Abstract
3-iodothyronamine (T1AM) is a novel endogenous relative of thyroid hormone, able to interact with trace amine-associated receptors, a class of plasma membrane G protein-coupled receptors, and to produce a negative inotropic and chronotropic effect. In the isolated rat heart 20–25 μM T1AM decreased cardiac contractility, but oxygen consumption and glucose uptake were either unchanged or disproportionately high when compared to mechanical work. In adult rat cardiomyocytes acute exposure to 20 μM T1AM decreased the amplitude and duration of the calcium transient. In patch clamped cardiomyocytes sarcolemmal calcium current density was unchanged while current facilitation by membrane depolarization was abolished consistent with reduced sarcoplasmic reticulum (SR) calcium release. In addition, T1AM decreased transient outward current (Ito) and IK1 background current. SR studies involving 20 μM T1AM revealed a significant decrease in ryanodine binding due to reduced Bmax, no significant change in the rate constant of calcium-induced calcium release, a significant increase in calcium leak measured under conditions promoting channel closure, and no effect on oxalate-supported calcium uptake. Based on these observations we conclude T1AM affects calcium and potassium homeostasis and suggest its negative inotropic action is due to a diminished pool of SR calcium as a result of increased diastolic leak through the ryanodine receptor, while increased action potential duration is accounted for by inhibition of Ito and IK1 currents.
Keywords: 3-iodothyronamine, ionic current, calcium, potassium, sarcoplasmic reticulum, ryanodine receptor, heart, thyroid
Introduction
3-iodothyronamine (T1AM) is a novel endogenously synthesized relative of thyroid hormone with significant physiological and behavioural effects in mammals [1]. In isolated rat hearts micromolar concentrations of T1AM produced a reversible, dose-dependent negative inotropic and chronotropic effect [1, 2]. In cultured rat cardiomyocytes decreased cellular shortening and increased action potential duration were reported upon exposure to T1AM [2]. These actions occur within minutes and consequently they appear to represent a non-genomic response. Interestingly, T1AM does not interact with nuclear thyroid hormone receptors. Rather in vitro it can activate heterologously expressed cloned rodent and human trace amine associated receptor 1 (TAAR1), and possibly other members of the same family of G-protein coupled receptors [3, 4]. Messenger RNAs coding for at least five different TAAR subtypes are expressed in rat heart and it has been suggested that one or more TAAR subtypes mediate T1AM’s diverse biological effects [2]. There is convincing evidence that transduction of T1AM-stimulated signalling involves changes in the phosphorylation state of tyrosine residues in yet-to-be-determined proteins, but the final effectors responsible for the functional effects have not been determined. Because myocardial function is largely dependent on the modulation of ionic currents and requires an adequate supply of metabolic energy, the present study attempted to understand T1AM’s effects on myocardial metabolism and ionic homeostasis.
Materials and methods
Chemicals and radionuclides
T1AM and thyronamine were synthesized as described elsewhere [5]. [3H]-ryanodine and [45Ca]-CaCl2 were obtained from New England Nuclear (Milan, Italy). Unless otherwise specified all other reagents were from Sigma-Aldrich (St. Louis, MO, USA).
Isolated heart perfusion
This investigation conforms to the Declaration of Helsinki and the Guiding Principles in the Care and Use of Animals. The project was approved by the Animal Care and Use committee of the University of Pisa. Male Wistar rats (275–300 g body weight), fed a standard diet, were anesthetized with a mixture of ether and air. The heart was then quickly excised and perfused according to the working heart technique, as described previously [2]. The height of the atrial chamber was set at 20 cm, corresponding to a filling pressure of 15 mmHg. To measure oxygen consumption the pulmonary artery was cannulated and samples of perfusate were collected from the aortic and pulmonary cannulas without exposure to air, as described previously [6]. Glucose was assayed in the recirculating perfusion buffer by the glucose oxidase technique using a commercial Glucose Assay Kit (GAHK-20, Sigma-Aldrich), and the rate of glucose uptake was calculated by linear regression.
Cardiomyocyte experiments
Left ventricular cardiomyocytes were prepared from male Wistar rat hearts by enzymatic digestion in a Langendorff apparatus. The isolated cells were resuspended in Tyrode’s solution, as described previously [7]. The following solutions were used (in mM): Tyrode’s solution, i.e. NaCl 140, KCl 5.4, MgCl2 1.2, glucose 10, HEPES-NaOH 5 (pH 7.3), CaCl2 1.8; modified Tyrode’s solution used for L-type calcium current recording, i.e. as above with the addition of 5.4 mM CsCl to block potassium currents; pipette solution, i.e. Cs-Aspartate 120, TEACl 10, Na2GTP 0.4, Na2ATP 5, MgCl2 2, CaCl2 5, EGTA 11, HEPES 10 (pH 7.2); perforated patch solution, i.e. KMeSO4 125, KCl 25, EGTA 1, amphotericin B 0.13, HEPES-KOH 5 (pH 7.0).
The experimental set-up for patch-clamp recording and data acquisition was similar to that described previously [8]. Dissociated cardiomyocytes grown in culture were placed in an experimental bath set on the stage of an inverted microscope (Nikon Diaphot TMD, Kawasaki, Japan). The patch-clamped cell was superfused by means of a temperature-controlled (36 ± 0.5°C) micro-superfuser that allows rapid changes in the cell’s surrounding solution to be made. Patch-clamp pipettes, prepared from glass capillary tubes by means of a two-stage horizontal puller (P-87 Flaming/Brown micropipette puller, Sutter Instrument, Novato, CA, USA), had a resistance of ∼2.5 MΩ when filled with pipette solution.
Recordings were performed with a patch amplifier (Axopatch 200B, Molecular Devices, Sunnyvale, CA, USA) in whole-cell configuration for current recording or by means of the perforated-patch technique for calcium transient recordings. Signals were digitized via a DAC/ADC interface (Digidata 1200B, Molecular Devices) and acquired by means of pClamp software. Currents were recorded in the voltage-clamp mode. Cell membrane capacitance was measured by applying a ±10 mV pulse starting from a holding potential of –70 mV, as previously reported [8]. Offline data analysis was performed with pClamp (vers. 9, Molecular Devices) and Origin 7.5 (GraphPad Software, San Diego, CA, USA).
The amplitudes of L-type calcium currents (ICa-L) were measured by applying a voltage protocol for steady-state activation (from –35 to +55 mV after a pre-step of –40 mV, to inactivate sodium currents) and inactivation (pre-steps in the range –75 to –5 mV followed by a test step at 0 mV to record maximal current amplitude). Bi- or mono-exponential fitting was used to determine the time constants of deactivation. Background currents were elicited by a ramp protocol from –120 to +50 mV and currents were measured at +40 mV and at –100 mV, chosen because they are representative of the ultra-rapid delayed rectifier current (IKur) and the inward rectifier current (IK1), respectively. Transient outward current (Ito) was measured by applying depolarizing steps from –40 to +60 mV and maximal current density was plotted versus step potential to obtain an activation curve.
To evaluate the effect of T1AM on calcium transient, left ventricular cardiomyocytes were incubated with Tyrode’s solution containing 1–5 μM fluo 3-acetoxymethyl ester (Fluo-3) and Pluronic F127 (Molecular Probes, Eugene, OR, USA) for 20 min. at 37°C to load Fluo-3. The preparation was set in the patch-clamp setup previously described, properly modified for fluorescence signals production and detection. Fluo-3 was excited with the 488 nm line of a xenon lamp (LPS 220, Photo Technology International, Birmingham, NJ, USA). Fluorescence was detected at 540 ± 15 nm with a photomultiplier tube (model 01–614, Photo Technology International) and signal was digitized by a DAC/ADC interfaces (Digidata 1200B, Molecular Devices). Oscilloscopes and computer screens allowed online data view. Experimental control, data acquisition and preliminary analysis were performed by means of the integrated software package pClamp. Calcium transient was recorded during a pulse at 0 mV from a holding potential at –40 mV.
Sarcoplasmic reticulum (SR) experiments
Ventricles were obtained from perfused hearts after 30 min. of exposure to T1AM or an equivalent period of control perfusion. The tissue was finely minced and homogenized in 5 volumes of 300 mM sucrose and 10 mM imidazole (pH 7.0) at 4°C by 15 + 15 passes in a Potter-Elvejheim homogenizer set at 800 rpm. A microsomal fraction enriched in SR was prepared by high-speed differential centrifugation as described elsewhere [9]. The techniques to assay SR function, previously described and validated [9–11], are summarized below. Measurements were typically performed on crude homogenates because SR purification has a low yield and may not be representative of the intact SR [12].
Active Ca2+ transport was evaluated on the basis of oxalate-supported Ca2+ uptake. Aliquots of homogenate (protein concentration 0.5–0.8 mg/ml) were pre-incubated at 37°C for 5 min. in the presence of 900 μM ryanodine to block the SR Ca2+ release channel. The uptake medium and the experimental technique were the same as described previously [9]. In a few experiments, different amounts of CaCl2 were added to obtain free Ca2+ concentrations ranging from 0.1 to 40 μM
SR Ca2+-induced Ca2+ release was determined after passive loading with 10 mM [45Ca]-CaCl2[10]. Ca2+ release was induced by washing the loaded vesicles with release buffer containing (in mM): HEPES potassium 20 (pH 6.8), KCl 100, CaCl2 1.01 and EGTA 1 (free calcium concentration was 18 μM) 1. A rapid filtration system with time resolution on the order of 10 msec. was used (RFS-04, Bio-Logics, Grenoble, France) and the rate constant of quick Ca2+ release was calculated over the first 100 msec. by exponential fitting.
SR Ca2+ leak was measured under conditions promoting SR channel closure. For this purpose SR vesicles were actively loaded by 2 min. incubation in the presence of 100 mM [45Ca]-CaCl2 and 2 mM acetylphosphate [11, 13]. The preparation was filtered using the RFS-04 equipment charged with the following buffer (in mM): Tris-MOPS (pH 7.0) 20, KCl 80, MgCl2 10, Tris-EGTA 1 and tetracaine 0.3. EGTA is used as a calcium chelator, while magnesium and tetracaine are known to produce channel blockade. Release rates were calculated by exponential fitting over 2 sec.
To determine high affinity ryanodine binding aliquots of homogenate were incubated at 37°C for 60 min. with 0.2 to 50 nM [3H]-ryanodine, in a buffer containing (mM): imidazole 25 (pH 7.4 at 37°C), KCl 1000, EGTA 0.950 and CaCl2 1.013 (free Ca2+ concentration: 20 μM) [9]. Saturation experiments were analysed by nonlinear fitting of a single binding site model.
Statistical analysis
Results are expressed as the mean ± S.E.M. Differences between groups were evaluated by ANOVA while individual groups were compared versus the control group using the Dunnett post hoc test. When only two groups were compared, the paired or unpaired t-test was used, as deemed appropriate. The threshold of statistical significance was set at P < 0.05. GraphPad Prism version 4.1 for Windows (GraphPad Software) was used for all data processing and statistical analysis.
Results
Functional and metabolic effects
In the isolated perfused working rat heart model baseline average values of the haemodynamic variables were as follows: cardiac output 62.9 ± 1.0 ml/min., aortic flow 43.4 ± 0.8 ml/min., coronary flow 19.6 ± 0.6 ml/min., systolic aortic pressure 177 ± 9 mmHg, heart rate 271 ± 14 beats per minute. In accordance with our previous findings, 2.5 μM T1AM did not modify contractile performance while 20 μM and 25 μM caused a significant decrease in cardiac output, aortic pressure, coronary flow and heart rate. The time course of these changes was the same as reported previously [2], and the maximum response occurred after 10–20 min. The values of the haemodynamic variables recorded after 20 min. are summarized in Table 1. However, these haemodynamic effects were not associated with comparable reductions of oxygen consumption or glucose uptake. Actually with 20 μM T1AM glucose uptake and oxygen consumption were not significantly modified (7.2 ± 0.8 versus 7.6 ± 1.1 μmol/min. per g and 37.1 ± 5.8 versus 40.0 ± 3.9 μmol/min. per g, respectively) in spite of 36% and 22% decrease in cardiac output and heart rate, respectively. A significant reduction in oxygen consumption was observed only with 25 μM T1AM, which determined 56% and 43% reduction of cardiac output and heart rate, respectively.
Table 1.
Haemodynamic and metabolic variables
| Group | HR bpm | AF ml/min. | CF ml/min. | CO ml/min. | AoP mmHg | Glucose uptake μmol/min. per g | Oxygen consumption μmol/min. per g |
|---|---|---|---|---|---|---|---|
| Control | 271 ± 14 | 43.4 ± 0.8 | 19.6 ± 0.6 | 62.9 ± 1.0 | 177 ± 9 | 7.6 ± 1.1 | 40.0 ± 3.9 |
| T1AM (2.5 μM) | 231 ± 18 | 37.3 ± 2.9 | 19.3 ± 2.4 | 56.7 ± 0.7 | 173 ± 7 | 7.3 ± 0.8 | 37.4 ± 3.5 |
| T1AM (20 μM) | 210 ± 12* | 22.7 ± 3.3† | 17.7 ± 1.0 | 40.4 ± 3.3† | 158 ± 8 | 7.2 ± 0.8 | 37.1 ± 5.8 |
| T1AM (25 μM) | 154 ± 16† | 13.2 ± 2.2† | 14.4 ± 0.8† | 27.6 ± 2.4† | 127 ± 18* | 4.5 ± 1.0 | 21.2 ± 3.2* |
Data represent mean ± S.E.M. of 10–16 hearts per group. Measurements were obtained after 20 min. of perfusion. HF, heart rate; AF, aortic flow; CF, coronary flow; CO, cardiac output and AoP, systolic aortic pressure.
P < 0.05
P < 0.01 versus control, by Dunnett’s test.
Electrophysiological effects
The electrophysiological effects of T1AM were studied in adult ventricular cardiomyocytes using the whole-cell patch-clamp technique. On the basis of our isolated heart results we decided to use 20 μM T1AM. T1AM had no effect on peak density of calcium currents but affected ICa-L inactivation kinetics (Fig. 1). Under control conditions ICa-L inactivated with a typical bi-exponential decay, characterized by two deactivation constants (τf: 9.0 ± 2.6 msec.; τs: 50.0 ± 10.0 msec.; n= 4). However, in the presence of T1AM ICa-L inactivated with a mono-exponential decay profile and the deactivation constant averaged 26.0 ± 2.0 msec. (n= 4, P < 0.01 versus control).
Figure 1.
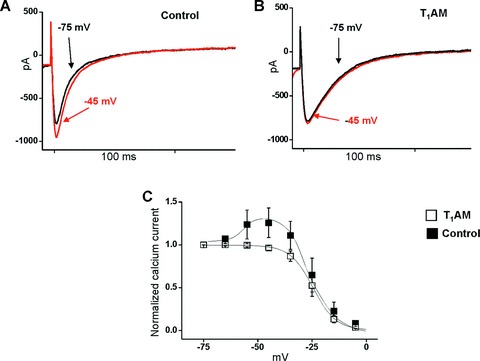
Effect of 20 μM T1AM on ICa-L current in single patch-clamped rat ventricular cardiomyocytes. (A) In control conditions during the inactivation protocol the current evoked from a holding potential (HP) of –45 mV was greater than that evoked from a HP of –75 mV, a process known as facilitation. (B) T1AM completely suppressed the facilitation phenomenon, and the current amplitude measured at HP –45 mV was 96% of that measured at HP of –75 mV. (C) Inactivation curves of ICa-L under control condition and after exposure to T1AM (n= 4 in each group). Maximal current amplitude was normalized and plotted versus pre-step potential. Further data analysis is reported in the text: under control conditions ICa-L inactivated with the typical bi-exponential decay, while in the presence of T1AM, ICa-L inactivated with a mono-exponential decay.
In addition, T1AM influenced the so-called L-type current facilitation, a typical feature of cardiac cells [14, 15]. This is illustrated in Fig. 1A: under control conditions, the current evoked from a holding potential of –45 mV was ≈30% greater than that evoked from a holding potential of –75 mV. T1AM completely abolished this phenomenon because the currents measured at –45 and –75 mV had the same amplitude (Fig. 1B). The steady state inactivation curve obtained by normalization of calcium current versus conditioning step potential (Fig. 1C) confirmed that T1AM abolished the overshoot observed in the control curve at holding potential between –50 and –30 mV. However, T1AM had no effect on the increase of the calcium current produced by exposure to 1 μM isoproterenol (data not shown), thus suggesting that the activity of protein kinase A is not hampered by exposure to T1AM.
Facilitation has been attributed to the effect of SR calcium release on the L-type calcium channel and it is abolished when the SR calcium pool is depleted [15, 16]. Therefore we investigated the influence of 20 μM T1AM on intracellular calcium transients. Cardiomyocytes were loaded with Fluo3 to obtain a fluorescence signal proportional to the intracellular concentration of free calcium. Figure 2 shows the results of a typical experiment in which the calcium transient was recorded during a pulse at 0 mV from a holding potential of –40 mV, and the substantial reduction of its amplitude that occurred in the presence of T1AM. In eight independent preparations T1AM produced significant decreases both in the peak of the signal (0.06 ± 0.01 versus 0.10 ± 0.02 arbitrary units, P < 0.05) and its decay time (86 ± 26 versus 176 ± 40 msec., P < 0.01).
Figure 2.
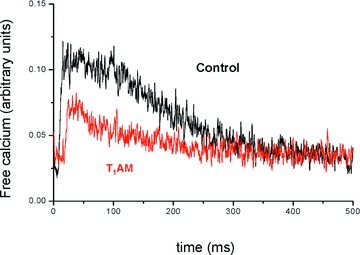
Effect of 20 μM T1AM on calcium transients in rat ventricular cardiomyocytes. T1AM (red trace) reduced the decay time and area of calcium transients with respect to control (black trace). See text for cumulative results obtained in eight different preparations.
We previously reported T1AM can produce a negative chronotropic effect which is associated with action potential prolongation [2]. This was confirmed in the present investigation. Cardiomyocytes exposed to 20 μM T1AM displayed an APD90 that increased from 84 ± 16 to 157 ± 29 msec. (n= 10, P < 0.05). To gain insight into the mechanism responsible for action potential prolongation we investigated the effects of 20 μM T1AM on Ito current, responsible for the first phase of the action potential repolarization, and on background currents (IK1 and IKur), responsible for the last phase of repolarization. Background currents were elicited by a ramp protocol and currents were measured at –100 and +40 mV, representative of IK1 and IKur, respectively (Fig. 3). IK1 was significantly reduced after exposure to 20 μM T1AM (pA/pF averaged –12.8 ± 1.5 versus–17.6 ± 2.2; n= 7, P < 0.05), whereas the changes in IKur did not reach statistical significance. The activation curve of Ito current, obtained by plotting maximal Ito density versus step potential (Fig. 4), revealed peak amplitude to be significantly reduced after exposure to T1AM at 20 mV to at 50 mV (n= 5, P < 0.05 in each case).
Figure 3.
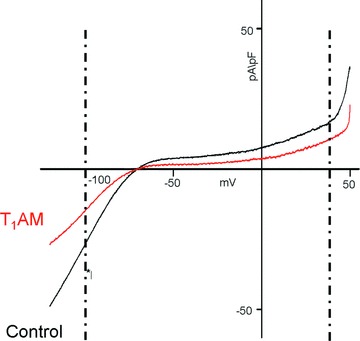
Effect of 20 μM T1AM on background currents in single patch-clamped rat ventricular cardiomyocytes. Typical ramp traces for controls (black line) and T1AM (red line) are shown. Currents measured at –100 mV and +40 mV (dotted lines) are representative of IK1 and IKur, respectively. See text for cumulative results obtained in seven different preparations.
Figure 4.
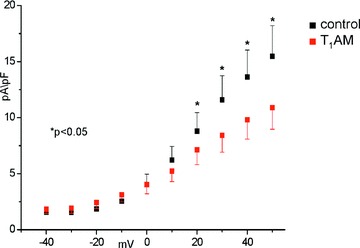
Effect of 20 μM T1AM on Ito current in single patch-clamped rat ventricular cardiomyocytes. The peak amplitude was significantly reduced after exposure to T1AM at 20–50 mV (*=P < 0.05). See text for analytical results.
Effects on SR function
To investigate whether the observed modifications of the calcium transient are associated with changes in SR function we determined the effects of perfusing with 20 μM T1AM on ryanodine binding, SR calcium release and oxalate-supported calcium uptake.
Ryanodine is a selective ligand of the SR calcium release channels and changes in ryanodine binding provide indirect evidence of changes in channel structure or gating [17]. Saturation binding curves obtained in control hearts and in hearts perfused with T1AM are shown in Fig. 5 (left panel). After perfusion with T1AM [3H]-ryanodine binding was significantly reduced, due to decreased Bmax (265 ± 19 versus 381 ± 19 fmol/mg of protein, P < 0.05), while the KD was not significantly altered (1.7 ± 0.5 versus 2.1 ± 0.2 nM). Incubation of control SR vesicles with 20 μM T1AM did not affect ryanodine binding (Fig. 5, right panel), demonstrating the observed effect was not due to direct interaction of T1AM with the SR calcium channel.
Figure 5.
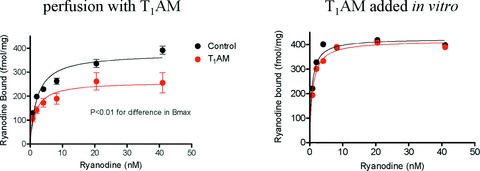
Effect of T1AM on ryanodine binding in crude cardiac homogenates. Left panel: saturation binding curves obtained in hearts perfused in the presence or in the absence of 20 μM T1AM. Symbols represent mean ± S.E.M. of four to five hearts per group. Data analysis yielded P < 0.05 for the difference in Bmax (see text for analytical results) Right panel: saturation binding curves obtained incubating cardiac honogenates with 20 μM T1AM for 2 hrs, and in the corresponding controls. Symbols represent average results obtained in two hearts per group, and results were virtually identical.
In other experiments SR vesicles obtained from hearts which had been perfused with T1AM were loaded with radiolabelled calcium and the kinetics of calcium induced calcium release were determined by a quick filtration technique, using a buffer containing 18 μM unlabelled calcium to determine full channel activation. As shown in the left panel of Fig. 6 no significant effect could be demonstrated, although there was a trend for the average value of the first-order release constant to be higher in the T1AM group (89 ± 18 versus 60 ± 15 sec.−1, P= 0.28).
Figure 6.
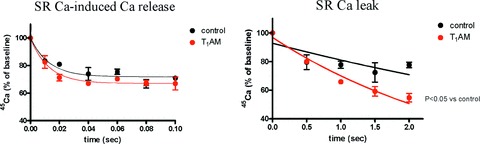
Effect of perfusion with 20 μM T1AM on SR calcium release determined using the quick filtration technique. Left panel: maximum release rate, measured after passive loading with 10 mM 45Ca and exposure to 18 μM unlabelled calcium. Right panel: passive leak measured after active loading with 45Ca and exposure to 1 mM EGTA, 10 mM MgCl2 and 0.3 mM tetracaine. The figures show vescicle radioactivity, expressed as percentage of the baseline value, at different filtration times (note the different time scale between the two panels). Data represent mean ± S.E.M. obtained in three different preparations. Exponential fitting yielded a significant difference in the rate constant for the right panel (P < 0.01). See text for analytical results.
Calcium release experiments were also performed under conditions which induce SR calcium channel closure in an effort to measure the so-called SR calcium leak. The results are shown in the right panel of Fig. 6. Under our experimental conditions the rate constant of calcium release was significantly increased following perfusion with 20 μM T1AM (0.32 ± 0.03 versus 0.13 ± 0.04 sec.−1, P < 0.01).
To evaluate active calcium transport by the SR calcium ATPase we measured oxalate-supported calcium uptake in the crude homogenate obtained from control hearts, and from hearts perfused with T1AM. With this technique oxalate precipitates calcium in the SR and allows the uptake rate to be linear for several minutes, so that it can be easily determined even in unfractionated preparations [9, 12]. As shown in Fig. 7, at a free calcium concentration of 10 μM the uptake rate was not modified by perfusion with 20 μM T1AM. In vitro incubation with 20 μM T1AM was also ineffective (data not shown). In additional experiments calcium uptake was measured at five different calcium concentrations in an effort to estimate its calcium-dependence. In two different preparations the EC50 for calcium uptake averaged 2.6 μM both in control hearts and in hearts which had been perfused with 20 μM T1AM. The maximum uptake rate was not significantly modified, as well (11.8 ± 2.5 versus 12.0 ± 0.4 nmol/min per mg, P= NS).
Figure 7.
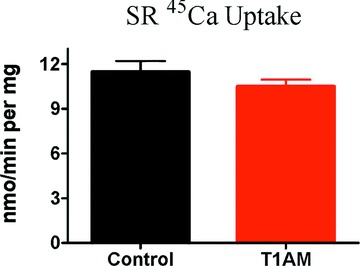
Effect of perfusion with 20 μM T1AM on oxalate-supported calcium uptake in the crude rat heart homogenate at 10 μM free calcium concentration. Data represent mean ± S.E.M. obtained in four different preparations. The difference between groups was not statistically significant by unpaired t-test.
Discussion
T1AM is a recently identified, naturally occurring compound that is most likely derived in vivo from thyroid hormone through decarboxylation and deiodination. In vitro evidence suggests T1AM activates a novel aminergic system coupled to the so-called TAARs [1, 2, 18]. Several functional effects of T1AM have already been described, and the focus of the present work was to unravel the molecular mechanism underlying its effects on contractility and action potential duration.
In the isolated rat heart a negative inotropic response to T1AM was observed before any decrease in oxygen consumption or glucose uptake is produced. Therefore, it seems unlikely that perfusion with T1AM impaired contractile performance because of energy shortage. On the other hand, cardiomyocyte experiments showed that T1AM substantially reduced the amplitude and duration of depolarization-induced calcium transients. A 20 μM concentration reduced the area under the calcium-time curve by more than 50%. On the basis of literature data and mathematical models of calcium homeostasis [19] this response is consistent with the observed reduction in cellular shortening, which averaged about 40% in our cardiomyocyte model [2]. Consequently, we hypothesize that the negative inotropic effect of T1AM is linked to reduced calcium mobilization.
The calcium transient is dependent on calcium influx through L-type sarcolemmal calcium channels and calcium release from the SR via the SR calcium channel–ryanodine receptor. Whole-cell patch-clamp experiments did not show any evidence of reduced L-type sarcolemmal calcium influx, because peak current density was unchanged. However, the phenomenon known as facilitation, namely increased current amplitude at more positive holding potentials (–50 and –30 mV), was abolished. Facilitation has generally been attributed to L-channel modulation by SR calcium release and is impaired after depletion of SR-sequestered calcium [15]. While facilitation may be also favoured by L-channel phosphorylation by protein kinase A [15], this mechanism can be ruled out in the present context, because isoproterenol-induced facilitation was not affected by T1AM. Therefore, our findings suggest that T1AM reduces SR calcium release.
Additional evidence that T1AM affects SR calcium release is provided by the observation that ryanodine binding was reduced after perfusion with T1AM. This does not appear to represent the consequence of a direct interaction between T1AM and the SR channel, because incubation of SR preparations with T1AM did not modify ryanodine binding. In general, a large number of physiological and pharmacological modulators of SR calcium release affect ryanodine binding, and altered ryanodine binding is usually, although not invariably, associated with altered calcium release [17]. When we determined the kinetics of SR calcium-induced calcium release by quick filtration experiments the first-order rate constant was not significantly modified after perfusion with T1AM. However, the so-called calcium leak, i.e. calcium release determined under conditions able to lock the SR channel closed, was significantly increased after perfusion with T1AM.
When interpreting these findings it should be emphasized that modulation of SR channel activation is less important than modulation of diastolic leak through the SR, insofar as inotropic status is concerned. Transmembrane calcium flux is the product of membrane permeability and concentration gradient; therefore, SR calcium release depends both on channel gating and on the extent of the SR calcium pool. Increased calcium release due to increased channel opening in systole would decrease SR calcium concentrations that over time would counteract the initial effect [20, 21]. This phenomenon is known as autoregulation, and it has been argued that modulation of calcium-induced calcium release cannot produce persistent changes in calcium transients, while the latter are largely dependent on the total SR calcium content [21]. The latter is determined by the difference between active uptake by the SR calcium-ATPase and diastolic calcium leak [19]. Because we did not observe any effect of T1AM perfusion on active SR calcium uptake, while calcium leak was increased, we posit that the latter may account for the negative inotropic response to T1AM.
This putative action would be similar to the action of ryanodine. As reviewed elsewhere [17] nanomolar concentrations of ryanodine lock the channel open. This state is associated to immediate calcium release, followed by a persistent reduction of the calcium transient. A similar negative inotropic effect resulting from an increased diastolic calcium leak is produced by dissociation of the complex consisting of the ryanodine receptor and the protein referred to as FKBP12.6, which has been proposed to play an important role in heart failure [22]. FKBP12.6–ryanodine receptor dissociation can also be produced by the drug FK-506. Notably, FK-506 has been reported to reduce ryanodine binding, owing to decreased Bmax with unchanged KD[17], as we observed to occur after exposure to T1AM.
Diastolic calcium leak leads to increased calcium cycling between SR and cytosol. As a consequence ATP consumption by ionic pumps should be higher than expected on the basis of contractile performance, which is consistent with what we observed in the isolated heart exposed to 20 μM T1AM, namely unchanged oxygen consumption and glucose uptake in spite of a significant reduction in cardiac output and heart rate.
At 25 μM T1AM concentration, substantial reductions in contractile performance, glucose uptake and oxygen consumption occurred. This observation suggests that the dose–response relationship for the effects on calcium homeostasis is rather steep, although it cannot be excluded that additional effects (e.g. primary metabolic effects) may develop at this concentration.
In cardiomyocytes exposure to T1AM prolongs action potential duration [2]. We therefore investigated its effect on the ionic currents responsible for repolarization. We observed that the Ito current and the IK1 inward rectifier current were both inhibited by T1AM. These effects can account for the observed changes of the action potential, and might also be involved in the negative chronotropic action of T1AM, although sinus node cells should be studied to test the latter hypothesis.
The results we present here demonstrate that the transduction system coupled to T1AM is able to modulate both calcium homeostasis and cardiac repolarizing currents. The molecular components of this pathway are still largely unknown. After the discovery of TAARs [3, 4, 23], and the observation that T1AM can activate heterologously expressed TAAR1 [1] TAARs emerged as prime candidates for mediating the biological actions of T1AM, although in vivo evidence is currently lacking, and will require the availability of genetic deletion models or of TAAR-selective antagonists. The mRNAs coding for at least five TAAR subtypes have been detected in rat heart tissue with TAAR8a, a completely uncharacterized member of the family, the predominant subtype [2]. Pharmacological experiments suggest the cardiac effects of thyronamines and certain trace amines are not mediated solely by TAAR1 but rather may well occur via a different TAAR subtype [18]. The identities of the G proteins which are coupled to these receptors and their downstream effectors have also not been established although there is growing evidence that changes in tyrosine phosphorylation may play a critical role [2]. Interestingly, tyrosine kinases and tyrosine phosphatases are known to modulate ionic channels in several cell types, including the heart, possibly by modulating the subcellular distribution of channel proteins and/or their association with ancillary proteins [24, 25]. In the current context it would be particularly interesting to assess whether T1AM can influence the interaction of the ryanodine receptor with FKBP12, a protein thought to regulate the diastolic calcium leak [19].
Several limitations of our investigation must be acknowledged. Elecrophysiological recordings were performed in a single cell type, and were not integrated with single channel recordings. In addition, we did not investigate sodium/calcium exchange nor mitochondrial calcium transport. Sodium/calcium exchange might indirectly affect the SR calcium pool because the exchanger competes with the SR calcium-ATPase for cytosolic calcium. Mitochondria are present in unfractionated preparations. They are known to both accumulate and release calcium although they are believed to play a minor role in the short-term regulation of calcium homeostasis in vivo[19], and their contribution to the calcium fluxes measured in our in vitro preparation should be negligible, because calcium uptake was measured in the presence of oxalate and calcium release was measured in the absence of sodium. Finally, we did not evaluate the calcium buffering capacity of the cytosol which could play a major role in determining free calcium concentration given that less than 1% of cytosolic calcium is unbound [19].
In conclusion, we have observed that T1AM signalling modulates calcium homeostasis and repolarizing potassium currents. These actions may account for its negative inotropic and possibly for its negative chronotropic action, leading to functional effects which are opposite those produced on a longer time scale by thyroid hormone. At present it is difficult to assess the physiological role of this aminergic system because the physiological concentration of myocardial T1AM is uncertain: pilot assays suggest average concentration to be in the mid-nanomolar range –i.e. 20-fold and 2-fold higher than 3,5,3′-triiodothyronine and thyroxine content – but the concentration at receptor level might be substantially higher [2]. Changes in local T1AM concentration might contribute to the cardiac manifestations of thyroid disease, and might be involved in pathological conditions such as heart failure, in which the role of thyroid hormone is certainly relevant but remains to be comprehensively clarified [26, 27]. In any case pharmacological manipulation of this interesting new signalling system could provide an exciting new approach to modulating cardiac function.
Acknowledgments
This work was supported by Ministero dell’Università e della Ricerca, Italy (Cofin 2005, G.C.) and by University of Pisa (Cofin 2007, R.Z.).
References
- 1.Scanlan TS, Suchland KL, Hart ME, et al. 3-Iodothyronamine is an endogenous and rapid-acting derivative of thyroid hormone. Nature Med. 2004;10:638–42. doi: 10.1038/nm1051. [DOI] [PubMed] [Google Scholar]
- 2.Chiellini G, Frascarelli S, Ghelardoni S, et al. Cardiac effects of 3-iodothyronamine: a new aminergic system modulating cardiac function. FASEB J. 2007;21:1597–608. doi: 10.1096/fj.06-7474com. [DOI] [PubMed] [Google Scholar]
- 3.Zucchi R, Chiellini G, Scanlan TS, et al. Trace amine-associated receptors and their ligands. Br J Pharmacol. 2006;149:967–78. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grandy DK. Trace amine-associated receptor 1 – family archetype or iconoclast? Pharmacol & Ther. 2007;116:355–90. doi: 10.1016/j.pharmthera.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hart ME, Suchland KL, Miyakawa M, et al. Trace amine-associated receptor agonists: synthesis and evaluation of thyronamines and related analogues. J Med Chem. 2006;49:1101–12. doi: 10.1021/jm0505718. [DOI] [PubMed] [Google Scholar]
- 6.Zucchi R, Limbruno U, Poddighe R, et al. Purine release from isolated rat heart: a new approach to the study of energy metabolism. J Mol Cell Cardiol. 1990;22:815–26. doi: 10.1016/0022-2828(90)90092-g. [DOI] [PubMed] [Google Scholar]
- 7.Cerbai E, Barbieri M, Mugelli A. Characterization of the hyperpolarization-activated current, If, in ventricular myocytes isolated from hypertensive rats. J Physiol. 1994;481:585–91. doi: 10.1113/jphysiol.1994.sp020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartiani L, Cerbai E, Lonardo G, et al. Prenatal exposure to carbon monoxide affects postnatal cellular electrophysiological maturation of the rat heart: a potential substrate for arrhythmogenesis in infancy. Circulation. 2004;109:419–23. doi: 10.1161/01.CIR.0000109497.73223.4D. [DOI] [PubMed] [Google Scholar]
- 9.Zucchi R, Ronca-Testoni S, Yu G, et al. Effect of ischemia and reperfusion on cardiac ryanodine receptors – sarcoplasmic reticulum Ca2+ channels. Circ Res. 1994;74:271–80. doi: 10.1161/01.res.74.2.271. [DOI] [PubMed] [Google Scholar]
- 10.Zucchi R, Ronca-Testoni S, Yu G, et al. Postischemic changes in cardiac sarcoplasmic reticulum Ca2+ channels: A possible mechanism of ischemic preconditioning. Circ Res. 1995;76:1049–56. doi: 10.1161/01.res.76.6.1049. [DOI] [PubMed] [Google Scholar]
- 11.Zucchi R, Gongyuan Y, Ghelardoni S, et al. Effect of MEN 10755, a new disaccharide analogue of doxorubicin, on sarcoplasmic reticulum Ca2+ handling and contractile function in rat heart. Br J Pharmacol. 2000;131:342–8. doi: 10.1038/sj.bjp.0703575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feher JJ, Lebolt WR, Manson NH. Differential effect of global ischemia on the ryanodine-sensiive and ryanodine-insensitive calcium uptake of cardiac sarcoplasmic reticulum. Circ Res. 1989;65:1400–8. doi: 10.1161/01.res.65.5.1400. [DOI] [PubMed] [Google Scholar]
- 13.Chu A, Submilla C, Scales D, et al. Trypsin digestion of junctional sarcoplasmic reticulum vesicles. Biochemistry. 1998;27:2827–33. doi: 10.1021/bi00408a025. [DOI] [PubMed] [Google Scholar]
- 14.Richard S, Charnet P, Nerbonne JM. Interconversion between distinct gating pathways of the high threshold calcium channel in rat ventricular myocytes. J Physiol. 1993;462:197–228. doi: 10.1113/jphysiol.1993.sp019551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrere-Lemaire S, Piot C, Leclercq F, et al. Facilitation of L-type calcium currents by depolarization in cardiac cells: impairment in heart failure. Cardiovasc Res. 2000;47:336–49. doi: 10.1016/s0008-6363(00)00107-3. [DOI] [PubMed] [Google Scholar]
- 16.Delgado C, Artiles A, Gómez AM, et al. Frequency-dependent increase in cardiac Ca2+ current is due to reduced Ca2+ release by the sarcoplasmic reticulum. J Mol Cell Cardiol. 1999;31:1783–93. doi: 10.1006/jmcc.1999.1023. [DOI] [PubMed] [Google Scholar]
- 17.Zucchi R, Ronca-Testoni S. The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states. Pharmacol Rev. 1997;49:1–51. [PubMed] [Google Scholar]
- 18.Frascarelli S, Ghelardoni S, Chiellini G, et al. Cardiac effects of trace amines: pharmacological characterization of trace amine-associated receptors. Eur J Pharmacol. 2008;587:231–6. doi: 10.1016/j.ejphar.2008.03.055. [DOI] [PubMed] [Google Scholar]
- 19.Bers Kluwer DM. 2001. Excitation-contraction coupling and cardiac contractile force. Dordrecht, The Netherlands:
- 20.Eisner DA, Trafford AW, Diaz ME, et al. The control of Ca release from the cardiac sarcoplasmic reticulum: regulation versus autoregulation. Cardiovasc Res. 1998;38:589–604. doi: 10.1016/s0008-6363(98)00062-5. [DOI] [PubMed] [Google Scholar]
- 21.Eisner DA, Choi HS, Diaz ME, et al. Integrative analysis od calcium cycling in cardiac muscle. Circ Res. 2000;87:1087–94. doi: 10.1161/01.res.87.12.1087. [DOI] [PubMed] [Google Scholar]
- 22.Marx SO, Reiken S, Hisamatsu Y, et al. PKA phosphorylation dissociates FKPB12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell. 2000;101:365–76. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- 23.Lindemann L, Ebeling M, Kratochwil NA, et al. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85:372–85. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Ogura T, Shuba LM, McDonald TF. L-type Ca2+ current in guinea pig ventricular myocytes treated with modulators of tyrosine phosphorylation. Am J Physiol. 1999;276:H1724–33. doi: 10.1152/ajpheart.1999.276.5.H1724. [DOI] [PubMed] [Google Scholar]
- 25.Wang WH, Lin DH, Sterling H. Regulation of ROMK channels by protein tyrosine kinase and tyrosine phosphatase. Trends Cardiovasc Med. 2000;12:138–42. doi: 10.1016/s1050-1738(02)00155-x. [DOI] [PubMed] [Google Scholar]
- 26.Kahaly GJ, Dillmann WH. Thyroid hormone action in the heart. Endocr Rev. 2005;26:704–28. doi: 10.1210/er.2003-0033. [DOI] [PubMed] [Google Scholar]
- 27.Klein I, Danzi S. Thyroid disease and the heart. Circulation. 2007;116:1725–35. doi: 10.1161/CIRCULATIONAHA.106.678326. [DOI] [PubMed] [Google Scholar]


