Abstract
Stem cells capable of generating neural differentiated cells are recognized by the expression of nestin and reside in specific regions of the brain, namely, hippocampus, subventricular zone and olfactory bulb. For other brain structures, such as leptomeninges, which contribute to the correct cortex development and functions, there is no evidence so far that they may contain stem/precursor cells. In this work, we show for the first time that nestin-positive cells are present in rat leptomeninges during development up to adulthood. The newly identified nestin-positive cells can be extracted and expanded in vitro both as neurospheres, displaying high similarity with subventricular zone–derived neural stem cells, and as homogeneous cell population with stem cell features. In vitro expanded stem cell population can differentiate with high efficiency into excitable cells with neuronal phenotype and morphology. Once injected into the adult brain, these cells survive and differentiate into neurons, thus showing that their neuronal differentiation potential is operational also in vivo. In conclusion, our data provide evidence that a specific population of immature cells endowed of neuronal differentiation potential is resident in the leptomeninges throughout the life. As leptomeninges cover the entire central nervous system, these findings could have relevant implications for studies on cortical development and for regenerative medicine applied to neurological disorders.
Keywords: neural stem cells, leptomeninges, stem cell niche, neuronal differentiation
Introduction
During recent years, embryonic and adult neural stem cells (NSCs) gained attention as major candidates for regenerative and cell replacement therapies in various neurodegenerative diseases. In this setting, stem cell-based therapies raised important ethical, technical and immunological concerns [1].
The clinical application of adult NSCs, despite their properties of self-renewal, neuro-glial differentiation potential and their possible use in autologous setting, is still of debate. Among technical concerns, of relevance is that NSCs have hardly accessible site of sampling, are difficult to expand in vitro as homogeneous stem cell population and show low rate of in vivo neuronal differentiation efficiency [2].
Both during development and in adulthood, neurogenesis from endogenous neural stem/precursor cells has been shown to occur in discrete areas of the brain where complex microenvironments, or niches, ensure a balance between proliferation and self-renewal [3, 4]. NSCs have been found in the main neurogenic regions of the brain, that is, hippocampus, subventricular zone (SVZ), olfactory bulb [5, 6] and in some non-neurogenic regions, that is, spinal cord [7]. In SVZ, NSCs are present up to adulthood and are in tight contact with astrocytes, neuroblasts, ependymal cells, endothelial cells and growth factor-rich basal lamina [8–11].
In this work, we asked whether other brain sites could host stem cell niches. To investigate the stem cell distribution in rat central nervous system (CNS), we analyzed the expression pattern in rat cortex of the stem/progenitor cells marker nestin. Nestin is an intermediate filament of neuroepithelial derivation [12] that has been detected in stem/progenitor cells of neural and non-neural tissues [13]. We found that a nestin-expressing cell population is present in rat leptomeninges during embryonic stages up to adulthood. Leptomeninges, which include arachnoid and pia mater, cover the entire CNS and are filled with cerebrospinal fluid produced by choroid plexi. All the major arteries supplying the brain pass through leptomeninges and form branches while penetrating the cortex [14]. Interestingly, every parenchymal vessels inside the CNS are surrounded by a perivascular space (Virchow–Robin space) formed by the extroflexions of leptomeninges (arachnoid and pia mater) filled with cerebrospinal fluid [15–17]. Thus, leptomeninges are widely spread inside the CNS parenchyma, including the choroid plexus.
Leptomeninges form a complex microenvironment that has important functions for the normal cortex development [18]. They are present since the very early embryonic stages of cortical development, when columnar neuroepithelium is located between ventricle surface and pial basal membrane. Leptomeninges are involved in multiple interactions among a large number of molecular and chemiotactic factors (e.g. SDF-1/CXCR4, reelin, oxidative state) [19–21], cell types (e.g. pia mater cells, radial glia, neural precursor cells, Cajal Retzius cells, glia limitans cells) [22, 23] and extracellular matrices (e.g. laminin, collagen IV, fibronectin) [24–26] that ensure correct cortical development. Abnormal function/structure of leptomeninges causes altered cortical histogenesis, as in the case of cobblestone lissencephaly (type II), where the fragmentation of pia mater basal membrane leads to the formation of cortical neurons protruding into the sub-arachnoid space [27].
The peculiar spatial relationships of leptomeninges in CNS, their role in cortex development and our serendipitous discovery of nestin-positive cells prompted us in determining whether leptomeninges could be a possible stem cell niche hosting stem/progenitor cells with neuronal differentiation potential.
In this work, we show that nestin-positive cells can be extracted from leptomeninges and expanded in vitro both as neurospheres, displaying high similarity with SVZ-derived NSCs, and as homogeneous cell population with stem cell features. In vitro expanded stem/progenitor cells can be induced to differentiate with very high efficiency in excitable cells with neuronal morphology and phenotype. When injected into adult brain, these cells survive and differentiate into neurons, thus showing that their neural differentiation potential is operational also in vivo.
Material and methods
Brain perfusion and immunofluorescence
The animals were perfused with 4% paraformaldehyde in PBS. Brains were dissected, fixed in 4% paraformaldehyde solution and then left in 10% and subsequently 30% sucrose solution. By freezing microtome 30-μm-thick coronal brain sections were cut and processed by immunofluorescence as previously described [28]. Briefly, brain slices were incubated for 2 hrs in blocking solution (PBS/5%FCS/3%BSA/0.3% Triton X-100). Slices were then incubated for 12 hrs at 4°C in floating, with antibodies. Primary antibodies were detected with appropriated secondary antibodies for 4 hrs at 4°C in blocking solution.
Antisera
The following primary antibodies were used: mouse monoclonal antibodies anti-nestin, anti-CD106 (VCAM-1), anti-CD90, anti-CD31, anti-CD45, anti-CD105 (endoglin) and anti-BrdU (all from Pharmingen/Becton Dickinson); anti-EGFP (rabbit, 1:2000, Invitrogen, San Giuliano Milanese, Milan, Italy), MAP2 (mouse, 1:1000, Sigma, Milan Italy), GFAP (mouse, 1:1000, BD Pharmigen, Buccinasco, Italy), Laminin (rabbit, 1:1000 Sigma), Nestin (mouse, 1:1000, BD Pharmigen), NG2 (rabbit, 1:500, Chemicon, Milan, Italy), Neurofilament 160 (mouse, 1:100, Sigma).
The following secondary antibodies were used: goat anti-mouse Ig/Alexa Fluor 488, IgM/FITC, IgG/PE and chicken anti-rabbit/Alexa Fluor 488 (all from Molecular Probes, San Giuliano Milanese, Milan, Italy), goat anti-mouse/Cy3, goat anti-rabbit/Cy3 (all from Amersham, Milan, Italy).
Cell cultures
Cells were harvested from the first cortical layers and overhead leptomeninges of Sprague–Dawley rats or from enhanced green fluorescent protein (EGFP) transgenic rats [29] at post-natal days 15 (n= 6 experiments, 10 animals each). Tissues were sampled with stereo-microscope from brain coronal sections. Mechanically and enzymatically dissociated tissue extracts from first cortical layers of P15 rats were cultured in neurosphere-inducing and adherent conditions.
1 Neurosphere culture: tissue extracts were seeded into 6-well plates (Falcon, Buccinasco, Italy) in 3 ml of neurosphere culture medium. Medium supplemented with fresh growth factors was added every 2–3 days. Neurospheres could be detected under phase optics after 7–10 days.
2 Adherent culture: tissue extracts were seeded into culture flasks (Falcon) with growing medium; after 72 hrs, non-adherent cells were removed. In 10 days, clear colony-forming units could be detected. Differentiation was performed after three to six passages by plating 1 × 105 cells/cm2 in poly-L-lysine (40 μg/ml, Sigma)-coated cover slip in the differentiation medium. For media composition, see below.
Media
Neurosphere culture medium: Neurobasal Medium, (Gibco, San Giuliano Milanese, Milan, Italy) containing 2% B27 supplements (Gibco), 1% N2 supplement (Gibco), 200 mM glutamine, 1% penicillin-streptomycin plus 20 ng/ml epidermal growth factor (EGF) and 10 ng/ml basic fibroblast growth factor (bFGF).
Growing medium: Dulbecco modified Eagle medium (DMEM), with high glucose concentration, GLUTAMAX I, 18% heat-inactivated fetal calf serum (FCS), 100 U/ml penicillin and 100 μg/ml streptomycin (all from GibcoBRL/Life Technologies, Milan, Italy). Differentiation medium: Neuron Chow (Neurobasal Medium [Gibco] containing 2% B27 supplements (Gibco), 200 mM glutamine, 10 mM glutamate and 1% penicillin–streptomycin–fungizole) plus 50 ng/ml brain-derived nerve growth factor (BDNF).
Flow cytometric analysis
Samples of fresh tissue extracts and cultured cells were analyzed by flow cytometry using standard methods [30].
Intracellular calcium imaging
Intracellular calcium levels (ratio 340/380) were evaluated by ratiometric imaging techniques. Cells were loaded with Fura2-AM for 30–45 min. at 37°C. The loading solution contained 5 μM Fura 2-AM in HEPES buffered solution. Loading solution was removed and the culture was incubated for another 10 min. at 37°C in HEPES buffered solution to allow de-esterification of Fura2-AM. The cover slips were then placed onto a recording chamber, connected by a Tygon tube to a 1-ml syringe used for substance fast application.
All image processing and analysis was performed using OpenLab software (Improvision, Tübingen, Deutschland, Europe) with an inverted Zeiss microscope (Zeiss, Oberkochen, Germany). Wavelength of 340 and 380 nm were used to excite Fura2 and the emitted light was collected at 510 nm.
Colony forming unit (CFU) assay
Dissociated tissue extracts were plated at five different cell concentrations. After 14 days of culture, every single cell colony with more than 50 cells was considered as CFU. CFU assay was performed every following passage by plating cells at four different concentrations. CFUs were stained with May–Grunwald Giemsa and then counted (mean ± S.D. of two different cultures).
Cells immunofluorescence
Cells on cover slips were immunostained as previously described [31]. Briefly, cells were first fixed, blocked and permeabilized. Slides were incubated for 10 min. with the nuclear dye DAPI (Sigma); then, immunostaining was performed with standard procedure using primary antibodies and fluorochrome-conjugated secondary antibodies.
5-bromo-2-deoxyuridine (BrdU) labelling
Cells were incubated for 12 hrs with BrdU (3 μM) before starting the differentiation protocol. After differentiation, cells were fixed on cover slips with 4% paraformaldehyde and rinsed with PBS. Cells were then treated for 15 min. in 2N HCl/0.5% Triton X-100 at room temperature, and the reaction were neutralized with 0.1 N B4Na2 (pH 8.5).
Stereotaxical surgery in brain
All in vivo experiments were in accordance with the Italian Legislative Decree N.116/92. Male Sprague–Dawley adult rats (Harlan, Milan, Italy) (n= 8) were anaesthetized and a guide cannula (26G, Plastics One, Roanoke, VA, USA) was stereotaxically (stereotaxic frame; Kopf Instruments, Tujunga, CA, USA) lowered to Hippocampus (AP -2.3mm, L 2.2 mm, V 3.4mm, incisor bar 3.0 below horizontal line; Paxinos and Watson). Either cell suspension or vehicle (PBS) were loaded in a Hamilton syringe connected with a Tygon tube (ID 0.020 mm, OD 0.060 mm, Saint-Gobain PPL Corp., Charny, France) and with a needle (33G, Plastics One). The needle was inserted in the guide cannula. A volume of 1 μl (3 × 103 EGFP + cells) was then infused with a syringe pump over 5 min. and left in place for additional 5 min.
Transplanted cell counts
Frequency estimation of transplanted EGFP+/MAP2+ leptomeningeal cells were performed in hippocampal slices of four rats injected with EGFP+ leptomeningeal cells and one not-injected rat as negative control. Five of serial coronal sections (30 μm) of hippocampus were randomly selected and stained with antibodies against EGFP and MAP2. Five random fields for each slice were acquired with a 40× objective in confocal microscopy. EGFP-positive cells and MAP2/EGFP double-positive cells were then counted. The number of MAP2-positive injected cells was expressed as percentage of EGFP-positive cells examined.
Quantitative real-time RT (reverse transcription)–PCR analysis (qPCR)
Total RNA was purified with Trizol reagent (Invitrogen) and retrotranscribed to cDNA by reverse transcriptase AMV contained in the First Strand cDNA Synthesis Kit (Roche, Milan, Italy). qPCR reactions were carried out in 20 μl total volume containing 10 ng of cDNA (RNA equivalent), 1× Power SYBR Green I Master Mix or Taqman Universal PCR Master Mix (Applied Biosystems, Monza, Milan, Italy), 0.4 μM primers forward and reverse or 1/20 Taqman probe. After a starting denaturation for 10 min. at 95°C, 40 PCR cycles (15 sec. 95°C and 1 min. 60°C) were carried out on ABI PRISM 7900HT SDS instrument (Applied Biosystems). The Taqman assays (Applied Biosystems) were as follows: Rn00566603_m1 for Gfap; Rn00565046_m1 for Mtap2; Rn00564394_m1 for Nes; Rn00578849_m1 for Cspg4; Rn00667869_m1 for Actb. Forward and reverse 5′-3′ primer sequences and PCR product lengths were as follows: Tdgf1, GCTGGTGAAGACCTCGACGT, CGGAAGGCACAAGCTGGA, 106 bp; Smad4, GCACTACCACCTGGACTGGAA, TGTGAACCGGCCAGTAATGTC, 126 bp; Pou5f1, GCCAAGCTGCTGAAACAGAAG, CTGGCTGAACACCTTTCCAAA, 96 bp; Nanog, GGCCTGACTCAGAAGGGCTC, TGCCCCATACTGGAAGGTTTC, 106 bp; Sox2, CGCCGAGTGGAAACTTTTGT, CGCGGCCGGTATTTATAATC, 111 bp; Klhl1, GCTCATAGGCTTGTCCTGAGCT, GCTTGGCTTCACAGACATCG-3′, 75 bp; Eng, GGACAGCCTCTCCTTCCAGC, TGCTCACCTGTACGAAGCCC, 101 bp; Col1a1, GCAGATTGAGAACATCCGCAG, CCAGTACTCTCCGCTCTTCCA, 106 bp; Cd44, CAACGCTATCTGTGCAGCCA, CAAGAGGAGCTGAGGCATTGA, 101 bp; Fgfr1, AAATTCAAATGCCCGTCGAG, GGCGTAACGAACCTTGTAGCC, 111 bp; Fgfr2, TTGGCAGCCAGAAATGTGC, CTTGACTGGAAGTCGCCCAT, 126 bp; Fgfr3, CAAGGTGTACAGCGACGCAC, GTGGTGTTAGCTCCTGCAGTCTT, 126 bp; Kit, GGCATCACCATCAAAAACGTG, GGGATAGCTTTGATGGCTGC, 131 bp; Cd34, CACCAGCCATCTCAGAGACCA, CAGTGTGACGGTTG-GGTAAGTC, 113 bp. The probe signal was normalized to an internal reference, and a cycle threshold (Ct) was taken significantly above the background fluorescence. The Ct value used for subsequent calculation was the average of three replicates. The relative expression level was calculated using transcript level of Actb as endogenous reference. Data analysis was done according to the comparative method following the User Bulletin No. 2 (Applied Biosystems).
Statistical analysis
Data were analyzed using GraphPad Prism4 software. Results were expressed as mean ± S.D. or S.E.M., when indicated. Differences between experimental conditions were analyzed using two-tailed Student’s t-test. P value <0.05 was considered statistically significant.
Results
Nestin-positive cells are present in rat leptomeninges during development up to adulthood
Immunofluorescence confocal microscopy with anti-nestin antibodies was used to identify potential stem cell sites in coronal sections of rat brain cortex at different stages of development. Figure 1 shows that nestin-positive cells were present in peripheral cell layers of the parietal cortex of brains obtained from embryos (embryonic day 20 [E20]), animals at different post-natal days (P1, P8, P15) and in adulthood. The distribution of nestin-positive cells within the cortical layers decreased over time but persisted in the superficial layer covering the cortex up to adulthood. As previously described by several groups, nestin-positive cells were present at high density in the SVZ at all the developmental stages and in adults [32] (positive control). Interestingly nestin-positive cells were also present in choroid plexus.
Figure 1.

Distribution of nestin-positive cells in rat cortex at different ages. Confocal images of coronal sections of parietal cerebral cortex: rats at embryonic day 20 (E20) (A), postnatal day +1 (P1) (B), +8 (P8) (C) and +15 (P15) (D) and adults (E). Immunolabelling with the anti-nestin (red) antibody. (F) Subventricular zone (SVZ) of P15 rats as positive control and choroid plexus. Scale bar 50 μm.
To better define and characterize this population of nestin-positive cells, we first analyzed the distribution of this marker in comparison with that of laminin (a specific marker of pia mater) [19] and glial fibrillary acidic proteins (GFAP, a marker of glial cells). DAPI staining was used to visualize cell nuclei. Figure 2 was obtained from P15 rat brains and indicates that the nestin-positive tissue was a layer of densely packed nucleated cells residing outside the pia mater boundary of the cortex (as shown in details in Fig. 2A and B) in the leptomeninges. This cell population was distinct from astrocytes (Fig. 2C).
Figure 2.
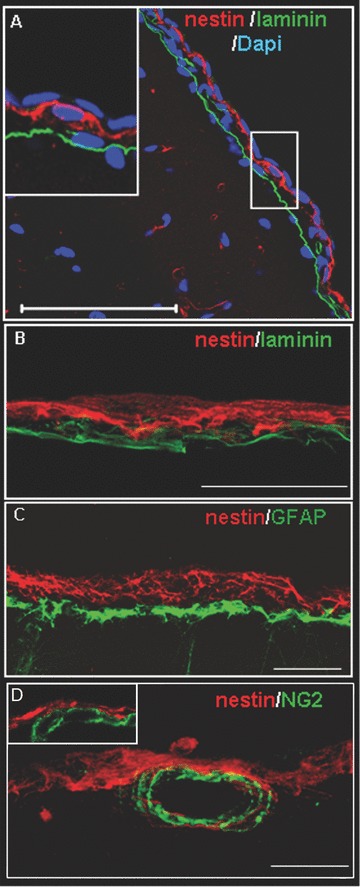
Localization of nestin-positive cells in leptomeningeal tissue. (A) Coronal section of the parietal cortex of P15 rat, stained with DAPI (blue), anti-laminin (green) and anti-nestin (red) antibodies. Scale bar 50 μm. (B, C, D) Z-stack reconstruction assembled from 10 serial 2 μm confocal sections. Sections were stained in red with anti-nestin antibodies and in green with either anti-laminin (B) or -GFAP (C) or -NG2 (D) antibodies; high magnification. None of these markers co-localizes with nestin. Scale bars 50 μm.
We then assessed whether the nestin-positive cell population included vascular cells. To this aim, we used antibodies against NG2 (integral membrane chondroitin sulphate proteoglycan, Cspg4) a marker of smooth muscle cells [33] and of microvascular pericytes [34]. As shown in Fig. 2D, the nestin-positive cell population did not include NG2-positive vascular cells.
In vitro expansion of the nestin-positive cell population derived from leptomeninges
Leptomeninges were stripped from the brain surface of P15 rats (n= 6 experiments, 10 animals each); the stripped samples also included the outermost layers of the cortex (Fig. 3A). Mechanically dissociated tissue extracts contained living nestin-positive cells (40.3%), as shown by FACS analysis (Fig. 3B).
Figure 3.

Tissue sampling. (A) Hematoxylin/eosin staining of a coronal section of P15 rat brain to show (arrows) the site and the extent of the biopsy. Scale bar 250 μm. (B) FACS analysis of tissue extracts from P15 rats. Viable cells, stained with Syto16, were gated and, among them, nestin-positive cells (red 40.3%) or negative (black) are shown.
Standard NSC growth condition. To assess whether cells with neural differentiation potential were present in the tissue extracts from this region, we first cultured these cells in the same conditions used to expand SVZ-NSCs. In NSC medium (see Materials and Methods), cells from tissue extract generated floating neurospheres in 9–11 days (Fig. 4A). Neurospheres could be expanded in vitro up to several months (data not shown). Similarly to SVZ-derived neurospheres [35], leptomeninges-derived neurospheres consisted of different cell types, including nestin-positive cells, neuronal MAP2-positive cells and glial GFAP-positive [36] (Fig. 4B and C). Quantitative gene expression assay was performed to analyze self-renewal regulators (Pou5f1/Oct4 [37], Nanog [38], Sox2 [39]), genes related to the undifferentiated state maintenance (Tdgf1/Cripto-1, Smad4, and Nestin) [40, 41] and genes related to neural differentiation (Klhl1, Gfap, Mtap2) [42]. Comparison of gene expression between SVZ- and leptomeninges-derived neurospheres were shown in Fig. 4D. Data were normalized for beta-actin expression level and were expressed as ratios between the two populations. A tight correlation between the gene expression pattern of SVZ-NSC-derived neurosphere and the leptomeninges-derived neurospheres (Fig. 4D) was observed. Statistically significant differences (P < 0.05) were observed for differentiation genes Gfap and Mtap2 only (Fig. 4D).
Figure 4.
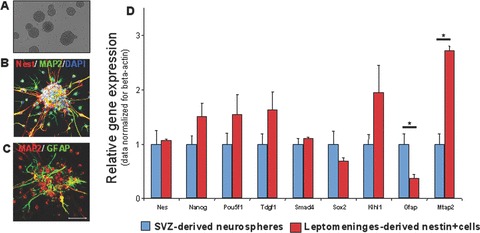
In vitro expansion of the tissue extract in neural stem cell growth conditions. Floating neurospheres were obtained, with morphology and phenotype comparable to those of SVZ-NSC-derived neurospheres. (A) Transmitted light of leptomeningeal-derived neurosphere. Confocal image of neurospheres stained with MAP2 (green), nestin (red) and DAPI (blue) (B), or MAP2 (red) and GFAP (green) (C), scale bar 100 μm. (D) Relative gene expression analysis. For each sample, expression levels of different genes were normalized to levels of beta-actin mRNA. The bars show fold change ± S.D. in transcription of normalized mRNA expression levels measured for leptomeninges-derived neurosphere compared to SVZ-NSC-derived neurosphere.
Adherent stem cell growth condition. We tried to overcome the complexity [36, 43, 44] of neurosphere assay by modifying the culture conditions. The whole tissue extract was plated in flasks with growing medium (see Materials and Methods). After 48 hrs, non-adherent cells were removed and the medium completely changed. In 1–3 weeks, cell colonies could be recognized. Only a minority of cells from the whole tissue extract adhered and gave rise to CFUs. Non-adherent cells underwent apoptosis or were removed by medium change. All the adherent cells proliferating after several days derived from CFU and displayed a homogeneous immunophenotype, as shown in Fig. 5A and E. This cell population was expanded for several passages and was clonogenic, since CFU were present at each passage (Fig. 5B). Time course experiments were performed to assess whether a cell population of nestin+/GFAP-/NG2-cells (similar to what found in vivo in the leptomeninges) was present in the culture. As shown in Fig. 5A, nestin+/NG2- cells were present as early as 5 hrs after plating (approximately 90% of attached cells). Similar results were obtained with GFAP staining (data not shown). From day 8, nestin+/GFAP–/NG2– cell colonies are detectable.
Figure 5.

In vitro expansion of the tissue extract in adherent cells culture conditions. (A) Time course experiment. Confocal images of nestin+/NG2- cells in adherent culture at 5 hrs, 1, 4, 8 and 12 days after plating. (B) CFU derived from leptomeningeal cells at different passages. Bars represent the CFU number/million of plated cells ± S.D. (B’) May–Grunwald Giemsa staining of a single colony of leptomeningeal adherent cells. (C) Transmitted light and (D) immunofluorescence, nestin (red), of adherent leptomeningeal cells. (E) FACS analysis, carried out on expanded cells obtained from adult rats, shows high expression of nestin and CD90. In vitro expanded cell population does not express neuro-glial (MAP2, GFAP and O4), leukocyte (CD45), endothelial (CD31, CD106) and haematopoietic stem cell markers (CD34). (F) Relative gene expression analysis. Leptomeningeal-derived nestin+ cells and BM-MSCs fold changes in transcription, normalized to actin mRNA, have been shown compared to SVZ-NSCs.
FACS analysis showed that in vitro expanded cells always retained nestin positivity, did not express markers of either neuronal (MAP2)/glial (GFAP) or hematopoietic (CD45, CD34) [45]/endothelial (CD105, CD31, CD106) [46] lineages and were positive for CD90, a non-specific marker of neural cells and stromal cells [47] (Fig. 5E). Immunofluorescence microscopy confirmed that expanded cells were a homogeneous population of nestin-positive cells (Fig. 5D) that did not express neuronal (MAP2), glial (GFAP), oligodendrocytic and pericytic markers (NG2) [48] (data not shown). Interestingly, a similar population of nestin-positive cells could be extracted and in vitro expanded also from choroid plexi, a tissue devoid of neural elements (data not shown). We studied by quantitative RT-PCR the expression of different MSC markers (see Table 1); we found that nestin-positive cells from leptomeninges displayed a different pattern of expression as compared with MSC. In addition, no evidence of adipocyte and osteocyte differentiation was achieved with the standard protocols used for MSC (data not shown).
Table 1.
Assessment by quantitative RT-PCR of MSC marker expression by leptomeningeal nestin-positive cells
| Protein | Gene | Leptomeningeal nestin-positive cells/BM-MSC fold change | P |
|---|---|---|---|
| Nestin | Nes | 4.9 | <0.01 |
| Collagen type1a | Col1a1 | 0.58 | <0.01 |
| NG2 | Cspg4 | 0.64 | <0.01 |
| CD34 | Cd34 | 0.2 | <0.01 |
| Hyaluronate binding protein (CD44) | Cd44 | 1.16 | 0.16 |
| Stem cell growth factor receptor kit (C-Kit) | Kit | 3.06 | <0.01 |
| Endoglin (CD105) | Eng | 1.24 | <0.01 |
| Fibroblast growth factor receptor 1 | Fgfr1 | 0.017 | <0.01 |
| Fibroblast growth factor receptor 2 | Fgfr2 | 1.93 | <0.01 |
| Fibroblast growth factor receptor 3 | Fgfr3 | 26.93 | <0.01 |
| Cripto-1 | Tdgf1 | 5.48 | 0.06 |
| Oct4 | Pou5f1 | 9.86 | <0.05 |
| Homeobox transcription factor Nanog | Nanog | 3.34 | <0.05 |
Gene expression analysis was performed to compare the stemness molecular signature and neural differentiation pattern of leptomeningeal nestin-positive cells with that of other known stem cell types derived from P15 rats, such as NSC from SVZ and bone marrow mesenchymal stem cell (BM-MSC). All these stem cell types were cultured in the same condition. Adherent/expanded leptomeninges-derived cells showed higher stemness-related gene expression, such as Pou5f1/Oct4, Tdgf1 (Cripto-1), Nanog, (P < 0.01) as compared with adherent cultured SVZ-NSC. By contrast, adherent/expanded leptomeningeal cells expressed lower neural differentiation genes, such as Mtap2 (MAP2) and Gfap as compared with adherent cultured SVZ-NSC (Fig. 5F). These findings were in line with data obtained by FACS and immunofluorescence, and they suggest a homogeneous phenotype of adherent/expanded leptomeningeal cells.
In vitro neuronal differentiation of expanded leptomeninges-derived cells
To determine whether the leptomeningeal adherent/expanded cells had neural differentiation potential, they were cultured with differentiating medium for at least 1 month and then analyzed for the presence of the neural marker MAP2. Real-time gene expression analysis of leptomeninges-derived cells in basal condition and after differentiation showed a statistically significant increase in Mtap2 (MAP2) and decrease in nestin expression levels (P < 0.01). Immunofluorescence analysis showed that cells differentiated in cultures into MAP2-positive neurons with high efficiency (30–50% of cells, n= 6 experiments; Fig. 6B–D). Most of MAP2-negative cells were nestin-positive. Some GFAP-positive astrocytes and rare NG2- or O4-positive oligodendrocytic precursors were also found in the differentiated cultures (data not shown). Moreover, MAP2-positive cells showed other features of neuronal phenotype, including distinct neuritic arborization, dendritic spines (arrows in Fig. 6C) and the expression of presynaptic protein synaptophysin (Fig. 6E). A fraction of the differentiated MAP2-positive cells also expressed the GluR2 sub-unit of the ionotropic AMPA-glutamate receptor (Fig. 6G) and the glutamate decarboxylase (GAD67), marker of GABAergic neurons (Fig. 6F).
Figure 6.
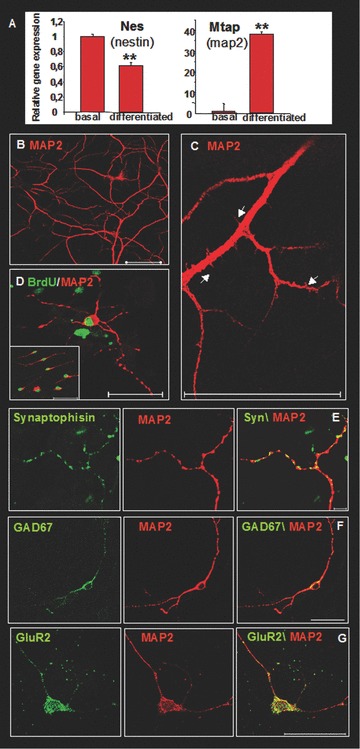
In vitro neural differentiation of leptomeningeal cells after adherent culture expansion (A) Real-time gene expression analysis of Nestin and Mtap2 (MAP2) in leptomeninges-derived cells in basal condition and after differentiation. The bars show folds change ± S.D. of normalized mRNA expression levels measured before and after differentiation (P < 0.01). (B, C, D) Differentiated cells, stained with antibodies against MAP2 (red). Arrows in (C) indicate dendritic spines. (D) BrdU staining (green) indicates that the MAP2-positive cells (red) derived from replicating cells. MAP2-positive cells also expressed components of the synaptic apparatus, including the presynaptic marker synaptophysin (E), the glutamate ionotropic receptor sub-unit GluR2 (G) and glutamate decarboxylase (GAD67) (F). Scale bars 50 μm.
These terminally differentiated cells were not residual adult neuronal cells from tissue extract. This was demonstrated by incubating nestin-positive cultures with BrdU for 9 hrs before inducing differentiation. As shown in Fig. 6D, MAP2-positive cells were also labelled by the anti-BrdU antibody, thus indicating that these neurons derived from replicating cells.
Responses to depolarizing agents were analyzed by calcium imaging after Fura-2 loading. Stimulation with 55 mM KCl caused increase of the intracellular calcium concentration indicated by the shift of the 340/380 ratio. Fast application of 55 mM KCl (approximately 40 sec.) produced a significant response in 77% of the cells studied (n= 36). Response reached a peak level after 9.7 ± 1.9 sec. of KCl application and was sustained and reversible Fig. 7A). Similar data were obtained from primary neuronal culture (Fig. 7B). These data suggest that leptomeningeal cells in vitro differentiated into neurones are excitable and express functional voltage-dependent calcium channels.
Figure 7.

Calcium imaging analysis of in vitro neural differentiated leptomeningeal cells. (A) Changes in intracellular free calcium are indicated by variation of 340 nm/380 nm fluorescence ratio in Fura-2-loaded cells. Depolarization induced by 55 mM KCl led to increase of 340/380 ratio in most of the cells present in the field. (B) Average responses (mean ± S.E.M.) expressed as peak and baseline values in differentiated cells and in primary neuronal cultures; ***P < 0.001.
In vivo neuronal differentiation of leptomeninges-derived cells
Transplantation studies were performed to determine whether adherent/expanded cells derived from leptomeninges of P15 rats could generate neural cell types in vivo. To recognize injected cells, expanded leptomeningeal nestin-positive cells were derived from EGFP-transgenic rats [29]. Cells were stereotaxically injected into the hippocampus of adult rats (n= 8). Immunofluorescence analysis with anti-EGFP antibody revealed the presence of EGFP+ cells in hippocampus 4–8 weeks after transplantation (Fig. 8). EGFP+ cells were not detected in the control rat (non-injected adult rats or rats injected with vehicle only). Real-time PCR confirmed that EGFP expression was restricted to injected rats (data not shown). To evaluate the differentiation of engrafted cells, 30-μm-thick sections were analyzed by confocal microscopy following immunostaining with anti-EGFP antibody and markers for either undifferentiated cells (nestin) or neurons (MAP2) or astrocytes (GFAP) or oligodendrocyte precursors (NG2). The injection needle track was recognizable in the CA2-CA3 hippocampal region (Fig. 8A). EGFP+ cells close to the injection point were surrounded by abundant GFAP-expressing astrocytes (Fig. 8B). Most of the transplanted cells at the injection site expressed nestin (Fig. 8C); EGFP+/GFAP+ and EGFP+/NG2+ double positive cells were rarely detected (Fig. 8D and E).
Figure 8.
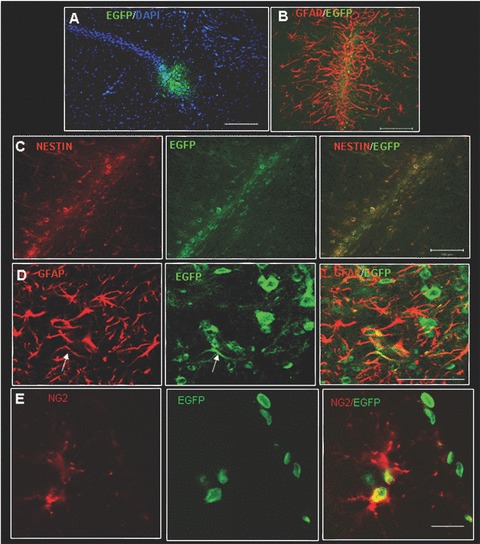
Confocal images of injection site of transplanted leptomeningeal cells. (A) GFP-positive cells (green) into the injection site in CA2 hippocampal region stained with nuclear marker DAPI (blue), scale bar 200 μm; (B) GFP-positive cells in the injection site surrounded by glia (GFAP red signal), scale bar 100 μm; (C) Colocalization of GFP-positive cells with nestin in the injection site (yellow signal), scale bar 100 μm; (D) Colocalization of GFP-positive cells with GFAP (yellow signal) near the injection site, scale bar 50 μm; (E) Colocalization of GFP-positive cells with NG2 (yellow signal), scale bar 50 μm.
Sixty days after injection, 49.8 ± 17.9% of the EGFP+ cells found in the hippocampus were also MAP2-positive (n= 4, see Materials and Methods). Most EGFP+/MAP2- cells were nestin-positive, undifferentiated cells. Most of the EGFP+ cells were located in the CA1 region (Fig. 9A–D). In this site, some of the grafted cells displayed complex morphology resembling the pyramidal neurons of the hippocampus (Fig. 9B’). EGFP+/MAP2+ engrafted cells were detected in the pyramidal layer and in the stratum oriens (Fig. 9C). Not all the EGFP+ cells observed in this layer also expressed MAP2, even if they displayed neuronal morphology and appeared to be well integrated within the CA1 (Fig. 9D and E). Transplanted cells displaying distinct neuronal morphology and MAP2 expression were also found in the hilus and in the sub-granular zone (SGZ) of the dentate gyrus (Fig. 9F).
Figure 9.

Confocal images of in vivo leptomeningeal cells neural differentiation. (A) Transplanted EGFP+ cells (green) are localized in CA1 region, scale bar 100 μm. (B) EGFP+ cells (green) showed complex phenotypes mimicking the pyramidal neurons of the hippocampus (CA1 pyramidal cell layer), scale bar 100 μm; (B’) high magnification, scale bar 20 μm. (C) Z-stack reconstruction assembled from 10 serial 1.63-μm confocal sections. EGFP (green)/MAP2 (red)/DAPI (blue)-positive cells in CA1 pyramidal layer, scale bar 50 μm. (C’, C’’, C’’’) confocal images pointing single cell (arrowhead) co-localizations (yellow) of EGFP (green) and MAP2 (red), scale bars 20 μm. EGFP+ cells co-localizing with MAP2 (yellow) (D) or not (E), scale bars 20 μm. Transplanted EGFP+ cells (green) co-localizing with MAP2 (yellow) in sub-granular zone (SGZ) and hilus of the dentatus gyrus, scale bar 200 μm (F). (F’) High magnification, scale bar 50 μm.
Discussion
In this work, we analyzed the leptomeningeal compartment of the rat brain to assess whether a stem cell population with neuronal differentiation potential is present in this structure. Indeed, we found that (i) nestin-positive cells are present in the leptomeningeal compartment at the embryonic stages and persist up to adulthood, (ii) leptomeningeal nestin-positive cells can be extracted and cultured as neurospheres with features similar to the NSC-derived neurospheres, (iii) leptomeningeal nestin-positive cells can be cultured as adherent cells and expanded in vitro as homogeneous population of nestin-positive cells that highly express many of the stemness-related genes, (iv) expanded nestin-positive cells can be induced to differentiate in vitro with high efficiency to generate excitable neurons and (v) expanded cells can differentiate into neurons when injected into brains of living rats.
Previous studies described the distribution of stem cell markers inside the brain [49], but there was no evidence so far that leptomeninges could be a stem cell niche. Nestin-positive cells were seen in the most superficial portion of the rat brains in embryos and post-natal puppies. These cells were neither glial nor muscle cells or pericytes. Number and distribution of the nestin-positive cells declined during development and become restricted to the leptomeninges from post-natal day 15. At this age, nestin-positive cells appeared as a layer associated to the leptomeninges and in contact but distinct from the pia mater. Other nestin-positive cells were abundant at known loci of neurogenesis, that is, SVZ, dentate gyrus and olfactory bulb [6]. Starting from these morphological observations, we decided to analyze leptomeninges as a potential source of nestin-positive stem/progenitor cells.
In this study, we report data from post-natal P15 rats. Preliminary data indicate that extraction efficiency is higher in younger animals and embryos. On the other hand, at P15 the cortical development is complete. This limits possible contamination by other potential sources of stem cells. This was confirmed by morphological examination of the stripped brains showing that SVZ, dentate gyrus and olfactory bulb remained intact. It is interesting to note that a similar population of nestin-positive cells could be extracted from choroid plexi, a site free of neural cells further confirming that the newly identified population of cultured nestin-positive cells did not originate from brain parenchyma. In addition, the number of cells that could be obtained from the biopsies was large enough to conduct extensive characterization of the leptomeningeal cells.
The nestin-positive cell population grown in vitro are likely to originate from the nestin-positive cells present in the stripped tissue and not from a process of transformation occurring in vitro. This was indicated by the presence of the nestin signal in cells as early as the cells become adherent to the flask following extraction. Previous work had shown that cells extracted from the whole brain express both NG2 and nestin antigens in vitro; these cells are endowed of neural differentiation potential [48]. The newly identified population of nestin-positive cells extracted from the leptomeninges were neither NG2- nor GFAP-positive cells, both in vivo and after expansion in vitro.
The extracted cells could be expanded as neurospheres or adherent cultures. Real-time PCR was used to show that leptomeninges-derived neurospheres had gene expression and multipotent differentiation potential comparable to those of SVZ-NSCs. Floating neurospheres are heterogeneous cultures containing multipotent stem cells, cell progenitors and many neuro-glial differentiating cells [35, 36]. We have used a different culturing protocol and extracted cells were grown as an adherent layer. At difference from the floating neurospheres, cells grew as a homogeneous cell population of nestin-positive/lineage-negative cells, characterized by clonogenicity and differentiation potential. This cell population showed high levels of stemness-related genes [40], such as Oct4, Nanog, Cripto-1 and Nestin, but it did not express differentiated neural genes. This procedure allowed a clear-cut differentiation between proliferative and differentiative stages of the cultured cells.
Leptomeninges-derived cells can be expanded and maintained in undifferentiated stage for up to several months; by switching medium, the cells can then be induced to differentiate in vitro with high efficiency into neuronal lineage. In 1 month, up to half of the cell population differentiated into MAP2-positive cells showing many features of terminally differentiated neurons, including dendritic spines, presynaptic proteins, receptors for neurotransmitters, enzymes involved in neurotransmitter synthesis and depolarization-induced changes of [Ca2+]i[50]. In conclusion, the nestin-positive stem/progenitor cells extracted from leptomeninges and grown in vitro have the potential to generate cells with several of the properties that identify mature and functional neurons.
As a further step of characterization of the newly identified cell population, we tested their neural differentiation potential in vivo. This was done by injecting expanded nestin-positive cells in hippocampus and following their fate for up to 60 days. To distinguish injected from resident cells, leptomeningeal cells were extracted and expanded from enhanced green fluorescent protein (EGFP) transgenic rats [29]. Following intra-brain transplant, the EGFP-positive cells persisted for up to 60 days into the hippocampus when approximately half of the transplanted cells in the hippocampus were MAP2-positive and had the morphological aspect of differentiated neurons. We cannot exclude that the EGFP+ engrafted cells may fuse with resident neurons. Fusion of stem cells with resident neurons has been shown to occur in some experimental setting when in vivo differentiation is rare [51]. This does not appear to be the case for leptomeningeal cells because up to 50% the engrafted cells express the neuronal MAP2 antigen; in addition, none of these cells appeared to be multi-nucleated. In conclusion, the data suggest that nestin-positive stem/progenitor cells extracted from leptomeninges may have capability of neural differentiation also in vivo.
Somatic stem cells have been described in many adult organs and are presumably responsible of the maintenance of tissue homeostasis [3]. Stem/progenitor cells normally reside in vivo in special microenvironments capable of regulating their persistence and differentiation [4]. The discovery that stem/progenitor cells are present in leptomeninges is new but not surprising. Indeed, this region is strictly associated to pia mater cells that secrete important chemotactic factors, such as SDF-1 [19], as well as several extracellular matrix components endowed of trophic functions [52]. In addition, it may be in contact/communication with other known NSC niches such as the ventricular zone mediated by the cerebrospinal fluid. Thus, this structure is a potentially favourable microenvironment, or niche, capable of hosting stem/precursor cells.
Other somatic stem cells displaying some of the characteristics of leptomeningeal nestin-positive cells, that is formation of neurospheres in liquid culture and neuronal differentiation, and not related to NSC have been previously described [53–58]. A transient wave of MSC originates during embryogenesis from Sox1+ neuroepithelium [59]. In adulthood, MSC appears to reside in multiple human organs as perivascular cells [60]. The origin and identity of the stem/progenitor cells we described is unknown; however, our data show that leptomeningeal nestin-positive cells are different from NG2-positive perivascular MSC and have different localization, as compared with all the other known somatic stem cells.
Further studies are needed to establish the in vivo role of leptomeningeal nestin-positive cells in embryonic, post-natal and adult CNS. As leptomeninges cover the entire CNS surface and also follow vessels into the brain mass, it is possible that the adult CNS has the potential to host regenerative stem cells over a large proportion of its volume. Finding factors that may expand migration, replication and differentiation properties of the resident population of stem cells may be relevant for the improvement of regenerative therapies for brain diseases.
Because of the persistence of these cells up to adulthood, their proliferation capability in vitro, and their differentiation potential into neuronal cells in vitro and in vivo, we suggest to name them leptomeningeal stem/progenitor cells (LeSC) as a new entity. Further characterization of LeSCs in normal and pathological conditions may help to better understand the cortical neurogenesis as well as their potential usefulness in the treatment of neurological diseases when used as source of NSC-like population.
Acknowledgments
We thank M. Schwartz, The Weizmann Institute of Science, Rehovot, Israel and E. Cattaneo, University of Milan, Italy, for useful suggestions and comments of the manuscript; F. Mosna and E. Formaggio for critical discussion of the experimental results; F. Rossi, University of Torino, Italy, for generously providing of enhanced green fluorescent protein (EGFP) transgenic rats (a generous gift from Dr. M. Okabe, Osaka University, Osaka, Japan); O. Perbellini for help with FACS analysis; A. Auber for assistance in sterotaxic surgery; D. Ferrari for technical help with histology; M. Dichio, V. Tedesco and L. Zangrandi for technical assistance; S. Marconi for providing valuable reagents and helping in protocol setting; C. Sala, Institute of Neuroscience, Milano, Italy, for help with confocal microscopy. This work was supported by Italian Ministry of University and Scientific Research (PRIN 2005) and Fondazione CARIVERONA.
References
- 1.Li JY, Christophersen NS, Hall V, et al. Critical issues of clinical human embryonic stem cell therapy for brain repair. Trends Neurosci. 2008;31:146–53. doi: 10.1016/j.tins.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Rosser AE, Zietlow R, Dunnett SB. Stem cell transplantation for neurodegenerative diseases. Curr Opin Neurol. 2007;20:688–92. doi: 10.1097/WCO.0b013e3282f132fc. [DOI] [PubMed] [Google Scholar]
- 3.Scadden DT. The stem-cell niche as an entity of action. Nature. 2006;441:1075–9. doi: 10.1038/nature04957. [DOI] [PubMed] [Google Scholar]
- 4.Xie T, Li L. Stem cells and their niche: an inseparable relationship. Development. 2007;134:2001–6. doi: 10.1242/dev.002022. [DOI] [PubMed] [Google Scholar]
- 5.Johansson CB, Momma S, Clarke DL, et al. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 6.Gould E. How widespread is adult neurogenesis in mammals. Nat Rev Neurosci. 2007;8:481–8. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- 7.Kehl LJ, Fairbanks CA, Laughlin TM, et al. Neurogenesis in postnatal rat spinal cord: a study in primary culture. Science. 1997;276:586–9. doi: 10.1126/science.276.5312.586. [DOI] [PubMed] [Google Scholar]
- 8.Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–84. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Garcion E, Halilagic A, Faissner A, et al. Generation of an environmental niche for neural stem cell development by the extracellular matrix molecule tenascin C. Development. 2004;131:3423–32. doi: 10.1242/dev.01202. [DOI] [PubMed] [Google Scholar]
- 10.Sirko S, von Holst A, Wizenmann A, et al. Chondroitin sulfate glycosaminoglycans control proliferation, radial glia cell differentiation and neurogenesis in neural stem/progenitor cells. Development. 2007;134:2727–38. doi: 10.1242/dev.02871. [DOI] [PubMed] [Google Scholar]
- 11.Temple S. The development of neural stem cells. Nature. 2001;414:112–7. doi: 10.1038/35102174. [DOI] [PubMed] [Google Scholar]
- 12.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 13.Wiese C, Rolletschek A, Kania G, et al. Nestin expression: a property of multi-lineage progenitor cells. Cell Mol Life Sci. 2004;61:2510–22. doi: 10.1007/s00018-004-4144-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reina-De La Torre F, Rodriguez-Baeza A, Sahuquillo-Barris J. Morphological characteristics and distribution pattern of the arterial vessels in human cerebral cortex: a scanning electron microscope study. Anat Rec. 1998;251:87–96. doi: 10.1002/(SICI)1097-0185(199805)251:1<87::AID-AR14>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Rodriguez-Baeza A, Reina-De La Torre F, Ortega-Sanchez M, et al. Perivascular structures in corrosion casts of the human central nervous system: a confocal laser and scanning electron microscope study. Anat Rec. 1998;252:176–84. doi: 10.1002/(SICI)1097-0185(199810)252:2<176::AID-AR3>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Jones EG. On the mode of entry of blood vessels into the cerebral cortex. J Anat. 1970;106:507–20. [PMC free article] [PubMed] [Google Scholar]
- 17.Nonaka H, Akima M, Nagayama T, et al. Microvasculature of the human cerebral meninges. Neuropathology. 2003;23:129–35. doi: 10.1046/j.1440-1789.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- 18.Halfter W, Dong S, Yip YP, et al. A critical function of the pial basement membrane in cortical histogenesis. J Neurosci. 2002;22:6029–40. doi: 10.1523/JNEUROSCI.22-14-06029.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borrell V, Marin O. Meninges control tangential migration of hem-derived Cajal-Retzius cells via CXCL12/CXCR4 signaling. Nat Neurosci. 2006;9:1284–93. doi: 10.1038/nn1764. [DOI] [PubMed] [Google Scholar]
- 20.Trommsdorff M, Gotthardt M, Hiesberger T, et al. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and ApoE receptor 2. Cell. 1999;97:689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 21.Madhavan L, Ourednik V, Ourednik J. Increased ‘vigilance’ of antioxidant mechanisms in neural stem cells potentiates their capability to resist oxidative stress. Stem Cells. 2006;24:2110–9. doi: 10.1634/stemcells.2006-0018. [DOI] [PubMed] [Google Scholar]
- 22.Marin-Padilla M. Cajal-Retzius cells and the development of the neocortex. Trends Neurosci. 1998;21:64–71. doi: 10.1016/s0166-2236(97)01164-8. [DOI] [PubMed] [Google Scholar]
- 23.Costa C, Harding B, Copp AJ. Neuronal migration defects in the Dreher (Lmx1a) mutant mouse: role of disorders of the glial limiting membrane. Cereb Cortex. 2001;11:498–505. doi: 10.1093/cercor/11.6.498. [DOI] [PubMed] [Google Scholar]
- 24.Haubst N, Georges-Labouesse E, De Arcangelis A, et al. Basement membrane attachment is dispensable for radial glial cell fate and for proliferation, but affects positioning of neuronal subtypes. Development. 2006;133:3245–54. doi: 10.1242/dev.02486. [DOI] [PubMed] [Google Scholar]
- 25.Grimpe B, Dong S, Doller C, et al. The critical role of basement membrane-independent laminin γ1 chain during axon regeneration in the CNS. J Neurosci. 2002;22:3144–60. doi: 10.1523/JNEUROSCI.22-08-03144.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duband JL, Dufour S, Thiery JP. The instructive role of fibronectins in cell migrations during embryonic development. Ann N Y Acad Sci. 1990;588:273–80. doi: 10.1111/j.1749-6632.1990.tb13216.x. [DOI] [PubMed] [Google Scholar]
- 27.Beggs HE, Schahin-Reed D, Zang K, et al. FAK deficiency in cells contributing to the basal lamina results in cortical abnormalities resembling congenital muscular dystrophies. Neuron. 2003;40:501–14. doi: 10.1016/s0896-6273(03)00666-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weimann JM, Johansson CB, Trejo A, et al. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5:959–66. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- 29.Okabe M, Ikawa M, Kominami K, et al. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–9. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 30.Krampera M, Glennie S, Dyson J, et al. Bone marrow mesenchymal stem cells inhibit the response of naïve and memory antigen-specific T cells to their cognate peptide. Blood. 2003;101:3722–9. doi: 10.1182/blood-2002-07-2104. [DOI] [PubMed] [Google Scholar]
- 31.Decimo I, Roncarati R, Grasso S, et al. SK3 trafficking in hippocampal cells: the role of different molecular domains. Biosci Rep. 2006;26:399–412. doi: 10.1007/s10540-006-9029-5. [DOI] [PubMed] [Google Scholar]
- 32.Zerlin M, Levison SW, Goldman JE. Early patterns of migration, morphogenesis, and intermediate filament expression of subventricular zone cells in the postnatal rat forebrain. J Neurosci. 1995;15:7238–49. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ozerdem U, Grako KA, Dahlin-Huppe K, et al. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev Dyn. 2001;222:218–27. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 34.Schrappe M, Klier FG, Spiro RC, et al. Correlation of chondroitin sulfate proteoglycan expression on proliferating brain capillary endothelial cells with the malignant phenotype of astroglial cells. Cancer Res. 1991;51:4986–93. [PubMed] [Google Scholar]
- 35.Bez A, Corsini E, Curti D, et al. Neurosphere and neurosphere-forming cells: morphological and ultrastructural characterization. Brain Res. 2003;993:18–29. doi: 10.1016/j.brainres.2003.08.061. [DOI] [PubMed] [Google Scholar]
- 36.Chojnacki A, Weiss S. Production of neurons, astrocytes and oligodendrocytes from mammalian CNS stem cells. Nat Protoc. 2008;3:935–40. doi: 10.1038/nprot.2008.55. [DOI] [PubMed] [Google Scholar]
- 37.Nichols J, Zevnik B, Anastassiadis K, et al. Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct4. Cell. 1998;95:379–91. doi: 10.1016/s0092-8674(00)81769-9. [DOI] [PubMed] [Google Scholar]
- 38.Loh YH, Wu Q, Chew JL, et al. The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat Genet. 2006;38:431–40. doi: 10.1038/ng1760. [DOI] [PubMed] [Google Scholar]
- 39.Avilion AA, Nicolis SK, Pevny LH, et al. Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 2003;17:126–40. doi: 10.1101/gad.224503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramalho-Santos M, Yoon S, Matsuzaki Y, et al. ‘Stemness’: transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 41.Adewumi O, Aflatoonian B, Ahrlund-Richter L, et al. Characterization of human embryonic stem cell lines by the International Stem Cell Initiative. Nat Biotechnol. 2007;25:803–16. doi: 10.1038/nbt1318. [DOI] [PubMed] [Google Scholar]
- 42.Jiang S, Seng S, Avraham HK, et al. Process elongation of oligodendrocytes is promoted by the Kelch-related protein MRP2/KLHL1. J Biol Chem. 2007;282:12319–29. doi: 10.1074/jbc.M701019200. [DOI] [PubMed] [Google Scholar]
- 43.Suslov ON, Kukekov VG, Ignatova TN, et al. Neural stem cell heterogeneity demonstrated by molecular phenotyping of clonal neurospheres. Proc Natl Acad Sci USA. 2002;99:14506–11. doi: 10.1073/pnas.212525299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pollard SM, Conti L, Sun Y, et al. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb Cortex. 2006;16:112–20. doi: 10.1093/cercor/bhj167. [DOI] [PubMed] [Google Scholar]
- 45.Krause DS, Ito T, Fackler MJ, et al. Characterization of murine CD34, a marker for hematopoietic progenitor and stem cells. Blood. 1994;84:691–701. [PubMed] [Google Scholar]
- 46.Khan SS, Solomon MA, McCoy JP. Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytometry B Clin Cytom. 2005;64:1–8. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 47.Gupta S, Verfaillie C, Chmielewski D, et al. Isolation and characterization of kidney-derived stem cells. J Am Soc Nephrol. 2006;17:3028–40. doi: 10.1681/ASN.2006030275. [DOI] [PubMed] [Google Scholar]
- 48.Dore-Duffy P, Katychev A, Wang X, et al. CNS microvascular pericytes exhibit multipotential stem cell activity. J Cereb Blood Flow Metab. 2006;26:613–24. doi: 10.1038/sj.jcbfm.9600272. [DOI] [PubMed] [Google Scholar]
- 49.Lein ES, Hawrylycz MJ, Ao N, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–76. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 50.Rowe EW, Jeftinija DM, Jeftinija K, et al. Development of functional neurons from postnatal stem cells in vitro. Stem Cells. 2005;23:1044–9. doi: 10.1634/stemcells.2005-0037. [DOI] [PubMed] [Google Scholar]
- 51.Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, et al. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- 52.Erickson AC, Couchman JR. Still more complexity in mammalian basement membranes. J Histochem Cytochem. 2000;48:1291–306. doi: 10.1177/002215540004801001. [DOI] [PubMed] [Google Scholar]
- 53.Ahmad I, Tang L, Pham H. Identification of neural progenitors in the adult mammalian eye. Biochem Biophys Res Commun. 2000;270:517–21. doi: 10.1006/bbrc.2000.2473. [DOI] [PubMed] [Google Scholar]
- 54.Tropepe V, Coles BL, Chiasson BJ, et al. Retinal stem cells in the adult mammalian eye. Science. 2000;287:2032–6. doi: 10.1126/science.287.5460.2032. [DOI] [PubMed] [Google Scholar]
- 55.Shechter R, Ronen A, Rolls A, et al. Toll-like receptor 4 restricts retinal progenitor cell proliferation. J Cell Biol. 2008;183:393–400. doi: 10.1083/jcb.200804010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murrell W, Fèron F, Wetzig A, et al. Multipotent stem cells from adult olfactory mucosa. Dev Dyn. 2005;233:496–515. doi: 10.1002/dvdy.20360. [DOI] [PubMed] [Google Scholar]
- 57.Murrell W, Wetzig A, Donnellan M, et al. Olfactory mucosa is a potential source for autologous stem cell therapy for Parkinson’s disease. Stem Cells. 2008;26:2183–92. doi: 10.1634/stemcells.2008-0074. [DOI] [PubMed] [Google Scholar]
- 58.Roisen FJ, Klueber KM, Lu CL, et al. Adult human olfactory stem cells. Brain Res. 2001;890:11–22. doi: 10.1016/s0006-8993(00)03016-x. [DOI] [PubMed] [Google Scholar]
- 59.Takashima Y, Era T, Nakao K, et al. Neuroepithelial cells supply an initial transient wave of MSC differentiation. Cell. 2007;129:1377–88. doi: 10.1016/j.cell.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 60.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–13. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]


