Abstract
The six copies of the response regulator CheY from Rhodobacter sphaeroides bind to the switch protein FliM. Phosphorylation by acetyl phosphate (AcP) was detected by tryptophan fluorescence quenching in three of the four CheYs that contain this residue. Autophosphorylation with Ac32P was observed in five CheY proteins. We also show that all of the cheY genes are expressed simultaneously; therefore, in vivo all of the CheY proteins could bind to FliM to control the chemotactic response. Consequently, we hypothesize that in this complex chemotactic system, the binding of some CheY proteins to FliM, does not necessarily imply switching of the flagellar motor.
The paradigm of the bacterial chemotactic response is the signal transduction pathway found in enteric bacteria such as Escherichia coli and Salmonella enterica serovar Typhimurium. This system regulates the direction of flagellar rotation through the interaction of the phosphorylated response regulator, CheY-P, with the switch protein FliM, which is a key process in bacterial chemotaxis (for reviews, see references 1, 7, 12, and 26). When most of the flagella of a cell rotate in the counterclockwise direction, they coalesce in a bundle that pushes the cell body in a linear trajectory called run. When flagella reverse the sense of rotation to clockwise, the bundle loses stability, and the uncoordinated motion of the filaments produces a tumble that reorients the cell. Therefore, the frequency of tumble determines the overall swimming direction of the cell. Mutational and genome analyses have identified multiple homologues of the signal transduction proteins in several bacterial species, i.e., Agrobacterium tumefaciens, Rhodobacter sphaeroides, and Sinorhizobium meliloti. S. meliloti has two CheY homologues: CheY2 modulates flagellar rotation, whereas CheY1 does not. CheA phosphorylates both CheY1 and CheY2 and, when CheA becomes inactive, CheY1 acts as the signal terminator by dephosphorylating CheY2, replacing the phosphatase CheZ and acting as a phosphate sink (23).
R. sphaeroides is a facultative non-sulfur-photosynthetic bacterium that belongs to the α-subgroup of the proteobacteria. It is able to grow photoheterotrophically and chemoheterotrophically, to utilize a wide range of organic acids for growth, and to fix nitrogen and CO2. Unlike E. coli and S. enterica, R. sphaeroides has a single subpolar flagellum that propels the cell by CW rotation stopping randomly producing reorientation of the bacterium. During the stop periods, the filament retracts into a coiled form (3). It has been suggested that the slow motion of the filament in this conformation helps reorient the cell (4). Multiple homologues of the chemotactic genes have been found in R. sphaeroides, including six cheY, four cheA and cheW, three cheR, and two cheB copies. The gene encoding the phosphatase cheZ has not been identified (11).
Bacterial strains, plasmids, and oligonucleotides are listed in Table 1. Luria broth was prepared as previously described (5). All chemicals used were reagent grade. R. sphaeroides cell cultures were grown in Sistrom's solid or liquid medium (21) under continuous illumination at 30°C for photoheterotrophic growth or in the dark with strong shaking. Recombinant DNA techniques were carried out by standard procedures (5). Reverse transcription-PCR (RT-PCR) was performed with specific primers that were synthesized by Sigma-Genosys.
TABLE 1.
Bacterial strains, plasmids, and oligonucleotides
| Strains, plasmids, and oligonucleotides | Relevant characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
| E. coli | ||
| JM103 | hsdR4 Δ(lac-pro) F′ traD36 proAB laclqZΔM15 | 5 |
| JM109 | hsdR17 Δ(lac-pro) F′ traD36 proAB laclqZΔM15 | 5 |
| XL1-Blue | supE44 hsdR17 recA1 endA1 gyrA46 thi relA1 lac F′ [proAB+laclqlacZΔM15 Tn10(Tetr)] | 5 |
| M15(pREP4) | thi lac ara+gal mtl F′ recA+uvr+lon+; pREP4 plasmid Kanr | Qiagen |
| R. sphaeroides WS8 | Wild type; spontaneous Nalr | 22 |
| Plasmids | ||
| pQE30 | Expression vector; Apr, N-terminal His6 tag | Qiagen |
| pQE60 | Expression vector; Apr, C-terminal His6 tag | Qiagen |
| pRSM | fliM cloned into the NcoI/BglII sites of pQE60 | This work |
| pRSMΔ13 | fliMΔ13 cloned into the NcoI/BglII sites of pQE60 | This work |
| pRSMΔ20 | fliMΔ20 cloned into the NcoI/BglII sites of pQE60 | This work |
| pRSY1 | cheY1 cloned into the NcoI/BamHI sites of pQE60 | This work |
| pRSY2 | cheY2 cloned into the SacI/PstI sites of pQE30 | This work |
| pRSY3 | cheY3 cloned into the BamHI/PstI sites of pQE30 | This work |
| pRSY4 | cheY4 cloned into the NcoI/BamHI sites of pQE60 | This work |
| pRSY5 | cheY5 cloned into the BamHI/PstI sites of pQE30 | This work |
| pRSY6 | cheY6 cloned into the BamHI/PstI sites of pQE30 | This work |
| Oligonucleotidesb | ||
| cheY1 fw | 5′-CATGCCATGGCGCCGCTGACCGTTCTTGCC-3′ | This work |
| cheY1 rev | 5′-CTCACGGACTGCTCGGATGAAGCC-3′ | This work |
| cheY2 fw | 5′-CCGAGCTCGTGGTCGATGACATGTCGACCAGC-3′ | This work |
| cheY2 rev | 5′-AAAACTGCAGTCATAGAGCGCCTACGACAGC-3′ | This work |
| cheY3 fw | 5′-CGCGGATCCAGCAGGACGGTTCTCGCCGTG-3′ | This work |
| cheY3 rev | 5′-AAAACTGCAGTCATCCGAGCACCTTCTTGAT-3′ | This work |
| cheY4 fw | 5′-CATGCCATGGCGACGAAAACCGTCCTCGCA-3′ | This work |
| cheY4 rev | 5′-CGCGGATCCGGCCAAGAAGCTTCTTCATCAC-3′ | This work |
| cheY5 fw | 5′-CGCGGATCCAGCAAGACGATCCTCGCGGTGGAT-3′ | This work |
| cheY5 rev | 5′-AAAACTGCAGTCAGGCGCCGGCCACCTTCTTGAC-3′ | This work |
| cheY6 fw | 5′-GCGGGATCCCCCTACAATGTCATGATC-3′ | This work |
| cheY6 rev | 5′-AAAACTGCAGTCAGGCGGCCATCAGCGT-3′ | This work |
Apr, ampicillin resistance; Kanr, kanamycin resistance; Nalr, nalidixic acid resistance.
fw, forward; rev, reverse.
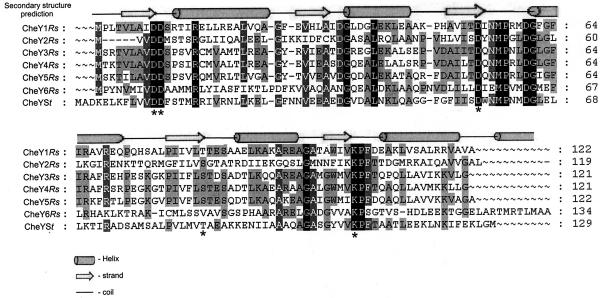
Multiple alignment of the R. sphaeroides CheY proteins (CheYRs) with CheY from S. enterica (CheYSt) shows extensive overall homology through the complete protein sequence. Conserved patterns of α-helix, β-strand, and random coil were predicted from the sequence by using the PSIPRED program (Fig. 1). This protein is a doubly wound α/β protein with a central five-stranded parallel β-sheet surrounded by five α-helices (25, 29). The phosphorylation site in CheYSt is Asp57 (19), which is located in the solvent-exposed loop between β3 and α3. This residue lies adjacent to other acidic residues, Asp12 and Asp13, which are involved in coordination of the Mg2+ ion required for phosphoryl transfer and dephosphorylation (9, 10, 15). Two residues, Thr87 and Lys109, complete the conserved cluster surrounding the active site of the regulatory domain and are thought to participate in the phosphorylation-induced conformational change in CheY (2, 9, 32). The six CheYRs proteins show these highly conserved amino acid residues that are present in Salmonella CheY. The phosphorylation site Asp57, in addition to Asp12, Asp13, and Lys109, is conserved in the six R. sphaeroides CheY proteins. Thr87 is the exception, given that it is present only in CheY1, whereas CheY2, CheY3, CheY4, and CheY5 contain Ser that conserves the neutral character of the Thr residue, as well as an OH group as a lateral chain. Apparently, CheY6 contains nonpolar Val in this position (Fig. 1), although it could be possible that the preceding Ser is out of register in our alignment.
FIG. 1.
Multiple alignment of the six CheY proteins from R. sphaeroides and CheY from S. enterica serovar Typhimurium. Sequence alignment was performed by using PILEUP (GCG-Wisconsin Package, version 9.1). Secondary structure prediction was carried out by using the program PSIPRED (8, 14). Identical residues are shaded in tones of black. Asterisks indicate relevant residues for the function of CheYSt.
Analysis of expression of the multiple cheY genes under two different metabolic conditions.
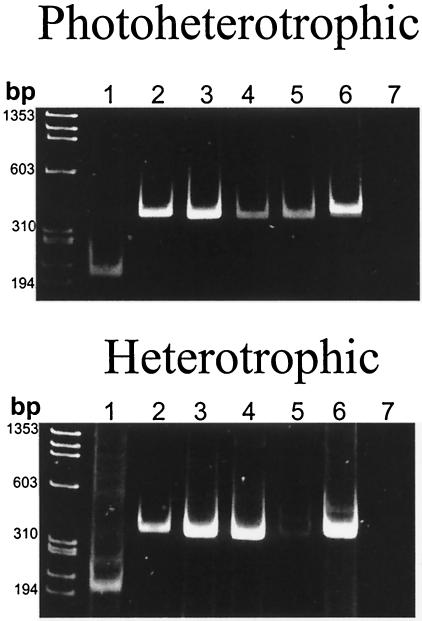
The expression of the mRNAs of the six cheY genes found in R. sphaeroides was addressed by using RT-PCR, and the results are shown in Fig. 2. Cells were grown either heterotrophically or photoheterotrophically, and total RNA was isolated by using standard reported procedures (5). Specific primers for each one of the cheY genes were tested to determine their expression (Table 1). Figure 2 shows that they are similarly expressed under the two growth conditions. Nevertheless, the expression of cheY5 under heterotrophic conditions is notably reduced, suggesting that its expression could be regulated by the prevailing environmental conditions. If no posttranscriptional control of the expression of these proteins exists, it can be assumed that the six proteins are present in the cytoplasm simultaneously, especially under phototrophic conditions. This could imply that a fine-tuning regulation of the chemotactic response in R. sphaeroides requires the simultaneous presence of all of the chemotactic response regulators.
FIG. 2.
RT-PCR of the six R. sphaeroides cheY transcripts. Total RNA was isolated from WS8 wild-type cells. One-fifth of each of the PCRs was loaded onto a 7.5% polyacrylamide gel. Lanes 1 to 6, show the amplification products of cheY1-6, respectively, and the expected sizes of these products are for 207 bp for cheY1, 354 bp for cheY2, 360 bp for cheY3, 360 bp for cheY4, 363 bp for cheY5, and 399 bp for cheY6. Lane 7 shows a control, with the oligonucleotides for amplification of cheY6, lacking avian myeloblastosis virus reverse transcriptase in the reaction.
Purification and characterization of the CheYRs proteins.
The cheY genes were amplified by PCR and cloned into pQE60 expression vector, which attaches a His6 tag at the C terminus of the protein (CheY1 and CheY4) or pQE30, which attaches a His6 tag at the N terminus of the protein (CheY2, CheY3, CheY5, and CheY6). These proteins were expressed in either JM103, XL1-Blue or M15(pREP4) strains (Table 1) and purified according to standard procedures (Qiagen, Inc., Valencia, Calif.). His-tagged CheY proteins were purified to homogeneity by Ni-nitrilotriacetic acid affinity chromatography. The six CheY proteins migrate as a single band of molecular mass of ∼14,400 Da (data not shown). The structural integrity of purified CheYRs proteins was assessed by analyzing their secondary structure by means of far UV circular dicroism (CD) spectra. Scans from 190 to 260 nm were performed on the purified CheY proteins. The CD spectra show that all CheY proteins maintained their structural integrity during purification and contain the secondary structure motifs that characterize these response regulators. It should be noted that CheY2 was the only protein recovered from inclusion bodies after denaturation and refolding. As a result of the refolding procedures, this protein shows a slight shift toward far UV wavelengths, which indicates that a fraction of refolded CheY2 acquires a different structural conformation (data not shown).
Effect of small-molecule phosphodonor AcP on the six CheY proteins of R. sphaeroides.
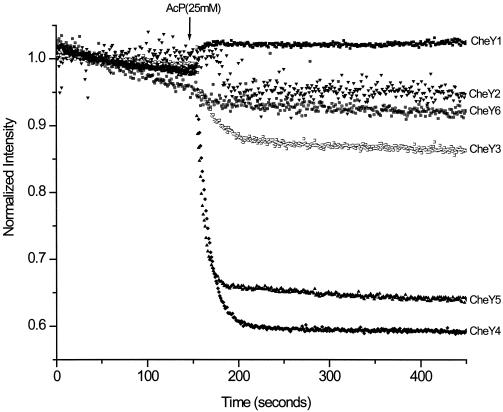
We tested the ability of the CheYRs proteins to become phosphorylated by the small-molecule phosphodonor acetyl phosphate (AcP). Since four of the six CheYRs proteins contain a tryptophan residue (Trp106) that can be used to report conformational changes, we determined intrinsic fluorescence changes due to phosphate binding to the conserved aspartic residue, Asp57. Figure 3 shows that fluorescence intensity decreases upon the addition of 25 mM AcP to the reaction medium in three of the four CheY proteins that contain Trp106 (CheY3, CheY4, and CheY5). CheY1 that also contains Trp106 behaves as CheY2 or CheY6 that contain phenylalanine or valine residues, respectively, in the same position. Since in CheYEc the substitution Tyr106Trp renders a tumbly phenotype (31), it would be interesting to determine whether Trp106 in CheYRs favors switching. Since fluorescence quenching of CheY3, CheY4, and CheY5 is dependent on the presence of Mg2+ (data not shown), this assay seems useful for detecting phosphorylation or changes associated with phosphorylation for at least these three CheY proteins. In addition, we determined in vitro phosphorylation by Ac32P of the CheYRs proteins according to procedures previously reported (27). The reactions were stopped after 1 min and subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and phosphorimaging. Radioactive labeling of five of the six CheY proteins was observed, indicating that AcP is indeed a phosphodonor for the CheYRs proteins (Fig. 4). As mentioned above, CheY2 showed an altered CD spectrum, due possibly to the fact that during renaturation from inclusion bodies the native α/β structures were partially lost, abolishing the ability to become phosphorylated by Ac32P. A lower level of 32P labeling of CheY3 and CheY6 was observed. This can be explained given that CheY3 has a lower affinity for AcP (data not shown) and CheY6 has a lower dephosphorylation half-time (17).
FIG. 3.
Intrinsic fluorescence of the R. sphaeroides CheY proteins. Purified CheY proteins were mixed with 25 mM AcP never exceeding 4% of the initial volume, and kinetic determinations were performed as described previously (13). Given that each CheY displays a different fluorescence intensity, emission was normalized for each protein to an arbitrary intensity of 1.0.
FIG. 4.
CheY phosphorylation by AcP. A phosphorimage of the six CheY proteins incubated with Ac32P for 1 min is shown. The reaction was stopped by the addition of 5 μl of 5× sample buffer for SDS-PAGE. Ac32P was synthesized as described previously (24).
Phospho-CheY binding to the switch protein FliM.
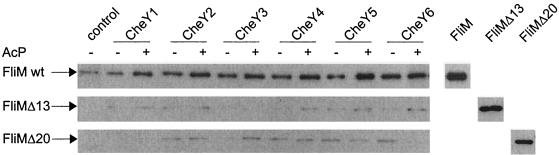
We tested whether these response regulators could interact with purified FliMRs. For this purpose, the coding region of fliMRs was amplified by PCR, cloned in pQE60, and expressed in E. coli JM109. Inclusion bodies were pelleted by centrifugation and solubilized in a buffer containing 5.5 M guanidine-HCl. His-tagged FliM was purified by Ni-nitrilotriacetic acid affinity chromatography under denaturing conditions and refolded by dilution in a buffer containing 100 mM Tris-HCl (pH 7.9), 500 mM NaCl, 2 M urea, 2.5 mM reduced and 0.5 mM oxidized glutathione, and 20% glycerol, followed by dialysis against TNG buffer (50 mM Tris-HCl [pH 7.9], 150 mM NaCl, 10% glycerol). Purified CheY proteins were immobilized on CNBr-activated Sepharose beads CL-4B (Pharmacia, Uppsala, Sweden) as previously described (30). Control beads contained covalently bound bovine serum albumin. CheY beads were incubated with FliMRs in the presence or absence of AcP (25 mM) and MgCl2 (5 mM) and subjected to SDS-PAGE and immunoblotting with polyclonal rabbit antibodies raised against FliMRs (the present study). Immunoblotting with anti-His antibodies confirmed that all of the experiments were performed with comparable amounts of CheY (data not shown). Figure 5 shows that the six CheY proteins bind FliM in the absence of AcP and that the addition of this phosphodonor results in a two- to threefold increase in FliM binding. This result indicates that the contact surface of CheY that interacts with FliM is well conserved in all of the CheY proteins. To explain the apparent contradiction between the absence of labeling of CheY2 by Ac32P (see Fig. 4) and the effect of AcP on FliM binding, we consider that covalent binding of CheY2 to CNBr-activated Sepharose beads possibly stabilizes a conformation that allows this protein to become phosphorylated by AcP. The specificity of binding of the different CheY proteins was addressed by using two deletion mutants lacking the first 13 or 20 residues of the N-terminal region of FliMRs (16). This region has been identified in FliMSt and FliM from Bacillus subtilis as the binding site of the response regulator CheY (6, 27, 28). Both fliMΔ13 and fliMΔ20 were cloned into pQE60, purified as for wild-type FliMRs, and subjected to the binding assay described above. We found that deletion of the N-terminal 13 or 20 amino acids of FliM reduces its binding capacity significantly. It should also be noted that anti-FliMRs antibodies recognize the two deletion mutants with equal efficiency (Fig. 5).
FIG. 5.
Binding of CheY and phospho-CheY to FliM. The six CheY proteins immobilized to Sepharose beads were assayed for binding to FliM, FliMΔ13, and FliMΔ20 in the presence or absence of AcP. Control lane shows nonspecific binding of FliM to bovine serum albumin-Sepharose beads. Immunoblots were probed with anti-FliMRs antibodies. The added purified FliM, FliMΔ13, and FliMΔ20 proteins are shown for reference.
Binding is the first step to control flagellar rotation. Studies carried out in E. coli indicated that switching is altered without modification of the binding affinity of CheY for FliM. From these studies Tyr106 has been identified as a relevant residue for signaling (31, 32). As mentioned above, CheY1, CheY3, CheY4, and CheY5 contain Trp in this position, whereas CheY2 and CheY6 contain Phe and Val residues, respectively. In spite of having Trp106, CheY1 did not show fluorescence quenching upon addition of AcP. It is possible that the conformational change provoked by phosphorylation does not affect the environment of Trp106; thus, the fluorescence remains unchanged. Further study should be carried out to determine whether Trp106 is critical for signaling in these response regulators.
The existence of at least one CheY acting as a phosphate sink is a central idea in the present model proposed to explain the chemotactic response in R. sphaeroides (18, 20). Our data do not contradict this idea, provided that the CheY proteins that act as phosphate sinks bind to the flagellar motor without promoting switching.
It can be hypothesized that in R. sphaeroides the chemotactic control is carried out by the concerted action of the different CheY proteins. By the same token, it is possible that some CheY proteins bind FliM without promoting switching thus competing for motor occupancy. This kind of control would require a complex balance among all of the reactions in which these response regulators are involved (i.e., binding to FliM and to CheA, phosphorylation and dephosphorylation rates, etc.). Therefore, the biochemical characterization of these parameters seems compulsory for understanding this complex response. In this regard, determination of the affinity of each CheY protein for FliM is currently one of our main interests.
It has been previously shown that R. sphaeroides strains expressing mutant FliM proteins (Δ13 or Δ20) showed a stopped phenotype. From these results it was suggested that the binding of CheY to FliM promotes CW rotation hence swimming, whereas the lack of interaction between FliM and CheY induces a stop event (16). In the light of the results reported here, it is conceivable that the stop events could also occur by the binding of a CheY protein that does not promote clockwise rotation. We are currently investigating this possibility.
Acknowledgments
We thank Michael Eisenbach, Diego González-Halphen, and Bertha González-Pedrajo for critical reading of the manuscript. We also thank Sebastian Poggio for many helpful discussions and the Molecular Biology Unit of this Institute for sequencing all of the PCR products and clones.
This study was supported by grant 38552-N from the Consejo Nacional de Ciencia y Tecnología of Mexico.
Footnotes
This study is dedicated to the memory of our dear friend Robert M. Macnab.
REFERENCES
- 1.Aizawa, S. I., I. B. Zhulin, L. Marquez-Magaña, and G. W. Ordal. 2002. Chemotaxis and motility, p. 437-452. In A. L. Sonenshein, R. Losick, and J. A. Hoch (ed.), Bacillus subtilis and its closest relatives: from genes to cells. American Society for Microbiology, Washington, D.C.
- 2.Appleby, J. L., and R. B. Bourret. 1998. Proposed signal transduction role for conserved CheY residue Thr87, a member of the response regulator active-site quintet. J. Bacteriol. 180:3563-3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armitage, J. P., and R. M. Macnab. 1987. Unidirectional, intermittent rotation of the flagellum of Rhodobacter sphaeroides. J. Bacteriol. 169:514-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armitage, J. P., T. P. Pitta, M. A. Vigeant, H. L. Packer, and R. M. Ford. 1999. Transformations in flagellar structure of Rhodobacter sphaeroides and possible relationship to changes in swimming speed. J. Bacteriol. 181:4825-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 6.Bren, A., and M. Eisenbach. 1998. The N terminus of the flagellar switch protein, FliM, is the binding domain for the chemotactic response regulator, CheY. J. Mol. Biol. 278:507-514. [DOI] [PubMed] [Google Scholar]
- 7.Bren, A., and M. Eisenbach. 2000. How signals are heard during bacterial chemotaxis: protein-protein interactions in sensory signal propagation. J. Bacteriol. 182:6865-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones, D. T. 1999. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 292:195-202. [DOI] [PubMed] [Google Scholar]
- 9.Lukat, G. S., B. H. Lee, J. M. Mottonen, A. M. Stock, and J. B. Stock. 1991. Roles of the highly conserved aspartate and lysine residues in the response regulator of bacterial chemotaxis. J. Biol. Chem. 266:8348-8354. [PubMed] [Google Scholar]
- 10.Lukat, G. S., A. M. Stock, and J. B. Stock. 1990. Divalent metal ion binding to the CheY protein and its significance to phosphotransfer in bacterial chemotaxis. Biochemistry 29:5436-5454. [DOI] [PubMed] [Google Scholar]
- 11.Mackenzie, C., M. Choudhary, F. W. Larimer, P. F. Predki, S. Stilwagen, J. P. Armitage, R. D. Barber, T. J. Donohue, J. P. Hosler, and S. Kaplan. 2001. The home stretch, a first analysis of the nearly completed genome of Rhodobacter sphaeroides 2.4.1. Photosynthesis Res. 70:19-41. [DOI] [PubMed] [Google Scholar]
- 12.Macnab, R. M. 1996. Flagella and motility, p. 123-145. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. American Society for Microbiology, Washington, D.C.
- 13.Mayover, T. L., C. J. Halkides, and R. C. Stewart. 1999. Kinetic characterization of CheY phosphorylation reactions: comparison of P-CheA and small-molecule phosphodonors. Biochemistry 38:2259-2271. [DOI] [PubMed] [Google Scholar]
- 14.McGuffin, L. J., K. Bryson, and D. T. Jones. 2000. The PSIPRED protein structure prediction server. Bioinformatics 16:404-405. [DOI] [PubMed] [Google Scholar]
- 15.Needham, J. V., T. Y. Chen, and J. J. Falke. 1993. Novel ion specificity of a carboxylate cluster Mg(II) binding site: strong charge selectivity and weak size selectivity. Biochemistry 32:3363-3367. [DOI] [PubMed] [Google Scholar]
- 16.Poggio, S., A. Osorio, G. Corkidi, G. Dreyfus, and L. Camarena. 2001. The N terminus of FliM is essential to promote flagellar rotation in Rhodobacter sphaeroides. J. Bacteriol. 183:3142-3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porter, S. L., and J. P. Armitage. 2002. Phosphotransfer in Rhodobacter sphaeroides chemotaxis. J. Mol. Biol. 324:35-45. [DOI] [PubMed] [Google Scholar]
- 18.Porter, S. L., A. V. Warren, A. C. Martin, and J. P. Armitage. 2002. The third chemotaxis locus of Rhodobacter sphaeroides is essential for chemotaxis. Mol. Microbiol. 46:1081-1094. [DOI] [PubMed] [Google Scholar]
- 19.Sanders, D. A., B. L. Gillece-Castro, A. M. Stock, A. L. Burlingame, and D. E. Koshland, Jr. 1989. Identification of the site of phosphorylation of the chemotaxis response regulator protein, CheY. J. Biol. Chem. 264:21770-21778. [PubMed] [Google Scholar]
- 20.Shah, D. S., S. L. Porter, A. C. Martin, P. A. Hamblin, and J. P. Armitage. 2000. Fine tuning bacterial chemotaxis: analysis of Rhodobacter sphaeroides behavior under aerobic and anaerobic conditions by mutation of the major chemotaxis operons and cheY genes. EMBO J. 19:4601-4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sistrom, W. R. 1962. The kinetics of the synthesis of photopigments in Rhodopseudomonas sphaeroides. J. Gen. Microbiol. 28:607-616. [DOI] [PubMed] [Google Scholar]
- 22.Sockett, R. E., J. A. C. Foster, and J. P. Armitage. 1990. Molecular biology of the Rhodobacter sphaeroides flagellum. FEMS Symp. 53:473-479. [Google Scholar]
- 23.Sourjik, V., and R. Schmitt. 1998. Phosphotransfer between CheA, CheY1, and CheY2 in the chemotaxis signal transduction chain of Rhizobium meliloti. Biochemistry 37:2327-2335. [DOI] [PubMed] [Google Scholar]
- 24.Stadtman, E. R. 1957. Preparation and assay of acetylphosphate. Methods Enzymol. 3:228-231. [Google Scholar]
- 25.Stock, A. M., J. M. Mottonen, J. B. Stock, and C. E. Schutt. 1989. Three-dimensional structure of CheY, the response regulator of bacterial chemotaxis. Nature 337:745-749. [DOI] [PubMed] [Google Scholar]
- 26.Stock, J. B., and M. G. Surette. 1996. Chemotaxis, p. 1103-1129. In F. C. Neidhart, R. I. Curtiss, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella: cellular and molecular biology. American Society for Microbiology, Washington, D.C.
- 27.Szurmant, H., M. W. Bunn, V. J. Cannistraro, and G. W. Ordal. 2003. Bacillus subtilis hydrolyzes CheY-P at the location of its action, the flagellar switch. J. Biol. Chem. 278:48611-48616. [DOI] [PubMed] [Google Scholar]
- 28.Toker, A. S., and R. M. Macnab. 1997. Distinct regions of bacterial flagellar switch protein FliM interact with FliG, FliN, and CheY. J. Mol. Biol. 273:623-634. [DOI] [PubMed] [Google Scholar]
- 29.Volz, K., and P. Matsumura. 1991. Crystal structure of Escherichia coli CheY refined at 1.7-Å resolution. J. Biol. Chem. 266:15511-15519. [DOI] [PubMed] [Google Scholar]
- 30.Welch, M., K. Oosawa, S. Aizawa, and M. Eisenbach. 1993. Phosphorylation-dependent binding of a signal molecule to the flagellar switch of bacteria. Proc. Natl. Acad. Sci. USA 90:8787-8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu, X., C. D. Amsler, K. Volz, and P. Matsumura. 1996. Tyrosine 106 of CheY plays an important role in chemotaxis signal transduction in Escherichia coli. J. Bacteriol. 178:4208-4215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu, X., J. Rebello, P. Matsumura, and K. Volz. 1997. Crystal structures of CheY mutants Y106W and T87I/Y106W: CheY activation correlates with movement of residue 106. J. Biol. Chem. 272:5000-5006. [DOI] [PubMed] [Google Scholar]