Abstract
We identify a novel contextual variable that alters the evaluation of delayed rewards in healthy subjects and those diagnosed with attention deficit/hyperactivity disorder (ADHD). When intertemporal choices are constructed of monetary outcomes with rounded values (e.g., $25.00), discount rates are greater than when the rewards have nonzero decimal values (e.g., $25.12). This finding is well explained within a dual system framework for temporal discounting in which preferences are constructed from separate affective and deliberative processes. Specifically, we find that round dollar values produce greater positive affect than do non-zero decimal values. This suggests that relative involvement of affective processes may underlie our observed difference in intertemporal preferences. Furthermore, we demonstrate that intertemporal choices with rounded values recruit greater brain responses in the nucleus accumbens to a degree that correlates with the size of the behavioral effect across subjects. Our demonstration that a simple contextual manipulation can alter self-control in ADHD has implications for treatment of individuals with disorders of impulsivity. Overall, the decimal effect highlights mechanisms by which the properties of a reward bias perceived value and consequent preferences.
Keywords: intertemporal choice, dual systems model, fMRI, reward, ADHD
Problems with self-control are some of the most detrimental for individuals as well as society, with obesity, excessive debt and substance abuse representing major health and economic concerns (Madden & Bickel, 2009; Madden, Petry, Badger, & Bickel, 1997; Reynolds, Leraas, Collins, & Melanko, 2009). These issues all have one feature in common: people opt for more immediately rewarding options and under-value future benefits to their overall detriment. To understand such phenomena, research has posited that future outcomes are evaluated using hyperbolic or quasi-hyperbolic discount functions, which effectively describe the tendency to over-value immediate rewards (Frederick et al., 2002). In these functions, value rapidly decreases as rewards are delayed from the present and decreases more slowly as rewards are delayed from future times.
The discount rate expressed in hyperbolic discounting is the critical factor determining relative preferences for immediate rewards. Discount rates depend on a wide variety of contextual and personal variables, such as the nature of the reward, its modality (Bickel & Marsch, 2001; McClure, Ericson, Laibson, Loewenstein, & Cohen, 2007), its magnitude (Green, Myerson, & McFadden, 1997; Thaler, 1981), and even the scent in the experimental room (Li, 2008). Individual factors which predict differences in delay discounting include age (Green, Fry, & Myerson, 1994; Sozou & Seymour, 2003; Steinberg, 2010), health (Chao, Szrek, Pereira, & Pauly, 2009), intelligence (Shamosh et al., 2008) and some psychiatric disorders (Ahn et al., 2011; Heerey, Robinson, McMahon, & Gold, 2007). Peters and Büchel (2011) refer to these dependencies as trait (immutable, e.g. person-related) and state (mutable, framing/context) factors that affect discounting rates. The prototypical disorder associated with greater discounting and poor self-control is Attention Deficit/Hyperactivity Disorder (ADHD; Marco et al., 2009; Paloyelis, Asherson, & Kuntsi, 2009; Rapport, Tucker, DuPaul, Merlo, & Stoner, 1986; Schweitzer & Sulzer-Azaroff, 1988, 1995; Tripp & Alsop, 1999).
Process theories of temporal discounting propose a dual systems model of decision-making to begin to capture the many influences on relative preferences for immediate reward (van den Bos & McClure, 2013). The first system is posited to be myopic in nature and is linked to positive emotional reactions to rewards. We use the term “affective” to represent this system (Loewenstein, 1996), which is thought to be subserved by brain areas including the nucleus accumbens (NAcc) in the ventral striatum, the ventromedial prefrontal cortex, and other areas involved in evaluating rewards (Kable & Glimcher, 2007; McClure et al., 2007; McClure, Laibson, Loewenstein, & Cohen, 2004). These brain reward regions have been linked to affective responses (Knutson & Greer, 2008; Panksepp, 2004), and are thought to signal reward value in a stereotyped manner acquired through associative learning (Daw, Niv, & Dayan, 2005; Schultz, Dayan, & Montague, 1997). The second process is hypothesized to be far sighted in nature, slow and rule-based in response, but flexible enough to adaptively control behavior. We refer to this as the “deliberative” system. It is thought to be subserved by the dorsolateral prefrontal cortex (dlPFC) and posterior parietal cortex (pPC; McClure et al., 2007; 2004).
Here we explore a novel effect on temporal discounting that appears to arise from differences in affective responses to reward prospects. The effect results from changing a seemingly innocuous feature of offered monetary rewards. Specifically, within-subject discount rates differ when choices are constructed from monetary rewards with rounded decimal values (e.g., $25.00) or numbers with non-zero decimal value (e.g., $25.12). Individuals tend to choose more impulsively when the choice is constituted of monetary rewards that are rounded numbers. We refer to this as the decimal effect. As rounded decimal amounts ($25.00) are more common in daily experience than are non-zero decimal values ($25.12; with .99 a possible exception), we speculate that this effect may result from greater familiarity and hence perceptual fluency with rounded dollar values (cf. Alter & Oppenheimer, 2006; Oppenheimer & Frank, 2008). Our primary aim is to provide a process account of the decimal effect. Based on data from several experiments, we will argue that non-zero decimal values in monetary rewards influence affective responses to the rewards and consequently influence how individuals trade off present for delayed rewards.
Our first study, Experiment 1, demonstrates the decimal effect. In Experiment 2 we show behavioral evidence that the decimal effect is related to increased positive affect to rounded monetary rewards. In Experiment 3 we provide fMRI evidence to support our main conclusions. In Experiment 4 we provide an extension of the decimal effect, testing whether rounded values have the ability to increase the value of delayed rewards. Our final study, Experiment 5, examines the decimal effect across a wide developmental period between typically developing controls and participants with ADHD.
Experiment 1
Affective processes may signal value in an automatic, stereotyped manner that is slowly acquired through experience. We hypothesized that differential experience with monetary rewards with rounded values relative to non-zero decimal values may bias how the rewards are processed by facilitating automatic responses and consequently influencing intertemporal preferences (Butterworth, 1999). We tested this prediction in our first experiment.
Method
Participants
We recruited 28 participants; 12 at Stanford University (8 men, mean age=20.26, range=18–22 years) and 16 from Baylor College of Medicine and the greater Houston area community (10 men, mean age=26.38, range=20–36 years). (See Table 1 for Inclusion/Exclusion criteria for all studies and Table 2 for demographic data for Experiments 1 to 4). We excluded one participant from each site because they failed to submit choices on all trials. Participants from Baylor College of Medicine completed the task while undergoing fMRI scanning (see Experiment 3).
Table 1.
Inclusion/Exclusion Criteria for all Experiments
| group | Expts 1 to 4 |
|---|---|
| HC | Inclusion Criteria |
| age 18 to 50 | |
| HC | Exclusion Criteria |
| clinical history of neurological, major medical or psychiatric disorder | |
| *fMRI contraindications | |
|
Expt 5 |
|
| Inclusion Criteria | |
| HC & ADHD | age 12 to 30 |
| IQ over 80 as per WASI | |
| HC | T score of 60 or lower on the total DSM Total ADHD score |
| HC | 3 or more inattentive and 3 or more hyperactive/impuslive DSM symtoms |
| ADHD | T score of 65 or higher on the total DSM Total ADHD score |
| ADHD | 6 or more inattentive and 6 or more hyperactive/impulsive DSM symtoms |
| significant symptoms before age 7 and across at | |
| ADHD | least two domains (e.g. home and school/work) |
| Exclusion Criteria | |
| HC & ADHD | Any Axis 1 disorder except for ADHD in the ADHD group |
| HC & ADHD | clinical history of neurological, major medical or psychiatric disorder |
| HC | history of treatment with psychoactive medication |
| HC* | fMRI contraindications |
Note: HC healthy control;
exclusion for Exp 3
Table 2.
Demographic and Clinical Characteristics for Participants in Experiments 1 to 4
| Expt | group | age | age range | gender | n |
|---|---|---|---|---|---|
| 1 | HC | 22.4 | 18–36 | 17m | 42 |
| 2 | HC | 29.5 | 19–50 | 19m | 40 |
| 3 | HC | 26.1 | 20–36 | 9m | 16 |
| 4 | HC | 35.5 | 19–45 | 92m | 183 |
Note: Data are summarized as mean for the continuous variables.
Material
Each participant was presented with 62 intertemporal choices offering an immediate reward and a larger but delayed reward. For half of the choice trials, rewards had rounded decimal values (e.g., $11.00 today or $21.00 in six weeks; rounded condition). The other half had only non-zero decimal values (e.g. $10.87 today or $20.74 in six weeks; decimal condition). We omitted decimal values of .25, .50, .75, and .99 as these are common numbers and may have intermediate effects between our rounded and non-zero decimal values. Trials were presented in random order.
The choice trials were derived from the hyperbolic discounting function (Mazur, 1987) that models subjective value as a function of delay according to the function:
| (1) |
where r is the magnitude of the reward, d is the delay until receipt, and V is the discounted value. For each trial, a unique discount rate, keq, implies indifference between the immediate reward and the discounted, delayed reward. Choices were constructed so that each trial in the rounded condition matched a trial in the decimal condition with an equal discount rate (keq) and delay. For the rounded value rewards, magnitudes spanned a range of $2 to $33; non-zero decimal values ranged from $2.14 to $32.90. Delayed rewards were available between 7 and 56 days in the future (in 7 day increments). Reward magnitudes could not be exactly equated thus half of the decimal values were slightly larger and the other half slightly smaller than their rounded pairs. As it was not possible to make the average magnitudes exactly the same, decimal values were on average 18¢ (±$1.33) smaller than rounded values. This design ensured that both conditions spanned the same range of intertemporal trade-offs, while controlling for any bias due to differences in reward magnitude (Thaler, 1981).
Procedure
Participants had unlimited time on each trial to make their choice. A 2,000 ms blank inter-trial interval (ITI) was used (see Fig. 1A). The 62 trials were split into four blocks of either 15 or 16 trials, with one 15 trial and one 16 trial block for both the rounded and decimal conditions. Block order was counterbalanced according to condition, with half of participants beginning with rounded and ending with decimal trials. Trial order within each block was randomly generated.
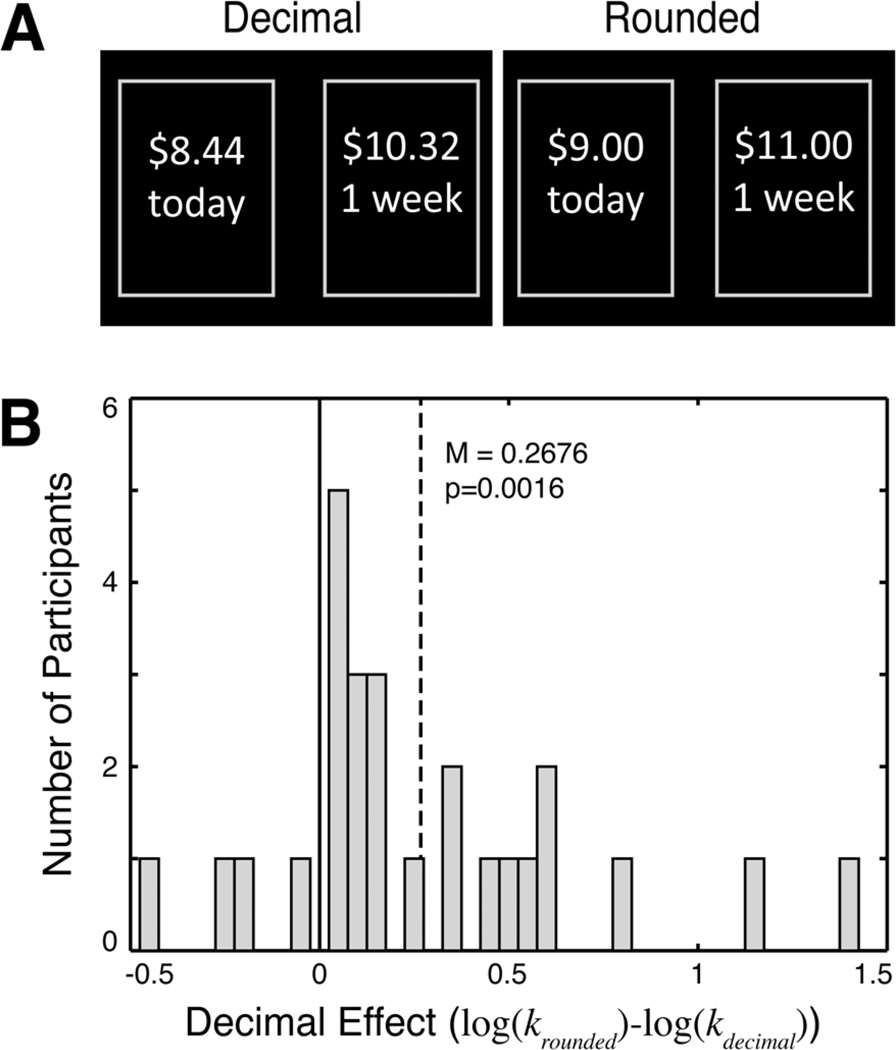
Figure 1.
(A) Intertemporal choices for monetary outcomes with non-zero and rounded decimal values elicit different temporal discount rates. (B) Discount rates are consistently higher for rounded dollar values across subjects, producing a robust mean decimal effect.
We used a lottery system in which one of the subject’s choices was randomly selected and paid to the subject according to the amount and delay of the selected choice. Subjects were instructed to consider each choice seriously as any one could potentially be paid according to their selection. This encouraged subjects to remain focused throughout the experiment and to treat all trials as equally determinant of their overall earnings.
Estimation of discount rates
For each subject and condition, discount rates were estimated by maximum likelihood. Participants’ binary choices between the immediate and delayed rewards were modeled with the exponential version of the Luce choice model (Luce, 2005). If we summarize the subjective value of the two alternatives as V1 and V2 for the immediate and delayed rewards, respectively, then the probability of choosing the immediate outcome for an arbitrary k is given by:
| (2) |
where VΔ(k)is the difference V1-V2 for some value of k. Likewise, the probability of choosing the delayed outcome is equal to 1-P(Choose V1). The parameter m captures how consistent choices are with the fitted discount function.
The likelihood of any set of choices per subject is the product of the probability for each observed choice. For each condition (c), we form the likelihood function:
| (3) |
where J=1 if the immediate reward is chosen and zero otherwise. We maximized Equation 3 with respect to k and m using a simulated annealing optimization algorithm. This yields condition-specific estimates for k and m. The standard errors of the estimates were obtained by invoking the asymptotic normality of the maximum likelihood estimators.
Results
Choices revealed the decimal effect: subjects made more impulsive decisions in the rounded relative to the decimal condition. We performed analyses on log-transformed discount rates using non-parametric tests since the distributions of log(k) were non-normal (Kolmogorov-Smirnov tests, p<0.001 for both decimal and rounded conditions). The decimal effect held among 22 of our 26 subjects (see Fig. 1B). Moreover, discount rates in both the decimal and rounded conditions were not significantly different across participants recruited from Stanford University and Baylor College of Medicine (Wilcoxon rank sum test; p>0.24 comparing discount rates in rounded and decimal conditions). We therefore analyzed data collectively across these two groups. Comparing the estimated discount rates across conditions within subjects, the mean of the differences between the log-discount rates in the rounded versus decimal conditions is positive (0.27) and significantly different from zero (sign test, p< 0.001).
We ruled out two potential confounds associated with the decimal effect. First, we found no difference in reaction time (RT) between the two conditions (mean RT rounded=3273.04 ms; mean RT decimal=3088.59; mean rounded - decimal = 184.45 ms, S.E. 138.59, t(25)=1.28, p>0.20). Second, choice consistency was not influenced by task condition. Comparing m values indicated no significant difference (Wilcoxon signed rank test p=0.67). Likewise, fitted k values predicted an average of 90.12% and 88.34% of choices in the decimal and rounded conditions, respectively (Wilcoxon signed rank test, p=0.17).
Reward magnitude is also known to influence discount rates (e.g., Thaler, 1981). To rule out an influence of magnitude on our results, we split choices (by median) into low and high magnitude trials, collapsing across decimal conditions. We then estimated k separately for low and high magnitude choices per subject. We performed a sign test on the difference in log(k) values across magnitudes and found no significant difference (p=0.33).
Discussion
Consistent with our hypothesis, we found that the nature of the decimal values in monetary rewards influenced intertemporal preferences. We suggested that monetary rewards containing rounded values would be more perceptually fluent and therefore trigger affective valuation processes to a greater degree than would non-zero decimal values. As affective processes are thought to be myopic in nature (Loewenstein, 1996), this would account for our observed differences in discount rates.
Experiment 2
Experiment 2 tested the hypothesis that rounded dollar values differ from nonzero decimal values on the basis of affective response. We primed affective processes by asking subjects to rate their emotional reaction (Hsee & Rottenstreich, 2004) to the prospect of winning different amounts of money to determine how rounded and non-rounded monetary rewards are evaluated using emotionally based valuation. We manipulated decimal values while holding magnitude comparable. We hypothesized that if valuation of round numbers involves more affective processing, round numbers would generate greater positive affect than comparable non-zero decimal numbers. The alternative hypothesis is that affective processes are unaffected by decimal value, in which case affect ratings between rounded and non-zero decimal values should not differ.
Method
Participants
A total of 54 volunteers were recruited (25 men; mean age=28.8 years) from the Stanford community and gave written informed consent to participate. Due to a technical error in conducting the experiment, 14 subjects did not complete all of the ratings and thus were excluded, leaving 40 participants for analyses.
Material and procedure
In accordance with the two-dimensional affective circumplex model of emotion (Watson & Tellegen, 1985; Watson, Wiese, Vaidy, & Tellegen, 1999), we separately assessed valence and arousal to measure the subjective emotional impact of rounded versus decimal monetary rewards. Participants received an online questionnaire asking them to make subjective assessments of 10 monetary rewards, five rounded and five with non-zero decimal values. Each rounded reward was matched to a decimal reward; in each pair the rounded number had a smaller objective value. Each of the ten numbers was presented in a random order and participants were asked the following questions:
“Imagine you have the chance to win $25.00. How Positive or Negative would you feel? How Activated/Aroused would you feel?”
Participants answered the questions using sliding scales numbered from 0 to 100 and anchored to 50 on presentation of the question.
Results
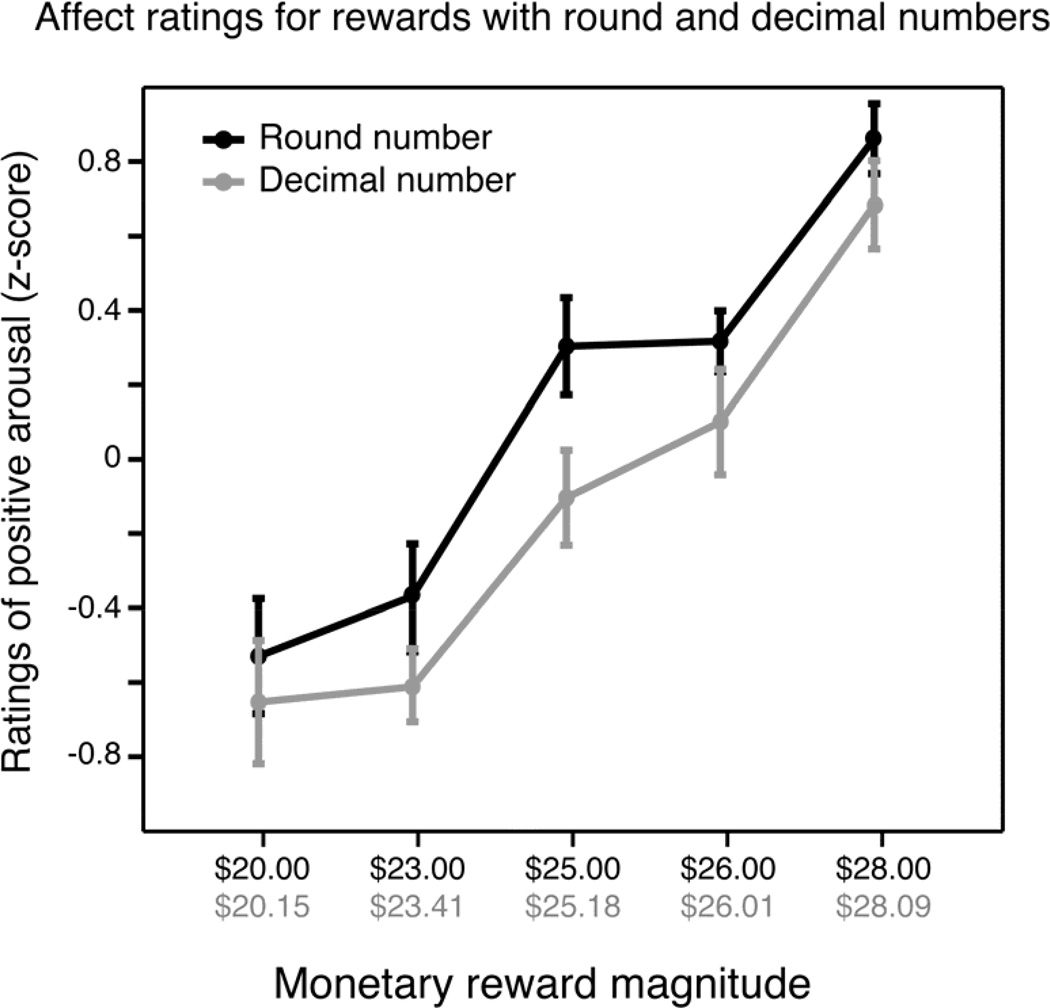
As valence (v) and arousal (a) ratings were significantly correlated in our data (r2=0.54; p<0.0001) we combined these measures on a single dimension of positive arousal as our primary variable of interest (; based on Knutson & Greer, 2008). A two-way, within-subjects ANOVA was conducted to compare the main effects of (1) condition (rounded vs. decimal values) and (2) reward magnitude for participants’ affect ratings for rewards. We found greater PA for rounded values with a significant main effect of condition (F(1,38)=5.48, p=0.03). We also found a significant main effect of reward magnitude on PA ratings (F(4,38)=29.82, p<0.001), with larger values eliciting more positive ratings. These results are shown in Figure 2, where we have plotted normalized ratings (z-score corrected within subject across conditions) as a function of reward amount. The interaction between condition and reward magnitude was not significant (p =0.18).
Figure 2.
Positive arousal reported for the prospect of earning a rounded dollar amount was larger than that reported for non-zero decimal values or marginally greater objective value. Data have been normalized within subjects (z-score transformed); error bars are standard errors of the mean.
Similar results held when valence or arousal were analyzed using similar ANOVAs. For valence there was a main effect of amount (F(4,38)=30.50; p<0.001) and condition (F(1,38)=4.98; p=0.03), but no significant interaction (p=0.38). For arousal, there was a main effect of amount (F(4,38)=23.96; p<0.001) and a trend for condition (F(1,38)=3.43; p=0.07) with no significant interaction (p=0.23).
Because of the large age range in our participants, we conducted additional ANOVA analyses looking for a main effect of age (split into quartiles) or an age × reward magnitude interaction. We found no significant differences on the basis of participants’ age (p>0.46 for both analyses).
Discussion
These results suggest that participants feel more positive arousal for monetary rewards with rounded compared to those with non-zero decimal values. Not surprisingly, they also reported feeling more positive arousal for greater magnitudes of monetary rewards. Importantly, this differential affective response overcomes the fact that rounded values were smaller in objective value.
Experiment 3
Properties linked to affective and deliberative processes distinguish the functions of the NAcc and dlPFC in intertemporal choice (McClure et al., 2004; Peters & Büchel, 2011). Affective responses to rewards and related NAcc activity, predict individual discount rates (Hariri et al., 2006). Cognitive ability correlates with dlPFC activity and lower discount rates (Shamosh et al., 2008; Shamosh & Gray, 2008). Furthermore, manipulating these systems either pharmacologically (Pine, Shiner, Seymour, & Dolan, 2010) or by direct stimulation (Figner et al., 2010) alters discount rates in the expected directions. In this study we measure correlates of affective and deliberative processing while subjects make intertemporal choices containing rounded or non-zero decimal values. Given the results from Experiment 2, we conjectured that rounded values would more effectively recruit the NAcc than would non-zero decimal values. fMRI also allows us to test whether rounded and decimal values differentially recruit deliberative processes by measuring activity in the dlPFC and pPC.
Method
Participants
Out of 28 participants in Experiment 1, the 16 participants from Baylor College of Medicine performed the task while undergoing fMRI scanning. The two subjects excluded from the analysis in Experiment 1 were from this group of 16.
Materials and procedure: behavioral task
Experimental materials and procedures were similar to Experiment 1, except that a 12 second ITI was included to accommodate the BOLD signal. Participants were paid as in Experiment 1 plus $20 base pay for the fMRI.
fMRI study procedure
Brain images were acquired using a 3 Tesla Siemens Trio MR Scanner at Baylor College of Medicine. A high-resolution (1×1×1 mm3) T1-weighted anatomical image was first acquired. For functional images, T2-weighted echo planar images (EPI) were acquired (TR = 2s, TE = 30ms, flip angle = 90°; data acquired approx. 30° off the AC-PC line, 37 slices with 2mm gap, 64×64 matrix, 3.0 mm3 isotropic voxels). Data preprocessing and linear regressions were conducted with SPM5. Region of interest (ROI) analysis was performed with AFNI using spherical masks of 12 mm diameter. Preprocessing included slice-time correction, realignment, spatial normalization, and smoothing with an 8 mm full-width at half maximum Gaussian kernel. Volumes were normalized to the Montreal Neurological Institute template, and re-sampled at 4×4×4 mm3 isotropic resolution.
Whole-brain GLM analyses fit hemodynamic responses with a boxcar activation function with RT indicating trial duration and onset given by choice presentation onset. Differences in RTs across choices were thus explicitly modeled. Movement parameters were modeled as covariates of no interest.
Results
Given that trials in the two conditions were paired, a subtraction of the mean brain response across the two conditions reveals the difference in brain activity in rounded versus decimal value choices. One confound with this subtraction is that choices themselves are different and may affect brain activity. We controlled for choice in two ways. Linear models were fit with a nuisance regressor that indicated the choice outcome (immediate or delayed reward). We also conducted hierarchical analyses where a linear model was first fit for choice outcome alone. The fitted choice-related responses were then subtracted from the original data and the residual signals were subjected to a linear model to fit average responses in rounded and decimal trials. Because the two approaches yielded qualitatively identical results, we only present the results with choice included as a nuisance regressor in this discussion.
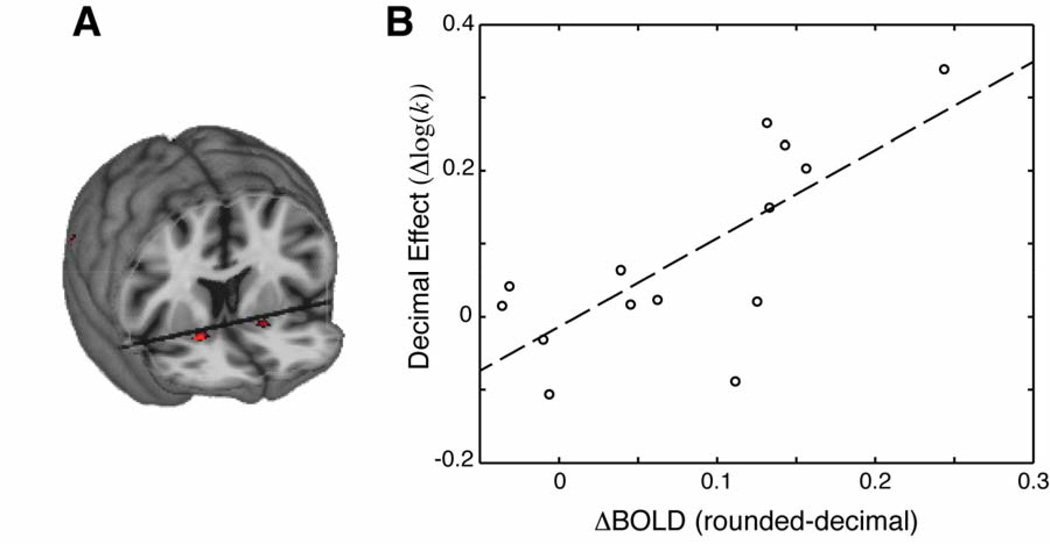
Two separate analyses were conducted to examine the effect of non-zero decimal versus rounded numbers on brain activity in intertemporal choice. First, an omnibus GLM analysis was performed on the whole brain, co-registered data. This analysis revealed three brain regions that had significantly greater activity in the rounded relative to the decimal condition (p<0.05, corrected for multiple comparisons by FDR). We omit from further discussion one region identified in the right ventrolateral temporal lobe that has not previously been associated with reward processing (peak MNI coordinates −60, −56, −4). The other two regions were in the left and right NAcc (Fig. 3A; 20, 10, −12 and −16, 10, −12, respectively).
Figure 3.
(A) Whole brain analyses indicate that, on average, the NAcc (bilaterally) is more activated as participants make intertemporal choices with rounded values compared to choices with non-zero decimal values. (B) The NAcc was identified in individual subjects using anatomical MRI images. Mean event-related responses in the bilateral NAcc correlated with the size of the decimal effect across individuals (Δlog(k) = log(kround)-log(kdecimal)).
Second, we created individual masks on non-normalized data to select the bilateral NAcc and directly analyzed the average activity within this anatomical region. The ROI analysis from subject-specific NAcc confirmed the results of the whole-brain analysis. The difference in NAcc activity measured across subjects correlated with the size of the behavioral decimal effect (i.e. Δlog(k) = log(kround)-log(kdecimal)). Subjects with greater NAcc activity in the rounded compared to the decimal condition showed larger increases in discounting rates in the rounded compared to the decimal condition (see Fig. 3B; r=0.74; p=0.002). The result holds when omitting the outlier subject at the top-right of the plot (r=0.62; p=0.02) and when performing a robust regression (p=0.002).
Finally, we had an a priori interest in the dlPFC and pPC given previous work (Figner et al., 2010; Hare, Camerer, & Rangel, 2009; McClure et al., 2007; McClure et al., 2004). No regions in either the dlPFC or pPC were significant in our whole brain analyses, even at the liberal threshold of p<0.1. We therefore specifically looked at average activity in ROIs of 12 mm diameter spheres based on regions identified in previous studies (dlPFC: McClure et al., 2004; 44, 44, 16, and Hare et al., 2009; −48, 15, 24; pPC: McClure et al., 2004; −8, −28, 32). There were no significant differences in rounded minus decimal values for any of these locations (dlPFC: p=0.45 and 0.37, respectively; pPC: p=0.55). Furthermore, the trend was for greater dlPFC and pPC activation for choices involving rounded numbers whereas the prediction from behavior would be less activation for rounded compared with non-zero decimal values.
Finally, other brain areas were of a priori interest because they have been implicated in reward processing in other studies. Thus ROI analyses were conducted on the ventromedial PFC (vmPFC), amygdala, and hippocampus. The vmPFC is commonly identified in fMRI studies of temporal discounting (see Peters & Büchel, 2010, for review; ROIs from McClure et al., 2004, 0, 44, 12; Hare et al., 2009, 3, 36, −12)). Likewise, the amygdala has been implicated in reward processing (ROI from Knutson et al., 2001) and the hippocampus is implicated in evaluating stimuli (ROI from Wimmer & Shohamy, 2012). We found no significant difference between conditions at either of the vmPFC locations (p>0.35 for responses averaged over 12 mm diameter spheres centered at the indicated locations). Similarly we found no significant differences in the hippocampus (p=0.22) or in the amygdala (p=0.31). In these latter two regions the trend was toward greater activity for choices involving decimal values relative to rounded values, contrary to our findings for the ventral striatum.
Discussion
Our prediction from examining choices between monetary outcomes was that intertemporal choices with rounded values would preferentially recruit brain reward areas, particularly the NAcc. This prediction was supported by the further finding that the degree of activity in the NAcc correlated with individual differences in the decimal effect.
“Affective” and “deliberative” modes of valuation are constructs intended to capture aspects of behavior. While there is certainly a link between the properties of these constructs and the function of the NAcc, dlPFC, and pPC, there are substantial differences as well (van den Bos & McClure, 2013). Nonetheless, fMRI allowed us to test for differential involvement of functionally disparate brain systems during intertemporal choices. We confirmed that the affect-related NAcc is differentially recruited during presentation of rounded values. Further, we find no evidence of differential recruitment of brain areas associated with deliberative processes. Conclusions from this latter finding should be tempered by acknowledging limited power (especially when asserting a null hypothesis); fMRI has relatively low signal-to-noise ratio. Additionally, the dlPFC and pPC are large brain regions whose organization is not well understood. We found no difference in activity in either of these cortical areas even at very liberal statistical thresholds, but additional work is necessary to confirm this finding.
The vmPFC may integrate multiple influences contributing to total subjective value (Rangel & Hare, 2010). The vmPFC receives (primarily indirect) inputs from both the NAcc and dlPFC (Hare et al., 2009) and activity in the vmPFC correlates with time-discounted value (Kable & Glimcher, 2007). Here, the vmPFC displayed a subtle dependence on rounded values in the subgenual cingulate cortex near that area associated with subjective value (Rangel & Hare, 2010). However, the effect in the vmPFC was notably weaker than in the NAcc, suggesting that the decimal value influences temporal discounting by influencing the type of primary motivations represented in the NAcc.
Experiment 4
In Experiment 1 we demonstrated that subjects were more likely to choose a larger delayed over a smaller sooner reward, when presented with non-zero decimal values. In Experiment 2 we established that participants did not feel as positively aroused to non-zero decimal values compared with rounded values. Therefore, it may be that the decimal effect arises from preferential affective responses to monetary rewards with rounded values. This effect may act in concert with the myopia generally assumed for the affective system in intertemporal choice to increase discount rates. The NAcc is preferentially activated by immediate rewards, but also maintains some response to delayed outcomes (Kable & Glimcher, 2007; McClure et al., 2004). Similarly, emotional responses are generally far greater to immediate over delayed outcomes (Loewenstein, 1996), but delayed rewards still induce positive affect. This raises the question of whether coupling rounded values to delayed rewards can enhance an otherwise diminished affective response to the benefit of more far-sighted decision-making. In Experiment 4, we test this idea by crossing decimal value (rounded vs. non-zero decimal) with time (immediate vs. delayed).
Method
Participants
We recruited a total of 200 participants using Amazon’s Mechanical Turk. Participants were restricted to be native English speakers and to reside in the United States. We obtained informed consent before participants completed the task. We excluded 17 subjects because they selected all smaller, sooner or larger, later choices. This left 183 eligible subjects (92 men; mean age = 35.52 years). Participants were randomly assigned to one of two conditions, the rounded-immediate (n=91) or the rounded-delayed condition (n=92).
Material and procedure
All subjects completed two temporal discounting questionnaires, presented via computer, with hypothetical reward choices. Each question offered a choice between a particular amount of money today and a larger amount of money after a certain number of days. Subjects were instructed to evaluate the questions as if they would actually receive the amount of money at the time specified in the choice. However, the choices were hypothetical in nature and did not influence payments. All subjects completed the same control questionnaire, which consisted of the same choices as constituted the nonzero decimal choices in Experiment 1. Subjects completed a second 31-item temporal discounting questionnaire that followed the same structure but differed slightly based on experimental condition. In the rounded-immediate condition, all of the monetary rewards offered today were round numbers (ranging from $2.00-$31.00), while the monetary rewards offered later had non-zero decimal values (ranging from $2.97-$38.34). In the rounded-delayed condition, all of the monetary rewards offered later were round numbers (ranging from $2.00 to $32.00), while the monetary values offered immediately had nonzero decimal values (ranging from $1.34 to $31.09). As in Experiment 1, values for immediate amounts, delayed amounts, and delay length were calculated according to Equation 1 to be matched between conditions on discounting rate, keq, and to share similar reward magnitudes and delays. Delay lengths ranged from 7 to 56 days as in Experiment 1. The order of control and experimental questionnaires was counterbalanced between subjects for both conditions and trials were presented in random order. Measures of temporal discounting were calculated by maximum likelihood as described for Experiment 1.
Results
Our dependent measure was the difference in the log-discount rates across experimental and control conditions. As the log-transformed values were not normally distributed (Kolmogorov-Smirnov test for normality; p<0.05 for both conditions), we performed non-parametric Wilcoxon signed rank tests. These analyses replicated our previous finding that immediately available rounded values increase discount rates (p=0.008; mean RT control=3075.3 ms; mean RT rounded 2735.2 ms; mean rounded – control 340.1 ms, SE. 383.3 ms). However, we find no change in discounting with rounded-delayed outcomes (p=0.90; mean RT control=2737.3 ms; mean RT rounded 2684.6 ms; mean rounded – control 52.7 ms, SE. 116.0 ms). A two-sided rank sum test indicates that the effect on discount rates was moderately greater for the rounded-immediate than the rounded-delayed condition (p=0.06). There was no difference in choice consistency across rounded-immediate and rounded-delayed conditions (rank sum test of m value estimates across rounded and control conditions, p=0.54).
Discussion
This experiment demonstrates a close coupling between the influence of the affective impact of rewards on temporal discounting and immediacy. In particular, we find that changing decimal values only impacts intertemporal preferences when the rounded value is available immediately. It is certainly possible that decimal value may influence the evaluation of delayed rewards and that this experiment simply suffers from lack of power. Thus, we hesitate to conclude that rounded decimal values have no effect on delayed rewards – but instead believe that rounded values preferentially impact the evaluation of immediate outcomes.
Experiment 5
Temporal discounting is tempered by individual and external contextual factors (Peters & Büchel, 2011; van den Bos & McClure, 2013). Individual factors that predict differences in behavior include age and the symptom domain of hyperactivity/impulsivity (Scheres & Hamaker, 2010; Scheres, Lee, & Sumiya, 2008; Scheres, Tontsch, Thoeny, & Kaczkurkin, 2010; Thorell, 2007). However, developmental findings in temporal discounting are inconsistent (Christakou, Brammer, & Rubia, 2011; Prencipe et al., 2010), perhaps because the age ranges studied tend to be wide and/or they do not systematically assess other contextual factors. Differential maturation rates of brain systems underlying decision-making may underlie changing self-control across lifespan. Some of these regions (e.g., NAcc, vmPFC and dlPFC) have also been linked to ADHD impairment (Costa Dias et al., 2013; Dickstein, Bannon, Castellanos, & Milham, 2006; Scheres, Milham, Knutson, & Castellanos, 2007). In this final experiment, we examined self-control across a crucial time of brain development where there are greater expectations for self-management (12 to 30 years). We hypothesized that decimal values would affect self-control choices in both control and ADHD groups. Moreover, we predicted that younger children, in general, would display less self-control, reflected by a greater tendency to select the smaller, sooner rewards, than would older participants.
Method
Participants
A group of 40 typically developing individuals and a group of 25 individuals diagnosed with ADHD, Combined Type (i.e., significant symptoms of inattention and hyperactivity/impulsivity) were recruited through the UC Davis MIND Institute. All participants gave written informed consent or verbal assent in addition to written consent from a parent or guardian in the case of minors (see Table 3 for demographic and clinical information). We included 12 years old as our minimum age because children younger than 12 are less likely to be able to fully appreciate monetary value and conceptualize the temporal delays presented within the paradigm. Participants were randomly assigned to one of two presentation orders, the rounded condition first (n=31) or the decimal condition first (n=34).
Table 3.
Demographic and Clinical Characteristics for Participants in Experiment 5
| ADHD (n = 25) |
Healthy Controls (n = 40) |
Total (n = 65) |
|
|---|---|---|---|
| Demographic Characteristics | |||
| Gender | |||
| Female | 13 (52%) | 17 (43%) | 30 (46%) |
| Male | 12 (48%) | 23 (58%) | 35 (54%) |
| Age | 18.6 (5.7) | 17.6 (4.1) | 18.0 (4.8) |
| Age Range | 12–30 | 12–28 | 12–30 |
| Clinical Characteristics | |||
| FSIQ1 | 115.2 (14.3) | 117.3 (11.1) | 116.4 (12.4) |
| Letter-Word Identification Score1 | 109.0 (12.1) | 110.6 (9.0) | 110.0 (10.3) |
| Math Calculation Score1 | 110.2 (12.5)* | 117.0 (12.6)* | 114.3 (12.9) |
| DSM Inattention Subscale Score2 | 79.3 (12.7)* | 45.6 (6.4)* | 58.8 (19.0) |
| DSM Hyperactive-Impulsive Subscale2 | 79.7 (12.8)* | 45.3 (4.2)* | 58.7 (18.9) |
Note: Data are summarized as mean (SD) for the continuous variables and frequency (%) for gender. FSIQ = Full Scale Wechsler Abbreviated Scale of Intelligence
Frequency missing in healthy control group = 2
Frequency missing in healthy control group = 1
Wilcoxon two-sample test p < 0.05
Material and procedure
A similar set of intertemporal choices was presented to subjects as in Experiment 1. As real rather than hypothetical rewards are thought to pose more of a challenge to self-control in ADHD (Scheres et al., 2008), we employed a lottery system as in Experiment 1. Each individual’s discount factor, k, was calculated as outlined above. Statistical analyses employed mixed effect models implemented in SAS Version 9.3. (using PROC MIXED), because they accounted for the correlated structure of the data due to repeated measures of delay discounting within subject (i.e., rounded and decimal trial types). This approach accommodated 3 instances of missing data (data excluded due to participants uniformly choosing either the immediate or delayed rewards). The core model predicting k included main effects for group (ADHD and control), condition (rounded and non-zero decimal), terms for age and gender, and a random effect for individual. Model assumptions were validated both graphically and analytically.
Results
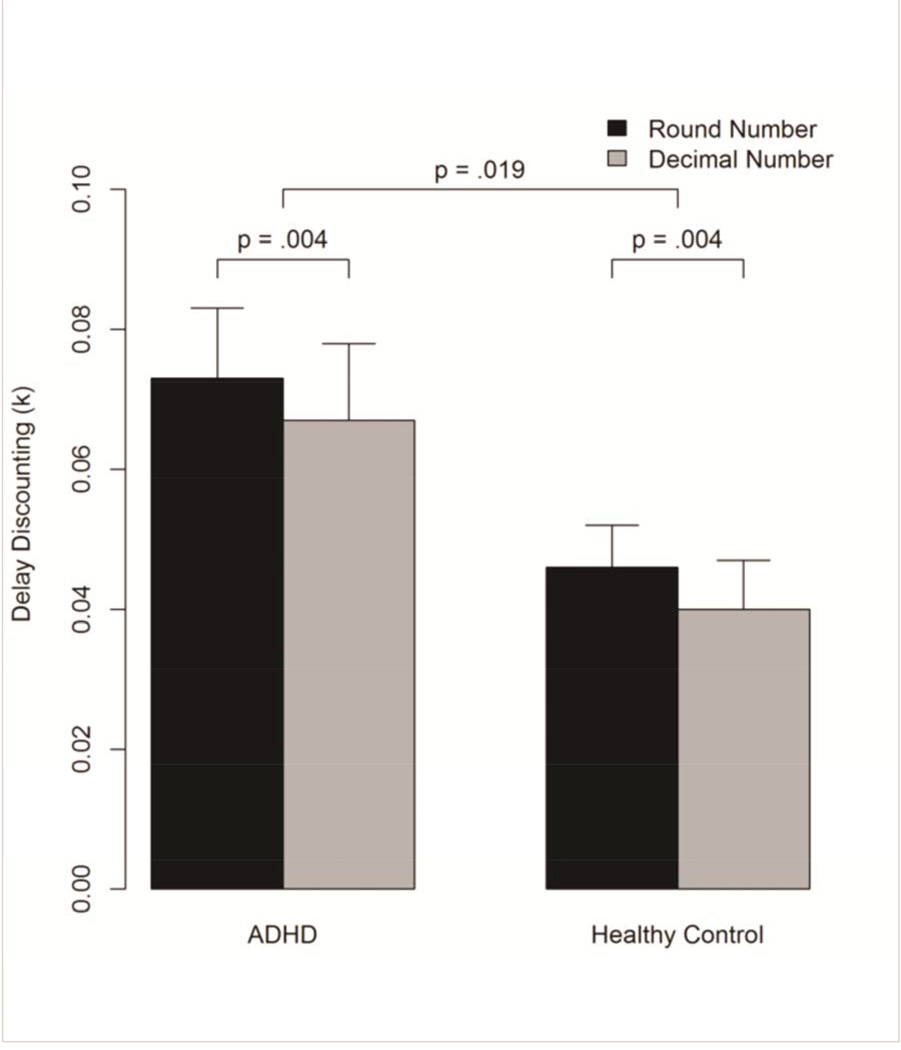
The analysis revealed a main effect of group (F(1,41.61)=5.99, p=0.02) with the ADHD group showing significantly greater discount rates (k) than the control group. There was also a main effect of condition (F(1,60.52)=8.82, p=0.004), with participants displaying the decimal effect (greater impulsivity in the rounded condition; see Fig. 4 and Table 4). As predicted, age was also significantly related to delay discounting, with younger age associated with larger discount rates (F(1,60.12)=5.17, p=0.03). There was neither a significant effect of gender on discount rates (F(1,57.45)=2.02, p=0.16) nor a significant group × condition interaction (p>0.7).
Figure 4.
Rates of impulsive decision-making (k) on a delay-discounting task using real rewards are displayed for individuals with ADHD and typically developing controls across two different conditions. In both conditions, subjects were presented with choices between a relatively small immediate monetary reward or a larger, delayed monetary reward. In the round number condition, monetary values were presented as a dollar amount only (e.g., $5.00) whereas in the decimal number condition these values were presented as dollars and cents (e.g., $5.03). The ADHD group made more impulsive choices than the typically developing control group overall, in that they chose the immediate reward over the larger, delayed reward more often. Introduction of the decimal condition reduced impulsivity in both the ADHD and control groups, meaning that in both groups, individuals tended to choose the larger, delayed reward more often when the amount was presented as dollars and cents rather than simply in dollars alone.
Table 4.
Summary of mixed effects model examining the relationship of group, condition, age, and gender to delay discounting
| Estimate (SE) | p- value | |
|---|---|---|
| Model term | ||
| Intercept | 0.054 (0.008) | < 0.001 |
| Group (ADHD) | 0.028 (0.011) | 0.019 |
| Condition (Non-zero decimal) | −0.009 (0.003) | 0.004 |
| Age | −0.003 (0.001) | 0.027 |
| Gender (Female) | −0.015 (0.011) | 0.161 |
Discussion
These results replicate our main finding that decimal values influence discount rates – even in those with elevated levels of impulsivity, such as ADHD. The tendency to favor immediately available rewards plays a central role in the delay aversion theory (Sonuga-Barke, Taylor, Sembi, & Smith, 1992) and the steeper and shorter delay-of-gratification gradient theory of ADHD (Sagvolden, Aase, Zeiner, & Berger, 1998). Our replication of the decimal effect in impulsive individuals is particularly significant for populations who display a greater tendency to select immediate rewards, such as adolescents and individuals with substance dependence (Madden & Bickel, 2009). Increased discounting is linked to poor health outcomes and reduced academic achievement and occupational success (Golsteyn, Gronqvist, & Lindahl, 2013). Attempting to improve self-control in individuals with heightened impulsivity by altering reward perception would be a novel approach for reducing the negative outcomes associated with impulsivity. Treatment of ADHD and substance use disorders currently involves contingency management in which rewards are given for appropriate behavior (e.g. Bickel, Jones, Landes, Christensen, Jackson and Mancino, 2010; Barkley, 2006). While the size and delay of the rewards are typically considered in developing a behavior plan, it has not been considered how to best frame or present rewards in these plans. Our findings suggest that future research should assess how framing effects could enhance the value of delayed rewards to increase self-control across conditions associated with impulsivity.
We also replicate the finding that younger individuals have higher discount rates than do older people, independent of the presence or absence of ADHD (Steinberg et al., 2009). Casey and colleagues (Casey, Duhoux, & Malter Cohen, 2010; Casey, Jones, & Hare, 2008) propose that an increase in risky behavior during adolescence is due to an imbalance between relatively more mature, subcortical brain systems versus less mature functioning in cortical regions linked to cognitive-control. Studies suggest impaired modulation of hyperactive reward-related striatal regions by cognitive control regions (i.e., dlPFC) in adolescence (Berns, Moore, & Capra, 2009; Christakou et al., 2011; Galvan et al., 2006; Van Leijenhorst et al., 2010). Brain regions linked to self-control and evaluation of future outcomes (Galvan et al., 2006) mature later in development (e.g., Christakou et al., 2011; Cohen et al., 2010; Olson et al., 2009). Optimal connectivity between dlPFC and other regions (pPC, vmPFC) to support more self-controlled behavior putatively occurs in adulthood (Luna, 2009). Regions such as the NAcc, which have been associated with more impulsive choices in Experiment 3, have also been consistently implicated in ADHD impairments (Hart, Radua, Nakao, Mataix-Cols, & Rubia, 2013; Scheres et al., 2007).
General Discussion
Emotional responses have long been hypothesized to underlie the short-sighted behavior evident in choices involving tempting immediate rewards (Loewenstein, 1996; Mischel, 1974). We identify a novel effect on delay discounting consistent with this assertion: subtle features of prospective rewards can change affective responses and impatience.
A large number of effects influence how intertemporal preferences are formed (van den Bos & McClure, 2013). One potential unifying framework for understanding these diverse influences may come from positing independent neuro-cognitive systems that underlie the evaluation of rewards. We refer to one common dichotomy of such systems herein as affective and deliberative. We have shown that such a framework can explain how a relatively innocuous feature of an intertemporal choice, the numbers following the decimal point, comes to influence discounting. We combined behavioral and neural measures to test how decimal values alter the affective responses that distinguish these two modes of valuation. Overall, we have established a pathway whereby properties of a reward influence consequent discount rates. Although it is possible that the decimal effect is better explained by other effects such as subtle differences in sensory processing or calculation of numerical differences between the rounded and decimal conditions, we believe this is less likely. We found no evidence in to support differences between the rounded and decimal conditions in visual or sensory brain regions nor in decision-related reaction times.
It remains to be seen whether the dual system framework will be sufficient to account for the number of factors known to influence intertemporal preferences. For example, people are more patient when the time of reward outcomes is expressed as an exact date as opposed to the duration of time from the present (Read, Frederick, Orsel, & Rahman, 2005). A recent fMRI study has shown that a similar manipulation, switching from delays to dates, modulates dlPFC activity, consistent with dual system theory (Peters & Büchel, 2010). Perhaps as interestingly, the dual system framework suggests novel effects. The idea for the decimal effect arose from considering ways in which we might modulate NAcc activity.
Positing two neuro-cognitive systems is almost certainly an oversimplification of how intertemporal preferences are actually constructed. The validity of dual system models of discounting is a source of much debate in the neuroscience literature (e.g., Hare et al., 2009; Kable & Glimcher, 2007). Nonetheless, such models have distinct advantages in accounting for numerous phenomena in delay discounting (van den Bos & McClure, 2013). One important future direction will be to relate dual system models to construal level theory (Trope & Liberman, 2003). Recent work by Fujita and colleagues has shown that priming people to think in broader, more abstract terms (high level construal) increases self-control (Fujita & Han, 2009). It is intriguing to hypothesize that thinking more abstractly depends on the dlPFC and priming this neural system increases that self-control but this is pure speculation at this point. We also acknowledge that there may be other plausible mechanisms than the dual-processing account, or the familiarity of rounded numbers, that may explain the downstream effect of an increased affective response to these rounded stimuli studied herein. However, our primary goal for this project was to document the outcome of altered affective responses. Future studies will attempt to determine the mechanism underlying the outcome.
The decimal effect also suggests one avenue for interventions aiming to ameliorate the effects of impulsivity. Our approach represents a novel attempt to shift impulsive behavior in populations associated with poor self-control by manipulating the choice context. ADHD is associated with problematic functioning in brain networks implicated in both cognitive (dlPFC/pPC) and affective/reward (vmPFC/NAcc) processes (Fassbender & Schweitzer, 2006). Despite this, attempts to modify self-control in ADHD and adolescents tend to focus on teaching deliberative strategies (Dawson & Guare, 2010). It should be possible to design choice environments in ways that decrease affective responses, reduce NAcc activity, and lead to more far-sighted choices. This suggestion is very similar to Mischel and colleagues’ demonstration that thinking of the abstract, physical qualities of a marshmallow increase ones’ ability to delay gratification and ultimately obtain more marshmallows (Mischel & Baker, 1975). The findings here suggest the neurobiological basis by which these framing effects may function. It may also be that differential neural activity relates to distinct symptom profiles in individuals with ADHD. For example, steeper discounting may be due to some combination of heightened sensitivity to immediate rewards, problems with response inhibition, or an ineffectiveness of future outcomes to influence current behavior.
Acknowledgements
The authors would like to thank all the participants and their families for their time. We would also like to thank Tadeus Hartanto, Lauren Boyle, Erin Calfee and Dorothy Yip for their assistance with data collection and Ruby Bhangoo and Murat Pakyurek for the assessment of research participants for Experiment 5. Funding was provided by NIMH 5 R01 MH066310 and NIMH R01 MH091068 (J.B.S).
References
- Ahn WY, Rass O, Fridberg DJ, Bishara AJ, Forsyth JK, Breier A, et al. Temporal discounting of rewards in patients with bipolar disorder and schizophrenia. J Abnorm Psychol. 2011;120(4):911–921. doi: 10.1037/a0023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alter AL, Oppenheimer DM. Predicting short-term stock fluctuations by using processing fluency. Proc Natl Acad Sci U S A. 2006;103(24):9369–9372. doi: 10.1073/pnas.0601071103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA. Attention-Deficit Hyperactivity Disorder. A Handbook for Diagnosis and Treatment. Third Edition. NY: The Guildford Press; 2006. [Google Scholar]
- Berns GS, Moore S, Capra CM. Adolescent engagement in dangerous behaviors is associated with increased white matter maturity of frontal cortex. PLoS One. 2009;4(8):e6773. doi: 10.1371/journal.pone.0006773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Marsch LA. Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction. 2001;96(1):73–86. doi: 10.1046/j.1360-0443.2001.961736.x. [DOI] [PubMed] [Google Scholar]
- Bickel WK, Jones BA, Landes RD, Christensen DR, Jackson L, Mancino M. Hypothetical intertemporal choice and real economic behavior: delay discounting predicts voucher redemptions during contingency-management procedures. Experimental and Clinical Psyhopharmacology. 2010;18(6):546–52. doi: 10.1037/a0021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterworth B. A head for figures. Science. 1999;284(5416):928–929. doi: 10.1126/science.284.5416.928. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Duhoux S, Malter Cohen M. Adolescence: what do transmission, transition, and translation have to do with it? Neuron. 2010;67(5):749–760. doi: 10.1016/j.neuron.2010.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LW, Szrek H, Pereira NS, Pauly MV. Time preference and its relationship with age, health, and survival probability. Judgm Decis Mak. 2009;4(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Rubia K. Maturation of limbic corticostriatal activation and connectivity associated with developmental changes in temporal discounting. Neuroimage. 2011;54(2):1344–1354. doi: 10.1016/j.neuroimage.2010.08.067. [DOI] [PubMed] [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, et al. A unique adolescent response to reward prediction errors. Nat Neurosci. 2010;13(6):669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa Dias TG, Wilson VB, Bathula DR, Iyer SP, Mills KL, Thurlow BL, et al. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol. 2013;23(1):33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw ND, Niv Y, Dayan P. Uncertainty-based competition between prefrontal and dorsolateral striatal systems for behavioral control. Nat Neurosci. 2005;8(12):1704–1711. doi: 10.1038/nn1560. [DOI] [PubMed] [Google Scholar]
- Dawson P, Guare R. Skills in children and Adolescents: A practical Guide to Assessment and Intervention. 2nd Edition. New York: Guilford Press; 2010. [Google Scholar]
- Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry. 2006;47(10):1051–1062. doi: 10.1111/j.1469-7610.2006.01671.x. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Schweitzer JB. Is there evidence for neural compensation in attention deficit hyperactivity disorder? A review of the functional neuroimaging literature. Clin Psychol Rev. 2006;26(4):445–465. doi: 10.1016/j.cpr.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Knoch D, Johnson EJ, Krosch AR, Lisanby SH, Fehr E, et al. Lateral prefrontal cortex and self-control in intertemporal choice. Nat Neurosci. 2010;13(5):538–539. doi: 10.1038/nn.2516. [DOI] [PubMed] [Google Scholar]
- Frederick S, Loewenstein G, O’Dohoghue T. Time discounting and time preference: A critical review. J Econ Lit. 2002;40(2):351–401. [Google Scholar]
- Fujita K, Han HA. Moving beyond deliberative control of impulses: the effect of construal levels on evaluative associations in self-control conflicts. Psychol Sci. 2009;20(7):799–804. doi: 10.1111/j.1467-9280.2009.02372.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golsteyn BHH, Gronqvist H, Lindahl L. Time Preferences and Lifetime Outcomes. IZA. 2013 [Google Scholar]
- Green L, Fry A, Myerson J. Discounting of delayed rewards: a life-span comparison. Psychological Science. 1994;5(1):33–36. [Google Scholar]
- Green L, Myerson J, McFadden E. Rate of temporal discounting decreases with amount of reward. Mem Cognit. 1997;25(5):715–723. doi: 10.3758/bf03211314. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(5927):646–648. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006;26(51):13213–13217. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart H, Radua J, Nakao T, Mataix-Cols D, Rubia K. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70(2):185–198. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- Heerey EA, Robinson BM, McMahon RP, Gold JM. Delay discounting in schizophrenia. Cogn Neuropsychiatry. 2007;12(3):213–221. doi: 10.1080/13546800601005900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsee CK, Rottenstreich Y. Music, pandas, and muggers: on the affective psychology of value. J Exp Psychol Gen. 2004;133(1):23–30. doi: 10.1037/0096-3445.133.1.23. [DOI] [PubMed] [Google Scholar]
- Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007;10(12):1625–1633. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philos Trans R Soc Lond B Biol Sci. 2008;363(1511):3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. The Effects of Appetitive Stimuli on Out-of-Domain Consumption Impatience. Journal of Consumer Research. 2008;34(5):649–656. [Google Scholar]
- Loewenstein G. Out of Control: Visceral Influences on Behavior. Organizational Behavior and Human Decision Processes. 1996;65(3):272–292. [Google Scholar]
- Luce RD. Individual Choice Behavior: A Theoretical Analysis (Dover Edition) 2005 [Google Scholar]
- Luna B. Developmental changes in cognitive control through adolescence. Adv Child Dev Behav. 2009;37:233–278. doi: 10.1016/s0065-2407(09)03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden GJ, Bickel WK. Impulsivity: The Behavioral and Neurological Science of Discounting. Washington, DC: APA Books; 2009. [Google Scholar]
- Madden GJ, Petry NM, Badger GJ, Bickel WK. Impulsive and self-control choices in opioid-dependent patients and non-drug-using control participants: drug and monetary rewards. Exp Clin Psychopharmacol. 1997;5(3):256–262. doi: 10.1037//1064-1297.5.3.256. [DOI] [PubMed] [Google Scholar]
- Marco R, Miranda A, Schlotz W, Melia A, Mulligan A, Muller U, et al. Delay and reward choice in ADHD: an experimental test of the role of delay aversion. Neuropsychology. 2009;23(3):367–380. doi: 10.1037/a0014914. [DOI] [PubMed] [Google Scholar]
- Mazur JE. An adjusting procedure for studying delayed reinforcement. In: Commons ML, Mazur JE, Nevin JA, Rachlin H, editors. Quantitative analysis of behavior: Vol. 5. The effect of delay and intervening events on reinforcement value. Hillsdale, NJ: Erlbaum; 1987. [Google Scholar]
- McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007;27(21):5796–5804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004;306(5695):503–507. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- Mischel W. Processes in delay of gratification. Advances in Experimental Social Psychology. 1974;7:249–292. [Google Scholar]
- Mischel W, Baker N. Cognitive appraisals and transformations in delay behavior. Journal of Personality and Social Psychology. 1975;31(2):254–261. [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: a diffusion tensor imaging study. J Cogn Neurosci. 2009;21(7):1406–1421. doi: 10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer DM, Frank MC. A rose in any other font would not smell as sweet: effects of perceptual fluency on categorization. Cognition. 2008;106(3):1178–1194. doi: 10.1016/j.cognition.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Paloyelis Y, Asherson P, Kuntsi J. Are ADHD symptoms associated with delay aversion or choice impulsivity? A general population study. J Am Acad Child Adolesc Psychiatry. 2009;48(8):837–846. doi: 10.1097/CHI.0b013e3181ab8c97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panksepp J. Affective neuroscience: the foundations of human and animal emotions. New York: Oxford University Press; 2004. [Google Scholar]
- Peters J, Büchel C. Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron. 2010;66(1):138–148. doi: 10.1016/j.neuron.2010.03.026. [DOI] [PubMed] [Google Scholar]
- Peters J, Büchel C. The neural mechanisms of inter-temporal decision-making: understanding variability. Trends Cogn Sci. 2011;15(5):227–239. doi: 10.1016/j.tics.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Pine A, Shiner T, Seymour B, Dolan RJ. Dopamine, time, and impulsivity in humans. J Neurosci. 2010;30(26):8888–8896. doi: 10.1523/JNEUROSCI.6028-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prencipe A, Kesek A, Cohen J, Lamm C, Lewis MD, Zelazo PD. Development of hot and cool executive function during the transition to adolescence. J Exp Child Psychol. 2010 doi: 10.1016/j.jecp.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Rangel A, Hare T. Neural computations associated with goal-directed choice. Curr Opin Neurobiol. 2010;20(2):262–270. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Rapport MD, Tucker SB, DuPaul GJ, Merlo M, Stoner G. Hyperactivity and frustration: the influence of control over and size of rewards in delaying gratification. J Abnorm Child Psychol. 1986;14(2):191–204. doi: 10.1007/BF00915440. [DOI] [PubMed] [Google Scholar]
- Read JP, Frederick S, Orsel B, Rahman J. Four Score and Seven Years from Now: The Date/Delay Effect in Temporal Discounting. Management Science. 2005;41(9):1326–1335. [Google Scholar]
- Reynolds B, Leraas K, Collins C, Melanko S. Delay discounting by the children of smokers and nonsmokers. Drug Alcohol Depend. 2009;99(1–3):350–353. doi: 10.1016/j.drugalcdep.2008.07.015. [DOI] [PubMed] [Google Scholar]
- Sagvolden T, Aase H, Zeiner P, Berger D. Altered reinforcement mechanisms in attention-deficit/hyperactivity disorder. Behav Brain Res. 1998;94(1):61–71. [PubMed] [Google Scholar]
- Scheres A, Hamaker EL. What we can and cannot conclude about the relationship between steep temporal reward discounting and hyperactivity-impulsivity symptoms in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2010;68(4):e17–e18. doi: 10.1016/j.biopsych.2010.05.021. [DOI] [PubMed] [Google Scholar]
- Scheres A, Lee A, Sumiya M. Temporal reward discounting and ADHD: task and symptom specific effects. J Neural Transm. 2008;115(2):221–226. doi: 10.1007/s00702-007-0813-6. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(5):720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Scheres A, Tontsch C, Thoeny AL, Kaczkurkin A. Temporal reward discounting in attention-deficit/hyperactivity disorder: the contribution of symptom domains, reward magnitude, and session length. Biol Psychiatry. 2010;67(7):641–648. doi: 10.1016/j.biopsych.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1598. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Sulzer-Azaroff B. Self-control: teaching tolerance for delay in impulsive children. J Exp Anal Behav. 1988;50(2):173–186. doi: 10.1901/jeab.1988.50-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer JB, Sulzer-Azaroff B. Self-control in boys with attention deficit hyperactivity disorder: effects of added stimulation and time. J Child Psychol Psychiatry. 1995;36(4):671–686. doi: 10.1111/j.1469-7610.1995.tb02321.x. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Deyoung CG, Green AE, Reis DL, Johnson MR, Conway AR, et al. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008;19(9):904–911. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- Shamosh NA, Gray JR. Delay Discounting and Intelligence: A meta-analysis. Intelligence. 2008;36(4):289–305. [Google Scholar]
- Sonuga-Barke EJ, Fairchild G. Neuroeconomics of attention-deficit/hyperactivity disorder: differential influences of medial, dorsal, and ventral prefrontal brain networks on suboptimal decision making? Biol Psychiatry. 2012;72(2):126–133. doi: 10.1016/j.biopsych.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Taylor E, Sembi S, Smith J. Hyperactivity and delay aversion--I. The effect of delay on choice. J Child Psychol Psychiatry. 1992;33(2):387–398. doi: 10.1111/j.1469-7610.1992.tb00874.x. [DOI] [PubMed] [Google Scholar]
- Sozou PD, Seymour RM. Augmented discounting: interaction between ageing and time-preference behaviour. Proc Biol Sci. 2003;270(1519):1047–1053. doi: 10.1098/rspb.2003.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L. A dual systems model of adolescent risk-taking. Dev Psychobiol. 2010;52(3):216–224. doi: 10.1002/dev.20445. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Graham S, O’Brien L, Woolard J, Cauffman E, Banich M. Age differences in future orientation and delay discounting. Child Dev. 2009;80(1):28–44. doi: 10.1111/j.1467-8624.2008.01244.x. [DOI] [PubMed] [Google Scholar]
- Thaler RH. Some Empirical Evidence on Dynamic Inconsistency. Economic Letters. 1981;8:201–207. [Google Scholar]
- Thorell LB. Do delay aversion and executive function deficits make distinct contributions to the functional impact of ADHD symptoms? A study of early academic skill deficits. J Child Psychol Psychiatry. 2007;48(11):1061–1070. doi: 10.1111/j.1469-7610.2007.01777.x. [DOI] [PubMed] [Google Scholar]
- Tripp G, Alsop B. Sensitivity to reward frequency in boys with attention deficit hyperactivity disorder. J Clin Child Psychol. 1999;28(3):366–375. doi: 10.1207/S15374424jccp280309. [DOI] [PubMed] [Google Scholar]
- Trope Y, Liberman N. Temporal construal. Psychol Rev. 2003;110(3):403–421. doi: 10.1037/0033-295x.110.3.403. [DOI] [PubMed] [Google Scholar]
- van den Bos W, McClure SM. Towards a general model of temporal discounting. J Exp Anal Behav. 2013;99(1):58–73. doi: 10.1002/jeab.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Watson D, Tellegen A. Toward a consensual structure of mood. Psychol Bull. 1985;98(2):219–235. doi: 10.1037//0033-2909.98.2.219. [DOI] [PubMed] [Google Scholar]
- Watson D, Wiese D, Vaidy J, Tellegen A. The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology. 1999;76(5):821–838. [Google Scholar]