Abstract
Neurofilaments are synthesised in neuronal cell bodies and then transported through axons. Damage to neurofilament transport is seen in amyotrophic lateral sclerosis (ALS). Here, we show that PKN1, a neurofilament head–rod domain kinase is cleaved and activated in SOD1G93A transgenic mice that are a model of ALS. Moreover, we demonstrate that glutamate, a proposed toxic mechanism in ALS leads to caspase cleavage and disruption of PKN1 in neurons. Finally, we demonstrate that a cleaved form of PKN1 but not wild-type PKN1 disrupts neurofilament organisation and axonal transport. Thus, deregulation of PKN1 may contribute to the pathogenic process in ALS.
Keywords: Neurofilament, PKN, Axonal transport, Amyotrophic lateral sclerosis, Motor neuron disease
1. Introduction
Protein kinase N (PKN) is a serine/threonine kinase that displays homology to PKC. PKN comprises three isoforms that each harbour a C-terminal located catalytic domain and unique N-terminal regulatory regions. PKN1 is the most abundant isoform in neurons and is implicated in a variety of functions including cytoskeletal organisation [1].
Neurofilaments, the intermediate filaments of neurons are one neuronal substrate for PKN1 [2]. Their major proteins are neurofilament light, middle and heavy chains (NFL, NFM and NFH). Neurofilaments are synthesised in neuronal cell bodies and then transported through axons where they control axonal calibre [3]. Dysfunction of neurofilament metabolism is strongly implicated in amyotrophic lateral sclerosis (ALS). Accumulations of neurofilaments are a pathology of ALS; mutations in NFL cause some forms of Charcot-Marie-Tooth disease and modulating neurofilament expression can model ALS and alter disease progression in mice expressing familial ALS-associated mutants of Cu/Zn superoxide dismutase-1 (SOD1) [4]. The mechanisms by which neurofilaments accumulate in ALS involve damage to their axonal transport and this is one of the earliest pathological changes seen in several transgenic mouse models of ALS [5-7].
NFL, NFM and NFH each contain a central alpha-helical rod domain that is flanked by globular N-terminal head domains and non-alpha-helical C-terminal domains. The head domains are believed to regulate filament assembly and PKN1 phosphorylates the head–rod domain of neurofilaments; this phosphorylation disrupts neurofilament assembly in vitro [2]. However, the effect of PKN1 on neurofilament organisation in vivo in neurons has not been reported and this is an important omission since neurofilaments display different assembly properties in vitro and in cells [8,9]. Moreover, how any PKN1-induced changes in neurofilament organisation influence their axonal transport is likewise unknown. Finally, any potential role of PKN1 in ALS has not yet been studied. Here, we have investigated these topics.
2. Materials and methods
All experiments were performed at least three times.
2.1. Reagents
Plasmids: rat GFP-NFM [10]; human FLAG-wild-type PKN1 (PKN1wt) and an N-terminal truncated constitutively-active form of PKN1 (amino acids 561–942) (PKN1561–942) [11]; pCINeo-CAT expressing the Escherichia coli chloramphenicol acetyl transferase gene was used as a control to balance the numbers and amounts of plasmids in experiments to investigate the effects of PKN1 on neurofilament transport. Antibodies: PKNαC6 that detects a C-terminally located epitope in PKN1 [11]; NA1216 antibody to NFM from Biomol, DM1a to tubulin and FLAG antibody M2 from Sigma; SOD1 antibody from Calbiochem. l-glutamate was obtained from Sigma and caspase inhibitors Z-VAD-FMK and Z-DEVD-FMK were obtained from Tocris. Inhibitors were prepared according to manufacturers instructions.
2.2. PKN1 kinase assays
PKN1 activity was determined using in vitro kinase assays with myelin basic protein as substrate essentially as described [12]. PKN1 was isolated by immunoprecipitation using antibody PKNαC6 or antibody to the FLAG tag on transfected PKN1. Immunoblots confirmed equal amounts of immunoprecipitated PKN1. Reactions were stopped after 20 min; pilot studies demonstrated that PKN1 activity was within the linear range at this time point. Control reactions included omission of primary antibody and demonstrated specificity of the assay. Samples were separated by SDS–PAGE and subjected to autoradiography. Signals were quantified as described [12].
2.3. Neuronal culture, transfection and assays of neurofilament axonal transport and microscopy
Primary cortical neurons were prepared and transfected as described[10,13]. Bulk axonal transport of neurofilaments was analysed as described [10,13,14]. Briefly, neurons cultured for 7 days were co-transfected with GFP-NFM and either PKN1wt, PKN1561–942 or pCINeo-CAT as a control and the cells then fixed at 180, 220, 240 and 260 min post-transfection. Images of GFP-NFM transfected cells (approximately 50 per time point) were collected via a CCD camera and the distances travelled by GFP-NFM through axons calculated using Metamorph image analysis software. Data from these measurements were then graphically plotted and statistically analysed as indicated so as to determine the speed of overall bulk neurofilament axonal transport. Previous studies have shown that the average length of axons in these neurons is approximately 780 μm and that they are no longer extending their primary axon; since measurements are made up to only approximately 150 μm this assay thus measures the rate of transport of neurofilaments through axons and is not linked to axonal growth [10]. Co-transfection of PKN1 was confirmed by immunostaining for FLAG and selected samples were additionally stained for tubulin to confirm that GFP-NFM transport was not limited by axonal length. Quantification of fluorescence signals from FLAG-tagged PKN1 in neurons was as described [15].
3. Results
3.1. Activation of PKN1 disrupts neurofilament assembly and axonal transport
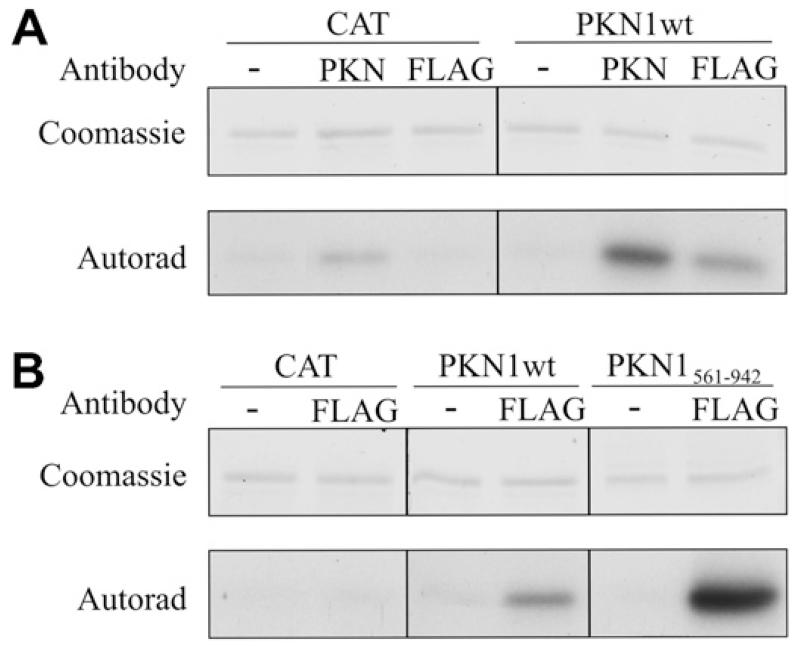
To investigate the effects of PKN1 on neurofilament organisation, we elevated PKN1 activity in neurons and studied neurofilament architecture by immunostaining. PKN1 activity was elevated by transfection of PKN1wt or a previously described constitutively-active isoform (PKN1561–942) [11]. Transfection of PKN1wt led to an overall increase in PKN1 activity that was demonstrated by in vitro kinase assays using antibody PKNαC6 to immunoprecipitate total (transfected plus endogenous) PKN1 and anti-FLAG to specifically immunoprecipitate transfected PKN1wt (Fig. 1A). However, transfected PKN1561–942 displayed higher levels of activity than PKN1wt (Fig. 1B).
Fig. 1.
Transfection of PKN1 elevates cellular PKN1 activity and PKN1561–942 displays greater activity than PKN1wt. (A) shows in vitro PKN1 assays of cells transfected with PKN1wt or CAT as a control. The higher level of activity seen with the PKN1 compared to the FLAG antibody may be because the PKN1 antibody pulls down endogenous + transfected whereas the FLAG antibody pulls down only transfected kinase. Also, the different antibodies may differentially immunoprecipitate active kinase. (B) shows assays from cells transfected with PKN1wt or PKN1561–942; cells transfected with CAT are again shown as a control. PKN1 was immunoprecipitated using either PKN antibody to isolate total (transfected plus endogenous PKN1) or an antibody to the FLAG tag on transfected PKN1 as indicated; − indicates omission of immunoprecipitating antibody as a control. Both the Coomassie stained gels showing substrate and corresponding autoradiographs are displayed.
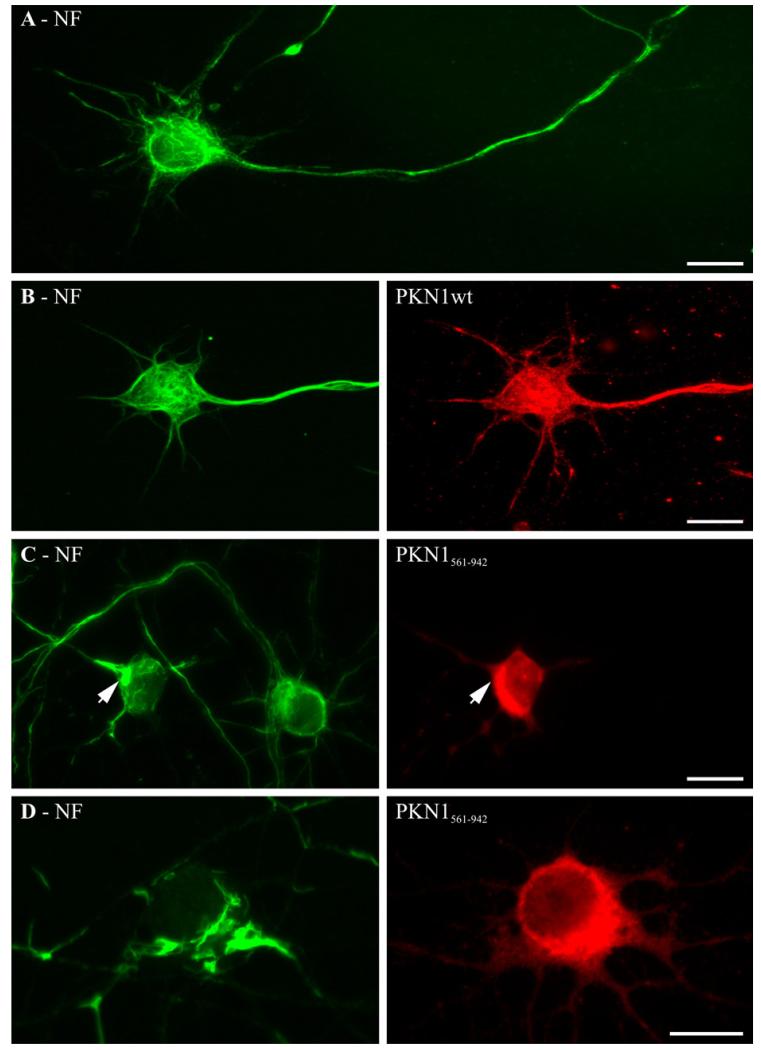
Despite the elevation of total PKN1 activity, transfection of PKN1wt had no detectable effect on neurofilament organisation; ordered arrays of neurofilaments were observed in both perikaryal regions and throughout axons and these were not noticeably different to those seen in non-transfected cells (Fig. 2A and B). However, transfection of PKN1561–942 induced a marked disruption to neurofilament architecture within perikaryal regions. In these neurons, cell body accumulation of neurofilaments in focal aggregates was readily apparent (Fig. 2C and D). To eliminate the possibility that this disruption to neurofilaments was an effect of different levels of PKN1561–942 and PKN1wt in the neurons (despite them being in the same vector) we quantified the levels of expression of transfected PKN1 in individual neurons by capturing the fluorescent signal from the FLAG tags. This revealed that there were no differences (one-way Anova, not shown). Thus, PKN1561–942 but not PKN1wt disrupts neurofilament architecture in neurons.
Fig. 2.
Expression of PKN1561–942 but not PKN1wt disrupts neurofilament organisation in rat cortical neurons. (A) shows normal distribution of neurofilaments in a non-transfected neuron. (B–D) show neurofilaments in neurons co-transfected with PKN1wt (B) or with PKN1561–942 (C, D). In (C) note the disruption to neurofilaments in the PKN1561–942 transfected neuron (arrowhead) but not in its non-transfected near neighbour. Neurofilaments were detected using antibody NA1216 and PKN1wt/PKN1561–942 with FLAG antibody. Scale bars = 20 μm.
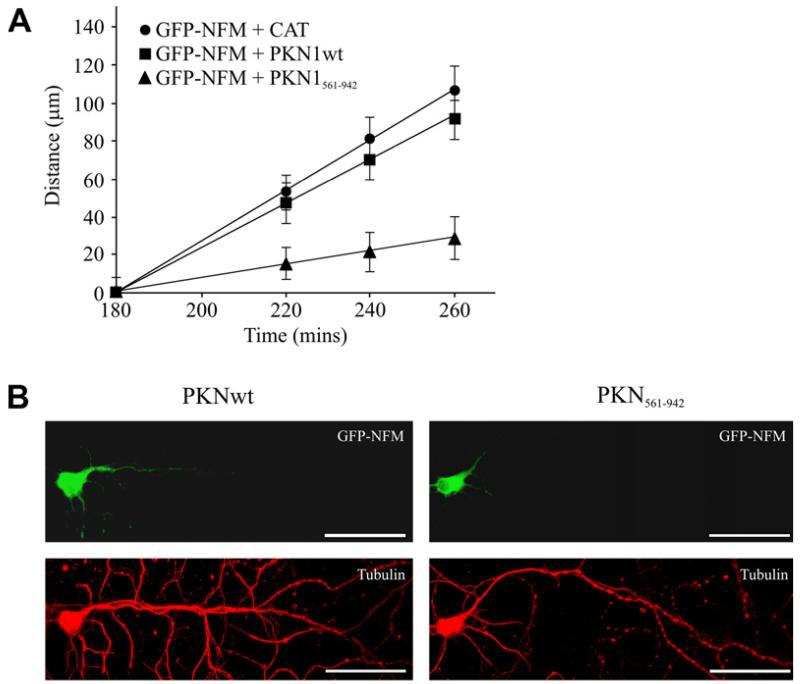
Neurofilament organisation is linked to their axonal transport; mutations that disrupt neurofilament organisation also disrupt their transport [13,16]. We therefore enquired whether expression of PKN1wt or PKN1561–942 altered axonal transport of neurofilaments. To do so, we used a previously described assay that involves monitoring movement of GFP-NFM through axons of transfected rat cortical neurons[10,13,14]. Whilst co-transfection of PKN1wt had no significant effect on neurofilament transport, co-transfection of PKN1561–942 induced a marked inhibition in the movement of neurofilaments in this assay (Fig. 3).
Fig. 3.
PKN1561–942 but not PKN1wt disrupts axonal transport of neurofilaments. (A) shows distances moved by GFP-NFM through axons of rat cortical neurons co-transfected with CAT (control), PKN1wt or PKN1561–942 at different time-points post-transfection as indicated. No significant differences were detected in GFP-NFM transport between CAT and PKN1wt co-transfected neurons at any time point. GFP-NFM in PKN1561–942 co-transfected neurons moved significantly less than both CAT and PKN1wt co-transfected neurons (P ≤ 0.05 at 220 min; P ≤ 0.001 at 240 and 260 min; n = 35–60, Mann–Whitney). (B) shows representative images of GFP-NFM labelling in neurons co-transfected with PKN1 or PKN1561–942 at 260 min post-transfection; cells were stained with tubulin to show presence of axon. Scale bar = 50 μm.
3.2. Glutamate induces caspase-mediated cleavage and activation of PKN1 in neurons
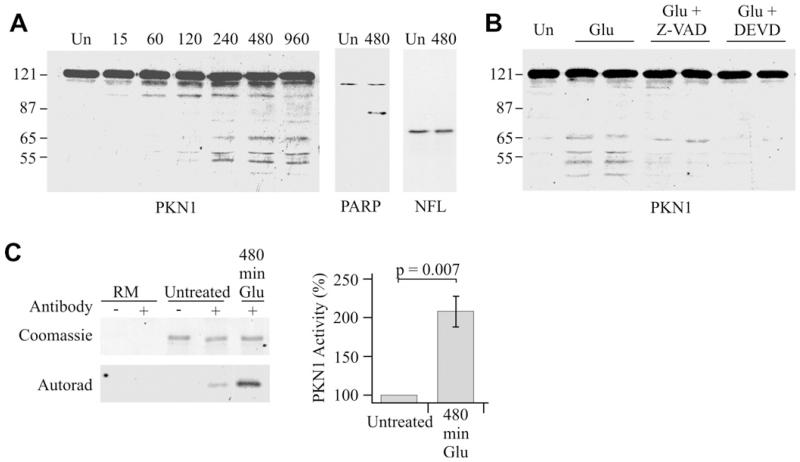
Glutamate excitotoxicity is strongly implicated in ALS [17]. We therefore monitored the effect of glutamate exposure on PKN1 in seven DIV cultured rat cortical neurons that are known to express glutamate receptors and to respond to glutamate treatment [10,18]. Short-term treatment with 100 μm glutamate had little effect on the electrophoretic mobility of PKN1. However, extended treatments induced proteolysis of PKN1 to generate an increasing number of N-terminally truncated fragments whose appearance were maximal at 480 min (Fig. 4A). LDH assays revealed no significant loss of viability of the neurons at his time point (data not shown) which is in agreement with previous studies [10,19].
Fig. 4.
Glutamate induces caspase-mediated cleavage and activation of PKN1 in cultured cortical neurons. (A) shows immunoblot of PKN1 in untreated neurons (Un) or neurons treated with 100 μm glutamate for 15–960 min as indicated. Also shown is cleavage of PARP, a known caspase substrate, but not NFL, which is not a substrate, following glutamate treatment. (B) shows inhibition of glutamate-induced PKN1 cleavage (480 min 100 μm glutamate treatment) by pre-treatment with caspase inhibitors. Z-VAD-FMK and Z-DEVD-FMK were used at 100 μm and applied 2 h prior to glutamate stimulation. (C) In vitro kinase assays for PKN1 activity in untreated neurons and neurons treated with 100 μm glutamate for 480 min. Both the Coomassie stained gel showing substrate and corresponding autoradiograph are shown along with reaction mix controls with no substrate (RM). + and − refer to presence or absence of the PKN1 antibody in the immunoprecipitations to isolate PKN1. Glutamate induces a 2.07-fold increase in activity (t-test; n = 7).
Caspase-3-induced cleavage of PKN1 to produce N-terminally truncated, constitutively-active isoforms including PKN1561–942 has been described in non-neuronal cells undergoing apoptosis [11]. To determine whether glutamate-induced cleavage in the cortical neurons also involved caspase-3, we treated the neurons with the broad-spectrum caspase inhibitor Z-VAD-FMK and the caspase-3 inhibitor Z-DEVD-FMK prior to glutamate exposure. Both of these compounds inhibited glutamate-induced cleavage of PKN1 in the neurons (Fig. 4B). Moreover, PARP a known caspase substrate was cleaved in the glutamate-treated neurons but NFL that is not a substrate was unaffected (Fig. 4A). We also investigated the effect of glutamate on PKN1 activity. To do so, we performed in vitro kinase assays on neurons following 480 min glutamate treatment. Antibody PKNαC6 that detects a C-terminal epitope was used to immunoprecipitate PKN1 so as to ensure that both full-length and cleaved isoforms containing the kinase domain were isolated. These assays revealed that PKN1 activity was elevated compared to untreated neurons (Fig. 4C).
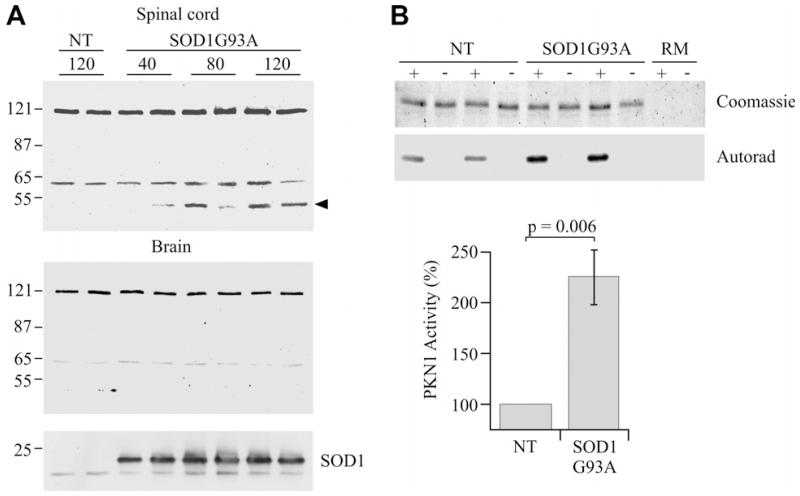
3.3. PKN1 activity is elevated and PKN1 is proteolytically cleaved in spinal cords of SOD1G93A transgenic mice
Mutations in SOD1 cause some familial forms of ALS. Transgenic mice expressing mutant SOD1 are models of ALS with SOD1G93A mice being the best characterized [20]. We enquired whether proteolytic cleavage of PKN1 similar to that observed in the glutamate-treated neurons occurs in SOD1G93A mice. To do so, we performed immunoblots for PKN1 in brain and spinal cords from SOD1G93A and control littermate mice at 40, 80 and 120 days. On the inbred C57Bl/6 background used, these ages correspond to prior to any clinical disease, early- and mid-stage disease.
We detected full-length PKN1 and also a further faster-migrating species (approximately 65 kDa) in brains and spinal cords of both SOD1G93A and control mice (Fig. 5A). We were unable to eliminate the appearance of this faster-migrating PKN1 species using a range of different methods to process the tissues. Thus, some cleavage of PKN1 occurs in nervous tissues under normal circumstances.
Fig. 5.
PKN1 is cleaved and activated in SOD1G93A transgenic mice. (A) Immunoblots to show PKN1 in spinal cord (upper) and brain (lower) from SOD1G93A and non-transgenic littermate (NT) mice at ages 40, 80 and 120 days. Full-length PKN1 (120 kDa) and an approx. 65 kDa species are seen in all samples. An additional approx. 55 kDa migrating species (arrowhead) is seen in spinal cord but not brains of SOD1G93A mice at 80 and 120 days; this species is not detected in non-transgenic littermates. The samples were also probed with an antibody to SOD1 to confirm the genotype (bottom panel). Two mice at each age are shown but an additional two mice were analysed with highly similar results. (B) shows in vitro kinase assays for PKN1 activity in spinal cords of SOD1G93A and non-transgenic littermate (NT) mice. Both the Coomassie stained gel showing substrate and corresponding autoradiograph are shown along with reaction mix controls with no substrate (RM). + and − refer to presence or absence of the PKN1 antibody in the immunoprecipitations to isolate PKN1. PKN1 activity is elevated approximately 2.25-fold in SOD1G93A mice (n = 8, t-test).
However, we observed the presence of an additional, 55 kDa PKN1 species in the spinal cords but not brains of SOD1G93A mice; this PKN1 species was not detected in non-transgenic mice (Fig. 5A). Moreover, the 55 kDa PKN1 species was clearly apparent in 80 and 120 day SOD1G93A mice but was only weakly labelled or absent in 40 day mice indicating that its appearance was linked to disease progression.
To enquire whether there were changes in PKN1 activity in the spinal cords of SOD1G93A mice, we performed in vitro kinase assays. These revealed that compared to non-transgenic littermates, PKN1 activity was elevated in 120-day-old SOD1-G93A mice (Fig. 5B). Whether this activation was directly linked to PKN1 cleavage is not clear but is consistent with this notion.
4. Discussion
Here, we demonstrate that excitotoxic glutamate exposure elevates PKN1 activity in neurons and also induces caspase-mediated cleavage of PKN1 to produce N-terminally truncated species. Such truncated PKN1 species behave as constitutively-active isoforms [11]. We also demonstrate that PKN1 activity is elevated in spinal cords of SOD1G93A transgenic mice and that PKN undergoes processing to produce additional N-terminally truncated PKN1 species in the spinal cords but not brains of these animals. Thus, both glutamate and ALS mutant SOD1 induce activation and cleavage of PKN1.
In cultured neurons, glutamate-induced cleavage of PKN1 involved caspase-3 type proteases. This is in agreement with earlier studies in non-neuronal cells [11]. Although the mechanisms by which mutant SOD1 causes ALS are not fully understood, there is strong evidence that disruption to glutamate handling contributes to the pathogenic process [17]. Moreover, caspase-3 is activated in SOD1G93A mice when animals first become symptomatic [21] and this is where we observe additional cleaved PKN1 species in the spinal cords of these animals. Thus, it seems likely that processing of PKN1 in the SOD1G93A mice also involves caspase-3. Interestingly, cleavage of PKN1 has also been observed in models of stroke [22].
Damage to axonal transport is one of the earliest pathological features in ALS and this includes disruption to transport of neurofilaments [5-7,15]. Increased phosphorylation of NFM/NFH side-arms is seen in cell bodies in ALS and phosphorylation of these domains slows neurofilament transport[10,14,23]. However, mutation of NFL head and rod domains (that does not involve changes to NFM/NFH side-arm phosphorylation) also perturbs neurofilament transport [13,16] and the proper assembly of neurofilaments is now known to be an essential requirement for their transport [24]. PKN1 phosphorylates the head–rod domains of neurofilaments in vitro [2] and phosphorylation of this region (in particular the head domain of NFL) regulates neurofilament assembly [3]. Our findings that PKN1561–942 markedly disrupts both neurofilament organisation and transport therefore suggest that at least part of these disruptions are via changes to neurofilament head–rod phosphorylation. It is also possible that PKN1561–942 targets other components of axonal transport, e.g. molecular motors. Future studies to identify the residues phosphorylated by PKN1 in neurofilaments will help clarify this issue.
It is noteworthy that whilst transfection of PKN1wt or PKN1561–942 both elevated cellular PKN1 activity, only PKN1561–942 induced damage to neurofilament organisation and transport. The N-terminal regions deleted in PKN1561–942 (and which are likewise deleted in the glutamate-treated neurons and SOD1G93A transgenic mice) are inhibitory to PKN1 activity. However, there is emerging evidence that they are also involved in targeting PKN1 to particular subcellular sites [25]. Thus, N-terminally deleted PKN1 isoforms may be targeted differently to neurofilaments and/or other substrates to induce damage to axonal transport. Interestingly, the cdk5 activator p35 (another neurofilament kinase) is also processed in mutant SOD1 transgenic mice and this processing removes an N-terminal myristylation signal that alters kinase distribution [26].
Clearly, the cellular transfection studies that demonstrate perturbation of neurofilament organisation and transport with PKN1561–942 but not PKN1wt involve overexpression and this is not the case in SOD1G93A transgenic mice where cleavage of endogenous levels of PKN1 occurs. Whether such endogenous levels of cleaved PKN1 are sufficient to generate disruption of neurofilaments is not clear. One possibility is that at least part of the disease process reflects a cumulative effect of low levels of cleaved PKN1 and this may account for the acute effects seen to neurofilaments in the transfected neurons whereas neurofilament disruption takes several months in SOD1G93A transgenic mice. Future studies to monitor the effects of PKN1 inhibitors (once specific ones are developed) on neurofilament organisation and transport, and disease progression in SOD1G93A transgenic might resolve this issue.
Despite the activation of caspase 3 in SOD1G93A transgenic mice, there is dispute over whether motor neurons die by apoptosis [27]. Indeed, damage to axons is now known to be a principal early feature of disease in these animals [28]. Our demonstration that deregulation of PKN1 can damage axonal transport therefore suggests a novel mechanism to link caspase 3 to ALS. Thus caspase-mediated processing of PKN1 induced by glutamate or other disease-associated insults may alter neurofilament head domain phosphorylation, organisation and transport. As such, abnormal PKN1 signalling may contribute to ALS.
Acknowledgements
This work was supported by grants from the Motor Neurone Disease Association, MRC, Wellcome Trust, European Union V1th Framework (NeuroNE) and Alzheimer’s Association. We thank Kurt De Vos for comments on the manuscript.
Abbreviations
- PKN
protein kinase N
- NFL, NFM and NFH
neurofilament light middle and heavy chains
- ALS
amyotrophic lateral sclerosis
References
- [1].Mukai H. The structure and function of PKN, a protein kinase having a catalytic domain homologous to that of PKC. J. Biochem. (Tokyo) 2003;133:17–27. doi: 10.1093/jb/mvg019. [DOI] [PubMed] [Google Scholar]
- [2].Mukai H, Toshimori M, Shibata H, Kitagawa M, Shimakawa M, Miyahara M, Sunakawa H, Ono Y. PKN associates and phosphorylates the head–rod domain of neurofilament protein. J. Biol. Chem. 1996;271:9816–9822. doi: 10.1074/jbc.271.16.9816. [DOI] [PubMed] [Google Scholar]
- [3].Sihag RK, Inagaki M, Yamaguchi T, Shea TB, Pant HC. Role of phosphorylation on the structural dynamics and function of types III and IV intermediate filaments. Exp. Cell Res. 2007;313:2098–2109. doi: 10.1016/j.yexcr.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xiao S, McLean J, Robertson J. Neuronal intermediate filaments and ALS: a new look at an old question. Biochim. Biophys. Acta. 2006;1762:1001–1012. doi: 10.1016/j.bbadis.2006.09.003. [DOI] [PubMed] [Google Scholar]
- [5].Williamson TL, Cleveland DW. Slowing of axonal transport is a very early event in the toxicity of ALS-linked SOD1 mutants to motor neurons. Nat. Neurosci. 1999;2:50–56. doi: 10.1038/4553. [DOI] [PubMed] [Google Scholar]
- [6].Collard J-F, Cote F, Julien J-P. Defective axonal transport in a transgenic mouse model of amyotrophic lateral sclerosis. Nature. 1995;375:61–64. doi: 10.1038/375061a0. [DOI] [PubMed] [Google Scholar]
- [7].Zhang B, Tu P, Abtahian F, Trojanowski JQ, Lee VM. Neurofilaments and orthograde transport are reduced in ventral root axons of transgenic mice that express human SOD1 with a G93A mutation. J. Cell Biol. 1997;139:1307–1315. doi: 10.1083/jcb.139.5.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ching GY, Liem RK. Assembly of type IV neuronal intermediate filaments in nonneuronal cells in the absence of preexisting cytoplasmic intermediate filaments. J. Cell Biol. 1993;122:1323–1335. doi: 10.1083/jcb.122.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee MK, Xu Z, Wong PC, Cleveland DW. Neurofilaments are obligate heteropolymers in vivo. J. Cell Biol. 1993;122:1337–1350. doi: 10.1083/jcb.122.6.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ackerley S, Grierson AJ, Brownlees J, Thornhill P, Anderton BH, Leigh PN, Shaw CE, Miller CCJ. Glutamate slows axonal transport of neurofilaments in transfected neurons. J. Cell Biol. 2000;150:165–175. doi: 10.1083/jcb.150.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takahashi M, Mukai H, Toshimori M, Miyamoto M, Ono Y. Proteolytic activation of PKN by caspase-3 or related protease during apoptosis. Proc. Natl. Acad. Sci. USA. 1998;95:11566–11571. doi: 10.1073/pnas.95.20.11566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kesavapany S, et al. Identification of a novel, membrane-associated neuronal kinase, cdk5/p35 regulated kinase (cprk) J. Neurosci. 2003;23:4975–4983. doi: 10.1523/JNEUROSCI.23-12-04975.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Brownlees J, et al. Charcot-Marie-Tooth disease neurofilament mutations disrupt neurofilament assembly and axonal transport. Hum. Mol. Genet. 2002;11:2837–2844. doi: 10.1093/hmg/11.23.2837. [DOI] [PubMed] [Google Scholar]
- [14].Ackerley S, Thornhill P, Grierson AJ, Brownlees J, Anderton BH, Leigh PN, Shaw CE, Miller CCJ. Neurofilament heavy chain side-arm phosphorylation regulates axonal transport of neurofilaments. J. Cell Biol. 2003;161:489–495. doi: 10.1083/jcb.200303138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].De Vos KJ, et al. Familial amyotrophic lateral sclerosis-linked SOD1 mutants perturb fast axonal transport to reduce axonal mitochondria content. Hum. Mol. Genet. 2007;16:2720–2728. doi: 10.1093/hmg/ddm226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Perez-Olle R, Jones ST, Liem RK. Phenotypic analysis of neurofilament light gene mutations linked to Charcot-Marie-Tooth disease in cell culture models. Hum. Mol. Genet. 2004;13:2207–2220. doi: 10.1093/hmg/ddh236. [DOI] [PubMed] [Google Scholar]
- [17].Van Den Bosch L, Van Damme P, Bogaert E, Robberecht W. The role of excitotoxicity in the pathogenesis of amyotrophic lateral sclerosis. Biochim. Biophys. Acta. 2006;1762:1068–1082. doi: 10.1016/j.bbadis.2006.05.002. [DOI] [PubMed] [Google Scholar]
- [18].Brownlees J, Yates A, Bajaj NP, Davis D, Anderton BH, Leigh PN, Shaw CE, Miller CCJ. Phosphorylation of neurofilament heavy chain side-arms by stress activated protein kinase-1b/Jun N-terminal kinase-3. J. Cell Sci. 2000;113:401–407. doi: 10.1242/jcs.113.3.401. [DOI] [PubMed] [Google Scholar]
- [19].Davis DR, et al. The phosphorylation state of the microtubule-associated protein tau as affected by glutamate, colchicine and b-amyloid in primary rat cortical neuronal cultures. Biochem. J. 1995;309:941–949. doi: 10.1042/bj3090941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu,Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- [21].Li M, et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- [22].Ueyama T, Ren Y, Sakai N, Takahashi M, Ono Y, Kondoh T, Tamaki N, Saito N. Generation of a constitutively active fragment of PKN in microglia/macrophages after middle cerebral artery occlusion in rats. J. Neurochem. 2001;79:903–913. doi: 10.1046/j.1471-4159.2001.00624.x. [DOI] [PubMed] [Google Scholar]
- [23].Shea TB, Yabe JT, Ortiz D, Pimenta A, Loomis P, Goldman RD, Amin N, Pant HC. Cdk5 regulates axonal transport and phosphorylation of neurofilaments in cultured neurons. J. Cell Sci. 2004;117:933–941. doi: 10.1242/jcs.00785. [DOI] [PubMed] [Google Scholar]
- [24].Yuan A, Rao MV, Kumar A, Julien JP, Nixon RA. Neurofilament transport in vivo minimally requires heterooligomer formation. J. Neurosci. 2003;23:9452–9458. doi: 10.1523/JNEUROSCI.23-28-09452.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Torbett NE, Casamassima A, Parker PJ. Hyperosmotic-induced protein kinase N 1 activation in a vesicular compartment is dependent upon Rac1 and 3-phosphoinositide-dependent kinase 1. J. Biol. Chem. 2003;278:32344–32351. doi: 10.1074/jbc.M303532200. [DOI] [PubMed] [Google Scholar]
- [26].Nguyen MD, Lariviere RC, Julien JP. Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron. 2001;30:135–147. doi: 10.1016/s0896-6273(01)00268-9. [DOI] [PubMed] [Google Scholar]
- [27].Migheli A, Atzori C, Piva R, Tortarolo M, Girelli M, Schiffer D, Bendotti C. Lack of apoptosis in mice with ALS. Nat. Med. 1999;5:966–967. doi: 10.1038/12381. [DOI] [PubMed] [Google Scholar]
- [28].Pun S, Santos AF, Saxena S, Xu L, Caroni P. Selective vulnerability and pruning of phasic motoneuron axons in motoneuron disease alleviated by CNTF. Nat. Neurosci. 2006;206:408–419. doi: 10.1038/nn1653. [DOI] [PubMed] [Google Scholar]