Abstract
Ganglioside GM1-bound cholera toxin–B sub-unit (CT-b) enters the cell via clathrin-coated pits and dynamin-independent non-caveolar raft-dependent endocytosis. Caveolin-1 (Cav1), associated with caveolae formation, is a negative regulator of non-caveolar raft-dependent endocytosis. In mammary epithelial tumour cells deficient for Mgat5, Cav1 is stably expressed at levels below the threshold for caveolae formation, forming stable oligomerized Cav1 microdomains or scaffolds that were shown to suppress EGFR signalling and reduce the plasma membrane diffusion rate of both EGFR and CT-b. Below threshold levels of Cav1 also inhibit the dynamin-dependent raft-mediated endocytosis of CT-b to the Golgi indicating that Cav1-negative regulation of raft-dependent endocytosis is caveolae independent. Inhibition of CT-b internalization does not require Cav1 phosphorylation but does require an intact Cav1 scaffolding domain. By flow cytometry, both over-expression of Cav1 and the dynamin K44A mutant block CT-b internalization from the plasma membrane defining a dynamin-dependent raft pathway for CT-b endocytosis in these cells. However, only minimal co-localization between CT-b and Cav1 is observed. These results suggest that Cav1 regulates raft-dependent internalization of CT-b indirectly via a mechanism that requires the Cav1 scaffolding domain and the formation of oligomerized Cav1 microdomains but not caveolae.
Keywords: caveolin-1, raft endocytosis, cholera toxin b subunit, dyanmin, caveolae
Introduction
Lipid rafts are small heterogeneous cholesterol and sphingolipid-enriched membrane domains involved in various biological processes [1]. Partition of molecules in plasma membrane lipid rafts favours their internalization via a process called raft-dependent endocytosis, generally characterized as clathrin-independent and sensitive to cholesterol depletion [2–4]. However, substantial heterogeneity in the regulation of raft-dependent endocytic pathways exists, in particular with respect to their dynamin dependence and the role of caveolae and the caveolar coat protein, caveolin-1 (Cav1) [4].
Dynamin-dependent caveolae-mediated endocytosis has been implicated in the internalization of simian virus 40 (SV40), lactosylceramide, albumin and GM1-ganglioside binding cholera toxin–B sub-unit (CT-b) [5–13]. However, more recent studies have proposed that CT-b is internalized from the cell surface predominantly via clathrin-dependent endocytosis and dynamin-independent raft endocytosis [14–16]. In Cav1−/– mouse embryo fibroblasts (MEFs), CT-b and SV40 are internalized via a dynamin independent, non-caveolar raft pathway that invokes tubular carriers [15, 17]. In wild-type MEFs, 50% of CT-b was internalized via clathrin-dependent endocytosis and essentially the rest by a dynamin-independent, non-caveolar pathway [15] that may involve flotillin-defined raft domains [16]. Interestingly, dynamin-regulated delivery of CT-b from the tubular carriers to the Golgi and not internalization at the cell surface questioning the extent to which CT-b is internalized via caveolar and dynamin-dependent raft endocytic pathways [15].
Cav1 over-expression prevents internalization of raft-dependent ligands such as CT-b, albumin, dysferlin and autocrine motility factor [15, 18–22]. Cav1 expression may negatively regulate raft-dependent endocytosis by sequestering ligands and their receptors in stable cell surface caveolae [3, 15, 20]. However, we recently demonstrated that Cav1 expression below the threshold required for caveolae formation can suppress EGFR signalling and cell surface diffusion of both EGFR and CT-b describing a regulatory function for Cav1 independently of caveolae [23]. Mammary epithelial tumour cells derived from wild-type PyMT transgenic mice express high levels of Cav1 and caveolae, whereas tumour cells from mice deficient for Golgi N-acetylglucosaminyltransferase V (Mgat5−/–) express Cav1 at levels below the threshold for caveolae formation. PyMT Mgat5−/– tumours generally grow more slowly, but a minority ‘escape’ the Mgat5 null phenotype (Mgat5−/–ESC) and show rapid growth and loss of Cav1 expression [23, 24]. In this cell model, oligomerized Cav1 microdomains, independently of caveolae formation, were found to be sufficient to impose growth restrictions on cells deficient for Mgat5 [23]. Here, using these cell lines, we show that Cav1 regulates the dynamin-dependent, raft-mediated internalization of CT-b under conditions where it is not associated with caveolae formation.
Materials and methods
Cell culture
Mgat5+/+(2.6 and 2.8), Mgat5−/– and Mgat5−/–ESC cells were from PyMT tumours as previously described [23–25]. Rescue and ESC-rescue cells were obtained by infection of Mgat5−/– and Mgat5−/–ESC cells with the pMX-PIE retrovirus encoding for murine Mgat5 and placed under puromycin selection [23, 25]. Cells were grown in DMEM containing non-essential amino acids, l-glutamine, penicillin–streptomycin, vitamins (Invitrogen, Carlsbad, CA, USA) and 10% foetal bovine serum (Medicorp, Montreal, QC, Canada) at 37°C in a humidified 5% CO2/95% air incubator. Adenoviruses expressing either the tetracycline-regulated chimeric transcription activator (tTA) or HA-tagged wild-type dynamin-1, HA-tagged dynK44A mutant and myc-tagged caveolin-1 under the control of the tetracycline-regulated promoter were as previously described [20]. Transfection with specific mouse caveolin-1 siRNA or control siRNA oligonucleotides (Dharmacon, Lafayette, CO, USA) or with Cav1-wt, Cav1Y14F and Cav1F92A/V94A plasmids was as previously described [23].
Cholera toxin–B sub-unit endocytosis
Five μg/ml CT-b-FITC was incubated with cells for 15 or 30 min. at 37°C, rinsed with medium, fixed with 3% paraformaldehyde in PBS, extensively rinsed with PBS and labelled with mouse mAb against Golgi GM130 (Transduction Laboratories, San Jose, CA, USA), polyclonal anti-Cav1 that recognizes both Cav1 and Cav2 (Santa Cruz sc-984, Santa Cruz, CA, USA) and species-specific Alexa568- and Alexa647-conjugated secondary antibodies (Molecular Probes, Invitrogen). Where indicated, cells were treated for 30 min. at 37°C with 5 mM mβCD before incubation with CT-b-FITC in the presence of mβCD. To label cell surface GM1, cells were incubated at 4°C with 5 μg/ml CT-b-FITC, fixed with paraformaldehyde and labelled with polyclonal anti-Cav1 antibody and Alexa568-conjugated secondary antibodies. Images were acquired with 488 (for FITC), 568 and 633 nm laser lines and 60χ Planapochromat objective using the Hi-Lo lookup table of an Olympus FV1000 (Olympus, Markham, ON, Canada) confocal microscope. CT-b-FITC endocytosis to the Golgi apparatus was quantified from at least 20 cells from six individual confocal images by determining the proportion of cellular CT-b-FITC labelling that overlapped with the GM130-labelled Golgi using the M1 function in Image Pro Plus software (Mediacybernetics, Bethesda, MD, USA). Cell surface GM1 and Cav1/2 expression were quantified by measuring the cell-associated fluorescence intensity of the respective channels of images acquired with equivalent acquisition settings using Image Pro Plus software. Data are presented as mean ± S.E. of at least three separate experiments.
Flow cytometry
Mgat5−/–ESC cells were treated with mβCD or infected with adenoviruses encoding for clathrin-hub, dynamin-wt, dynamin K44A and Cav1 [20]. After 48 hrs, cells were incubated for 30 min. at 37°C with either 1 μg/ml CT-b-FITC or 15 μg/ml Tf-FITC. To remove surface-bound fluorescent ligand, cells were rapidly chilled on ice and washed with ice-cold acidic buffer (0.5 mM NaCl, 0.2 mM acetic acid, pH 2) [26]. For flow cytometry, at least 50,000 cells were acquired and analyzed using FACSCalibur and Cellquest software (BD Biosciences, San Jose, CA, USA). Data are the means ± S.E. of triplicates and are representative of three separate experiments.
Results
Reduced Cav1 increases dynamin-dependent, raft-mediated endocytosis of CT-b to the Golgi
As previously reported [23], quantitative immunofluorescence shows that Mgat5−/– cells show approximately 50% reduction in Cav1 levels, below the threshold for caveolae formation, compared with Mgat5+/+ cells, whereas Mgat5−/–ESC cells display significantly reduced Cav1 expression compared with both cell lines (Fig. 1A). Rescue of Mgat5−/– cells (Rescue) with an Mgat5 expressing retrovirus restored Cav1 expression to levels observed in Mgat5+/+ cells. However, upon rescue, Mgat5−/–ESC cells (ESC-Rescue) still showed reduced Cav1 levels (Fig. 1A). GM1 expression impacts on CT-b uptake [27] and we verified that all cell lines displayed similar surface GM1 levels by labelling with CT-b-FITC at 4°C (Fig. 1A). However, following addition of CT-b at 37°C, only Mgat5−/–ESC and ESC-Rescue cells, which express reduced Cav1 levels, showed increased CT-b endocytosis to the Golgi (Fig. 1B). These results are consistent with our previous demonstration that loss of Cav1 in Mgat5−/–ESC cells was associated with increased cell surface diffusion of CT-b [23]. The fact that ESC-Rescue cells displayed the same elevated CT-b uptake as Mgat5−/–ESC cells indicates that Mgat5 expression does not impact on CT-b internalization (Fig. 1B).
Figure 1.
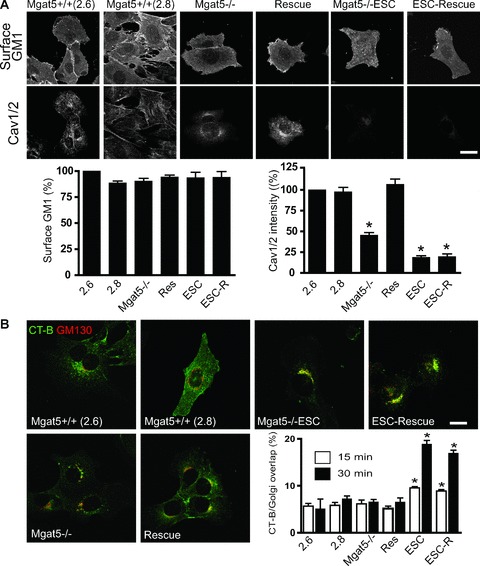
Reduced caveolin expression in Mgat5−/–ESC and ESC-Rescue cells is associated with increased CT-b uptake to the Golgi. (A) Mgat5+/+ (2.6 and 2.8), Mgat5−/–, Rescue, Mgat5−/–ESC and ESC-Rescue cells were probed for cell surface expression of GM1 with CT-b at 4°C (top) or labelled with polyclonal anti-Cav1 antibody that recognizes both Cav1 and Cav2 (Cav1/2) (bottom). Fluorescent intensity of cell-associated CT-b-FITC and total anti-Cav1/2 immunolabelling was quantified. (B) Mgat5+/+ (2.6 and 2.8), Mgat5−/–, Rescue, Mgat5−/–ESC and ESC-Rescue cells were incubated with CT-b-FITC for 15 and 30 min. at 37°C, and the cells were then fixed and labelled for the Golgi marker GM130 and CT-b delivery to the Golgi quantified. Merged images are presented for CT-b-FITC endocytosis at 30 min. (green) and the GM130 labelled Golgi (red). CT-b-FITC co-localization with the Golgi apparatus at 15 and 30 min. incubation was quantified. Bars = 20 μm. *P < 0.01 relative to Mgat5+/+ (2.6) cells.
Internalization of CT-b to the Golgi in Mgat5−/–ESC cells was sensitive to cholesterol depletion with methyl-β-cyclodextrin (mβCD), inhibited by adenoviral expression of the dynamin K44A mutant but not wild-type dynamin and not affected by over-expression of the clathrin-hub (Fig. 2A). CT-b is therefore delivered to the Golgi apparatus of Mgat5−/–ESC cells via a clathrin-independent, dynamin-dependent raft pathway. Over-expression of Cav1 has previously been shown to inhibit CT-b internalization [15, 19]. Consistent with these results, CT-b internalization to the Golgi was greatly reduced in Mgat5−/–ESC cells infected with Cav1-expressing adenovirus (Fig. 2A).
Figure 2.
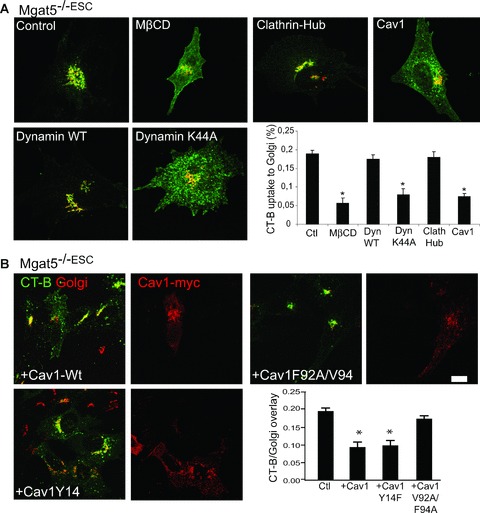
Cav1 regulation of CT-b endocytosis requires an intact scaffolding domain but not phosphorylation on tyrosine 14. (A) Mgat5−/–ESC cells were treated with mβCD for 30 min. or infected with adenoviruses encoding for clathrin-hub, dynamin-wt, dynamin K44A or Cav1 and then incubated with CT-b-FITC (green) for 30 min. at 37°C. Cells were then fixed and labelled with GM130 (red). Co-localization of CT-b and Golgi was quantified for the indicated experimental conditions. (B) Mgat5−/–ESC cells were transfected with Cav1-wt or Cav1 Y14F and F92A/V94A mutants and processed for endocytosis. Cells were then fixed and labelled with anti-GM130 and anti-myc antibodies to identify transfected cells and CT-b-FITC/Golgi co-localization was quantified. *P < 0.01 relative to control cells.
Cav1-negative regulation of raft-dependent endocytosis is caveolae-independent and scaffolding domain-dependent
Cav1 contains a scaffolding domain implicated in Cav1 oligomerization and receptor sequestration as well as a Src-kinase tyrosine phosphorylation site (Y14) that is associated with EGF-induced caveolae formation and focal adhesion turnover [28–31]. The ability of Cav1 to interact with EGFR and restrict the EGF signalling response was found to be dependent on the scaffolding domain and not Cav1 tyrosine phosphorylation [23]. Mgat5−/–ESC cells transfected with Cav1 mutant in the Y14 tyrosine phosphorylation site (Cav1Y14F) mutant displayed a similar reduction in CT-b internalization to the Golgi as cells expressing wild-type Cav1 (Fig. 2B). However, the Cav1F92A/V94A scaffolding domain mutant was unable to significantly reduce CT-b internalization in Mgat5−/–ESC cells (Fig. 2B). These data indicate that the Cav1 scaffolding domain, but not its tyrosine phosphorylation, is critical for Cav1-negative regulation of CT-b endocytosis.
Conversely, siRNA-mediated knock-down of Cav1 in Mgat5+/+ and Mgat5−/– cells, resulting in decreased Cav1 expression, significantly increased uptake of CT-b to the Golgi (Fig. 3A and B). Uptake of CT-b in Mgat5−/– cells following Cav1 siRNA treatment was dynamin- and cholesterol-dependent and clathrin-independent (Fig. 3C) showing that Cav1 is negatively regulating the raft-dependent endocytosis of CT-b. The fact that reduction of Cav1 levels in Mgat5−/– cells, lacking caveolae [23], increases CT-b uptake demonstrates that Cav1 regulates raft-dependent endocytosis independently of caveolae.
Figure 3.
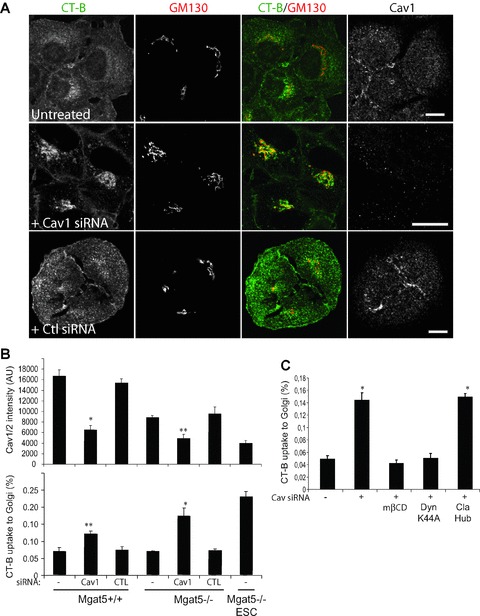
Cav1 siRNA knock-down increases raft-dependent endocytosis of CT-b-FITC to the Golgi. (A) Mgat5−/– cells were treated with non-specific or Cav1 siRNA and incubated with CT-b-FITC for 30 min. prior to fixation and labelling for Cav1 and GM130. Individual images of CT-b-FITC, GM130 and Cav1 as well as a merged image of CT-b and GM130 are presented. (B) Quantification of Cav1/2 expression (top) and CT-b uptake to the Golgi (bottom) following treatment of Mgat5+/+ and Mgat5−/– with Cav1 siRNA is presented in graphic form and compared with the levels of Mgat5−/–ESC cells. (C) Mgat5−/– cells were treated with Cav1 siRNA and either treated with mβCD for 30 min. or infected with adenoviruses encoding for dynamin K44A or Cav1 prior to incubation with CT-b-FITC for 30 min., fixation and labelling for GM130 and quantification of CT-b uptake to the Golgi. Bars = 20 μm. *P < 0.01, **P < 0.05 relative to control cells.
Cav1 regulation of CT-b endocytosis occurs indirectly at the cell surface
CT-b has been shown to follow a two-step path to the Golgi apparatus via caveosomes with dynamin blocking CT-b delivery to the Golgi at an intracellular site [11, 15]. We therefore established whether inhibition of CT-b endocytosis by dynamin and Cav1 occurs at the cell surface or intracellularly. After incubation with CT-b-FITC at 37°C, cells were acid washed to eliminate any cell surface label and intracellular CT-b-FITC measured by flow cytometry. CT-b-FITC labelling at 4°C provided a control for efficacy of cell surface removal of CT-b-FITC by acid washing and use of the same conditions on clathrin-dependent transferrin (Tf) uptake showed specificity for raft-dependent endocytosis (Fig. 4). mβCD treatment and over-expression of Cav1 and dynamin K44A completely inhibited cellular uptake of CT-b to levels observed at 4°C. Conversely, infection with the clathrin hub adenovirus did not impact on intracellular accumulation of CT-b-FITC but did block Tf-FITC uptake (Fig. 4B).
Figure 4.
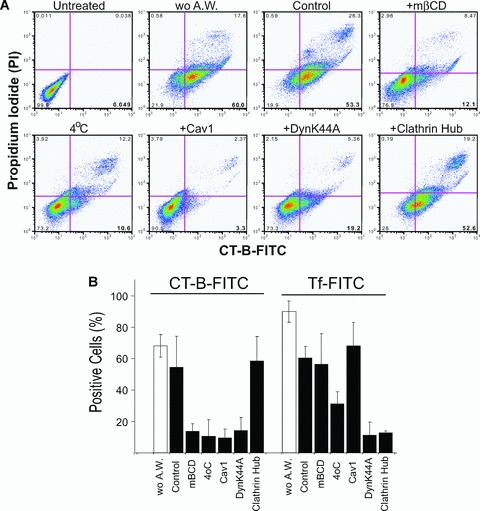
Cav1 and dynamin regulate raft-dependent CT-b endocytosis at the cell surface. (A) Mgat5−/–ESC cells were not treated (Control), treated with mβCD or infected with adenoviruses encoding for clathrin-hub, dynamin K44A and Cav1 and incubated with CT-b-FITC at 37°C for 30 min. and cell surface CT-b removed by acid washing. Alternatively, cells were not incubated with CT-b (Untreated), not acid-washed (wo A.W.) or incubated with CT-b-FITC at 4°C. Prior to flow cytometry, cells were incubated with propidium iodide (PI) to identify dead cells. Representative flow cytometry profiles are shown for PI and CT-b-FITC intensities under the various conditions, as indicated. (B) Percent positive cells was determined following incubation with CT-b-FITC (left) or Tf-FITC (right) for the conditions indicated earlier. Cells were acid washed in order to remove surface-bound CT-b-FITC or Tf-FITC (black bars) except where indicated (wo A.W., white bars).
No significant colocalization was observed between Cav1 and CT-b either in caveolae-expressing Mgat5+/+ cells or in Mgat5−/– cells expressing oligomerized Cav1 microdomains but not caveolae (Fig. 5). Although some co-localization between CT-b and Cav1 could be observed in Mgat5−/–ESC cell transfected with Cav1-mRFP, the fact that the inhibition of CT-b endocytosis by Cav1 over-expression was not associated with recruitment of CT-b to cell surface Cav1 domains is indicative of an indirect effect of Cav1 on CT-b endocytosis (Fig. 5).
Figure 5.
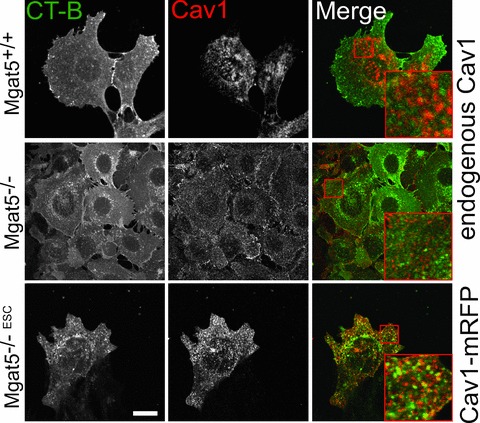
Minimal co-localization of cell surface CT-b and Cav1. Mgat5+/+ and Mgat5−/– cells were incubated with CT-b-FITC (green) for 30 min. at 37°C, fixed and labelled for Cav1 (red). Alternatively, Mgat5−/–ESC cells transfected with Cav1-mRFP (red) were incubated with CT-b-FITC (green) for 30 min. at 37°C (bottom row). Regions boxed in red are enlarged to reveal the incidence of co-localization. Bar = 20 μm.
Discussion
In the mammary epithelial tumour cells used here, CT-b is shown to be internalized predominantly via a cholesterol- and caveolin-1-sensitive, dynamin-dependent raft pathway. Inhibition of CT-b endocytosis by mβCD, the dynamin K44A mutant and Cav1 was shown to occur at the plasma membrane. This suggests that CT-b internalization in these cells follows an endocytic route different from the dynamin-independent pathway involving tubular intermediates reported in MEFs [15]. This study therefore further highlights the varied routes of endocytosis followed by CT-b in different cell types (reviewed in [4]). The basis for the choice of major endocytic route followed by CT-b in different cells remains uncertain. The internalization of CT-b via caveolae [10, 12–14, 32], the fact that Cav1−/– MEFs show a switch in CT-b uptake from the clathrin pathway to a dynamin-independent raft pathway [15] and the ability of Cav1 expression to regulate the dynamin-dependent raft uptake of CT-b shown in this study argues that expression of Cav1 is one, but certainly not the only, determinant of the endocytic route followed by CT-b.
The ability of siRNA-mediated reduction of Cav1 levels in Mgat5−/– cells, that express few or no caveolae [4], to increase CT-b delivery to the Golgi indicates that Cav1 regulation of endocytosis is independent of caveolae formation. Although Mgat5−/– cells express Cav1 at levels below the threshold for caveolae formation, they still express oligomeric Cav1 that recruits EGFR and functionally regulates cell surface CT-b and EGFR diffusion [23]. Indeed, the scaffolding domain of Cav1 was crucial for Cav1-negative regulation of CT-b endocytosis as a scaffolding domain mutant, but not mutation of the Y14 tyrosine phosphorylation site, inhibited CT-b uptake to the Golgi apparatus. Similarly, mutation of the Cav1 scaffolding domain, but not the Y14 tyrosine phosphorylation site, inhibited EGFR signalling [4]. However, minimal CT-b co-localization with Cav1 contrasts the recruitment of EGFR to Cav1 microdomains previously observed in the Mgat5−/– cells [23]. Similarly, negative regulation by caveolin of dysferlin endocytosis requires the scaffolding domain but not dysferlin association with caveolins or caveolae [22]. The Cav1 scaffolding domain mediates interaction with various receptors, the formation of Cav1 oligomers and Cav1 interaction with cholesterol [29]. The ability of reduced levels of Cav1 to regulate raft-dependent CT-b internalization together with its minimal co-localization with uninternalized cell surface CT-b leads us to believe that expression of Cav1 and of oligomerized Cav1 microdomains impact at distance on raft functionality.
Cav1 interacts with dynamin-2 [33] and Cav1 could potentially regulate raft endocytosis by sequestering dynamin-2 away from rafts. Although such sequestration of dynamin-2 should also affect clathrin-dependent uptake, Cav1 does not affect Tf uptake (Fig. 4). Alternatively, Cav1 may function as a store of lipid raft components such as cholesterol or sphingolipids that may be released under specific conditions [34]. Indeed, cholesterol and glycosphingolipids can over-ride Cav1-dependent negative regulation of the caveolar uptake of lactosyl ceramide [21] and plasma membrane sphingomyelin is required for CT entry into cells [35]. Cav1 is a cholesterol-binding protein [36] and its sequestration of cholesterol may impact on the functionality, including the endocytic potential, of non-caveolar raft domains. Indeed, whether raft domains are small, transient and dynamic or exist as a continuum [37, 38], stable Cav1 expressing microdomains with the capacity to interact with and sequester raft components will necessarily impact on their composition, functionality and endocytic potential. Indirect regulation of raft-dependent endocytosis by oligomerized Cav1 microdomains, or scaffolds, therefore represents a regulatory mechanism distinct from sequestration of endocytic ligand by invaginated caveolae. This provides a novel functional role for oligomerized Cav1 microdomains outside caveolae.
References
- 1.Pike LJ. Rafts defined: a report on the Keystone symposium on lipid rafts and cell function. J Lipid Res. 2006;47:1597–8. doi: 10.1194/jlr.E600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 2.Kirkham M, Parton RG. Clathrin-independent endocytosis: new insights into caveolae and non-caveolar lipid raft carriers. Biochimica et Biophysica Acta (BBA) – Molecular Cell Research. 2005;1745:273–86. doi: 10.1016/j.bbamcr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 3.Nabi IR, Le PU. Caveolae/raft-dependent endocytosis. J Cell Biol. 2003;161:673–7. doi: 10.1083/jcb.200302028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lajoie P, Nabi IR. Regulation of raft-dependent endocytosis. J Cell Mol Med. 2007;11:644–53. doi: 10.1111/j.1582-4934.2007.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Minshall RD, Tiruppathi C, Vogel SM, et al. Endothelial cell-surface gp60 activates vesicle formation and trafficking via Gi-coupled Src kinase signaling pathway. J Cell Biol. 2000;150:1057–70. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pelkmans L, Kartenbeck J, Helenius A. Caveolar endocytosis of simian virus 40 reveals a new two-step vesicular-transport pathway to the ER. Nat Cell Biol. 2001;3:473–83. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 7.Sharma DK, Choudhury A, Singh RD, et al. Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J Biol Chem. 2003;278:7564–72. doi: 10.1074/jbc.M210457200. [DOI] [PubMed] [Google Scholar]
- 8.Puri V, Watanabe R, Singh RD, et al. Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J Cell Biol. 2001;154:535–48. doi: 10.1083/jcb.200102084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shajahan AN, Timblin BK, Sandoval R, et al. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J Biol Chem. 2004;279:20392–400. doi: 10.1074/jbc.M308710200. [DOI] [PubMed] [Google Scholar]
- 10.Henley JR, Krueger EW, Oswald BJ, et al. Dynamin-mediated internalization of caveolae. J Cell Biol. 1998;141:85–99. doi: 10.1083/jcb.141.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nichols BJ. A distinct class of endosome mediates clathrin-independent endocytosis to the Golgi complex. Nat Cell Biol. 2002;4:374–8. doi: 10.1038/ncb787. [DOI] [PubMed] [Google Scholar]
- 12.Oh P, McIntosh DP, Schnitzer JE. Dynamin at the neck of caveolae mediates their budding to form transport vesicles by GTP-driven fission from the plasma membrane of endothelium. J Cell Biol. 1998;141:101–14. doi: 10.1083/jcb.141.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parton RG, Joggerst B, Simons K. Regulated internalization of caveolae. J Cell Biol. 1994;127:1199–215. doi: 10.1083/jcb.127.5.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Torgersen ML, Skretting G, van Deurs B, et al. Internalization of cholera toxin by different endocytic mechanisms. J Cell Sci. 2001;114:3737–47. doi: 10.1242/jcs.114.20.3737. [DOI] [PubMed] [Google Scholar]
- 15.Kirkham M, Fujita A, Chadda R, et al. Ultrastructural identification of uncoated caveolin-independent early endocytic vehicles. J Cell Biol. 2005;168:465–76. doi: 10.1083/jcb.200407078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glebov OO, Bright NA, Nichols BJ. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. 2006;8:46–54. doi: 10.1038/ncb1342. [DOI] [PubMed] [Google Scholar]
- 17.Damm EM, Pelkmans L, Kartenbeck J, et al. Clathrin- and caveolin-1-independent endocytosis: entry of simian virus 40 into cells devoid of caveolae. J Cell Biol. 2005;168:477–88. doi: 10.1083/jcb.200407113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kojic LD, Joshi B, Lajoie P, et al. Raft-dependent endocytosis of autocrine motility factor is phosphatidylinositol-3-kinase-dependent in breast carcinoma cells. J Biol Chem. 2007;282:29305–13. doi: 10.1074/jbc.M704069200. [DOI] [PubMed] [Google Scholar]
- 19.Le PU, Nabi IR. Distinct caveolae-mediated endocytic pathways target the Golgi apparatus and the endoplasmic reticulum. J Cell Sci. 2003;116:1059–71. doi: 10.1242/jcs.00327. [DOI] [PubMed] [Google Scholar]
- 20.Le PU, Guay G, Altschuler Y, et al. Caveolin-1 is a negative regulator of caveolae-mediated endocytosis to the endoplasmic reticulum. J Biol Chem. 2002;277:3371–9. doi: 10.1074/jbc.M111240200. [DOI] [PubMed] [Google Scholar]
- 21.Sharma DK, Brown JC, Choudhury A, et al. Selective stimulation of caveolar endocytosis by glycosphingolipids and cholesterol. Mol Biol Cell. 2004;15:3114–22. doi: 10.1091/mbc.E04-03-0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hernandez-Deviez DJ, Howes MT, Laval SH, et al. Caveolin regulates endocytosis of the muscle repair protein, dysferlin. J Biol Chem. 2008;283:6476–88. doi: 10.1074/jbc.M708776200. [DOI] [PubMed] [Google Scholar]
- 23.Lajoie P, Partridge E, Guay G, et al. Plasma membrane domain organization regulates EGFR signaling in tumor cells. J Cell Biol. 2007;179:341–56. doi: 10.1083/jcb.200611106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granovsky M, Fata J, Pawling J, et al. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nat Med. 2000;6:306–12. doi: 10.1038/73163. [DOI] [PubMed] [Google Scholar]
- 25.Partridge EA, Le Roy C, Di Guglielmo GM, et al. Regulation of cytokine receptors by Golgi N-glycan processing and endocytosis. Science. 2004;306:120–4. doi: 10.1126/science.1102109. [DOI] [PubMed] [Google Scholar]
- 26.Davy A, Robbins SM. Ephrin-A5 modulates cell adhesion and morphology in an integrin-dependent manner. EMBO J. 2000;19:5396–405. doi: 10.1093/emboj/19.20.5396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pang H, Le PU, Nabi IR. Ganglioside GM1 levels are a determinant of the extent of caveolae/raft-dependent endocytosis of cholera toxin to the Golgi apparatus. J Cell Sci. 2004;117:1421–30. doi: 10.1242/jcs.01009. [DOI] [PubMed] [Google Scholar]
- 28.Goetz J, Joshi B, Lajoie P, et al. Concerted regulation of focal adhesion dynamics by galectin-3 and tyrosine phosphorylated caveolin-1. J Cell Biol. 2008;180:1261–75. doi: 10.1083/jcb.200709019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goetz JG, Lajoie P, Wiseman SM, et al. Caveolin-1 in tumor progression: the good, the bad and the ugly. Cancer Metastasis Rev. 2008;27:715–35. doi: 10.1007/s10555-008-9160-9. [DOI] [PubMed] [Google Scholar]
- 30.Joshi B, Strugnell SS, Goetz JG, et al. Phosphorylated caveolin-1 regulates Rho/ROCK-dependent focal adhesion dynamics and tumor cell migration and invasion. Cancer Res. 2008;68:8210–20. doi: 10.1158/0008-5472.CAN-08-0343. [DOI] [PubMed] [Google Scholar]
- 31.Orlichenko L, Huang B, Krueger E, et al. Epithelial growth factor-induced phosphorylation of caveolin 1 at tyrosine 14 stimulates caveolae formation in epithelial cells. J Biol Chem. 2006;281:4570–9. doi: 10.1074/jbc.M512088200. [DOI] [PubMed] [Google Scholar]
- 32.Tadokoro S, Shattil SJ, Eto K, et al. Talin binding to integrin {beta} tails: a final common step in integrin activation. Science. 2003;302:103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 33.Yao K, Gietema JA, Shida S, et al. In vitro hypoxia-conditioned colon cancer cell lines derived from HCT116 and HT29 exhibit altered apoptosis susceptibility and a more angiogenic profile in vivo. Br J Cancer. 2005;93:1356–63. doi: 10.1038/sj.bjc.6602864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parton RG, Simons K. The multiple faces of caveolae. Nat Rev Mol Cell Biol. 2007;8:185–94. doi: 10.1038/nrm2122. [DOI] [PubMed] [Google Scholar]
- 35.Saslowsky DE, Lencer WI. Conversion of apical plasma membrane sphingomyelin to ceramide attenuates the intoxication of host cells by cholera toxin. Cell Microbiol. 2008;10:67–80. doi: 10.1111/j.1462-5822.2007.01015.x. [DOI] [PubMed] [Google Scholar]
- 36.Murata M, Peranen J, Schreiner R, et al. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–43. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meder D, Moreno MJ, Verkade P, et al. Phase coexistence and connectivity in the apical membrane of polarized epithelial cells. Proc Natl Acad Sci USA. 2006;103:329–34. doi: 10.1073/pnas.0509885103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma P, Varma R, Sarasij RC, et al. Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell. 2004;116:577–89. doi: 10.1016/s0092-8674(04)00167-9. [DOI] [PubMed] [Google Scholar]


