (Figure 1B), a two-way RM ANOVA for data from vapor days 6-15 indicated a significant day x dependence interaction effect F(9,90)=2.54, p=0.012. Post-hoc analyses revealed that, relative to controls, change in active lever presses from baseline was greater in nicotine-dependent rats on days 14 and 15 (p<0.05 in both cases).
Figure 1.
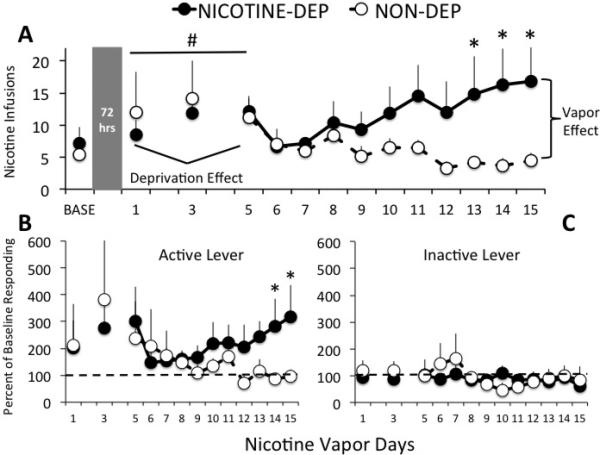
[A] Mean ± SEM nicotine infusions (FR-1 schedule, 20 s timeout) by nicotine vapor-exposed (black circles) and air-exposed (white circles) rats during 2-hr operant sessions prior to (baseline = 3-day mean) and during chronic intermittent (12 hrs/day) nicotine vapor exposure. Rats were tested intermittently during the first 5 days of vapor exposure (72 hrs between end of baseline & day 1 test, 48 hrs between day 1 & 3 tests, and 48 hrs between day 3 & 5 tests). Rats were then tested daily during days 6-15 of nicotine vapor exposure. All tests occurred at 8-12 hrs following removal from nicotine vapor (i.e., withdrawal). Data suggest a tendency toward a nicotine deprivation effect on days 1-5 regardless of nicotine vapor condition, # p=0.057 main effect of day. Also, nicotine vapor-exposed rats exhibited significant elevations in nicotine responding on days 13, 14, and 15 of vapor exposure, * p<0.02 relative to non-dependent controls. Also shown are [B] active and [C] inactive levers by nicotine vapor-exposed (black circles) and air-exposed (white circles) rats over days, expressed as percent of baseline (3-day mean) prior to the start of vapor exposure. Change from baseline active lever responding by individual rats confirms raw data and statistical analyses. Nicotine vapor-exposed rats exhibited no change from baseline inactive lever responding, nor did non- dependent controls with the exception of a slight increase on days 6 & 7 of the protocol, confirming that the difference between groups in inactive lever responding was due to baseline differences after rats were split into groups matched for active lever responding. * p<0.05 relative to non-dependent controls.
Nicotine vapor-exposed rats responded more than controls on the inactive lever over days during both intermittent, F(1,10)=12.38, p=0.006, and consecutive days, F(1,10)=11.23, p=0.007, testing (data not shown). However, relative to baseline, inactive lever responding did not change over time for either group (Figure 1C), confirming that rats exposed to nicotine vapor had higher baseline inactive lever pressing than air- exposed controls, Mann–Whitney U = 2.50, n1 = n2 = 6, p = 0.009.
As shown in Figure 2, a two-way RM ANOVA for data collected in Experiment 2 indicated a significant dose x dependence interaction effect on somatic withdrawal signs, F(1,9)=7.55, p=0.023. Main effects showed that nicotine-dependent rats exhibited higher somatic WD scores, F(1,9)=6.49, p=0.031, and somatic WD scores were higher after mecamylamine injections relative to saline injections, F(1,9)=13.82, p=0.005. Post-hoc analyses revealed that nicotine-dependent rats injected with mecamylamine exhibited significantly higher somatic WD scores relative to mecamylamine-injected controls (p=0.002) and also relative to their own scores following vehicle injections (p=0.001).
Figure 2.
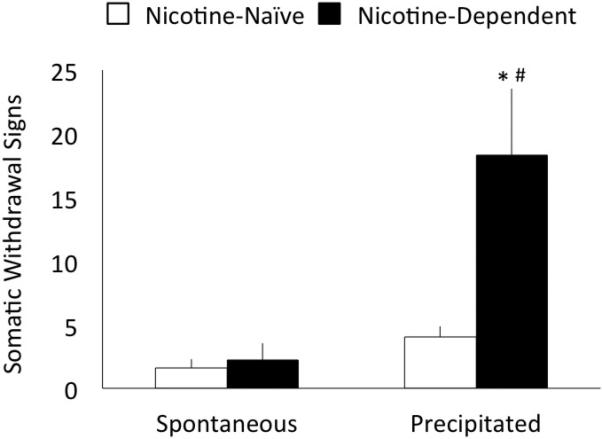
Mean ± SEM somatic withdrawal scores by nicotine vapor-exposed (black bars) and air-exposed (white bars) rats injected with saline (vapor day 13) or mecamylamine (1.5 mg/kg, vapor day 14) at ~8 hrs withdrawal from nicotine vapor. Scores from the 10-min observation period represent a summation of counts for behavioral signs that include blinks, gasps, writhes, head shakes, ptosis, teeth chattering, and yawns (Malin et al., 1992). Nicotine vapor-exposed rats exhibited higher scores overall than controls (p<0.05), and nicotine vapor-exposed rats injected with mecamylamine exhibited robust withdrawal relative to both saline injections and nicotine-naïve rats. * p<0.01 relative to non-dependent controls, # p<0.01 relative to saline injection.
Finally, as shown in Figure 3, measurement of nicotine in the air demonstrated a robust positive correlation between nicotine flow rate and the concentration of nicotine in the air (r2=0.99, p<0.001).
Figure 3.
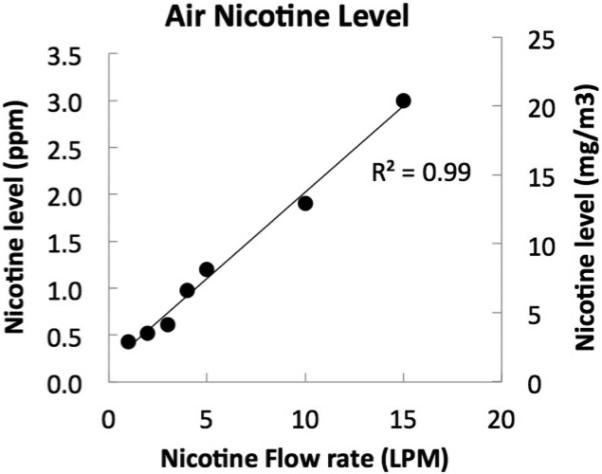
Air-nicotine concentrations expressed as parts per million (ppm; left y-axis) and mg/m3 (right y-axis). Air-Nicotine concentrations are shown as a function of nicotine flow rate, expressed in liter per minute (LPM). There was a strong positive correlation between nicotine flow rate and air-nicotine concentrations (r2=0.99). Rats in Experiments 1 and 2 were exposed to a nicotine flow rate of 5 LPM, which produces air- nicotine concentrations similar to those experienced by human smokers.
DISCUSSION
A major challenge in the field of pre-clinical nicotine addiction research has been the effort to produce escalated nicotine self-administration in rats. In this study, we achieve escalation of nicotine self-administration during daily limited-access (2-hr) operant sessions by exposing rats to chronic intermittent (12 hrs/day) nicotine vapor and testing rats for nicotine self-administration during withdrawal (8-12 hrs) from vapor. Early in the vapor exposure procedure (days 1-5), rats were tested for nicotine self-administration on non-consecutive days (48-72 hrs between sessions), and all rats exhibited a tendency toward a nicotine deprivation effect, regardless of nicotine vapor condition. Following 12 days of chronic intermittent nicotine vapor inhalation, rats exhibited increases in operant nicotine responding relative to non-dependent controls, and also relative to their own baseline both in terms of mean responding and percent change from baseline.
Baseline nicotine self-administration rates during short-access operant sessions vary greatly based on whether rats are previously trained to perform an operant response for food followed by substitution of an i.v. nicotine infusion for the food reinforcer. In our study, rats were never food-deprived and were never trained to perform an operant response for food. The baseline infusion rates observed in our study were slightly lower (per hour) but comparable to infusion rates previously observed in male Wistar rats trained in this way (e.g., Cohen et al., 2012).
Extended access to many drugs of abuse (e.g., stimulants, opiates) produces escalation of self-administration in rats (Koob & Kreek, 2007). However, simply allowing rats longer periods of access to operant nicotine, for example 6 hrs (Paterson & Markou, 2004) or 23 hrs (O'Dell et al., 2007) per day, is not sufficient to produce escalation of nicotine self-administration. A recent study showed that long-access self-administration levels are increased over time by (1) intermittence of testing and (2) co-administration of a monoamine oxidase inhibitor (MAOI), although one-hour intakes were not compared between long-access and short-access groups in that study (Cohen et al., 2012). In this study, we show that chronic intermittent nicotine vapor produces robust escalation of operant nicotine self-administration during short-access sessions, without co-administration of other drugs, and without the use of food substitution training. In the present study, all operant sessions occurred at 8-12 hrs withdrawal from levels of nicotine vapor that did not produce any observable signs of spontaneous somatic withdrawal, but did facilitate mecamylamine-precipitated somatic withdrawal.
There does not appear to be a one-to-one relationship between somatic signs of nicotine withdrawal and escalation of nicotine self-administration. Long-access operant nicotine sessions reliably produce mecamylamine-precipitated (but not spontaneous) somatic withdrawal signs (O'Dell et al., 2007, Paterson & Markou, 2004). Furthermore, severity of mecamylamine-precipitated nicotine withdrawal is positively correlated with mean total nicotine intake (but not escalation per se; O'Dell et al., 2007). However, in the Paterson & Markou (2004) study, rats exhibited spontaneous and precipitated nicotine withdrawal in the absence of escalation. In another study, long-access rats with both daily and intermittent access to nicotine exhibited spontaneous and precipitated withdrawal, but only rats with intermittent access exhibited escalation of intake (Cohen et al., 2012). In our study, nicotine vapor-exposed rats exhibited mecamylamine- precipitated, but not spontaneous, withdrawal (similar to George et al., 2010) and also exhibited escalation of short-access nicotine self-administration. The sum of these results plus the fact that rats are not injected with mecamylamine prior to self-administration sessions, suggests that physical withdrawal may contribute to, but is not solely responsible for escalated nicotine self-administration, and that there is likely a neural dissociation between physical and motivational signs of nicotine dependence (Koob, 2008).
In this study, rats were exposed to a constant air-nicotine concentration of ~7.5 mg/m3 over a 12-hr period. The average dose of nicotine per puff by a heavy smoker is ~75-200 μg (Xie et al., 2006), and the average daily nicotine intake in smokers is ~42 mg/day (Djordjevic et al., 2000). Considering that the total volume of air entering the lungs per minute in a healthy adult is 5-8 LPM at rest, the average human smoker is exposed to a range of air-nicotine concentrations between 4-12 mg/m3, similar to air-nicotine concentrations observed in the present study. This averaged daily concentration in humans does not take into consideration the smoking pattern and associated spike in nicotine level after each puff and cigarettes, although puff-associated spikes in brain-nicotine concentrations appear to be dampened in dependent smokers due to slower release from the lungs (Rose et al., 2009). These results suggest that chronic exposure to ~7.5 mg/m3 nicotine in rats may mimic the human condition.
Rats with long access (21-23 hrs/day) to operant nicotine exhibit a nicotine deprivation effect following 3 days without nicotine access (Cohen et al., 2012; George et al., 2007; O'Dell & Koob, 2007). Conversely, prior studies report that rats with short access to operant nicotine in 1-hr sessions do not show any increase in nicotine self-administration, transient or otherwise, when operant sessions are spaced by 48 or 72 hrs (Cohen et al., 2012; George et al., 2007). In the present study, we show that rats with short access to operant nicotine in 2-hr sessions exhibit increases in nicotine self-administration when operant sessions are spaced by 48 or 72 hrs, but this increase in responding is transient and fades upon resumption of consecutive-days testing. It is not clear what produced the different patterns of results in this experiment vs. previous studies, since both studies used the same rat strain and gender, the same unit dose, and the same deprivation durations. Two possible causes for these differential effects are (1) the length of operant nicotine self-administration sessions (2 hrs in this study vs. 1 hr in previous studies) or (2) slightly lower baseline levels of responding (per hour) in this study vs. previous studies.
In conclusion, the present investigation shows that chronic intermittent nicotine vapor inhalation produces escalation of nicotine self-administration, as well as physical dependence in rats. Furthermore, we report that rats exhibit a nicotine deprivation effect when 2-hr operant nicotine self-administration sessions are spaced by 48-72 hrs. It will be important for future studies to explore both the negative affective components of nicotine withdrawal as well as dose-response curves of i.v. nicotine self-administration in rats made dependent on nicotine via vapor inhalation. Overall, these data suggest that nicotine vapor can be used to induce escalation of nicotine self-administration, a long-standing obstacle in the field of nicotine addiction research.
ACKNOWLEDGEMENTS
The authors thank Dr. Ami Cohen and Ilham Polis for their assistance with nicotine vapor and nicotine self-administration methodologies. This work was funded by AA018400 (NWG), LSUHSC SOM Faculty Start-Up Funds (NWG), AA021097 (LSUHSC T35 training grant), AA007577 (LSUHSC T32 training grant).
REFERENCES
- Backup Data Report No. S-293, Nicotine. Documentation of the NIOSH Validation Tests. Department of Health, Education, and Welfare (NIOSH); 1977. Publication No. 77-185. Prepared for NIOSH under Contract #CDC-99-74-45. [Google Scholar]
- Cohen A, Koob GF, George O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacol. 2012;37:2153–60. doi: 10.1038/npp.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic MV, Stellman SD, Zang E. Doses of nicotine and lung carcinogens delivered to cigarette smokers. J Natl Cancer Inst. 2000;92:106–11. doi: 10.1093/jnci/92.2.106. [DOI] [PubMed] [Google Scholar]
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–60. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- George O, Ghozland S, Azar MR, Cottone P, Zorrilla EP, Parsons LH, et al. CRF- CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc Natl Acad Sci USA. 2007;104:17198–203. doi: 10.1073/pnas.0707585104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Grieder TE, Cole M, Koob GF. Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacol Biochem Behav. 2010;96:104–7. doi: 10.1016/j.pbb.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, Koob GF. Vapor inhalation of alcohol in rats. Curr Protoc Neurosci. 2008 doi: 10.1002/0471142301.ns0929s44. Chapter 9:Unit 9.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith RB, Standafer S. Simultaneous mainstream-sidestream smoke exposure systems II. The rat exposure system. Toxicol. 1985;35:13–24. doi: 10.1016/0300-483x(85)90128-3. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Fleming CB, Catalano RF. Individual and social influences on progression to daily smoking during adolescence. Pediatrics. 2009;124:895–902. doi: 10.1542/peds.2008-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59:11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G, Kreek MJ. Stress, dysregulation of drug reward pathways, and the transition to drug dependence. Am J Psychiatry. 2007;164:1149–59. doi: 10.1176/appi.ajp.2007.05030503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin DH, Lake JR, Newlin-Maultsby P, Roberts LK, Lanier JG, Carter VA, et al. Rodent model of nicotine abstinence syndrome. Pharmacol Biochem Behav. 1992;43:779–84. doi: 10.1016/0091-3057(92)90408-8. [DOI] [PubMed] [Google Scholar]
- NIOSH Manual of Analytical Methods. (2nd Edition) 1977 Apr;3 Method S293 DHHS (NIOSH) Publication 77-157-C. [Google Scholar]
- O'Dell LE, Chen SA, Smith RT, Specio SE, Balster RL, Paterson NE, et al. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J Pharmacol Exp Ther. 2007;320:180–193. doi: 10.1124/jpet.106.105270. [DOI] [PubMed] [Google Scholar]
- O'Dell LE, Koob GF. ‘Nicotine deprivation effect’ in rats with intermittent 23-hour access to intravenous nicotine self-administration. Pharmacol Biochem Behav. 2007;86:346–353. doi: 10.1016/j.pbb.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson NE, Markou A. Prolonged nicotine dependence associated with extended access to nicotine self-administration in rats. Psychopharmacol. 2004;173:64–72. doi: 10.1007/s00213-003-1692-7. [DOI] [PubMed] [Google Scholar]
- Rose JE, Mukhin AG, Lokitz SJ, Turkington TG, Herskovic J, Behm FM, Garg S, Garg PK. Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proc Natl Acad Sci U S A. 2010;107:5190–5. doi: 10.1073/pnas.0909184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie JP, Sun SH, Wang HY, Zong YL, Nie C, Guo YL. Determination of nicotine in mainstream smoke on the single puff level by liquid-phase microextraction coupled to matrix-assisted laser desorption/ionization Fourier transform mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:2573–8. doi: 10.1002/rcm.2636. [DOI] [PubMed] [Google Scholar]


