Abstract
The aim of this study was to examine the levels of endoplasmic reticulum (ER) stress in minor salivary glands, to investigate the interplay between ER stress-induced autophagy and apoptosis in human salivary gland (HSG) cells and to test the effect of ER stress-induced apoptosis on the cellular redistribution of the two major Sjögren’s syndrome (SS) autoantigens Ro/Sjögren’s syndrome-related antigen A (SSA) and La/Sjögren’s syndrome-related antigen B (SSB). Minor salivary gland biopsies from SS patients and sicca controls were examined by immunohistochemistry for the expression of 78 kDa glucose-regulated protein/binding immunoglobulin protein (GRP78/BiP) as an indicator of unfolded protein response (UPR). HSG cells were treated with thapsigargin (TG) and cell viability, autophagy and apoptosis were assessed. Immunoblot was applied to detect the conversion of LC3I to LC3II and the protein levels of GRP78/BiP and X-box binding protein-1 (XBP-1). Apoptosis was evaluated by a single-stranded DNA enzyme-linked immunosorbent assay (ELISA). Ro/SSA and La/SSB localization was visualized using immunofluorescence. GRP78/BiP was expressed by acinar and ductal epithelial cells in salivary glands of patients and sicca controls. TG treatment induced autophagy, as indicated by enhanced protein expression of LC3II. The protein levels of UPR marker XBP-1 were increased after TG treatment, while GRP78/BiP levels were decreased. TG treatment resulted in induction of HSG apoptosis. Ro/SSA and La/SSB autoantigens were localized predominantly to the cytoplasm in resting cells, while they were redistributed to cell membrane and blebs in the apoptotic cells. In conclusion, ER stress is activated in minor salivary gland epithelial cells from SS patients and controls. ER stress-induced apoptosis in HSG cells leads to cell surface and apoptotic blebs relocalization of Ro/SSA and La/SSB autoantigens.
Keywords: autophagy, apoptosis, autoimmunity, ER stress, epithelial cells, La/SSB, Ro/SSA, Sjogren’s syndrome
Introduction
Sjögren’s syndrome (SS) is an autoimmune disorder in which the main targets of immune injury are specific secretory epithelial tissues, such as salivary and lacrymal glands and, less frequently, bronchioles, cholangia and distal kidney tubules 1.
A hypothesis for the initiation and perpetuation of the immune attack is that activation of secretory epithelial cells, following a chronic or acute external or internal insult, leads to surface expression of autoantigens such as Ro/Sjögren’s syndrome-related antigen A (SSA) and La/Sjögren’s syndrome-related antigen B (SSB) which trigger an autoimmune response.
The relocalization of the autoantigens is a major pathophysiological event, which has been observed in situ and in vitro after activation of epithelial cells 2 and may reflect the local production 3 and secretion of relevant autoantibodies in the saliva 4. The presence of autoantibodies in the sera of patients can be found many years before the clinical presentation of the disease is apparent 5. All these facts and hypotheses guide investigators to further dissect the aetiology of epithelial cell activation and direct their studies to identify markers for preclinical diagnosis of the disease and thus early therapeutic intervention.
Previous experimental data showed that apoptotic keratinocytes lead to relocalization of Ro/SAA and La/SSB to the cell surface 6, while recent data showed that enhanced apoptosis, by disruption of signal transducer and activator of transcription-3 (STAT-3)-IκB-ζ signalling pathway in mouse epithelial cells, induces Sjögren’s syndrome-like autoimmune disease with the characteristic tissue lesion as well as the presence of autoantibodies to Ro/SSA and La/SSB autoantigens 7. Therefore, apoptosis seems to be a key mechanism for the initiation of an autoimmune response.
The epithelial cells of the targeted tissues of SS have a common characteristic. They constantly secrete fluid rich of different proteins, and this process is supported by extended endoplasmic reticulum machinery. Studies over the past four decades have demonstrated that Ca2+ plays a crucial role in the control of salivary gland function and salivary fluid secretion 8.
The endoplasmic reticulum (ER) is a dynamic intracellular cell compartment for the synthesis and folding of secreted and transmembrane proteins as well as for regulating calcium balance. In response to cellular stress, a signalling cascade, the unfolded protein response (UPR), is activated to re-establish cellular homeostasis 9. The UPR initially facilitates the folding or degradation of unfolded proteins and elicits a maximum response to ER stress. If the UPR is not able to correct the misfolded or unfolded proteins, autophagy is induced leading to degradation of terminally misfolded ER proteins. However, if the UPR is overwhelmed, apoptosis is initiated 10. Induction of ER stress can be produced in different cells by various danger signals including, among others, energy deprivation, viral infection and catecholamine effects 9.
Our hypothesis is that, in SS, non-immunological factors that can induce ER stress in secretory epithelial cells may lead to autophagic and apoptotic processes rendering them immunogenic, as the autoantigens Ro/SSA and La/SSB may be relocated from the nucleus and the cytoplasm to the cell surface.
In the present study we attempted to examine the levels of ER stress activation in minor labial salivary gland tissue from SS patients and control individuals, to identify the interplay between ER stress-induced autophagy and apoptosis and to test the effect of ER stress-induced apoptosis on the cellular redistribution of the two major SS autoantigens Ro/SSA and La/SSB. We tested the changes of the UPR-related signals, the cell-death time profile and the cell redistribution of autoantigens on a human salivary gland (HSG) epithelial cell line exposed to thapsigargin (TG), an ER stress inducer.
Materials and methods
Cell cultures and chemicals
The HSG cell line was purchased from European Collection of Cell Cultures (ECACC no. 95031024; ECACC, Salisbury, UK). HSG cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS), 1% penicillin/streptomycin and 2 mM L-glutamine in a humidified 5% CO2 atmosphere at 37°C. The compounds TG and 3-methyladenine (3-MA) were from Calbiochem (La Jolla, CA, USA) and 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium (MTT) was from Applichem (Darmstadt, Germany). Protease inhibitor cocktail was from Sigma (P8340). Anti-78 kDa glucose-regulated protein/binding immunoglobulin protein (GRP78/BiP) antibody was from Cell Signaling (Beverly, MA, USA), anti-XBP1 antibody and anti-LC3 I/II antibody were from Novus Biologicals (Littleton, CO, USA). Primary polyclonal antibodies against Ro52/SSA1 (PA5-22294) and La/SSB (PA5-29763) were from ThermoScientific Pierce (Rockford, IL, USA). All other reagents were obtained from Sigma-Aldrich unless noted otherwise.
Tissue samples
Minor salivary gland specimens were obtained by biopsy (after informed consent was received) from 12 individuals undergoing diagnostic evaluation of sicca symptoms indicative of SS at the outpatient Autoimmune Diseases Clinic at Athens Euroclinic. In eight patients (all women) in this study, primary SS was diagnosed on the basis of the American–European Consensus criteria for primary SS 11. The control group comprised four individuals (all women) who had subjective xerostomia but normal labial minor salivary gland biopsy and who did not fulfil the classification criteria for SS 11. None of the patients or controls had evidence of IgG4 syndrome, lymphoma, sarcoidosis, essential mixed cryoglobulinaemia or infection by hepatitis B or C virus or human immunodeficiency virus.
Immunohistochemical analysis
The in-situ expression of GRP78/BiP in paraffin-embedded labial minor salivary gland biopsy specimens was analysed by standard technique, using the EnVision system (Dako, Glostrup, Denmark) and anti-GRP78/BiP monoclonal antibody in a dilution 1/400. Antigen retrieval was performed by microwaving in 10 mM citrate buffer (pH 6·0). Normal non-immune fetal bovine serum and 0·5% H2O2 in methanol were used to block non-specific antibody binding and endogenous peroxidase activity, respectively. Negative control stainings were performed by replacing primary antibody with irrelevant isotype-matched antibody. Staining was developed using diaminobenzidine tetrahydrochloride. All sections were counterstained with haematoxylin.
Viability assay
The viability of the cultured cells was measured using the MTT assay. HSG cells were plated in 96-well plates at 1·0 × 105 cells/ml cell density 48 h prior to induction of ER stress. HSG cells were treated with TG at 10 μM for the indicated times to induce ER stress. The cells were then incubated with 5 mg/ml MTT added directly in the medium for 4 h at 37°C. The medium was then aspirated and the formazan crystals were solubilized with dimethylsulphoxide (DMSO). The formazan solution intensity is proportional to the number of living cells present in culture. Absorbance was determined in a microplate reader at 540 nm, and results are presented as the percentage of optical density in the treated wells versus the untreated controls.
Protein isolation
After treatment with the appropriate agents, cells grown in six-well plates were washed twice with ice-cold phosphate-buffered saline (PBS) and scraped into chilled PBS with protease inhibitors. The cells were pelleted and the pellets were resuspended in cell lysis buffer containing protease inhibitor cocktail. Cells were passed through a 21-gauge needle, followed by incubation on ice for 20 min. After centrifugation, the supernatants were collected and assayed for protein concentration using the Bradford method (BioRad, Richmond, CA, USA).
Western blot analysis
Twenty μg of each protein sample was resolved on 4–15% sodium dodecyl sulphate–polyacrylamide gel electrophoresis gels and transferred electrophoretically to nitrocellulose (BioRad) for incubation with the appropriate antibodies. Blots were then probed with peroxidase-conjugated species-appropriate secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and visualized by enhanced chemiluminescence (Amersham Biosciences, Roosendaal, the Netherlands) and exposed to an X-ray film (Kodak, Stuttgart, Germany). Equal protein loading was verified using an anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) monoclonal antibody (Santa Cruz Biotechnology).
Evaluation of apoptosis
After induction of ER stress, HSG cell apoptosis was measured using a commercial single-stranded DNA (ssDNA) enzyme-linked immunosorbent assay kit (Millipore Chemicon, Temecula, CA, USA), which detects ssDNA, corresponding to the most specific apoptosis end-product.
Immunofluorescence
Cells were cultured in chamber slides (Lab-Tek; Nunc, Roskilde, Denmark), fixed with 4% paraformaldehyde (PFA) and permeabilized with PBS containing 0·1% Triton X-100. Non-specific binding was blocked with 2% dry milk in Tris-buffered saline and Tween 20 (TBST) for 1 h. Slides were incubated overnight at 4°C with primary polyclonal antibodies against Ro52/SSA1 and La/SSB or with anti-LC3 I/II antibodies. After washing, slides were incubated for 1 h with AlexaFluor-488-conjugated secondary antibody (ThermoScientific) and nuclei were stained with propidium iodide (PI) (Sigma-Aldrich). Slides were covered with fluorescent mounting medium (Dako) and analysed with a fluorescence microscope (Carl Zeiss, Zena, Germany).
Statistical analysis
Results are expressed as the mean ± standard deviation (s.d.) of three independent experiments. Statistical significance was determined using t-test analysis (Graphpad Prism 6). P-values less than 0·05 were considered significant.
Results
ER stress-induced chaperone protein GRP78/BiP was expressed selectively by acinar and ductal epithelial cells of salivary glands from both SS patients and sicca controls
Glucose-regulated protein 78 kD GRP78/BiP is one of the best-characterized ER luminal proteins required for the folding of misfolded or unfolded proteins 9 within the ER. During ER stress and unfolded protein accumulation, GRP78/BiP is released from the proteins that normally repress each of the three arms of the UPR signalling pathway protein kinase receptor (PKR)-like ER kinase (PERK), activating transcription factor 6 (ATF6), and inositol requiring-1 (IRE-1), resulting in UPR activation. Using immunohistochemistry, GRP78/BiP was detected in the cytoplasm of acinar and ductal epithelial cells of labial minor salivary glands of all SS patients and sicca controls (Fig. 1a,b). The percentage of epithelial cells expressing the protein was 30–50%. There were no differences on GRP78/BiP expression in the tissues of sicca controls and SS patients. In labial minor salivary gland epithelial cells of patients, the percentage of positive epithelial cells did not correlate with the intensity of the lymphocytic infiltrates evaluated by Chisholm grading score 12. The infiltrating cells were rarely positive for GRP78/BiP in seven of eight patient biopsies, while it was intense and extensive in one (data not shown).
Figure 1.
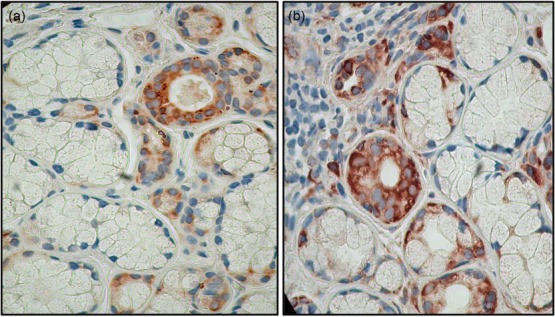
78 kDa glucose-regulated protein/binding immunoglobulin protein (GRP78/BiP) is expressed by some acinar and ductal labial salivary gland epithelial cells from Sjögren’s syndrome (SS) patients and controls. (a). Salivary gland biopsy from a sicca control. GRP78/BiP is detected in acinar and ductal cells. (b). Salivary gland biopsy from a SS patient. Selective acinar and ductal cells express the protein in their cytoplasm. The intensity as well the number of cells that express GRP78/BiP did not show any correlation with the severity of the infiltrates. A minority of infiltrating cells also expressed GRP78/BiP (magnification ×200).
ER stressor TG induces the UPR in HSG cells
Treatment with TG leads to emptying of ER Ca2+ stores and induces a profound ER stress response 13. To identify the appropriate TG concentration, we performed a cell viability assay using MTT. TG was found to decrease the number of viable cells after 24 h of treatment in a dose-dependent manner (Fig. 2a). The viability in the TG-treated cells decreased significantly to 77·1 ± 2% at 0·1 μM and to 51·56 ± 3·5% at 10 μM after 24 h (Fig. 2a). A concentration of 10 μM of TG was further used for testing its effect on UPR and autophagy.
Figure 2.
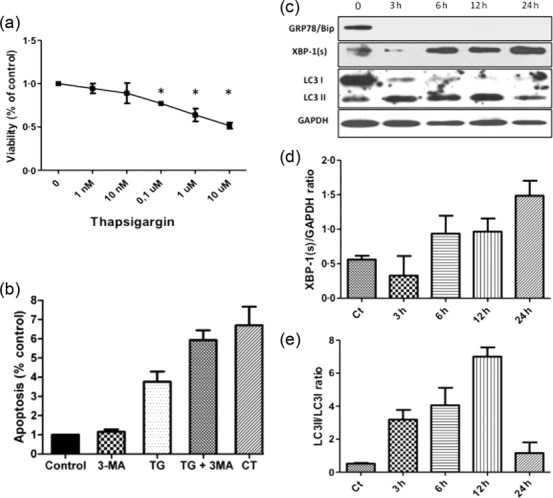
Endoplasmic reticulum (ER) stressor thapsigargin (TG) induces the unfolded protein response (UPR), activates autophagy, and induces apoptosis in human salivary gland (HSG) cells. (a) Cell viability was assessed by the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium (MTT) assay. HSG cells were exposed to the indicated concentrations of TG for 24 h. Data are expressed as the mean ± standard deviation percentage of the control (0). Control indicates HSG viability without TG (100%). Results are representative of three independently performed experiments. *P < 0.05 versus control, by t-test. (b) Cell apoptosis was evaluated using ssDNA detection kit. Untreated HSG cells served as negative control, indicating only spontaneous apoptosis, and HSG cells treated with camptothecin 5 μM (CT) was used as positive control. (c) Following incubation with 10 μM thapsigargin for 3, 6, 12 and 24 h protein lysates were subjected to immunoblot analysis using antibodies against 78 kDa glucose-regulated protein/binding immunoglobulin protein (GRP78/BiP), spliced X-box binding protein 1 (XBP-1), light chain 3 (LC3)I/II and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The relative protein levels of XPP-1s to GAPDH (d) and LC3II to LC3I (e) were quantified with Image Lab software (BioRad). Graphs represent means and standard deviation of three experiments. Ct indicates 0 time-point without treatment.
The protein levels of X-box binding protein-1 (XBP-1) and GRP78/BiP were determined by immunoblot analysis. XBP-1 is a ubiquitously expressed transcription factor that increases the transcription of UPR target genes. XBP-1 becomes transcriptionally active when ER transmembrane proteins PERK, ATF6 and IRE1 are released from GRP78/Bip 14,15. As shown in Fig. 2b,c, TG increased spliced XBP-1 protein levels in HSG cells in a time-dependent manner with the maximum effect observed at 24 h. GRP78/BiP protein levels were decreased rapidly and were not detectable as early as 3 h after TG treatment.
ER stressor TG induces autophagy in HSG cells
To investigate whether a conventional ER stress inducer elicits autophagy, HSG cells were treated with 10 μM TG (TG) for 3, 6, 12 or 24 h. Immunoblotting was employed to analyse conversion of the cytoplasmic form of microtubule-associated protein 1 light chain 3 (LC3-I) to the membrane-bound form (LC3-II), which reflects the number of autophagosomes formed, and this is indicative of autophagy induction. After treatment with TG, LC3-I was converted to LC3-II in a time-dependent manner (Fig. 2c,e), indicating the induction of autophagy. After 24 h of treatment with TG autophagy was attenuated. This may reflect the induction of cell death under constant ER stress conditions. Immunofluorescence staining demonstrated the distribution and appearance of LC3 punctae in untreated HSG and in cells exposed to 10 μM TG for 24 h, as shown in Fig. 3a,d, respectively. Remarkably, this effect was attenuated after treatment with the autophagy inhibitor 3-MA, an inhibitor of the class III phosphoinositide 3-kinase. As shown in Fig. 3g, 3-MA inhibited the formation of vacuoles in TG-treated cells. These data demonstrate clearly that ER stressor TG activates autophagy in HSG cells.
Figure 3.
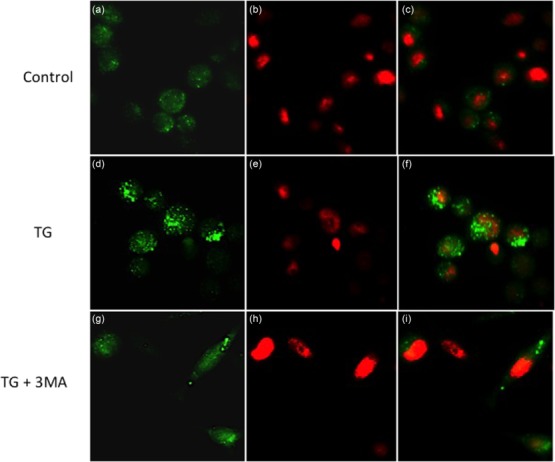
Endoplasmic reticulum (ER) stressor thapsigargin (TG) activates autophagy in human salivary gland (HSG) cells. Cells were cultured in chamber slides with or without 10 μM TG and/or the autophagy inhibitor 3-MA for 24 h and were then analysed by immunofluorescence microscopy using anti-light chain 3 (LC3)I/II antibody (green). Nuclear DNA was stained with PI (red). Note the increased LC3-positive punctae formation in the TG-treated cells (d). Magnification × 200.
ER stress induces apoptosis in HSG cells
In order to investigate the effect of UPR activation on cell death, we first tested the HSG cells for apoptosis by testing the production of ssDNA. The levels of ssDNA were increased by 3·75 ± 0·54-fold after treatment with 10 μM TG for 24 h (Fig. 2b), indicating that UPR activation induces a number of secondary events that over a period of 24 h leads to the demise of the cells. This observation highlights a role for apoptotic pathways after UPR activation in salivary gland epithelial cells
The effect of autophagy inhibition on apoptosis after ER stress
To investigate further the role of autophagy in cell death after ER stress induction, HSG cells were treated with TG in the presence or absence of the autophagy inhibitor 3-MA. Treatment of HSG with 3-MA increased tremendously the percentage of TG-induced apoptosis by 5·9 ± 0·5-fold (Fig. 2b). 3-MA alone did not affect the apoptotic process (Fig. 2b), and did not produce additional damage to TG-treated cells. These data indicate that autophagy might partially compensate for an impaired endoplasmic reticulum, as an active autophagic pathway was protective during TG treatment.
Cellular relocalization of Ro52/SSA and La/SSB autoantigen in non-apoptotic and apoptotic HSG cells
We next sought to investigate the possible effect of ER stress-induced apoptosis in the cellular distribution of the two major autoantigens Ro52/SSA and La/SSB. Immunofluorescence staining demonstrated that Ro52/SSA and La/SSB autoantigens are expressed in a relatively diffuse cytoplasmic pattern in the untreated HSG cells with low and partial staining of the nucleus (Figs 4a and 5a). In the same cells, nuclei stained positive for PI and showed a normal round-matrix pattern.
Figure 4.
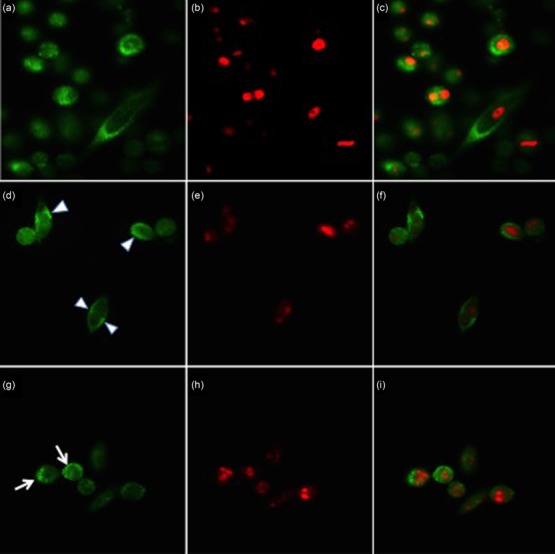
Subcellular localization of La/Sjögren’s syndrome-related antigen B (SSB) antigen under normal conditions and apoptosis in vitro, as shown by immunofluorescence staining of human salivary gland (HSG) cells. La/SSB (SSB) was stained with polyclonal antibody followed by AlexaFluor-488 secondary antibody (green) and nuclear DNA was stained with propidium iodide (PI) (red). In (a), under normal conditions La/SSB is increased in the cytoplasm showing a diffuse staining pattern and in a lower level in the nucleus. In (c) a merged image of (a) and (b) indicates that La/SSB is partially intermingled with PI-positive DNA (yellow, indicating overlapping green and red pixels) in untreated cells. Following treatment with 10 μM thapsigargin for 24 h La/SSB showed a striking pattern of relocalization to the periphery, forming thin eccentric rims or crescents inside the cell membrane (d,f). At later stages of apoptosis, La/SSB was found to be exposed on the surface of apoptotic cells, present in blebs budding from the membrane and on apoptotic bodies (g,i).
Figure 5.
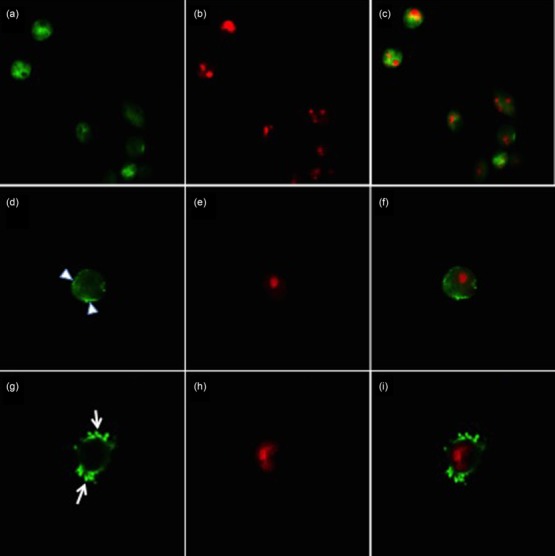
Subcellular localization of Ro52/Sjögren’s syndrome-related antigen A (SSA) antigen in normal and apoptotic human salivary gland (HSG) in vitro, as shown by immunofluorescence staining. Ro52/SSA was stained with polyclonal antibody followed by AlexaFluor-488 secondary antibody (green) and nuclear DNA was stained with propidium iodide (PI) (red). In (a), under normal conditions Ro52/SSA is increased in the cytoplasm showing a diffuse staining pattern and at a lower level in the nucleus. In (c) a merged image of (a) and (b) indicates that Ro52/SSA is partially intermingled with PI-positive DNA (yellow, indicating overlapping green and red pixels) in untreated cells. In (d), after treatment of HSG with 10 μM thapsigargin for 24 h Ro52/SSA1 can be seen as an eccentric rim at the surface of the cells (arrowhead). At later stages of apoptosis, Ro52/SSA1 accumulates in clusters around and at the surface (arrowhead) of the apoptotic bodies (green).
After ER stress induction both Ro52/SSA and La/SSB showed a striking pattern of relocalization in HSG cells at different stages of apoptosis. In cells at a relatively early stage of apoptosis after TG treatment, a reduced cytoplasmic staining of the two autoantigens was observed. Ro52/SSA and La/SSB were relocalized in these cells to the periphery and formed thin eccentric rims or crescents inside the cell membrane (Figs 4d and 5d). During the final stages of apoptosis, where HSG cells were in the process of forming apoptotic blebs, Ro52/SSA and La/SSB were found abundantly in these blebs (Figs 4g and 5g).
Discussion
In this study we show that many acinar and ductal salivary gland epithelial cells are in ER stress-activated status. This activation induces, under certain conditions, relocalization of the autoantigens Ro/SSA and La/SSB to the surface of the salivary gland epithelial cells, while cells are in apoptotic phase. In other words, apoptosis induced by ER stress results in surface accessibility of Ro/SSA and La/SSB autoantigens in the salivary gland epithelial cells.
During past years, significant progress has been accomplished which led to the increase in our understanding of the cellular responses to ER stress. This type of endogenous stress and the subsequent activation of UPR have received a great deal of attention, due to its critical implication in various diseases such as diabetes mellitus, cardiovascular, neurodegenerative and neoplastic disorders 9. In contrast to the ample data for the involvement of ER stress in autophagy and apoptosis, limited data are available for its possible role in autoimmunity and particularly in the pathogenesis of SS. Conversely, apoptosis and autophagy have been implicated in pathogenetic processes of autoimmune diseases.
Autophagy was recognized recently as a process involved in antigen presentation, of extracellular as well as intracellular cell components, through major histocompatibility complex (MHC) II molecules, in modulation of cytokine secretion leading to T helper type 1 (Th1) and T17 polarization, as well as in secretory processes of immune and non-immune cells. In addition, non-specific autophagy-lysosomal inhibitors, such as chloroquine, have been used to treat clinical manifestations of different autoimmune diseases 16.
When the protein content in the saliva of SS patients was evaluated, it is of great interest that cathepsin D, a lysosomal proteinase, was among the proteins with the higher concentration 17. It is known that this proteinase increases under autophagic conditions and the cells over-expressing cathepsin D undergo apoptosis more rapidly 18. This finding suggests that many salivary gland epithelial cells in SS patients are in an autophagic state.
In the past, Casciola-Rosen et al. have shown that during apoptosis the autoantigens Ro/SSA and LA/SSB, as well as the chaperon protein GRP78/BiP, are redistributed to the surface of the keratinocytes 6. Relocalization of the cellular autoantigens Ro/SSA and La/SSB on apoptotic cardiac myocytes, and their recognition by the corresponding autoantibodies, was described later 19. This interaction provided a possible explanation for the cardiac conductive system lesion observed in neonatal lupus 20. However, ER stress was never investigated as a possible mechanism that leads to apoptosis of the targeted cells.
The UPR represents a final common pathway in the response to a variety of stresses promoting ER functions 9. In our study, TG was used as the inducer of ER stress, autophagy and apoptosis of salivary epithelial cells. In real life, other inducers of ER stress can be responsible for this phenomenon. Epithelial cell viral infection can be one of the best candidates for this process. Epstein–Barr virus (EBV) 21 and Coxsackie viruses 22 have been described as possible inducers of salivary gland epithelial cell activation and apoptosis in SS patients.
Supporting evidence that ER stress may play a detrimental role towards epithelial cell autoimmune reaction in SS is the activation of the type I interferon (IFN) pathway in the pathogenesis of SS. IFN ‘signature’ is evident in minor salivary gland biopsies from these patients 23,24. Interestingly, Smith et al. have shown that ER stress significantly augments type I IFN immune response in the setting of pathogen challenge, such as viral infection or endogenous damage 25,26.
Another candidate for the induction of ER stress on salivary gland epithelial cells may be the chronic or severe acute stress that SS patients experience before sicca manifestations arise 27. Induction of ER stress as well as apoptosis induced by noradrenalin have been described in cardiomyocytes in autoimmune myocardiopathy 28. Thus, a combination of viral as well as sympathetic hormone insults may lead to severe ER stress, cell autophagy and apoptosis. These phenomena, leading to translocation of autoantigens and autoimmune initiation reflected at least by autoantibodies, may take place years before the clinical manifestations of the disease become evident, time that may allow an effective therapeutic intervention.
The other interesting observation of our experimental system was that the chaperone protein GRP78/BiP is not detectable early after ER stress, a phenomenon which can be explained either by down-regulation of its production or by binding to another cellular protein which does not allow its recognition by antibody. It is of great interest that translocation of this chaperone protein to the cell membrane and its binding with human leucocyte antigen (HLA) class I molecules serves as a co-receptor for Coxsackie virus A-9 29. Additionally, Rosengren et al. have shown that GRP78/BiP is down-regulated after ER stress in pancreatic β cells, implying an increased vulnerability of these cells to ER stress 30. Furthermore, Pirot et al. have shown that GRP78/BiP expression is suppressed after ER stress in pancreatic β cells, and this effect was enhanced when cells were pretreated with IFN-γ 31. These observations need further evaluation to delineate the role of this chaperone protein in autophagy and apoptosis, as well as in increased susceptibility of already stressed secretory epithelial cells to Coxsackie viral infection.
Our experiments were performed using HSG cells. The observed changes may be representative of those occurring in human patient tissues. Ro and La autoantigens were found in ductal epithelial cells in minor salivary gland biopsies from SS patients 32. Furthermore, relocalization of La autoantigen together with heat shock proteins were observed in activated in in-situ conjunctival cells from SS patients 2. It would be of great interest if the observations presented in our work regarding the relocalization of autoantigens by ER stress could be extended using cultured salivary gland epithelial cells from SS patients and controls. The findings of future studies will delineate the role of ER stress on autoantigen relocalization and thus induction and perpetuation of autoimmune reactivity.
Acknowledgments
The authors wish to thank Professor H. M. Moutsopoulos for his valuable comments to the manuscript and for continuing inspiration. This study was supported by unrestricted educational grants.
Disclosure
The authors declare that there are no conflicts of interest.
References
- Moutsopoulos HM. Sjögren’s syndrome: autoimmune epithelitis. Clin Immunol Immunopathol. 1994;72:162–5. doi: 10.1006/clin.1994.1123. [DOI] [PubMed] [Google Scholar]
- Yannopoulos DI, Roncin S, Lamour A, Pennec YL, Moutsopoulos HM, Youinou P. Conjunctival epithelial cells from patients with Sjögren’s syndrome inappropriately express major histocompatibility complex molecules, La(SSB) antigen, and heat-shock proteins. J Clin Immunol. 1992;12:259–65. doi: 10.1007/BF00918149. [DOI] [PubMed] [Google Scholar]
- Halse A, Harley JB, Kroneld U, Jonsson R. Ro/SSA reactive B lymphocytes in salivary glands and peripheral blood of patients with Sjogren’s syndrome. Clin Exp Immunol. 1999;115:203–7. doi: 10.1046/j.1365-2249.1999.00778.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Vissink A, Arellano Metal. Identification of autoantibody biomarkers for primary Sjögren’s syndrome using protein microarrays. Proteomics. 2011;11:1499–507. doi: 10.1002/pmic.201000206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson R, Theander E, Sjostrom B, Brokstad K, Henriksson G. Autoantibodies present before symptom onset in primary Sjögren syndrome. JAMA. 2013;310:1854–5. doi: 10.1001/jama.2013.278448. [DOI] [PubMed] [Google Scholar]
- Casciola-Rosen LA, Anhalt G, Rosen AJ. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–30. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuma A, Hoshino K, Ohba Tetal. Enhanced apoptosis by disruption of the STAT3-IκB-ζ signaling pathway in epithelial cells induces Sjogren’s syndrome-like autoimmune disease. Immunity. 2013;38:450–60. doi: 10.1016/j.immuni.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Castle D, Castle A. Intracellular transport and secretion of salivary proteins. Crit Rev Oral Biol Med. 1998;9:4–22. doi: 10.1177/10454411980090010301. [DOI] [PubMed] [Google Scholar]
- Bravo R, Parra V, Gatica D, et al. Endoplasmic reticulum and the unfolded protein response: dynamics and metabolic integration. Int Rev Cell Mol Biol. 2013;301:215–90. doi: 10.1016/B978-0-12-407704-1.00005-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- Vitali C, Bombardieri S, Jonsson R, et al. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American–European Consensus Group. Ann Rheum Dis. 2002;61:554–8. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm D. Manson D. Labial salivary gland biopsy in Sjogren’s disease. J Clin Pathol. 1968;21:656–60. doi: 10.1136/jcp.21.5.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Hanley MR. Thapsigargin inhibits the sarcoplasmic or endoplasmic reticulum Ca-ATPase family of calcium pumps. J Biol Chem. 1991;266:17067–71. [PubMed] [Google Scholar]
- Bertolotti A. Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2:326–32. doi: 10.1038/35014014. [DOI] [PubMed] [Google Scholar]
- Kohno K. Stress-sensing mechanisms in the unfolded protein response: similarities and differences between yeast and mammals. J Biochem. 2010;147:27–33. doi: 10.1093/jb/mvp196. [DOI] [PubMed] [Google Scholar]
- Bhattacharya A, Eissa NT. Autophagy and autoimmunity cross-talks. Front Immunol. 2013;4:88. doi: 10.3389/fimmu.2013.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Gao K, Pollard R, et al. Preclinical validation of salivary biomarkers for primary Sjögren’s syndrome. Arthritis Care Res. 2010;62:1633–8. doi: 10.1002/acr.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchiyama Y. Autophagic cell death and its execution by lysosomal cathepsins. Arch Histol Cytol. 2001;64:233–46. doi: 10.1679/aohc.64.233. [DOI] [PubMed] [Google Scholar]
- Miranda ME, Tseng CE, Rashbaum W, et al. Accessibility of SSA/Ro and SSB/La antigens to maternal autoantibodies in apoptotic human fetal cardiac myocytes. J Immunol. 1998;161:5061–9. [PubMed] [Google Scholar]
- Miranda-Carús ME, Askanase AD, Clancy RM, et al. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-alpha by macrophages. J Immunol. 2000;165:5345–51. doi: 10.4049/jimmunol.165.9.5345. [DOI] [PubMed] [Google Scholar]
- Fox RI, Chilton T, Scott S, Benton L, Howell FV, Vaughan Potential role of Epstein–Barr virus in Sjögren’s syndrome JH. Rheum Dis Clin North Am. 1987;13:275–92. [PubMed] [Google Scholar]
- Triantafyllopoulou A, Tapinos N, Moutsopoulos HM. Evidence for coxsakie virus infection in primary Sjogren’s syndrome. Arthritis Rheum. 2004;50:2897–902. doi: 10.1002/art.20463. [DOI] [PubMed] [Google Scholar]
- Mavragani CP, Crow MK. Activation of the type I interferon pathway in primary Sjogren’s syndrome. J Autoimmun. 2010;35:225–31. doi: 10.1016/j.jaut.2010.06.012. [DOI] [PubMed] [Google Scholar]
- Hall JC, Casciola-Rosen L, Berger AE, et al. Precise probes of type II interferon activity define the origin of interferon signatures in target tissues in rheumatic diseases. Proc Natl Acad Sci. 2012;109:17609–14. doi: 10.1073/pnas.1209724109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Turner MJ, DeLay ML, Klenk EI, Sowders DP, Colbert RA. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-beta induction via X-box binding protein 1. Eur J Immunol. 2008;38:1194–203. doi: 10.1002/eji.200737882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YP, Zeng L, Tian A, et al. Endoplasmic reticulum stress regulates the innate immunity critical transcription factor IRF3. J Immunol. 2012;189:4630–9. doi: 10.4049/jimmunol.1102737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaiskos D, Mavragani CP, Makaroni S, et al. Stress, coping strategies and social support in patients with primary Sjögren’s syndrome prior to disease onset: a retrospective case–control study. Ann Rheum Dis. 2009;68:40–6. doi: 10.1136/ard.2007.084152. [DOI] [PubMed] [Google Scholar]
- Mao W, Fukuoka S, Iwai C, et al. Cardiomyocyte apoptosis in autoimmune cardiomyopathy: mediated via endoplasmic reticulum stress and exaggerated by norepinephrine. Am J Physiol Heart Circ Physiol. 2007;293:H1636–45. doi: 10.1152/ajpheart.01377.2006. [DOI] [PubMed] [Google Scholar]
- Triantafilou K, Fradelizi D, Wilson K, Triantafilou M. GRP78, a coreceptor for coxsackie virus A9, interacts with major histocompatibility complex class I molecules which mediate virus internalization. J Virol. 2002;76:633–43. doi: 10.1128/JVI.76.2.633-643.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosengren V, Johansson H, Lehtiö J, Fransson L, Sjöholm A, Ortsäter H. Thapsigargin down-regulates protein levels of GRP78/BiP in INS-1E cells. J Cell Biochem. 2012;113:1635–44. doi: 10.1002/jcb.24032. [DOI] [PubMed] [Google Scholar]
- Pirot P, Eizirik DL, Cardozo AK. Interferon-gamma potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defense mechanisms. Diabetologia. 2006;49:1229–36. doi: 10.1007/s00125-006-0214-7. [DOI] [PubMed] [Google Scholar]
- Barcellos KS, Nonogaki S, Enokihara MM, Teixeira MS, Andrade LE. Differential expression Ro/SSA and La/SSB 52kDa, mRNA and protein in minor salivary glands from patients with primary Sjogren’s syndrome. J Rheumatol. 2007;34:1283–92. [PubMed] [Google Scholar]


