Abstract
The ability to name objects or abstract entities is an essential feature of speech and language, being commonly considered a central component of normal neurologic function. For this reason, the bedside testing of naming performance is part of the neurologic examination, especially since naming impairments can signify the early onset of a progressive disease or the occurrence of a more established problem. Modern neuroscience research suggests that naming relies on specific and distributed networks that operate in concert to support various processing stages, spanning from object recognition to spoken words. Likewise, studies evaluating the types of naming impairments in patients with neurologic conditions have contributed to the understanding of acquired forms of naming impairments and the underlying stages during normal language processing. In this article, we review the neurobiological mechanisms supporting naming, with a focus on the clinical application of these concepts. We provide an overview of the stages of cognitive processing that are hypothesized to support naming. For each stage, we explore the evidence revealing its neural basis, drawing parallels to clinical syndromes that commonly disrupt each stage. We review the patterns of naming impairment across various neurologic conditions, including classic language disorders, such as poststroke aphasia or primary progressive aphasia, as well as other diseases where language impairments may be subtle but helpful for the appropriate diagnosis. In this context, we provide a structured and practical guide for the bedside naming assessments rooted in modern neuroscience, aimed at supporting the evaluation and diagnosis of neurologic conditions that affect language.
The ability to name objects or abstract entities is essential in everyday speech production. Retrieving the correct word that denotes a specific something relies on an orchestrated sequence of brain processes, ranging from perception of a stimulus to the physical articulation of the sounds used to speak its name. While intimately related, each stage of this complex process relies on the activation of distinct neural circuits. Accordingly, naming impairments may emerge following disruption of one or more of the stages thought to support naming.
The process of naming most commonly occurs during discourse, when we are constantly required to retrieve abstract concepts to either understand or deliver a message, or by our need to identify an object perceived in the environment. Thus, naming can relate to an object that was seen, smelled, touched, heard, tasted, or any combination of these modalities. Stimulus processing results in the recognition of the stimulus as a familiar entity. From there, a series of processing stages supports the eventual production of an output, i.e., the name, from a given input. Whichever the output modality, i.e., the spoken word, the written form, or gesturing, an ordered motor sequence is executed to deliver a coherent symbol that may be understood by someone else (table).
Table.
Summary of processing stages involved in naming (input: visual, output: spoken word)
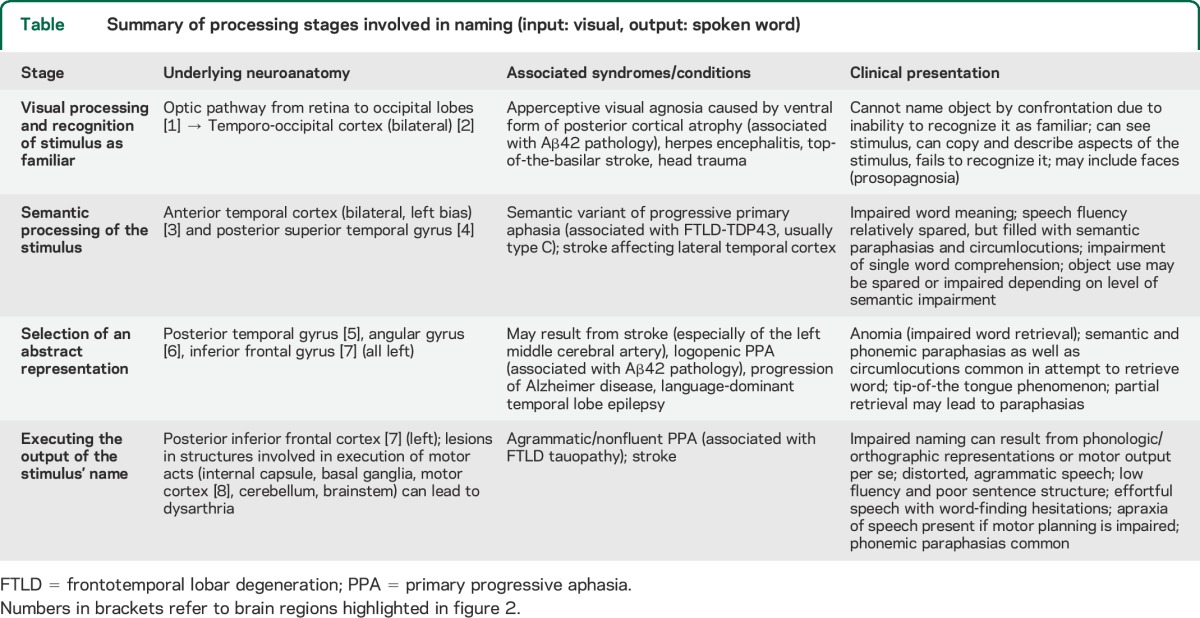
We review the process of naming by providing an ordered and sequential overview of the different stages involved in this cognitive ability, exploring imaging and clinical evidence revealing their neural mechanisms, and placing a strong focus on the clinical syndromes emerging due to impairments at each stage. We refer to the example of an object (a spoon) being perceived by the visual system (input) eventually spoken out loud (output), as summarized by figure 1.
Figure 1. Stages involved in the process of naming.
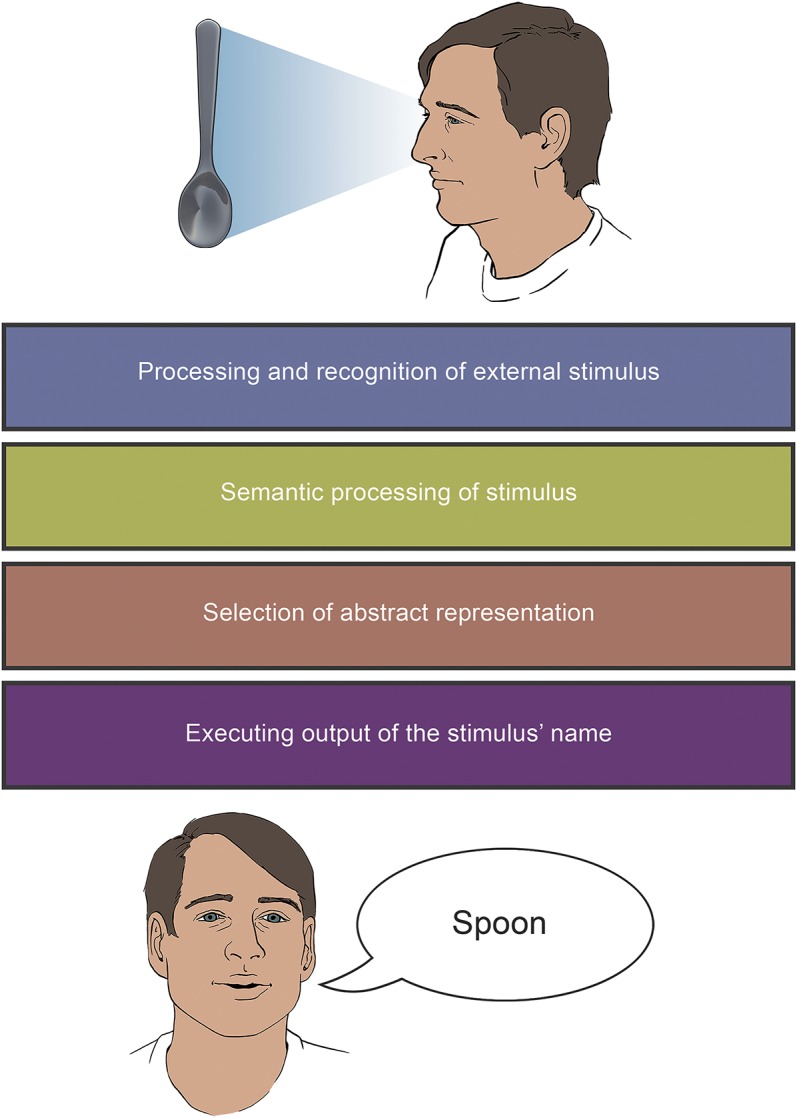
Schematic representation of the process of speaking out (output) the name of an object (in this case, a spoon) perceived by the visual system (input modality).
PROCESSING AND RECOGNITION OF AN EXTERNAL STIMULUS
The visual perception of an object in the environment starts with the absorption of light in the retinal photoreceptors and its transduction into signals that travel to the occipital regions of the braine1 (figure 2, area 1). Cortical processing of the stimulus results in a representation that can be recognized as familiar based on previously stored information. This involves occipito-parietal connections, mostly devoted to object localization and attention, as well as occipito-temporal connections, involved in object recognition.1 This initial process has been extensively studied by cognitive neuroscientists, characterizing numerous substages to identify a familiar object by processing its shape, depth, color, and edges, to match it to similar visual representations from memory.e2 The use of large datasets has also allowed for the development of computational models to understand speech production and naming errors.e3
Figure 2. Main brain areas involved in the process of naming.
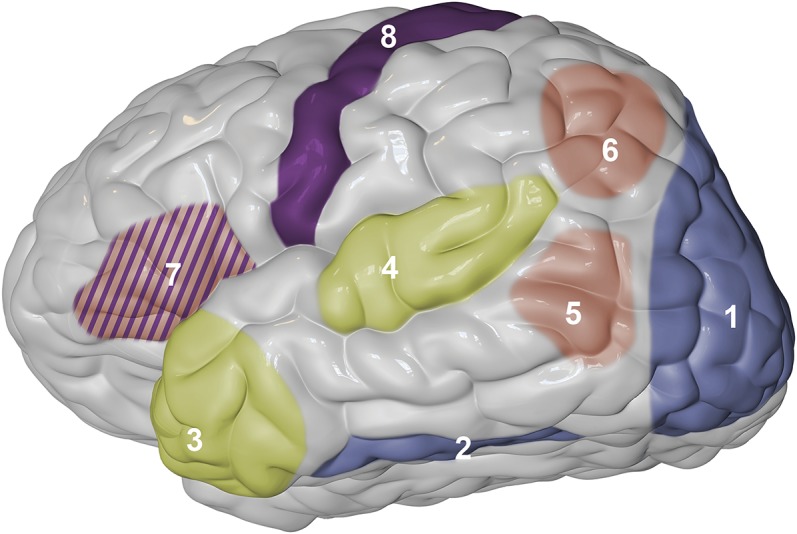
Left lateral view of the brain highlights regions that play an important role in recognizing an external stimulus as familiar (blue), obtaining semantic information about such stimulus (green), accessing its abstract mental representation (orange), and executing its name (purple). Notice that the inferior frontal gyrus (area 7) is crucial for the 2 latter stages, and is thus filled with a striped pattern of both colors.
Studies have shown that the occipitotemporal/fusiform regions (figure 2, area 2) mediate core aspects of object perception and, particularly, their recognition as familiar entities.2,3 Evidence for the involvement of these areas comes from clinical accounts of apperceptive visual agnosia. Patients with this syndrome have an intact visual pathway and may successfully copy or even describe portions of the object; however, they are unable to recognize the object. Thus, naming by confrontation is severely impaired.4
Neurologic conditions that affect the occipitotemporal areas can limit the ability to name objects because the recognition of the stimulus is impaired. Posterior cortical atrophy (PCA) is a neurodegenerative condition presenting with progressive visuoperceptual deficits.5 PCA typically involves dorsal (occipito-parietal) or ventral (occipito-temporal) networks. Even though Alzheimer disease (AD) pathology accounts for a vast majority of PCA cases,6 patients with this condition show brain degeneration in occipital and temporal areas that extend beyond the atrophy typically seen in patients in the earlier stages of AD.7 Injury of occipitotemporal regions, for example due to infectious (e.g., herpes encephalitise4) or vascular (e.g., top-of-the-basilar syndromee5) causes, can also disrupt the ability to recognize a visually perceived object as familiar, especially when occipitotemporal involvement is bilateral.8
SEMANTIC PROCESSING OF THE STIMULUS
Having recognized the stimulus, the next stage involves accessing semantic information to obtain a contextual meaning for the object in question. Some authors have described different levels of semantic information, ranging from more general and universal information about the object (i.e., conceptual knowledge) to the specific features that define each object as unique and different from others (i.e., lexical semantics).8,9 These 2 levels of information processing may sometimes be selectively impaired in different disorders,8 and comprehensive language and neuropsychological testing may aid in identifying what specific component of semantic information is impaired. In everyday neurologic practice, however, their dissociation can prove challenging. Patients with naming impairment due to difficulties in accessing semantic information will often make semantic paraphasias (e.g., calling a spoon a knife). Among the structures relevant in accessing meaning, both anterior temporal lobes (figure 2, area 3), possibly with a more prominent role of the left hemisphere, have been consistently implicated in semantic processing.10,11 Patients with progressive neurodegeneration of these areas exhibit marked impairment in naming and single word comprehension, producing relatively fluent speech, albeit filled with semantic paraphasias and circumlocutions.12 A prime example of this condition is the semantic variant of primary progressive aphasia (PPA-S), which is usually associated with frontotemporal lobar degeneration (FTLD)–TDP pathology.13
Impaired naming and semantic errors are also characteristic of patients with damage to the left posterior temporal cortex, especially in the superior gyrus (figure 2, area 4), most frequently as a result of stroke.8,e6,e7 This observation is in line with recently proposed models of speech processing, which identify 2 distinct streams able to map phonologic and sensory representations onto articulatory motor ones (dorsal stream) and onto lexical conceptual representations (ventral stream). The latter demands a pivotal role of the anterior temporal cortex in semantic computation and a role of the posterior temporal cortex as a gateway to access meaning from sensory representations.14 This ventral stream of speech processing, connecting the temporal lobes with the temporo-parietal junction, allows for the processing of speech signals for comprehension. Here, comprehension can be understood in a broader sense than classic oral speech comprehension (i.e., mapping phonologic representations to meaning); that is, in order to name an object perceived in our environment, one must also comprehend its meaning. PPA-S may also exhibit degeneration of the fusiform gyrus15,e8 and white matter loss in uncinate (temporo-frontal) and inferior longitudinal (temporo-occipital) fasciculi.16,e9
SELECTION OF AN ABSTRACT REPRESENTATION: LEXICAL ACCESS
Once the perceived object is processed semantically, an abstract representation can be selected, without a specific modality (sequence of sounds, letters, or gestures). This is referred to as lexical access, or lemma,17 and its role as a necessary and distinct stage in naming has been contentious,e10–e12 but most models agree that this mental representation of the object acts as a gateway to execute the output. For instance, the well-described tip-of-the-tongue (TOT) phenomenon reflects the role of abstract, amodal representations in retrieving a name: one knows exactly what the object is or does (i.e., semantic processing has been completed) but is unable to name it. TOT phenomenon has been shown to occur in healthy individuals, especially during aging or with the use of infrequent words.18 This modality-independent representation is evidenced in bilingualism (and multilingualism): a person speaking about a spoon in one language will access the same mental representation as when naming a spoon in a different language, even if the actual name (and thus, the sequences of sounds) is radically different. Indeed, the overlap in brain activity when speaking different languages has been consistently shown in the literature.19,20
Given the complexity of these abstract representations, involvement of several different brain regions can lead to anomia, the inability to name an object because of impaired word retrieval (i.e., due to an inaccessible abstract representation). Vascular lesions to posterior portions of the temporal cortex (figure 2, area 5), angular gyrus (figure 2, area 6), and inferior frontal gyrus, which includes Broca area (figure 2, area 7), have all been associated with anomia.8,21–23 Furthermore, likely due to several pathways connecting cortical areas to the diencephalon, stroke lesions affecting the thalamus can also lead to anomia.24
Involvement of these cortical areas by other mechanisms can also result in anomia. For example, atrophy of the temporoparietal region due to neurodegeneration is a classic finding in patients with logopenic PPA (PPA-L).25,26 These patients present with mild loss of fluency (exacerbated by longer utterances), characterized by word-retrieval pauses and anomia.26,27 Their difficulty resides in accessing the mental representations to name objects they know. The pathology underlying this variant of PPA is similar to findings typical of AD,12,13 which can also present with word retrieval difficulties. Likewise, naming impairment in patients with AD is thought to result from degeneration of temporal and parietal circuitry.28–31
Naming difficulties due to impaired lexical access are also well-documented among patients with medial temporal lobe epilepsy (TLE), particularly when affecting the language-dominant hemisphere. Typically described as a material-specific verbal memory problem, it can be as debilitating as seizures.32,e13 Naming impairments in TLE are thought to arise from abnormal networks linking medial temporal with frontal and posterior temporal structures.32 Other neurologic conditions compromising lateral temporal circuitry, either of neoplastice14 or traumatic nature,e15,e16 have also been described to produce anomic aphasia.
Anomia is frequently associated with paraphasias. Being unable to access the abstract representation, and thus, the name of the stimulus in question, a person may try to elicit similarly sounding words, generating phonemic paraphasias (e.g., spook instead of spoon), or semantically related words, thus producing semantic paraphasias.
EXECUTING THE OUTPUT OF THE STIMULUS' NAME
From the mental representation, a specific route can be selected to elicit the name. Importantly, one can name an object via spoken word (phonologic representation), written word (orthographic representation), or by means of gestures (either as an adjunct to discourse, or more thoroughly through established forms of sign language). A patient unable to retrieve a name will be unable to speak, write, or gesticulate it. However, if the retrieval stage is completed, and all prior stages also successfully executed, patients may still show impairments in naming because of a selective inability to plan the execution of the output. Phonologic and orthographic representations must be effectively computed for proper object naming. The impairment at this stage can in fact occur in one modality but not the other, with some patients able to speak the name of an object but not write it out, and vice versa.33,34 This stage is key, because all output modalities require a representation featuring the planned sequenced necessary for execution of motor commands. The inferior frontal gyrus (figure 2, area 7), the motor cortex (figure 2, area 8), and the insula35 are all essential in this stage. They are also fundamental for the subsequent and final stage, which is the actual execution of the motor sequence for sounds, words, or gestures. This final motor execution stage is conceived by modern models of speech processing as part of a comprehensive dorsal pathway that maps mental representations onto articulatory motor representations, necessary for execution of sounds.14,36
Patients with the agrammatic/nonfluent variant of PPA show marked atrophy of the language-dominant inferior frontal gyrus, usually associated with FTLD-tau pathology.26 This leads to impoverished fluency, typically agrammatic and characterized by effortful speech filled with sound errors.12 Patients who have stroke of the language-dominant hemisphere, especially affecting the superior division of the middle cerebral artery, will likely develop Broca aphasia from dysfunction of the inferior frontal gyrus or insula, even though lesions usually extend to Wernicke area as well.37 Their speech is also effortful, nonfluent, and agrammatic.9,38
Following our main example, oral articulation of the word spoon requires a sequence of movements of the oral and upper respiratory apparatus that must be planned and executed such that they will be comprehensible and coherent. This planning stage can itself be impaired, leading to a condition known as apraxia of speech (AoS). AoS can arise in association with stroke or PPA.39 Recent studies have also proposed a primary progressive AoS syndrome, characterized by progressive, pure motor programming deficits, and associated with peak atrophy in superior lateral premotor and supplementary motor areas.40 These patients present with speech sound errors and distortions in articulation, especially in longer words.41
AoS must be distinguished from dysarthria, a condition characterized by motor execution deficits in the context of spared motor planning. Patients with dysarthria present with consistent distortions across words, irrespective of length, and have difficulties with different aspects of movement (strength, amplitude) of oral and laryngeal organs. Because dysarthria results from altered execution, it may arise from lesions in any of the brain regions involved in motor acts, including the motor areas, internal capsule, basal ganglia, brainstem, or cerebellum.42–44
CLINICAL APPROACH TO NAMING IMPAIRMENTS
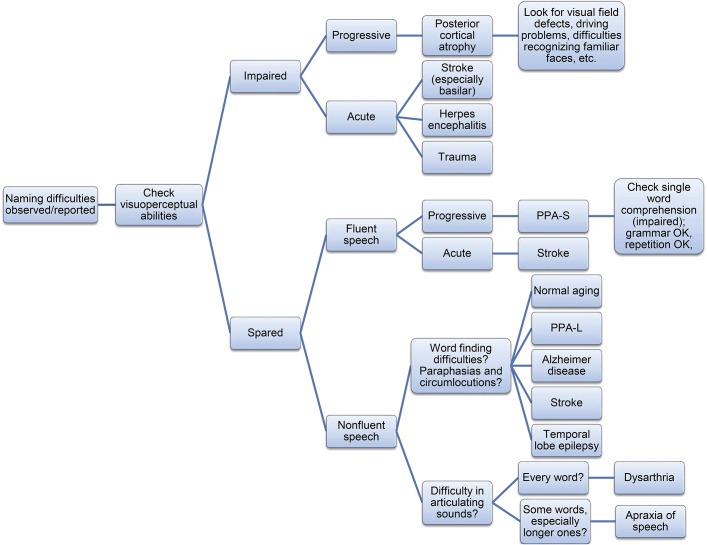
Naming difficulties can be reported by patients themselves, or more frequently, by their closest caregivers. Clinicians may also detect difficulties with naming in spontaneous speech during the patient interview. Cognitive screening tests routinely used in neurology services may hint at a naming impairment. The Mini-Mental State Examination requires the patient to name a pencil and a watch.45 The more comprehensive Addenbrooke's Cognitive Examination III, originally designed as a global screening tool for dementia, tests naming by also asking patients to name 12 pictures.46 The Boston Naming Test, designed specifically to assess naming by picture confrontation, is a tool widely used by clinicians, as it is easy to administer and score.47 Initial information gathered about naming performance from these sources (i.e., subjective reports, clinical impression, performance on screening/naming tests) can help the clinician in narrowing the differential diagnoses before requesting further tests, which may include blood chemistry, neuroimaging, neurophysiology, or specialized neuropsychological tests. Figure 3 summarizes a schematic algorithm for one of many approaches that could guide clinicians in approaching naming impairments.
Figure 3. Clinical approach to naming impairments.
Schematic decision tree in order to explore differential diagnoses for a patient presenting to the clinic with naming impairments. PPA-L = logopenic variant of primary progressive aphasia; PPA-S = semantic variant of primary progressive aphasia.
A summary of this algorithm can be presented as follows. Once naming difficulties are suspected, a first initial helpful step is to investigate visuospatial deficits. Accounts of visual perception defects, difficulties navigating familiar environments, decreased ability to recognize familiar faces, problems with orderly organizing objects in drawers or shelves, and difficulty reading are all hints of potential occipito-temporal involvement. If positive, an insidious onset and progressive worsening may be indicative of PCA. Most frequently, however, these deficits will be associated with the progression of AD, typically accompanied by chief memory complaints and deterioration of activities of daily living. An acute onset, instead, may suggest traumatic, infectious, or vascular mechanisms.
Evaluating speech fluency is a common next step in the examination process. This is not without controversy, however, as experts define fluency in a wide variety of ways and taking into account numerous aspects of speech. In general terms, loss of fluency is a deviation from the expected smooth, continuous, rhythmic, and effortless speech that we all encounter in communication with the neurotypical population. Fluent speech may nonetheless contain irrelevant content. When characterized by numerous semantic paraphasias it can orient differential diagnosis to problems in the semantic processing stage, although not exclusively, as semantic paraphasias may also be elicited as a result of impaired word retrieval. In this latter case, however, speech will most likely be effortful and also feature phonemic paraphasias. Insidious onset and progressive nature points at PPA-S, whereas acute naming impairment is indicative of stroke, likely in the inferior division of the middle cerebral artery, affecting lateral temporal areas. Single word comprehension will likely be impaired.
If speech is not fluent, 2 major explanations must be explored: the difficulty lies in retrieving the word (anomia), or it is related to articulatory problems. If the former, this will be evident from TOT behavior and sometimes the presence of paraphasias—both phonologic (most commonly) and semantic—as well as frequent circumlocutions in an attempt to compensate for the lack of the object's name. Clinicians must conduct a thorough review of systems keeping in mind any signs for progressive worsening (PPA-L, AD) and elements suggesting abnormal brain activity (TLE) and vascular history (stroke). Many patients may present with complaints of increased frequency of TOT phenomena, posing a diagnostic challenge among normal aging, mild cognitive impairment, and the early onset of a neurodegenerative disorder. Conversely, speech dysfluency, especially when characteristically effortful, indicates articulatory problems. Clinicians must evaluate the consistency with which speech sound errors and distortions occur. If they are present in most words, independently of their length, this favors the diagnosis of dysarthria. If, instead, longer, polysyllabic words pose a greater challenge for the patient, AoS should be considered. The latter is especially true for cases in which the same sound is properly executed in certain words but not in others.48 It is important to consider whether these occur in isolation or whether they are accompanied by other language or motor deficits. For AoS, a lack of other language deficits supports the diagnosis of primary progressive AoS; in other cases, for example, AoS may accompany nonfluent PPA.
There are many possibilities to approach naming deficits from the clinical perspective. The example offered in this review merely attempts to highlight the importance of identifying key elements in the patient history and assessment in order to better orient differential diagnosis. We also present this model as a way to review the majority of the concepts introduced by this review. The use of complementary forms of behavioral testing can also help identify the stage at which naming may be impaired. Object comprehension can help test semantic knowledge; writing can help determine whether it is the lemma that is affected, or the orthographic/phonologic representations.
TREATMENT OF NAMING IMPAIRMENTS
Naming impairment is frequently reported as one of the most debilitating consequences of neurologic Disease.e17 Its severity is a strong predictor of poor quality of life in chronic neurologic patients.49,50 One of the most frequent approaches for the treatment of anomia is based on the cueing hierarchy paradigm.51 According to this strategy, patients are initially confronted with colored pictures and asked to name them. An incorrect response is followed by an initial cue aimed at facilitating word retrieval. For example, for a patient unable to name the word spoon, an initial semantic cue would ask to identify the function of the object: “What is this used for?” Failure to name the object would be followed by another cue; for instance, by having the examiner demonstrate the way the object is used. If the patient is still unable to retrieve the appropriate name, the examiner may offer a sentence to be completed with the corresponding word; for example, “John is drinking soup with a ____.” The examiner will offer cues that progressively approximate the actual name of the object, often times providing the patient with the first 1 or 2 phonemes of the name, e.g., “sp____,” and, in the most challenging cases, asking the patient to repeat the whole word (“Say spoon”). Different alternatives to the order of this progressive hierarchy have been implemented in patients with anomia, focusing mainly on the retrieval of either lexical-phonologic levels or, as in the aforementioned example, lexical-semantic levels.52,53
Another approach frequently used to treat naming impairments is semantic feature analysis, which aims to access the target name by demanding patients to generate words and phrases that are related to that target by means of pictures, written labels, or verbal prompts.54,55 Continuing with our example, eliciting words like knife, fork, plate, table, and so forth may help access the word spoon by activating, and thus strengthening, the semantic network surrounding the target. Phonologic feature presents patients with pictures of objects and asks them to identify phonologic features, including first sound, number of syllables, and words with which it rhymes, among other components.56 This strategy may be more efficient across different etiologies given that semantic feature analysis is not beneficial for patients with semantic deficits.57
While speech therapy may be effective in many patients, others continue to have major language impairment despite treatment. Baseline behavioral testing is nonetheless a poor indicator of anomia prognosis.58 Structural neuroimaging before and after speech therapy using cueing hierarchies has determined that naming improvement is hindered when patients exhibit lesions involving areas commonly associated with lexical retrieval and phonologic processing, such as Brodmann areas 37 and 39.59 Brain activation of the perilesional left frontal and left temporal cortices, on the other hand, is associated with an increase in the number of items named correctly after treatment.60 Some patients with apparently intact temporal or frontal cortical structures, however, fail to improve in naming abilities.37 This is most likely related to changes occurring at the level of white matter tracts connecting brain structures related to language. Thus, research studies employing whole-brain connectivity are needed, as they may be especially helpful in identifying prognostic markers of recovery that will help tailor interventions for each patient.
DISCUSSION
From the perception and recognition of a stimulus as familiar, to obtaining semantic information about this stimulus, accessing a mental representation, and finally executing its name, our brain engages in a series of dissociable yet interacting stages in order to name objects. Different neuropathologic mechanisms, both of progressive nature or of acute onset, can disrupt these stages, impairing a patient's naming ability. The characteristics of these naming difficulties can provide important hints for clinicians to understand the underlying reasons for such changes. Therefore, assessing naming ability constitutes a central part of the neurologic examination and understanding the neurobiological bases of naming impairments across different neurologic conditions can be helpful in everyday clinical settings.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Emma Vought for help in designing the figures.
GLOSSARY
- AD
Alzheimer disease
- AoS
apraxia of speech
- FTLD
frontotemporal lobar degeneration
- PCA
posterior cortical atrophy
- PPA-L
logopenic variant of primary progressive aphasia
- PPA-S
semantic variant of primary progressive aphasia
- TLE
temporal lobe epilepsy
- TOT
tip of the tongue
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Ezequiel Gleichgerrcht: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, accepts responsibility for conduct of research and final approval. Julius Fridriksson: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, study supervision, obtaining funding. Leonardo Bonilha: drafting/revising the manuscript, study concept or design, accepts responsibility for conduct of research and final approval, study supervision, obtaining funding.
STUDY FUNDING
Supported by grants from NIH/NIDCD (DC009571 and DC014021).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Van Essen DC, Anderson CH, Felleman DJ. Information processing in the primate visual system: an integrated systems perspective. Science 1992;255:419–423. [DOI] [PubMed] [Google Scholar]
- 2.Minnebusch DA, Suchan B, Koster O, Daum I. A bilateral occipitotemporal network mediates face perception. Behav Brain Res 2009;198:179–185. [DOI] [PubMed] [Google Scholar]
- 3.Tarr MJ, Gauthier I. FFA: a flexible fusiform area for subordinate-level visual processing automatized by expertise. Nat Neurosci 2000;3:764–769. [DOI] [PubMed] [Google Scholar]
- 4.Barton JJ. Higher cortical visual deficits. Continuum 2014;20:922–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beh SC, Muthusamy B, Calabresi P, et al. Hiding in plain sight: a closer look at posterior cortical atrophy. Pract Neurol 2015;15:5–13. [DOI] [PubMed] [Google Scholar]
- 6.Borruat FX. Posterior cortical atrophy: review of the recent literature. Curr Neurol Neurosci Rep 2013;13:406. [DOI] [PubMed] [Google Scholar]
- 7.Alves J, Soares JM, Sampaio A, Goncalves OF. Posterior cortical atrophy and Alzheimer's disease: a meta-analytic review of neuropsychological and brain morphometry studies. Brain Imaging Behav 2013;7:353–361. [DOI] [PubMed] [Google Scholar]
- 8.DeLeon J, Gottesman RF, Kleinman JT, et al. Neural regions essential for distinct cognitive processes underlying picture naming. Brain 2007;130:1408–1422. [DOI] [PubMed] [Google Scholar]
- 9.Hillis AE. Naming and language production. Continuum 2010;16:29–44. [DOI] [PubMed] [Google Scholar]
- 10.Humphries C, Binder JR, Medler DA, Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. J Cogn Neurosci 2006;18:665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsapkini K, Frangakis CE, Hillis AE. The function of the left anterior temporal pole: evidence from acute stroke and infarct volume. Brain 2011;134:3094–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mesulam MM, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol 2014;10:554–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, Bigio EH. Asymmetry and heterogeneity of Alzheimer's and frontotemporal pathology in primary progressive aphasia. Brain 2014;137:1176–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hickok G, Poeppel D. The cortical organization of speech processing. Nat Rev Neurosci 2007;8:393–402. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Pan P, Song W, Shang HF. Quantitative meta-analysis of gray matter abnormalities in semantic dementia. J Alzheimer's Dis 2012;31:827–833. [DOI] [PubMed] [Google Scholar]
- 16.Agosta F, Scola E, Canu E, et al. White matter damage in frontotemporal lobar degeneration spectrum. Cereb Cortex 2012;22:2705–2714. [DOI] [PubMed] [Google Scholar]
- 17.Kempen G, Huijbers P. The lexicalization process in sentence production and naming: indirect election of words. Cognition 1983;14:185–209. [Google Scholar]
- 18.Brown AS. A review of the tip-of-the-tongue experience. Psychol Bulletin 1991;109:204–223. [DOI] [PubMed] [Google Scholar]
- 19.Correia J, Formisano E, Valente G, Hausfeld L, Jansma B, Bonte M. Brain-based translation: fMRI decoding of spoken words in bilinguals reveals language-independent semantic representations in anterior temporal lobe. J Neurosci 2014;34:332–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kovelman I, Baker SA, Petitto LA. Bilingual and monolingual brains compared: a functional magnetic resonance imaging investigation of syntactic processing and a possible “neural signature” of bilingualism. J Cogn Neurosci 2008;20:153–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Antonucci SM, Beeson PM, Labiner DM, Rapcsak SZ. Lexical retrieval and semantic knowledge in patients with left inferior temporal lobe lesions. Aphasiology 2008;22:281–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sebastian R, Gomez Y, Leigh R, Davis C, Newhart M, Hillis AE. The roles of occipitotemporal cortex in reading, spelling, and naming. Cogn Neuropsychol 2014;31:511–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tate MC, Herbet G, Moritz-Gasser S, Tate JE, Duffau H. Probabilistic map of critical functional regions of the human cerebral cortex: Broca's area revisited. Brain 2014;137:2773–2782. [DOI] [PubMed] [Google Scholar]
- 24.Hebb AO, Ojemann GA. The thalamus and language revisited. Brain Lang 2013;126:99–108. [DOI] [PubMed] [Google Scholar]
- 25.Gorno-Tempini ML, Dronkers NF, Rankin KP, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurology 2004;55:335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76:1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henry ML, Gorno-Tempini ML. The logopenic variant of primary progressive aphasia. Curr Opin Neurol 2010;23:633–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huff FJ, Corkin S, Growdon JH. Semantic impairment and anomia in Alzheimer's disease. Brain Lang 1986;28:235–249. [DOI] [PubMed] [Google Scholar]
- 29.Nelissen N, Vandenbulcke M, Fannes K, et al. Abeta amyloid deposition in the language system and how the brain responds. Brain 2007;130:2055–2069. [DOI] [PubMed] [Google Scholar]
- 30.Pekkala S, Wiener D, Himali JJ, et al. Lexical retrieval in discourse: an early indicator of Alzheimer's dementia. Clin Linguist Phon 2013;27:905–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moreaud O, David D, Charnallet A, Pellat J. Are semantic errors actually semantic? Evidence from Alzheimer's disease. Brain Lang 2001;77:176–186. [DOI] [PubMed] [Google Scholar]
- 32.McDonald CR, Ahmadi ME, Hagler DJ, et al. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology 2008;71:1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillis AE, Rapp BC, Caramazza A. When a rose is a rose in speech but a tulip in writing. Cortex 1999;35:337–356. [DOI] [PubMed] [Google Scholar]
- 34.Piras F, Marangolo P. Independent access to phonological and orthographic lexical representations: a replication study. Neurocase 2004;10:300–307. [DOI] [PubMed] [Google Scholar]
- 35.Ibanez A, Gleichgerrcht E, Manes F. Clinical effects of insular damage in humans. Brain Struct Funct 2010;214:397–410. [DOI] [PubMed] [Google Scholar]
- 36.Hickok G. Computational neuroanatomy of speech production. Nat Rev Neurosci 2012;13:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fridriksson J, Bonilha L, Rorden C. Severe Broca's aphasia without Broca's area damage. Behav Neurol 2007;18:237–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Freitas GR. Aphasia and other language disorders. Front Neurol Neurosci 2012;30:41–45. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler W, Aichert I, Staiger A. Apraxia of speech: concepts and controversies. J Speech Lang Hear Res 2012;55:S1485–S1501. [DOI] [PubMed] [Google Scholar]
- 40.Josephs KA, Duffy JR, Strand EA, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain 2012;135:1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ogar J, Slama H, Dronkers N, Amici S, Gorno-Tempini ML. Apraxia of speech: an overview. Neurocase 2005;11:427–432. [DOI] [PubMed] [Google Scholar]
- 42.Enderby P. Disorders of communication: dysarthria. Handb Clin Neurol 2013;110:273–281. [DOI] [PubMed] [Google Scholar]
- 43.Javalkar V, Khan M, Davis DE. Clinical manifestations of cerebellar disease. Neurol Clin 2014;32:871–879. [DOI] [PubMed] [Google Scholar]
- 44.Zenon A, Olivier E. Contribution of the basal ganglia to spoken language: is speech production like the other motor skills? Behav Brain Sci 2014;37:576. [DOI] [PubMed] [Google Scholar]
- 45.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh S, Schubert S, Hoon C, Mioshi E, Hodges JR. Validation of the Addenbrooke's Cognitive Examination III in frontotemporal dementia and Alzheimer's disease. Dement Geriatr Cogn Disord 2013;36:242–250. [DOI] [PubMed] [Google Scholar]
- 47.Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- 48.Forrest K. Diagnostic criteria of developmental apraxia of speech used by clinical speech-language pathologists. Am J Speech Lang Pathol 2003;12:376–380. [DOI] [PubMed] [Google Scholar]
- 49.Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? A systematic review. Arch Phys Med Rehabil 2012;93:S86–S95. [DOI] [PubMed] [Google Scholar]
- 50.Code C, Hemsley G, Herrmann M. The emotional impact of aphasia. Semin Speech Lang 1999;20:19–31. [DOI] [PubMed] [Google Scholar]
- 51.Linebaugh CW, Lehner LH. Cueing hierarchies and word retrieval: a therapy program. Aphasiology 1977;7:19–31. [Google Scholar]
- 52.Wambaugh J. A comparison of the relative effects of phonologic and semantic cueing treatments. Aphasiology 2003;17:433–441. [Google Scholar]
- 53.Wambaugh J, Linebaugh CW, Doyle PJ, Martinez AL, Kalinyak-Fliszar M, Spencer KA. Effects of two cueing treatments on lexical retrieval in aphasic speakers with different levels of deficit. Aphasiology 2001;15:933–950. [Google Scholar]
- 54.Boyle M, Coelho CA. Application of semantic feature analysis as a treatment for aphasic dysnomia. Am J Speech Lang Pathol 1995;4:94–98. [Google Scholar]
- 55.Coelho CA, McHugh RE, Boyle M. Semantic feature analysis as a treatment for aphasic dysnomia: a replication. Aphasiology 2000;14:133–142. [Google Scholar]
- 56.Leonard C, Rochon E, Laird L. Treating naming impairments in aphasia: findings from a phonological components analysis treatment. Aphasiology 2008;22:923–947. [Google Scholar]
- 57.van Hees S, Angwin A, McMahon K, Copland D. A comparison of semantic feature analysis and phonological components analysis for the treatment of naming impairments in aphasia. Neuropsychol Rehabil 2013;23:102–132. [DOI] [PubMed] [Google Scholar]
- 58.Hillis AE. Treatment of naming disorders: new issues regarding old therapies. J Int Neuropsychol Soc 1998;4:648–660. [DOI] [PubMed] [Google Scholar]
- 59.Fridriksson J. Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci 2010;30:11558–11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. Neuroimage 2012;60:854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.