Abstract
Rationale
Ethanol and nicotine are frequently co-abused. The biological basis for the high co-morbidity rate is not known. Alcohol-preferring (P) rats will self-administer EtOH or nicotine directly into the posterior ventral tegmental area (pVTA).
Objective
The current experiments examined whether sub-threshold concentrations of EtOH and nicotine would support the development of self-administration behaviors if the drugs were combined.
Methods
Rats were implanted with a guide cannula aimed at the pVTA. Rats were randomly assigned to groups that self-administered sub-threshold concentrations of EtOH (50 mg%) or nicotine (1 μM) or combinations of ethanol (25 or 50 mg%) and nicotine (0.5 or 1.0 μM). Alterations in gene expression downstream projections areas (nucleus accumbens shell, AcbSh) were assessed following a single, acute exposure to EtOH (50 mg%), nicotine (1 μM) or ethanol and nicotine (50 mg% + 1 μM) directly into the pVTA.
Results
The results indicated that P rats would co-administer EtOH and nicotine directly into the pVTA at concentrations that did not support individual self-administration. EtOH and nicotine directly administered into the pVTA resulted in alterations in gene expression in the AcbSh (50.8-fold increase in BDNF, 2.4-fold decrease in GDNF, 10.3-fold increase in Vglut1) that were not observed following microinjections of equivalent concentrations/doses of ethanol or nicotine.
Conclusion
The data indicate that ethanol and nicotine act synergistically to produce reinforcement and alter gene expression within the mesolimbic dopamine system. The high rate of co-morbidity of alcoholism and nicotine dependence could the result of the interactions of EtOH and nicotine within the mesolimbic dopamine system.
Introduction
Ethanol and nicotine co-use/abuse is common. Individuals that are nicotine dependent are 10 times more likely to be diagnosed with alcoholism than non-smokers in their lifetime (DiFranza and Guerrera 1990). The rate of smoking is high (between 80 and 97%; John et al. 2003a,b) in alcoholics, and has remained constant while the overall smoking rate has reduced (John et al. 2003a,b). Co-abuse is associated with higher rates of drug craving, increased levels of drug consumption during relapse, reduction in clinical outcomes, and an increased rate of high levels of drug consumption (c.f., Gilbertson et al. 2011). In non-addicted individuals, nicotine and alcohol co-use increases consumption of both drugs (Ati-Daoud et al. 2005). The initiation of nicotine and ethanol co-use typically begins in adolescence, which leads to greater detrimental adult outcomes than if a single drug is used during adolescence (Hingson et al. 2008).
Despite the extensive clinical evidence that ethanol and nicotine co-abuse is pervasive, there has been insufficient basic research conducted to understand the biological basis of the co-abuse. The mesolimbic dopamine system has been hypothesized to be a critical neurocircuit for drug reward/reinforcement. Independently, ethanol and nicotine are directly self-administered into the posterior ventral tegmental area (pVTA) at pharmacologically relevant concentrations (Rodd et al. 2004a; Ikemoto et al. 2006; Hauser et al. 2013). The sensitivity of the pVTA to the reinforcing properties of nicotine and ethanol is influenced by genetic background. P rats self-infused nicotine in the range of 10–200 μM while Wistar rats require a 50 μM concentration to support self-administration (Hauser et al. 2013). Ethanol was self-infused into the posterior VTA by P rats at concentrations as low as 75 mg%, while Wistar rats require an ethanol concentration of 125 mg% to support self-administration (Rodd et al. 2004a). Systemically, P rats will establish intravenous nicotine self-administration at a lower concentration than non-alcohol preferring (NP) rats (Le et al. 2006). Le et al. (2010) reported that unselected rats will simultaneously self-administer nicotine (iv) and EtOH (oral), but blood ethanol and/or nicotine levels were not reported.
Ethanol and nicotine interact within the mesolimbic dopamine system to alter self-administration, dopamine neurochemistry, and gene expression. Microinjection of nicotine acetylcholine receptor antagonists into the VTA decreases ethanol self-administration, while nicotine can increase ethanol self-administration and -seeking (c.f., Hauser et al. 2012). Systemic administration of nicotine and ethanol increases dopamine levels in the nucleus accumbens significantly more than following equivalent ethanol or nicotine exposure (Tizabi et al. 2002; 2007; Doyon et al. 2013). Co-perfusion of ethanol and nicotine into the VTA results in stimulation of dopamine neurons that is not observed following equivalent exposure to ethanol or nicotine alone (Clark and Little 2004). Most human genetic studies examining ethanol and nicotine have ignored co-abuse issues. In the VTA, chronic alcohol and nicotine use produced unique alterations in gene expression of glutamatergic transmission (Flatscher-Bader et al. 2008). The current experiments determined whether a solution containing sub-threshold concentrations of ethanol and nicotine would be self-administered directly into the pVTA, and whether alterations in gene expression in the nucleus accumbens shell (AcbSh) occurred following microinjection of a solution containing equivalent subthreshold concentrations of ethanol, nicotine, and ethanol and nicotine into the pVTA.
Methods
Animals
P rats (71st and 72rd generations) weighing 250–320 g at time of surgery were used. Animals were double-housed upon arrival and maintained on a 12-hr reverse light-dark cycle (lights off at 0900 hr). Food and water were freely available except in the test chamber. All research protocols were approved by the Institutional Animal Care and Use Committee and are in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institute on Drug Abuse, NIH, and the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
Data for subjects that did not complete all experimental test sessions were eliminated from the analyses. The number of animals indicated for each experiment represents 98% of the total number that underwent surgery; 2% of the animals were not included for analyses mainly due to the loss of the guide cannula before completion of all experimental sessions. The data for these animals were not used because their injection sites could not be verified.
Drug and Vehicle
The artificial cerebrospinal fluid (aCSF) vehicle consisted of 120.0 mM NaCl, 4.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM Mg SO4, 25.0 mM NaHCO3, 2.5 mM CaCl2, and 10.0 mM d-glucose. Nicotine tartrate (Sigma-Aldrich, St. Louis, MO USA) and ethyl alcohol (190 proof; McCormick Distilling Co., Weston, MO) were dissolved in the aCSF solution. When necessary, 0.1 M HCl or 0.1 M NaOH was added to the solutions to adjust pH levels to 7.4 ± 0.1.
Apparatus
The test chambers and electrolytic microinfusion transducer (EMIT) system have been previously described (Rodd-Henricks et al. 2003; Rodd et al. 2004a,b) and were used to control microinfusions of nicotine, ethanol, ethanol and nicotine, or vehicle.
Animal Preparation
Animal surgery was conducted as outlined in previous manuscripts (Rodd-Henricks et al. 2003; Rodd et al. 2004a,b). Animals were handled for at least 5 min daily following the fourth recovery day. Subjects were not acclimated to the test chamber prior to the commencement of data collection, nor were they trained on any other operant paradigm.
General Test Condition
For testing, subjects were brought to the testing room, the stylet was removed, and the injection cannula screwed into place. Rats were placed individually in the test chamber. To avoid trapping air at the tip of the injection cannula, the infusion current was delivered for 5 sec during insertion of the injector that resulted in one non-contingent administration of nicotine or aCSF at the beginning of the session. Injection cannula extended 1.0 mm beyond the tip of the guide. The test chamber was equipped with two levers. Depression of the ‘active lever’ (FR1 schedule of reinforcement) caused the delivery of a 100-nl bolus of infusate over a 5-sec period followed by a 5-sec time-out period. During both the 5-sec infusion period and 5-sec time-out period, responses on the active lever did not produce further infusions. The assignment of active and inactive lever with respect to the left or right position was counterbalanced among subjects. However, the active and inactive levers remained the same for each rat throughout the experiment. No shaping technique was used to facilitate the acquisition of lever responses. The number of infusions and responses on the active lever was recorded. Responses on the ‘inactive lever’ were recorded, but did not result in infusions. The duration of each test session was 4 hr and sessions occurred every other day.
Experiment 1 – Ethanol + Nicotine Co-Administration in Female P rats in the Posterior VTA
Female rats were randomly assigned to one of 7 groups (n = 58; 8–9/group). Rats were allowed to self-administer aCSF, ethanol alone (50 mg%), nicotine alone (1 μM), or combinations of ethanol and nicotine (12.5 mg% ethanol + 1 μM nicotine, 25 mg% ethanol + 1 μM nicotine, 50 mg% ethanol + 1 μM nicotine, and 50 mg% ethanol + 0.5 μM nicotine). The original infusate solution was available for self-administration during the first four sessions (acquisition). During the fifth and sixth sessions (extinction), all animals received infusions of aCSF. On the seventh session, rats were allowed to respond for their originally assigned infusate.
Experiment 2 - Ethanol + Nicotine Co-Administration in Male P rats in the Posterior VTA
Male rats were randomly assigned to one of 4 groups (n = 27; 6–8/group). Rats were allowed to self-administer aCSF, ethanol alone (50 mg%), nicotine alone (1 μM), or ethanol and nicotine (50 mg% ethanol + 1 μM nicotine). The 7 sessions were conducted as described for Experiment 1.
Experiment 3 – Alteration in Gene Expression in the AcbSh Produced by Co-Administration of Ethanol + Nicotine in the Posterior VTA
To standardize the amount of ethanol and/or nicotine received between groups, a passive microinjection protocol was employed using the same EMIT units described in Experiments 1 and 2. Female P rats had cannulas aimed at the pVTA to receive the passive microinjections in a pattern that mirrored ICSA self-administration. Microinjections occurred over a 10 minute period. A pattern of a 5-sec pulse microinjection followed by a 15-sec timeout period was used to produce 30 total microinjections (a total obtained by some self-administering rats). Rats were randomly assigned to one of four groups (n = 25; 5–7/group); aCSF, ethanol alone (50 mg%), nicotine alone (1 μM), or ethanol and nicotine (50 mg% ethanol + 1 μM nicotine). Previous research indicated that microinjection of the low concentration of ethanol (50 mg%) into the pVTA would not induce a significant alteration in dopamine levels in the Acb (Ding et al. 2009).
Tissue Collection
Three hours after the passive microinjection (aCSF, nicotine, ethanol or combination of nicotine and EtOH), rats were euthanized, brains were removed and frozen in isopentane chilled with dry ice (approximately −50° C). Brains were then stored at −80° C until processed.
Tissue preparation and micropunch for RT-PCR
All equipment and working surfaces were kept RNase free during dissection of the regions of interest. Frozen brains were sliced at 300μm (coronal), using a Leica cryostat, and slices were placed onto microscope slides. Locations of injectors were verified in the slices using a Leica dissecting microscope set to 4 X magnification. The shell of nucleus accumbens, ipsilateral to the injection, was dissected out of 7 adjacent 300 μm coronal brain slices (from approximately 2.76 – 0.96mm from bregma; Paxinos and Watson 2005) using a 1.0 mm Harris Micro-punch (Electron Microscopy Sciences, Hatfeild, PA) after first removing the accumbens core. The AcbSh tissue was immediately placed into 75μl of SurePrep™ TrueTotal™ RNA purification buffer (Fisher Scientific) and samples were vortexed and frozen on dry ice and kept at −80 °C overnight.
RNA was isolated using SurePrep™ TrueTotal™ RNA Purification Kit (Fisher Scientific). Total RNA was determined using Nanodrop 1000. Extracted RNA was then reverse transcribed using the GeneAmp Gold RNA PCR kit (Applied Biosystems) at the following reaction conditions: 2.5 μM Oligo-dT primer, 2.5 mM magnesium, 250 mM of each deoxynucleotide triphosphate, 0.5 U/ml of RNase inhibitor and final concentration of 0.75 U/μl of MuLV reverse transcriptase. The reverse transcription conditions were 10 min at room temperature, 15 min at 42°C, 10 min at 68°C and 5 min at 95°C and produced approximately 25μl of product.
Taqman low-density arrays (TLDA)
Each microfluidic card of the custom designed GABA/Glutmate Taqman low-density array has 8 separate loading ports (2 ports per sample), each with 48 separate wells per loading port, making 96 wells per sample. Each 2-μl well contains specific, user-defined primers and probes, capable of detecting a single gene. In this study, the mRNA levels of eighty-nine genes associated with amino acid neurotransmitters, as well as the neuronal plasticity markers BDNF and GDNF were included.
A cDNA sample (100 μl) isolated from the AcbSh from each rat was added to an equal volume of TaqMan® universal PCR master mix (Applied Biosystems). After gentle mixing and centrifugation, the mixture was transferred to a loading port on a TLDA card. The array was centrifuged twice for 1 min, each at 1200 rpm, to distribute the samples from the loading port to each well. The card was then sealed and PCR amplification performed using an Applied Biosystems Prism 7900HT sequence detection system (equipped with a TaqMan® low density array upgrade). Thermal cycler conditions were as follows: 2 min at 50 °C, 10 min at 94.5 °C, 30 s at 97 °C, and 1 min at 59.7 °C for 40 cycles. A common threshold was set for all genes using RQ manager software (ABI). The Ct values obtained from the RQ manager (ABI) were then imported into RealTime StatMiner v4.5 (Integromics) for further analyses.
Reference gene selection
The TLDAs included 18S, Gapdh, Hprt1, Rplp2 and Ubc as reference genes based on their proven role as housekeeping genes and their uniform expression in preliminary TLDA rat endogenous control assays from rat AcbSh (data not shown). The geNORM procedure, also known as the pairwise approach, included in the RealTime StatMiner (Integromics) software, was utilized to determine the stability of the selected reference genes, as described previously (Vandesompele et al. 2002). Using this approach, the following genes were selected for normalization of gene expression levels: Gapdh, Hprt1 and Ubc.
Data analysis
Expression of each gene relative to the control (aCSF) treatment group was calculated using the delta delta Ct method. These values were then converted to log10 (log10(ΔΔCt)) for statistical analyses and graphing.
Results
Experiment 1 - Ethanol + Nicotine Co-Administration in Female P rats in the Posterior VTA
Two analyses were performed on the data set. The first analysis examined the average number of infusions self-administered during the first 4 sessions. An ANOVA indicated that there was a significant effect of Drug Condition (F7,50 = 8.6; p < 0.001; Fig. 1). Post-hoc comparisons (Tukey’s) indicated that female P rats self-administering 25 mg% ethanol + 1 μM nicotine (38.2 ± 4.5 infusions/session) or 50 mg% ethanol + 1 μM nicotine (44.1 ± 6.1 infusions/session) administered significantly more infusions than all other groups (range from 8.7 ± 3.5 to 15.6 ± 6.8).
Figure 1.
Depicts the average number of infusions (± SEM) across the initial 4 sessions (acquisition) as a function of infusate concentration in female (left panel) and male (right panel) alcohol-preferring (P) rats. * indicates infusions significantly higher number of infusions than aCSF, 50 mg% EtOH, and 1 μM nicotine (p < 0.05; Tukey’s b post-hoc).
The second analysis examined the number of lever responses (active and inactive) as a function of Drug Condition across all sessions (Mixed Factor ANOVA with between subject factor of Drug Condition and within subject factors of Lever and Sessions). The analysis revealed a significant Drug Condition X Lever X Session interaction (F42,300 = 2.2; p < 0.001). The interaction term was decomposed by examining lever responses across sessions within each Drug Condition. In female P rats self-administering aCSF, 1 μM nicotine, 50 mg% ethanol, or 50 mg% ethanol + 0.5 μM nicotine there were no significant terms (p values > 0.33). In female P rats self-administering 25 mg% ethanol + 1 μM nicotine, or 50 mg% ethanol + 1 μM nicotine (Figs. 2 and 3), there were significant Lever X Session interactions (p values < 0.001). For these groups, there was lever discrimination during sessions 1–4 and 7 (p values < 0.001). In addition, during aCSF substitution (extinction, sessions 5 and 6) there were significant reductions in the number of active lever responses (compared to session 4) and a loss of discrimination between active and inactive levers (p values < 0.001). During reinstatement (session 7), the number of active lever responses returned to levels observed prior to extinction and there was again lever discrimination. In female P rats self-administering 50 mg% ethanol + 0.5 μM nicotine and 1 μM nicotine (Figs. 2 and 3), there was also an effect of session (p values < 0.021). The effect of session in both groups was based upon an increase in responding during session 7, which was significantly higher than that observed during session 4 and 6 (t-test; p values < 0.007).
Figure 2.
Depicts the mean (± SEM) active and inactive lever presses for female P rats given 50 mg% ethanol (EtOH; top panel), 50 mg% EtOH + 1 μM nicotine (Nic; middle panel), or 1 μM Nic (bottom panel) directly into the posterior VTA. * Indicates significantly greater responding on the active lever than responding for aCSF, and significantly more responding on the active than inactive lever. Responses on the active and inactive levers for aCSF for all 7 sessions are not shown but are similar to responses on the active and inactive lever for 50 mg% EtOH (top panel).
Figure 3.
Depicts the mean (± SEM) active and inactive lever presses for female P rats given ethanol + nicotine (50 mg% EtOH + 0.5 μM Nic or 25 mg% EtOH + 1 μM Nic) to self-infuse into the posterior VTA. * Indicates significantly greater responding on the active lever than responding for aCSF, and significantly more responding on the active than inactive lever.
Experiment 2 - Ethanol + Nicotine Co-Administration in Male P rats in the Posterior VTA
The statistical analyses were conducted identically for the male data as those performed on the female data. Similarly, there was a significant effect of Drug Condition (F3,22 = 14.9; p < 0.001; Fig. 1) on the average number of infusions self-administered during sessions 1–4. Post-hoc comparisons (Tukey’s) indicated that male P rats self-administering 50 mg% ethanol + 1 μM nicotine (43.3 ± 6.8 infusions/session) administered significantly more infusions than all other groups (range from 9.4 ± 3.8 to 12.3 ± 5.5). A significant Drug Condition X Lever X Session interaction (F18,132 = 2.4; p < 0.001) was revealed in the analysis examining active and inactive lever responses. In male P rats self-administering 50 mg% ethanol + 1 μM nicotine, the number of responses was significantly higher than all other groups during sessions 1–4 (Fig. 4), and these rats discriminated between the active and inactive levers (p values < 0.003). Similar to the female rats, male P rats self-administering 50 mg% ethanol + 1 μM nicotine displayed extinction during aCSF substitution and reinstated during session 7 (p values < 0.002). In male P rats self-administering 1 μM nicotine, there was also an increase in responding during session 7 compared to sessions 4 and 6 (p values < 0.001).
Figure 4.
Depicts the mean (± SEM) active and inactive lever presses for male P rats given 50 mg% ethanol (EtOH; top panel), 50 mg% EtOH + 1 μM nicotine (Nic; middle panel), or 1 μM Nic (bottom panel) to self-infuse into the posterior VTA. * Indicates significantly greater responding on the active lever than responding for aCSF, and significantly more responding on the active than inactive lever. Responses on the active and inactive levers for aCSF for all 7 sessions are not shown but are similar to responses on the active and inactive lever for 50 mg% EtOH (top panel).
Experiment 3 - Alteration in Gene Expression in the AcbSh Produced by Co-Administration of Ethanol + Nicotine in the Posterior VTA
Overall, the expression of 9 genes was altered in the AcbSh following microinjections of drugs into the pVTA (Figs. 5 and 6). For interpretation purposes, we used a strict statistical approach in defining additive and synergistic effects. Additive effects were defined as statistical findings that would indicate the aCSF was significantly different from the EtOH alone or Nic alone group(s), while the EtOH + nicotine group was significantly different from the EtOH alone or Nic alone groups. Synergistic effects were defined as statistical findings indicating that only the EtOH + nicotine group was significantly different from the aCSF controls. In addition, the y-axis scale in Figs. 5 and 6 are in log10. Therefore, there is a visual bias to ‘observe’ additive effects instead of synergistic effects in the figures (detailed for BDNF).
Figure 5.
Panel showing neurotrophic genes that significantly changed in the Acbsh following administration of 50 mg% ethanol + 1 μM nicotine into the pVTA (Bdnf, Gdnf). * indicates significantly higher expression in the 50 mg% ethanol + 1 μM nicotine compared to all other groups.
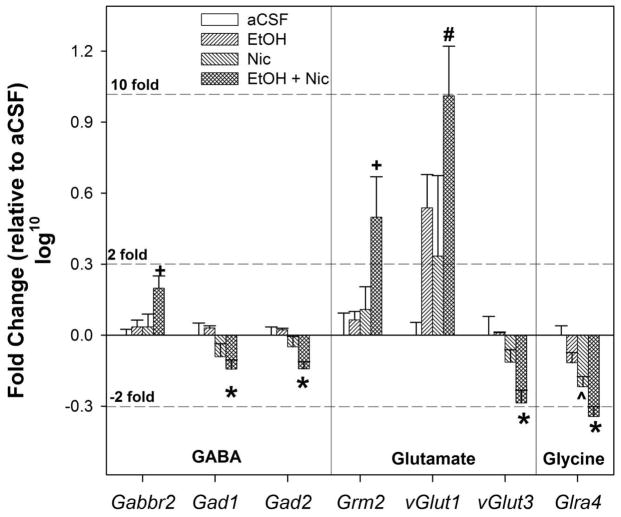
Figure 6.
Panels showing GABA-ergic, glutamatergic, and glycine related genes that significantly changed in the Acbsh following administration of 50 mg% ethanol + 1 μM nicotine into the pVTA. + symbols indicated significantly higher expression in the 50 mg% ethanol + 1 μM nicotine compared to all other groups (Post-hoc; SNK). * indicates significantly lower expression in the 50 mg% ethanol + 1 μM nicotine compared to water and 50 mg% ethanol groups. # symbol indicates significantly higher expression in the 50 mg% ethanol + 1 μM nicotine compared to water. ^ symbol indicates significantly lower expression in the 1 μM nicotine compared to water.
Neurotrophic Factors
Overall, the data indicated that acute microinjections of 50 mg% ethanol + 1 μM nicotine into the pVTA resulted in unique alterations in gene expression in the AcbSh that were not observed following equivalent exposure to ethanol or nicotine (Figure 5). This phenomenon was clearly indicated by the analyses performed on the neurotrophic factors Bdnf and Gdnf. Compared to aCSF injections (ANOVA F3,15 = 4.428, p = 0.026; F3,15 = 4.418, p = 0.030, respectively: Fig. 5) the 50 mg% ethanol + 1 μM nicotine group had a 50-fold increase (SEM ± 1.3) in Bdnf expression compared to controls (SNK p = 0.003), and significantly lower expression of Gdnf compared to controls (−2.4 fold) and EtOH only treated rats (p = 0.005, 0.036, respectively). Bdnf levels in some members of the aCSF, 50 mg% ethanol (5.9 ± 2.8 fold change compared to aCSF), and 1 μM nicotine (8.9 ± 4.1 fold change compared to aCSF) groups were near detection limits. For Gdnf, the 50 mg% ethanol + 1 μM nicotine group was the only group with expression values near detection limits, while Gdnf expression in all other groups was readily detected.
GABA receptor and receptor subunits
The only gene encoding GABA receptors or receptor subunits that was significantly altered by drug injection into the pVTA was the GABA B2 receptor (Gabbr2, ANOVA F3,15 = 4.46, p = 0.025; Figure 6). Expression of Gabbr2 was significantly increased (1.6 fold versus control) compared to control, EtOH only and Nic only groups (p = 0.006, 0.018, and 0.018, respectively). Expression of genes encoding the subunits of GABAA receptors in the AcbSh were not significantly altered by the acute exposure to either drug or the combination of drugs, although a trend was observed for GABA A receptor alpha3 (Gabra3) and alpha 4 (Gabra4) subunits (ANOVA F3,15 = 3.35, p = 0.056 and F3,15 = 3.00, p = 0.073), with the combined treatment trending towards an increased expression of Gabra3 and reduced expression of Gabra4 compared to controls. The expression of the three genes encoding the GABA rho receptor did not reach detection limits in our assay (data not shown).
GABA related genes
In addition to GABA receptors and subunits, the effects of pVTA microinjections of ethanol, nicotine or ethanol + nicotine on expression of GABA related genes in the AcbSh was also determined. Expression of both of the GABA synthesizing enzymes genes, glutamic acid decarboxylase 1 and 2 (Gad1 and Gad2) was significantly reduced (Gad1 - 1.4; Gad2 -1.4, ANOVA F3,15 = 3.56, p = 0.048, and F3,15 = 5.24, p = 0.015; Fig. 6). Specifically, expression of Gad1 and Gad2 was significantly reduced compared to control group (p = 0.035, 0.008), and EtOH group (p = 0.014, 0.003). No significant changes in gene expression were observed for GABA transporters or GABAA binding proteins (data not shown).
Glutamate receptor and receptor subunits
The only gene encoding glutamate receptors or receptor subunits that was significantly altered by drug injection into the pVTA was metabotropic glutamate receptor 2 (Grm2, ANOVA F3,15 = 4.18, p = 0.031; Figure 6). Expression of Grm2 was significantly increased (3.2 versus control) in the ethanol + nicotine group compared to control, ethanol, and nicotine groups (p = 0.04, 0.016, and 0.028 respectively). In addition to Grm2, there was a trend towards reduction of the metabotropic glutamate receptor Grm4 (ANOVA F3,15 = 2.98, p = 0.074) in the combined group compared to controls. Expression of genes encoding the subunits of ionotropic glutamate receptors (NMDA, AMPA & Delta, and Kainate; data not shown) in the AcbSh was not significantly altered by the acute pVTA exposure to drug(s).
Glutamate related genes
In addition to glutamate receptors and subunits, the effects of pVTA injection of ethanol, nicotine or ethanol + nicotine on expression of glutamate-related genes in the AcbSh were also determined. Expression of two of the vesicular glutamate transporters, 1 and 3 (Vglut1 and Vglut3), was significantly altered by pVTA microinjections (ANOVA F3,15 = 3.56, p = 0.048 and F3,15 = 5.24, p = 0.015; Figure 6), and vesicular glutamate transporter 2 (Vglut2) expression had a strong trend (ANOVA F3,15 = 3.48, p = 0.0502). In rats administered 50 mg% ethanol + 1 μM nicotine into the pVTA, expression of Vglut1 was increased 10.3 relative to aCSF controls, and was significantly greater than aCSF controls and the nicotine group (p = 0.006, 0.045, respectively). Expression of Vglut3 was significantly reduced (−1.9 versus control) by combined ethanol + nicotine microinjections into the pVTA compared to all other groups (p = 0.003, 0.004, and 0.046). No significant changes in gene expression were observed for plasma membrane glutamate transporter genes, AMPA receptor trafficking genes, or glutamate receptor binding proteins (data not shown).
Glycine receptor
There was only one significant alteration in the glycine receptor system, the glycine receptor alpha subunit 4 (gl4a). Statistically, there was a significant reduction in the gl4a subunit in the AcbSh in nicotine administered rats compared to the aCSF and EtOH groups (p values < 0.03), and ethanol and nicotine co-microinjections further enhanced this reduction (significantly different from all other groups; p values < 0.02).
Discussion
The current results support the hypothesis that the pVTA is one site where ethanol and nicotine interact in the brain to initiate and promote their co-use, as a result of their synergistic interactions at low doses. The reinforcing effects of the mixture of ethanol + nicotine is supported by the findings that clear lever discrimination occurred during the first 4 sessions, lever discrimination was lost and responses on the active lever decreased when aCSF alone was given in sessions 5 and 6, and responding on the active lever increased when the mixture was returned (Figs. 2–4). Furthermore, the acute exposure of the pVTA to the mixture of low concentrations of ethanol and nicotine produced significant changes in the expression of genes that could alter glutamate and GABA receptor synaptic function in the AcbSh, suggesting that this feed forward pathway for processing the rewarding effects of ethanol and nicotine may have been activated.
The synergistic interaction of ethanol and nicotine within the pVTA may be a result of their acting at different receptor sites, and/or at the same receptor, where the presence of nicotine (or ethanol) alters the functional response of the receptor to ethanol (or nicotine). Nicotinic and 5-HT3 receptors have been found to be co-localized on nerve terminals (Nayak et al. 2000). Therefore, it is possible that these 2 receptors may be co-localized on VTA dopamine neurons; local perfusion of nicotine (Rahman et al. 2004) or a 5-HT3 agonist (Liu et al. 2006) stimulated somatodendritic dopamine release in the VTA.
Ethanol and nicotine act directly at the 5-HT3 receptor (nicotine binds at a lower affinity to the 5-HT3 receptor than any cholinergic nicotinic receptor; Jackson and Yakel 1995; Lovinger and Zhou 1988). The reinforcing properties of both ethanol and nicotine are mediated by the 5-HT3 receptor (Rodd-Henricks et al. 2003; Hauser et al. 2013). Therefore, it is possible that the synergistic actions of ethanol and nicotine are mediated in part by the activation of 5-HT3 receptors.
The synergistic interaction of ethanol and nicotine in the pVTA to produce reinforcement is most likely predicated upon the ability of the mixture to stimulate dopamine neurons that are not observed following exposure to equivalent concentrations of ethanol or nicotine alone (Clark and Little 2004). Evidence supports the idea that the reinforcing effects of ethanol or nicotine within the posterior VTA is mediated by activating local dopamine neurons (Pidoplichko et al. 2004; Rodd et al. 2004b). Neither 50 mg% ethanol nor 1 μM nicotine is self-administered into the pVTA, suggesting this concentration of each drug is not sufficient to activate VTA dopamine neurons. On the other hand, the mixture of these inactive concentrations of ethanol and nicotine is readily self-infused into the pVTA, suggesting activation of local DA neurons is occurring.
Smokers can readily obtain blood nicotine concentrations that exceed the levels that were used in the current experiment (Benowitz 1997). The ethanol concentration used is below the level of intoxication in humans. P rats will orally self-administer solutions of ethanol and nicotine (15% and 0.14 mg/ml) daily at rates that produce blood ethanol and nicotine concentrations that approach those used in the current experiments (>80 mg% and 27 ng/ml, respectively; Hauser et al. 2012). The actual concentration of nicotine in the brain following microinjection is probably lower than that emitted in the current ICSA experiments, and may approach the levels observed following oral EtOH + nicotine self-administration in P rats (167 nM). Therefore, biologically relevant levels of ethanol and nicotine can act synergistically within the pVTA to produce reinforcement.
Ikemoto et al. (2006) reported that nicotine was self-administered directly into the posterior VTA at concentrations starting at 25 mM in Wistar rats. These levels are approximately 4500-fold higher than clinically relevant levels of nicotine, and are well beyond the neurotoxic levels of nicotine (Toledano et al. 2010; Ferrara and Winterer 2009). Similarly, the actual levels of nicotine established in the pVTA is probably lower than 25 mM, but are likely still toxic. The toxicity of the nicotine levels employed could partial explain the failure to replicate self-administration of 25 mM nicotine into the pVTA in naïve and nicotine ‘sensitized’ rats (Farquhar et al. 2012). In mice (Exley et al. 2011), nicotine is self-administered directly into the pVTA at concentrations comparable to those used in the current experiments and by Hauser et al. (2013).
The results also indicate that a single, acute exposure to ethanol and nicotine together in the pVTA produces alterations in gene expression in the AcbSh that are not observed following equivalent microinjections of only ethanol or nicotine (Figs. 5 and 6). Alterations in gene expression in other brain regions produced by microinjections of EtOH + nicotine into the pVTA are likely to occur, but were not assessed in the current experiments. Increases in BDNF levels are associated with the promotion of synaptic plasticity that underlies the development of appetitive and aversive emotional learning (Choi et al. 2012). BDNF levels are increased in the VTA and Acb following chronic drug exposure (Bolanos and Nestler 2004). BDNF infusions into the VTA create a neurological state that mimics opiate addiction (Vargas-Perez et al. 2009). Increases in BDNF levels in the Acb or amygdala are associated with chronic ethanol (Moonat et al. 2011), cocaine (Li et al. 2013), and nicotine (Perna and Brown 2013) use, and the promotion of drug-seeking (Li et al. 2013). In general, Bdnf expression was low in the Acb for the aCSF, ethanol, and nicotine groups, but transcription was dramatically increased (50-fold) by co-administration of ethanol and nicotine in the pVTA. In contrast, Gdnf levels in the aCSF, ethanol, and nicotine groups were readily detected, but the expression level in the ethanol and nicotine group decreased to detection level thresholds.
GDNF levels in the mesolimbic dopamine system are positively associated with a reduction in drug-use/-seeking. GDNF has neuroprotective properties within the mesolimbic dopamine system through regulation of neuronal excitability and transmitter release (Beck et al. 1995; Wang et al. 2001; 2003). Intra-VTA infusions of GDNF reduced cocaine-induced conditioned place preference, reduced alterations in the VTA produced by chronic morphine administrations, and can reduce a number of ethanol self-administration behaviors (Messer et al. 2000; He et al. 2005; Carnicella et al. 2008; 2009). The general inhibitory effect of GDNF on drug-use/-seeking has lead to the promotion of GDNF as a potential pharmacotherapy for the treatment of drug addiction (Carnicella and Ron 2009).
In post-mortem brains of alcoholics (no exclusion for nicotine use) and cocaine users, expression levels of GABA-B receptors were altered, and the expression of GAD1 and GAD2 was reduced (Enoch et al. 2012). The observation that similar effects on GABA-B and GAD expression levels were detected following exposure to multiple drugs of abuse suggests the possibility that these components of the GABA system are potential pharmacological targets for the treatment of drug addiction (Enoch et al. 2012). Alterations in the expression levels of GABA-related genes in the AcbSh following pulse microinjections of ethanol, nicotine, or ethanol + nicotine were limited (Fig. 6). Yet, the current findings also indicate a significant reduction in Gad1 and Gad2 expression levels in the AcbSh following microinjections of only ethanol and nicotine into the pVTA.
The alterations in the glutamatergic corticostriatal pathway are thought to produce neuroplasticity that is the basis of addiction (glutamate homeostasis hypothesis; Kalivas 2009). Glutamate homeostasis, the regulation of glutamate levels within the synaptic cleft, is determined by glutamatergic release and glial and synaptic uptake (Diamon and Jahr 1997). Extracellular levels of glutamate are primarily regulated by the cystine-glutamate transport (C-GT) and the vesicular glutamate transporters (VGlut1-3; c.f. Kalivas 2009). Activation of the C-GT in the Acb enhances mGlu2 receptors’ reduction of synaptic glutamate release (Moran et al. 2005). VGlut1 levels are reduced in the Acb core following nicotine or cocaine self-administration (Knackstedt et al. 2009, 2010). The current data indicate only the ethanol + nicotine microinjections into the pVTA increased the expression of mGlu2 and VGlut1, and a decrease in VGlut3 (Figs. 6). VGlut3 is located on cholinergic neurons, not GABA medium spiny neurons (MSN) in the AcbSh, and is co-localized with neurokinin 1 receptors (Commons and Serock 2009). Therefore, the data suggest that following microinjections of ethanol and nicotine into the pVTA there was an increase in activity in MSN, and a reduction in the inhibitory cholinergic system within the AcbSh.
The current experiments did not exhaustively examine the interaction between ethanol and nicotine on reinforcement within the pVTA (e.g., determining if adding a subthreshold concentration of nicotine would increase established ethanol self-administration), therefore other ethanol-nicotine interactions within the pVTA, and other brain regions, are possible. In addition, the object of the current experiment was not designed to determine alterations in gene expression in the AcbSh produced by microinjections of reinforcing concentrations of ethanol or nicotine (determination of unique effects of ethanol and nicotine).
In humans, nicotine usage can increase alcohol consumption, and alcohol consumption can increase nicotine usage (c.f. Ati-Daoud et al. 2005). Self-reports from alcoholics and nicotine dependent individuals indicated that there is a positive correlation between the number of cigarettes smoked and amount of alcohol consumed (True et al. 1999). The dual-propagation of ethanol and nicotine use in humans may be based upon the ability of these drugs to act synergistically, or in other promoting manners, to decrease reward thresholds or to enhance reward experience. The current experiments indicate that the pVTA is a neuroanatomical site where ethanol and nicotine can synergistically act to produce reinforcement, and that alterations in gene expression in the mesolimbic dopamine system (AcbSh) produced by ethanol and nicotine exposure may enhance future drug use.
Acknowledgments
The skillful technical assistance of Tylene Pommer is gratefully acknowledged. This research was supported in part by AA07462, AA07611, and AA019366.
Footnotes
None of the authors has a conflict of interest associated with this research.
The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
References
- Abruzzo LV, Lee KY, Fuller A, Silverman A, Keating MJ, Medeiros LJ, Coombes KR. Validation of oligonucleotide microarray data using microfluidic low-density arrays: a new statistical method to normalize real-time RT-PCR data. Biotechniques. 2005;38:785–792. doi: 10.2144/05385MT01. [DOI] [PubMed] [Google Scholar]
- Ait-Daoud N, Wiesback GA, Bienkowski P, Li MD, Pfutzer RH, Singer MV, Lesch OM, Johnson BA. Comorbid alcohol and nicotine dependence: from the biomolecular basis to clinical consequences. Alcohol Clin Exp Res. 2005;29:1541–1549. doi: 10.1097/01.alc.0000174692.20933.49. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Systemic absorption and effects of nicotine from smokeless tobacco. Adv Dent Res. 1997;11:336–341. doi: 10.1177/08959374970110030501. [DOI] [PubMed] [Google Scholar]
- Bolanos CA, Nestler EJ. Neurotrophic mechanisms in drug addiction. Neuromolecular Med. 2004;5:69–83. doi: 10.1385/NMM:5:1:069. [DOI] [PubMed] [Google Scholar]
- Carnicella S, Kharazia V, Jeanblanc J, Janak PH, Ron D. GDNF is a fast-acting potent inhibitor of alcohol consumption and relapse. Proc Natl Acad Sci USA. 2008;105:8114–8119. doi: 10.1073/pnas.0711755105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Ron D. GDNF - A potential target to treat addiction. Pharmacol Ther. 2009;122:9–18. doi: 10.1016/j.pharmthera.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DC, Gourley SL, Ressler KJ. Prelimbic BDNF and TrkB signaling regulates consolidation of both appetitive and aversive emotional learning. Transl Psychiatry. 2012;2:e205. doi: 10.1038/tp.2012.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A, Little HJ. Interaction between low concentrations of ethanol and nicotine on firing rate of ventral tegmental dopamine neurones. Drug Alcohol Depend. 2004;16:199–206. doi: 10.1016/j.drugalcdep.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Commons KG, Serock MR. Coincidence of neurokinin 1 receptors with the vesicular glutamate transporter (VGLUT3) in the rat forebrain. Neurosci Lett. 2009;464:188–192. doi: 10.1016/j.neulet.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond JS, Jahr CE. Transporters buffer synaptically release glutamate on a submillisecond time scale. J Neurosci. 1997;17:4672–4687. doi: 10.1523/JNEUROSCI.17-12-04672.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFranza JR, Guerrera MP. Alcoholism and smoking. J Stud Alcohol. 1990;51:130–5. doi: 10.15288/jsa.1990.51.130. [DOI] [PubMed] [Google Scholar]
- Doyon WM, Thomas AM, Ostroumov A, Dong Y, Dani JA. Potential substrates for nicotine and alcohol interactions: a focus on the mesocorticolimbic dopamine system. Biochem Pharmacol. 2013;86:1181–1193. doi: 10.1016/j.bcp.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enoch MA, Zhou Z, Kimura M, Mash DC, Yuan Q, Goldman D. GABAergic gene expression in postmortem hippocampus from alcoholics and cocaine addicts; corresponding findings in alcohol-naïve P and NP rats. PloS One. 2011;7:e29369. doi: 10.1371/journal.pone.0029369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exley R, Maubourguet N, David V, Eddine R, Evrard A, Pons S, Marti F, Threlfell S, Cazala P, McIntosh JM, Changeux J-P, Maskos W, Cragg SJ, Faure P. Distinct contributions of nicotinic acetylcholine receptor subunit α4 and subunit α6 to the reinforcing effects of nicotine. PNAS. 2011;108:7577–7582. doi: 10.1073/pnas.1103000108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar MJ, Latimer MP, Winn P. Nicotine self-administered directly into the VTA by rats is weakly reinforcing but has strong reinforcement enhancing properties. Psychopharmacology. 2012;220:43–54. doi: 10.1007/s00213-011-2452-8. [DOI] [PubMed] [Google Scholar]
- Ferrea S, Winterer G. Neuroprotective and neurotoxic effects of nicotine. Pharmacopsychiatry. 2009;42:255–265. doi: 10.1055/s-0029-1224138. [DOI] [PubMed] [Google Scholar]
- Flatscher-Bader T, Zuvela N, Landis N, Wilce PA. Smoking and alcoholism target genes associated with plasticity and glutamate transmission in the human ventral tegmental area. Human Mol Genet. 2008;17:38–51. doi: 10.1093/hmg/ddm283. [DOI] [PubMed] [Google Scholar]
- Gilbertson R, Frye RF, Nixon SJ. Nicotine as a factor in stress responsiveness among detoxified alcoholics. Alcohol Alcohol. 2011;46:39–51. doi: 10.1093/alcalc/agq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Ding ZM, Getachew B, Toalston JE, Oster SM, McBride WJ, Rodd ZA. The posterior ventral tegmental area mediates alcohol-seeking behavior in alcohol-preferring rats. J Pharmacol Exp Ther. 2011;336:857–865. doi: 10.1124/jpet.110.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Katner SN, Deehan GA, Jr, Toalston JE, Scott BJ, Bell RL, McBride WJ, Rodd ZA. Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012;36:1963–1972. doi: 10.1111/j.1530-0277.2012.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Bracken AL, Deehan GA, Jr, Toalston JE, Ding ZM, Truitt WA, Bell RL, McBride WJ, Rodd ZA. Selective breeding for high alcohol preference increases the sensitivity of the posterior VTA to the reinforcing effects of nicotine. Addict Biol. 2013 doi: 10.1111/adb.12048. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Edwards EM. Age at drinking onset, alcohol dependence, and their relation to drug use and dependence, driving under the influence of drugs, and motor-vehicle crash involvement because of drugs. J Stud Alcohol Drugs. 2008;69:192–201. doi: 10.15288/jsad.2008.69.192. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu ZH. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson MB, Yakel JL. The 5-HT3 receptor channel. Ann Rev Physiol. 1995;57:447–468. doi: 10.1146/annurev.ph.57.030195.002311. [DOI] [PubMed] [Google Scholar]
- John U, Hill A, Rumpf HJ, Hapke U, Meyer C. Alcohol high risk drinking, abuse, and dependence among tobacco smoking medical care patients and the general population. Drug Alcohol Depend. 2003a;64:233–241. doi: 10.1016/s0376-8716(02)00315-0. [DOI] [PubMed] [Google Scholar]
- John U, Meyer C, Rumpf HJ, Schumann A, Thyrian JR, Hapke U. Strength of the relationship between tobacco smoking, nicotine dependence and the severity of alcohol dependence syndrome criteria in a population-based sample. Alcohol Alcohol. 2003b;38:606–612. doi: 10.1093/alcalc/agg122. [DOI] [PubMed] [Google Scholar]
- Lê AD, Li Z, Funk D, Shram M, Li T-K, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naïve offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Lo S, Harding S, Juzytsch W, Marinelli PW, Funk D. Coadministration of intravenous nicotine and oral alcohol in rats. Psychopharmacology. 2010;208(3):475–486. doi: 10.1007/s00213-009-1746-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, DeJoesph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Ford KA, Ferrario CR, Loweth JA, Wolf ME. Different roles of BDNF in the nucleus accumbens core versus shell during the incubation of cue-induced cocaine craving and its long-term maintenance. J Neurosci. 2013;33:1130–1142. doi: 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Thielen RJ, Rodd ZA, McBride WJ. Activation of serotonin-3 receptors increases dopamine release within the ventral tegmental of Wistar and alcohol-preferring (P) rats. Alcohol. 2006;40:167–176. doi: 10.1016/j.alcohol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Zhou Q. Alcohol effects on the 5-HT3 ligand-gated ion channel. Toxicol Lett. 1998;100–101:239–246. doi: 10.1016/s0378-4274(98)00191-x. [DOI] [PubMed] [Google Scholar]
- Moonat S, Sakharkar AJ, Zhang H, Pandey SC. The role of amygdaloid brain-derived neurotrophic factor, activity-regulated cytoskeleton-associated protein and dendritic spines in anxiety and alcoholism. Addict Biol. 2011;16:238–250. doi: 10.1111/j.1369-1600.2010.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak SV, Ronde P, Spier AD, Lummis SCR, Nichols RA. Nicotinic receptors co-localize with 5-HT3 serotonin receptors on striatal nerve terminals. Neuropharmacology. 2000;39:2681–2690. doi: 10.1016/s0028-3908(00)00109-x. [DOI] [PubMed] [Google Scholar]
- Paxinos GW, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; Oxford, UK: 2005. [Google Scholar]
- Perna MK, Brown RW. Adolescent nicotine sensitization and effects of nicotine on accumbal dopamine release in a rodent model of increased dopamine D(2) receptor sensitivity. Behav Brain Res. 2013;242:102–109. doi: 10.1016/j.bbr.2012.12.037. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li T-K, Murphy JM, McBride WJ. Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring (P) and Wistar rats. Alcohol Clin Exp Res. 2004a;28:1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Melendez RI, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of ethanol within the ventral tegmental area of male Wistar rats: evidence for involvement of dopamine neurons. J Neurosci. 2004b;24(5):1050–1057. doi: 10.1523/JNEUROSCI.1319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Oster SM, Toalston JE, Pommer TJ, McBride WJ, Murphy JM. Serotonin-3 receptors in the posterior ventral tegmental area regulate ethanol self-administraton of alcohol-preferring (P) rats. Alcohol. 2010;44:245–255. doi: 10.1016/j.alcohol.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. The effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol into the posterior VTA of Wistar rats. Psychopharmacology. 2003;165:252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- Sanchez-Espiridion B, Sanchez-Aquilera A, Montalban C, Martin C, Martinez R, Gonzalez-Carrero J, Poderos C, Bellas C, et al. A TaqMan low-density array to predict outcome in advanced Hodgkin’s lymphoma using paraffin-embedded samples. Clin Cancer Res. 2009;15:1367–1375. doi: 10.1158/1078-0432.CCR-08-1119. [DOI] [PubMed] [Google Scholar]
- Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Effects of systemic alcohol and central nicotine administration into the ventral tegmental area on dopamine release in the nucleus accumbens. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- Tizabi Y, Bai L, Copeland RL, Jr, Taylor RE. Combined effects of systemic alcohol and nicotine on dopamine release in the nucleus accumbens shell. Alcohol Alcohol. 2007;42:413–416. doi: 10.1093/alcalc/agm057. [DOI] [PubMed] [Google Scholar]
- Toledano, Alvarez MI, Toledano-Diaz A. Diversity and variability of the effects of nicotine on different cortical regions of the brain – therapeutic and toxicological implications. Cent Nerv Sst Agents Med Chem. 2010;1:180–206. doi: 10.2174/1871524911006030180. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas-Perez H, Ting-A Kee R, Walton CH, Hansen DM, Razavi R, Clarke L, Bufalino MR, Allison DW, Stefffensen SC, van der Kooy C. Ventral tegmental area BDNF induces an opiate-dependent-like reward state in naïve rats. Science. 2009;324:1732–1734. doi: 10.1126/science.1168501. [DOI] [PMC free article] [PubMed] [Google Scholar]