Figure 4.
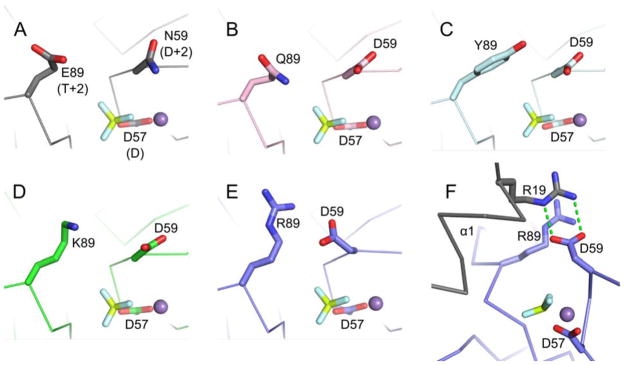
Positioning of residues D+2 and T+2 in CheYs complexed with BeF3− and Mn2+. In wild type CheY (A, PDB entry 1FQW), the side chain of Asn59 at position D+2 is directed away from Glu89 at position T+2 and is not positioned to interact with the leaving group atom (in autophosphorylation) or the nucleophilic water molecule (in autodephosphorylation). Similar orientations are observed for the Asp at D+2 in CheY DQ·BeF3− ·Mn2+ (B, pink carbons), CheY DY·BeF3− ·Mn2+ (C, cyan carbons), and CheY DK·BeF3− ·Mn2+ (D, green carbons). The active site of CheY DR·BeF3− ·Mn2+ (E/F, slate carbons) showed a reorientation of the Asp at D+2 toward the Arg at 89 and in position to aid catalysis. The Asp at D+2 in the CheY DR structure does not hydrogen bond with the Arg at T+2 as seen in the MO42− and WO42− structures, but instead interacts with Arg19 from helix α1 (grey) of a symmetry related molecule (F). In all panels, the BeF3− is shown as yellow and white sticks and the M2+ as a purple sphere.
