Abstract
Background
Deep brain stimulation (DBS) for treatment-resistant depression (TRD) is the focus of great interest and numerous studies. Given the state of this research, the risks of DBS, the uncertainty of direct benefits, and the potential for therapeutic misconception (TM), examination of research participants’ perspectives is critical to addressing concerns about the adequacy of consent among people with TRD.
Methods
Among 31 participants considering DBS studies at two sites, self-report questionnaires were used to examine three dimensions of TM (eight true/false items). Additional Likert-scale items assessed perceptions of risks, potential benefits, and altruistic motivations.
Results
Participants correctly identified the surgery itself as the riskiest study procedure, although only four participants rated the surgery as “high risk.” Most participants rated the entire DBS study as “moderate” or lower risk. Participants rated the likelihood of others benefiting in the future more strongly than they did the likelihood of personal benefit. Participants held positive attitudes toward research, and were moderately altruistic. Nearly two-thirds of the 31 participants (64.5%) answered at least one of the true/false TM items incorrectly.
Conclusions
Individuals considering DBS studies for TRD demonstrated reasonable perceptions of risks and benefits, distinguished among procedural risks, and expressed hopes for personal benefit as well as altruism. Findings related to TM were mixed: Participants understood the experimental stage of DBS for depression and endorsed the possibility of no personal benefit, yet there was some evidence for TM. Although these findings are reassuring, investigators must nevertheless remain vigilant about identifying and addressing potential misconceptions.
Keywords: informed consent, major depression, research benefits, research risks, therapeutic misconception
Depression is one of the most disabling medical conditions in the world (Moussavi et al. 2007; World Health Organization [WHO] 2008). Depression is associated with worse health outcomes, social problems, and up to 60% of deaths by suicide. It accounts for more than 11% of the total disease burden worldwide, with functional disabilities exceeded only by cardiovascular disease and cancer (Greden 2001; WHO World Mental Health Survey Consortium 2004). Up to 50% of patients with major depression continue to suffer from significant and disabling symptoms despite trying multiple available treatments (Fava 2003; Rush et al. 2006), making depression a prime example of the need for advances in psychiatric treatment.
Deep brain stimulation (DBS) has the potential to ameliorate the suffering of many individuals with diverse neurological, psychiatric, and medical conditions. DBS, a Food and Drug Administration (FDA)-approved treatment of Parkinson’s disease and several other movement disorders, is an invasive procedure that carries numerous risks (e.g., bleeding in the brain, infection, and complications from anesthesia, all of which could lead to permanent physical disability, neurological impairment, or death). DBS is currently in early-phase trials for a number of severe psychiatric disorders. Relatively small studies of DBS for treatment-resistant depression (TRD), obsessivecompulsive disorder (OCD), and Tourette’s syndrome have shown preliminary efficacy (Bewernick et al. 2010; Flaherty et al. 2005; Goodman et al. 2010; Greenberg et al. 2006; Holtzheimer and Mayberg 2011; Kennedy et al. 2011; Lozano et al. 2008; Malone et al. 2009; Mayberg et al. 2005). However, expansion of DBS as treatment for psychiatric disorders, without a sufficient evidence base from rigorously designed trials, is unwarranted at this time (Fins et al. 2011).
Early research on the use of DBS for psychiatric conditions has generated a number of ethical concerns (Bell, Mathieu, and Racine 2009; Dunn et al. 2011; Fins and Schiff 2010; Glannon 2010; Rabins et al. 2009; Schlaepfer, Lisanby and Pallanti 2010; Synofzik and Schlaepfer 2008; Wolpe, Ford and Harhay 2007). A conference convened in 2007 that sought to clarify scientific and ethical issues in DBS for mood and behavioral disorders produced 16 points for the field to consider, several of which highlighted the importance of the process by which consent is obtained from potential participants (Rabins et al. 2009). To date, however, few data have been available to inform these discussions. Some have argued that investigators’ or ethicists’ judgments and moral intuitions, while undoubtedly valuable, can nevertheless be usefully augmented by empirical data (Miller 2004; Roberts 2002). In the context of psychiatric research, data on the perspectives of research participants themselves have provided important insights into the decisional abilities, perceptions of risks and benefits, and influences on research enrollment decision making (Dunn, Candilis, and Roberts 2006; Roberts and Roberts 1999; Roberts et al. 2004). Such data can help inform debate, guidelines, and policymaking regarding research on DBS and other emerging technologies for psychiatric disorders.
One particularly salient issue in clinical studies involving technologies that are perceived as “cutting edge” by the research community and public alike (Churchill et al. 1998; Schlaepfer, Lisanby, and Pallanti 2010; Wind and Anderson 2008) is the blurring of the distinctions between research and clinical care—a form of “therapeutic misconception” (TM) (Appelbaum, Roth, and Lidz 1982; Henderson et al. 2007). TM continues to be discussed and debated in the bioethics literature (Appelbaum, Lidz, and Grisso 2004; Charuvastra and Marder 2008). Studies have indicated that a propensity for TM appears to occur across the spectrum of clinical research endeavors, although there is no gold-standard method of assessing its presence (Appelbaum et al. 2004; Appelbaum et al. 1987; Dunn et al. 2006; Durand-Zaleski et al. 2008; Henderson et al. 2006; Henderson et al. 2007; Misra et al. 2008).
A second ethical concern is the possibility that desperation—resulting from long-term, intractable illness—or other variables, such as severe depression itself, could affect patients’ abilities to judge appropriately the purposes, risks, and benefits of research studies, thereby influencing their willingness to enroll. Patients who are screened and who consent to early-phase DBS trials in psychiatry represent a subgroup of people with depression who have exhausted other treatment modalities or have few remaining, realistic options. This raises the important question of whether desperation could lead people to confuse research with clinical treatment, to judge risks inaccurately, or to have overly optimistic ideas about the potential for personal benefit (Jansen 2006; Jansen et al. 2011).
Therapeutic misconception and perceptions of risks and benefits have not been examined in patients with TRD who are considering enrolling in DBS research. This study evaluated these perceptions among a unique sample of patients being screened for enrollment in two early-phase DBS trials. General attitudes toward biomedical research, as well as the degree of altruistic motivation for participation in the DBS trial, were also assessed. Given the relatively small sample size and the paucity of previous research in this area, the study was exploratory and descriptive; the little literature that exists, however, led us to expect at least some evidence for TM in this sample. We also expected that patients with depression, similar to other patients with serious mental illness (Dunn, Candilis, and Roberts 2006; Roberts et al. 2002), would express both the hope for personal benefit, and altruism, and that, as a group, they would distinguish among levels of risk of different study procedures.
METHODS
Participants
Participants were pooled from a group of individuals considering two studies of DBS for TRD at two urban medical schools. Key inclusion criteria at Site A were: 18–70 years old; reliably diagnosed major depressive disorder (MDD) or bipolar II disorder; current major depressive episode of at least 12 months duration, not responding to at least four adequate antidepressant treatments; and lifetime failure of electroconvulsive therapy (ECT)—a procedure used for a variety of psychiatric conditions, including depression, in which seizures are electrically induced in anesthetized patients—or inability to receive ECT. Key exclusion criteria were: clinically significant medical or psychiatric comorbidity; recent substance use disorder; and active suicidal ideation with plan or intent (at time of screening and during the preoperative assessment period), or history of recent suicide attempts (within the past 2–3 years). At Site B, key inclusion and exclusion criteria were similar to those at site A, with the following additions: Age range was limited to 21–70 years old; diagnosis was restricted to MDD; and a prior trial of psychotherapy of known efficacy was required.
Patients who passed an initial screening for the DBS studies and participated in the consent procedure for the DBS study itself—which included an initial presurgical observation period to ensure that patients continued to meet severity criteria, based on the Hamilton Depression Rating Scale [HDRS-17 (Hamilton 1960)] or the Montgomery-Asberg Depression Rating Scale [MADRS (Montgomery and Asberg 1979)]—were asked to participate in this ancillary study of ethical issues in DBS research. As part of this ancillary study, these participants were administered, immediately following the fomal consent procedure, the MacArthur Competence Assessment Tool–Clinical Research [MacCAT-CR (Appelbaum and Grisso 2001)], a semistructured, open-ended interview designed to aid in the assessment of capacity to consent to participate in clinical research (data to be reported elsewhere). As part of the MacCAT-CR administration procedures, information pertaining to items that were answered incorrectly was reviewed again, providing the opportunity to clarify any misunderstandings.
At Site A, informed consent was obtained by a study psychiatrist; at Site B, informed consent was obtained by a research coordinator or study psychiatrist. The study design at each site included DBS surgery (during which two burr holes are drilled into a patient’s skull, bilateral DBS electrodes are implanted, and a battery pack is implanted subcutaneously in the chest wall). Additionally, both studies included a sham control period (1 month of a sham [stimulation off] lead-in at Site A, 6 months of either sham (stimulation off) or active [stimulation on] at Site B) followed by chronic, open-label DBS. Beyond the DBS surgery and long-term follow-up, patients at Site A were asked to participate in a number of ancillary studies (e.g., positron emission tomography [PET], a sleep study, and electroencephalography).
This study was approved by the institutional review boards at all participating institutions, and all subjects provided written informed consent for this ancillary study.
Measures
The following measures were completed by self-report after the consent and MacCAT-CR interview.
Therapeutic misconception
Based on prior work by Appelbaum, Lidz, and others on TM (Dunn et al. 2006; Lidz and Appelbaum 2002; Lidz et al. 2004), eight true/false statements were used to evaluate misconceptions regarding personal benefit from the study, beliefs about the individualization of treatment, and beliefs about the purpose of the study.
Perceptions of risks and benefits and altruistic motivations
Eight questions examined perceptions about overall study risks, potential for personal benefit, potential to benefit others in the future (i.e., advance scientific knowledge of treatment for depression), and desire to help others as a motivating factor for participation. Each item was scored on a 5-point Likert-type scale. Participants were also asked to rate the level of risk of each of the specific study procedures using the following scale: “Minimal risk or less (risks involved in everyday activities),” “Minor increase over minimal risk,” “Moderate risk,” or “High risk.”
General research attitudes
The Research Attitudes Questionnaire (RAQ) (Kim et al. 2005; Muroff, Hoerauf, and Kim 2006) assessed general attitudes toward biomedical research. The nine items on this scale include four negatively valenced (e.g., “Medical researchers are mainly motivated by personal gain”) and five positively valenced items, rated on a 5-point Likert scale (“strongly disagree” to “strongly agree”). An overall RAQ score was calculated by summing responses to all items, with negative items reversescored.
Data analysis
As this was an exploratory study, descriptive statistics were used to characterize the sample’s responses. Spearman rho (ρ) correlations were used to describe relationships between variables.
RESULTS
At Site A, 24 individuals passed the initial screening and reached the consent process; of these, three did not complete the full set of questionnaires, leaving 21 individuals with usable data. At Site B, data were available from all ten participants who reached the consent process for the DBS study. Therefore, data from 31 participants from both sites were available for the analyses presented here (some questions were left unanswered by some participants; in those cases the results represent data from fewer than 31 participants, as noted).
Demographic and clinical characteristics of participants are presented in Table 1. Participants ranged from 27 to 63 years old (mean 43.3, SD = 9.1), the majority (60.7%) were women, and 42.9% reported never having been married. Participants were well educated, with an average of 16.7 (SD = 3.0) years of education, and with 89.3% having at least some college education. Site differences were observed in the participant’s marital status (chi-square = 9.78, p =.04).
Table 1.
Demographic and clinical characteristics of participants (n = 31)
| Site A (n = 21) | Site B (n = 10) | Test statistic and p value | |
|---|---|---|---|
| Gender (% female) | 13 (61.9%) | 4 (57.1%) | Fisher's exact test, p = 1.00 |
| Age | 42.7 (9.6) | 44.4 (8.0) | t(26) = −0.39, p = .70 |
| Percent never married | 8 (38.1%)* | 4 (57.1%)* | Chi-square = 9.78, p = .04 |
| Percent at least some college education | 18 (85.7%) | 7 (100%) | Chi-square = 3.33, p = .50 |
| Years of education | 16.8 (3.4) | 16.6 (1.5) | t(25) = 0.17, p = .87 |
| Age of depression onset | 21.0 (10.5) | 20.1 (4.0) | t(25) = 0.21, p = .84 |
| Number of depressive episodes | 7.11 (9.0) | 4.3 (3.2) | t(24) = 0.80, p = .43 |
| Number of hospitalizations | 4.9 (5.3)* | 1.3 (0.5)* | t(18.81) = 2.93, p =.01 |
| Number of suicide attempts | 1.68 (2.9) | 1.29 (2.2) | t(24) = 0.33, p = .74 |
| HDRS-17 score at baseline | 24.0 (3.4)* | 20.3 (2.3)* | t(24) = 0 = 2.66, p = .02 |
| Percent family history of bipolar | 42.1% | N/A | N/A |
| Number of hypomanic episodes | 4.53 (11.6) | N/A | N/A |
Note. t-Tests degrees of freedom vary due to missing data. The variance of number of hospitalizations was unequal between the two sites (Levene's F = 5.21, p < .04); therefore, a t-test without the equal variance assumption was conducted. Asterisk indicates significant difference.
In terms of clinical characteristics, participants reported a mean of 6.4 (SD = 7.9; range: 1–40) depressive episodes, with the mean age of the onset being 20.8 years (SD = 9.2; range: 4–42). They reported a mean of 1.6 (SD = 2.7; range: 0–12) lifetime suicide attempts and 3.9 (SD = 4.8; range: 0–20) hospitalizations due to depressive symptoms. At baseline, participants had a mean HDRS score of 22.9 (SD = 3.5, range: 17–30). Site differences were found in the number of previous hospitalizations for depression (m = 4.9 (SD = 5.3) vs. m = 1.3 (SD = 0.5), t(18.81) = 2.93, p = .01) and the mean HDRS-17 score (m = 24.0 (SD = 3.4) vs. m = 20.3 (SD = 2.3), t(24) = 2.66, p = .02). Fifteen of the 20 participants providing family history data (75%) indicated a history of depression. Six participants met criteria for other concurrent psychiatric diagnoses; of these, anxiety disorders were the most prevalent. Two additional participants met criteria for other past psychiatric diagnoses (in full remission). At Site A, patients with a diagnosis of bipolar disorder, type II, were included; eight (38%) participants had that diagnosis and reported a mean of 4.5 hypomanic episodes (SD = 11.6; range: 0–50). Eight participants in the total sample (42% of those providing data) indicated a family history of bipolar disorder.
Of the 31 participants in the present study, eight did not go on to participate in the DBS studies. At Site A, three were excluded for not meeting the severity criteria, one was excluded for psychiatric comorbidity, and one participant withdrew due to lack of interest. At Site B, one participant was excluded for not meeting the severity criteria, one was excluded for suicidality severity, and one withdrew. No participant was excluded due to impaired capacity to provide informed consent, based either on MacCAT-CR scores or on responses to the therapeutic misconception true/false items.
Performance on therapeutic true/false items (Table 2). Of the 31 participants, 11 (35.5%) answered all items correctly, 12 (38.7%) missed one item, 7 (22.6%) missed two items, and 1 (3.2%) missed three items. Taken together, nearly twothirds (64.5%) of participants incorrectly answered at least one item related to the study’s purpose, likelihood of personal benefit, or individualization of treatment. Seven participants endorsed both of the items intended to be mutually exclusive (“The study is mostly intended to learn more about DBS” and “The study is mostly intended to help me”).
Table 2.
Performance on therapeutic misconception true/false items (n = 31)
| (Dimension of therapeutic misconception) Specific item | Participants responding incorrectly, n (%) |
|---|---|
| (Purpose of the study) | |
| The study is mostly intended to learn more about DBS as a treatment for depression. (T) |
2 (6.5%) |
| The study is mostly intended to help me. (F) | 7 (22.6%) |
| (Likelihood of personal benefit) | |
| There is a chance that I will not benefit from being in the study. (T) | 0 (0%) |
| My own treatment for depression will certainly be improved as a result of this study. (F) |
6 (19.4%) |
| DBS is still an experimental treatment for depression. (T) | 0 (0%) |
| (Individualization of treatment) | |
| The research physician cannot add any other medication for depression during the first seven months of the study, even if he or she thinks it would help me. (T) |
7 (22.6%) |
| During the first month after surgery, the research physicians will know whether my DBS stimulator is ON or OFF. (T) |
3 (9.7%) |
| During the first month after surgery, I will definitely receive active DBS. (F) | 4 (12.9%) |
Note. (T)–correct response is "True"; (F)–correct response is "False."
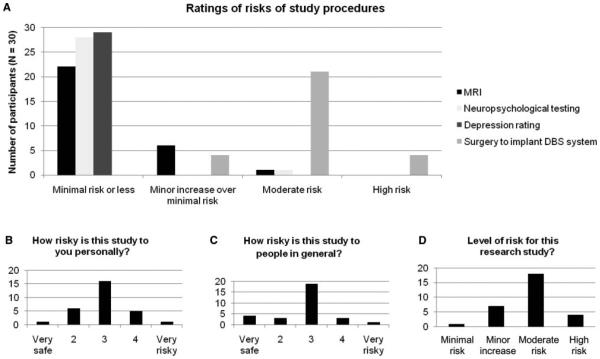
Perception of risks and benefits (Figures 1 and 2). Participants’ ratings of the risks of the various study procedures are shown in Figure 1A. Two procedures (depression rating scales and neuropsychological testing) were rated as minimally risky by all or almost all participants. DBS surgery was identified as the riskiest procedure (modal rating: “moderate risk,” n = 21; “high risk,” n = 4; “minor increase over minimal risk,” n = 4). No participants rated the surgery as “minimal risk,” and all but one participant rated it higher than all other procedures (this participant rated the magnetic resonance imaging [MRI], neuropsychological testing, and DBS surgery as “moderate risk”).
Figure 1.
(A) Perceived risks of specific study procedures. (B) Perceived risk of study to participant personally. (C) Perceived risk of study to people with TRD in general. (D) Participants’ ratings of overall level of risk of study.
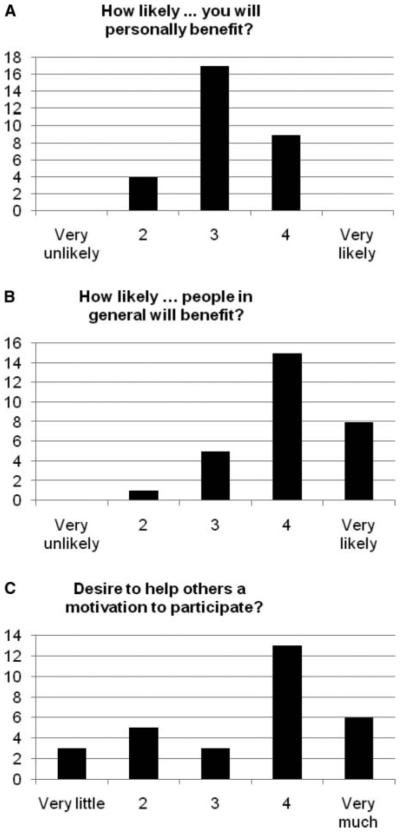
Figure 2.
(A) Participants’ ratings of likelihood of personal benefit. (B) Participants’ ratings of likelihood of benefit to people with TRD in general. (C) Participants’ ratings of altruism as a motivating factor in participation.
The distributions of risk ratings for personal risk and for risk to people generally, scaled from 1 = very safe to 5 = very risky, are shown in Figures 1B and 1C. The mean risk ratings were both moderate and did not differ for personal and general (3.0 (SD = 0.8) vs. 2.8 (SD = 0.9), paired t(29) = -1.04, p = .31). When asked to rate the level of risk on a categorical, four-level scale, the majority of participants (n = 18, 58.1%) rated the overall risk of the study as “moderate” (Figure 1D), and only one participant (3.3%) rated it as “minimal risk.”
The majority of participants rated the likelihood of personal benefit as a 3 on the 5-point scale (Figure 2A). However, the modal rating of the likelihood that others would benefit was a “4,” and the mean rating for likelihood of others benefiting was significantly greater than the mean rating for the likelihood of personal benefit (4.0 (SD = 0.8) vs. 3.21 (SD = 0.6), paired t(27) = –4.53, p < .001).
Although ratings of personal risk correlated with ratings of risks to people in general (ρ = 0.45, p = .01), they were not related to the ratings of personal benefit (ρ = .09, p = .63). No participant disagreed with the statement “In my opinion, the possible benefits to me outweigh the personal risks of being in this study.” Five participants were neutral regarding this statement; 10 agreed, and 14 strongly agreed.
General research attitudes. Positive and negative research attitude scores were calculated by summing the scores on the five positively valenced and four negatively valenced items, respectively, on the RAQ. Participants tended to be positively predisposed toward research; the mean score on the Positive attitude subscale was 19.8 (SD = 2.4; possible range: 5–25; higher score = more positive) while the mean on the Negative attitude subscale was 14.0 (SD = 2.2; possible range: 4–20; higher score = less negative). The mean overall score was 32.3 (SD = 3.2, possible range: 9–45), reflecting generally positive attitudes toward research.
Altruism (Figure 2C). Participants were moderately altruistic regarding participation in the study, as assessed by the response to the question “To what degree is the desire to help others in the future a motivation for your participating in this study?” with an average rating of 3.47 (SD = 1.3, range: 1 “very little” to 5 “very much”). Altruism was significantly correlated with both the interest in enrolling in research (ρ = 0.39, p = .04) and a generally positive attitude toward research (ρ = 0.46, p = .02), as well as with greater perceived likelihood that other people might benefit from the study (ρ = 0.32, p = .09), at a level of a nonsignificant trend. Altruism was not related to the expectation of personal benefit (ρ = 0.09, p = .66).
DISCUSSION
The purpose of this study was to characterize the perceptions of risks and benefits and the prevalence and nature of TM among a unique sample of people considering enrollment in early phase DBS clinical trials for TRD. As a group, these participants distinguished reliably among risk levels of different study procedures, expressed hopes for personal benefit, and demonstrated moderate levels of altruism on average. However, they also showed some evidence of TM, which was consistent with our expectations.
Of note was that these participants did not consider DBS, a key component of which involves implantation of electrodes in a deep structure of the brain (in this case, the anterior cingulate), especially risky. This finding may reflect, at least in part, the population sampled—that is, patients who had sought out the DBS trials, undergone detailed screening procedures, and considered the risks and benefits of the DBS study for some time before the present questionnaire was administered. Moreover, in many cases, patients had researched DBS on their own and were knowledgeable about prior studies. Thus, this sample was already favorably predisposed to considering DBS as an experimental treatment for their depression. Their views therefore may not generalize to those of the larger population of people with TRD.
Another explanation for this finding is that, as definitions were not provided for “high,” “moderate,” or “minimal risk,” respondents were free to assign their own “anchoring” for these terms. In their open-ended responses (Christopher et al. In Press), some participants explicitly contrasted the risks of participating in the study with the risks of staying depressed. Thus, compared to the latter, the risks of the DBS study may not have seemed high. Nevertheless, these findings underscore the importance of reviewing serious risks with potential research participants repeatedly and in detail to ensure that participants are truly informed and do not unreasonably minimize potential risks.
As pointed out by Lipsman and colleagues (2010), “Although DBS is minimally invasive neurosurgery, it is the most maximally invasive psychiatric treatment available.” Thus, the “moderate” risk rating may indeed be a fairly accurate compromise between the status of DBS in the neurosurgical field, and the novelty and invasiveness of DBS in psychiatry. Indeed, it appeared that the participants understood the nature of DBS surgery, as they assigned higher risk ratings to DBS surgery than to any other study procedures.
How participants viewed the likelihood of benefit was also noteworthy. Overall, participants expressed a certain degree of ambivalence regarding the likelihood of personal benefit. This may have reflected a mixture of both hope for improvement and some degree of hopelessness. Hopelessness is a defining feature of patients with severe depression, and given that these patients had tried so many treatments without success, this emotional response may have tempered a cognitive ability to consider the possibility of benefit. At the same time, they were significantly more likely to agree with the prospect that the study would benefit others than that it would benefit themselves, which suggests that they see considerable promise in DBS as a treatment for depression. Taken together, these findings are reassuring. They suggest that in spite of media portrayals of DBS outcomes, which tend to highlight the likelihood of benefit while minimizing ethical concerns and associated risks (Racine et al. 2007; Schlaepfer, Lisanby and Pallanti 2010), most prospective participants hold a cautious outlook regarding whether DBS will benefit them directly.
These participants were positively disposed toward research in general and moderately altruistic regarding their motivation to participate in the DBS trial. As with all questions of this type, some responses may have been the result of demand characteristics—that is, participants may have given what they perceived as “appropriate” responses. On the other hand, respondents who reported an altruistic motive to participate were also more likely to believe that the DBS trial would benefit others, without being more likely to expect personal benefit, suggesting their altruistic motives are, in fact, genuine.
Consistent with their views on the likelihood of personal benefit, all participants correctly answered TM items regarding the possibility that DBS would not benefit them personally and regarding the experimental nature of the treatment. This finding suggests that participants grasped that the treatment was not proven and that improvement for themselves personally was not guaranteed. However, given that some participants also incorrectly answered other items regarding personalization of treatment within the trial, the implication of this finding is not entirely clear. It is possible that these two items most closely reflected what patients recalled from the consent discussions, for example. It is also possible that participants did not expect personal benefit, while still hoping for personal benefit. Further work is warranted that explores in more depth potential participants’ expectations of personal benefit in the context of experimental trials involving novel therapies or applications.
A majority of respondents gave incorrect responses to at least one of the other TM items, with more than onethird of respondents answering two or more incorrectly. This high prevalence is consistent with prior work examining TM across a variety of research settings, including several early-phase trials (Appelbaum et al. 2004; Daugherty et al. 1995; Daugherty et al. 2000; Henderson et al. 2006). In this study, the TM items with the most frequent incorrect responses had to do with the participant’s understanding of the therapeutic orientation of the trial in relation to him- or herself. Thus, while these participants do not appear to have unrealistic expectations that DBS will benefit them personally (as noted earlier), a significant minority incorrectly answered items regarding the trial being intended primarily to help them, the likelihood of their own depression treatment improving by being in the study, or the research physician’s ability to tailor the treatment to their individual needs. Other indicators of TM—failing to identify DBS as an experimental intervention and the primary purpose of the trial as learning more about DBS—were notably absent from this group. This apparent discrepancy should not be surprising. Rather, it reflects the multidimensional nature of TM (Appelbaum et al. 2004) and the fact that indicators of TM are likely to vary across subjects and across clinical research setting, type, and primary motivation.
Such heterogeneity also highlights the challenges that researchers face in seeking to identify and minimize TM among their subjects. As these data demonstrate, despite the long and detailed nature of the informed consent process for these two DBS trials (consistent with early-phase research), an accurate understanding of the likelihood of risk and benefit could not be taken as evidence that potential subjects do not have any manifestations of TM. Thus, the best efforts to reduce TM require careful probing and clarification regarding each TM dimension, using questions tailored to the details of the study protocol. As there is no gold-standard instrument for measuring TM, investigators need to take care to consider what aspects of the study are most critical for subjects to grasp, what differences exist between clinical care and the study, and how these may impact subjects’ ongoing care.
This investigation had several limitations. The small sample size limited the type of analyses that could be performed, as well as the interpretations that could be made. However, the small sample size reflected the available pool of participants—namely, the subgroup of people with TRD who were willing to participate in DBS and passed the rigorous screening process for these early phase trials. Several of the findings were based on single-item responses. In selecting our instruments, we erred on the side of reducing the questionnaire burden on depressed participants, and selected fewer items that were face-valid and easily comprehensible. Finally, participants’ depressed status may have skewed the responses to some questions, especially those related to the hopes of direct benefit. However, these responses likely reflect the subjective perceptions of very depressed individuals facing a difficult choice of whether to undergo a complex and still-experimental intervention for their depression.
DBS may eventually be shown to offer the possibility of improvement for many individuals suffering from TRD. However, given the infancy of this field, valid informed consent in this context will necessarily need to be comprehensive and dense, given the complexity of the intervention being studied, the potential risks, and the many unknowns. Overall, the persons considering enrollment in these two DBS for TRD trials exhibited a reasonable understanding of the risks and likelihood of personal benefit. Of course, these findings may not necessarily be true of participants in other DBS trials. Consistent with other early-phase clinical research, most potential participants demonstrated a vulnerability to mistaking aspects of the DBS research for those of traditional clinical treatment, although not the experimental nature of the trial or the possibility that DBS would not be personally beneficial. Future empirical ethics research in this area should include a more detailed examination of the manifestations of therapeutic misconception to which participants with depression (and other psychiatric disorders) may be vulnerable, as well as its antecedents, and should explore efforts to reduce TM within informed consent discussions. •
Contributor Information
Yan Leykin, University of California–San Francisco.
Paul P. Christopher, University of Massachusetts Medical School
Paul E. Holtzheimer, Emory University
Paul S. Appelbaum, Columbia University
Helen S. Mayberg, Emory University
Sarah H. Lisanby, Duke University
Laura B. Dunn, University of California–San Francisco
REFERENCES
- Appelbaum PS, Grisso T. MacCAT-CR: MacArthur Competence Assessment Tool for Clinical Research. Professional Resource Press; Sarasota, FL: 2001. [Google Scholar]
- Appelbaum PS, Lidz CW, Grisso T. Therapeutic misconception in clinical research: Frequency and risk factors. IRB. 2004;26(2):1–8. [PubMed] [Google Scholar]
- Appelbaum PS, Roth LH, Lidz. C. The therapeutic misconception: informed consent in psychiatric research. International Journal of Law and Psychiatry. 1982;5(3–4):319–329. doi: 10.1016/0160-2527(82)90026-7. [DOI] [PubMed] [Google Scholar]
- Appelbaum PS, Roth LH, Lidz CW, Benson P, Winslade W. False hopes and best data: Consent to research and the therapeutic misconception. Hastings Center Report. 1987;17(2):20–24. [PubMed] [Google Scholar]
- Bell E, Mathieu G, Racine E. Preparing the ethical future of deep brain stimulation. Surgical Neurology. 2009;72(6):577–586. doi: 10.1016/j.surneu.2009.03.029. [DOI] [PubMed] [Google Scholar]
- Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biological Psychiatry. 2010;67(2):110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- Charuvastra A, Marder SR. Unconscious emotional reasoning and the therapeutic misconception. Journal of Medical Ethics. 2008;34(3):193–197. doi: 10.1136/jme.2006.018960. [DOI] [PubMed] [Google Scholar]
- Christopher PP, Leykin Y, Holtzheimer PE, et al. Enrolling in deep brain stimulation research for depression: Influences on potential subjects’ decision making. Depression and Anxiety. doi: 10.1002/da.20916. In press. [DOI] [PubMed] [Google Scholar]
- Churchill LR, Collins ML, King NM, Pemberton SG, Wailoo KA. Genetic research as therapy: Implications of “gene therapy” for informed consent. Journal of Law, Medicine & Ethics. 1998;26(1):38–47. doi: 10.1111/j.1748-720x.1998.tb01904.x. 3. [DOI] [PubMed] [Google Scholar]
- Daugherty C, Ratain MJ, Grochowski E, et al. Perceptions of cancer patients and their physicians involved in phase I trials. Journal of Clinical Oncology. 1995;13(5):1062–1072. doi: 10.1200/JCO.1995.13.5.1062. [DOI] [PubMed] [Google Scholar]
- Daugherty CK, Banik DM, Janish L, Ratain MJ. Quantitative analysis of ethical issues in phase I trials: A survey interview of 144 advanced cancer patients. IRB. 2000;22(3):6–14. [PubMed] [Google Scholar]
- Dunn LB, Candilis PJ, Roberts LW. Emerging empirical evidence on the ethics of schizophrenia research. Schizophrenia Bulletin. 2006;32(1):47–68. doi: 10.1093/schbul/sbj012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LB, Holtzheimer PE, 3rd, Hoop JG, Mayberg H, Roberts LW, Appelbaum PS. Ethical issues in deep brain stimulation research for treatment-resistant depression: Focus on risk and consent. AJOB Neuroscience. 2011;2:29–36. doi: 10.1080/21507740.2010.533638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LB, Palmer BW, Keehan M, Jeste DV, Appelbaum PS. Assessment of therapeutic misconception in older schizophrenia patients with a brief instrument. American Journal of Psychiatry. 2006;163(3):500–506. doi: 10.1176/appi.ajp.163.3.500. [DOI] [PubMed] [Google Scholar]
- Durand-Zaleski IS, Alberti C, Durieux P, et al. Informed consent in clinical research in France: Assessment and factors associated with therapeutic misconception. Journal of Medical Ethics. 2008;34(9):e16. doi: 10.1136/jme.2007.023473. [DOI] [PubMed] [Google Scholar]
- Fava M. Diagnosis and definition of treatment-resistant depression. Biological Psychiatry. 2003;53(8):649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- Fins JJ, Mayberg HS, Nuttin B, et al. Misuse of the FDA’s humanitarian device exemption in deep brain stimulation for obsessive-compulsive disorder. Health Affairs. 2011;30(2):302–311. doi: 10.1377/hlthaff.2010.0157. [DOI] [PubMed] [Google Scholar]
- Fins JJ, Schiff ND. Conflicts of interest in deep brain stimulation research and the ethics of transparency. Journal of Clinical Ethics. 2010;21(2):125–132. [PubMed] [Google Scholar]
- Flaherty AW, Williams ZM, Amirnovin R, et al. Deep brain stimulation of the anterior internal capsule for the treatment of Tourette syndrome: Technical case report. Neurosurgery. 2005;57(4 suppl.):E403. doi: 10.1227/01.neu.0000176854.24694.95. discussion E403. [DOI] [PubMed] [Google Scholar]
- Glannon W. Consent to deep brain stimulation for neurological and psychiatric disorders. Journal of Clinical Ethics. 2010;21(2):104–111. [PubMed] [Google Scholar]
- Goodman WK, Foote KD, Greenberg BD, et al. Deep brain stimulation for intractable obsessive compulsive disorder: Pilot study using a blinded, staggered-onset design. Biological Psychiatry. 2010;67(6):535–542. doi: 10.1016/j.biopsych.2009.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greden JF. The burden of recurrent depression: Causes, consequences, and future prospects. Journal of Clinical Psychiatry. 2001;62(suppl. 22):5–9. [PubMed] [Google Scholar]
- Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31(11):2384–2393. doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery & Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GE, Churchill LR, Davis AM, et al. Clinical trials and medical care: Defining the therapeutic misconception. PLoS Medicine. 2007;4(11):1735–1738. doi: 10.1371/journal.pmed.0040324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson GE, Easter MM, Zimmer C, et al. Therapeutic misconception in early phase gene transfer trials. Social Science & Medicine. 2006;62(1):239–253. doi: 10.1016/j.socscimed.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS. Deep brain stimulation for psychiatric disorders. Annual Review of Neuroscience. 2011;34:289–307. doi: 10.1146/annurev-neuro-061010-113638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen LA. The problem with optimism in clinical trials. IRB. 2006;28(4):13–19. [PubMed] [Google Scholar]
- Jansen LA, Appelbaum PS, Klein WM, et al. Unrealistic optimism in early-phase oncology trials. IRB. 2011;33(1):1–8. [PMC free article] [PubMed] [Google Scholar]
- Kennedy SH, Giacobbe P, Rizvi SJ, et al. Deep brain stimulation for treatment-resistant depression: Follow-up after 3 to 6 years. American Journal of Psychiatry. 2011;168(5):502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- Kim SYH, Kim HM, McCallum C, Tariot PN. What do people at risk for Alzheimer disease think about surrogate consent for research? Neurology. 2005;65:1395–1401. doi: 10.1212/01.wnl.0000183144.61428.73. [DOI] [PubMed] [Google Scholar]
- Lidz CW, Appelbaum PS. The therapeutic misconception: Problems and solutions. Medical Care. 2002;40(9 suppl.):V55–V63. doi: 10.1097/01.MLR.0000023956.25813.18. [DOI] [PubMed] [Google Scholar]
- Lidz CW, Appelbaum PS, Grisso T, Renaud M. Therapeutic misconception and the appreciation of risks in clinical trials. Social Science & Medicine. 2004;58(9):1689–1697. doi: 10.1016/S0277-9536(03)00338-1. [DOI] [PubMed] [Google Scholar]
- Lipsman N, Bernstein M, Lozano AM. Criteria for the ethical conduct of psychiatric neurosurgery clinical trials. Neurosurgery Focus. 2010;29(2):E9. doi: 10.3171/2010.4.FOCUS09327. [DOI] [PubMed] [Google Scholar]
- Lozano AM, Mayberg HS, Giacobbe P, Hamani C, Craddock RC, Kennedy SH. Subcallosal cingulate gyrus deep brain stimulation for treatment-resistant depression. Biological Psychiatry. 2008;64(6):461–467. doi: 10.1016/j.biopsych.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Malone DA, Jr., Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biological Psychiatry. 2009;65(4):267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Miller FG. Research ethics and misguided moral intuition. Journal of Law, Medicine & Ethics. 2004;32(1):111–116. doi: 10.1111/j.1748-720x.2004.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Misra S, Socherman R, Hauser P, Ganzini L. Appreciation of research information in patients with bipolar disorder. Bipolar Disorders. 2008;10(5):635–646. doi: 10.1111/j.1399-5618.2008.00609.x. [DOI] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Moussavi S, Chatterji S, Verdes E, Tandon A, Patel V, Ustun B. Depression, chronic diseases, and decrements in health: Results from the World Health Surveys. Lancet. 2007;370(9590):851–858. doi: 10.1016/S0140-6736(07)61415-9. [DOI] [PubMed] [Google Scholar]
- Muroff JR, Hoerauf SL, Kim SY. Is psychiatric research stigmatized? An experimental survey of the public. Schizophrenias Bulletin. 2006;32(1):129–136. doi: 10.1093/schbul/sbj003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabins P, Appleby BS, Brandt J, et al. Scientific and ethical issues related to deep brain stimulation for disorders of mood, behavior, and thought. Archives of General Psychiatry. 2009;66(9):931–937. doi: 10.1001/archgenpsychiatry.2009.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine E, Waldman S, Palmour N, Risse D, Illes J, U.K. print media “Currents of hope”: Neurostimulation techniques in U.S. Cambridge Quarterly of Healthcare Ethics. 2007;16(3):312–316. [PubMed] [Google Scholar]
- Roberts L, Roberts B. Psychiatric research ethics: an overview of evolving guidelines and current ethical dilemmas in the study of mental illness. Biological Psychiatry. 1999;46(8):1025–1038. doi: 10.1016/s0006-3223(99)00205-x. [DOI] [PubMed] [Google Scholar]
- Roberts LW. Ethics and mental illness research. Psychiatric Clinics of North America. 2002;25(3):525–545. doi: 10.1016/s0193-953x(01)00014-4. [DOI] [PubMed] [Google Scholar]
- Roberts LW, Hammond KA, Warner TD, Lewis R. Influence of ethical safeguards on research participation: Comparison of perspectives of people with schizophrenia and psychiatrists. American Journal of Psychiatry. 2004;161(12):2309–2311. doi: 10.1176/appi.ajp.161.12.2309. [DOI] [PubMed] [Google Scholar]
- Roberts LW, Warner TD, Brody JL, Roberts B, Lauriello J, Lyketsos C. Patient and psychiatrist ratings of hypothetical schizophrenia research protocols: Assessment of harm potential and factors influencing participation decisions. American Journal of Psychiatry. 2002;159(4):573–584. doi: 10.1176/appi.ajp.159.4.573. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR∗ D report. American Journal of Psychiatry. 2006;163(11):1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Lisanby SH, Pallanti S. Separating hope from hype: some ethical implications of the development of deep brain stimulation in psychiatric research and treatment. CNS Spectrums. 2010;15(5):285–287. doi: 10.1017/s1092852900027504. [DOI] [PubMed] [Google Scholar]
- Synofzik M, Schlaepfer TE. Stimulating personality: Ethical criteria for deep brain stimulation in psychiatric patients and for enhancement purposes. Biotechnology Journal. 2008;3(12):1511–1520. doi: 10.1002/biot.200800187. [DOI] [PubMed] [Google Scholar]
- WHO World Mental Health Survey Consortium Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. Journal of the American Medical Association. 2004;291:2581–2590. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- Wind JJ, Anderson DE. From prefrontal leukotomy to deep brain stimulation: The historical transformation of psychosurgery and the emergence of neuroethics. Neurosurgical Focus. 2008;25(1):E10. doi: 10.3171/FOC/2008/25/7/E10. [DOI] [PubMed] [Google Scholar]
- Wolpe PR, Ford PJ, Harhay M. Ethical issues in deep brain stimulation. In: Baltuch GH, Stern MB, editors. Deep brain stimulation for Parkinson’s disease. Informa Healthcare; New York: 2007. pp. 323–338. [Google Scholar]
- World Health Organization . The global burden of disease. 2004 Update. WHO Press; Geneva: 2008. [Google Scholar]