Abstract
Haploinsufficiency is a state of genetic disease, which is caused by hemizygous mutations of functional alleles. Lissencephaly is a typical example of haploinsufficiency disorders characterized by a smooth cerebral surface, thick cortex and dilated lateral ventricules associated with mental retardation and seizures due to defective neuronal migration. LIS1 was the first gene cloned in an organism, which was deleted or mutated in patients with lissencephaly in a heterozygous fashion. Series of studies uncovered that LIS1 is an essential regulator of cytoplasmic dynein. In particular, we reported that LIS1 is essential for dynein transport to the plus-end of microtubules by kinesin, which is essential for maintain proper distribution of cytoplasmic dynein within the cell. Fortuitously, we found that a substantial fraction of LIS1 is degraded by the cystein protease, calpain after reaching the plus-end of microtubules. We further demonstrated that inhibition of calpain-mediated LIS1 degradation increased LIS1 level at the cortex of the cell, resulting in therapeutic benefit using genetic mouse models with reduced levels of LIS1. Our work might provide a potential therapeutic approach for the treatment of a fraction of haploinsufficiency disorders through augmenting reduced proteins by the targeting inhibition of degradation machinery.
Keywords: Genetic disease, Haploinsufficiency, Lissencephaly, Calpain inhibitor, Protein augmentation
1. Introduction
Towards treatment of genetic diseases, numerous approaches have been designed as gene therapy, including the insertion of genes into an individual's cells and tissues or suppression of gene function by siRNA. Currently, most gene therapy studies are aimed at cancer and hereditary diseases linked to a genetic defect. However, this has proven difficult, primarily because of the problems involved in carrying large sections of DNA or delivering them to large mass of tissues with therapeutic efficiency. Although the technology is still in its infancy, scientific breakthroughs continue to move gene therapy toward mainstream medicine. One of feasible approaches to address this hard task is the usage of small chemical compounds targeting to the critical point where mutated genes are involved.
Lissencephaly is a severe human developmental disorder of brain due to defective neuronal migration (Dobyns, 1989, Dobyns, et al., 1991). In lissencephaly patients, mutation of two genes, LIS1 or doublecortin (DCX), account for the majority of classical lissencephaly (LIS) (Gleeson, et al., 1998, Pilz, et al., 1998, Reiner, et al., 1993). LIS1 encodes a protein carrying seven WD repeats(Reiner, et al., 1993) that was first identified as a non-catalytic subunit of platelet activating factor-acetylhydrolase (Pafah1b1) (Hattori, et al., 1994). Intensive studies were performed to address the molecular function of LIS1, which led to the conclusion that LIS1 is an essential molecule for proper regulation of cytoplasmic dynein (Vallee, et al., 2001, Wynshaw-Boris, 2007, Yamada, et al., 2008). In the series of experiments, we found that substantial fraction of LIS1 is degraded after reaching the plus-end of microtubules (Yamada, et al., 2009). We probed for molecules that were involved in LIS1 degradation using selective inhibitors, and found that calpain inhibitors efficiently prevented the degradation of LIS1, suggesting that LIS1 is degraded by calpain dependent proteolysis (Yamada, et al., 2009). We also showed that presence of calpain inhibitors improves neuronal migration of Lis1+/- mice (Yamada, et al., 2009). Our findings suggest that inhibiting degradation machinery of protein or mRNA using small chemical compound will be a novel strategy towards therapeutic intervention of lissencephaly. In a former report, inhibition of MMP-2 and -9 (matrix metalloproteinase-2 and -9) by doxycycline had been shown to prevent thoracic aortic aneurysm in Marfan syndrome (Chung, et al., 2007). In addition, the design of MMP inhibitors has been subjected to extensive investments and clinical trials have been performed with these inhibitors in several types of cancers exhibiting both sporadic and familial forms. Here, we propose that prevention of protein degradation would be a potential approach towards therapeutic intervention of a fraction of genetic diseases.
2. Overview of neuronal migration during cortical development
The human brain is the most elaborate structure known and comprised of an integrated network of more than 100 billion neuronal cells. The fine tuning of proliferation and neurogenesis, neuronal migration and differentiation and connectivity underlies the proper development of the cerebral cortex. In this part, we will focus on an underlying mechanism by which neuronal migration is regulated.
During development of the central nervous system (CNS) in vertebrates, far-ranging cell migrations deploy young neurons toward the surface of the developing brain. Neurons settle into six neuronal laminae within the forebrain, and in those laminae they interact with ingrown axons to form the neuronal circuitry of brain. As specific classes of cells come to reside in specific layers, migration also reflects a program of neuronal fate. This program of neurogenesis occurs in an inside-out manner, with the earliest-generated neurons positioned in the deepest layers and later-generated neurons occupying the superficial layers(Hatten, 1985, 1999). Molecular genetic studies indicate that CNS migrations fall within three-step program of development that includes (1) establishment of guidance cues for migration, (2) signal transductions for migrating neurons, and (3) sequential movement of migrating neurons into compact layers. Although these steps are oversimplified and more sophisticated regulations in specific regions are present, they are helpful for understanding of the basic mechanism of neuronal migration. As guidance cues for migration, identification and characterization of the reelin gene (Reln) is an important milestone (D'Arcangelo, et al., 1995, Hirotsune, et al., 1995). Reelin was found to be present in a relatively small population of cells located in the marginal zone of the cerebral cortex. These cells were identified as Cajal-Retzius neurons by their position and morphology. REELIN is thought to provide a guidance cue to migrating neurons, and also may function to be a stop signal (Hack, et al., 2002). Defective migration associated with reeler mutant is not cell-automonous, which is rescued by chimeric formation (Mikoshiba, et al., 1986) or ectopic expression of REELIN by transgene (Magdaleno, et al., 2002).
Signals for migration from outside are transduced into the neuron. Scrambler was isolated as a mutant that is phenotypically indistinguishable with reeler. Mutated gene in Scrambler encodes disabled-1 (Dab1) that is a cytoplasmic protein containing a motif known as a protein interaction/phosphotyrosine binding (PI/PTB) domain (Howell, et al., 1997, Sheldon, et al., 1997). Mammalian Dab1 was originally identified as a Src-binding protein in a yeast two-hybrid screen (Howell, et al., 1997). It is expressed at high levels in the developing CNS and it is phosphorylated on tyrosine residues during brain development. Tyrosine phosphorylation of Dab1 promotes an interaction with several nonreceptor tyrosine kinases, including Src, Fyn, and Abl through their SH2 domains, implying Dab1 functions in kinase signaling cascades during development (Howell, et al., 1997). Further study disclosed that VLDLR and ApoER2 participate in transmitting the extracellular Reelin signal to intracellular signaling processes initiated by mDab1 (Trommsdorff, et al., 1999). Another breakthrough has been the discovery that the proline-directed serine/threonine kinase Cdk5 plays an essential role in the developing brain (Ohshima, et al., 1996, Tsai, et al., 1994). Li-Huei Tsai et al. also revealed that in migrating neurons nuclear movement is regulated by a specialized microtubule (MT) structure, the organization of which depends on focal adhesion kinase (FAK) phosphorylation by Cdk5 (Xie, et al., 2003). These findings are important because, together with the data of Hoshino and colleagues (Kawauchi, et al., 2006), they demonstrate that the role of signaling proteins, such as kinase cascades in the developing cortex is a key regulator of neuronal migration.
Neuronal migration results from a repeating two distinct events, including neurite outgrowth and nucleokinesis. First there is rapid extension and retraction of the leading neurite, which stabilizes tens of microns ahead of the soma. This is followed by forward displacement of the nucleus and soma into the leading process with concurrent retraction of the trailing process. For efficient neuronal migration in the neocortex, newly born cortical neurons undergo dramatic shifts in their cytoskeletal structure as they journey to the cortical plate, with both reversals in migration direction and temporary adoption of a multipolar shape (Noctor, et al., 2004). Over the past few years, the importance of cytoskeleton components in cellular processes crucial for cortical development has emerged from a body of functional data. This was reinforced by the association of mutations in the LIS1 and DCX genes, which both encode proteins involved in microtubule homeostasis. In the series of remodeling cytoskeletal structure, microtubule organizing center (MTOC), centrosome is located ahead of the nucleus in the direction of migration (Gregory, et al., 1988, Rakic, 1972). Subsequent time-lapse experiments have shown that neuronal migration occurs as a stepwise process where the leading process extends forward along the substrate, followed by centrosome movement into the leading process (Solecki, et al., 2004). The nucleus then rapidly translocates through the leading process towards the centrosome. The shortened leading process subsequently elongates and the cycle repeats (Solecki, et al., 2004). Proposed model is: LIS1, dynein and Par6α couple the centrosome and nucleus during nuclear translocation and dynein motor movement generates force on MTs, whereas DCX stabilizes the MTs of the perinuclear cage. Extension of the leading process generates tension between the centrosome and nucleus, pulling it forward. In particular, LIS1 and DCX function with dynein to mediate nucleus–centrosome (N-C) coupling in neuronal migration, which is critical in neuronal migration (Tanaka, et al., 2004). Further, myosin constriction at the trailing edge squeezes the nucleus forward into the leading process. Through these pathways, extracellular guidance cues are transmitted to determine positioning of the centrosome.
3. Genetical feature of lissencephaly
The term lissencephaly covers a group of rare malformations sharing the common feature of anomalies in the appearance of brain convolutions (characterized by simplification or absence of folding) associated with abnormal organization of the cortical layers as a result of neuronal migration defects during embryogenesis. Children with lissencephaly have feeding and swallowing problems, muscle tone anomalies (early hypotonia and subsequently limb hypertonia), seizures (in particular, infantile spasms) and severe psychomotor retardation. Multiple forms of lissencephaly have been described and their current classification is based on the associated malformations and underlying etiology. Two large groups can be distinguished: classical lissencephaly (and its variants) and cobblestone lissencephaly. In classical lissencephaly (or type I), the cortex appears thickened, with four more or less disorganized layers rather than six normal layers. In the variants of classical lissencephaly, extra-cortical anomalies are also present (total or subtotal agenesis of the corpus callosum and/or cerebellar hypoplasia). The classical lissencephalies and the variant forms can be further divided into several subgroups. Four forms can be distinguished on the basis of their genetic etiology: anomalies in the LIS1 gene (isolated lissencephaly and Miller-Dieker syndrome (MDS)) (Reiner, et al., 1993), anomalies in the alpha-tubulin, TUBA1A (Keays, et al., 2007), beta-tubulin, TUBB2B (Jaglin, et al., 2009) and DCX genes (des Portes, et al., 1998, Gleeson, et al., 1998), and lissencephalies caused by mutations in the ARX gene (XLAG syndrome, X-linked lissencephaly with agenesis of the corpus callosum)(Kitamura, et al., 2002). The incidence of all forms of type I lissencephaly is around 1 in 100,000 births. In addition to these four entities, isolated lissencephalies without a known genetic defect, lissencephalies with severe microcephaly (microlissencephaly) and lissencephalies associated with polymalformative syndromes are also included in the group of classical lissencephalies. Cobblestone lissencephaly (formally referred to as type II) is present in three entities: the Walker-Warburg, Fukuyama and MEB (Muscle-Eye-Brain) syndromes (Spalice, et al., 2009). It is characterized by global disorganization of cerebral organogenesis with an uneven cortical surface (with a pebbled or cobblestone appearance).
Functional links between the genes are beginning to be elucidated. For example, the importance of motor proteins and cytoskeleton components in cellular processes crucial for cortical development has emerged from a body of functional data. This was reinforced by the association of mutations in the LIS1 and DCX genes, which both encode proteins involved in regulation of cytoplasmic dynein and microtubule homeostasis (see below). In addition, the discovery of patients with lissencephaly and bilateral asymmetrical polymicrogyria carrying mutations in TUBA1A and TUBB2B further supports this view. On the other hand, mutations of ARX displayed defective neuronal migration, which is prone to GABAergic inter-neurons supplemented from lateral- or medial- ganglionic eminence. ARX is thought to be implicated in the pathway of Dlx homeobox gene family, which controls large numbers of downstream effector genes that are essential for neuronal differentiation. These series of evidences indicate that the fine tuning of proliferation, differentiation, neuronal migration and connectivity underlies the proper development of the cerebral cortex.
4. Molecular mechanism of defective neuronal migration in lissencephaly by LIS1 mutation
The molecular basis for neuronal migration has been an area of intense investigation, in part because many human neurological diseases are either directly or indirectly linked to disordered migration. Among mutated genes in lissencephaly, LIS1 is occupying a unique position. MDS (100%) (Toyo-oka, et al., 2003) and some cases of isolated lissencephaly sequence (ILS) (40%) are the result of haploinsufficiency at human chromosome 17p13.3 (Dobyns, 1989, Dobyns, et al., 1991, Dobyns, et al., 1993). LIS1 is an atypical microtubule associated protein. LIS1 consists of seven spaced WD-40 repeats, and probably assumes the structure of a ‘propeller wheel’ similar to other WD-40 repeat proteins. Bovine and murine LIS1 are almost identical in amino acid sequence. In mammals, LIS1 has at least two identified functions. LIS1 is a non-catalytic subunit of platelet-activating factor acetylhydrolase (PAFAH) isoform Ib, an inactivating enzyme for platelet-activating factor (PAF)(Hattori, et al., 1994). Mouse models for these disorders have aided in the understanding of the function of LIS1 and the pathways associated with it during brain development. Mice were produced with graded reduction in LIS1 dosage. Mice with decreased levels of Lis1 exhibited dose-dependent disorganized cortical layers, hippocampus, cerebellum and olfactory bulb because of cell autonomous neuronal migration defects (Hirotsune, et al., 1995) and are a good model for the human disorder. Complete loss of LIS1 results in peri-implantation lethality, a result confirmed in another Lis1 knockout (Hirotsune, et al., 1995), demonstrating that Lis1 is an essential gene.
One of important clues to address LIS1 function was provided from Aspergillus nidulans, a filamentous fungus (Morris, et al., 1998, Morris, et al., 1998). LIS1 has a non-enzymatic function; it is part of a highly conserved pathway that regulates nuclear migration in fungi (Morris, 2000). The LIS1 homologue in the slime mold Aspergillus nidulans, NudF is part of a signaling pathway that regulates nuclear migration along microtubules via the regulation of dynein motor function. In this organism, nuclei migrate toward the growing tip of hyphae to establish regular spacing, a process termed nucleokinesis (Oakley and Morris, 1980, Xiang and Morris, 1999). Several nuclear distribution mutants (nud mutants) have been generated in this organism (Morris, 1975, Xiang and Morris, 1999). One of these, nudF is the fungal homologue of LIS1 http://www3.interscience.wiley.com/cgi-bin/fulltext/117984457/main.html,ftx_abs - b27 (Xiang, et al., 1995), while other nud mutants directly implicated cytoplasmic dynein and dynactin in this process (Beckwith, et al., 1998, Xiang, et al., 1994). These observations suggest that LIS1 could be involved in a regulation of cytoplasmic dynein. For example, Lis1 is enriched in neurons relative to levels in other cell types, and that Lis1 interacts with the microtubule motor cytoplasmic dynein. Production of more Lis1 in non-neuronal cells increases retrograde movement of cytoplasmic dynein and leads to peripheral accumulation of microtubules (Smith, et al., 2000). In addition, LIS1 protein co-immunoprecipitates with cytoplasmic dynein and dynactin, and localizes to the cell cortex and to mitotic kinetochores, which are known sites for binding of cytoplasmic dynein. Overexpression of LIS1 in cultured mammalian cells interferes with mitotic progression and leads to spindle misorientation. Injection of anti-LIS1 antibody interferes with attachment of chromosomes to the metaphase plate, and leads to chromosome loss (Faulkner, et al., 2000). Furthermore, mosaic analysis of Drosophila shows that neurons containing a mutated cytoplasmic-dynein heavy chain (Dhc64C) exhibit phenotypes similar to Lis1 mutants (Liu, et al., 2000). Another nud gene family, NDEL1 cooperatively regulates cytoplasmic dynein with LIS1 (Niethammer, et al., 2000, Sasaki, et al., 2000). These underlying mechanisms are deeply involved in neurogenesis (Yingling, et al., 2008) and neuronal migration.
5. LIS1 is required for proper positioning of cytoplasmic dynein
The cytoplasmic dynein complex is composed of a heavy chain, an ATPase, which powers motility along microtubules, and several non-catalytic subunits (Vallee, 1990, 1991). The cytoplasmic dynein heavy chain is a member of the ATPase associated with various cellular activities (AAA+ ATPase) superfamily (Kardon and Vale, 2009, Karki and Holzbaur, 1999). We previously reported that LIS1 and NDEL1 cooperatively regulate anterograde transport of cytoplasmic dynein in a kinesin dependent fashion (Figure 1)(Yamada, et al., 2008). In addition, NDEL1 also regulates microtubule organization with Aurora-A, which is essential for neurite extension (Mori, et al., 2009). Among the nud gene family, NUDC has been implicated in the regulation of dynein-mediated nuclear migration. We further reported that mNUDC, mammalian homologue of NUDC is an essential molecule for anterograde transport of cytoplasmic dynein and dynactins (Yamada, et al., 2010). We demonstrated that mNUDC co-migrated with cytoplasmic dynein, dynactins and kinesin-1. Inhibition of mNUDC by a blocking antibody against mNUDC severely perturbed anterograde transport of cytoplasmic dynein and dynactins, whereas kinesin-1 movement remained intact, suggesting that mNUDC functions as an adaptor molecule of kinesin-1 for anterograde transport of cytoplasmic dynein and dynactins (Yamada, et al., 2010). In the series of experiments, half of LIS1 is degraded via calpain-dependent proteolysis, and that inhibition or knockdown of calpains protects LIS1 from proteolysis, which leads to rescue of the phenotypes in Lis1+/- mouse (Yamada, et al., 2009)(see below). The recent report suggests not mutually exclusive possibility, in which LIS1 and NDEL1 cooperatively regulate a persistent-force dynein state that improves ensemble function of multiple dyneins for transport under high-load conditions (McKenney, et al., 2010).
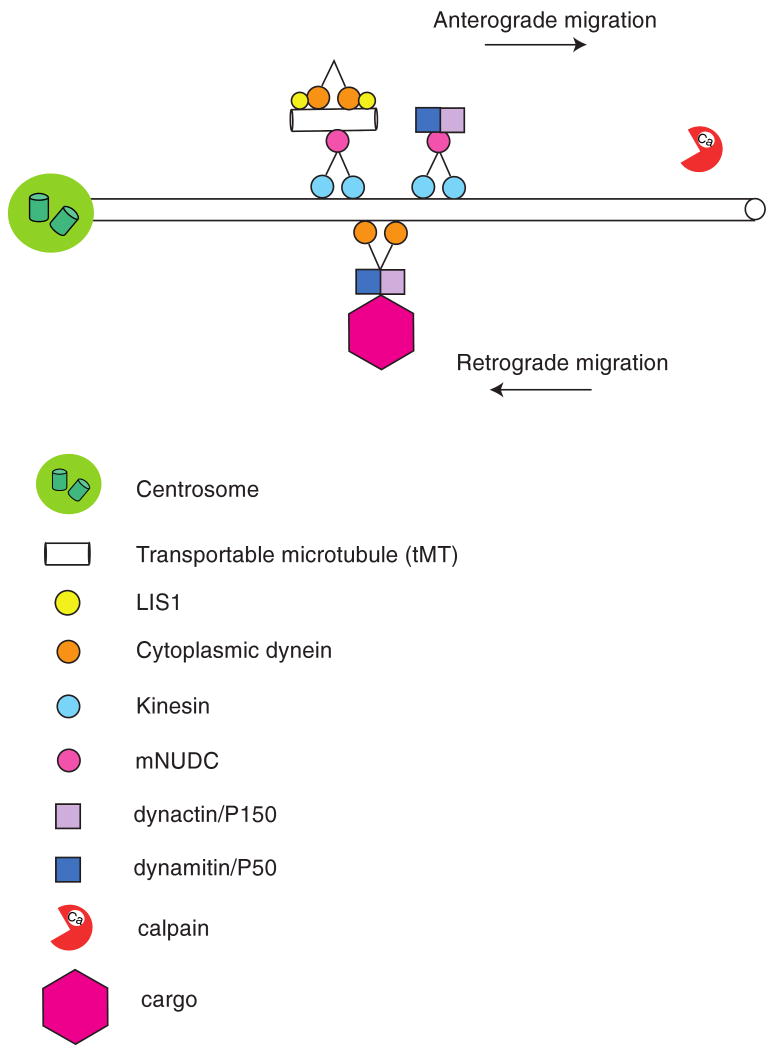
Figure 1. Model for the regulation of cytoplasmic dynein by LIS1 and mNUDC.
Schematic representation of the role of LIS1 for anterograde transport of cytoplasmic dynein, a dynactin complex and mNUDC as an adaptor molecule transport by kinesin-1. mNUDC mediates the connection between MTs and dynactins. Cytoplasmic dynein of the idling state will be somehow activated and will bind a dynactin complex for cargo transport. Substantial fraction of LIS1 is degraded by calpain(s) after the transport to the plus-end of microtubules.
6. Physiological role of protein degradation in the cell
Eukaryotic cells have evolved complex machineries for protein degradation, and the precise regulation of degradation underlies many fundamental cellular processes (Ciechanover, 2005). Some proteases in eukaryotic cells are confined to specialized protein complexes (proteasomes) and organelles (lysosomes) to prevent nonspecific proteolysis (Ciechanover, 2005). In other cases, proteases are functioning within the cytosol, including calpains or caspases.
The proteasome is an intricate molecular machine, which serves to degrade proteins following their conjugation to ubiquitin. Substrates dock onto the proteasome at its 19-subunit regulatory particle via a diverse set of ubiquitin receptors and are then translocated into an internal chamber within the 28-subunit proteolytic core particle, where they are hydrolyzed. Proteasome dependent protein degradation is essential not only for quality control by removing mis-folded and damaged proteins but also for the regulation of Wnt signaling (Petersen and Reddien, 2009) or cell cycle regulation (Sullivan and Morgan, 2007). On the other hand, lysosomes are membrane-bound cytoplasmic organelles involved in intracellular protein degradation. The degradative function of these organelles is carried out by more than 50 acid-dependent hydrolases (e.g., proteases, lipases, glycosidases) contained within its lumen (Dell'Angelica, et al., 2000, Kornfeld and Mellman, 1989). In addition to lysosomal proteins, these organelles contain cell type-specific components that are responsible for their specialized functions (Bird, et al., 2009).
Proteases working within the cytosol also display unique functions. For example, apoptosis can be ascribed to the actions of caspases, a family of cysteinyl aspartate-directed proteases that cleave a wide range of cellular proteins (for a compiled list of published caspase substrates, refer to the CASBAH online database http://bioinf.gen.tcd.ie/casbah/, Lüthi and Martin, 2007). Calpains are Ca2+-dependent cystein proteases (proteolytic enzymes with cystein in the catalytic site) that modulate cellular function (Franco, et al., 2004). In humans, 14 independent genes encode members of the calpain superfamily. Some calpain proteases are confined to specific tissues, whereas others are ubiquitous. Tissue-specific calpains have been implicated in diabetes, cataracts, multiple sclerosis, cancer, Duchenne's muscular dystrophy, and Alzheimer's disease and are known to cause at least one disorder, autosomal recessive limb-girdle muscular dystrophy type 2A (LGMD2A).
7. Inhibition of protein degradation as a therapeutic intervention for lissencephaly
LIS1 is essential for targeting of cytoplasmic dynein to the plus-end of microtubules, thereby maintaining proper positioning of cytoplasmic dynein in the cell (Yamada, et al., 2008). Interestingly, we found that substantial fraction of LIS1 is degraded by calpain after reaching to the plus-end of microtubules (Yamada, et al., 2009). This gave us an idea: LIS1 protein could be increased by inhibiting the calpain-mediated degradation at the cortex of the cell, and this would result in any therapeutic benefit. Recently, we demonstrated that inhibition of calpain restored LIS1 protein to normal level using mouse embryonic fibroblast (MEF) cells or dorsal root gangalia (DRG) neurons established from Lis1+/- mice. We further demonstrated that inhibition of calpain led to rescue of the aberrant distribution of cytoplasmic dynein and intracellular components including mitochondria and β-COP positive vesicles in Lis1+/- MEF cells, and improved neuronal migration of Lis1+/- cerebellar granular neurons. Intraperitoneal injection of a calpain inhibitor to pregnant Lis1+/- dams rescued apoptotic neuronal cell death, reduction of brain weight and neuronal migration defects in Lis1+/- offspring. Lis1+/- mice displayed abnormal behavior and impaired in the spatial learning, including hindpaw clutching responses, a rotarod test and the Morris water maze task (Paylor, et al., 1999). Impaired performance on the rotarod test in the ALLN (a calpain inhibitor)-minus group was significantly improved in the ALLN-plus group (Yamada, et al., 2009). Analysis of gait dynamics revealed that stride length variability of forelimbs was increased in the ALLN-minus group. In the ALLN-plus group, this variability was improved (Yamada, et al., 2009). Restoration of normal parameters in gait analysis also supported the functional improvement of Lis1+/- mice after injection of ALLN.
Here, we used our knowledge of the pathogenesis and mechanism of action of LIS1 and its pathway to directly assess the effect of inhibition of calpain with quantitative phenotypic measures in vivo and in vitro. This approach provides proof-of-principle for a potential therapeutic intervention to a severe developmental cortical dysplasia, lissencephaly. Although extensive work has been performed to understand the molecular and cell biological basis of lissencephaly due to haploinsufficiency of LIS1, therapeutic strategies for lissencephaly have not been addressed. This is probably because the treatment of lissencephaly is a daunting task for several reasons. First, in lissencephaly early brain development is affected, so one would have to treat throughout development. Second, all neurons throughout the brain would have to be treated, since lissencephaly results in global defects in brain development. Third, LIS1 mutations in humans are de novo, presenting a problem of detection at an early enough time point to allow therapy to commence when it can still make a difference. In spite of these difficulties, though, there are some advantages to considering how to treat lissencephaly that results from LIS1 haploinsufficiency. First, LIS1 protein is present since individuals have heterozygous loss of LIS1, so one can consider augmenting the already present but low levels of LIS1 protein in affected individuals. In addition, there are dosage dependent effects of LIS1, so any augmentation of LIS1 protein levels will likely have a beneficial effect. Finally, a great deal is known about the pathogenesis and mechanism of action of LIS1 and its pathway, so the effects of any therapeutic modality can be accessed directly with quantitative measures in vivo and in vitro.
8. Future perspectives
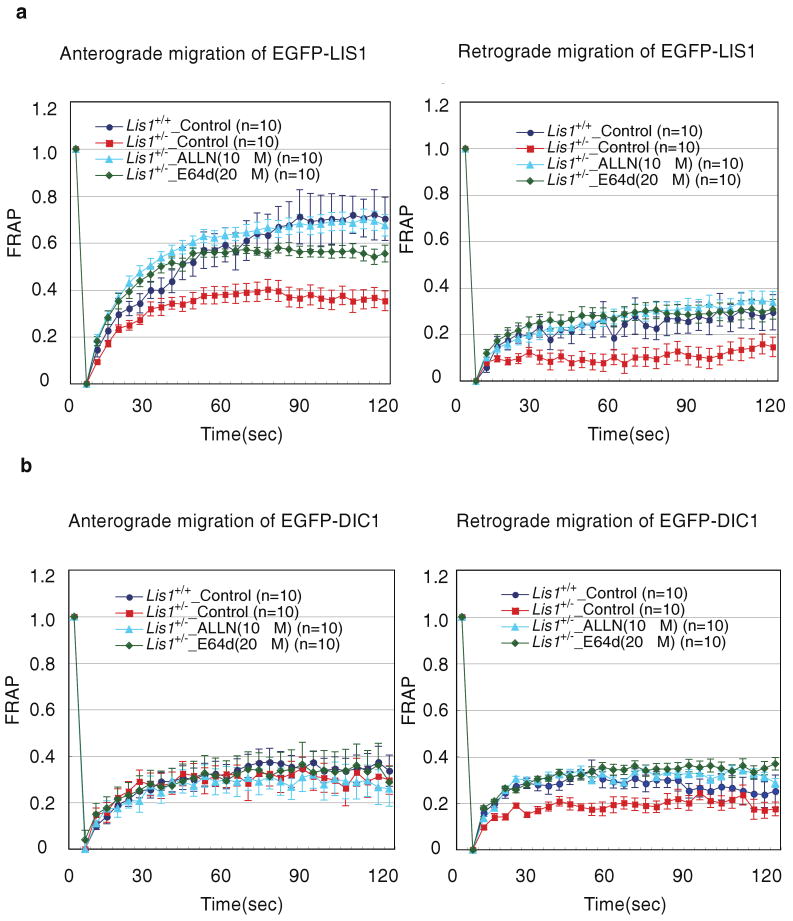
Several problems remain and must be overcome if this promising avenue of therapy can be eventually tested in human lissencephaly. In particular, demonstration of effectiveness by postnatal administration of calpain inhibitors is essential. Because the treatment soon after birth should be considered as an initial approach. This approach might be less effective than prenatal treatment. However, in a rat model of subcortical band heterotopia (SBH) generated by in utero RNA interference of the Dcx gene, that aberrantly positioned neurons can be stimulated to migrate by reexpressing Dcx after birth. Restarting migration in this way both reduces neocortical malformations and restores neuronal patterning (Manent, et al., 2009). The capacity to reduce SBH continues into early postnatal development. These evidences suggest that disorders of neuronal migration may be eventually treatable by reengaging developmental programs both to reduce the size of cortical malformations and to rescue clinical manifestations. In addition, Lis1+/- mice revealed defective axonal transport, which might be responsible for severe mental retardation. This might be attributed to the improper dynein distribution, which would be reversed by LIS1 augmentation by calpain inhibitors. Therefore, we examined the dynamics of LIS1 and cytoplasmic dynein after the treatment of a calpain inhibitor, ALLN or E64d by fluorescence recovery after photobleach (FRAP) analysis (Yamada, et al., 2010, Yamada, et al., 2008). After two hours of the treatment, significant increase of anterograde and retrograde transport of LIS1 was observed in Lis1+/- DRG neurons (Figure 2a). These observations suggest that prevention of LIS1 degradation results in facilitation of LIS1 recycle rather than terminal accumulation. In fact, LIS1 did not exhibit terminal accumulation in excess by the ALLN or E64d treatment. Regarding cytoplasmic dynein transport, there was no obvious difference in the anterograde transport after two hours of the ALLN or E64d treatment, whereas retrograde transport was significantly increased, which is the same level with wild type (Figure 2b). We assume that the enhancement of retrograde transport of cytoplasmic dynein might be attributable partially to prevention of terminal degradation of cytoplasmic dynein and partially to increase of the supply of cytoplasmic dynein by anterograde transport. These observations suggest that functional defect of each neuron might be reversible by treatment of calpain inhibitors, thereby such rescue may increase the mentality and decrease seizure risk.
Figure 2. FRAP analysis of LIS1 or dynein intermediate chain1 (DIC1) transport after administration of ALLN or E64d using dorsal root gangalia (DRG) neurons.
We examined the effect of ALLN or E64d on LIS1 transport after two hours (a) using DRG neurons. Note: ALLN or E64d augments anterograde and retrograde transport of LIS1 in Lis1+/- DRG neurons to the wild type level. We also examined the effect of ALLN or E64d on DIC1 transport after two hours (b). Note: ALLN or E64d augments retrograde transport of DIC1 in Lis1+/- DRG neurons to the wild type level. DRGs from postnatal mice were dissociated using a previously described method (Lindsay, 1988). During dissociation of cells, D-MEM was used with 10% heat-inactivated bovine serum, 200 ng/ml 2.5s mNGF (Sigma) and 5 mM uridine/deoxfluorouridine (Sigma). The cells were plated onto poly-D-lysine coated dishes (MatTek) and cultured in the above medium for 48 hrs. DRGs were transfected with vectors to express EGFP-Lis1 or EGFP-DIC1 immediately after dissection using the Basic Nucleofector kit for primary neurons (Amaxa Biosystems). FRAP analyses were performed to extended axons from DRGs using the 510 META system (Carl Zeiss) 48 hours after transfection as described (Mochizuki, et al., 2001).
Human beings suffer from more than 5000 different diseases caused by single gene mutations. Gene therapy by introduction of a normal functional gene into cells or siRNA for the suppression of toxic gene products has been challenged. Due to the limitation of the method for gene transfer, currently those approaches have not been applied for the practical therapeutic interventions. On the other hand, therapeutic intervention using chemical compounds with low molecular weight will be beneficial for drug delivery and the cost of treatments. Polycystic kidney diseases (PKDs) are primarily characterized by the growth of fluid-filled cysts in renal tubules leading to end-stage renal disease. Mutations in the PKD1 or PKD2 genes lead to autosomal dominant PKD (ADPKD), a slowly developing adult form. Treatment with the cyclin-dependent kinase (CDK) inhibitor (R)-roscovitine does indeed yield effective arrest of cystic disease in juvenile cystic kidneys (jck) and autosomal recessive polycystic kidney (cpk) mouse models of PKD (Bukanov, et al., 2006). Haploinsufficiency is defined as a dominant phenotype in diploid organisms that are heterozygous for a loss-of-function allele. The total level of a gene product (a particular protein) produced by the cell is about half of the normal level and that level is not sufficient to permit the cell to function normally. Many of the haploinsufficient mutations in humans are observed in transcription factors (Seidman and Seidman, 2002). For example, mutations in the gene encoding for the cAMP-responsive element binding protein (CREB) binding protein (CBP) result in Rubinstein-Taybi syndrome (RTS), a rare condition that occurs in 1/125,000 births and is characterized by mental retardation and skeletal abnormalities (Petrij, et al., 1995). Loss of function of CBP may therefore interfere with the transcriptional activation of target genes in two ways: by preventing recruitment to the promoter of the basal transcription machinery and by blocking chromatin remodeling. Marfan syndrome (MFS) and Ehlers-Danlos syndrome type IV (EDS IV) are clinical entities characterized by vascular abnormalities that result from mutations of structural components of the extracellular matrix (ECM), which are another examples of haploinsufficiency (Dietz, et al., 1991, Toriello, et al., 1996). The relevance of haploinsufficiency in human disease has become increasingly apparent, and any human diseases that result from haploinsufficiency are potential targets of our strategy to boost levels of the haploinsufficient protein. Prevention of protein degradation or inhibition of mRNA degradation will be an effective method to increase mutated protein in each disease. In addition to treatment of lissencephaly, calpain inhibitors hold promise for the treatment of neuromuscular and neurodegenerative diseases in which calpains have been shown to be upregulated (e.g. Parkinson's disease and Duchenne muscular dystrophy) (Saez, et al., 2006). Application of calpain inhibitors to these diseases might have a more direct effect, since the human genome has utrophin that has similar function with dystrophin (Banks and Chamberlain, 2008). Sporadic morbidity to neurodegenerative diseases such as Parkinson disease or Alzheimer disease might be attributable to hemizygous mutations of responsible genes. Prevention of protein or mRNA degradation will be a potential strategy for therapeutic intervention in such cases as well.
Acknowledgments
We thank Toshio Yanagida and Michiyuki Matsuda for generous support and encouragement. We also thank Takako Takitoh and Yuko Yoshida for technical support. This work was supported by Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan to Shinji Hirotsune. This work was also supported by The Mother and Child Health Foundation, The Naito Foundation, Japan Brain Foundation and The Uehara Memorial Foundation to Shinji Hirotsune, and NIH grants NS41030 and HD47380 to Anthony Wynshaw-Boris.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banks, Chamberlain The value of mammalian models for duchenne muscular dystrophy in developing therapeutic strategies. Curr Top Dev Biol. 2008;84:431–53. doi: 10.1016/S0070-2153(08)00609-1. [DOI] [PubMed] [Google Scholar]
- Beckwith, et al. The “8-kD” cytoplasmic dynein light chain is required for nuclear migration and for dynein heavy chain localization in Aspergillus nidulans. J Cell Biol. 1998;143:1239–47. doi: 10.1083/jcb.143.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, et al. Endolysosomal proteases and their inhibitors in immunity. Nat Rev Immunol. 2009;9:871–82. doi: 10.1038/nri2671. [DOI] [PubMed] [Google Scholar]
- Bukanov Long-lasting arrest of murine polycystic kidney disease with CDK inhibitor roscovitine. Nature. 2006;444:949–52. doi: 10.1038/nature05348. [DOI] [PubMed] [Google Scholar]
- Chung, et al. Loss of elastic fiber integrity and reduction of vascular smooth muscle contraction resulting from the upregulated activities of matrix metalloproteinase-2 and -9 in the thoracic aortic aneurysm in Marfan syndrome. Circ Res. 2007;101:512–22. doi: 10.1161/CIRCRESAHA.107.157776. [DOI] [PubMed] [Google Scholar]
- Ciechanover Proteolysis: from the lysosome to ubiquitin and the proteasome. Nat Rev Mol Cell Biol. 2005;6:79–87. doi: 10.1038/nrm1552. [DOI] [PubMed] [Google Scholar]
- D'Arcangelo, et al. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–23. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica, et al. Lysosome-related organelles. FASEB J. 2000;14:1265–78. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- des Portes, et al. doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH) Hum Mol Genet. 1998;7:1063–70. doi: 10.1093/hmg/7.7.1063. [DOI] [PubMed] [Google Scholar]
- Dietz, et al. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991;352:337–9. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Dobyns The neurogenetics of lissencephaly. Neurol Clin. 1989;7:89–105. [PubMed] [Google Scholar]
- Dobyns, et al. Clinical and molecular diagnosis of Miller-Dieker syndrome. Am J Hum Genet. 1991;48:584–94. [PMC free article] [PubMed] [Google Scholar]
- Dobyns, et al. Lissencephaly. A human brain malformation associated with deletion of the LIS1 gene located at chromosome 17p13. Jama. 1993;270:2838–42. doi: 10.1001/jama.270.23.2838. [DOI] [PubMed] [Google Scholar]
- Faulkner, et al. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat Cell Biol. 2000;2:784–91. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- Franco, et al. Calpain-mediated proteolysis of talin regulates adhesion dynamics. Nat Cell Biol. 2004;6:977–83. doi: 10.1038/ncb1175. [DOI] [PubMed] [Google Scholar]
- Gleeson, et al. Doublecortin, a brain-specific gene mutated in human X-linked lissencephaly and double cortex syndrome, encodes a putative signaling protein. Cell. 1998;92:63–72. doi: 10.1016/s0092-8674(00)80899-5. [DOI] [PubMed] [Google Scholar]
- Gregory, et al. Cytology and neuron-glial apposition of migrating cerebellar granule cells in vitro. J Neurosci. 1988;8:1728–38. doi: 10.1523/JNEUROSCI.08-05-01728.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack, et al. Reelin is a detachment signal in tangential chain-migration during postnatal neurogenesis. Nat Neurosci. 2002;5:939–45. doi: 10.1038/nn923. [DOI] [PubMed] [Google Scholar]
- Hatten Neuronal regulation of astroglial morphology and proliferation in vitro. J Cell Biol. 1985;100:384–96. doi: 10.1083/jcb.100.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatten Central nervous system neuronal migration. Annu Rev Neurosci. 1999;22:511–39. doi: 10.1146/annurev.neuro.22.1.511. [DOI] [PubMed] [Google Scholar]
- Hattori, et al. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase [corrected] Nature. 1994;370:216–8. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- Hirotsune, et al. The reeler gene encodes a protein with an EGF-like motif expressed by pioneer neurons. Nat Genet. 1995;10:77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- Howell, et al. Mouse disabled (mDab1): a Src binding protein implicated in neuronal development. EMBO J. 1997;16:121–32. doi: 10.1093/emboj/16.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell, et al. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–7. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- Jaglin, et al. Mutations in the beta-tubulin gene TUBB2B result in asymmetrical polymicrogyria. Nat Genet. 2009 doi: 10.1038/ng.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardon, Vale Regulators of the cytoplasmic dynein motor. Nat Rev Mol Cell Biol. 2009;10:854–65. doi: 10.1038/nrm2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karki, Holzbaur Cytoplasmic dynein and dynactin in cell division and intracellular transport. Curr Opin Cell Biol. 1999;11:45–53. doi: 10.1016/s0955-0674(99)80006-4. [DOI] [PubMed] [Google Scholar]
- Kawauchi, et al. Cdk5 phosphorylates and stabilizes p27kip1 contributing to actin organization and cortical neuronal migration. Nat Cell Biol. 2006;8:17–26. doi: 10.1038/ncb1338. [DOI] [PubMed] [Google Scholar]
- Keays, et al. Mutations in alpha-tubulin cause abnormal neuronal migration in mice and lissencephaly in humans. Cell. 2007;128:45–57. doi: 10.1016/j.cell.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura, et al. Mutation of ARX causes abnormal development of forebrain and testes in mice and X-linked lissencephaly with abnormal genitalia in humans. Nat Genet. 2002;32:359–69. doi: 10.1038/ng1009. [DOI] [PubMed] [Google Scholar]
- Kornfeld, Mellman The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Lindsay Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8:2394–405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, et al. Drosophila Lis1 is required for neuroblast proliferation, dendritic elaboration and axonal transport. Nat Cell Biol. 2000;2:776–83. doi: 10.1038/35041011. [DOI] [PubMed] [Google Scholar]
- Magdaleno, et al. Rescue of ataxia and preplate splitting by ectopic expression of Reelin in reeler mice. Neuron. 2002;33:573–86. doi: 10.1016/s0896-6273(02)00582-2. [DOI] [PubMed] [Google Scholar]
- Manent, et al. Dcx reexpression reduces subcortical band heterotopia and seizure threshold in an animal model of neuronal migration disorder. Nat Med. 2009;15:84–90. doi: 10.1038/nm.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney, et al. LIS1 and NudE induce a persistent dynein force-producing state. Cell. 2010;141:304–14. doi: 10.1016/j.cell.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoshiba, et al. Studies on the development and growth of the mammalian nervous system by aggregation chimeras: analysis of corticohistogenesis in the cerebrum by reeler mutant mice. Prog Clin Biol Res. 1986;217B:137–40. [PubMed] [Google Scholar]
- Mochizuki, et al. Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature. 2001;411:1065–8. doi: 10.1038/35082594. [DOI] [PubMed] [Google Scholar]
- Mori, et al. An essential role of the aPKC-Aurora A-NDEL1 pathway on neurite elongation by modulation of microtubule dynamics. Nat Cell Biol. 2009 doi: 10.1038/ncb1919. [DOI] [PubMed] [Google Scholar]
- Morris Mitotic mutants of Aspergillus nidulans. Genet Res. 1975;26:237–54. doi: 10.1017/s0016672300016049. [DOI] [PubMed] [Google Scholar]
- Morris Nuclear migration. From fungi to the mammalian brain. J Cell Biol. 2000;148:1097–101. doi: 10.1083/jcb.148.6.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris, et al. The lissencephaly gene product Lis1, a protein involved in neuronal migration, interacts with a nuclear movement protein, NudC. Curr Biol. 1998;8:603–6. doi: 10.1016/s0960-9822(98)70232-5. [DOI] [PubMed] [Google Scholar]
- Morris, et al. Nuclear migration, nucleokinesis and lissencephaly. Trends Cell Biol. 1998;8:467–70. doi: 10.1016/s0962-8924(98)01389-0. [DOI] [PubMed] [Google Scholar]
- Niethammer, et al. NUDEL is a novel Cdk5 substrate that associates with LIS1 and cytoplasmic dynein. Neuron. 2000;28:697–711. doi: 10.1016/s0896-6273(00)00147-1. [DOI] [PubMed] [Google Scholar]
- Noctor, et al. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–44. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Oakley, Morris Nuclear movement is beta--tubulin-dependent in Aspergillus nidulans. Cell. 1980;19:255–62. doi: 10.1016/0092-8674(80)90407-9. [DOI] [PubMed] [Google Scholar]
- Ohshima, et al. Targeted disruption of the cyclin-dependent kinase 5 gene results in abnormal corticogenesis, neuronal pathology and perinatal death. Proc Natl Acad Sci U S A. 1996;93:11173–8. doi: 10.1073/pnas.93.20.11173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paylor, et al. Impaired learning and motor behavior in heterozygous Pafah1b1 (Lis1) mutant mice. Learn Mem. 1999;6:521–37. doi: 10.1101/lm.6.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen, Reddien Wnt signaling and the polarity of the primary body axis. Cell. 2009;139:1056–68. doi: 10.1016/j.cell.2009.11.035. [DOI] [PubMed] [Google Scholar]
- Petrij, et al. Rubinstein-Taybi syndrome caused by mutations in the transcriptional co-activator CBP. Nature. 1995;376:348–51. doi: 10.1038/376348a0. [DOI] [PubMed] [Google Scholar]
- Pilz, et al. LIS1 and XLIS (DCX) mutations cause most classical lissencephaly, but different patterns of malformation. Hum Mol Genet. 1998;7:2029–37. doi: 10.1093/hmg/7.13.2029. [DOI] [PubMed] [Google Scholar]
- Rakic Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145:61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- Reiner, et al. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–21. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- Saez, et al. The therapeutic potential of the calpain family: new aspects. Drug Discov Today. 2006;11:917–23. doi: 10.1016/j.drudis.2006.08.009. [DOI] [PubMed] [Google Scholar]
- Sasaki, et al. A LIS1/NUDEL/cytoplasmic dynein heavy chain complex in the developing and adult nervous system. Neuron. 2000;28:681–96. doi: 10.1016/s0896-6273(00)00146-x. [DOI] [PubMed] [Google Scholar]
- Seidman, Seidman Transcription factor haploinsufficiency: when half a loaf is not enough. J Clin Invest. 2002;109:451–5. doi: 10.1172/JCI15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon, et al. Scrambler and yotari disrupt the disabled gene and produce a reeler-like phenotype in mice. Nature. 1997;389:730–3. doi: 10.1038/39601. [DOI] [PubMed] [Google Scholar]
- Smith, et al. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol. 2000;2:767–75. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- Solecki, et al. Par6alpha signaling controls glial-guided neuronal migration. Nat Neurosci. 2004;7:1195–203. doi: 10.1038/nn1332. [DOI] [PubMed] [Google Scholar]
- Spalice, et al. Neuronal migration disorders: clinical, neuroradiologic and genetics aspects. Acta Paediatr. 2009;98:421–33. doi: 10.1111/j.1651-2227.2008.01160.x. [DOI] [PubMed] [Google Scholar]
- Sullivan, Morgan Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol. 2007;8:894–903. doi: 10.1038/nrm2276. [DOI] [PubMed] [Google Scholar]
- Tanaka, et al. Lis1 and doublecortin function with dynein to mediate coupling of the nucleus to the centrosome in neuronal migration. J Cell Biol. 2004;165:709–21. doi: 10.1083/jcb.200309025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toriello, et al. A translocation interrupts the COL5A1 gene in a patient with Ehlers-Danlos syndrome and hypomelanosis of Ito. Nat Genet. 1996;13:361–5. doi: 10.1038/ng0796-361. [DOI] [PubMed] [Google Scholar]
- Toyo-oka, et al. 14-3-3epsilon is important for neuronal migration by binding to NUDEL: a molecular explanation for Miller-Dieker syndrome. Nat Genet. 2003;34:274–85. doi: 10.1038/ng1169. [DOI] [PubMed] [Google Scholar]
- Trommsdorff, et al. Reeler/Disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- Tsai, et al. p35 is a neural-specific regulatory subunit of cyclin-dependent kinase 5. Nature. 1994;371:419–23. doi: 10.1038/371419a0. [DOI] [PubMed] [Google Scholar]
- Vallee Mitosis: dynein and the kinetochore. Nature. 1990;345:206–7. doi: 10.1038/345206a0. [DOI] [PubMed] [Google Scholar]
- Vallee Cytoplasmic dynein: advances in microtubule-based motility. Trends Cell Biol. 1991;1:25–9. doi: 10.1016/0962-8924(91)90066-i. [DOI] [PubMed] [Google Scholar]
- Vallee, et al. LIS1: cellular function of a disease-causing gene. Trends Cell Biol. 2001;11:155–60. doi: 10.1016/s0962-8924(01)01956-0. [DOI] [PubMed] [Google Scholar]
- Wynshaw-Boris Lissencephaly and LIS1: insights into the molecular mechanisms of neuronal migration and development. Clin Genet. 2007;72:296–304. doi: 10.1111/j.1399-0004.2007.00888.x. [DOI] [PubMed] [Google Scholar]
- Xiang, et al. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc Natl Acad Sci U S A. 1994;91:2100–4. doi: 10.1073/pnas.91.6.2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang, Morris Hyphal tip growth and nuclear migration. Curr Opin Microbiol. 1999;2:636–40. doi: 10.1016/s1369-5274(99)00034-x. [DOI] [PubMed] [Google Scholar]
- Xiang, et al. NudF, a nuclear migration gene in Aspergillus nidulans, is similar to the human LIS-1 gene required for neuronal migration. Mol Biol Cell. 1995;6:297–310. doi: 10.1091/mbc.6.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, et al. Serine 732 phosphorylation of FAK by Cdk5 is important for microtubule organization, nuclear movement, and neuronal migration. Cell. 2003;114:469–82. doi: 10.1016/s0092-8674(03)00605-6. [DOI] [PubMed] [Google Scholar]
- Yamada, et al. mNUDC is required for plus-end-directed transport of cytoplasmic dynein and dynactins by kinesin-1. EMBO J. 2010;29:517–31. doi: 10.1038/emboj.2009.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, et al. LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. Embo J. 2008;27:2471–83. doi: 10.1038/emboj.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada, et al. Inhibition of calpain increases LIS1 expression and partially rescues in vivo phenotypes in a mouse model of lissencephaly. Nat Med. 2009;15:1202–7. doi: 10.1038/nm.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingling, et al. Neuroepithelial stem cell proliferation requires LIS1 for precise spindle orientation and symmetric division. Cell. 2008;132:474–86. doi: 10.1016/j.cell.2008.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]