Abstract
Rationale
Alcohol and nicotine co-use can reciprocally promote self-administration and drug-craving/drug-seeking behaviors. To date, the neurocircuitry in which nicotine influences ethanol (EtOH) seeking has not been elucidated. Clinical and preclinical research has suggested that the activation of the mesolimbic dopamine system is involved in the promotion of drug seeking. Alcohol, nicotine, and serotonin-3 (5-HT3) receptors interact within the posterior ventral tegmental area (pVTA) to regulate drug reward. Recently, our laboratory has reported that systemic administration of nicotine can promote context-induced EtOH seeking.
Objectives
The goals of the current study were to (1) determine if microinjections of pharmacologically relevant levels of nicotine into the pVTA would enhance EtOH seeking, (2) determine if coadministration of nicotinic cholinergic receptor antagonist (nACh) or 5-HT3 receptor antagonists would block the ability of nicotine microinjected into the pVTA to promote EtOH seeking, and (3) determine if 5-HT3 receptors in the pVTA can modulate EtOH seeking.
Results
Nicotine (100 and 200 µM) microinjected into the pVTA enhanced EtOH seeking. Coinfusion with 200 µM mecamylamine (nACh antagonist) or 100 and 200 µM zacopride (5-HT3 receptor antagonist) blocked the observed nicotine enhancement of EtOH seeking. The data also indicated that microinjection of 1 µM CPBG (5-HT3 receptor agonist) promotes context-induced EtOH seeking; conversely microinjection of 100 and 200 µM zacopride alone reduced context-induced EtOH seeking.
Conclusions
Overall, the results show that nicotine-enhanced EtOH-seeking behavior is modulated by 5-HT3 and nACh receptors within the pVTA and that the 5-HT3 receptor system within pVTA may be a potential pharmacological target to inhibit EtOH-seeking behaviors.
Keywords: Alcohol-seeking behavior, Serotonin-3 receptors, Nicotinic cholinergic receptors, Alcohol-preferring P rat, Pavlovian Spontaneous Recovery, Zacopride, Mecamylamine
Introduction
Alcohol and nicotine are also commonly used together, and the co-abuse of both drugs can increase use and relapse more than either drug alone. The severity of nicotine dependency is linked to more severe levels of alcohol relapse (Abrams et al. 1992; Gulliver et al. 1995) and impairs the likelihood that an individual with alcohol dependence will succeed in becoming abstinent if they continue to smoke during this period (Gulliver et al. 1995; Sobell et al. 1995; Daeppen et al. 2000). Preclinical studies have provided evidence that nicotine can enhance relapse behaviors, such as ethanol (EtOH) seeking (Le et al. 2003; Hauser et al. 2012a) and EtOH relapse drinking (Lopez-Moreno et al. 2004; Alen et al. 2009; Hauser et al. 2012a).
The activation of dopamine (DA) neurons in the ventral tegmental area (VTA) plays a critical role in drug reward/reinforcement and compulsive drug-seeking behaviors; both EtOH (Brodie et al. 1990; Brodie et al. 1999) and nicotine (Calabresi et al. 1989; Nisell et al. 1994) can activate VTADA neurons. Nicotinic acetylcholine (nACh) receptors within the VTA are thought to be one of the molecular targets underlying EtOH and nicotine co-abuse. The reinforcing effects of nicotine are modulated via stimulation of nACh receptors within the VTA (Corrigall et al. 1994; Nisell et al. 1994); studies have shown that the reinforcing effects of EtOH may be partially modulated by nACh receptors (Blomqvist et al. 1996; Ericson et al. 2003; Soderpalm et al. 2000). EtOH intake (Le et al. 2000; Hendrickson et al. 2009) and nicotine-stimulated EtOH drinking (Smith et al. 1999; Sajja and Rahman 2012) can be reduced by mecamylamine (Mec), a nonselective nACh receptor antagonist.
A few studies have examined the involvement of nACh receptors in EtOH-seeking behavior. Local infusion of α-conotoxin MII (α3β2, β3, and α6 nicotinic antagonists) into the VTA and systemic administration of Mec can reduce cue-induced EtOH seeking, whereas dihydro-β-erythroidine (α4β2 nicotinic antagonist) did not have an effect (Lof et al. 2007). In addition, varenicline, an α4β2 nicotinic receptor partial agonist, reduced EtOH-seeking behavior (Steensland et al. 2007).
The nACh and serotonin-3 (5-HT3) receptors are members of the Cys-loop ligand-gated ion channel superfamily; 5-HT3 receptors share up to 30 % sequence homology of nACh receptors. Nicotine binds with a higher affinity to the 5-HT3 receptor compared to any nACh receptor (Jackson and Yakel 1995; Breitinger et al. 2001; Gurley and Lanthorn 1998). In addition, the nACh receptor antagonist Mec and epibatidine (nicotinic agonist) have at least a fourfold greater affinity for the 5-HT3 receptor than the nACh receptor (Drisdel et al. 2008). In contrast, zacopride (Zac), a 5-HT3 receptor antagonist, does not have an appreciable affinity for nicotinic receptors (Kidd et al. 1993).
The posterior VTA (pVTA) mediates the reinforcing actions of EtOH (Rodd-Henricks et al. 2000) and nicotine (Ikemoto et al. 2006; Hauser et al. 2013). The activation of 5-HT3 receptors is involved in mediating the reinforcing effects of EtOH (Rodd-Henricks et al. 2003) and nicotine (Hauser et al. 2013) within the pVTA. The pVTA is also involved in mediating context-induced EtOH-seeking behavior via activation of local DA neurons (Hauser et al. 2011). Because of the similarities in structure between nACh and 5-HT3 receptors (Mascia et al. 2000; Peters et al. 2006), and the potential interactions of these receptors and of nicotine at the 5-HT3 receptor (Bianchi et al. 1995; Gurley and Lanthorn 1998; Nayak et al. 2000; Dougherty and Nichols 2009), it is possible that some of the effects of nicotine on EtOH seeking within the pVTA may be mediated in part through activation of 5-HT3 receptors.
The selectively bred alcohol-preferring (P) line of rats appears to be a useful animal model to study EtOH and nicotine use. P rats will intravenously (i.v.) self-administer more nicotine as well as express greater nicotine-seeking behavior than the alcohol-nonpreferring (NP) rats (Le et al. 2006a). Nicotine also has greater reinforcing effects in P than NP rats (Le at al. 2006a). Intracranial self-administration studies have provided further support that EtOH and nicotine may share common genetic risk factors because P rats are more sensitive to the reinforcing effects of EtOH (Rodd et al. 2003) and nicotine (Hauser et al. 2013) within the pVTA compared to Wistar rats.
Context has been shown to influence extinction learning and reinstatement of previously learned behaviors (Bouton 2002). In a spontaneous recovery paradigm, subjects are allowed to self-administer in a specific environment, the behavior is extinguished in the same environment, the subjects are withheld from that environment for a certain time, and behavior is recorded when the animals are returned to the original environment. Since spontaneous recovery is defined in the alcohol clinical literature as the cessation of alcohol consumption in human alcoholics without treatment intervention, we have altered the term to Pavlovian Spontaneous Recovery (PSR). PSR is a unique phenomenon in that it is time dependent (Bouton 1988), directly correlated to reward saliency (Robbins 1990), and contextual cues associated with first-learned signals and the amount of first- and second-learned associations (Brooks 2000).
The objectives of the current study were to test the hypothesis that local application of nicotine within the pVTA would enhance context-induced EtOH-seeking behavior and that nicotine’s stimulating effects are mediated in part via activation of 5-HT3 and nACh receptors in the pVTA. In addition, we tested the hypothesis that the activation of 5-HT3 receptors alone in the pVTA is involved in mediating context-induced EtOH-seeking behavior.
Methods
Animals
Adult EtOH-naïve female P rats from the 70th generation weighing 250–325 g at the start of the experiment were used. Female rats were used in the present study because they maintain their body and head size better than male rats for more accurate and reliable stereotaxic placements. Rats were maintained on a 12-h reversed light dark cycle (lights off at 0900 hours). Food and water were available in the home cage ad libitum throughout the experiment. All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, National Institutes of Health, and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council 1996).
Chemical agents and vehicle
Nicotine HCl (Sigma, St. Louis, MO), Mec (Sigma, St. Louis, MO), Zac (Tocris Bioscience, Ellisville, MO), and 1-(m-chlorophenyl)-biguanide (CPBG, Tocris Bioscience, Ellisville, MO) were dissolved in artificial cerebrospinal fluid (aCSF), and the pH was adjusted to 7.4±0.1. The aCSF consisted of (in millimolar) 120.0 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25.0 NaHCO3, 2.5 CaCl2, and 10.0 d-glucose.
Operant response training
P rats were placed in the standard two-lever operant-conditioning chamber, as previously described (Rodd-Henricks et al. 2002a, b; Rodd et al. 2006; Hauser et al. 2011, 2012a). Operant-conditioning sessions were 60 min in duration and occurred daily for 10 weeks. The EtOH concentration used during self-administration was 15% (v/v). During the initial 4 weeks of daily access to operant responding, both solutions (water and EtOH) were reinforced on a fixed ratio 1 (FR1) schedule. The response requirement for EtOH was increased to FR3 for 3 weeks, and then to FR5 for 3 weeks. After the P rats had established stable levels of responding on the FR5 schedule for EtOH and FR1 for water, they underwent seven sessions of extinction training (60 min/session), when neither water nor EtOH was available.
Stereotaxic surgeries
After extinction training, all rats were maintained in the home cages for 14 days. Previous research has shown that 2 weeks of home caging produced robust expression of EtOH seeking (Rodd-Henricks et al. 2002a, b; Rodd et al. 2006). Stereotaxic implantation was performed after 7 days in the home cage. While under isoflurane anesthesia, rats were prepared for bilateral stereotaxic implantation of 22-gauge guide cannula (Plastics One, Roanoke, VA) into the pVTA; the guide cannula was aimed 1.0 mm above the target region. Coordinates (Paxinos and Watson 1998) for placements to target the pVTA were −5.8 mm posterior to bregma, +2.1 mm lateral to the midline, and −8.5 mm ventral from the surface of the skull at a 10° angle to the vertical. A 28-gauge stylet was placed into the guide cannula and extended 0.5 mm beyond the tip of the guide. After surgery, rats were individually housed and allowed to recover for 7 days. Animals were handled for at least 5 min daily beginning on the fourth recovery day and were habituated for 2 consecutive days to the handling procedures necessary for microinjections.
Context-induced EtOH seeking: Pavlovian Spontaneous Recovery (PSR) testing
After 14 days in the home cages, rats were returned to the operant chambers for PSR testing in 60-min sessions. The FR5-FR1 schedule lever contingencies and dipper functioning were maintained, but EtOH and water were absent for the four consecutive PSR sessions (Hauser et al. 2012a; Rodd et al. 2006). There are four consecutive PSR sessions because previous studies have shown that exposure to EtOH odor cues or EtOH priming (Rodd-Henricks et al. 2002a, b) and some drugs (Dhaher et al. 2010) may enhance PSR responding for more than one session.
Experiment 1: effects of nicotine in the pVTA on EtOH seeking
The first set of rats was microinjected bilaterally with vehicle (aCSF) or nicotine (50, 100, or 200 µM, n=7–8/group). Nicotine was administered using the electrolytic microinfusion transducer system, as previously described (Hauser et al. 2011). Nicotine was administered consecutively to both sides of the pVTA for 10 min/side using three 5-s pulses per minute; each 5-sec pulse infused 100 nl. These concentrations of nicotine have been previously shown to be self-infused into the pVTA (Hauser et al. 2013). In this and all subsequent experiments, the micro-injections were given prior to only the first PSR session, starting 25–30 min before the session. The use of the electrolytic microinfusion transducer system has been shown to give good neuroanatomical specificity (Ikemoto et al. 2006; Rodd-Henricks et al. 2000, 2002a; Rodd et al. 2007).
Experiment 2: coinfusion of nicotine with Mec into the pVTA on EtOH seeking
The second set of rats was microinjected bilaterally with vehicle (aCSF), 100 µM nicotine+100 µMMec, or 100 µM nicotine+200 µMMec (n=6–7/group). The 100-µM nicotine data from experiment 1 were used to compare the effects of Mec+nicotine. The concentrations of Mec used have been reported to effectively reduce EtOH drinking (Ericson et al. 1998) and EtOH-associated cue-induced DA levels within the VTA (Lof et al. 2007).
Experiment 3: coinfusion of nicotine with Zac into the pVTA on EtOH seeking
The third set of rats was microinjected bilaterally with vehicle (aCSF), 100 µM nicotine+100 µM Zac, 100 µM nicotine+ 200 µM Zac (n=5–7/group). The 100-µM nicotine data from experiment 1 were used to compare the effects of Zac+nicotine. These concentrations of Zac were previously used in intracranial self-administration (ICSA) studies to inhibit the self-infusion of EtOH into the pVTA (Rodd-Henricks et al. 2003).
Experiment 4: effects of Zac in the pVTA on EtOH seeking
The fourth set of rats (n=5–8/group) was microinjected bilaterally with aCSF, or 100 or 200 µM Zac.
Experiment 5: effects of CPBG in the pVTA on EtOH seeking
The fifth set of rats (n=4–5/group) was microinjected bilaterally with aCSF, or 1 or 10 µM CPBG. The concentrations of CPBG were selected based on previous research that showed P rats will self-administer 1 and 10 µM CPBG into the pVTA (Rodd et al. 2007).
Histology
At the termination of the experiment, 1 % bromophenol blue (0.5 µl) was injected into the infusion site. Subsequently, the animals were given a fatal dose of pentobarbital and then decapitated. The brains were removed and immediately frozen at −70 °C. The frozen brains were subsequently equilibrated at −15 °C in a cryostat microtome and then sliced into 40-µm sections. Sections were then stained with cresyl violet and examined under a light microscope for verification of the injection site using the rat brain atlas of Paxinos and Watson (1998).
Statistical analysis
Overall operant responding (60 min) data were analyzed with a mixed factorial ANOVA with a between-subjects factor of dose and a repeated measure of “session”. For the PSR tests, the baseline measure for the factor of session was the average number of responses on the EtOH or water lever for the last three extinction sessions. Post hoc Tukey’s b was used to determine individual differences. All analyses with p≤0.05 were considered significant.
Results
Histology placements
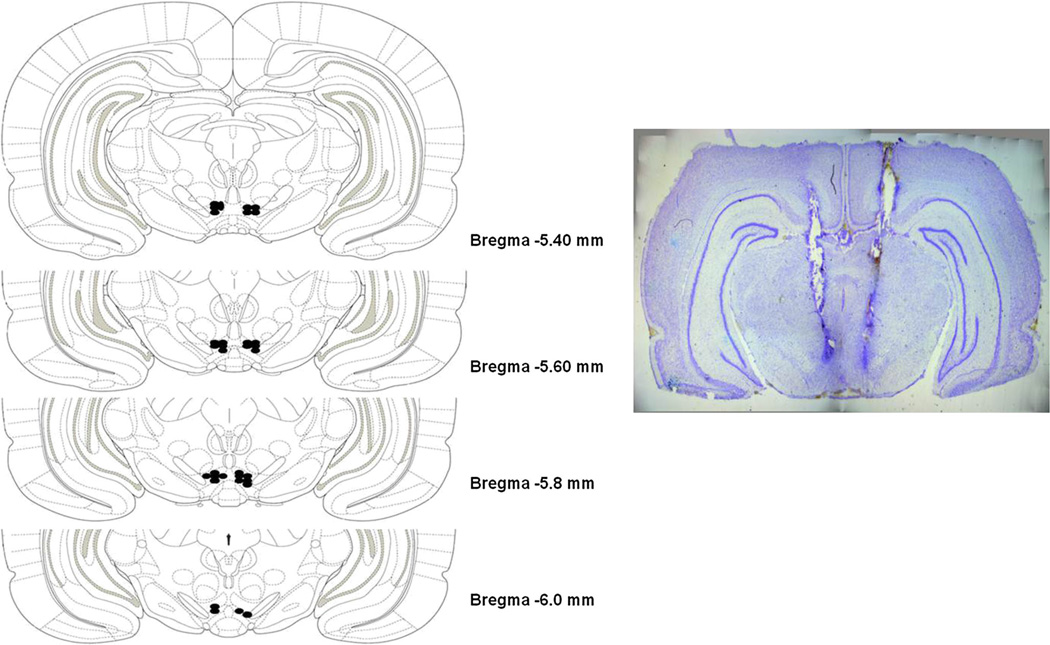
The pVTA is defined as the VTA region at the level of the interpeduncular nucleus at 5.4–6.0 mm posterior to bregma (Rodd-Henricks et al. 2000). Only animals that had correct injector placements were used in data analysis. As seen in Fig. 1, the pVTA injector placements were at 5.4–6.0 mm posterior to bregma. The success rate for dual pVTA placements was around 85 %. Incorrect injection sites were located in the anterior VTA or red nucleus.
Fig. 1.
Representative dual placements for the microinfusions of aCSF, nicotine, zacopride, or CPBG into pVTA of P rats are shown. Filled circles represent placements of injection sites within the pVTA (defined as −5.4 to −6.0 mm bregma)
Responses on the EtOH and water levers during self-administration and extinction
Average responses on the EtOH lever during the last five sessions of the EtOH self-administration part of the procedure ranged from 168 to 218 responses/session across the five experiments (Table 1). Average responses on the water lever during these sessions were usually less than 20 responses/session. Average EtOH intakes during these five sessions ranged from 1.2 to 1.5 g/kg/session. Responses on the EtOH lever were markedly reduced across extinction sessions, whereas the low responses on the water lever were not significantly altered across extinction sessions (Table 1).
Table 1.
Representation of EtOH and water lever responses (mean± SEM) during the last 5 days of maintenance, the first day of extinction, and last 3 days of extinction that were used for baseline prior to the first PSR session
| EtOH Lever (FR5) | Water Lever (FR1) | EtOH (g/kg) | |
|---|---|---|---|
| Experiment 1 (Nicotine) | |||
| EtOH Maintenance | 202±22 | 10±2 | 1.4±0.2 |
| Extinction (Day 1) | 146±11 | 10±2 | |
| Base Extinction (last 3 days) | 26±3 | 9±3 | |
| Experiment 2 (Nicotine+Mecamylamine) | |||
| EtOH Maintenance | 204±15 | 15±3 | 1.5±0.1 |
| Extinction (Day 1) | 180±11 | 22±3 | |
| Base Extinction (last 3 days) | 29±3 | 13±2 | |
| Experiment 3 (Nicotine+Zacopride) | |||
| EtOH Maintenance | 182±15 | 19±3 | 1.3±0.1 |
| Extinction (Day 1) | 144±11 | 8±4 | |
| Base Extinction (last 3 days) | 27±3 | 14±3 | |
| Experiment 4 (Zacopride) | |||
| EtOH Maintenance | 168±18 | 14±3 | 1.2±0.1 |
| Extinction (Day 1) | 151±15 | 33±9 | |
| Base Extinction (last 3 days) | 31±6 | 17±4 | |
| Experiment 5(CPBG) | |||
| EtOH Maintenance | 218±19 | 9±2 | 1.5±0.1 |
| Extinction (Day 1) | 147±10 | 12±2 | |
| Base Extinction (last 3 days) | 35±7 | 11±4 |
Effects of nicotine in the pVTA on EtOH seeking
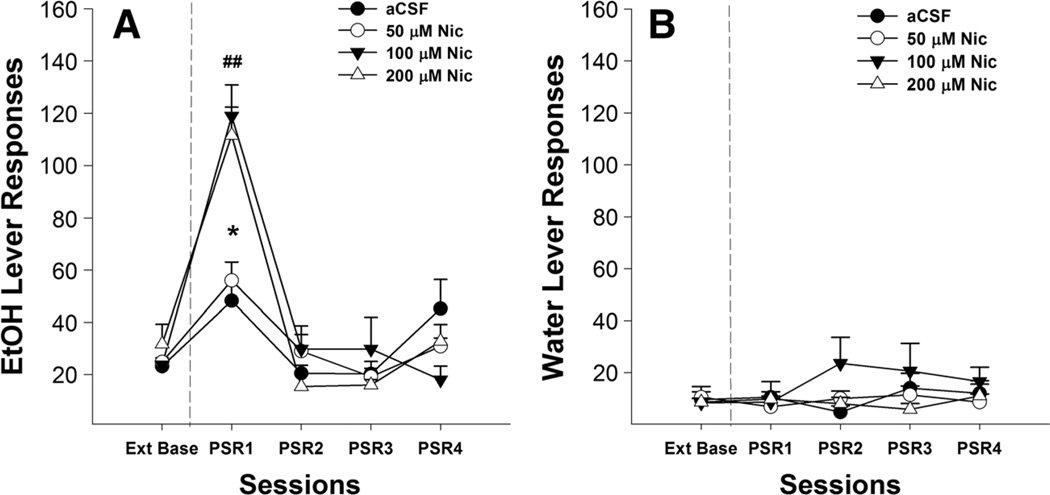
Examining the effects of nicotine alone on the number of responses on the lever previously associated with the delivery of EtOH (Fig. 2a) indicated a significant effect of session (F4,13=30.10, p<0.001), “dose” (F3,16=13.98, p<0.001), and session by dose interaction (F12,45=3.18, p<0.01). Post hoc comparisons indicated that P rats given 100 or 200 µM nicotine directly into the pVTA responded more than P rats microinjected with aCSF or 50 µM nicotine during the first PSR session. Comparison to extinction baseline values revealed that responding on the lever previously associated with EtOH was significantly increased during the first PSR session for rats given aCSF or 50,100, or 200 µMnicotine (p values <0.05). Nicotine did not significantly alter responses on the lever previously associated with water (Fig. 2b).
Fig. 2.
Mean (±SEM) responses per session on the lever previously associated with the delivery of EtOH (a) or water (b) by P rats (n=6–7/group) microinjected with aCSF or 50, 100, or 200 µM nicotine into the pVTA. Asterisk (*) indicates that rats that administered aCSF or 50, 100, or 200 µM nicotine responded significantly (p<0.05) more on the EtOH lever during the first PSR session compared to extinction baseline levels. Double pound (##) indicates that 100 or 200 µM nicotine increased responding on the EtOH lever during the first PSR session compared to the aCSF and 50 µM nicotine (p<0.05)
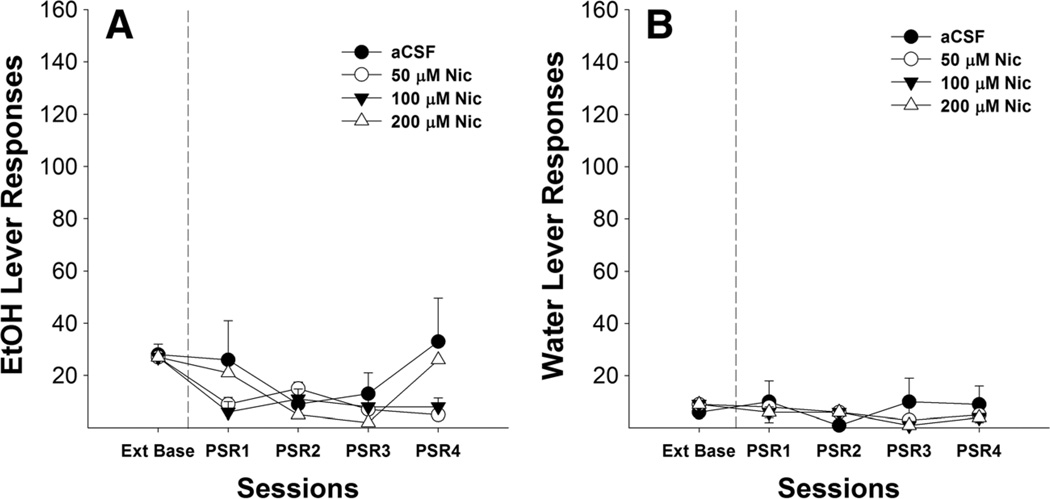
Microinjection of nicotine into areas outside of the pVTA (i.e., the anterior VTA and red nucleus) did not appear to significantly alter responses on the EtOH or water levers during the first or subsequent PSR sessions (Fig. 3).
Fig. 3.
Mean (±SEM) responses per session on the lever previously associated with the delivery of EtOH (a) or water (b) by P rats (n= 1–6/group), with placements in the anterior VTA and red nucleus, microinjected with aCSF or 50, 100, or 200 µM nicotine. No significant differences were detected
Effects of co-infusion of nicotine with Mec into the pVTA on EtOH-seeking
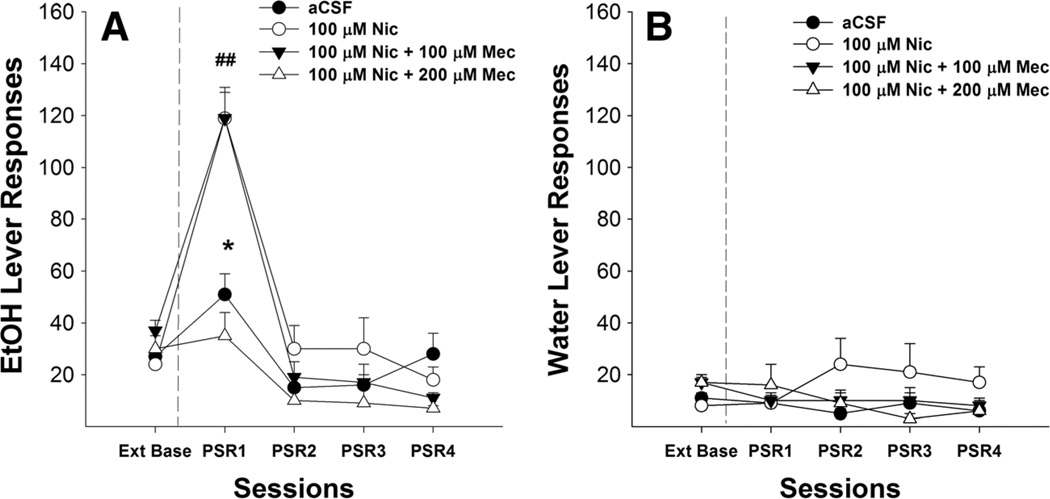
The number of responses on the lever previously associated with the delivery of EtOH for rats given aCSF, 100 µM nicotine, or 100 µM nicotine with Mec (Fig. 4a) indicated a significant effect of session (F4,14=25.19, p<0.001), dose (F3,16= 7.08, p<0.01), and session by dose interaction (F12,45=2.71, p<0.01). Post hoc comparisons indicated that P rats given 100 µM nicotine+200 µMMec responded significantly less than P rats microinjected with 100 µMnicotine, 100 µM nicotine+100 µMMec, or aCSF. P rats given 100 µM nicotine or 100 µMnicotine+100 µM Mec responded more on the EtOH lever during the first PSR session compared to the aCSF group. Comparison to extinction baseline values indicated responding on the lever previously associated with EtOH was significantly increased during the first PSR testing for rats given aCSF, 100 µM nicotine and 100 µM nicotine+100 µMMec (p values<0.05). There were no significances in the number of responses on the lever previously associated with the delivery of water during the first PSR session (Fig. 4b).
Fig. 4.
Mean (±SEM) responses per session on the lever previously associated with the delivery of EtOH (a) or water (b) by P rats (n=6–7/group) microinjected with aCSF or 100 µM nicotine, 100 µM nicotine+ 100 µM mecamylamine (a nonselective nicotinic receptor antagonist), or 100 µM nicotine+µM mecamylamine into the pVTA. Asterisk (*) indicates that rats that administered aCSF, 100 µM nicotine, or 100 µM nicotine+100 µM mecamylamine responded significantly (p<0.05) more on the EtOH lever during the first PSR session compared to extinction baseline levels. Double pound (##) indicates that 100 µM nicotine or 100 µM nicotine+100 µM mecamylamine increased responding on the EtOH lever during the first PSR session compared to the aCSF, whereas 100 µM nicotine+200 µM mecamylamine decreased responding on the EtOH lever during the first PSR session compared to 100 µM nicotine or 100 µM nicotine+100 µM mecamylamine (p<0.05)
Effects of coinfusions of nicotine with Zac into the pVTA on EtOH seeking
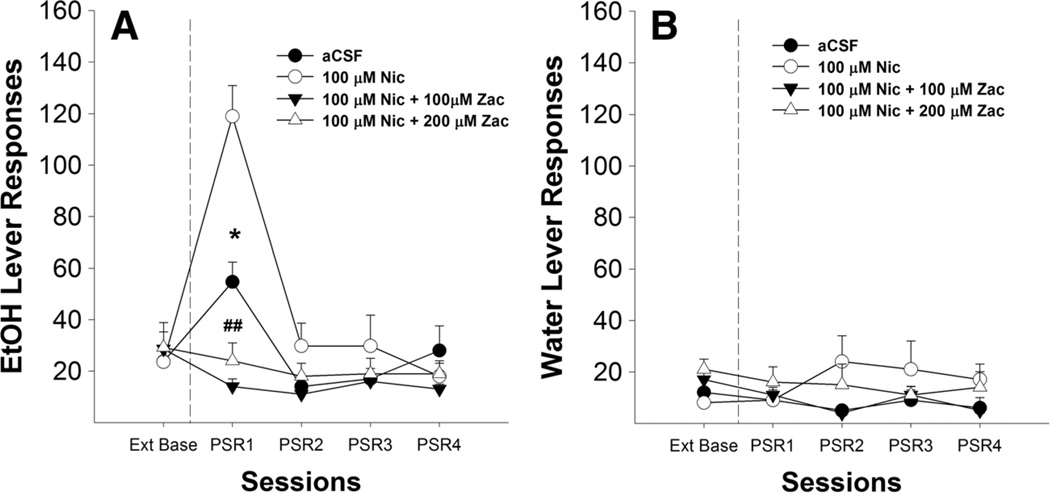
Analysis of the number of responses on the lever previously associated with the delivery of EtOH for rats microinjected with aCSF, 100 µM nicotine, 100 µMnicotine+100 µM Zac, or 100 µM nicotine+200 µM Zac (Fig. 5a) indicated a significant effect of session (F4,15= 11.790, p<0.001), dose (F3,18=6.975, p<0.01), and session by dose interaction (F12,51=2.788,p<0.005). Post hoc comparisons indicated that P rats given 100 µMnicotine +100 or 200 µM Zac responded significantly less than P rats given aCSF or nicotine alone. P rats given 100 µM nicotine responded more during the first PSR session compared to the aCSF group. P rats given aCSF or nicotine alone responded more during the first PSR session compared to extinction baseline (p value <0.01). Microinjection of 100 µM nicotine+100 µM Zac (but not nicotine+ 200 µM Zac) reduced the number of EtOH lever responses during the first PSR session compared to extinction baseline (p values <0.01). The results indicated that there were no significant group differences for the number of responses on the lever previously associated with water (Fig. 5b).
Fig. 5.
Mean (±SEM) responses per session on the lever previously associated with the delivery of EtOH (a) or water (b) by P rats (n=5–7/group) microinjected with aCSF, 100 µM nicotine, 100 µM nicotine+ 100 µM zacopride (a 5-HT3 receptor antagonist), or 100 µM nicotine+ 200 µM zacopride into the pVTA. Asterisk (*) indicates that rats that administered aCSF or 100 µM nicotine responded significantly (p<0.05) more on the EtOH lever during the first PSR session compared to extinction baseline levels. Double pound (##) indicates that 100 µM nicotine+100 µM zacopride, or 100 µM nicotine+200 µM zacopride decreased responding on the EtOH lever during the first PSR session compared to the aCSF or 100 µM nicotine (p<0.05)
Effects of Zac in the pVTA on EtOH-seeking
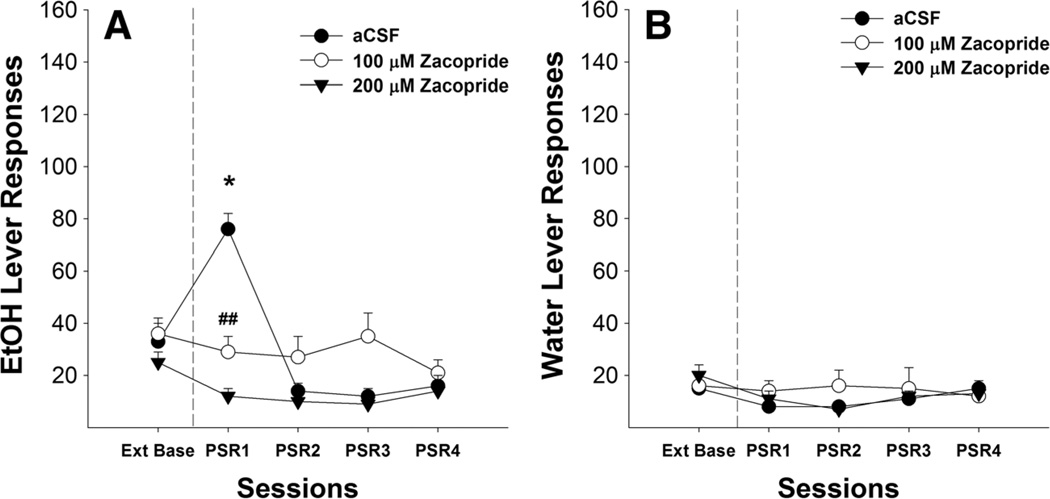
For Zac alone, the number of responses on the lever previously associated with the delivery of EtOH (Fig. 6a) indicated a significant effect of session (F4,12= 17.69, p<0.001), dose (F2,15=3.70, p=0.049), and session by dose interaction (F8,26=4.47,p<0.01). Post hoc comparisons indicated that P rats given 100 and 200 µM Zac responded significantly less than P rats given aCSF. Comparison to extinction baseline values revealed that responding on the lever previously associated with EtOH was significantly increased during the first PSR testing for rats that administered aCSF (p<0.01), where-as there were no differences between 100 or 200 µM Zac compared to extinction baseline. There were no significant group differences in the number of responses on the lever previously associated with water during the first PSR session (Fig. 6b).
Fig. 6.
Mean (±SEM) responses per session on the lever previously associated with the delivery of EtOH (a) or water (b) by P rats (n=5–8/group) microinjected with aCSF, or 100 or 200 µM zacopride (a 5-HT3 receptor antagonist) into the pVTA. Asterisk (*) indicates that rats that administered aCSF responded significantly (p<0.01) more on the EtOH lever during the first PSR session compared to extinction baseline levels. Double pound (##) indicates that 100 or 200 µM zacopride significantly decreased responding on the EtOH lever during the first PSR session compared to aCSF (p<0.01)
Effects of CPBG in the pVTA on EtOH seeking
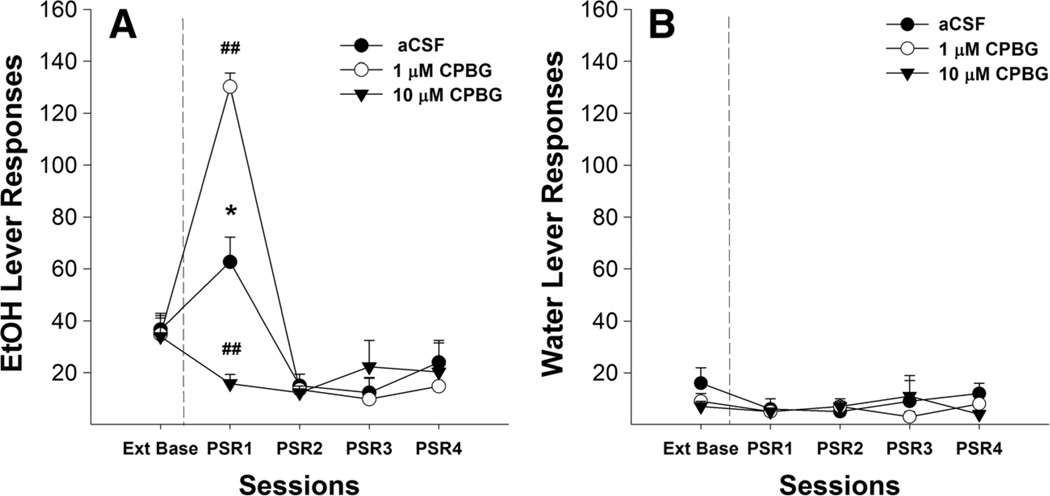
For CPBG, examining the number of responses on the lever previously associated with the delivery of EtOH (Fig. 7a) indicated a significant effect of session (F4,6 = 42.99, p<0.001), dose (F2,9=4.07, p=0.055), and session by dose interaction (F8,12=6.78, p<0.01). Individual ANOVAs performed for each session indicated a significant effect of dose during the first PSR test session (F2,9=68.40,p<0.001). Post hoc comparisons indicated that P rats given 1 µM CPBG directly into the pVTA responded significantly more during the first PSR session than P rats given aCSF, whereas those that administered 10 µM CPBG responded significantly less during the first PSR compared to the aCSF group. In addition, EtOH lever responses were higher in the aCSF and 1-µM CPBG groups during the first PSR test session compared to extinction baseline (p values <0.05). There were no significant differences between 10-µM CPBG and extinction baseline values. There were no significant group differences in the number of responses on the lever associated with water during the first PSR session (Fig. 7b).
Fig. 7.
Mean (±SEM) responses per session on the lever previously associated with the delivery of EtOH (a) or water (b) by P rats (n=4–5/group) microinjected with aCSF, or 1 or 10 µM CPBG (a 5-HT3 receptor agonist) into the pVTA. Asterisk (*) indicates that rats that administered aCSF or 1 µM CPBG responded significantly (p<0.05) more on the EtOH lever during the first PSR session compared to extinction baseline levels. Double pound (##) indicates that 1 µM CPBG increased responding on the EtOH lever, and 10 µM CPBG decreased responding on the EtOH lever during the first PSR session compared to the aCSF (p<0.01)
Discussion
The major findings of this study are that physiologically relevant levels of nicotine microinjected into the pVTA can promote context-induced EtOH seeking and that 5-HT3 and nACh receptors are involved. The 5-HT3 receptor antagonist Zac microinjected into the pVTA reduced responding on the EtOH lever in PSR test at both doses (Fig. 6a), suggesting that activating 5-HT3 receptors within the pVTA are involved in the expression of context-induced EtOH-seeking behavior in P rats. 5-HT3 receptors play a role in increasing the activity of VTA DA neurons (Campbell et al. 1996). In addition, there are 5-HT3 receptors found within the pVTA (Herve et al. 1987). Inhibition of 5-HT3 receptors within the VTA can inhibit EtOH-induced extracellular DA release in the VTA (Campbell et al. 1996). A previous study (Hauser et al. 2011) indicated that activation of DA neurons in the pVTA, but not the anterior VTA, mediates context-induced EtOH-seeking behavior of P rats. Overall, these results suggest that activation of 5-HT inputs at 5-HT3 receptors in the pVTA, resulting in the stimulation of VTA DA neurons, may be involved in the expression of EtOH-seeking behavior.
The current results are in agreement with the findings of Le et al. (2006b) who report that intraperitoneal injections of the 5-HT3 receptor antagonists, ondansetron and tropisetron, reduced footshock-induced EtOH-seeking behavior in Wistar rats. In addition, the current findings also support clinical findings that administration of a 5-HT3 receptor antagonist can reduce alcohol craving in alcohol-dependent subjects (Johnson 2004; Johnson et al. 2002; Sellers et al. 1994).
In contrast, other studies have found that the 5-HT3 receptor antagonist ondansetron did not alter nicotine-induced decreases in self-stimulation threshold within the VTA (Ivanova and Greenshaw 1997) nor did the 5-HT3 antagonists ICS 205–930 and MDL-72222 alter intravenous nicotine self-administration or nicotine-induced stimulation of activity (Corrigall and Coen 1994). The apparent disagreement between the present study and those mentioned above may be due to a combination of factors, including different mechanisms that may underlie the different behaviors that were measured, as well as differences in the drug histories of the animals and the routes of administration of the antagonists.
If activation of 5-HT3 receptors in the pVTA is involved in regulating context-induced EtOH-seeking behavior, as suggested by the results with zacopride (Fig. 6), then local microinfusion of a 5-HT3 receptor agonist into the pVTA should increase expression of context-induced EtOH-seeking behavior. The microinjection of 1 µM CPBG into the pVTA increased responding on the EtOH lever approximately twofold over aCSF levels of responding (Fig. 7a), providing additional support for the involvement of 5-HT3 receptors in mediating context-induced EtOH-seeking behavior. This effect did not appear to be due to an increase in general motor activity since responses on the water lever were not altered (Fig. 7b). However, in contrast to the effects observed with 1 µM CPBG, the microinjection of 10 µM CPBG into the pVTA significantly reduced responding on the EtOH lever compared to aCSF levels (Fig. 7a). This concentration of CPBG should still be having an effect on DA release as suggested by reverse microdialysis studies (Campbell et al. 1996; Liu et al. 2006), and ICSA results showed that the 10 µM CPBG was the optimal concentration self-infused (Rodd et al. 2007). Therefore, it is possible that 10 µM effects of CPBG, due to its enhanced reinforcing effects and higher DA release in pVTA, are producing a euphoric effect leading to the reduction of context-induced EtOH seeking in the current study. Alternatively, it is possible that the current experimental approach may be producing a local higher-CPBG concentration than the ICSA and reverse microdialysis procedures, resulting in inhibition of DA reuptake and higher concentrations of DA-activating D2 autoreceptors, which would reduce VTA DA neuronal activity and inhibit EtOH-seeking behavior (Hauser et al. 2011).
Varenicline, an α4β2 nACh receptor partial agonist and a potent 5-HT3 receptor agonist (Lummis et al. 2011), reduced EtOH-seeking behavior in rats (Steensland et al. 2007). The apparent differences between the current 5-HT3 agonist results and Steensland et al. (2007) findings may be due to differences in the route of administration, e.g., with systemic administration; varenicline may be acting at sites outside the VTA to reduce EtOH seeking.
Nicotinic receptors within the pVTA appear to be involved in mediating EtOH-seeking behavior. Microinfusion of 100 and 200 µM nicotine increased responding on the EtOH lever twofold over control values (Fig. 2a). These concentrations did not alter responding on the water lever (Fig. 2b), indicating that the increased responding on the EtOH lever was not due to an increase in general motor activity. Concentrations of 10 to 200 µM nicotine were self-infused into the pVTA by P rats (Hauser et al. 2013), suggesting that the concentrations of nicotine used in the present study can produce positive effects. Local application of 100 µMnicotine within the VTA has been shown to increase somatodendritic (Rahman et al. 2004) and terminal (Ding et al. 2012) DA release. Overall, the results are consistent with effects of nicotine on EtOH-seeking behavior being mediated by increased VTA DA neuronal activity. These results are in agreement with previous studies that demonstrated (a) the involvement of pVTA DA neuronal activity (Hauser et al. 2011) in regulating EtOH seeking and (b) that systemically administered nicotine significantly enhanced EtOH-seeking behavior in P rats (Hauser et al. 2012a). The nicotine concentrations in the current study approximate levels attained by human smokers (15–150 ng/ml; Benowitz and Jacob 1984) and of P rats consuming nicotine (Hauser et al. 2012b).
The enhancing effect of nicotine on EtOH-seeking behavior was inhibited by coinfusion with Mec (Fig. 4a) and Zac (Fig. 5a), suggesting that nicotine may be acting at both nACh and 5-HT3 receptors. These results are consistent with the effects of 5-HT3 receptor antagonists inhibiting the self-infusion of nicotine into the pVTA (Hauser et al. 2013), and with the high affinity of nicotine for the 5-HT3 receptor (Jackson and Yakel 1995). Responding on the water lever was not altered by any of the treatments (Figs. 4b and 5b); because of the low responses on the water lever, a floor effect may have prevented observing any reduced responding due to general motor impairment. However, previous studies indicated that microinjection of a 5-HT3 receptor antagonist into the pVTA did not impair operant responding (Rodd et al. 2010); neither systemic (Ford et al. 2009) nor VTA (Ericson et al. 1998) administration of Mec appeared to impair motor activity. Interestingly, it has been reported that Mec (10 µM) can inhibit 5-HT-activated currents by acting as an antagonist at 5-HT3 receptors (Drisdel et al. 2008), thus suggesting that Mec may be a 5-HT3 receptor antagonist as well as a nACh receptor antagonist in the current study on EtOH seeking. However, it is not clear if the results of the present study are selective for EtOH-seeking behavior or if similar effects could also be observed for other drugs.
The α4 nicotinic receptor and 5-HT3 receptor coexist on striatal nerve terminals (Dougherty and Nichols 2009; Nayak et al. 2000), although it is not known if a similar coexistence also occurs within the VTA. Activation of nACh or 5-HT3 receptors within the pVTA produces reinforcing effects (Hauser et al. 2013; Rodd et al. 2007) and stimulates DA release (Liu et al. 2006; Rahman et al. 2004), suggesting that both types of receptors may be located on VTADA neurons. Antagonism of nACh receptors with Mec can reduce nicotine-induced DA release within VTA (Nisell et al. 1994; Rahman et al. 2004). Although there are no current reports on Zac’s effects on nicotine-induced DA release, it has been reported that the 5-HT3 antagonist ICS 205–930 can attenuate systemic nicotine-induced DA release in the Acb, thus suggesting that some of nicotine’s stimulating effects on DA may be mediated in part by 5-HT3 receptors (Carboni et al. 1989). The α4 and α6 nicotinic receptors have been shown to be necessary for nicotine-induced DA neuronal activity in the VTA (Zhao-Shea et al. 2011; Gotti et al. 2010); α7 nicotinic receptors located on the presynaptic glutamate terminals (Albuquerque et al. 2009; Gotti and Clementi 2004; Nayak et al. 2000; Wonnacott 1997) are thought to prolong DA neurotransmission by enhancing glutamatergic excitation in the presence of nicotine (Pidoplichko et al. 2004). 5-HT3 receptors also appear to be involved in mediating the release of glutamate (Dong et al. 2009). Overall, these latter findings suggest that the interactions of nicotinic and 5-HT3 receptors within the pVTA are likely complex and involve more than just their interactions directly on DA neurons.
In conclusion, the ability of local applications of nicotine into the pVTA to promote context-induced EtOH seeking suggests that the pVTA is a neuroanatomical site in which drugs of abuse can activate seeking behavior for another drug of abuse. The current findings also provide evidence that the actions of nicotine appear to occur through its effects at both nACh and 5-HT3 receptors in the pVTA and that activation of 5-HT3 receptors may play a role in mediating context-induced EtOH seeking.
Acknowledgments
The skillful technical assistance of Tylene Pommer and Victoria McQueen is gratefully acknowledged. This project was supported by research grants AA07611, AA022287, AA020908, AA07462, and AA019366 from NIAAA. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIAAA or NIH.
Footnotes
Conflict of interest I certify that there is no actual or potential conflict of interest in relation to this article.
References
- Abrams DB, Rohsenow DJ, Niaura RS, Pedraza M, Longbaugh R, Beatties MC, Binkoff JA, Noel NE, Monti PM. Smoking and treatment outcome for alcoholics: effects on coping skills, urge to drink and drinking rats. Behav Ther. 1992;23:283–297. [Google Scholar]
- Albuquerque EX, Pereira EFR, Alkondon M, Rogers SW. Mammalian nicotinic acetylcholine receptors: from structure to function. Physiol Rev. 2009;89:73–120. doi: 10.1152/physrev.00015.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alen F, Gomez R, Gonzalez-Cuevas G, Navarro M, Lopez-Moreno JA. Nicotine causes opposite effects on alcohol intake: Evidence in an animal experimental model of abstinence and relapse from alcohol. Nicotine Tob Res. 2009;11:1304–1311. doi: 10.1093/ntr/ntp139. [DOI] [PubMed] [Google Scholar]
- Bianchi C, Ferraro L, Tanganelli S, Morari M, Spalluto G, Simonato M, Beani L. 5-Hydroxytryptamine-mediated effects of nicotine on endogenous GABA efflux from guinea-pig slices. Br J Pharmacol. 1995;116:2724–2728. doi: 10.1111/j.1476-5381.1995.tb17233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat: Effects of nicotinic acetylcholine receptor blockade or subchronic nicotine treatment. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- Breitinger H-GA, Geetha N, Hess GP. Inhibition of the serotonin 5-HT3 receptor by nicotine, cocaine and fluoxetine investigated by rapid chemical kinetic techniques. Biochem. 2001;40:8419–8429. doi: 10.1021/bi0106890. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brooks DC. Recent and remote extinction cues reduce spontaneous recovery. Q. J. Exp Psychol. 2000;153:25–58. doi: 10.1080/027249900392986. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Lacey MG, North RA. Nicotinic excitation of rat ventral tegmental neurones in vitro studied by intracellular recording. Br J Pharmacol. 1989;98:135–140. doi: 10.1111/j.1476-5381.1989.tb16873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell AD, Kohl RR, McBride WJ. Serotonin-3 receptor and ethanol-stimulated somatodendritic dopamine release. Alcohol. 1996;13:569–574. doi: 10.1016/s0741-8329(96)00069-9. [DOI] [PubMed] [Google Scholar]
- Carboni E, Acquas E, Frau R, Di Chiara G. Differential inhibitory effects of a 5-HT3 antagonist on drug-induced stimulation of dopamine release. Eur J Pharmacol. 1989;164:515–519. doi: 10.1016/0014-2999(89)90259-8. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine self-administration and loco-motor activity are not modified by the 5-HT3 antagonists ICS 205-930 and MDL 72222. Pharmacol Biochem Behav. 1994;49:67–71. doi: 10.1016/0091-3057(94)90457-x. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Adamson KL. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 1994;653:278–284. doi: 10.1016/0006-8993(94)90401-4. [DOI] [PubMed] [Google Scholar]
- Daeppen JB, Smith TL, Danko GP, Gordon L, Landi NA, Nurnberger JI, Jr, Bucholz KK, Raimo E, Schuckit MA. Clinical correlates of cigarette smoking and nicotine dependence in alcohol-dependent men and women. The Collaborative Study Group on the Genetics of Alcoholism. Alcohol Alcohol. 2000;35:171–175. doi: 10.1093/alcalc/35.2.171. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Hauser SR, Getachew B, Bell RL, McBride WJ, McKinzie DL, Rodd ZA. The orexin-1 receptor antagonist SB-334867 reduces alcohol relapse drinking, but not alcohol-seeking, in Alcohol-Preferring (P) rats. J Addict Med. 2010;4:153–159. doi: 10.1097/ADM.0b013e3181bd893f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Z-M, Katner SN, Rodd ZA, Truitt W, Hauser SR, Deehan GA, Jr, Engleman EA, McBride WJ. Repeated exposure of the posterior ventral tegmental area to nicotine increases the sensitivity of local dopamine neurons to the stimulating effects of ethanol. Alcohol. 2012;46:217–223. doi: 10.1016/j.alcohol.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L, Zhu Y, Dong Y, Yang J, Zhao Y, Qi Y, Wu P, Zhu Y, Zheng P. Neuroactive steroid dehydroepiandrosterone sulfate inhibits 5 hydroxytryptamine (5-HT)-evoked glutamate release via activation of sigma-1 receptors and then inhibition of 5-HT3 receptors in rat prelimbic cortex. J Pharmacol Exp Ther. 2009;330:494–501. doi: 10.1124/jpet.109.154294. [DOI] [PubMed] [Google Scholar]
- Dougherty JJ, Nichols RA. Cross-regulation between colocalized nicotinic acetylcholine and 5-HT3 serotonin receptors on presynaptic nerve terminals. Acta Pharmacol Sin. 2009;30:788–794. doi: 10.1038/aps.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drisdel RC, Sharp D, Henderson T, Hales TG, Green WN. High affinity binding of epibatidine to serotonin type 3 receptors. J Biol Chem. 2008;283:9659–9665. doi: 10.1074/jbc.M703672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson M, Blomqvist O, Engel JA, Soderpalm B. Voluntary ethanol intake in the rat and the associated accumbal dopamine overflow are blocked by ventral tegmental mecamylamine. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- Ericson M, Molander A, Lof E, Engel JA, Soderpalm B. Ethanol elevates accumbal dopamine levels via indirect activation of ventral tegmental nicotinic acetylcholine receptors. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- Ford MM, Fretwell AM, Nickel JD, Mark GP, Strong MN, Yoneyama N, Finn DA. The influence of mecamylamine on ethanol and sucrose self-administration. Neuropharmacology. 2009;57:250–258. doi: 10.1016/j.neuropharm.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotti C, Clementi F. Neuronal nicotinic receptors: from structure to pathology. Prog Neurobiol. 2004;74:363–396. doi: 10.1016/j.pneurobio.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Gotti C, Guiducci S, Tedesco V, Corbioli S, Zanetti L, Moretti M, Zanardi A, Rimondini R, Mugnaini M, Clementi F, Chiamulera C, Zoli M. Nicotinic acetylcholine receptors in the mesolimbic pathway: primary role of ventral tegmental area alpha6beta2* receptors in mediating systemic nicotine effects on dopamine release, locomotion, and reinforcement. J Neurosci. 2010;30:5311–5325. doi: 10.1523/JNEUROSCI.5095-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulliver SB, Rohsenow DJ, Colby SM, Dey AN, Abrams DB, Niaura RS, Monti PM. Interrelationship of smoking and alcohol dependence, use and urges to use. J Stud Alcohol. 1995;56:202–206. doi: 10.15288/jsa.1995.56.202. [DOI] [PubMed] [Google Scholar]
- Gurley DA, Lanthorn TH. Nicotinic agonists competitively antagonize serotonin at mouse 5-HT3 receptors expressed in Xenopus oocytes. Neurosci Lett. 1998;247:107–110. doi: 10.1016/s0304-3940(98)00306-1. [DOI] [PubMed] [Google Scholar]
- Hauser SR, Ding ZM, Getachew B, Toalston JE, Oster SM, McBride WJ, Rodd ZA. The posterior ventral tegmental area mediates alcohol-seeking behavior in alcohol-preferring rats. J Pharmacol Exp Ther. 2011;336:857–865. doi: 10.1124/jpet.110.168260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Getachew B, Oster SM, Dhaher R, Ding ZM, Bell RL, McBride WJ, Rodd ZA. Nicotine modulates alcohol-seeking and relapse by alcohol-preferring (P) rats in a time-dependent manner. Alcohol Clin Exp Res. 2012a;36:43–54. doi: 10.1111/j.1530-0277.2011.01579.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Katner SN, Deehan GA, Jr, Ding ZM, Toalston JE, Scott BJ, Bell RL, McBride WJ, Rodd ZA. Development of an oral operant nicotine/ethanol co-use model in alcohol-preferring (P) rats. Alcohol Clin Exp Res. 2012b;36:43–54. doi: 10.1111/j.1530-0277.2012.01800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser SR, Bracken AL, Deehan GA, Jr, Toalston JE, Ding ZM, Truitt WA, Bell RL, McBride WJ, Rodd ZA. Selective breeding for high alcohol preference increases the sensitivity of the posterior VTA to the reinforcing effects of nicotine. Addict Biol. 2013 doi: 10.1111/adb.12048. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson LM, Zhao-Shea R, Tapper AR. Modulation of ethanol drinking-in-the-dark by mecamylamine and nicotinic acetylcholine receptor agonists in C57BL/6 J mice. Psychopharmacology (Berlin) 2009;204:563–572. doi: 10.1007/s00213-009-1488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D, Pickel VM, Joh TH, Beaudet A. Serotonin axon terminals in the ventral tegmental area of the rat: fine structure and synaptic input to dopaminergic neurons. Brain Res. 1987;435:71–83. doi: 10.1016/0006-8993(87)91588-5. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Qin M, Liu Z-H. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. J Neurosci. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanová S, Greenshaw AJ. Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mec-amylamine but not scopolamine or ondansetron. Psychopharmacology (Berlin) 1997;134:187–192. doi: 10.1007/s002130050441. [DOI] [PubMed] [Google Scholar]
- Jackson MB, Yakel JL. The 5-HT3 receptor channel. Ann Rev Physiol. 1995;57:447–468. doi: 10.1146/annurev.ph.57.030195.002311. [DOI] [PubMed] [Google Scholar]
- Johnson BA. Role of the serotonergic system in the neurobiology of alcoholism: implications for treatment. CNS Drugs. 2004;18:1105–1118. doi: 10.2165/00023210-200418150-00005. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Roache JD, Ait-Daoud N, Zanca NA, Velazquez M. Ondansetron reduces the craving of biologically predisposed alcoholics. Psychopharmacology (Berlin) 2002;160:408–413. doi: 10.1007/s00213-002-1002-9. [DOI] [PubMed] [Google Scholar]
- Kidd FJ, Levy JC, Nielsen M, Hamon M, Gozlan H. Characterisation of the non-5-HT3 high-affinity ‘R’ binding site for (R)-zacopride in brain and other tissues. Eur J Pharmacol. 1993;247:45–56. doi: 10.1016/0922-4106(93)90136-w. [DOI] [PubMed] [Google Scholar]
- Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Involvement of nicotinic receptors in alcohol self-administration. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Wang A, Harding S, Juzytsch W, Shaham Y. Nicotine increases alcohol self-administration and reinstates alcohol seeking in rats. Psychopharmacology (Berlin) 2003;168:216–221. doi: 10.1007/s00213-002-1330-9. [DOI] [PubMed] [Google Scholar]
- Le AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naϊve offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006a;26:1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Funk D, Harding S, Juzytsch W, Fletcher PJ, Shaham Y. Effects of dexfenfluramine and 5-HT3 receptor antagonists on stress-induced reinstatement of alcohol seeking in rats. Psychopharmacology (Berlin) 2006b;186:82–92. doi: 10.1007/s00213-006-0346-y. [DOI] [PubMed] [Google Scholar]
- Liu W, Thielen RJ, Rodd ZA, McBride WJ. Activation of serotonin-3 receptors increases dopamine release within the ventral tegmental area of Wistar and alcohol-preferring (P) rats. Alcohol. 2006;40:167–176. doi: 10.1016/j.alcohol.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lof E, Olausson P, deBejczy A, Stomberg R, Mclntosh JM, Taylor JR, Söderpalm B. Nicotinic acetylcholine receptors in the ventral tegmental area mediate the dopamine activating and reinforcing properties of ethanol cues. Psychopharmacology (Berlin) 2007;195:333–343. doi: 10.1007/s00213-007-0899-4. [DOI] [PubMed] [Google Scholar]
- Lopez-Moreno JA, Trigo-Diaz JM, de Rodriguez FF, Gonzalez CG, de Gomez HR, Crespo GI, Navarro M. Nicotine in alcohol deprivation increases alcohol operant self-administration during reinstatement. Neuropharmacology. 2004;47:1036–1044. doi: 10.1016/j.neuropharm.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Lummis SC, Thompson AJ, Bencherif M, Lester HA. Varenicline is a potent agonist of the human 5-hydroxytryptamine3 receptor. J Pharmacol Exp Ther. 2011;339:125–131. doi: 10.1124/jpet.111.185306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci U S A. 2000;97:9305–9310. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayak SV, Ronde P, Spier AD, Lummis SCR, Nichols RA. Nicotinic receptors co-localize with 5-HT3 serotonin receptors on striatal nerve terminals. Neuropharmacology. 2000;39:2681–2690. doi: 10.1016/s0028-3908(00)00109-x. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4th edn. New York: Academic Press; 1998. [Google Scholar]
- Peters JA, Carland JE, Cooper MA, Livesey MR, Deeb TZ, Hales TG, Lambert JJ. Novel structural determinants of single-channel conductance in nicotinic acetylcholine and 5-hydroxytryptamine type-3 receptors. Biochem Soc Trans. 2006;34:882–886. doi: 10.1042/BST0340882. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, Noguchi J, Areola OO, Liang Y, Peterson J, Zhang T, Dani JA. Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learn Mem. 2004;11:60–69. doi: 10.1101/lm.70004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Zhang J, Corrigall WA. Local perfusion of nicotine differentially modulates somatodendritic dopamine release in the rat ventral tegmental area after nicotine pre-exposure. Neurochem Res. 2004;29:1687–1693. doi: 10.1023/b:nere.0000035803.64724.17. [DOI] [PubMed] [Google Scholar]
- Robbins SJ. Mechanisms underlying spontaneous recovery in authoshaping. J Exp Psychol Anim Behav Process. 1990;16:235–249. [Google Scholar]
- Rodd ZA, McKinzie DL, Bell RL, McQueen VK, Murphy JM, Schoepp DD, McBride WJ. The metabotropic glutamate 2/3 receptor agonist LY404039 reduces alcohol-seeking but not alcohol self-administration in alcohol-preferring (P) rats. Behav Brain Res. 2006;171:207–215. doi: 10.1016/j.bbr.2006.03.032. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Gryszowka VE, Toalston JE, Oster SM, Ji D, Bell RL, McBride WJ. The reinforcing actions of a serotonin-3 receptor agonist within the ventral tegmental area: evidence for subregional and genetic differences and involvement of dopamine neurons. J Pharmacol Exp Ther. 2007;321:1003–1012. doi: 10.1124/jpet.106.112607. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Oster SM, Toalston JE, Pommer TJ, McBride WJ, Murphy JM. Serotonin-3 receptors in the posterior ventral tegmental area regulate ethanol self-administration of alcohol-preferring (P) rats. Alcohol. 2010;44:245–255. doi: 10.1016/j.alcohol.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology. 2000;149:217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcohol Clin Exp Res. 2002a;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, Li TK. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcohol Clin Exp Res. 2002b;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology. 2003;165:252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- Sajja RK, Rahman S. Neuronal nicotinic receptor ligands modulate chronic nicotine-induced ethanol consumption in C57BL/6 J mice. Pharmacol Biochem Behav. 2012;102:36–43. doi: 10.1016/j.pbb.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, Sobell MB. Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcohol Clin Exp Res. 1994;18:879–885. doi: 10.1111/j.1530-0277.1994.tb00054.x. [DOI] [PubMed] [Google Scholar]
- Smith BR, Horan JT, Gaskin S, Amit Z. Exposure to nicotine enhances acquisition of ethanol drinking by laboratory rats in a limited access paradigm. Psychopharmacology (Berlin) 1999;142:408–412. doi: 10.1007/s002130050906. [DOI] [PubMed] [Google Scholar]
- Sobell MB, Sobell LC, Kozolowski LT. Dual recoveries from alcohol and smoking problems. In: Fertig JB, Allen JS, editors. Alcohol and tobacco: from basic science to clinical practice. Bethesda: NIAAA; 1995. [Google Scholar]
- Soderpalm B, Ericson M, Olausson P, Blomqvist O, Engel JA. Nicotinic mechanisms involved in the dopamine activating and reinforcing properties of ethanol. Behav Brain Res. 2000;113:85–96. doi: 10.1016/s0166-4328(00)00203-5. [DOI] [PubMed] [Google Scholar]
- Steensland P, Simms JA, Holgate J, Richards JK, Bartlett SE. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, selectively decreases ethanol consumption and seeking. Proc Natl Acad Sci U S A. 2007;104:12518–12523. doi: 10.1073/pnas.0705368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997;20:92–98. doi: 10.1016/s0166-2236(96)10073-4. [DOI] [PubMed] [Google Scholar]
- Zhao-Shea R, Liu L, Soll LG, Improgo MR, Meyers EE, Mclntosh JM, Grady SR, Marks MJ, Gardner PD, Tapper AR. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology. 2011;36:1021–1032. doi: 10.1038/npp.2010.240. [DOI] [PMC free article] [PubMed] [Google Scholar]