Abstract
Background
The aim of this study was to assess the association between the clinical and neurobiological markers of neurodevelopmental impairments and early-onset schizophrenia spectrum psychosis.
Methods
A sample of 36 patients with early-onset schizophrenia spectrum psychosis was compared to a control sample of 36 patients with migraine. We assessed early childhood neurodevelopmental milestones using a modified version of the General Developmental Scale, general intellectual ability using the Wechsler Intelligence Scale for Children–Revised or Leiter International Performance Scale–Revised for patients with speech and language abnormalities, and neurological soft signs with specific regard to subtle motor impairment.
Results
Subjects with early-onset psychosis had a higher rate of impaired social development (P=0.001), learning difficulties (P=0.04), enuresis (P=0.0008), a lower intelligence quotient (P<0.001), and subtle motor impairments (P=0.005) than control subjects.
Conclusion
We suggest that neurodevelopment in early-onset psychosis is characterized by a global impairment of functional and adaptive skills that manifests from early childhood, rather than a delay or limitation in language and motor development. The current evidence is based on a small sample and should be investigated in larger samples in future research.
Keywords: early-onset psychosis, early-onset schizophrenia, neurodevelopment, social cognition, intellectual disabilities
Introduction
Schizophrenia is a severe disabling disorder that affects approximately 1% of the worldwide population, but its causes and pathogenesis remain not completely known.1,2 The neurodevelopmental model is, to date, the most widely accepted pathogenetic hypothesis of schizophrenia, although contributions from the neurodegenerative model are not entirely excluded.3 Rapoport et al4 published selective review updates of recent clinical, epidemiological, brain imaging, and genetic studies in support of the hypothesis that schizophrenia is primarily a neurodevelopmental disorder. Altered brain development in schizophrenia may be accounted for by the abnormal expression of genes that are essential for neurodevelopmental processes. Such genetic mechanisms may significantly interact with prenatal and/or perinatal environmental insults to enhance the risk of developing schizophrenia.1 So, early neurodevelopmental anomalies may interact with typical brain maturation processes throughout childhood and adolescence, causing a wide range of relatively benign markers of developmental disruption.5 Delayed childhood development may precede adult psychosis,6,7 and aberrant neurodevelopment may be even more salient in cases of schizophrenia with very early onset, but the rarity of childhood-onset schizophrenia has limited studies of such patients.8 Childhood-onset schizophrenia, in fact, is a rare disorder with a prevalence rate of one child in 10,000 before the age of 12 years, and a remarkable increase around puberty and early adolescence.9,10 On the other hand, early-onset schizophrenia, in biological continuity with adult forms of the illness, continues to provide unique neurodevelopmental data, as probands are younger, and developmental brain changes are less influenced by the illness or its treatment. For these reasons, investigation of childhood and early-onset schizophrenia has become a major research area during recent years, thus contributing to a better understanding of schizophrenia at all ages.4,9 Language abnormalities, motor impairment and poor coordination, delays in the onset of urinary continence, difficulty reading, and broadly defined premorbid social impairments, have been individually described in early-onset schizophrenia.8–10 However, studies to date on neurodevelopmental impairments in early-onset psychosis (EOP) are few and not readily comparable, mainly due to the different methods used to intercept “development abnormalities” retrospectively, thus leading to heterogeneous results.8–10 Several questions remain open, and a better understanding of the evolutionary trajectories of neurodevelopment would be important since these premorbid impairments are not specific to schizophrenia and may occur in other neurodevelopmental disorders, including attention deficit hyperactivity disorder, intellectual deficiency, and autism spectrum disorders.4 This study was based on the hypothesis that in patients with EOP, we can define evolutionary trajectories characterized by specific clinical and neurobiological markers of premorbid neurodevelopmental impairment arising during the early years of life. The aim was to assess early childhood neurodevelopmental milestones, general intellectual ability, and neurological soft signs (NSS) in a sample of patients with early-onset schizophrenia spectrum psychosis compared to a control sample of patients with migraine. We referred jointly to childhood-onset and adolescent-onset schizophrenia spectrum psychosis as “early-onset psychosis”, defined as the manifestation of psychotic symptoms before the age of 18 years.
Methods
Subjects
The study sample consisted of 36 patients of both sexes who were under 18 years of age. They were consecutively referred since 2007, over a 5-year period, among the inpatients of the Child Neuropsychiatry Unit, Department of Basic Medical Sciences, Neurosciences and Sense Organs, University “Aldo Moro” of Bari, Italy. These patients were diagnosed as suffering from early-onset schizophrenia spectrum psychosis (schizophrenia, schizophreniform disorder, schizoaffective disorder, psychosis not otherwise specified) in accordance with the Diagnostic and Statistical Manual for Mental Disorders, 4th Edition–Text Revision (DSM-IV-TR) criteria.11 Patients were excluded if they had comorbid attention deficit hyperactivity disorder and autism spectrum disorder. The control sample consisted of 36 patients of both sexes, recruited among the inpatients of the same child neuropsychiatry unit with a diagnosis of migraine in accordance with the International Classification of Headache Disorders–II edition (ICHD-II). These patients were more easily recruitable for the control group instead of the healthy subjects, because they had already been included in our headache protocol. These individuals were selected based on age and sex, which were similar to those of the patients (age within 12 months); the control subjects were excluded if they had a history of current and lifetime major psychiatric disorder, as determined through the clinical assessment of psychopathological conditions and an anamnestic interview. The ethical committee of the Hospital Consortium Policlinico of Bari, Italy approved the study. All of the patients parents included in the study provided written consent.
Assessment
Experienced child psychiatrists made a diagnosis of EOP on the basis of the patient’s clinical evaluations, information obtained from family members, and a review of the patient’s past medical records. Clinical diagnoses were supported using the Italian version of the semistructured diagnostic interview, Kiddie-Schedule for Affective Disorders and Schizophrenia–Present and Lifetime Version (K-SADS-PL), conducted by child psychiatrists trained in the use of the instrument. Premorbid and actual functioning was assessed using the Premorbid Adjustment Scale (PAS)12 and the Children’s Global Assessment Scale (CGAS).13 Both cases and controls were subjected to a comprehensive clinical assessment, including a physical, neurological, and psychological examination, as well as an instrumental evaluation by laboratory blood tests and electrocardiogram.
Early childhood neurodevelopment
At least one of the parents of both the case and control group subjects was interviewed to record early childhood neurodevelopmental milestones, using a modified version of the General Developmental Scale.14 Developmental skills were examined by binary test items (attained/failed), which were expected to be solved by a large majority of children at a given age. Five areas of early development were assessed: motor; speech; sphincter control; social skills; and school learning. Each broad area of impairment was scored as present or absent on the basis of the following items: delay in motor development, including first unsupported sitting (>8 months) and/or walking (>18 months); delay in speech/language development, including the first word other than “mama/dada” (>24 months) and/or first meaningful two- or three-word phrases (>36 months); problems with sphincter control, including enuresis (if wetting at least once a week over the age of 5 years) and encopresis (if occurring at least once a week over the age of 4 years), for a minimum of 6 months; and school learning disabilities (reading, writing, and calculation), which were confirmed by either school report or information gathered from the child’s parents. Impaired social development between the ages of 0–6 years required a definite history of the lack of one or more gestures when communicating, the lack of reciprocal social communication, stereotyped or idiosyncratic use of language, abnormal prosody, the lack of imaginative/imitative play, the failure to regulate gaze/facial expression/posture in social communication, the failure to make friends and share interests, and the failure to seek comfort or share pleasure. From the Hollis version of General Developmental Scale, we deleted the item “any neurodevelopmental disorder (eg, hyperkinesis, tics, autism, learning disabilities, IQ <70)” because it maybe overinclusive, referring to clinical conditions that deserve specific attention in the field of neurodevelopmental disorders. Therefore, we replaced the item “reading difficulties” with “difficulties in school learning”, to include disabilities in writing and arithmetic as well. The intelligence quotient (IQ) was assessed separately, and patients with comorbid attention deficit hyperactivity disorder and autism spectrum disorder were excluded from the study group.
IQ measures
All subjects underwent intelligence testing to measure IQ. The first choice was the Wechsler Intelligence Scale for Children–Revised (WISC-R),15 which provides a measure of verbal performance and full-scale IQs. For patients with an abnormality of speech and language, according to the clinical judgment, we used a nonverbal test – the Leiter International Performance Scale–Revised (Leiter-R)16 – which is a battery of visualization and reasoning. Scores below 75 were considered an indication of cognitive impairment.
Subtle motor impairment
NSS, with specific regard to subtle motor impairment, were assessed by asking the patients to perform some motor tasks for the examination of motor coordination and motor sequencing; based on the normal or impaired execution of these motor tasks, each subject was evaluated for the presence or absence of subtle motor impairment.
Statistical analysis
To assess the relations between EOP and childhood developmental abnormalities, IQ impairment, and subtle motor impairment, 2×2 tables were constructed, and a chi-squared test (or Fisher’s exact test, when appropriate) was calculated. When it was possible, the odds ratio (OR) and its 95% confidence interval (CI) were calculated. A significance level of P<0.05 was selected. Statistical analysis was performed by Stata MP for Mac OS 10.
Results
The study sample consisted of 23 males and 13 females with a mean age at assessment of 13 years (range: 7–17 years). There was no significant difference in the mean age of onset according to sex (10 years for males; 11 years for females), with a range of onset ranging from 5 to 16 years in both cases. The control group consisted of 20 males and 16 females with a mean age of 12 years (range: 7–17 years). The main sociodemographic and clinical features of the study and control samples are summarized in Table 1. Childhood developmental abnormalities, totally considered, were significantly associated with EOP (OR: 14.8; 95% CI: 4–60.9; P<0.001), as can be seen in Table 2. Specifically, subjects with EOP had a higher rate of impaired social development (P=0.001), school learning difficulties (P=0.04), and enuresis (P=0.0008) than control subjects (Figure 1). Significant differences were also observed between cases and controls with regard to the IQ evaluation (P<0.001), showing an association between cognitive impairment and EOP. In the study group, we found five individuals with borderline IQ, nine with mild cognitive impairment, and five with medium cognitive impairment. Three patients were unable to complete the IQ testing because of their clinical conditions. In the control group, we found one case of mild cognitive impairment and two cases of medium cognitive impairment. Patients with EOP also had a significantly higher frequency of subtle motor impairments than the control subjects (P=0.005) with reference to both motor coordination and motor sequencing.
Table 1.
Sociodemographic and clinical features of EOP
| EOP | Controls | |
|---|---|---|
| Sex | ||
| Male, n (%) | 23/36 (63.8) | 20/36 (55.5) |
| Female, n (%) | 13/36 (36.1) | 16/36 (44.5) |
| Family history of psychiatric disorders, n (%)a | 24/36 (68.6) | 14/36 (38.8) |
| Obstetric complications, n (%) | 15/36 (44.1) | 11/36 (30.5) |
| Professional help sought for one or more developmental area, n (%)b | 19/36 (52.8) | 3/36 (8.3) |
| Age at first psychotic episode (years) | 11 (5–16) | N/A |
| PASc | 0.5 | N/A |
| Diagnosis | ||
| Schizophrenia, n (%) | 14/36 (38.9) | N/A |
| Schizophreniform disorder, n (%) | 7/36 (19.4) | N/A |
| Schizoaffective disorder, n (%) | 3/36 (8.3) | N/A |
| Psychosis not otherwise specified, n (%) | 12/36 (33.3) | N/A |
| CGAS | 37 | 85 |
Notes:
Schizophrenia and related disorders, mood disorders, anxiety disorders, substance abuse/dependence, personality disorders, and mental retardation;
psychologist, speech therapist, physical therapist, special education teacher;
data missing for two cases at baseline. Data are represented as mean total score CGAS (mean total score). CGAS total score is expressed as number from 0 to 100.
Abbreviations: EOP, early-onset psychosis; PAS, Premorbid Adjustment Scale; CGAS, Children’s Global Assessment Scale; N/A, not applicable.
Table 2.
Markers of neurodevelopmental impairments in cases and controls
| Cases (n, %) | Controls (n, %) | OR (95% CI) | P-value | |
|---|---|---|---|---|
| Childhood neurodevelopmental abnormalities | 24 (70.6) | 5 (13.4) | 14.8 (4–60.9) | 0.000* |
| Impaired motor development | 1 (2.9) | 3 (8.3) | 0.3 (0.1–4.5) | 0.3290 |
| Impaired language development | 9 (26.5) | 5 (13.8) | 2.2 (0.6–9.5) | 0.1548 |
| Delays in sphincter control | 9 (26.5) | 0 (0) | N/A | 0.0008* |
| Impaired social development | 11 (32.3) | 0 (0) | N/A | 0.0001* |
| Difficulties in school learning | 14 (41.2) | 4 (11) | 5.6 (1.4–26.1) | 0.0042* |
| IQ impairment | 19 (57.6) | 3 (8.3) | 14.9 (3.4–87.3) | 0.001* |
| Subtle motor impairment | 12 (46.1) | 7 (19.4) | 3.5 (1.005–12.9) | 0.0050* |
Note:
P<0.05. Data are presented as number and percent of patients with presence of abnormalities in each neurodevelopmental area considered.
Abbreviations: OR, odds ratio; CI, confidence interval; IQ, intelligence quotient; N/A, not applicable.
Figure 1.
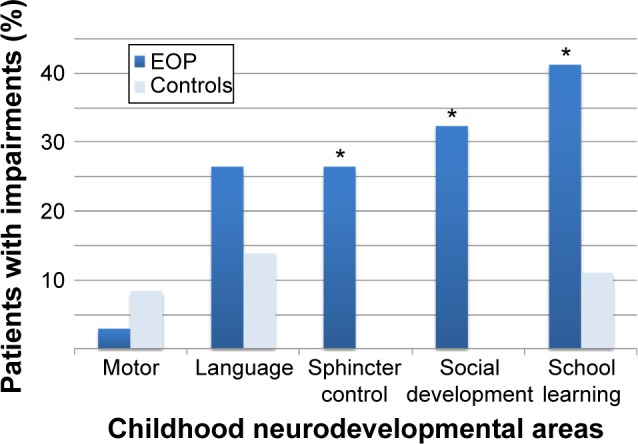
Early childhood developmental delays in case and control groups.
Note: *P<0.05.
Abbreviation: EOP, early-onset psychosis.
Discussion
In this study, we examined the clinical markers of neurobiological impairment across different areas of childhood development in a sample of EOP patients that were compared with a control group of patients with migraine. It is known that children who become schizophrenic later in life have more developmental problems than controls, but the following questions remain: which developmental factors are more specifically associated with the illness? And, is it possible to define abnormal evolutionary trajectories that more typically predict the onset of psychosis? Our data suggested that childhood neurodevelopment in EOP is characterized by a global impairment of functional and adaptive skills, rather than a specific limitation or delay in the age of achievement of some developmental landmarks, such as learning to stand, walk, or speak. One of the main findings of this study, in fact, is that an impairment of social skills evident before the age of 6 years was significantly associated with EOP rather than migraine. This finding is shared by most of the previous studies conducted on childhood neurodevelopment in early-onset schizophrenia, which compared schizophrenia patients with nonpsychotic psychiatric subjects and healthy subjects as well.14,17–20
We can infer by the results of our and previous studies that early premorbid social impairment could represent all of the markers of neurodevelopmental impairment that are more specifically associated with EOP. Our data also point out that the sample of EOP had significant difficulties in school learning. This had already been suggested by other studies, where poor premorbid educational attainment might be a putative predictive factor for the development of schizophrenia spectrum disorders.21,22 This finding is in continuity with the decrease of functioning observed in the premorbid phase of EOP, and it repurposes the need to define the level of cognitive functioning in these subjects. We found a significantly higher frequency of lower IQ in patients with EOP than control subjects (P<0.001), which was in accordance with the neuropsychological evidence for the association between cognitive impairment and EOP.23–26 Cognitive impairment has been considered a core feature of schizophrenia;27 thus, the addition of cognitive impairment as a diagnostic criterion for schizophrenia in the fifth edition of the DSM (DSM-5)28 was carefully considered. No change was made with respect to DSM-IV-TR because cognitive deficits have not been found to sufficiently distinguish schizophrenia from several other “boundary disorders”. While the nature of cognitive impairment at the time of diagnosis may not be discriminating of schizophrenia, the developmental pattern of declining cognition over the years prior to the onset of psychosis may be relevant for differential diagnosis.29 Moreover, all the neurocognitive domains impaired in schizophrenia, such as verbal memory, working memory, motor function, attention, executive functions, and verbal fluency, have been related to adaptive and social skills, so that a recent and inspiring field of research in schizophrenia is represented by social cognition.27,30,31 In line with these considerations, our data showed an impairment of the neurodevelopmental trajectory, but only in the areas of social and cognitive skills. This finding recalls the idea of a clinical and neurobiological connection between these two domains, and supports the hypothesis that an impairment in social cognition, evident from the earliest stages of neurodevelopment, may be a specific marker of the premorbid phase of schizophrenia.
Conversely, more discrepancies are noted in the findings pertaining to other developmental areas. We found no significant association between speech delays, motor development delays, and EOP, suggesting that the age of achievement of some developmental landmarks is not associated with EOP. With regard to the delay of motor development, some authors of previous studies found significantly higher rates of patients with motor impairment (from 31% to 64%), including both delayed milestones and poor coordination, symptoms of restlessness, and abnormal repetitive movements.17,18 We found, instead, no statistically significant difference in the acquisition of specific milestones of postural–motor development. Our results pertaining to statistical significance and prevalence rates (3%–7%) were similar to those reported by Vourdas et al32 and Hollis,14 likely because we used structured methods of data collection. On the other hand, we found that subtle motor impairment, considered separately, was present in 46% of the sample of patients, and this was significantly different from the control group. It can thus be inferred that subtle motor abnormalities are most significantly associated with the development path of patients with EOP, while clearly identifiable impairment in postural–motor development is noticeable only in a small minority. As suggested by Heuser et al33 motor NSS may be related to abnormalities in the frontal and parietal cortices and in the cerebellum of patients with first-episode psychosis.
Moreover, we found a significant association between delays in sphincter control and EOP. Epidemiological and clinical studies conducted to date show that children with special needs, such as those with low IQ, Fragile X and Rett syndromes, have higher rates of urinary incontinence than children without developmental physical or cognitive impairments.34,35 However, there is less evidence for delayed or impaired bladder control in childhood-/adolescent-onset schizophrenia.14,32 Hyde et al36 highlighted that there is a heritable component to childhood enuresis in the families of patients with schizophrenia and, based on data obtained by volumetric brain magnetic resonance imaging scans and neuropsychological testing, it was suggested that a history of childhood enuresis may be a marker of frontal cortical maldevelopment, potentially related to genetic risk factors.
Conclusion
In conclusion, although this study had limitations associated with a small sample size and the use of migraine patients as a control group, we propose that the contemporary presence of difficulties in social skills evident before the age of 6 years and a low IQ score may be considered as an early manifestation of developmental trajectories that evolve into impairments in social cognition, and later into emerging schizophrenia. Impairments in motor coordination and enuresis may be considered more generically associated with a genetic risk for developing this illness. Further longitudinal studies of larger samples are needed to support these data. Since cognitive deficits are significantly associated with poor premorbid adjustment and functional outcomes, there is a clear rationale for further research to clarify the relationship between neurocognition and social competence, as well as to examine the relationship between these specific domains and the negative symptoms of EOP.
Footnotes
Author contributions
All authors contributed toward data analysis, drafting and revising the paper and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Meyer U, Feldon J. Epidemiology-driven neurodevelopmental animal models of schizophrenia. Prog Neurobiol. 2010;90(3):285–326. doi: 10.1016/j.pneurobio.2009.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “Just the Facts”: what we know in 2008 part 1: overview. Schizophr Res. 2008;100(1–3):4–19. doi: 10.1016/j.schres.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 3.Gross G, Huber G. Schizophrenia: neurodevelopmental disorder or degenerative brain process? Fortschr Neurol Psychiatr. 2008;76(Suppl 1):S57–S62. doi: 10.1055/s-2008-1038153. German. [DOI] [PubMed] [Google Scholar]
- 4.Rapoport JL, Giedd JN, Gogtay N. Neurodevelopmental model of schizophrenia: update 2012. Mol Psychiatry. 2012;17(12):1228–1238. doi: 10.1038/mp.2012.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Golembo-Smith S, Walder DJ, Daly MP, et al. The presentation of dermatoglyphic abnormalities in schizophrenia: a meta-analytic review. Schizophr Res. 2012;142(1–3):1–11. doi: 10.1016/j.schres.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isohanni M, Jones PB, Moilanen K, et al. Early developmental milestones in adult schizophrenia and other psychoses. A 31-year follow-up of the Northern Finland 1966 Birth Cohort. Schizophr Res. 2001;52(1–2):1–19. doi: 10.1016/s0920-9964(00)00179-1. [DOI] [PubMed] [Google Scholar]
- 7.Niemi LT, Suvisaari JM, Tuulio-Henriksson A, Lönnqvist JK. Childhood developmental abnormalities in schizophrenia: evidence from high-risk studies. Schizophr Res. 2003;60(2–3):239–258. doi: 10.1016/s0920-9964(02)00234-7. [DOI] [PubMed] [Google Scholar]
- 8.Nicolson R, Lenane M, Singaracharlu S, et al. Premorbid speech and language impairments in childhood-onset schizophrenia: association with risk factors. Am J Psychiatry. 2000;157(5):794–800. doi: 10.1176/appi.ajp.157.5.794. [DOI] [PubMed] [Google Scholar]
- 9.Remschmidt H. Early-onset schizophrenia as a progressive-deteriorating developmental disorder: evidence from child psychiatry. J Neural Transm. 2002;109(1):101–107. doi: 10.1007/s702-002-8240-3. [DOI] [PubMed] [Google Scholar]
- 10.Vyas NS, Patel NH, Puri BK. Neurobiology and phenotypic expression in early onset schizophrenia. Early Interv Psychiatry. 2011;5(1):3–14. doi: 10.1111/j.1751-7893.2010.00253.x. [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, 4th Edition–Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 12.Cannon-Spoor HE, Potkin SG, Wyatt RJ. Measurement of premorbid adjustment in chronic schizophrenia. Schizophr Bull. 1982;8(3):470–484. doi: 10.1093/schbul/8.3.470. [DOI] [PubMed] [Google Scholar]
- 13.Shaffer D, Gould MS, Brasic J, et al. A children’s global assessment scale (CGAS) Arch Gen Psychiatry. 1983;40(11):1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 14.Hollis C. Developmental precursors of child- and adolescent-onset schizophrenia and affective psychoses: diagnostic specificity and continuity with symptom dimensions. Br J Psychiatry. 2003;182:37–44. doi: 10.1192/bjp.182.1.37. [DOI] [PubMed] [Google Scholar]
- 15.Weschsler D. WISC-III: Weschsler Intelligence Scale for Children. New York, NY: The Psychological Corporation; 1991. [Google Scholar]
- 16.Roid GMM LJ. International Performance Scale–Revised: Examiners Manual. Wood Dale, IL: Stoelting Co; 1997. [Google Scholar]
- 17.Arango C, Fraguas D, Parellada M. Differential neurodevelopmental trajectories in patients with early-onset bipolar and schizophrenia disorders. Schizophr Bull. 2014;40(Suppl 2):S138–S146. doi: 10.1093/schbul/sbt198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hollis C. Child and adolescent (juvenile onset) schizophrenia. A case control study of premorbid developmental impairments. Br J Psychiatry. 1995;166(4):489–495. doi: 10.1192/bjp.166.4.489. [DOI] [PubMed] [Google Scholar]
- 19.Shannon C, Douse K, McCusker C, Feeney L, Barrett S, Mulholland C. The association between childhood trauma and memory functioning in schizophrenia. Schizophr Bull. 2011;37(3):531–537. doi: 10.1093/schbul/sbp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payá B, Rodríguez-Sánchez JM, Otero S, et al. Premorbid impairments in early-onset psychosis: differences between patients with schizophrenia and bipolar disorder. Schizophr Res. 2013;146(1–3):103–110. doi: 10.1016/j.schres.2013.01.029. [DOI] [PubMed] [Google Scholar]
- 21.Allen DN, Frantom LV, Strauss GP, van Kammen DP. Differential patterns of premorbid academic and social deterioration in patients with schizophrenia. Schizophr Res. 2005;75(2–3):389–397. doi: 10.1016/j.schres.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Chong SA. Inclusion of cognitive impairment in the DSM diagnosis of schizophrenia: if not now, when? World Psychiatry. 2008;7(1):37–38. doi: 10.1002/j.2051-5545.2008.tb00150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bralet MC, Navarre M, Eskenazi AM, Lucas-Ross M, Falissard B. Interest of a new instrument to assess cognition in schizophrenia: The Brief Assessment of Cognition in Schizophrenia (BACS) Encephale. 2008;34(6):557–562. doi: 10.1016/j.encep.2007.12.005. French. [DOI] [PubMed] [Google Scholar]
- 24.Frangou S. Neurocognition in early-onset schizophrenia. Child Adolesc Psychiatr Clin N Am. 2013;22(4):715–726. doi: 10.1016/j.chc.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Stefanopoulou E, Manoharan A, Landau S, Geddes JR, Goodwin G, Frangou S. Cognitive functioning in patients with affective disorders and schizophrenia: a meta-analysis. Int Rev Psychiatry. 2009;21(4):336–356. doi: 10.1080/09540260902962149. [DOI] [PubMed] [Google Scholar]
- 26.Woodberry KA, Giuliano AJ, Seidman LJ. Premorbid IQ in schizophrenia: a meta-analytic review. Am J Psychiatry. 2008;165(5):579–587. doi: 10.1176/appi.ajp.2008.07081242. [DOI] [PubMed] [Google Scholar]
- 27.Nutt D, Gispen-de Wied CC, Arango C, et al. Cognition in schizophrenia: summary Nice Consultation Meeting 2012. Eur Neuropsychopharmacol. 2013;23(8):769–778. doi: 10.1016/j.euroneuro.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 28.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington, VA: American Psychiatric Association; 2013. [Google Scholar]
- 29.Tandon R, Bruijnzeel D, Rankupalli B. Does change in definition of psychotic symptoms in diagnosis of schizophrenia in DSM-5 affect caseness? Asian J Psychiatr. 2013;6(4):330–332. doi: 10.1016/j.ajp.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 30.Adolphs R. The neurobiology of social cognition. Curr Opin Neurobiol. 2001;11(2):231–239. doi: 10.1016/s0959-4388(00)00202-6. [DOI] [PubMed] [Google Scholar]
- 31.Mehta UM, Thirthalli J, Subbakrishna DK, Gangadhar BN, Eack SM, Keshavan MS. Social and neuro-cognition as distinct cognitive factors in schizophrenia: a systematic review. Schizophr Res. 2013;148(1–3):3–11. doi: 10.1016/j.schres.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 32.Vourdas A, Pipe R, Corrigall R, Frangou S. Increased developmental deviance and premorbid dysfunction in early onset schizophrenia. Schizophr Res. 2003;62(1–2):13–22. doi: 10.1016/s0920-9964(02)00429-2. [DOI] [PubMed] [Google Scholar]
- 33.Heuser M, Thomann PA, Essig M, Bachmann S, Schröder J. Neurological signs and morphological cerebral changes in schizophrenia: An analysis of NSS subscales in patients with first episode psychosis. Psychiatry Res. 2011;192(2):69–76. doi: 10.1016/j.pscychresns.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 34.Nevéus T, Läckgren G, Tuvemo T, Hetta J, Hjälmås K, Stenberg A. Enuresis – background and treatment. Scand J Urol Nephrol Suppl. 2000;(206):1–44. [PubMed] [Google Scholar]
- 35.von Gontard A. Urinary incontinence in children with special needs. Nat Rev Urol. 2013;10(11):667–674. doi: 10.1038/nrurol.2013.213. [DOI] [PubMed] [Google Scholar]
- 36.Hyde TM, Deep-Soboslay A, Iglesias B, et al. Enuresis as a premorbid developmental marker of schizophrenia. Brain. 2008;131(Pt 9):2489–2498. doi: 10.1093/brain/awn167. [DOI] [PMC free article] [PubMed] [Google Scholar]


