Abstract
The aim of this study was to elucidate more clearly the role of interleukin (IL)-18 in modulating the IL-22 pathway in primary Sjögren’s syndrome (pSS) patients and in pSS-associated lymphomas. Minor salivary glands (MSGs) from patients with pSS and non-specific chronic sialoadenitis (nSCS), parotid glands biopsies from non-Hodgkin lymphomas (NHL) developed in pSS patients, were evaluated for IL-18, IL-22, IL-22 receptor 1 (IL-22R1), IL-22 binding protein (IL-22BP) and signal transducer and activator of transcription-3 (STAT-3) expression. MSGs IL-22R1-expressing cells were characterized by confocal microscopy and flow cytometry in pSS, nSCS and healthy controls. The effect of recombinant IL-18 and IL-22 on peripheral blood mononuclear cells (PBMCs) from pSS and nSCS was studied by flow cytometry and reverse transcription–polymerase chain reaction (RT-PCR). MSGs of pSS and NHL were characterized by an imbalance between IL-22 and IL-22BP protein expression, with IL-18 and IL-22BP being expressed in a mutually exclusive manner and IL-18 and IL-22R1 being correlated directly. Aberrant expression of IL-22R1, induced by IL-18, was observed only among tissue and circulating myeloid cells of pSS patients and macrophages of NHL tissues of pSS patients, but not nSCS. IL-22R1 expression on PBMC of pSS was functional, as its stimulation with recombinant IL-22 significantly up-regulated the expression of STAT-3, IL-17 and IL-22. An IL-18-dependent aberrant expression of IL-22R1 on cells of haematopoietic origin seems to be a specific immunological signature of patients with pSS and pSS-associated lymphomas.
Keywords: IL-18, IL-22, IL-22BP, IL-22R1, non-Hodgkin lymphoma, Sjögren’s syndrome
Introduction
Primary Sjögren’s syndrome (pSS) is a chronic systemic autoimmune disease characterized by dysregulated immune responses and a higher rate of non-Hodgkin lymphoma (NHL) risk 1–3.
We have recently shown the occurrence in pSS patients of a local and systemic over-expression of interleukin (IL)-18 4 and IL-22 5, highlighting the role of dysregulated innate and adaptive immune responses in the pathogenesis of pSS. Both IL-18 and IL-22 are immune-stimulatory cytokines participating in the pathogenesis of chronic inflammatory diseases. IL-22, however, does not play a role in immune cells cross-talk because, unlike the IL-10R2 which is expressed constitutively in many human tissues, IL-22R1 is not detectable in immune cells 6. In physiological conditions, in fact, IL-22R1 is expressed on hepatocytes, keratinocytes, intestinal epithelial cells and pancreatic cells (reviewed in 7). In this context, IL-22 seems to act mainly eliciting proinflammatory responses to defend rapidly against invading pathogens (reviewed in 7). Dysregulation of IL-22 signalling is, however, described in human inflammatory disorders other than pSS, such as psoriasis, and in the pathogenesis of solid tumours of the skin, lung, brain, gastrointestinal tract and of haematological malignancies (reviewed in 7).
The final functional outcome of both IL-18 and IL-22 seems to depend upon the levels of the respective binding proteins 6,8, which are secreted glycoproteins that antagonize cytokine activity by specifically blocking their interaction with the cell surface receptors. Recent evidence indicates that IL-18 can synergize with IL-12 to enhance IL-22 levels in T helper type 1 (Th1 cells) and also to influence the IL-22BP in both humans and mice 9,10, suggesting the occurrence of a immunological link between inflammasome-IL-18 axis and IL-22, and thus between the early step of innate immunity and the adaptive immunity.
The aim of the present study was to investigate the relationship between the IL-18 and IL-22 pathway in pSS and in pSS-associated non-Hodgkin lymphoma.
Materials and methods
Patients and samples
Thirty patients fulfilling the American–European Consensus Group criteria for pSS 11 were enrolled consecutively. The occurrence of lymphoma was carefully excluded in all pSS patients by clinical and radiological [sonography, computerized tomography (CT), positron emission tomography (PET)/CT] examinations. Fifteen patients also displaying subjective complaints of dry mouth or eyes who did not meet the American–European Consensus Group criteria for pSS were included as non-specific chronic sialoadenitis (nSCS) and evaluated as controls. The occurrence of autoimmune or chronic inflammatory diseases was carefully excluded in nSCS subjects. Paraffin-embedded samples obtained from patients with a previous diagnosis of pSS-associated lymphoma (n = 10) were selected from the biopsy bank of the Pathology Unit of the Ospedale Cervello (Palermo, Italy). Histologically, lymphomas were diagnosed by the presence of sheets or halos of monocytoid B cells and the presence of clonal populations was confirmed by polymerase chain reaction (PCR) analysis for immunoglobulin (Ig)H and/or IgL chain restriction. Blood from 10 healthy subjects (HC) [eight female, mean age ± standard deviation (s.d.) 43 ± 5·6] was also obtained. Clinical characteristics of the patients and controls are shown in Table1. Minor labial salivary glands (MSG) biopsies from pSS and nSCS patients, obtained during routine diagnostic procedures, were placed into formalin fixative, RNA later or RPMI. All the patients and controls gave their informed consent and the study was approved by the ethical committee of the University of Palermo. Paraffin-embedded sections of 5-μm thickness were stained with haematoxylin and eosin (H&E) for histological evaluation and MSG were assessed for the presence of lymphocytic infiltrates and/or foci (defined as aggregates of ≥ 50 lymphocytes) and the focus score was reported as the number of foci per 4 mm2 of tissue 12.
Table 1.
Clinical characteristics of patients and controls
| pSS (n = 30) | nSCS (n = 15) | NHL* (n = 10) | |
|---|---|---|---|
| Age (years) (range) | 43 (25–60) | 54 (33–68) | 59 (49–67) |
| Female sex, n (%) | 26 (86) | 12 (80) | 5 (100) |
| Disease duration, months (range) | 70 (12–240) | 96 (22–300) | 103 (60–180) |
| Anti-nuclear antibodies, n (% of patients) | (21) 70 | – | 100 |
| Anti-Ro and/or anti-LA antibodies (% of patients) | 18 (60) | – | 80 |
| Rheumatoid factor (% of patients) | 13 (44) | – | 80 |
| ESR mm/h, mean (s.d.) | 33 (14) | 15 (4) | 70 (21) |
| C-reactive protein, mg/l, mean (s.d.) | 11 (3) | 4 (1·5) | 23 (7) |
| Low C4 level n (%) | 2 (6·6) | – | 5 (50) |
| Cryoglubulinaemia n (%) | 2 (6·6) | – | 5 (50) |
| Focus score 0–1 n (%) | 7 (23·3) | – | – |
| Focus score 2 n (%) | 5 (16·6) | – | – |
| Focus score 3 n (%) | 7 (16·6) | – | – |
| Focus score 4 n (%) | 11 (36·6) | – | – |
| Germinal centre n (%) | 8 (26·6) | – | – |
| Extraglandular involvement n (%) | |||
| Synovitis | 2 (6·6%) | – | – |
| Vasculitis | 3 (10) | – | – |
| Auto-immune cytopaenia | 1 (3·3) | – | – |
| Cutaneous involvement | 5 (16) | – | – |
| Renal involvement | 2 (6·6) | – | – |
| Pulmonary involvement | 1 (3·3) | – | – |
| Neurological involvement | 2 (6·6) | – | – |
Clinical data of patients with primary Sjögren’s syndrome (pSS) patients who developed non-Hodgkin lymphoma (NHL) are referred to the time of the onset of lymphoma. nSCS = non-specific chronic sialoadenitis.
RNA isolation and quantitative real-time reverse transcription–polymerase chain reaction (RT–PCR)
RT–PCR was performed on whole SGs or isolated SG mononuclear cells (SGMCs), as described previously 5. Master mix and Taqman® gene expression assays for glyceraldehyde 3-phosphate dehydrogenase (GAPDH) control and target genes were obtained from Applied Biosystems (Foster City, CA, USA) (Table2). Final values were expressed as fold of induction (FOI).
Table 2.
List of primers for reverse transcription–polymerase chain reaction (RT–PCR) and primary and secondary antibodies used
| GAPDH | Hs99999905_m1 |
| IL-18 | Hs01038788_m1 |
| IL-22 | Hs01574154_m1 |
| IL-22R1 | Hs00222035_m1 |
| IL-22BP | Hs00364814_m1 |
| IL-17 | Hs00174383_m1 |
| IL-23p19 | Hs00372324_m1 |
| RORc | Hs01076122_m1 |
| STAT-3 | Hs00234174_m1 |
| Rabbit anti-human IL-22 (1 : 100 dilution) | Novus Biological, Littleton, CO, USA |
| Rabbit anti-human IL-22R1 (1 : 100 dilution) | Novus Biological, Littleton, CO, USA |
| Rabbit anti-human IL-18 (1 : 100 dilution) | Novus Biological, Littleton, CO, USA |
| Rabit anti-human IL-22BP (1 : 100 dilution) | Novus Biological, Littleton, CO, USA |
| Mouse anti-human CD3 (1 : 100 dilution) | Dako, Glostrup, Denmark |
| Mouse anti-human CD68 (1 : 100 dilution) | Dako, Glostrup, Denmark |
| Mouse anti-human CD19 (1 : 100 dilution) | Dako, Glostrup, Denmark |
| Alexa Fluor® 488 Goat Anti-Rabbit IgG | Life Technologies, Europe, BV, Stockholm, Sweden |
| Alexa Fluor® 555 Goat Anti-Mouse IgG | Life Technologies, Europe, BV, Stockholm, Sweden |
| Anti-human-CD3-APC-conjugated | BD Biosciences, San Jose, CA, USA |
| Anti-human-CD19-FITC-conjugated | BD Biosciences, San Jose, CA, USA |
| Anti-human-CD14-FITC-conjugated | BD Biosciences, San Jose, CA, USA |
| Anti-human-CD68-FITC-conjugated | BD Biosciences, San Jose, CA, USA |
| Anti-human-IL-22R1-PE-conjugated | R&D Systems, Minneapolis, MN, USA |
| Anti-human-CD4-APC-conjugated | BD Biosciences, San Jose, CA, USA |
| Anti-human-IL-17-FITC-conjugated | R&D Systems, Minneapolis, MN, USA |
APC = allophycocyanin; FITC = fluorescein isothiocyanate; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; Ig = immunoglobulin; PE = phycoerythrin; STAT-3 = signal transducer and activator of transcription-3.
Immunohistochemistry
Immunohistochemistry was performed on 5-μm-thick paraffin-embedded sections from salivary glands, tonsils (used as positive controls) and from parotids of non-Hodgkin lymphomas from pSS patients, as described previously 5. A list of the primary and secondary antibodies used is provided in Table 2.
Flow cytometry
Minor SGMC and PBMCs were obtained as described previously 5 from 20 pSS patients and eight nSCS subjects. PBMCs were also obtained from 10 healthy subjects. For SGMC isolation, salivary gland tissues were washed in saline buffer and enzymatic digestion was performed using collagenase (1·5 mg/ml; Life Technologies, Carlsbad, CA, USA) in Dulbecco’s modified Eagle’s medium (DMEM) containing antibiotics for 2 h. Recovered cells were then cultured with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (0·5 µg/ml) and incubated at 37°C in 5% CO2. Cell viability (trypan blue dye exclusion) was always > 95%. After 2 h of incubation brefeldin A (10 µg/ml; Sigma, St Louis, MO, USA) was added and after 16 h of incubation cells were collected and stained with monoclonal antibodies (mAb). A list of the antibodies used is shown in Table2. Four-colour flow cytometry analysis was performed using a fluorescence activated cell sorter (FACS)Calibur (BD Biosciences, San Jose, CA, USA). At least 50 000 cells (events), gated on the lymphocytes or monocytes/macrophages region, were acquired for each sample.
Cell cultures with recombinant IL-22 and IL-18
PBMCs isolated from heparinized blood samples of 10 pSS and 10 nSCS patients by centrifugation over Ficoll-Hypaque (Sigma) density gradients and SGMC obtained from five pSS with focus score 1–2 were cultured in 24 flat-bottomed plates (Becton Dickinson Labware, Franklin Lakes, NJ, USA) at a density of 1 × 106 cells in 1 ml of RPMI-1640 medium with 10% fetal calf serum (FCS), 2 mM L-glutamine, 20 nM HEPES and 100 U/ml penicillin/streptomycin with or without recombinant IL-22 (rIL-22) (0·1 or 10 ng/ml) (R&D Systems, Minneapolis, MN, USA) and IL-18 (10 or 20 ng/ml) (MBL International, Woburn, MA, USA). Cells were incubated at 37°C in a humidified atmosphere containing 5% CO2 for 48 h and for 5 days; after incubation cells were characterized by flow cytometry and reverse transcription–polymerase chain reaction (RT-PCR) for IL-17, IL-22, IL-23, RAR-related orphan receptor (ROR)c and signal transducer and activator of transcription-3 (STAT-3) was also performed.
Statistical analysis
Parametric and non-parametric statistical analysis was been performed calculating the mean ± standard deviation (s.d.) and median, respectively. For comparison of parametric and non-parametric data, t-test and Mann–Whitney rank-sum test were used where appropriate. Spearman’s correlation analysis was utilized to quantify the expression associations between the genes of interest. Data are expressed as mean ± standard error of the mean (s.e.m.). P-values less than 0·05 were considered significant.
Results
Histology
Biopsy specimens from pSS patients, but not from nSCS subjects, were characterized by the presence of periductal lymphocytic infiltration, sometimes leading to the formation of ectopic lymphoid structures and germinal centre-like structures, accompanied by various degrees of atrophy or severe destruction of the acini (not shown). An additional 10 patients with NHL, all women affected by primary SS, were also considered. The diagnosis of pSS was established 6–20 years before the diagnosis of NHL. Seven cases were low-grade marginal zone B cell lymphoma with lymphoepithelial lesions, two were nodal lymphomas and the last was a high-grade extranodal diffuse large B cell lymphoma involving the retroperitoneal area. The B phenotype of each lymphoma was confirmed using the pan-B antibody CD20.
IL-22R1 in pSS patients
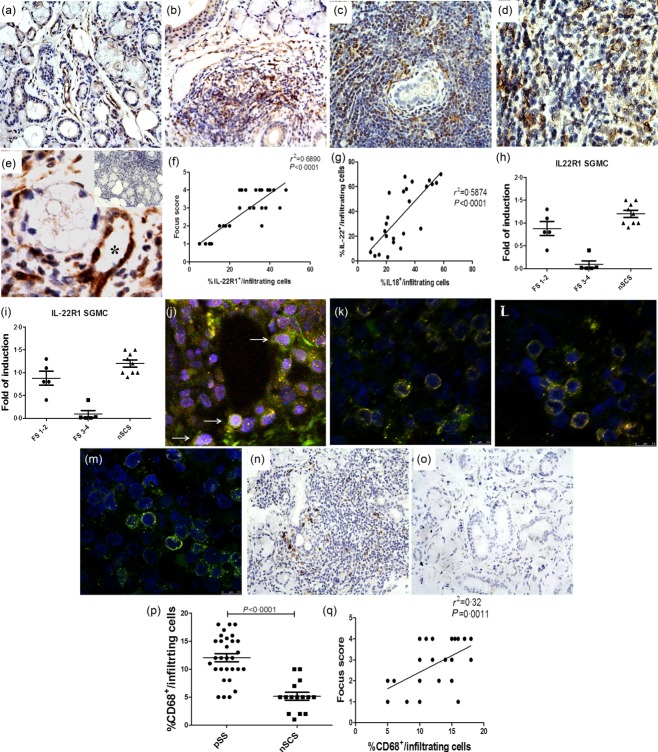
The MSG over-expression of IL-22 and IL-18 was confirmed by RT–PCR and immunohistochemistry (IHC) in this new cohort of pSS patients (Supporting information, Fig. S1a–h, respectively). In particular, IL-22 was expressed mainly among infiltrating mononuclear cells of pSS with no significant epithelial expression (Supporting information, Fig. S1a–c). Conversely, IL-18 was expressed strongly in both infiltrating mononuclear and epithelial cells (Supporting information, Fig. S1d–f). No significant co-expression of IL-22 and IL-18 was observed in pSS (data not shown). We next evaluated the expression of the inducible IL-22R1 subunit. IL-22R1 expression was detected among infiltrating mononuclear cells (Fig. 1b–e), endothelial cells of vessels scattered through the inflamed salivary glands (Fig. 1e, asterisk) and acinar epithelial cells (Fig. 1b). Conversely, only rare acinar and ductal epithelial positivity was observed in the salivary glands of nSCS, IL-22R1 being observed essentially among endothelial cells (Fig. 1a). The percentage of infiltrating IL-22R1-expressing cells/infiltrating mononuclear cells was correlated significantly with the lymphocytic focus score (Fig. 1f) and related directly to the percentage of IL-18-expressing cells/infiltrating mononuclear cells (Fig. 1g). IL-22R1 m-RNA was also evaluated by RT–PCR in both whole SGs and isolated SGMC. In pSS patients with focus score (FS) 1 and 2 the m-RNA levels of IL-22R1 was not significantly different from the controls (Fig. 1h,i). Conversely, patients with higher FS (3–4) displayed a significant down-regulation of the mRNA levels (Fig. 1h,i) compared to nSCS. In order to clarify which cells express IL-22R1, double immunostaining by confocal laser scanning microscopy was performed. Epithelial cells expressed IL-22R1 (Fig. 1j), only a very small percentage of CD3+ cells displayed IL-22R1 positivity (Fig. 1l) and no IL-22R1 expression was found among CD19+ cells (Fig. 1m), the majority of tissue IL-22R1-expressing cells also being CD68+ (Fig. 1k). CD68+ cells were observed to infiltrate the salivary glands of pSS patients compared significantly with nSCS, their number being correlated strongly and directly with the focus score (Fig. 1n–q). Finally, the functionality of IL-22R1 expression on immune cells of pSS was confirmed by the demonstration of a significant co-localization of IL-22R1 and pSTAT-3 (Fig. 2a–d).
Figure 1.
Interleukin (IL)-22R1 expression in the salivary glands of patients with primary Sjögren’s syndrome (pSS patients). (a–e) Representative microphotographs showing IL-22R1 immunostainings in non-specific chronic sialoadenitis (nSCS) (a) and pSS patients (b–d). IL-22R1 aberrant expression was observed among infiltrating inflammatory cells (b–e) and endothelial cells of microvessels distributed in the inflammatory infiltrates (asterisk, e) in pSS. Insert in e: representative paraffin sections of salivary glands of pSS patients stained with rabbit immunoglobulins (isotype control). (f) Correlation of IL-22R1 with the focus scores and with IL-18-expressing cells (g). Percentage of IL-22R1-expressing cells/infiltrating mononuclear cells was correlated with the focus score of minor salivary glands of pSS and with the number of IL-18-expressing cells/infiltrating mononuclear cells. The r2 and P (P < 0·0001)-values were determined with Spearman’s correlation coefficient. Relative m-RNA quantification of IL-22R1 was assessed by quantitative reverse transcription–polymerase chain reaction (qRT–PCR) in salivary glands obtained from 30 pSS and nSCS patients (h,i) and salivary gland mononuclear cells (SGMC) from 10 pSS and 10 nSCS patients. (j–m) Representative images of confocal analysis of IL-22RA1 localization in pSS patients. (j) Merged double-staining for MNF-116 (red) and IL-22RA1 green. (k) Merged double-staining of CD68 (red) and IL-22RA1 (green). (l) Merged double-staining of CD3 (red) and IL-22RA1 (green). (m) Merged double-staining of CD19 (red) and IL-22RA1 (green). (n,o) Representative microphotographs showing CD68 immunostainings in pSS (n) and non-Sjögren’s syndrome patients (nSS) patients (o). (p) Numbers of CD68+ cells in pSS patients and controls. (q) Correlation of CD68+ cells with the focus scores. (a,b,e,n,o) Original magnification ×250; (c) original magnification ×400; (d,e,j–m) original magnification ×630.
Figure 2.
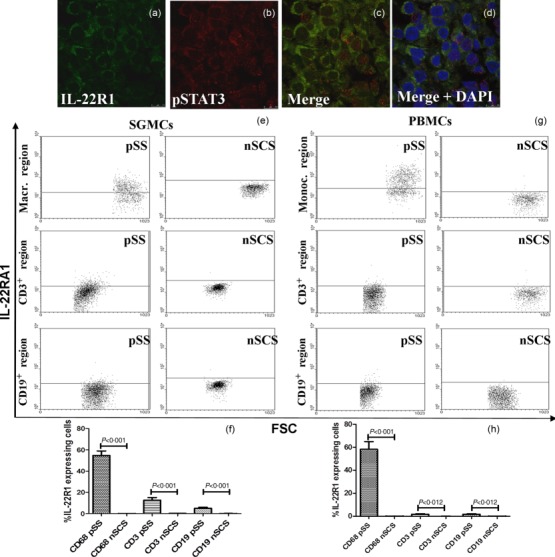
Interleukin (IL)-22R1 and p-signal transducer and activator of transcription-3 (pSTAT-3) in salivary glands and IL-22R1 expression on isolated mononuclear cells from salivary gland mononuclear cells (SGMC) and peripheral blood mononuclear cells (PBMC) of primary Sjögren’s syndrome (pSS) and non-Sjögren’s syndrome patients (nSS) subjects. (a–d) Representative images of confocal analysis of IL-22R1 and pSTAT-3 co-localization in pSS patients. (a) Single-staining for IL-22RA1 and pSTAT-3 (b). (c) Merged double-staining of IL-22R1 (red) and pSTAT-3 (green). (d) Merged double-staining of IL-22R1 and pSTAT-3 with 4’,6-diamidino-2-phenylindole (DAPI). (e) Representative dot-plot of IL-22R1 expression among SGMC from patients and controls. (f) Percentages of IL-22R1-expressing cells among SG CD68+, CD3+ and CD19+ cells. (g) Representative dot-plot of IL-22R1 expression among peripheral blood mononuclear cells (PBMCs) from patients and controls. (h) Percentages of IL-22R1-expressing cells among SG CD68+, CD3+ and CD19+ cells. IL-22R1 expression was observed strongly in circulating monocytes and tissue macrophages only in pSS patients.
The expression of IL-22R1 in MSG of pSS patients was also confirmed by flow cytometric analysis on salivary gland isolated mononuclear cells (SGMC) and on circulating blood mononuclear cells. As shown in Fig. 2e,f, IL-22R1 was expressed on the surface of macrophages (54·8 ± 14%) isolated from the SGMC of pSS but not nSCS subjects. A significant percentage of circulating monocytes (61 ± 18%) also expressed IL-22R1 in pSS (Fig. 2g,h), its expression being virtually absent in nSCS, and in healthy controls (not shown).
IL-18 and IL-22 modulates the in-vitro expression of IL-22R1
Because IL-22 was expressed mainly in MSG with the highest FS, we next evaluated whether the different modulation of IL-22R1 observed might be related to a negative feedback regulation. MSG-derived cells obtained from five patients with FS 1–2 were stimulated with recombinant IL-22 and the expression of IL-22R1 was assessed by RT–PCR. As shown in Fig. 3a, in the presence of high concentrations of IL-22 the m-RNA levels of IL-22R1 were reduced significantly. Because of the role of IL-18 in modulating the IL-22 axis 10 and the correlation between IL-22R1 and IL-18 expression in pSS, we investigated whether IL-18 might also be involved in the regulation of the IL-22 axis in salivary glands of pSS. IL-18 stimulation significantly up-regulated the expression of IL-22R1 m-RNA (Fig. 3b–d), but not of IL-22 (Fig. 3c–e), in only pSS patients, and was accompanied by a significant increase of the percentage of IL-22R1-expressing cells, evaluated by flow cytometry (Fig. 3f).
Figure 3.
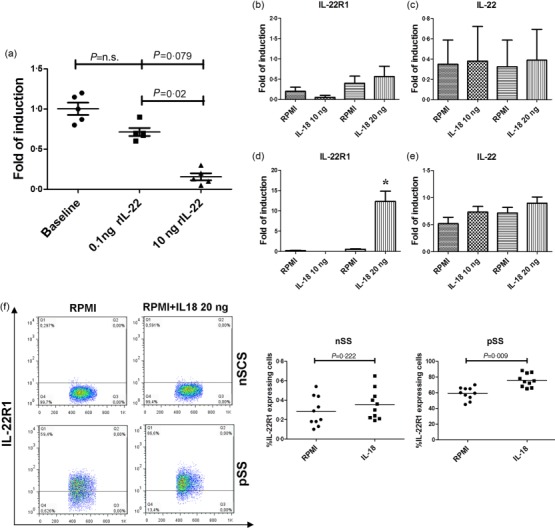
Effect of recombinant interleukin (IL)-22 on salivary gland mononuclear cells (SGMCs) and IL-18 on peripheral blood mononuclear cells (PBMCs) of primary Sjögren’s syndrome (pSS). (a) IL-22R1 mRNA expression was down-regulated after incubation of SGMCs with recombinant IL-22. PBMCs from 10 pSS and 10 non-specific chronic sialoadenitis (nSCS) subjects were isolated and cultured with 10 and 20 ng/ml of recombinant IL-18. The percentage of IL-22R1-expressing cells and the m-RNA expression levels of IL-22R1 and IL-22 were assessed by flow cytometry and reverse transcription–polymerase chain reaction (RT–PCR). (b,c) Relative m-RNA quantification of IL-22R1 (b) and IL-22 (c) was assessed in isolated PBMCs from non-Sjögren’s syndrome patients (nSS) before and after incubation with recombinant IL-18. (d,e) Relative m-RNA quantification of IL-22R1 (d) and IL-22 (e) was assessed in isolated PBMCs from pSS before and after incubation with recombinant IL-18. (f) representative dot-plot showing percentages of IL-22R1-expressing cells before and after exposure to recombinant IL-18. *P = 0·00013.
IL-22BP in the MSG of pSS patients
As the functional outcome of IL-22 seems to depend upon the levels of the soluble inhibitor IL-22BP, its expression was next assessed. IL-22BP m-RNA was significantly over-expressed (Fig. 4a) in pSS compared to nSCS. Interestingly, the highest levels of IL-22BP were observed in those pSS patients displaying the lowest FS (Fig. 4a). Conversely, IL-22BP immunoreactivity was weakly detectable in immune cells scattered among the inflammatory infiltrate, being observable only in few mononuclear cells distributed in the proximity of ducts (Fig. 4b–d) and among ductal epithelial cells (Fig. 4d). No expression of IL-22BP was observed in nSCS MSG (Fig. 4e).
Figure 4.
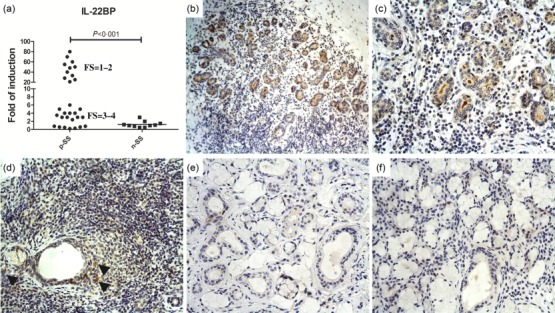
Interleukin (IL)-22BP expression in the salivary glands of patients with primary Sjögren’s syndrome (pSS patients). Relative m-RNA quantification of IL-22BP (a) was assessed by quantitative reverse transcription–polymerase chain reaction (qRT–PCR) in salivary glands obtained from 30 pSS patients and non-Sjögren’s syndrome patients (nSS). (b–d) Representative microphotographs showing IL-22BP immunostainings in pSS (b–d) and nSS (e) subjects. IL-22BP expression was weakly observed among infiltrating mononuclear cells scattering in the inflammatory infiltrates, being observable only in few mononuclear cells distributed in the proximity of ducts (d, arrows). A clear IL-22BP expression was observed in ductal epithelial cells and in the lumen ducts (b–d). (e) No significant IL-22BP immunoreactive cells were observed in nSS subjects. (f) Representative paraffin sections of salivary glands of pSS patients stained with rabbit immunoglobulins (isotype control). (b) Original magnification ×100; (c–f) Original magnification ×250.
Effect of in-vitro stimulation of PBMC and SGMC of pSS patients with IL-22
We next investigated the role of recombinant IL-22 (rIL22) in the expansion of Th17 cells in vitro stimulation on PBMC and SGMC isolated from five pSS and fice nSCS patients. rIL-22 induced a significant expansion of Th17 cells (from 0·76 ± 0·13 to 6 ± 2·4, P < 0·05) (Fig. 5a) only in pSS, and was accompanied by the significant up-regulation of IL-22, IL-17, RORc and STAT-3 m-RNA levels (Fig. 5b–e).
Figure 5.
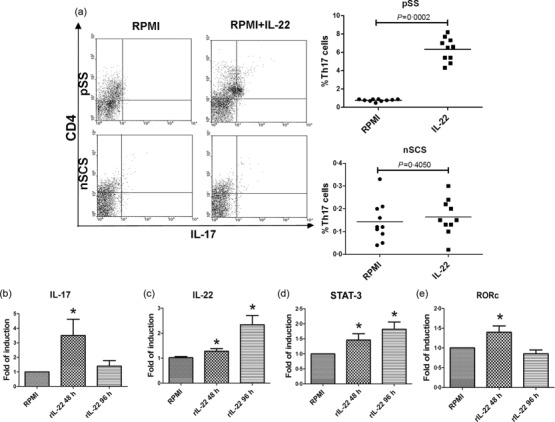
Effect of recombinant interleukin (IL)-22 on peripheral blood mononuclear cells (PBMCs) of pSS. PBMCs from 10 primary Sjögren’s syndrome (pSS) patients were isolated and cultured with 0·1 ng/ml of recombinant IL-22. The percentage of IL-17-expressing cells and the m-RNA expression levels of IL-17, IL-22, IL-22R1, signal transducer and activator of transcription-3 (STAT-3) and RAR-related orphan receptor (ROR)c were assessed by flow cytometry and reverse transcription–polymerase chain reaction (RT–PCR). (a) Representative dot plot showing the percentage of IL-17 expressing cells before and after exposure to recombinant IL-22. (b–e) Relative m-RNA quantification of IL-17 (b), IL-22 (c), STAT-3 (d) and RORc (e) was assessed in isolated PBMCs from pSS before and after incubation with recombinant IL-22. *P < 0·05.
IL 18/IL-22 axis in pSS-associated non-Hodgkin’s lymphoma
As the IL-22/IL-22R1 axis is implicated in the pathogenesis of B and T cells lymphomas with IL-22R1 expressed aberrantly on the surface of neoplastic cells 13,14, we next evaluated the expression of IL-18 and IL-22 pathways on patients with pSS who developed non-Hodgkin’s lymphoma. A similar expression of IL-22 (Fig. 6a,b) was observed in the mucosa-associated lymphoid tissue (MALT) lymphoma tissues of pSS patients compared to MSG of pSS. IL-22R1 (Fig. 6c–e) was over-expressed in the lymphoma tissues of pSS patients, mainly on the surface of B cells and tissue macrophages (Supporting information, Fig. S2d–i), and was accompanied by significant pSTAT-3 increased expression (Fig. 6f,g). Because the activated STAT-3 promotes cell proliferation and survival in B cell lymphomas 15 we next evaluated whether IL-22R1-expressing cells also co-express pSTAT-3. As shown in Fig. 6, pSTAT-3 and IL-22R1 strongly co-localize. The procancerogenic activity of IL-22 in a murine model of colon cancer has been related to the reduced levels of its natural inhibitor IL-22BP 10. IL-22BP-expressing cells were increased compared to pSS (Fig. 6l) and located exclusively in the context of lymphoepithelial lesions and virtually absent in the remaining the tissue specimens (Fig. 6i –k). Concerning the role of IL-18 in modulating the IL-22 axis in pSS patients, we next evaluated the expression of IL-18 and the relationship between IL-18 and IL-22 in pSS-associated non-Hodgkin lymphoma. IL-18 was expressed significantly in MALT lymphoma tissues of pSS at levels similar to pSS, particularly in the context of myoepithelial lesions (MESA) and in inflammatory mononuclear cells scattered throughout the neoplastic tissues (Fig. 6m–o), being correlated directly with the percentage of IL-22- and IL-22R1-positive cells/infiltrating cells (r2 = 0·45 and r2 = 0·56, respectively, P < 0·05). Conversely, IL-18-positive cells were never observed in the context of lymphoepithelial lesions and in particular in the areas of IL-22BP expression.
Figure 6.
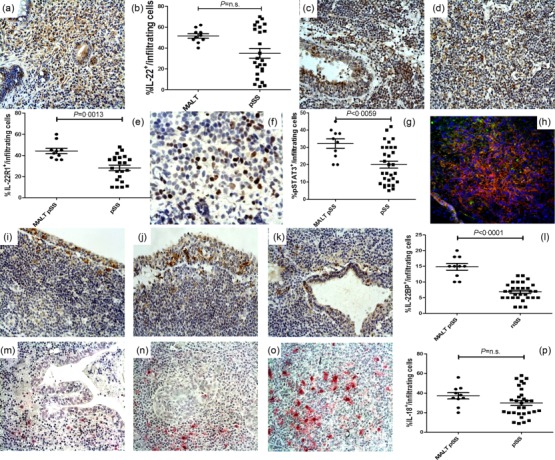
Interleukin (IL)-22R1 p-signal transducer and activator of transcription-3 (pSTAT-3) and IL-18 expression in non-Hodgkin lymphomas (NHL) of primary Sjögren’s syndrome (pSS) patients. (a,b) Representative microphotographs showing IL-22 (a), IL-22R1 (c–d), pSTAT-3 (e), IL-22BP (i–k) and IL-18 (m–o) immunostainings in NHL of pSS patients. Intense immunostainings of IL-22 (a,b) and IL-22RA1 (c–e) was observed in NHL tissues of pSS and was accompanied by the intense expression of pSTAT-3 (e,f). (h) Representative images of confocal analysis of IL-22RA1 and pSTAT-3 co-localization in NHL tissues of pSS patients. i–k: IL-22BP was expressed only in the context of lymphoepithelial lesions in NHL tissues. l: quantification of IL-22BP+ cells. (m–o) IL-18 expression was weakly observed among lymphoepithelial lesions (m) but expressed strongly in the context of myoepithelial lesion (n) and in the neoplastic tissues (o). P = quantification of IL-18+ cells a, c–d, h, i–k and m–o = original magnification ×250; (e) original magnification ×630.
Discussion
In this study we confirmed, in a new cohort of patients, the increased expression levels of IL-18 and IL-22 and provided the first evidence, to our knowledge, that (i) in pSS IL-22R1 is expressed aberrantly on haematopoietic cells at both systemic and salivary gland level; (ii) there is a tissue deficit in the expression of the natural inhibitor of IL-22, IL-22BP and (iii) the IL-22 axis seems to be functionally dependent upon IL-18 signalling. We also demonstrated that IL-18 and IL-22/IL-22R1 but not IL-22BP are over-expressed in the non-Hodgkin lymphoma tissues of pSS patients.
Primary Sjögren’s syndrome is a chronic autoimmune disease characterized by altered cytokine networks 1,5, with IL-22 over-expressed in the serum and salivary glands of pSS patients and correlated with hyposalivation, the presence of autoantibodies and the lymphocytic focus score 5,16. IL-22 is a member of the IL-10 family of proteins whose functional outcome depends upon the presence of the balance between the inducible IL-22R1 subunit and the levels of IL-22 binding protein (IL-22BP) 6. In the salivary glands of pSS, we observed a relative imbalance of IL-22/IL-22BP because the strong amount of IL-22 in the context of inflammatory infiltrate was not accompanied by a concomitant expression of IL-22BP. IL-22BP immunoreactivity was, in fact, observed predominantly in the ducts lumen of pSS, suggesting its predominant epithelial production and lumen secretion, while IL-22 positivity among mononuclear cells correlated inversely with that of IL-18. The level of IL-22BP seems to be controlled by IL-18 10, a cytokine that belongs to the IL-1 superfamily 8 and that synergizing with IL-12 and IL-23 also enhances IL-22 levels in Th1 and γδ T cells, respectively 9. IL-18 is released as an immature form and then processed into its mature forms through the activation of the NLRP3 inflammasome via the purinergic P2X7 receptor 8. In pSS, both IL-18 and P2X7R are over-expressed and correlated with local and systemic clinical indicators of the degree of the disease 4,17, suggesting a direct role of the P2X7R–inflammasome complex and of IL-18 in the pathogenesis of pSS.
Here we speculate that increased IL-18 expression, observed in the MSG of pSS, may be implicated in the dysfunctional behaviour of the IL-22 axis. IL-18 was correlated significantly with the expression level of IL-22 and IL-22R1 in the inflammatory infiltrates of pSS patients. Furthermore, IL-18 stimulation seems to be sufficient to up-regulate IL-22R1 significantly on PBMCs of pSS, without apparent effect on IL-22 levels; this is related possibly to the absence, in our in-vitro experiments, of other proinflammatory cytokines required for the expression of IL-22 in immune cells. IL-18 has been demonstrated previously to reduce the levels of IL-22BP in isolated PBMC of normal subjects 9. In our study, an inverse distribution was found between IL-18 and IL-22BP in MSG of pSS patients, apparently confirming the important role of IL-18 in also negatively modulating the IL-22BP behaviour in pSS.
The suggested IL-18-dependent increased expression of IL-22R1 might be relevant in the pathogenesis of pSS. IL-22R1 expression has been demonstrated to be restricted, in physiological conditions, only to non-haematopoietic cells 6. Using immunohistochemistry and flow cytometry, we were able to demonstrate that IL-22R1 aberrant expression occurs on haematopoietic cells of pSS patients at both local and systemic levels and that IL-18 significantly modulated the expression of IL-22R1 on these cells. Over-expression of IL-22R1 in the SG seems to be specific to pSS and not related to the presence of a significantly higher proportion of macrophages compared to controls. Using RT–PCR, however, we observed a different regulation of its expression. In patients with lower FS, IL-22R1 expression was not different compared to controls, being down-regulated significantly in those with higher FS. This finding was surprising, as the strong protein expression of IL-22R1 was confirmed analysing the whole SG rather than the isolated SGMC of pSS. Dissociation of messenger RNA and protein expression in human lymphoid tissues has been demonstrated previously 18, and the contrasting behaviour of IL-22R1 protein and RNA in pSS tissues may suggest that translational and post-translational control mechanisms play a significant role in regulating IL-22R1 levels. We could not exclude, however, the occurrence of a counter-regulation mechanism due to the excessive IL-22/IL-22R1 interaction, as demonstrated by the significant reduction of IL-22R1 mRNA levels we observed in SGMCs cultured in the presence of increasing amounts of IL-22. High infiltration levels of macrophages has been shown in the autoimmune lesions of pSS with lymphoma 19 and, in our study, macrophages located in close proximity to CD20+ cells were the main cells expressing IL-22R1. As IL-22 seems to be involved in the germinal centre formation, probably by driving and maintaining CXCL13 production 20, IL-22R1-expressing macrophages could participate strategically in the organization of immune response in pSS. Interestingly, the abnormal expression of IL-22R1 on haematopoietic cells was accompanied by a concomitant expression of pSTAT-3 suggesting strongly, in our opinion, the existence of an autocrine IL-22 stimulatory loop in pSS. Notably, IL-22R1 aberrant expression was also observed on peripheral monocytes and, to a lesser extent, on T lymphocytes from pSS patients. Conversely, IL-22R1 was never expressed on the surface of immune cells of PBMC isolated from NSCS patients and normal controls. Taken together, these results seem to suggest that IL-22R1 aberrant expression at both local and systemic level is a specific feature of pSS patients.
In the present study, we also examined the immunological consequences of the atypical expression of IL-22R1 in pSS. In-vitro stimulation of PBMCs with IL-22 only in pSS resulted in a significant up-regulation of the m-RNA levels of IL-17, IL-22, ROR-c and STAT-3, accompanied by a significant expansion of IL-17-producing CD3-expressing cells. These results are in agreement with those obtained in previously published studies that analysed patients with ALK+ anaplastic large cell lymphoma (ALCL) and IL-22R1 transgenic mice 13,21. Patients with ALK+ALCL, who abnormally express the IL-22R1 on lymphomatous T cells, immunologically display increased serum levels of IL-22 and IL-17 13. Similarly, IL-22R1 transgenic animals that express IL-22R1 on lymphocytes develop a systemic inflammatory disease characterized by increased levels of circulating IL-17, granulocyte colony-stimulating factor and IL-22 21. Taken together, these findings suggest that the atypical expression of IL-22R1 on haematopoietic cells generates a strong IL-22-polarized immune response in a positive autoregulatory loop. The differential IL-22R1 expression observed in NSCS patients and controls highlights, in our opinion, the importance of a tightly regulated IL-22R1 expression on immune cells in preventing systemic inflammatory reactions. In physiological conditions, IL-22 acts as a proinammatory cytokine but only on epithelial linings of mucosal barriers (the only cells expressing IL-22R1) to defend rapidly against pathogens 7.
Sjögren’s syndrome is associated with highly increased risk factors for the development of salivary gland lymphoma 2. The IL-22/IL-22R1/STAT-3 axis has been demonstrated previously to play a pivotal role in human tumorigenesis, with IL-22R1 being expressed aberrantly on the surface of B and T lymphomatous cells 13,14,22–25. Interestingly, it seems that the balance between IL-22 and its soluble antagonist IL-22BP, more than the expression of IL-22 per se, critically regulates tumorigenesis 10. Although the comparison between MSGs and parotid gland expression of cytokine and cytokine receptor might not, necessarily, be biologically significant, in our study IL-22 was expressed consistently in the lymphoma tissues of pSS patients and was accompanied by an aberrant IL-22R1 over-expression on lymphoma-infiltrating cells. The abnormal IL-22R1 expression was also associated with the co-expression of pSTAT-3, suggesting that a persistent IL-22 stimulation may be of biological importance in the growth of non-Hodgkin lymphoma. Furthermore, activation of IL-22 pathway seemed to be not counter-balanced sufficiently by a parallel increased expression of IL-22BP, as the latter was localized exclusively in correspondence of lymphoepithelial lesions, and virtually absent in the remaining parts of the neoplastic tissue. Interestingly, the IL-22 axis activation in lymphoma tissues of pSS was correlated directly and strongly with IL-18. Tissue expression of IL-18 has been suggested to be a histopathological indicator of the risk of lymphomagenesis in pSS being correlated with the occurrence of clinical and serological indicators of a high risk of B cell lymphoma, such as persistent C4 hypocomplementaemia and salivary gland enlargement 26. Although IL-18 cannot be considered a direct causative agent of lymphoma in pSS, our observation of the mutually exclusive distribution of IL-18 and IL-22BP in lymphoma tissues may be relevant, IL-22BP being absent when IL-18 was present and vice versa.
In conclusion, we have provided evidence of an imbalance between IL-22 and IL-22BP and of the aberrant expression of IL-22R1 in MSG and PBMC of pSS patients, also providing demonstration of the IL-22R1 functional relevance. We have also demonstrated that specific aberrant activation, IL-18-driven, of the IL-22R1/STAT-3 pathway occurs in the inflamed salivary glands and NHL lymphoma of pSS patients. Blocking the aberrant IL-18/IL-22 pathway may be a useful therapeutic strategy for pSS and pSS-associated lymphomas.
Disclosure
The authors declare no financial conflicts of interest.
Acknowledgments
We thank Dr Francesca Raiata for her technical support in the immunohistochemical experiments. This work was supported in part by a grant from the Ministry of the University and Scientific Research of Italy.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Fig. S1. Interleukin (IL)-18 and IL-22 expression in primary Sjögren’s syndrome (pSS) and non-specific chronic sialoadenitis (nSCS) patients. (a–c) Representative microphotographs showing IL-22 immunostainings in nSCS (a) and pSS (b,c) patients. (d–f) Representative microphotographs showing IL-18 immunostainings in nSCS (d) and pSS (e,f) patients. (g,h) Relative m-RNA quantification of IL-22 (g) and IL-18 (h) was assessed by quantitative reverse transcription–polymerase chain reaction (qRT–PCR) in salivary glands obtained from 30 pSS and nSCS patients. (a–f) Original magnification ×250.
Fig. S2. Interleukin (IL)-22R1 expressing cells in mucosa-associated lymphoid tissue (MALT) lymphoma tissues of primary Sjögren’s syndrome (pSS) patients. (a–c) Representative images of confocal analysis of IL-22R1 and CD3 co-localization in MALT tissues. (a,b) Single-staining for IL-22RA1 (a) and CD3 (b). (c) Merged double-staining of CD3 (red) and IL-22RA1 (green). (d–f) Representative images of confocal analysis of IL-22R1 and CD19 co-localization in MALT tissues. (d,e) Single-staining for IL-22RA1 (d) and CD3 (e). (f) Merged double-staining of CD19 (red) and IL-22RA1 (green). (g–i) Representative images of confocal analysis of IL-22R1 and CD68 co-localization in MALT tissues. (g,h) Single-staining for IL-22RA1 (g) and CD68 (h). (i) Merged double-staining of CD68 (red) and IL-22RA1 (green). (a–i) Original magnification ×250.
References
- Roescher N, Tak PP, Illei GG. Cytokines in Sjogren’s syndrome: potential therapeutic targets. Ann Rheum Dis. 2010;69:945–8. doi: 10.1136/ard.2009.115378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias C, Isenberg DA. Susceptibility of patients with rheumatic diseases to B-cell non-Hodgkin lymphoma. Nat Rev Rheumatol. 2011;7:360–8. doi: 10.1038/nrrheum.2011.62. [DOI] [PubMed] [Google Scholar]
- Liang Y, Yang Z, Qin B, Zhong R. Primary Sjogren’s syndrome and malignancy risk: a systematic review and meta-analysis. Ann Rheum Dis. 2014;73:1151–6. doi: 10.1136/annrheumdis-2013-203305. [DOI] [PubMed] [Google Scholar]
- Bombardieri M, Barone F, Pittoni V. Increased circulating levels and salivary gland expression of interleukin-18 in patients with Sjögren’s syndrome: relationship with autoantibody production and lymphoid organization of the periductal inflammatory infiltrate. Arthritis Res Ther. 2004;6:R447–56. doi: 10.1186/ar1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccia F, Guggino G, Rizzo A, et al. Potential involvement of IL-22 and IL-22-producing cells in the inflamed salivary glands of patients with Sjogren’s syndrome. Ann Rheum Dis. 2012;71:295–301. doi: 10.1136/ard.2011.154013. [DOI] [PubMed] [Google Scholar]
- Rutz S, Eidenschenk C, Ouyang W, et al. IL-22, not simply a Th17 cytokine. Immunol Rev. 2013;252:116–32. doi: 10.1111/imr.12027. [DOI] [PubMed] [Google Scholar]
- Lin C, Savan R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev. 2014;25:257–71. doi: 10.1016/j.cytogfr.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney AL. IL-22, a Th1 cytokine that targets the pancreas and select other peripheral tissues. Int Immunopharmacol. 2004;4:669–77. doi: 10.1016/j.intimp.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Zenewicz LA. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259–63. doi: 10.1038/nature11535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjögren’s syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan JS, Daniels TE, Talal N, Sylvester RA, et al. The histopathology of Sjogren’s syndrome in labial salivary gland biopsies. Oral Surg Oral Med Oral Pathol. 1974;37:217–29. doi: 10.1016/0030-4220(74)90417-4. [DOI] [PubMed] [Google Scholar]
- Bard JD, Gelebart P, Anand M, Amin HM, Lai R. Aberrant expression of IL-22 receptor 1 and autocrine IL-22 stimulation contribute to tumorigenicity in ALK+ anaplastic large cell lymphoma. Leukemia. 2008;22:1595–603. doi: 10.1038/leu.2008.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelebart P, Zak Z, Dien-Bard J, Anand M, Lai R. Interleukin 22 signaling promotes cell growth in mantle cell lymphoma. Transl Oncol. 2011;4:9–19. doi: 10.1593/tlo.10172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding BB, Yu JJ, Yu RY. Constitutively activated STAT3 promotes cell proliferation and survival in the activated B-cell subtype of diffuse large B-cell lymphomas. Blood. 2008;111:1515–23. doi: 10.1182/blood-2007-04-087734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie TN, Stewart CM, Berg KM, Li Y, Nguyen CQ, et al. Expression of interleukin-22 in Sjögren’s syndrome: significant correlation with disease parameters. Scand J Immunol. 2011;74:377–82. doi: 10.1111/j.1365-3083.2011.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldini C, Rossi C, Ferro F. The P2X7 receptor-inflammasome complex has a role in modulating the inflammatory response in primary Sjögren’s syndrome. J Intern Med. 2013;274:480–9. doi: 10.1111/joim.12115. [DOI] [PubMed] [Google Scholar]
- Chleq-Deschamps CM, LeBrun DP, Huie P, et al. Topographical dissociation of BCL-2 messenger RNA and protein expression in human lymphoid tissues. Blood. 1993;81:293–8. [PubMed] [Google Scholar]
- Christodoulou MI, Kapsogeorgou EK, Moutsopoulos HM, et al. Characteristics of the minor salivary gland infiltrates in Sjögren’s syndrome. J Autoimmun. 2010;34:400–7. doi: 10.1016/j.jaut.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Khader SA, Guglani L, Rangel-Moreno J. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol. 2011;187:5402–7. doi: 10.4049/jimmunol.1101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savan R, McFarland AP, Reynolds DA, et al. A novel role for IL-22R1 as a driver of inflammation. Blood. 2011;117:575–84. doi: 10.1182/blood-2010-05-285908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZJ, Zhou Q, Yin W, et al. Interleukin 22-producing CD4+ T cells in malignant pleural effusion. Cancer Lett. 2012;326:23–32. doi: 10.1016/j.canlet.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Curd LM, Favors SE, Gregg RK, et al. Pro-tumour activity of interleukin-22 in HPAFII human pancreatic cancer cells. Clin Exp Immunol. 2012;168:192–9. doi: 10.1111/j.1365-2249.2012.04570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagaki T, Sugaya M, Suga H. IL-22, but not IL-17, dominant environment in cutaneous T-cell lymphoma. Clin Cancer Res. 2011;17:7529–38. doi: 10.1158/1078-0432.CCR-11-1192. [DOI] [PubMed] [Google Scholar]
- Jiang R, Tan Z, Deng L, et al. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900–9. doi: 10.1002/hep.24486. [DOI] [PubMed] [Google Scholar]
- Manoussakis MN, Boiu S, Korkolopoulou P, et al. Rates of infiltration by macrophages and dendritic cells and expression of interleukin-18 and interleukin-12 in the chronic inflammatory lesions of Sjögren’s syndrome: correlation with certain features of immune hyperactivity and factors associated with high risk of lymphoma development. Arthritis Rheum. 2007;56:3977–88. doi: 10.1002/art.23073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Interleukin (IL)-18 and IL-22 expression in primary Sjögren’s syndrome (pSS) and non-specific chronic sialoadenitis (nSCS) patients. (a–c) Representative microphotographs showing IL-22 immunostainings in nSCS (a) and pSS (b,c) patients. (d–f) Representative microphotographs showing IL-18 immunostainings in nSCS (d) and pSS (e,f) patients. (g,h) Relative m-RNA quantification of IL-22 (g) and IL-18 (h) was assessed by quantitative reverse transcription–polymerase chain reaction (qRT–PCR) in salivary glands obtained from 30 pSS and nSCS patients. (a–f) Original magnification ×250.
Fig. S2. Interleukin (IL)-22R1 expressing cells in mucosa-associated lymphoid tissue (MALT) lymphoma tissues of primary Sjögren’s syndrome (pSS) patients. (a–c) Representative images of confocal analysis of IL-22R1 and CD3 co-localization in MALT tissues. (a,b) Single-staining for IL-22RA1 (a) and CD3 (b). (c) Merged double-staining of CD3 (red) and IL-22RA1 (green). (d–f) Representative images of confocal analysis of IL-22R1 and CD19 co-localization in MALT tissues. (d,e) Single-staining for IL-22RA1 (d) and CD3 (e). (f) Merged double-staining of CD19 (red) and IL-22RA1 (green). (g–i) Representative images of confocal analysis of IL-22R1 and CD68 co-localization in MALT tissues. (g,h) Single-staining for IL-22RA1 (g) and CD68 (h). (i) Merged double-staining of CD68 (red) and IL-22RA1 (green). (a–i) Original magnification ×250.