Abstract
Objective:
To ascertain whether characteristics of ventricular tachycardia/fibrillation (VT/VF) differed between people with epilepsy and those without and which individuals with epilepsy were at highest risk.
Methods:
We ascertained 18 people with active epilepsy identified in a community-based registry of sudden cardiac arrest (SCA) with ECG-confirmed VT/VF (cases). We compared them with 470 individuals with VT/VF without epilepsy (VT/VF controls) and 54 individuals with epilepsy without VT/VF (epilepsy controls). Data on comorbidity, epilepsy severity, and medication use were collected and entered into (conditional) logistic regression models to identify determinants of VT/VF in epilepsy.
Results:
In most cases, there was an obvious (10/18) or presumed cardiovascular cause (5/18) in view of preexisting heart disease. In 2 of the 3 remaining events, near–sudden unexpected death in epilepsy (SUDEP) was established after successful resuscitation. Cases had a higher prevalence of congenital/inherited heart disease (17% vs 1%, p = 0.002), and experienced VT/VF at younger age (57 vs 64 years, p = 0.023) than VT/VF controls. VT/VF in cases occurred more frequently at/near home (89% vs 58%, p = 0.009), and was less frequently witnessed (72% vs 89%, p = 0.048) than in VT/VF controls. Cases more frequently had clinically relevant heart disease (50% vs 15%, p = 0.005) and intellectual disability (28% vs 1%, p < 0.001) than epilepsy controls.
Conclusion:
Cardiovascular disease rather than epilepsy characteristics is the main determinant of VT/VF in people with epilepsy in the community. SCA and SUDEP are partially overlapping disease entities.
In a recent community-based study, people with epilepsy had a threefold higher risk of sudden cardiac arrest (SCA) from cardiac causes (ECG-documented ventricular tachycardia/fibrillation [VT/VF]) than those without, irrespective of traditional cardiac risk factors.1 In another community-based study, SCA in epilepsy occurred at younger age and was less frequently witnessed than in those without epilepsy, suggesting differing etiologies.2 Only 26% of people with SCA in this study had VT/VF, while most (74%) had bradycardia/asystole, or pulseless electrical activity (PEA).2 Bradycardia/asystole or PEA are the final outcome of every SCA and do not necessarily prove a cardiac cause.3 No control group with epilepsy without VT/VF was analyzed.2 It remains unknown what causes SCA in people with epilepsy and who is at highest risk. SCA in the community is estimated to result from cardiac causes in 60%–80%,4,5 while no underlying cause is identified at postmortem examination in approximately 30%.6 Negative postmortem investigations are the hallmark of sudden unexpected death in epilepsy (SUDEP).7–9 Sporadic video-EEG recordings in SUDEP suggest that a minority of patients have VT/VF following an epileptic seizure.7,8,10 It is, however, difficult to translate these figures to the general community, as these recordings were obtained in a highly selected group of people with severe epilepsy. Our primary aim was to determine whether the characteristics and causes of SCA differed between people with and without epilepsy in the community and to identify the determinants of SCA in epilepsy. As a secondary aim, we analyzed whether patients with SCA with epilepsy fulfilled SUDEP criteria, to assess whether SUDEP contributes to SCA in people with epilepsy in the community.
METHODS
Design and setting.
We conducted 2 case-control studies using 1 case group and 2 control groups of individuals ≥12 years of age who were drawn from 2 community-based databases. Cases were defined as people with epilepsy who had VT/VF. They were compared to people who had VT/VF without epilepsy (VT/VF controls) and to individuals with epilepsy who had not had VT/VF (epilepsy controls). Cases and VT/VF controls were drawn from the Amsterdam Resuscitation Studies (ARREST) registry, while epilepsy controls were drawn from the Out-Patient Population-based Epilepsy Cohort (OPPEC).
Standard protocol approvals, registrations, and patient consents.
ARREST was approved by the Medical Ethics Committee of the Academic Medical Centre, and OPPEC by the Medical Ethics Committee of the University Medical Centre Utrecht. In ARREST, written informed consent was obtained from all survivors of VT/VF and the use of observational data from nonsurvivors was allowed by the medical ethics committee. In OPPEC, all participants provided written informed consent.
ARREST registry.
ARREST is an ongoing, prospective, community-based registry of out-of-hospital SCA designed to establish the survival and the clinical and genetic determinants of VT/VF in a contiguous urban and rural area in the Netherlands with a population of about 2.4 million.1 Details of the study design have been reported elsewhere.1,11–14 In short, complete coverage of the study region and an inclusion rate >95% of all out-of-hospital episodes of VT/VF is obtained through a mandatory multiple-source notification system in which all emergency dispatch centers, ambulance services, first responders, and hospitals in the study region collaborate. Resuscitation parameters are collected according to Utstein criteria,15 including a continuous ECG. If an automated external defibrillator is used, the research center collects the device, so that the ECG recording of each resuscitation attempt can be extracted. Obvious noncardiac causes of VT/VF (for example, trauma, drowning, suicide, intoxication) are excluded.
OPPEC database.
OPPEC is a cross-sectional, community-based study, designed to assess the clinical, demographic, genetic, and pharmacologic determinants of antiepileptic drug (AED) treatment response in an outpatient cohort of people with epilepsy in a (sub)urban region in the center of the Netherlands (het Gooi-Utrecht).16,17 Participants were recruited during an 18-month period (July 2010–December 2011) from the databases of 30 pharmacies, covering a population of about 250,000 inhabitants. Those who had at least 2 prescriptions for any AED dispensed in the previous 2 years (indicating long-term use) were asked to participate: response rate was 30%.16 The hospital and general practitioner (GP) records were reviewed to confirm a diagnosis of epilepsy (see Definition of epilepsy).
Cases.
Of all participants enrolled in ARREST with complete GP and hospital records during the 7-year study period (July 2005–July 2012), we first extracted those with a presumed diagnosis of epilepsy based on the terms epilepsy or epileptic seizure in their GP or hospital records. Only those individuals with a confirmed diagnosis of active epilepsy were subsequently included in the study. To determine whether individuals with a confirmed diagnosis of active epilepsy and VT/VF could be classified as SUDEP, clinical and event characteristics were evaluated by 2 neurologists with a special interest in epilepsy (J.W.S., R.D.T.) and 1 cardiologist (H.L.T.). In case of disagreement, the reviewers discussed to reach consensus. SUDEP was defined as sudden, unexpected, witnessed or unwitnessed, nontraumatic and nondrowning death, occurring in benign circumstances, in an individual with epilepsy, with or without evidence for a seizure and excluding documented status epilepticus (seizure duration ≥30 minutes or seizures without recovery in between), in which postmortem examination did not reveal a cause of death.9 Cases without autopsy results were classified as either probable or possible SUDEP depending on whether a potentially competing cause of death was found after cardiac evaluation. Near-SUDEP was defined as “an individual with epilepsy who survived resuscitation for more than 1 hour after a cardiorespiratory arrest and who had no structural cause identified after investigation.”9
Control groups.
The VT/VF control group consisted of all individuals with VT/VF without epilepsy in ARREST with complete GP and hospital records (survivors and nonsurvivors) in 2007–2008 (yielding a sufficient number of controls). Each case was also matched by age (±5 years) and sex to 3 epilepsy controls from OPPEC.
Definition of epilepsy.
The information of all people with a presumed diagnosis of epilepsy in ARREST and of all participants in OPPEC was reviewed by 2 members of the OPPEC diagnostic confirmation team who are neurologists with a special interest in epilepsy (F.S.L. and G.-J.d.H.).16,17 They independently rated the likelihood of this diagnosis on a visual analogue scale of 0–100. People with an average score of ≥80 were considered to have epilepsy. Only those individuals with active epilepsy were selected. Active epilepsy was defined as current treatment with AEDs or a seizure within the previous 2 years.18
Collection of clinical information.
The following clinical data were obtained from GP, pharmacy, and hospital records in ARREST and OPPEC: epilepsy characteristics (etiology, seizure freedom in the last 2 years, age at onset, duration of epilepsy), cardiovascular risk factors (hypertension, hypercholesterolemia, diabetes mellitus, stroke/TIA), presence/absence of intellectual disability, and clinically relevant heart disease (ischemic, valvular, or congenital/inherited heart disease with or without cardiac arrhythmia). Complete medication histories of the year before VT/VF (ARREST) or inclusion in the study (OPPEC) were obtained from community pharmacies. We defined 3 drug categories: (1) QT prolonging medication (www.azcert.org), including the AEDs phenytoin and felbamate; (2) depolarization-blocking drugs (www.brugadadrugs.org), including the AEDs carbamazepine, oxcarbazepine, phenytoin, and lamotrigine; and (3) cardiovascular drugs (β-adrenoreceptor blockers, calcium channel antagonists, angiotensin-converting enzyme inhibitors, diuretics, angiotensin II receptor blockers, nitrates, platelet aggregation inhibitors, or statins). Individual drugs may belong to more than one category.
Statistical analysis.
Patient and event characteristics were described and compared between cases and VT/VF controls using χ2 statistics (Pearson/Fisher exact where appropriate) for categorical data and the Student t test/Mann-Whitney U test for continuous data. Variables found significant in univariable analysis were included in a logistic regression model to determine whether the characteristics of VT/VF in cases and controls differed. To identify risk factors for VT/VF (cases vs epilepsy controls), univariable and multivariable conditional logistic regression was employed, thereby accounting for matched data. In this model, Firth correction for rare events was used for some seldom occurring variables (intellectual disability). p Values <0.05 were considered to be significant. Statistics were performed in SPSS (cases vs VT/VF controls; version 17.0 for Windows, Chicago, IL) and in R (cases vs epilepsy controls; R statistical package, version 3.10, package clogit and logistf, version 1.10).
RESULTS
Cases.
During the study period, 2,584 people with VT/VF were included, 39 of whom had a presumed diagnosis of epilepsy (figure 1). After exclusions due to inaccurate diagnosis, insufficient information to validate a diagnosis, or inactive epilepsy, 18 cases (mean age 57 years, 67% male) with VT/VF and active epilepsy were included in the final analysis (tables 1 and 2). An underlying cardiovascular cause of VT/VF was found in 10 cases (56%): acute myocardial infarction, hypertrophic cardiomyopathy, and co-incidence of transient cardiac ischemia and drug-induced QTc prolongation. In 5 others (28%), an underlying cardiovascular cause was presumed, as they had recent-onset cardiac symptoms (severe recurrent chest pains <1 week before VT/VF) or clinically relevant preexisting heart disease. These suspicions could not be confirmed as all died before hospital admission and had no postmortem investigation. In the 3 remaining cases, no definite cardiac cause of VT/VF could be established after further investigations: in the first case, VT/VF was attributed to a high fever (42°C) secondary to autopsy-confirmed bilateral pneumonia. Therefore, it did not fulfill SUDEP criteria. The remaining 2 individuals died a few days later in the hospital after initial successful resuscitation, and did not have autopsy: one of these was classified as probable near-SUDEP in view of a normal comprehensive diagnostic workup. In this case, prolonged convulsive movements were reported by witnesses and rectal diazepam was administered. The second case, apparently non-seizure-related, was classified as possible near-SUDEP as slight ECG abnormalities without cardiac enzyme changes were found that were considered insufficient evidence for a diagnosis of myocardial infarction.
Figure 1. Identification of cases.
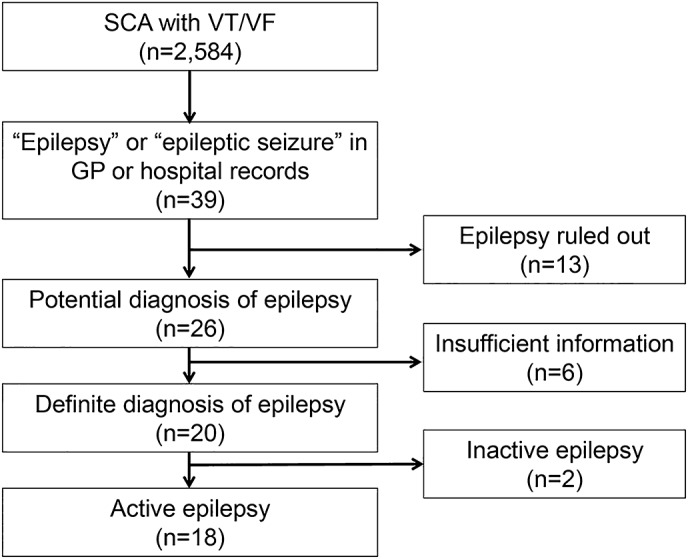
A total of 2,584 individuals ≥12 years of age with sudden cardiac arrest (SCA) due to ventricular tachycardia/fibrillation (VT/VF) and complete general practitioner (GP) or hospital records were enrolled in Amsterdam Resuscitation Studies in the period from July 1, 2005, to July 31, 2012. From this cohort, 18 cases with active epilepsy were selected for this study after exclusions for various reasons. In 13 people, a diagnosis of epilepsy was ruled out: data entry errors (n = 3), alcohol-induced seizures (n = 3), nonepileptic transient loss of consciousness (n = 3), single seizure (n = 3), revision of epilepsy diagnosis (n = 1). In some individuals (n = 6), there was insufficient information to either confirm or rule out a diagnosis of epilepsy. In these cases, either the general practitioner or neurologist could not be located (n = 4), or the diagnosis was established ≥20 years ago and full medical records were no longer available (n = 2).
Table 1.
Characteristics of sudden cardiac arrest due to ventricular tachycardia/fibrillation in cases (n = 18)

Table 2.
Comparison of cases and VT/VF controls

VT/VF characteristics in people with and without epilepsy.
In the 18 cases and 470 VT/VF controls, general and event characteristics were analyzed (table 2). Myocardial infarction was the most common cause of VT/VF in both groups. The prevalence of congenital/inherited heart disease (aortic coarctation, hypertrophic cardiomyopathy, long QT syndrome) was higher in cases than in VT/VF controls (17% vs 1%) and VT/VF in cases generally occurred at a younger age (mean age 57 vs 64 years). In cases, VT/VF was also more likely to occur at/near home (89% vs 58%); accordingly, VT/VF was less likely to be witnessed (72% vs 89%). In multivariable analysis, these 4 variables were independently associated with VT/VF in epilepsy (table 2).
Epilepsy characteristics in people with and without VT/VF.
General and epilepsy characteristics were compared between 18 cases and 54 epilepsy controls (table 3). Cases were more likely to have clinically relevant heart disease (50% vs 15%) and intellectual disability (28% vs 2%) than epilepsy controls. In multivariable analysis, these variables were independently associated with VT/VF in epilepsy (table 3).
Table 3.
Comparison of cases and epilepsy controls

DISCUSSION
We analyzed the characteristics, etiology, and risk factors of VT/VF in epilepsy in the community. Myocardial infarction was the most common cause of VT/VF both in people with and without epilepsy.
Our study confirms and adds to findings from a previous community-based study on SCA in people with or without epilepsy that combined various presenting rhythms2: people with epilepsy were younger and had a higher prevalence of congenital/inherited heart disease. Younger age, occurrence at/near home, and absence of witnesses are also characteristics associated with SUDEP.7,8 This further suggests partial overlap between VT/VF in epilepsy and SUDEP. Taken together, cardiovascular disease events constitute the predominant cause of VT/VF in epilepsy, whereas in a minority VT/VF was unexplained and a diagnosis of (near) SUDEP was established (figure e-1 on the Neurology® Web site at Neurology.org). This may explain why common SUDEP risk factors such as the presence of recent seizures were not seen in our cases.7,8
Cardiovascular disease rather than epilepsy characteristics was the strongest risk factor for VT/VF in epilepsy. Cases were also more likely to have intellectual disability than epilepsy controls. Shared genetics, shared etiology, or shared (cardiovascular) comorbidity are potential explanations for the increased risk of VT/VF in people with epilepsy.1 A single ion channel mutation expressed both in the brain and in the heart may confer intellectual disability, a propensity for epilepsy, and an innate vulnerability to cardiac arrhythmias (especially in the presence of new-onset or preexistent heart disease).19,20
Iatrogenic blockade of sodium ion channels might also contribute to a higher risk of cardiac arrhythmia, as the use of AEDs with these properties was associated with an increased risk of sudden cardiac death in people with and without epilepsy.21 We did not confirm this finding, as our study was neither powered nor designed to assess this. Future studies need to replicate the association between sodium channel blocking AEDs and cardiac arrhythmias. Prudence and careful monitoring of presence of additional sodium channel blocking factors (such as cardiac ischemia or heart failure) is warranted when treating people with epilepsy with these AEDs.
Congenital/inherited heart disease, epilepsy, and intellectual disability may result from a multiple malformation syndrome affecting the heart and brain.22 Epilepsy may also be associated with an increased prevalence of acquired cardiovascular comorbidity.23,24 People with epilepsy were found to have a worse cardiovascular risk profile23–25 and a higher risk of recurrent life-threatening cardiac arrhythmias or cardiovascular death than the general population.26–29 Chronic inflammation (for example, the inflammatory cytokine interleukin-6 [IL-6]) may represent a link between epilepsy and cardiovascular comorbidity by promoting atherosclerosis or arrhythmogenesis.30 Levels of IL-6 appear to be chronically elevated in people with epilepsy,31 those with intellectual disability,32 and to an even further extent in people with both conditions.32 High concentrations of IL-6 were also found to be associated with an increased risk of sudden cardiac death in a prospective community-based study after correction for traditional cardiac risk factors.30
A major strength of our study was that we included only SCA cases with ECG-confirmed VT/VF. This is more rigorous than in previous studies that included all types of cardiac arrest regardless of presenting rhythm, in which no distinction between primary and secondary cardiac arrest could be made.2,3
Our approach allowed us to demonstrate that, in epilepsy, VT/VF and SUDEP may partially overlap and that ventricular arrhythmias are one of the underlying mechanisms of (near) SUDEP not only in ictal recordings of people with severe epilepsy,7,8,10 but also in people with epilepsy in the community. We did not assess the full spectrum of sudden death in epilepsy and only identified a few (near) SUDEP cases. Thus, we are unable to speculate on the overall relevance of VT/VF as a SUDEP pathomechanism in the community. VT/VF in epilepsy may result from a spectrum of triggers including cardiovascular disease, epileptic seizures (SUDEP), or combination of both.
The category SUDEP plus has been promoted in the recent unified SUDEP criteria to avoid neglecting the potential effects of coexisting disease.9 A cardiovascular cause was presumed in 5 of our cases but not proven due to lack of diagnostic evaluation. Our study thus demonstrates that the boundaries between SCA and SUDEP are difficult to delineate in a community-based setting. We therefore only classified those with a full cardiovascular workup as near SUDEP. Cases and epilepsy controls were drawn from 2 different community-based cohorts. Inclusion of epilepsy controls was based on AED prescription records; this may have introduced bias, since this did not apply to cases. Epilepsy controls could therefore potentially have had a higher prevalence of AED use and more severe epilepsy. In practice, however, all cases and epilepsy controls used AEDs and the proportions of people who had recent seizures (in the last 2 years) did not significantly differ.
We found that VT/VF risk in epilepsy is mainly determined by cardiovascular disease. Careful attention to cardiovascular risk factors in people with epilepsy may reduce the rate of sudden death in this population.
Supplementary Material
ACKNOWLEDGMENT
The authors thank the participating emergency dispatch centers, ambulance services, first responders, and hospitals for their cooperation and support; L.E. Bekkers, P.C. Homma, E.M. Landman, and R. Stieglis for ARREST data collection, data entry, and patient follow-up; the following members of the OPPEC study group: J.A. Carpay, A.C. Egberts, D.G. Kasteleijn-Nolst Trenité, B.P. Koeleman, P. van der Linden, D. Lindhout, K.G. Moons, S.G. Uijl, and I. Wilting; M. Tanck for statistical assistance; and G.S. Bell for reviewing the manuscript.
GLOSSARY
- AED
antiepileptic drug
- ARREST
Amsterdam Resuscitation Studies
- GP
general practitioner
- IL-6
interleukin-6
- OPPEC
Out-Patient Population-based Epilepsy Cohort
- PEA
pulseless electrical activity
- SCA
sudden cardiac arrest
- SUDEP
sudden unexpected death in epilepsy
- VT/VF
ventricular tachycardia/fibrillation
Footnotes
Editorial, page 208
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
The study was conceptualized and designed by R.J.L., M.T.B., A.B., J.W.S., R.D.T., and H.L.T. Data were collected by R.J.L., M.T.B., M.W., and A.B. Data analysis and interpretation was by R.J.L., M.T.B., M.W., F.S.L., G.-J.d.H., J.W.S., H.L.T., and R.D.T. The paper was drafted by R.J.L., M.T.B., J.W.S., R.D.T., and H.L.T. All authors approved the submitted version. R.D.T. and H.L.T. are the guarantors.
STUDY FUNDING
Supported by the Dutch National Epilepsy Fund (project number 10-07), Christelijke Vereniging voor de Verpleging van Lijders aan Epilepsie (Nederland), and Netherlands Organization for Scientific Research (NWO, grant ZonMW Vici 918.86.616).
DISCLOSURE
R. Lamberts, M. Blom, M. Wassenaar, A. Bardai, F. Leijten, and G. de Haan report no disclosures relevant to the manuscript. J. Sander receives research support from the Dr. Marvin Weil Epilepsy Research Fund, Eisai, GSK, EU FP7, and WHO, and has been consulted by and received fees for lectures from GSK, Eisai, Lundbeck, Teva, and UCB. R. Thijs receives research support from NUTS Ohra Fund, Medtronic, and AC Thomson Foundation, and has received fees for lectures from Medtronic, UCB Pharma, and GSK. H. Tan reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bardai A, Lamberts RJ, Blom MT, et al. Epilepsy is a risk factor for sudden cardiac arrest in the general population. PLoS One 2012;7:e42749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stecker EC, Reinier K, Uy-Evanado A, et al. Relationship between seizure episode and sudden cardiac arrest in patients with epilepsy: a community-based study. Circ Arrhythm Electrophysiol 2013;6:912–916. [DOI] [PubMed] [Google Scholar]
- 3.Deasy C, Bray JE, Smith K, et al. Out-of-hospital cardiac arrests in young adults in Melbourne, Australia: adding coronial data to a cardiac arrest registry. Resuscitation 2011;82:1302–1306. [DOI] [PubMed] [Google Scholar]
- 4.Pell JP, Sirel JM, Marsden AK, et al. Presentation, management, and outcome of out of hospital cardiopulmonary arrest: comparison by underlying aetiology. Heart 2003;89:839–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adabag AS, Luepker RV, Roger VL, et al. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol 2010;7:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puranik R, Chow CK, Duflou JA, et al. Sudden death in the young. Heart Rhythm 2005;2:1277–1282. [DOI] [PubMed] [Google Scholar]
- 7.Tomson T, Nashef L, Ryvlin P. Sudden unexpected death in epilepsy: current knowledge and future directions. Lancet Neurol 2008;7:1021–1031. [DOI] [PubMed] [Google Scholar]
- 8.Surges R, Thijs RD, Tan HL, et al. Sudden unexpected death in epilepsy: risk factors and potential pathomechanisms. Nat Rev Neurol 2009;5:492–504. [DOI] [PubMed] [Google Scholar]
- 9.Nashef L, So EL, Ryvlin P, et al. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 2012;53:227–233. [DOI] [PubMed] [Google Scholar]
- 10.Espinosa PS, Lee JW, Tedrow UB, et al. Sudden unexpected near death in epilepsy: malignant arrhythmia from a partial seizure. Neurology 2009;72:1702–1703. [DOI] [PubMed] [Google Scholar]
- 11.Bardai A, Berdowski J, van der Werf C, et al. Incidence, causes, and outcomes of out-of-hospital cardiac arrest in children: a comprehensive, prospective, population-based study in the Netherlands. J Am Coll Cardiol 2011;57:1822–1828. [DOI] [PubMed] [Google Scholar]
- 12.Berdowski J, Blom MT, Bardai A, et al. Impact of onsite or dispatched automated external defibrillator use on survival after out-of-hospital cardiac arrest. Circulation 2011;124:2225–2232. [DOI] [PubMed] [Google Scholar]
- 13.Blom MT, Warnier MJ, Bardai A, et al. Reduced in-hospital survival rates of out-of-hospital cardiac arrest victims with obstructive pulmonary disease. Resuscitation 2013;84:569–574. [DOI] [PubMed] [Google Scholar]
- 14.Blom MT, van Hoeijen DA, Bardai A, et al. Genetic, clinical and pharmacological determinants of out-of-hospital cardiac arrest: rationale and outline of the Amsterdam Resuscitation Studies (ARREST) registry. Open Heart 2014;1:e000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cummins RO, Chamberlain DA, Abramson NS, et al. Recommended guidelines for uniform reporting of data from out-of-hospital cardiac arrest: the Utstein Style: a statement for health professionals from a task force of the American Heart Association, the European Resuscitation Council, the Heart and Stroke Foundation of Canada, and the Australian Resuscitation Council. Circulation 1991;84:960–975. [DOI] [PubMed] [Google Scholar]
- 16.Wassenaar M, van Heijl I, Leijten FS, et al. Treatment of epilepsy in daily clinical practice: have outcomes improved over the past 10 years? J Neurol 2013;260:2736–2743. [DOI] [PubMed] [Google Scholar]
- 17.Wassenaar M, Kasteleijn-Nolst Trenité DG, de Haan GJ, et al. Seizure precipitants in a community-based epilepsy cohort. J Neurol 2014;261:717–724. [DOI] [PubMed] [Google Scholar]
- 18.Fisher RS, van Emde Boas W, Blume W, et al. Epileptic seizures and epilepsy: definitions proposed by the International League Against Epilepsy (ILAE) and the International Bureau for Epilepsy (IBE). Epilepsia 2005;46:470–472. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JN, Hofman N, Haglund CM, et al. Identification of a possible pathogenic link between congenital long QT syndrome and epilepsy. Neurology 2009;72:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glasscock E. Genomic biomarkers of SUDEP in brain and heart. Epilepsy Behav 2014;38:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bardai A, Blom MT, van Noord C, et al. Sudden cardiac death is associated both with epilepsy and with use of antiepileptic medications. Heart 2015;101:17–22. [DOI] [PubMed] [Google Scholar]
- 22.Miller G, Vogel H. Structural evidence of injury or malformation in the brains of children with congenital heart disease. Semin Pediatr Neurol 1999;6:20–26. [DOI] [PubMed] [Google Scholar]
- 23.Gaitatzis A, Carroll K, Majeed A, et al. The epidemiology of the comorbidity of epilepsy in the general population. Epilepsia 2004;45:1613–1622. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention (CDC). Comorbidity in adults with epilepsy: United States, 2010. MMWR Morb Mortal Wkly Rep 2013;62:849–853. [PMC free article] [PubMed] [Google Scholar]
- 25.Elliott JO, Moore JL, Lu B. Health status and behavioral risk factors among persons with epilepsy in Ohio based on the 2006 Behavioral Risk Factor Surveillance System. Epilepsy Behav 2008;12:434–444. [DOI] [PubMed] [Google Scholar]
- 26.Janszky I, Hallqvist J, Tomson T, et al. Increased risk and worse prognosis of myocardial infarction in patients with prior hospitalization for epilepsy: the Stockholm Heart Epidemiology Program. Brain 2009;132:2798–2804. [DOI] [PubMed] [Google Scholar]
- 27.Badheka A, Rathod A, Kizilbash MA, et al. Epileptic patients who survived sudden cardiac death have increased risk of recurrent arrhythmias and death. J Cardiovasc Med 2010;11:810–814. [DOI] [PubMed] [Google Scholar]
- 28.Olesen JB, Abildstrøm SZ, Erdal J, et al. Effects of epilepsy and selected antiepileptic drugs on risk of myocardial infarction, stroke, and death in patients with or without previous stroke: a nationwide cohort study. Pharmacoepidemiol Drug Saf 2011;20:964–971. [DOI] [PubMed] [Google Scholar]
- 29.Ding D, Wang W, Wu J, et al. Premature mortality risk in people with convulsive epilepsy: long follow-up of a cohort in rural China. Epilepsia 2013;54:512–517. [DOI] [PubMed] [Google Scholar]
- 30.Hussein AA, Gottdiener JS, Bartz TM, et al. Inflammation and sudden cardiac death in a community-based population of older adults: the Cardiovascular Health Study. Heart Rhythm 2013;10:1425–1432. [DOI] [PubMed] [Google Scholar]
- 31.Nowak M, Bauer S, Haag A, et al. Interictal alterations of cytokines and leukocytes in patients with active epilepsy. Brain Behav Immun 2011;25:423–428. [DOI] [PubMed] [Google Scholar]
- 32.Lehtimäki KA, Liimatainen S, Peltola J, et al. The serum level of interleukin-6 in patients with intellectual disability and refractory epilepsy. Epilepsy Res 2011;95:184–187. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


