Abstract
Background
Regulatory T cells (Treg) are potentially a useful therapeutic option for the treatment of immunopathological conditions including graft-versus-host disease. Umbilical cord blood (UCB) offers certain advantages over adult peripheral blood (APB) as a source of Treg for cellular therapy but yields far fewer Treg per unit. Pooling of Treg from multiple donors may overcome this challenge.
Methods
In this study, we assessed the in vitro and in vivo efficacy of multiple donor pooled UCB or APB-derived Treg.
Results
In vitro, pooled freshly isolated UCB-derived Treg were as suppressive as APB-derived Treg. However, in a mouse model of human skin allodestruction, pooled UCB-derived Treg were more potent at suppressing alloresponses and prolonging skin survival compared with pooled APB-derived Treg. Improved survival of UCB Treg in an in vivo cell survival assay and their lower expression of human leukocyte antigen-ABC suggested that lower immunogenicity may account for their superior efficacy in vivo.
Conclusion
Multiple-unit UCB is therefore a viable source of human Treg for cellular therapy, and pooling of Treg from multiple donors offers a useful strategy for achieving required therapeutic doses.
Keywords: Regulatory T cells, Treg, Graft-versus-host disease, Skin, Transplantation, Umbilical cord blood, Multiple unit cord blood transplantation
Regulatory T cells (Treg) are capable of suppressing the proliferation and function of various effector cell populations that contribute to innate and adaptive immune responses (1, 2). The application of Treg as a cellular therapy to treat autoimmune diseases, transplant rejection, and graft-versus-host disease (GVHD) has great potential (3, 4). Adoptive cellular therapy would permit infusion of Treg at a known dose and an appropriate time point, after assessment for purity and functional activity (5). Such a cellular therapy may integrate with existing biological control mechanisms in vivo and therefore be associated with fewer side effects than current immunosuppressive therapies (6). The most appropriate source of Treg for different clinical applications is debated, and both adult peripheral blood (APB) and umbilical cord blood (UCB) have been suggested as sources for Treg for clinical application. There are significant differences in the phenotype of T cells derived from APB compared with UCB (7–10). In UCB, there is a clear delineation of CD25hi Treg from predominantly nonactivated CD25− effector T cells (Teffs), allowing the isolation of Treg with a high purity purely on the basis of CD25hi expression (11, 12). In contrast, APB contains a large proportion of activated CD25+ Teffs, therefore requiring depletion of CD127+ cells (13). More importantly, Treg derived from UCB exhibit a predominantly naïve phenotype, with only a small memory population (10, 14). This naïve phenotype is associated with a significantly enhanced proliferation potential (15). Proliferating naïve Treg developing under the influence of a specific immunological environment may develop into memory Treg with the appropriate tissue-specific homing and functional molecules (16). The activation status of Treg may have a significant impact on their suppressive capacity. Freshly isolated UCB CD4+CD25+ cells have been reported to be incapable of suppressing the proliferation or activation of autologous responder cells in vitro in some systems (8, 17–19), while in vitro-expanded UCB Treg demonstrate suppressive functions in vitro (19–21) and in GVHD models in vivo (21, 22). Nevertheless, in other systems where polyclonally stimulated adult responders are used, or where responders are stimulated with adult-derived antigen-presenting cells (APC), freshly isolated UCB Treg have demonstrated effective in vitro suppression (11, 23–25). The performance of UCB Treg in clinical studies reported to date is promising. Early data from a phase I dose-escalation trial for the infusion of UCB-derived Treg in patients receiving double UCB transplants for the treatment of hematological malignancies demonstrated a reduced incidence of grade II to IV acute GVHD in patients treated with Treg (26).
While the frequency of CD25+ cells among the CD4+ fraction is significantly higher in UCB than in APB (7, 10, 27), the absolute number of Treg that can be obtained from a single unit of UCB is substantially lower than the average yield from a standard APB unit. UCB Treg are therefore expanded ex vivo in order to achieve the large numbers required for in vivo use (26). Importantly, however, expanded UCB Treg do not maintain their largely naïve phenotype and therefore may behave less efficiently in vivo where differentiation into a specific Treg subtype is beneficial. We therefore investigated an alternative approach whereby multiple allogeneic units of UCB are pooled in order to achieve therapeutic Treg doses. We show that although the in vitro suppressive ability of multiple-unit pooled human UCB-derived Treg was at least as effective as APB-derived Treg, the in vivo efficacy was significantly better on a per-cell basis. Differential immunogenicity, and subsequent survival of Treg after adoptive transfer, contributes to this difference in functional potency of UCB versus APB-derived Treg in vivo.
RESULTS
Purity and Phenotype of UCB-Derived Treg
We assessed the purity and phenotype of UCB Treg obtained using a single-step magnetic bead isolation protocol. As a benchmark, we compared the isolated UCB Treg population with Treg isolated from APB using a two-step CD4+CD25+CD127lo magnetic bead protocol that we have found to yield high Treg purity (28). Flow cytometric analysis revealed that UCB-derived cells isolated by CD25+ selection contained a proportion of CD4+CD25+CD127lo cells similar to that obtained from APB by CD4+CD25+CD127lo selection (see Figure S1, SDC, http://links.lww.com/TP/A743). However, the proportion of FOXP3+CD127lo cells was higher in Treg derived from APB compared with UCB (Fig. 1A). Meanwhile, FOXP3 was expressed with a lower median intensity in UCB CD4+CD25+ isolates compared with APB CD4+CD25+ isolates (Fig. 1B). Thus, FOXP3 was expressed at a lower density, on a per-cell basis, and at lower frequency among freshly isolated CD4+ UCB-derived Treg versus CD4+CD25+ CD127lo APB-derived Treg. Consistent with previous reports, demonstrating a predominantly naïve phenotype among UCB-derived Treg (7–9, 29), we found the expression of CD45RA significantly higher in UCB-derived Treg compared with APB-derived Treg (Fig. 1C). Next, we compared the ability of resting and stimulated UCB-derived and APB-derived Treg to express molecules described to confer Treg suppressive function. First, we assessed the expression of ectonucleotidase CD39, which has been described to mediate Treg suppression by hydrolysis of extracellular ATP and has been attributed to a memory Treg subset (30). Approximately 50% of APB-derived Treg cells expressed ectonucleotidase CD39, whereas only 15% of unstimulated and 20% to 30% of activated UCB-derived Treg expressed CD39 (Fig. 1D). We analyzed the ability of Treg to express perforin, which has been linked with the ability of human Treg to induce death of autologous target cells (31). Interestingly, although resting Treg did not express perforin, αCD3 and αCD28 stimulation resulted in the induction of perforin expression in Treg, with on average 60% of UCB-derived Treg expressing perforin compared to 30% of APB-derived Treg (Fig. 1E). Finally, we measured Treg production of the anti-inflammatory cytokines IL-10 and TGFβ, which have been demonstrated to convey suppressive function in both mouse and human Treg (32, 33). TGFβ levels were assessed by measurement of latency-associated peptide (LAP), which forms noncovalent bonds with TGFβ and therefore allows the measurement of total TGFβ present in the sample without acidification. The levels of LAP measured in both unstimulated and stimulated samples from either Treg population were below test detection levels (data not shown). Interestingly, stimulated APB-derived Treg produced significantly more IL-10 than stimulated UCB-derived cells (Fig. 1F). Taken together, our results suggest that UCB-derived and APB-derived human Treg vary in the degree and range of utilization of molecular mechanisms of regulation and therefore may differ in efficacy of suppression.
FIGURE 1.
Phenotype of UCB-derived and APB-derived CD25+ Treg. Treg freshly isolated from UCB or APB were immunostained for CD4, CD25, CD127, FOXP3, and CD45RA (A–C). The percentage of cells or mean fluorescence intensity (MFI) values are plotted for each donor, with means indicated by horizontal lines. Percentage of CD4+ cells within each isolate expressing FOXP3+CD127lo plotted for 10 UCB donors and nine adult donors (A). MFI associated with FOXP3 expression among freshly isolated CD4+ T cells plotted for 10 UCB donors and nine adult donors (B). Percentage of freshly isolated Treg expressing CD45RA plotted for seven UCB donors and eight adult donors, gated on CD25+CD127lo cells (C). Expression of CD39 (D) and perforin (E) by αCD3 and αCD28 bead stimulated or unstimulated UCB-derived or APB-derived Treg cultured for 48 hr in the presence of IL-2. IL-10 levels in supernatants from 48-hr cultures of αCD3 and αCD28 bead stimulated or unstimulated UCB-derived or APB-derived Treg (F). Four different donors with up to four repeats were tested in each group (D–F). *P<0.05.
UCB-Derived Treg and APB-Derived Treg Display Similar In Vitro Suppressive Activity But Differential In Vivo Effects
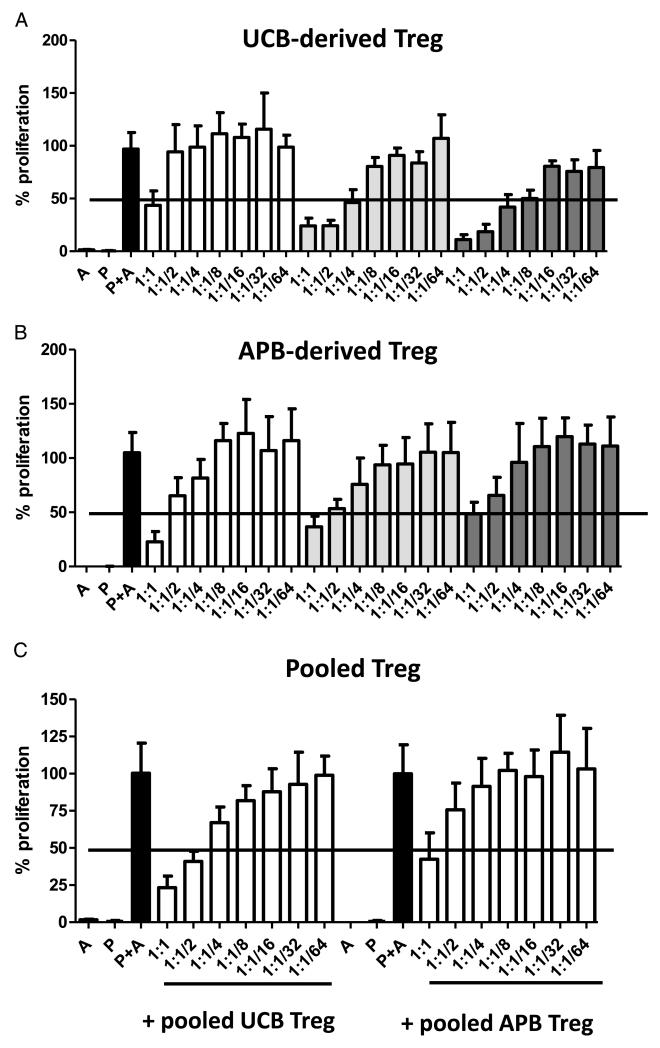
We first compared the ability of single-unit UCB and APB-derived Treg to suppress the proliferation of allogeneic responder peripheral blood monocyte cells (PBMCs) in vitro and found the suppression mediated by both Treg populations to be equivalent (Fig. 2A and B). As discussed earlier, the pooling of multiple units of UCB-derived or APB-derived Treg is a potentially useful method for obtaining greater numbers of therapeutic Treg without altering the phenotype of cells by ex vivo stimulation and expansion. In order to confirm that the functionality of pooled Treg is not compromised, we compared the in vitro suppressive efficacy of triple-unit UCB-derived and APB-derived Treg. Interestingly, both types of pooled Treg were equivalent in their suppressive capabilities, with suppression levels of allogeneic PBMCs similar to that achieved with single-unit Treg preparations (Fig. 2C). Hence, pooling of UCB units constitutes a viable approach to source sufficient Treg while maintaining the benefits of using UCB-derived Treg directly after isolation.
FIGURE 2.
Suppressive capacity of UCB-derived versus APB-derived Treg. Treg freshly isolated from UCB were cultured with 5×104 irradiated allogeneic adult PBMCs (P) at various Treg-to-responder ratios, in the presence of allogeneic stimulators (A). Responder proliferation was measured by a 3H-thymidine incorporation assay. Percentage proliferation is taken as the number of responders undergoing proliferation in the presence of Treg as a percentage of the number of stimulated responders proliferating in the absence of Treg. Mean values±standard deviation (SD) from six replicates at each Treg-to-responder ratio are plotted for three UCB donors. Treg freshly isolated from APB were cultured in conditions identical to those used for UCB-derived Treg (B). Percentage proliferation of responders, relative to stimulated responders without Treg, is plotted as the mean of six replicates at each Treg-to-responder ratio, from one representative experiment including three adult donors. Data are represented as mean values±SD. Treg from the same three UCB or APB samples as those depicted in (A) and (B), respectively, were pooled and subjected to the in vitro suppression assay under identical conditions as Treg of single-donor origin (C). Data are represented as mean±SD for six replicates at each Treg-to-responder ratio.
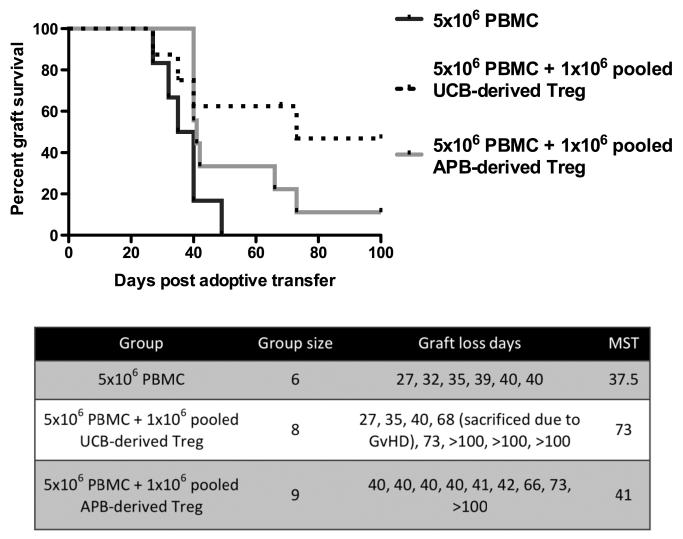
The ability of populations of Treg to suppress effectively in vitro does not necessarily confirm their in vivo efficacy. We therefore assessed the ability of Treg pooled from multiple APB or UCB donors to prevent the rejection of a human skin allograft in a PBMC-humanized mouse model (34). Untreated mice and pooled APB Treg-treated mice displayed similar human skin allograft rejection kinetics, with median survival times (MST) of 37.5 and 41 days, respectively (Fig. 3). In contrast, mice treated with pooled UCB-derived Treg displayed enhanced engraftment of the human skin transplant, achieving a MST of 73 days compared with 41 days using APB-derived Treg, indicating that freshly isolated UCB-derived Treg confer an advantageous effect in vivo compared to their APB-derived counterparts (P=0.0323 between UCB-derived Treg and PBMC alone) (Fig. 3). Importantly, both UCB-derived and APB-derived Treg were randomly selected and therefore displayed a high degree of human leukocyte antigen (HLA) mismatch with the responder PBMCs (mean match of 1.7 out of 6 HLA loci for UCB-derived Treg and mean match of 1 out of 6 HLA loci for APB-derived Treg). Pooled Treg were also highly mismatched between themselves (mean match of 1.3 out of 6 HLA loci within the UCB group and mean match of 2.3 out of 6 HLA loci between APB Treg, data from one representative experiment), providing evidence that even highly mismatched UCB-derived Treg are able to effectively control allograft rejection.
FIGURE 3.
Prolongation of human skin allograft survival by adoptively transferred UCB-derived and APB-derived Treg in a humanized mouse model. BALB/c Rag2−/−cγ−/− mice were transplanted with human skin and 35 days later received 5×106 PBMCs alone or together with 1×106 freshly isolated UCB-derived or APB-derived Treg, pooled from multiple donors. Percentage of grafts surviving (i.e., exhibiting no macroscopic indicators of rejection) is plotted over time. Treatment with UCB-derived Treg extended human skin allograft survival time significantly in comparison to PBMC alone (P=0.0323), achieving a MST of 73 days (two independent in vivo assays, Treg pooled from four to six donors).
UCB-Derived Treg Survive for Longer Than APB-Derived Treg After Adoptive Transfer In Vivo
The presence and persistence of therapeutic Treg in vivo is an important predictor of allograft survival (35). The differential in vivo effects of freshly isolated APB and UCB may therefore be related to differences in their survival after adoptive transfer. Importantly, in an HLA mismatched model such as that used in our study, some Treg cells are likely to be killed by allogeneic PBMC. The extent and susceptibility of different Treg populations to be effectively recognized and killed by allogeneic leukocytes has not previously been studied. To address this, we examined the persistence of UCB-derived and APB-derived Treg in vivo. Carboxyfluorescein succinimidyl ester (CFSE)-labeled Treg, pooled from multiple UCB or APB donors, were co-transferred with allogeneic adult PBMCs into BALB/c Rag2−/−cγ−/− mice by intraperitoneal injection. Seven days after adoptive transfer, CFSE-positive human cells were enumerated from peritoneal lavages. Interestingly, the number of surviving UCB-derived Treg was significantly greater than the number of surviving APB-derived Treg (Fig. 4A). In order to determine whether this was caused by reduced killing of UCB Treg in vivo, we examined the expression of HLA molecules on UCB-derived and APB-derived Treg. While the expression of HLA-DR was similar between UCB-derived and APB-derived Treg (Fig. 4B), the expression of HLA-ABC was significantly lower on UCB-derived Treg compared with APB-derived Treg (Fig. 4C) indicating that UCB-derived Treg have a reduced susceptibility to CD8+ T-cell-mediated killing.
FIGURE 4.
Survival of APB-derived and UCB-derived Treg in vivo after adoptive transfer. 5×106 PBMCs were injected intraperitoneally into BALB/c Rag2−/−cγ−/− mice alone (n=5 mice) or with 1×106 freshly isolated, CFSE-stained Treg pooled from either five adult donors (n=3 mice) or five UCB donors (n=5 mice) (A). Cells were extracted by peritoneal lavage on day 7 after adoptive transfer and Treg (identified as CFSE+ cells) were enumerated. Mean cell numbers are plotted±SD. Freshly isolated Treg from UCB and APB were immunostained for HLA-DR (B) and HLA-ABC (C), and the MFI among CD4+ cells from each source was determined. Data are represented as mean values from three UCB and three APB Treg donors±SD.
DISCUSSION
There is great interest in cellular therapies such as Treg that may engage inherent immune control mechanisms to prevent the development of specific immune responses. While it has long been recognized that Treg may be present in their thymically derived or inducible types, it is now increasingly clear that Treg heterogeneity may mirror that of effector T cells, with naïve-like and memory-like subtypes (15, 16). The expression of markers of cellular immaturity such as CD45RA on Treg has been associated with improved in vivo proliferation and suppression, with UCB-derived Treg showing a predominance of a naïve Treg phenotype. However, the isolation and expansion of naïve Treg to obtain adequate numbers for therapy alters the naïve phenotype and may therefore prove counterproductive. Transfection of naïve CD4+ cells with FOXP3 or the pooling of Treg from multiple donors are therefore the only two methods of generating nonexpanded Treg. Pooling of Treg is a potentially rapid method for producing Treg that may be used as an “off-the-shelf” therapy. The current study is the first investigating the feasibility and efficacy of pooled Treg for in vivo use. Our results indicate that, in spite of containing a lower proportion of FOXP3+CD127lo cells after isolation, freshly isolated UCB-derived Treg are at least as suppressive as Treg freshly isolated from APB. Importantly, pooling from multiple donors neither enhanced nor compromised the suppressive capabilities of Treg compared with using Treg from a single allogeneic donor. Contrary to previous reports that UCB CD4+CD25+ cells are incapable of suppressing responder cell proliferation or activation in vitro (8, 17, 18), we observed comparable inhibition of responder cell proliferation in vitro by freshly isolated UCB-derived and APB-derived Treg. More importantly, UCB-derived Treg controlled human skin allograft rejection in vivo more effectively than APB-derived Treg.
Pooling of Treg necessarily implies that the cells will be nonautologous to the recipient. These Treg are therefore potential targets of an allogeneic killing response. The superior performance of UCB-derived over APB-derived Treg in our in vivo transplantation model may be attributable to the greater persistence of these cells in the early phase after adoptive transfer, as indicated by the in vivo cell survival assay. This explanation is consistent with the observation that suppression of xenogeneic GVHD in a mouse model correlates with the persistence of Treg in the peripheral blood (22). Differential immunogenicity between APB-derived and UCB-derived Treg populations, suggested by the higher levels of HLA class I molecules expressed by APB-derived Treg, compared with UCB-derived Treg, provides a mechanistic explanation for the observed disparity in in vivo cell survival. However, it is also possible that inherent functional differences in suppressive mechanisms utilized by these Treg populations are present, although in vitro a difference in the suppressive capability between the two populations was not observed.
Cellular composition might be a contributing factor determining the overall potency of UCB-derived versus APB-derived Treg. Treg in UCB are enriched for subpopulations exhibiting a resting naïve CD25+CD45RA+FoxP3lo phenotype, as opposed to an activated CD45RA−FoxP3hi phenotype (7–9, 29). Resting naïve Treg are quiescent, but proliferate strongly upon TCR stimulation, a property associated with maturation into an activated Treg phenotype and acquisition of robust suppressive function (15). Interestingly, CD45RA+ Treg are more resistant to apoptosis than CD45RA− Treg (15), which may also contribute to the enhanced survival of UCB Treg in vivo. In vitro, CD45RA+ cells have been found to be more suppressive than CD45RA− Treg (36), suggesting an inherent functional superiority of naïve versus activated Treg. Whole UCB transfusions induce GVHD less frequently, and less severely, than adult BM transplants in the treatment of hematological malignancies (37, 38). The naivety of the effector T-cell population in UCB has been offered as an explanation for this mild disease severity (14, 38, 39). However, it is also possible that the functionality of the Treg compartment within UCB is more pronounced than in adults. Additionally, the specific fate (governing attributes such as homing properties, repertoire of effector molecules, and principal cellular targets) acquired by naïve Treg during their functional maturation is determined by the context in which these cells encounter antigen in the periphery. These fate determinants reflect current immunological status, allowing the repertoire of Treg effector functions to be adapted according to the prevailing demands upon Treg function (40). Naïve Treg maturing in the presence of a graft may therefore be directed towards a fate conducive to graft protection. Conversely, the virtue of adaptability among naïve Treg might be accompanied by less favorable consequences of phenotypic plasticity leading to pro-inflammatory activity (41), although previous studies suggest that CD45RA+ Treg exhibit a more stable phenotype and function than CD45RA− Treg (36).
UCB offers practical benefits over APB as a source of human Treg for cellular therapy. One of the major impediments to the development of Treg as a cellular therapy is the paucity of reliable cell surface markers for sorting human Treg. In mice, CD25 is used as a surrogate surface marker for the expression of foxp3. However, in humans, CD4+CD25+ activated effector T cells constitute a substantial population in the peripheral blood and, hence, a substantial contaminant in CD4+CD25hi isolates. As such, adult human Treg can be isolated with consistently high purity only by depletion of CD127+ cells, in addition to CD25+ enrichment (13, 24, 42). In contrast, because activated T effector cells are relatively scarce in UCB (43, 44), effector populations are predominantly CD25− and therefore delineated clearly from CD25hi Treg populations. Thus, using a protocol with relatively low labor and economic costs, utilizing good manufacturing practice-compatible reagents, Treg may be isolated from UCB purely upon the criterion of CD25hi expression by magnetic bead isolation (11, 12). Rather than comparing Treg isolated from UCB and APB by an identical procedure, we reasoned that our findings would have greater clinical relevance if Treg were isolated using protocols optimized for the blood source in each instance. Specifically, APB-derived Treg were isolated using a magnetic-bead-based CD4+CD25+CD127lo selection kit, regarded by many as a suitable protocol for this purpose. For the isolation of Treg from UCB, we adopted a protocol developed by Figuero-Tentori and colleagues (11), which relies solely upon magnetic-bead-based positive selection of CD25+ cells. Because the efficacy of this protocol applied to UCB has been demonstrated to be equal to the standard protocol applied to APB, we judge that this less manually intensive protocol is likely to be adopted as a standard for UCB Treg isolation in clinical practice. As an implication of this methodology, it is noteworthy that the function of UCB Treg was found to be superior even when compared with adult Treg purified by a more stringent isolation procedure than that used to isolate UCB Treg. Thus, our results offer further validation of this single-step isolation procedure for UCB Treg. The relative ease of isolation and the regulatory functionality of freshly isolated multiple-unit UCB Treg in vivo imply that this is a useful method for the generation of therapeutic human Treg for clinical use.
MATERIALS AND METHODS
Ethics Statement
Protocols were approved by the Committee on Animal Care and Ethical Review at the University of Oxford in accordance with the UK Animals (Scientific Procedures) Act 1986. Collection of human tissue samples was performed with full informed written consent and ethical approval from the Oxfordshire Research Ethics Committee (REC B), study number 07/H0605/130.
PBMC and Treg Isolation
Adult human PBMCs were isolated from buffy coats of healthy donors (provided by the NHS Blood and Transplant [NHSBT] UK). Adult CD4+CD25+ CD127dim/− Treg cells were isolated using a magnetic-bead-based CD4+CD25+ CD127lo Regulatory T Cell Isolation Kit (Miltenyi Biotech) and LS columns (Miltenyi Biotech), as per manufacturer’s guidelines. Cord blood mononuclear cells were obtained from whole UCB units (NHSBT UK) by density gradient centrifugation using Ficoll-Paque Premium (GE Healthcare). Cord blood CD25+ cells were isolated by labeling cells with MACS CD25+ Microbeads II (Miltenyi Biotech) and passing through two LS columns (Miltenyi Biotech) in succession, according to the manufacturer’s guidelines. Treg from APB and UCB were freshly isolated and not subjected to any period of cell culture prior to use in in vitro and in vivo assays.
In Vitro Suppression Tests
Freshly isolated human Treg were cultured for 7 days with cryopreserved allogeneic human PBMCs in the presence of irradiated third-party PBMCs (i.e., allogeneic to both the responder PBMCs and the Treg), as a source of stimulation. Cells were cultured in complete medium, in 96-well round-bottomed plates at densities of 5×104 PBMCs and 1×105 allogeneic stimulators per well, with Treg at ratios from 1 Treg:1 responder to 1/64 Treg:1 responder. Treg derived from each of three cord bloods and three adult donors, or pooled (in equal proportions) from the same three cord blood or adult donors, were tested against PBMC responders from the same nonautologous adult donor. Six replicates were performed for each Treg donor, or combination of donors, at each Treg-to-responder ratio. Proliferation of PBMCs was determined by incorporation of 3H-thymidine (Perkin Elmer), added for the last 16 hour of culture.
In Vitro Activation
To test functional marker expression upon activation, 1×105 isolated Treg were cultured for 48 hr in the presence of 250 U/mL recombinant IL-2 (Chiron) and stimulated with 2×104 αCD3 and αCD28 beads (Invitrogen) or left unstimulated. Supernatants were collected for analysis of IL-10 and LAP levels using a FlowCytomix Simplex beads assay (eBioscience). Cells were stained for functional markers as described below.
Flow Cytometry
For phenotypic analyses, αCD3-eFluor450 (eBioscience), αCD4-ECD (Beckmann Coulter), αCD8-APC-Cy7 (BD), αCD25-PECy7 (BD), and αCD45-APC (Invitrogen) were used. 7-AAD viability staining solution (eBioscience) was used to eliminate dead cells from the analysis. FOXP3 intracellular staining was performed using a FOXP3 staining buffer set and αFoxP3-FITC antibody (both eBioscience). For analysis of functional markers, Treg after culture were stained with αCD39-PE-Cy7, αCD3-eFluor450 (both eBioscience), αCD4-ECD (Beckmann Coulter), αCD25-APC-Cy7 (BD) antibodies, and 7-AAD dye, followed by fixation and permeabilization with FOXP3 staining buffers and staining with αFOXP3-PE and αPerforin-APC (both eBioscience). For analysis of HLA expression, αHLA-ABC-FITC and αHLA-DR-PerCP (BD) were used. Acquisition was performed using a BD FACSCanto II instrument and data analyzed using BD FACSDiva software.
Mice
BALB/c Rag2−/−cγ−/− (H2d) mice were housed under specific pathogen-free conditions in the Biomedical Services Unit of the John Radcliffe Hospital (Oxford, UK).
In Vivo Skin Survival Assay
Skin transplantation was performed as previously described (34). Only mice displaying greater than 1% splenic human leukocyte chimerism were included in analyses. Recipients of human skin grafts, 35 days posttransplantation, received 5×106 human PBMC in pure RPMI via intraperitoneal injection, with or without 1×106 freshly isolated human CD4+CD25+CD127lo Treg pooled from four APB donors or CD25+ Treg pooled from six UCB donors. Treg were nonautologous to both the PBMCs and the skin graft donor.
In Vivo Cell Survival Assay
BALB/c Rag2−/−cγ−/− mice received 5×106 cryopreserved and thawed PBMC intraperitoneally, with or without 1×106 freshly isolated, CFSE-labeled Treg pooled from either five APB or five UCB donors. Cells were extracted by peritoneal lavage at day 7 after adoptive transfer and Treg (identified as CFSE-positive cells) enumerated by flow cytometry.
Statistical Analysis
All statistical analyses were conducted using GraphPad Prism 5.00 (GraphPad Software, San Diego, CA, USA). Cell numbers and percentages of cellular populations were analyzed using two-tailed one-sample t tests. Log-rank tests were applied to graft survival data.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank Mr. T.E.E. Goodacre and the staff of the plastic surgery theaters for their assistance with the procurement of human skin, the staff of the Biomedical Services Unit at the John Radcliffe Hospital for their expert animal care, the Oxford Transplant Centre for molecular human leukocyte antigen typing, and A. Bushell for his invaluable advice.
This work was supported by grants from The Wellcome Trust, Medical Research Council UK, European Union Integrated Projects, RISET and the ONE Study, the Royal College of Surgeons of England, the Dunhill Medical Trust, and the Oxford Health Services Research Committee Charitable Trust.
Footnotes
The authors declare no conflicts of interest.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
REFERENCES
- 1.Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417. doi: 10.1038/nri3227. [DOI] [PubMed] [Google Scholar]
- 2.Wood KJ, Goto R. Mechanisms of rejection: current perspectives. Transplantation. 2012;93:1. doi: 10.1097/TP.0b013e31823cab44. [DOI] [PubMed] [Google Scholar]
- 3.Wood KJ, Bushell A, Jones ND. Immunologic unresponsiveness to alloantigen in vivo: a role for regulatory T cells. Immunol Rev. 2011;241:119. doi: 10.1111/j.1600-065X.2011.01013.x. [DOI] [PubMed] [Google Scholar]
- 4.Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol Rev. 2008;223:371. doi: 10.1111/j.1600-065X.2008.00637.x. [DOI] [PubMed] [Google Scholar]
- 5.Safinia N, Sagoo P, Lechler R, et al. Adoptive regulatory T cell therapy: challenges in clinical transplantation. Curr Opin Organ Transplant. 2010;15:427. doi: 10.1097/MOT.0b013e32833bfadc. [DOI] [PubMed] [Google Scholar]
- 6.Taylor AL, Watson CJ, Bradley JA. Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit Rev Oncol Hematol. 2005;56:23. doi: 10.1016/j.critrevonc.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 7.Wing K, Ekmark A, Karlsson H, et al. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106:190. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thornton CA, Upham JW, Wikstrom ME, et al. Functional maturation of CD4+CD25+CTLA4+CD45RA+ T regulatory cells in human neonatal T cell responses to environmental antigens/allergens. J Immunol. 2004;173:3084. doi: 10.4049/jimmunol.173.5.3084. [DOI] [PubMed] [Google Scholar]
- 9.Valmori D, Merlo A, Souleimanian NE, et al. A peripheral circulating compartment of natural naive CD4 Tregs. J Clin Invest. 2005;115:1953. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takahata Y, Nomura A, Takada H, et al. CD25+CD4+ T cells in human cord blood: an immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Exp Hematol. 2004;32:622. doi: 10.1016/j.exphem.2004.03.012. [DOI] [PubMed] [Google Scholar]
- 11.Figueroa-Tentori D, Querol S, Dodi IA, et al. High purity and yield of natural Tregs from cord blood using a single step selection method. J Immunol Methods. 2008;339:228. doi: 10.1016/j.jim.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Bresatz S, Sadlon T, Millard D, et al. Isolation, propagation and characterization of cord blood derived CD4+ CD25+ regulatory T cells. J Immunol Methods. 2007;327:53. doi: 10.1016/j.jim.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown JA, Boussiotis VA. Umbilical cord blood transplantation: basic biology and clinical challenges to immune reconstitution. Clin Immunol. 2008;127:286. doi: 10.1016/j.clim.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miyara M, Yoshioka Y, Kitoh A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30:899. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Duhen T, Duhen R, Lanzavecchia A, et al. Functionally distinct subsets of human FOXP3+ Treg cells that phenotypically mirror effector Th cells. Blood. 2012;119:4430. doi: 10.1182/blood-2011-11-392324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wing K, Lindgren S, Kollberg G, et al. CD4 T cell activation by myelin oligodendrocyte glycoprotein is suppressed by adult but not cord blood CD25+ T cells. Eur J Immunol. 2003;33:579. doi: 10.1002/eji.200323701. [DOI] [PubMed] [Google Scholar]
- 18.Chang CC, Satwani P, Oberfield N, et al. Increased induction of allogeneic-specific cord blood CD4+CD25+ regulatory T (Treg) cells: a comparative study of naive and antigenic-specific cord blood Treg cells. Exp Hematol. 2005;33:1508. doi: 10.1016/j.exphem.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Fujimaki W, Takahashi N, Ohnuma K, et al. Comparative study of regulatory T cell function of human CD25CD4 T cells from thymocytes, cord blood, and adult peripheral blood. Clin Dev Immunol. 2008;2008:305859. doi: 10.1155/2008/305859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Godfrey WR, Spoden DJ, Ge YG, et al. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood. 2005;105:750. doi: 10.1182/blood-2004-06-2467. [DOI] [PubMed] [Google Scholar]
- 21.Fan H, Yang J, Hao J, et al. Comparative study of regulatory T cells expanded ex vivo from cord blood and adult peripheral blood. Immunology. 2012;136:218. doi: 10.1111/j.1365-2567.2012.03573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hippen KL, Harker-Murray P, Porter SB, et al. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112:2847. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wing K, Larsson P, Sandstrom K, et al. CD4+ CD25+ FOXP3+ regulatory T cells from human thymus and cord blood suppress antigen-specific T cell responses. Immunology. 2005;115:516. doi: 10.1111/j.1365-2567.2005.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seddiki N, Santner-Nanan B, Tangye SG, et al. Persistence of naive CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 25.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee CC, Lin SJ, Cheng PJ, et al. The regulatory function of umbilical cord blood CD4(+) CD25(+) T cells stimulated with anti-CD3/anti-CD28 and exogenous interleukin (IL)-2 or IL-15. Pediatr Allergy Immunol. 2009;20:624. doi: 10.1111/j.1399-3038.2008.00843.x. [DOI] [PubMed] [Google Scholar]
- 28.Peters JH, Preijers FW, Woestenenk R, et al. Clinical grade Treg: GMP isolation, improvement of purity by CD127 depletion, Treg expansion, and Treg cryopreservation. PLoS One. 2008;3:e3161. doi: 10.1371/journal.pone.0003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ng WF, Duggan PJ, Ponchel F, et al. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 30.Borsellino G, Kleinewietfeld M, Di Mitri D, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood. 2007;110:1225. doi: 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 31.Grossman WJ, Verbsky JW, Barchet W, et al. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 32.Tang Q, Boden EK, Henriksen KJ, et al. Distinct roles of CTLA-4 and TGF-beta in CD4+CD25+ regulatory T cell function. Eur J Immunol. 2004;34:2996. doi: 10.1002/eji.200425143. [DOI] [PubMed] [Google Scholar]
- 33.Oida T, Xu L, Weiner HL, et al. TGF-beta-mediated suppression by CD4+CD25+ T cells is facilitated by CTLA-4 signaling. J Immunol. 2006;177:2331. doi: 10.4049/jimmunol.177.4.2331. [DOI] [PubMed] [Google Scholar]
- 34.Issa F, Hester J, Goto R, et al. Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation. 2010;90:1321. doi: 10.1097/TP.0b013e3181ff8772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen VH, Zeiser R, Dasilva DL, et al. In vivo dynamics of regulatory T-cell trafficking and survival predict effective strategies to control graft-versus-host disease following allogeneic transplantation. Blood. 2007;109:2649. doi: 10.1182/blood-2006-08-044529. [DOI] [PubMed] [Google Scholar]
- 36.Hoffmann P, Eder R, Boeld TJ, et al. Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T-cell lines upon in vitro expansion. Blood. 2006;108:4260. doi: 10.1182/blood-2006-06-027409. [DOI] [PubMed] [Google Scholar]
- 37.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. Eurocord Transplant Group and the European Blood and Marrow Transplantation Group. N Engl J Med. 1997;337:373. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 38.Madrigal JA, Cohen SB, Gluckman E, et al. Does cord blood transplantation result in lower graft-versus-host disease? It takes more than two to tango. Hum Immunol. 1997;56:1. doi: 10.1016/s0198-8859(97)00125-0. [DOI] [PubMed] [Google Scholar]
- 39.Canto E, Rodriguez-Sanchez JL, Vidal S. Distinctive response of naive lymphocytes from cord blood to primary activation via TCR. J Leukoc Biol. 2003;74:998. doi: 10.1189/jlb.0303098. [DOI] [PubMed] [Google Scholar]
- 40.Li L, Boussiotis VA. Molecular and functional heterogeneity of T regulatory cells. Clin Immunol. 2011;141:244. doi: 10.1016/j.clim.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hori S. Developmental plasticity of Foxp3+ regulatory T cells. Curr Opin Immunol. 2010;22:575. doi: 10.1016/j.coi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Nadig SN, Wieckiewicz J, Wu DC, et al. In vivo prevention of transplant arteriosclerosis by ex vivo expanded human regulatory T cells. Nat Med. 2010;16:809. doi: 10.1038/nm.2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li L, Godfrey WR, Porter SB, et al. CD4+CD25+ regulatory T-cell lines from human cord blood have functional and molecular properties of T-cell anergy. Blood. 2005;106:3068. doi: 10.1182/blood-2005-04-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen L, Cohen AC, Lewis DB. Impaired allogeneic activation and T-helper 1 differentiation of human cord blood naive CD4 T cells. Biol Blood Marrow Transplant. 2006;12:160. doi: 10.1016/j.bbmt.2005.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.