Abstract
The purpose of the present study was to determine the relative amount of S-thiolated proteins (i.e. S-homocysteinylated, S-cysteinylglycinylated, S-glutathionylated and S-cysteinylated proteins) to the total protein thiols (i.e. the sum of reduced protein sulphydryl groups (PSHs) and protein mixed disulphides with homocysteine [HcySH], cysteinylglycine, cysteine [CysSH] and glutathione) in the plasma of healthy individuals aged 20 to 93. After plasma separation, total protein thiols, S-thiolated proteins, as well as CysSH, cystine, HcySH and homocystine were measured by high-performance liquid chromatography (HPLC) with fluorescence determination of the thiol-monobromobimane conjugate. Determination of plasma levels of protein thiols was performed by spectrophotometry with 5,5′-dithiobis(2-nitrobenzoic acid) as a titrating agent. The present study demonstrates an age-dependent reduction in the amount of PSHs, and an age-dependent increase in cysteinylated and homocysteinylated plasma proteins in healthy human beings. This indicates that the efficiency of the reduced protein thiol pool as an antioxidant defence system decreases with age, possibly causing an increased risk of irreversible oxidation (i.e. further oxidation to sulphinic and sulphonic acids, which are usually not reducible by thiol reducing agents) of sulphydryl groups of plasma proteins. The drop in the plasma level of protein sulphydryl groups suggests depletion and/or impairment of the antioxidant capacity of plasma, likely related to an alteration of the delicate balance between the different redox forms of thiols.
Keywords: ageing, biomarkers of oxidative stress, plasma antioxidant capacity, protein thiols, S-thiolated proteins
Introduction
According to the ‘free radical hypothesis’, the ageing process is a progressive accumulation of oxidative changes leading to disease and death [1, 2]. Studies also show age-related declines in both exogenous and endogenous antioxidant systems including vitamins C and E, glutathione (GSH) and antioxidant enzymes [3–6].
Intracellular thiols such as GSH are essential in maintaining the highly reduced environment inside the cell, whereas extracellular thiols also constitute an important component in antioxidant defence. Because the response of a cell to oxidative stress typically involves alterations in thiol content, plasma thiol concentrations are increasingly utilized for clinical and translational research involving oxidative stress [7–13], and for routine clinical diagnosis and monitoring of various human diseases and metabolic disorders [14–17]. Human plasma contains homocysteine (HcySH), cysteinylglycine (CysGlySH), cysteine (CysSH) and GSH as reduced thiols. These thiols are also found as low-molecular-mass (symmetrical) disulphides, i.e. homocystine ([HcyS]2), cystinilglycine ([CysGlyS]2), cystine ([CysS]2) and glutathione disulphide (GSSG). The measurement of these thiols in human plasma is of interest because most of them are metabolically related and disturbances of their concentrations can correspond to disorders of metabolism that occurs during ageing [18]. Ageing has recently been correlated to a decrease in human plasma GSH and CysGlySH and the concomitant increase in most oxidized forms of thiols as well as to a parallel increase in total Cys and total Hcy, probably due to an augmented efflux of these amino acids from various organs [19].
In human plasma, concentration of protein sulphydryl groups (PSHs) is in the 0.4–0.5 mM range, whereas that of low-molecular-mass thiols is in the 0.1–20 μM range [19, 20]. PSHs are common targets of oxidative modifications [21–23]. They are present as reduced thiols and/or mixed disulphides with HcySH, CysGlySH, CysSH and GSH (as a whole referred to as S-thiolated proteins) [19, 24, 25], through a reaction of S-thiolation, in which protein thiols form a mixed disulphide bond with low-molecular-mass thiols. This process plays both a regulatory and an antioxidant role, because it protects protein cysteinyl thiols against irreversible oxidation (i.e. further oxidation to sulphinic and sulphonic acids) [26] and also has a role in protein redox regulation [27].
There exist reports on the level of GSH, GSSG, malondialdehyde, protein carbonyls, 4-hydroxy-2,3-trans-nonenal, uric acid and the redox couples GSH/GSSG and CysSH/(CysS)2, as well as on antioxidant enzyme activities (superoxide dismutases and GSH peroxidases) in blood and plasma of healthy populations during ageing [6, 19, 28–32]. In these studies, tendencies for some parameters and also significant correlations were established, showing an increase in pro-oxidative effects and a decrease in antioxidative ones during ageing.
S-thiolated plasma proteins have been detected in healthy human beings, but increase in patients with cardiovascular diseases and in uremic patients on haemodialysis [33, 34]. Although increased protein S-thiolation is considered an in vivo marker of oxidative injury, scarce attention has been paid to the significance and quantification of S-thiolated proteins in blood plasma of healthy individuals during ageing. The purpose of the present study was to determine the relative amount of S-thiolated proteins (i.e. homocysteinylated, cysteinylglycinylated, glutathionylated and cysteinylated proteins) to the total protein thiols (i.e. the sum of protein sulphydryl groups and protein mixed disulphides with HcySH, CysGlySH, CysSH and GSH) in the plasma of healthy individuals aged 20 to 93 years. The results indicate a significant age-dependent decrease in reduced plasma PSHs, as well as an age-dependent increase in plasma concentrations of total S-thiolated proteins. In particular, we observed a little increase in S-homocysteinylated proteins as well as a large increase in S-cysteinylated proteins during ageing in healthy elderly individuals. The present results suggest that protection from oxidative damage by plasma thiol redox buffer becomes less efficient with age; this concerns not only decrease in plasma GSH [19, 28, 32] and increase in total Cys and total Hcy [19], but also cysteinylation and homocysteinylation of plasma proteins.
Materials and methods
Materials
Monobromobimane (mBrB) was obtained from Calbiochem (La Jolla, CA, USA). Acivicin, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), N-ethylmaleimide (NEM) and all the other reagents used in this study were of analytical grade from Sigma-Aldrich (Milan, Italy). HPLC Spherisorb C18 column was purchased from Varian, Inc. (Palo Alto, CA, USA). HPLC separation was performed with an Agilent series 1100 HPLC equipped with fluorescence detector. Spectrophotometric analyses were performed with a Jasco V/530 instrument (Jasco Corporation, Tokyo, Japan).
Study participants
The study participants included 63 healthy individuals (38 females, 25 males) aged 20–93 years. Each participant declared not to have taken any medicine in the last 7 days, and was screened for diagnosed diseases. Individuals with diabetes or age-related cardiovascular, renal, pulmonary, hepatic or ocular disease were excluded, and none of them had any abnormality on physical examination nor in routine laboratory blood tests (serum glutamic oxaloacetic transaminase [SGOT], serum glutamic piruvate transaminase [SGPT], γ-glutamyltransferase [γ-GT], uraemia, creatininemia, erythrocytes sedimentation rate, blood cell number, platelet number, mean cellular volume, haematocrit, haemoglobin concentration, plasma protein concentration, albumin concentration). In particular, none of the individuals had any alterations in renal function (evaluated by the serum levels of creatinine and urea levels) nor in hepatic function (evaluated by activity of γ-GT and transaminases). Furthermore, the selection excluded not only individuals with acute infections or chronic diseases, but also excluded healthy individuals undergoing supplementation with antioxidant substances. The individuals were divided into four age groups (Table 1).
Table 1.
Characteristics of study groups
| Age groups | No. of individuals | Mean age | Females | Males |
|---|---|---|---|---|
| 20–40 years | 18 | 32.3 | 10 | 8 |
| 41–60 years | 18 | 52.1 | 11 | 7 |
| 61–80 years | 16 | 67.5 | 9 | 7 |
| 81–93 years | 11 | 84.3 | 8 | 3 |
All human experimentation was conducted in conformance with the ‘Principles for Research Involving Animals and Human Beings’ and approved by the Local Ethical Committee of the University of Siena. Informed consent was obtained from all the individuals after the purpose and nature of the study had been explained.
Plasma collection and protein analysis
Blood samples were drawn in the morning after 10–12 hrs of starving from the antecubital vein, collected in plastic tubes containing K3EDTA, acivicin (50 μg/ml) and either mBrB or NEM to alkylate free sulphydryls. Samples were centrifuged at 10,000 ×g for 20 sec., at 4°C, for plasma separation; this rapid centrifugation was applied in order to avoid, as much as possible, the efflux of mBrB-GSH conjugate from red blood cells that could artefactually increase the measured levels of plasmatic GSH [35]. After plasma separation, S-thiolated proteins (i.e. the sum of S-homocysteinylated, S-cysteinylglycinylated, S-glutathionylated and S-cysteinylated proteins), total protein thiols (i.e. the sum of PSH +S-thiolated proteins; tPS), as well as CysSH, (CysS)2, HcySH and (HcyS)2, were measured by HPLC with fluorescence determination of the thiol-mBrB conjugate as previously reported [36]. The relative amounts of S-homocysteinylated (HcySSP), S-cysteinylglycinylated (CysGlySSP), S-glutathionylated (GSSP) and S-cysteinylated proteins (CysSSP) were calculated to the total amount of protein cysteinyl thiols (tPS, i.e. PSH +S-thiolated proteins). A summary of the procedures followed for accurate thiol/disulphide determination in human plasma is reported in Fig. 1.
Figure 1.
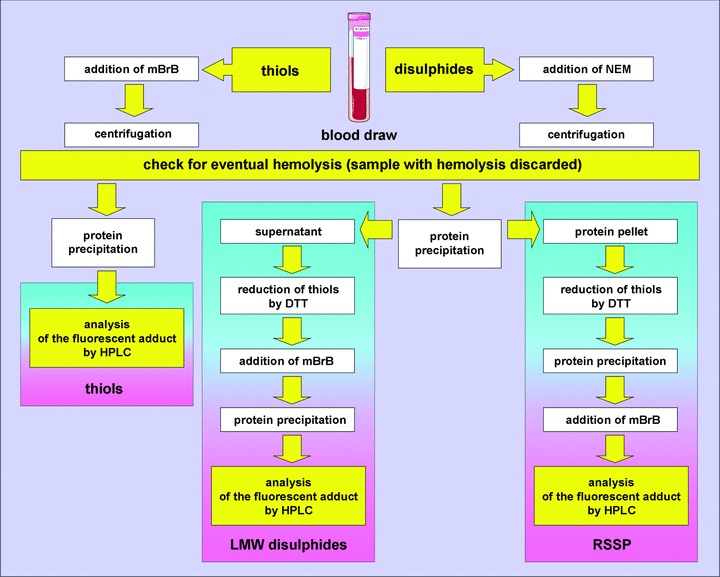
Schematic representation of the main analytical steps making the procedures we adopted for accurate thiol/disulphide determination in human plasma. mBrB: monobromobimane; NEM: N-ethylmaleimide; DTT: dithiothreitol; LMW: low molecular weight and RSSP: protein/thiol mixed disulphides.
For determination of plasma levels of PSH, aliquots of blood samples were collected in evacuated plastic tubes containing only K3EDTA and acivicin (50 μg/ml) and then immediately centrifuged at 10,000 ×g for 20 sec., at 4°C; plasma was then stored at –80°C until the analyses were carried out. PSH determination was performed by spectrophotometry with DTNB as a titrating agent [37].
Statistical analysis
For each age group, data are presented as the mean ± S.D. Statistical analysis among different age groups was performed by applying the one-way ANOVA test and the Bonferroni’s test as multiple comparison method for all pairwise. Linear regression was performed by applying the Pearson product moment correlation test.
Results
Human red blood cells contain approximately 500 times higher GSH concentration than plasma [26] so that minor haemolysis can result in erroneously high plasma GSH values. Furthermore, thiols can be lost by oxidation, which occurs with a half time of about 5 min. in plasma at room temperature [38]. Here, we have used a procedure that we have optimized to collect blood with minimal haemolysis, to prevent thiol oxidation and degradation during sample processing and to provide a specific and sensitive measure of plasma thiols [19, 35, 36, 39].
In all measured plasma samples, independently of age, mean values of PSH and S-thiolated proteins (i.e. S-cysteinylglycinylated +S-glutathionylated +S-homocysteinylated +S-cysteinylated proteins = RSSP) were 417.10 ± 29.23 μM and 182.9 ± 29.30 μM, respectively. On the basis of the total concentration for each low molecular mass thiol species, i.e. the concentration of reduced thiol + low-molecular-mass (symmetrical or unmixed) disulphides +S-thiolated proteins (i.e. protein/thiol mixed disulphides, RSSP), in all measured plasma samples, independently of age, tCys is present at the highest concentration (∼237 μM), with tCys > tCysGly > tHcy > tGSH (Table 2). These results are in agreement with other reports [19, 20, 40]. In this context, it is worth noting that reported concentrations of low-molecular-mass disulphides presented in Table 2 and throughout the manuscript are thiols measured after reduction of disulphides (Fig. 1); thus, what reported, for example, as GSSG is actually the sum of GSSG and asymmetrical disulphides, i.e. CysSSG, HcySSG and CysGlySSG. Hence, values reported by some authors, who discriminate between GSSG and asymmetrical disulphides [29], may be lower.
Table 2.
Plasma thiol levels in healthy individuals, independently of age
| Thiols | Plasma levels (μM) |
|---|---|
| GSH | 2.23 ± 0.78 |
| GSSG | 1.23 ± 0.44 |
| GSSP | 2.31 ± 1.18 |
| Total GSH | 5.79 ± 1.80 |
| HcySH | 0.23 ± 0.14 |
| (HcyS)2 | 1.71 ± 1.16 |
| HcySSP | 9.33 ± 3.57 |
| Total Hcy | 11.25 ± 4.44 |
| CysGlySH | 2.04 ± 0.74 |
| (CysGlyS)2 | 5.69 ± 1.48 |
| CysGlySSP | 11.96 ± 2.96 |
| Total CysGly | 19.74 ± 3.93 |
| CysSH | 9.96 ± 1.29 |
| (CysS)2 | 67.74 ± 13.59 |
| CysSSP | 156.82 ± 28.44 |
| Total Cys | 237.11 ± 36.61 |
Data are presented as the mean ± S.D.
Figure 2 shows micromolar plasma concentrations of total protein thiols (tPS = PSH + RSSP) for each age group. The distribution of the total protein thiol levels during ageing ranged from about 589 to 618 μM. There was neither significant age-related tendency within this range nor significant difference between males and females (not shown). Differently, results illustrated in Fig. 3 show age-dependent diminution in plasma concentration of PSH (Fig. 3A) as well as age-dependent increase in plasma concentrations of S-thiolated proteins (Fig. 3B). PSH declined with age from about 449 to 397 μM (–12%) (Fig. 3A), whereas the level of S-thiolated proteins increased with age from about 163 to 221 μM (+35%) (Fig. 3B and C). Results illustrated in Fig. 3D show age-dependent decrease in the plasma PSH/RSSP ratio from about 2.7 to 1.8, most, if not all, of decrease becoming evident over 60 years.
Figure 2.
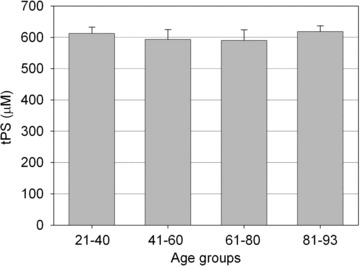
Plasma levels of total protein thiols (tPS = reduced protein sulphydryl groups +S-thiolated proteins) for each age group. Data are means ± S.D.
Figure 3.
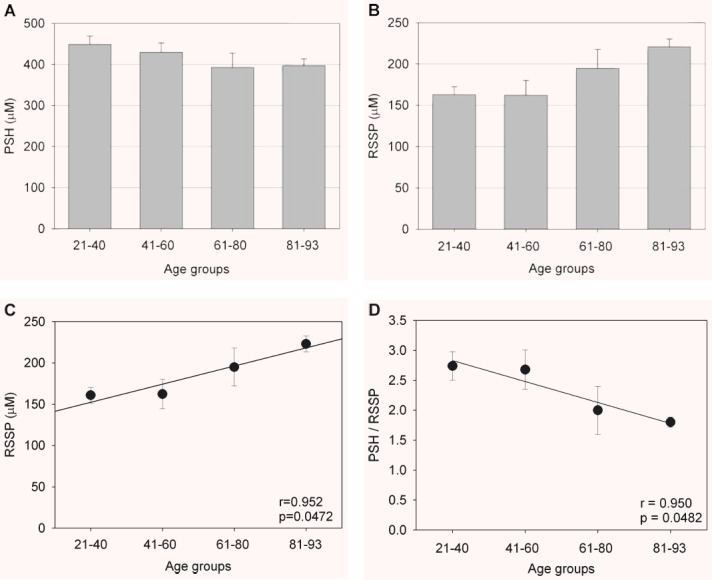
Plasma levels of reduced protein sulphydryl groups (PSH) (A) and S-thiolated proteins (RSSP) (B, C) for each age group. (D): Shows the age-dependent decrease in the plasma reduced protein thiols/S-thiolated proteins (PSH/RSSP) ratio for each age group. Data are means ± S.D. Statistical significance (one-way ANOVA, P < 0.05): (A): 21–40 versus 61–80 and versus 81–93; 41–60 versus 81–93; (B): 21–40 and 41–60 versus 81–93. (C): 21–40 versus 61–80 and 81–93; 41–60 versus 61–80 and 81–93.
Measurements of S-cysteinylglycinylated and S-glutathionylated proteins, as the relative amount of S-thiolated proteins to total protein thiols and as a function of age, are illustrated in Fig. 4A and B, respectively. These S-thiolated proteins do not change significantly in correlation with age.
Figure 4.
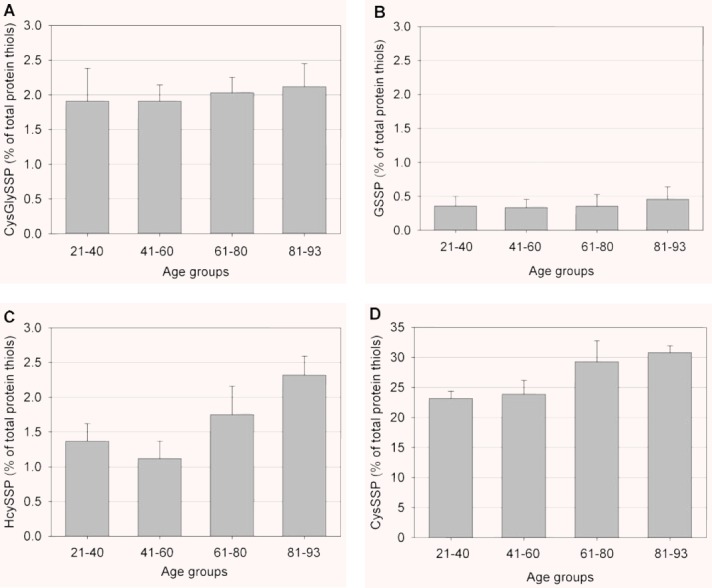
Plasma levels of S-cysteinylglycinylated (CysGlySSP) (A), S-glutathionylated (GSSP) (B), S-homocysteinylated (HcySSP) (C), and S-cysteinylated (CysSSP) and (D) proteins for each age group. All values are expressed as the percentage to total plasma protein thiols. Data are means ± S.D. Statistical significance (one-way ANOVA, P < 0.05): (C): 21–40 versus 81–93; 41–60 versus 61–80 and 81–93; 61–80 versus 81–93; (D): 21–40 versus 61–80 and 81–93; 41–60 versus 61–80 and 81–93.
Measurements of S-homocysteinylated and S-cysteinylated proteins, as the relative amount of S-thiolated proteins to total protein thiols and as a function of age, are illustrated in Fig. 4C and D, respectively. An apparent, age-related minute increase (about 1%) was measured for S-homocysteinylated proteins, but variation of data also increased with age (Fig. 4C); however, the presence of S-homocysteinylated proteins, compared with that occurring in the youngest individuals (first group of age), increased by 25% and 75%, respectively, in the individuals aged 61–80 years and more than 80 years of age (Fig. 4C). Differently, a positive correlation was determined between S-cysteinylated proteins and the ageing process: results illustrated in Fig. 4D show a clear age-dependent increase, ∼8%, in plasma concentrations of S-cysteinylated proteins. In comparison with the group of the youngest individuals, the percentage increase in S-homocysteinylated proteins was about 26% and 35%, respectively, in the third and fourth group of age. Notwithstanding there is evidence for an association between S-cysteinylated proteins and age, there is also considerable variation among individuals of the same age group. However, the data reported in Fig. 4C and D clearly show that, until approximately 60 years of age, there is no significant association between S-homocysteinylated and S-cysteinylated proteins and age, whereas the ratio of S-homocysteinylated and S-cysteinylated proteins to total protein thiols increase with age over 60 years.
The CysSH/(CysS)2 redox couple quantitatively represents the largest pool of low-molecular-weight thiols and disulphides in plasma [41], and the mean value of plasma CysSH/(CysS)2 pool in human beings shows a significant oxidative shift between the third and the ninth decade of life [6, 42]. Results illustrated in Fig. 5A show that the plasma HcySH/(HcyS)2 ratio changes significantly during the whole age range. Differently, we measured only a slight age-dependent decrease (note the different scale range on the y-axis between Fig. 5A and B) in the plasma CysSH/(CysS)2 ratio (Fig. 5B).
Figure 5.
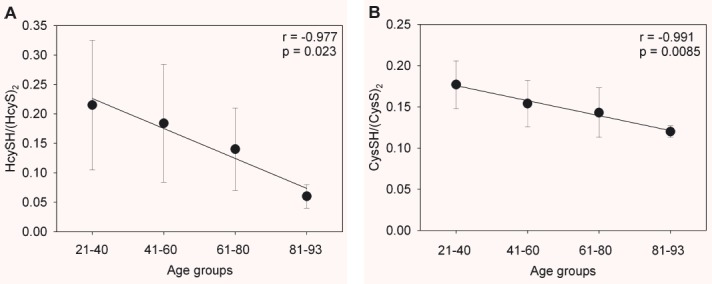
Effect of age on the homocysteine/homocystine, HcySH/(HcyS)2, (A) and the cysteine/cystine, CysSH/(CysS)2, (B) redox couple in healthy individuals. Data are means ± S.D.
Discussion
Major differences between cellular and extracellular compartments exist both in terms of the concentrations of sulphydryl/disulphide systems and their relative redox states [41, 43]. Within cells, the major low-molecular-weight sulphydryl/disulphide pool, GSH/GSSG, is principally in the reduced form. Differently, the CysSH/(CysS)2 pool, mainly in the disulphide form, quantitatively represents the largest pool of low-molecular-weight thiols and disulphides in plasma and the extracellular compartment on the whole. Therefore, intracellular proteins may be prevalently S-glutathionylated, whereas extracellular proteins may be predominantly S-cysteinylated. For example, although the small fraction of S-thiolated haemoglobin in red blood cells is S-glutathionylated [44–47], plasma proteins such as albumin are mainly S-cysteinylated [13, 26, 48].
In human plasma, concentration of PSHs is mostly due to cysteinyl residues of albumin, which reaches concentrations of more than 500 μM. As for the low-molecular-mass thiols, plasma concentration of GSH is generally in the range of 2–4 μM [19, 20, 26], CysSH is in the range of 8–10 μM and that of (CysS)2 is higher than 40 μM [29]. Although representing only a minor component as compared with protein cysteinyl thiols and free CysSH, GSH is the most extensively studied extracellular thiol/disulphide component in the human plasma during ageing [28, 32].
The present study shows no significant changes in the plasma concentrations of total protein thiols (PSH + RSSP) for each age group (Fig. 2). On the other hand, the age-related decrease in the plasma level of PSHs is accompanied by a significant increase in the concentration of S-thiolated proteins (Fig. 3). These data suggest that there is some increase in oxidative events targeting protein thiols throughout adult life, the phenomenon becoming clearly manifest as from 60–70 years. The pools of S-thiolated proteins differ in their relationships with age. There are scarce or no differences in the relative amount (as compared to total plasma protein thiols) of
S-cysteinylglycinylated and S-glutathionylated proteins between younger and older age groups of healthy individuals (Fig. 4A and B). On the contrary, S-homocysteinylated and S-cysteinylated proteins increase with age in healthy individuals (Fig. 4C and D). The age-related homocysteinylation is accompanied by a significative decrease in the plasma HcySH/(HcyS)2 ratio (Fig. 5A). In agreement with other reports [6, 42], our results suggest that the age-related cysteinylation is accompanied by a decrease in the plasma CysSH/(CysS)2 pool in healthy human beings (Fig. 5B).
The most abundant reduced sulphydryl group in plasma is that of human serum albumin (HSA), given its high concentrations. The single free cysteinyl thiol of HSA, Cys34, accounts for ∼80% of reduced thiols in human plasma and is an important scavenger of reactive oxygen and nitrogen species (ROS/RNS) in the vascular compartment, thus resulting to be a quantitatively important redox buffer of blood [49–51]. Although albumin circulates mostly (∼70%) in the reduced state, an important fraction (∼25%) of HSA circulates as mixed disulphides with low-molecular-mass thiols, mainly CysSH, likely via a stable sulphenic acid intermediate, whereas a small fraction (∼5%) is oxidized to higher, irreversible oxidation states (i.e. sulphinic and/or sulphonic acids) [50–52]. This balance between reduced and oxidized forms of HSA can shift in pathological states, and during ageing [51, 52]. Thus, decrease in plasma PSHs, as seen in aged groups (Fig. 3A), has important physiological implications. Reduced PSHs are important scavenger of ROS/RNS, thus homocysteinylation and cysteinylation of plasma protein sulphydryls that occur in healthy elderly individuals over the age of 60 years (Fig. 4C and D) could contribute to the decrease in the antioxidant capacity of plasma PSH. This involves an increased risk of irreversible oxidation (i.e. further oxidation to sulphinic and sulphonic acids) of protein thiols, which, because of the emerging data suggesting an important role for the extracellular thiol/disulphide redox environment in inducing and/or affecting intracellular signals that activate intracellular redox-sensitive pathways, such as cellular proliferation and matrix expression [41, 53, 54], may be critically important on signal transduction and transcription events that utilize proteins containing sulphydryl groups in reactive sites. Thus, the associated risk of adverse effects during ageing may increase (Fig. 6).
Figure 6.
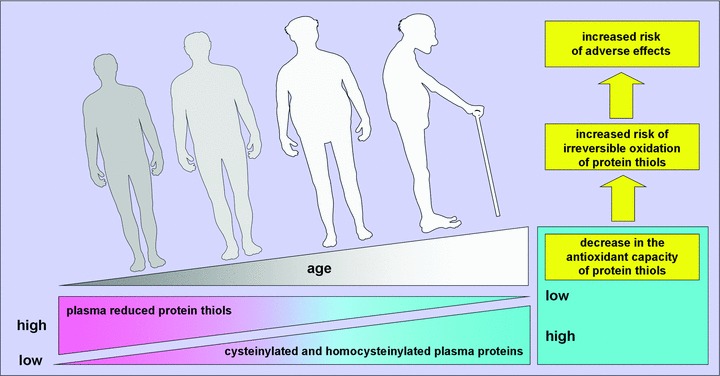
Mean plasma PSH redox state becomes progressively oxidized with age after 60 years. Measurements of homocysteinylated and cysteinylated plasma proteins in healthy individuals show that little variation occurs among younger individuals but, after about 60 years, plasma reduced protein sulphydryl groups become progressively S-thiolated with age, contributing to the decrease in the antioxidant capacity of plasma. Thus, the associated risk of adverse effects during ageing may increase. In principle, S-thiolation in plasma protein thiols is reversible. Thus, if oxidative stress contributes to oxidant-mediated changes of ageing and age-related diseases, nutritional modulation or therapeutic interventions might provide a potentially useful strategy to restore redox state of human plasma.
Protein-bound HcySH accounts for 70% to 80% of plasma total HcySH in healthy individuals [55, 56]. Homocysteinylation of accessible CysSH residues in different cellular and plasma proteins could alter their structure or function or both [57]. In vitro studies have shown that homocysteinylation of the Cys9 residue of annexin II, the endothelial cell surface docking protein for tissue plasminogen activator, inhibits binding [58]. HcySH also binds the fibrin binding domain of plasma fibronectin, inhibiting its ability to bind fibrin [59], as well as factor Va, making it resistant to inactivation by activated protein C [60]. Furthermore, the amyloidogenic protein transthyretin (pre-albumin) undergoes S-homocysteinylation at its single CysSH residue both in vitro and in vivo[61] and homocysteinylation of LDL induces an increase in oxidative damage and a decreased viability on human endothelial cells [62]. Intriguing, recent findings provide the first evidence of S-homocysteinylation of intracellular metallothionein, a ubiquitous intracellular zinc-chaperone and superoxide anion radical scavenger, which results in loss of protein function, thus suggesting a novel mechanism for disruption of intracellular zinc and redox homeostasis [63]. These studies thus provide novel evidence as to how HcySH may affect multiple cellular stress pathways [63].
Taken together, these studies suggest a possible link between increased oxidative stress, decrease in plasma GSH and PSH [19, 28, 32], increase in S-thiolated proteins and ageing, thus suggesting that antioxidant supplementation might be beneficial during ageing. On the other hand, a few clinical investigations have shown that CysSH supplementation exerts positive effects on certain functional and biochemical parameters, which become typically compromised in the course of ageing [64], supporting the hypothesis that oxidative changes may contribute to the ageing process.
In conclusion, several lines of evidence indicate that oxidative stress increases with age. Plasma redox values are dynamic indicators of the systemic balance between oxidative and antioxidant processes [6, 19, 41, 43]. The cross-talk among the various thiol pools in plasma (redox thiol status) has been proposed to be an element of the extracellular antioxidant defence system [6, 41, 65]. In this study, we show significant age-related changes of important biomarkers of oxidative stress in human plasma of healthy individuals. The increasing oxidative stress during ageing is accompanied by a decrease in reduced protein sulphydryls, and increase in S-homocysteinylated and S-cysteinylated proteins. This finding does not only underline the ‘free radical hypothesis’ of ageing, but is also important for the evaluation of further clinical studies with regard to basic (normal) values and with regard to the usage of age matched healthy control groups. In principle, analyses of reduced protein sulphydryls, S-homocysteinylated and S-cysteinylated protein levels in human plasma can be used to assess efficacy of intervention strategies against oxidative stress prior to or early after onset of clinical symptoms in ageing and age-related diseases.
Acknowledgments
This work was supported by Fondazione Monte dei Paschi di Siena and by FIRST 2007 (Fondo Interno Ricerca Scientifica e Tecnologica), University of Milan. Figure 6 included in this article was prepared using and combining medical cliparts and illustrations available within the Servier Medical Art section, by courtesy of Servier International.
References
- 1.Beckman KB, Ames BN. The free radical theory of aging matures. Physiol Rev. 1998;78:547–81. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 2.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 3.Lenton KJ, Therriault H, Cantin AM, et al. Direct correlation of glutathione and ascorbate and their dependence on age and season in human lymphocytes. Am J Clin Nutr. 2000;71:1194–200. doi: 10.1093/ajcn/71.5.1194. [DOI] [PubMed] [Google Scholar]
- 4.Mecocci P, Polidori MC, Troiano L, et al. Plasma antioxidants and longevity: a study on healthy centenarians. Free Radic Biol Med. 2000;28:1243–8. doi: 10.1016/s0891-5849(00)00246-x. [DOI] [PubMed] [Google Scholar]
- 5.Macmillan-Crow LA, Cruthirds DL. Manganese superoxide dismutase in disease. Free Radic Res. 2001;34:325–36. doi: 10.1080/10715760100300281. [DOI] [PubMed] [Google Scholar]
- 6.Jones DP, Mody VC, Carlson JL, et al. Redox analysis of human plasma allows separation of prooxidant events of aging from decline in antioxidant defenses. Free Radic Biol Med. 2002;33:1290–300. doi: 10.1016/s0891-5849(02)01040-7. [DOI] [PubMed] [Google Scholar]
- 7.Himmelfarb J, Hakim RM. Oxidative stress in uremia. Curr Opin Nephrol Hypertens. 2003;12:593–8. doi: 10.1097/00041552-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Moriarty SE, Shah JH, Lynn M, et al. Oxidation of glutathione and cysteine in human plasma associated with smoking. Free Radic Biol Med. 2003;35:1582–8. doi: 10.1016/j.freeradbiomed.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 9.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–80. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 10.Ashfaq S, Abramson JL, Jones DP, et al. The relationship between plasma levels of oxidized and reduced thiols and early atherosclerosis in healthy adults. J Am Coll Cardiol. 2006;47:1005–11. doi: 10.1016/j.jacc.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 11.Aslam S, Santha T, Leone A, et al. Effects of amlodipine and valsartan on oxidative stress and plasma methylarginines in end-stage renal disease patients on hemodialysis. Kidney Int. 2006;70:2109–15. doi: 10.1038/sj.ki.5001983. [DOI] [PubMed] [Google Scholar]
- 12.Zinellu A, Zinellu E, Sotgia S, et al. Factors affecting S-homocysteinylation of LDL apoprotein B. Clin Chem. 2006;52:2054–9. doi: 10.1373/clinchem.2006.071142. [DOI] [PubMed] [Google Scholar]
- 13.Ogasawara Y, Mukai Y, Togawa T, et al. Determination of plasma thiol bound to albumin using affinity chromatography and high-performance liquid chromatography with fluorescence detection: ratio of cysteinyl albumin as a possible biomarker of oxidative stress. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;845:157–63. doi: 10.1016/j.jchromb.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 14.Ducros V, Demuth K, Sauvant MP, et al. Methods for homocysteine analysis and biological relevance of the results. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;781:207–26. doi: 10.1016/s1570-0232(02)00497-x. [DOI] [PubMed] [Google Scholar]
- 15.Ozkan Y, Ozkan E, Simsek B. Plasma total homocysteine and cysteine levels as cardiovascular risk factors in coronary heart disease. Int J Cardiol. 2002;82:269–77. doi: 10.1016/s0167-5273(02)00010-4. [DOI] [PubMed] [Google Scholar]
- 16.Pastore A, Federici G, Bertini E, et al. Analysis of glutathione: implication in redox and detoxification. Clin Chim Acta. 2003;333:19–39. doi: 10.1016/s0009-8981(03)00200-6. [DOI] [PubMed] [Google Scholar]
- 17.Zinellu A, Pinna A, Zinellu E, et al. High-throughput capillary electrophoresis method for plasma cysteinylglycine measurement: evidences for a clinical application. Amino Acids. 2008;34:69–74. doi: 10.1007/s00726-007-0590-4. [DOI] [PubMed] [Google Scholar]
- 18.Andriollo-Sanchez M, Hininger-Favier I, Meunier N, et al. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: the ZENITH study. Eur J Clin Nutr. 2005;59:S58–62. doi: 10.1038/sj.ejcn.1602300. [DOI] [PubMed] [Google Scholar]
- 19.Giustarini D, Dalle-Donne I, Lorenzini S, et al. Age-related influence on thiol, disulphide and protein mixed disulphide levels in human plasma. J Gerontol A. 2006;61:1030–8. doi: 10.1093/gerona/61.10.1030. [DOI] [PubMed] [Google Scholar]
- 20.Mansoor MA, Svardal AM, Ueland PM. Determination of the in vivo redox status of cysteine, cysteinylglycine, homocysteine, and glutathione in human plasma. Anal Biochem. 1992;200:218–29. doi: 10.1016/0003-2697(92)90456-h. [DOI] [PubMed] [Google Scholar]
- 21.Dalle-Donne I, Scaloni A, Giustarini D, et al. Proteins as biomarkers of oxidative/nitrosative stress in diseases. The contribution of redox proteomics. Mass Spectrom Rev. 2005;24:55–99. doi: 10.1002/mas.20006. [DOI] [PubMed] [Google Scholar]
- 22.Dalle-Donne I, Giustarini D, Colombo R, et al. S-glutathionylation in human platelets by a thiol-disulphide exchange-independent mechanism. Free Radic Biol Med. 2005;38:1501–10. doi: 10.1016/j.freeradbiomed.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 23.Dalle-Donne I, Rossi R, Colombo R, et al. Biomarkers of oxidative damage in human disease. Clin Chem. 2006;52:601–23. doi: 10.1373/clinchem.2005.061408. [DOI] [PubMed] [Google Scholar]
- 24.Eaton P. Protein thiol oxidation in health and disease: techniques for measuring disulphides and related modifications in complex protein mixtures. Free Radic Biol Med. 2006;40:1889–99. doi: 10.1016/j.freeradbiomed.2005.12.037. [DOI] [PubMed] [Google Scholar]
- 25.Hortin GL, Seam N, Hoehn GT. Bound homocysteine, cysteine, and cysteinylglycine distribution between albumin and globulins. Clin Chem. 2006;52:2258–64. doi: 10.1373/clinchem.2006.074302. [DOI] [PubMed] [Google Scholar]
- 26.Dalle-Donne I, Milzani A, Gagliano N, et al. Molecular mechanisms and potential clinical significance of S-glutathionylation. Antioxid Redox Signal. 2008;10:446–73. doi: 10.1089/ars.2007.1716. [DOI] [PubMed] [Google Scholar]
- 27.Dalle-Donne I, Rossi R, Giustarini D, et al. S-Glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–98. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- 28.Samiec PS, Drews-Botsch C, Flagg EW, et al. Glutathione in human plasma: decline in association with aging, age-related macular degeneration, and diabetes. Free Radic Biol Med. 1998;24:699–704. doi: 10.1016/s0891-5849(97)00286-4. [DOI] [PubMed] [Google Scholar]
- 29.Jones DP, Carlson JL, Mody VC, et al. Redox state of glutathione in human plasma. Free Radic Biol Med. 2000;28:625–35. doi: 10.1016/s0891-5849(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 30.Erden-Inal M, Sunal E, Kanbak G. Age-related changes in the glutathione redox system. Cell Biochem Funct. 2002;20:61–6. doi: 10.1002/cbf.937. [DOI] [PubMed] [Google Scholar]
- 31.Junqueira VB, Barros SB, Chan SS, et al. Aging and oxidative stress. Mol Aspects Med. 2004;25:5–16. doi: 10.1016/j.mam.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Gil L, Siems W, Mazurek B, et al. Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res. 2006;40:495–505. doi: 10.1080/10715760600592962. [DOI] [PubMed] [Google Scholar]
- 33.Undas A, Perla J, Lacinski M, et al. Autoantibodies against N-homocysteinylated proteins in humans: implications for atherosclerosis. Stroke. 2004;35:1299–304. doi: 10.1161/01.STR.0000128412.59768.6e. [DOI] [PubMed] [Google Scholar]
- 34.Perna AF, Satta E, Acanfora F, et al. Increased plasma protein homocysteinylation in hemodialysis patients. Kidney Int. 2006;69:869–76. doi: 10.1038/sj.ki.5000070. [DOI] [PubMed] [Google Scholar]
- 35.Rossi R, Dalle-Donne I, Milzani A, et al. Hematic oxidized forms of glutathione: a powerful biomarker of oxidative stress status. Clin Chem. 2006;52:1406–14. doi: 10.1373/clinchem.2006.067793. [DOI] [PubMed] [Google Scholar]
- 36.Giustarini D, Dalle-Donne I, Colombo R, et al. An improved HPLC mesurement for GSH and GSSG in human blood. Free Radic Biol Med. 2003;35:1365–72. doi: 10.1016/j.freeradbiomed.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 37.Ellman GL. Tissue sulfhydryl goups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 38.Jones DP, Carlson JL, Samiec PS, et al. Glutathione measurement in human plasma. Evaluation of sample collection, storage and derivatization conditions for analysis of dansyl derivatives by HPLC. Clin Chim Acta. 1998;275:175–84. doi: 10.1016/s0009-8981(98)00089-8. [DOI] [PubMed] [Google Scholar]
- 39.Giustarini D, Dalle-Donne I, Colombo R, et al. Interference of plasmatic glutathione and hemolysis on glutathione disulphide levels in human blood. Free Radic Res. 2004;38:1101–6. doi: 10.1080/10715760400008854. [DOI] [PubMed] [Google Scholar]
- 40.Tsikas D. Incorrect and inconsistent use of homocysteine’s nomenclature: a potential source of misunderstandings. Eur J Clin Invest. 2003;33:1095–6. doi: 10.1111/j.1365-2362.2003.01265.x. [DOI] [PubMed] [Google Scholar]
- 41.Jones DP. Redefining oxidative stress. Antioxid Redox Signal. 2006;8:1865–79. doi: 10.1089/ars.2006.8.1865. [DOI] [PubMed] [Google Scholar]
- 42.Hildebrandt W, Kinscherf R, Hauer K, et al. Plasma cysteine concentration and redox state in ageing and physical exercise. Mech Ageing Dev. 2002;123:1269–81. doi: 10.1016/s0047-6374(02)00013-1. [DOI] [PubMed] [Google Scholar]
- 43.Moriarty-Craige SE, Jones DP. Extracellular thiols and thiol/disulphide redox in metabolism. Annu Rev Nutr. 2004;24:481–509. doi: 10.1146/annurev.nutr.24.012003.132208. [DOI] [PubMed] [Google Scholar]
- 44.Niwa T, Naito C, Mawjood AH, et al. Increased glutathionyl hemoglobin in diabetes mellitus and hyperlipidemia demonstrated by liquid chromatography/electrospray ionization-mass spectrometry. Clin Chem. 2000;46:82–8. [PubMed] [Google Scholar]
- 45.Giustarini D, Dalle-Donne I, Colombo R, et al. Protein glutathionylation in erythrocytes. Clin Chem. 2003;49:327–30. doi: 10.1373/49.2.327. [DOI] [PubMed] [Google Scholar]
- 46.Mawatari S, Murakami K. Different types of glutathionylation of hemoglobin can exist in intact erythrocytes. Arch Biochem Biophys. 2004;421:108–14. doi: 10.1016/j.abb.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 47.Sampathkumar R, Balasubramanyam M, Sudarslal S, et al. Increased glutathionylated hemoglobin (HbSSG) in type 2 diabetes subjects with microangiopathy. Clin Biochem. 2005;38:892–9. doi: 10.1016/j.clinbiochem.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 48.Bar-Or D, Curtis CG, Sullivan A, et al. Plasma albumin cysteinylation is regulated by cystathionine beta-synthase. Biochem Biophys Res Commun. 2004;325:1449–53. doi: 10.1016/j.bbrc.2004.10.191. [DOI] [PubMed] [Google Scholar]
- 49.Halliwell B, Gutteridge MC. The antioxidants of human extracellular fluids. Arch Biochem Biophys. 1990;280:1–8. doi: 10.1016/0003-9861(90)90510-6. [DOI] [PubMed] [Google Scholar]
- 50.Carballal S, Radi R, Kirk MC, et al. Sulfenic acid formation in human serum albumin by hydrogen peroxide and peroxynitrite. Biochemistry. 2003;42:9906–14. doi: 10.1021/bi027434m. [DOI] [PubMed] [Google Scholar]
- 51.Carballal S, Alvarez B, Turell L, et al. Sulfenic acid in human serum albumin. Amino Acids. 2007;32:543–51. doi: 10.1007/s00726-006-0430-y. [DOI] [PubMed] [Google Scholar]
- 52.Era S, Kuwata K, Imai H, et al. Age-related change in redox state of human serum albumin. Biochim Biophys Acta. 1995;1247:12–6. doi: 10.1016/0167-4838(94)00166-e. [DOI] [PubMed] [Google Scholar]
- 53.Anderson CL, Iyer SS, Ziegler TR, et al. Control of extracellular cysteine/cystine redox state by HT-29 cells is independent of cellular glutathione. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1069–75. doi: 10.1152/ajpregu.00195.2007. [DOI] [PubMed] [Google Scholar]
- 54.Ramirez A, Ramadan B, Ritzenthaler JD, et al. Extracellular cysteine/cystine redox potential controls lung fibroblast proliferation and matrix expression through upregulation of transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L972–81. doi: 10.1152/ajplung.00010.2007. [DOI] [PubMed] [Google Scholar]
- 55.Refsum H, Helland S, Ueland PM. Radioenzymic determination of homocysteine in plasma and urine. Clin Chem. 1985;31:624–8. [PubMed] [Google Scholar]
- 56.Refsum H, Smith AD, Ueland PM, et al. Facts and recommendations about total homocysteine determinations: an expert opinion. Clin Chem. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 57.Glushchenko AV, Jacobsen DW. Molecular targeting of proteins by L-homocysteine: mechanistic implications for vascular disease. Antioxid Redox Signal. 2007;9:1883–98. doi: 10.1089/ars.2007.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Giles WH, Croft JB, Greenlund KJ, et al. Association between total homocyst(e)ine and the likelihood for a history of acute myocardial infarction by race and ethnicity: results from the Third National Health and Nutrition Examination Survey. Am Heart J. 2000;139:446–53. doi: 10.1016/s0002-8703(00)90088-7. [DOI] [PubMed] [Google Scholar]
- 59.Majors AK, Sengupta S, Willard B, et al. Homocysteine binds to human plasma fibronectin and inhibits its interaction with fibrin. Arterioscler Thromb Vasc Biol. 2002;22:1354–9. doi: 10.1161/01.atv.0000023899.93940.7c. [DOI] [PubMed] [Google Scholar]
- 60.Undas A, Williams EB, Butenas S, et al. Homocysteine inhibits inactivation of factor Va by activated protein C. J Biol Chem. 2001;276:4389–97. doi: 10.1074/jbc.M004124200. [DOI] [PubMed] [Google Scholar]
- 61.Lim A, Sengupta S, McComb ME, et al. In vitro and in vivo interactions of homocysteine with human plasma transthyretin. J Biol Chem. 2003;278:49707–13. doi: 10.1074/jbc.M306748200. [DOI] [PubMed] [Google Scholar]
- 62.Ferretti G, Bacchetti T, Moroni C, et al. Effect of homocysteinylation of low density lipoproteins on lipid peroxidation of human endothelial cells. J Cell Biochem. 2004;92:351–60. doi: 10.1002/jcb.20069. [DOI] [PubMed] [Google Scholar]
- 63.Barbato JC, Catanescu O, Murray K, et al. Targeting of metallothionein by L-homocysteine: a novel mechanism for disruption of zinc and redox homeostasis. Arterioscler Thromb Vasc Biol. 2007;27:49–54. doi: 10.1161/01.ATV.0000251536.49581.8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dröge W, Schipper HM. Oxidative stress and aberrant signaling in aging and cognitive decline. Aging Cell. 2007;6:361–70. doi: 10.1111/j.1474-9726.2007.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ueland PM, Mansoor MA, Guttorsmen AB, et al. Reduced, oxidized and proteinbound forms of homocysteine and other aminothiols in plasma comprise the redox thiol status – a possible element of the extracellular antioxidant defense system. J Nutr. 1996;126:1281S–4S. doi: 10.1093/jn/126.suppl_4.1281S. [DOI] [PubMed] [Google Scholar]


