Abstract
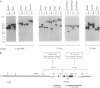
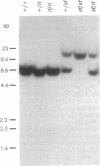
Reeler (rl) is an autosomal recessive mutation that affects migration of postmitotic neurons in the mouse central nervous system. The reeler (rl/rl) mouse displays a disruption of laminar structures in both the cerebellum and the forebrain and it exhibits tremors, dystonia, and ataxia. The molecular basis of the reeler phenotype is unknown because the gene involved has not yet been identified. We report here the isolation and characterization of an allele of rl, reelertransgene (rltg). This allele was generated by the fortuitous insertion of a transgene, supfos (sf), into the mouse rl locus. Crosses between rl/+ and rltg/+ mice yielded offspring that exhibited the reeler phenotype, indicating that rl and rltg are allelic. We cloned the genomic sequences flanking the transgene insertion site from the rltg/rltg mouse genome. Chromosomal mapping studies revealed that the 5' flanking cellular sequence maps to a locus, D5Gmr1, that lies in a region of mouse chromosome 5 that also contains the rl locus. Southern blot analysis using a probe derived from the D5Gmr1 locus revealed no gross structural rearrangement in the rl locus. Thus, unlike the two rl alleles described previously, rltg provides a molecular probe that can now be used to identify and isolate the rl gene.
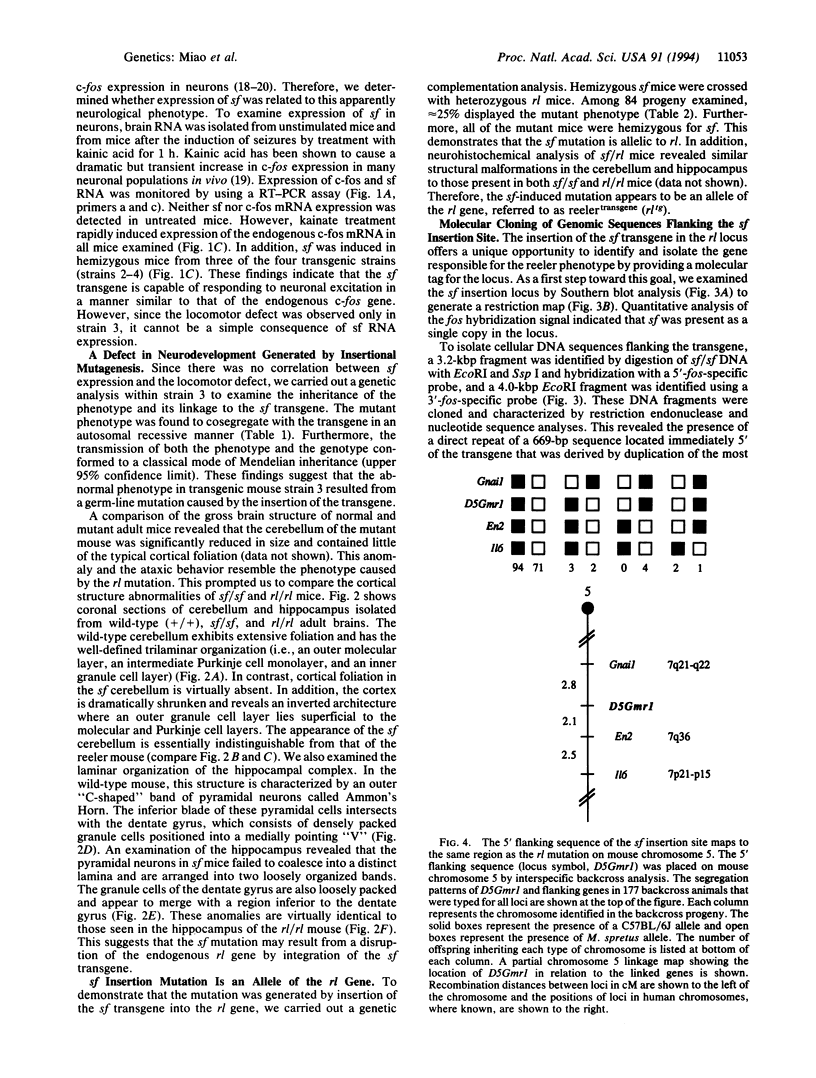
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angevine J. B., Jr, Sidman R. L. Autoradiographic study of cell migration during histogenesis of cerebral cortex in the mouse. Nature. 1961 Nov 25;192:766–768. doi: 10.1038/192766b0. [DOI] [PubMed] [Google Scholar]
- BERRY M., ROGERS A. W., EAYRS J. T. PATTERN OF CELL MIGRATION DURING CORTICAL HISTOGENESIS. Nature. 1964 Aug 8;203:591–593. doi: 10.1038/203591b0. [DOI] [PubMed] [Google Scholar]
- Caviness V. S., Jr, Rakic P. Mechanisms of cortical development: a view from mutations in mice. Annu Rev Neurosci. 1978;1:297–326. doi: 10.1146/annurev.ne.01.030178.001501. [DOI] [PubMed] [Google Scholar]
- Copeland N. G., Jenkins N. A. Development and applications of a molecular genetic linkage map of the mouse genome. Trends Genet. 1991 Apr;7(4):113–118. doi: 10.1016/0168-9525(91)90455-y. [DOI] [PubMed] [Google Scholar]
- Gadisseux J. F., Evrard P. Glial-neuronal relationship in the developing central nervous system. A histochemical-electron microscope study of radial glial cell particulate glycogen in normal and reeler mice and the human fetus. Dev Neurosci. 1985;7(1):12–32. doi: 10.1159/000112273. [DOI] [PubMed] [Google Scholar]
- Goffinet A. M. The reeler gene: a clue to brain development and evolution. Int J Dev Biol. 1992 Mar;36(1):101–107. [PubMed] [Google Scholar]
- Miao G. G., Curran T. Cell transformation by c-fos requires an extended period of expression and is independent of the cell cycle. Mol Cell Biol. 1994 Jun;14(6):4295–4310. doi: 10.1128/mcb.14.6.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuno H., Suzuki T., Yoshida T., Hashimoto Y., Curran T., Iba H. Inhibition of jun transformation by a mutated fos gene: design of an anti-oncogene. Oncogene. 1991 Sep;6(9):1491–1497. [PubMed] [Google Scholar]
- Pinto-Lord M. C., Evrard P., Caviness V. S., Jr Obstructed neuronal migration along radial glial fibers in the neocortex of the reeler mouse: a Golgi-EM analysis. Brain Res. 1982 Aug;256(4):379–393. doi: 10.1016/0165-3806(82)90181-x. [DOI] [PubMed] [Google Scholar]
- Rakic P. Neurons in rhesus monkey visual cortex: systematic relation between time of origin and eventual disposition. Science. 1974 Feb 1;183(4123):425–427. doi: 10.1126/science.183.4123.425. [DOI] [PubMed] [Google Scholar]
- Schilling K., Luk D., Morgan J. I., Curran T. Regulation of a fos-lacZ fusion gene: a paradigm for quantitative analysis of stimulus-transcription coupling. Proc Natl Acad Sci U S A. 1991 Jul 1;88(13):5665–5669. doi: 10.1073/pnas.88.13.5665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeyne R. J., Schilling K., Robertson L., Luk D., Oberdick J., Curran T., Morgan J. I. fos-lacZ transgenic mice: mapping sites of gene induction in the central nervous system. Neuron. 1992 Jan;8(1):13–23. doi: 10.1016/0896-6273(92)90105-m. [DOI] [PubMed] [Google Scholar]
- Smeyne R. J., Vendrell M., Hayward M., Baker S. J., Miao G. G., Schilling K., Robertson L. M., Curran T., Morgan J. I. Continuous c-fos expression precedes programmed cell death in vivo. Nature. 1993 May 13;363(6425):166–169. doi: 10.1038/363166a0. [DOI] [PubMed] [Google Scholar]
- Stanfield B. B., Cowan W. M. The development of the hippocampus and dentate gyrus in normal and reeler mice. J Comp Neurol. 1979 Jun 1;185(3):423–459. doi: 10.1002/cne.901850303. [DOI] [PubMed] [Google Scholar]
- Terashima T., Inoue K., Inoue Y., Yokoyama M., Mikoshiba K. Observations on the cerebellum of normal-reeler mutant mouse chimera. J Comp Neurol. 1986 Oct 8;252(2):264–278. doi: 10.1002/cne.902520209. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Xanthoudakis S., Miao G., Wang F., Pan Y. C., Curran T. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992 Sep;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuasa S., Kitoh J., Oda S., Kawamura K. Obstructed migration of Purkinje cells in the developing cerebellum of the reeler mutant mouse. Anat Embryol (Berl) 1993 Oct;188(4):317–329. doi: 10.1007/BF00185941. [DOI] [PubMed] [Google Scholar]