Abstract
Abstract
Microglia
are resident immune cells in the central nervous system that become activated and produce pro-inflammatory and neurotrophic factors upon activation of various cell-surface receptors. The P2X4 receptor (P2X4R) is a sub-type of the purinergic ion-channel receptors expressed in microglia. P2X4R expression is up-regulated under inflammatory or neurodegenerative conditions, and this up-regulation is implicated in disease pathology. However, the molecular mechanism underlying up-regulation of P2X4R in microglia remains unknown. In the present study, we investigated the intracellular signal transduction pathway that promotes P2X4R expression in microglia in response to fibronectin, an extracellular matrix protein that has previously been shown to stimulate P2X4R expression. We found that in fibronectin-stimulated microglia, activation of phosphatidylinositol 3-kinase (PI3K)–Akt and mitogen-activated protein kinase kinase (MAPK kinase, MEK)–extracellular signal-regulated kinase (ERK) signalling cascades occurred divergently downstream of Src-family kinases (SFKs). Pharmacological interference of PI3K–Akt signalling inhibited fibronectin-induced P2X4R gene expression. Activation of PI3K–Akt signalling resulted in a decrease in the protein level of the transcription factor p53 via mouse double minute 2 (MDM2), an effect that was prevented by MG-132, an inhibitor of the proteasome. In microglia pre-treated with MG-132, fibronectin failed to up-regulate P2X4R expression. Conversely, an inhibitor of p53 caused increased expression of P2X4R, implying a negative regulatory role of p53. On the other hand, inhibiting MEK–ERK signalling activated by fibronectin suppressed an increase in P2X4R protein but interestingly did not affect the level of P2X4R mRNA. We also found that fibronectin stimulation resulted in the activation of the translational factor eIF4E via MAPK-interacting protein kinase-1 (MNK1) in an MEK–ERK signalling-dependent manner, and an MNK1 inhibitor attenuated the increase in P2X4R protein. Together, these results suggest that the PI3K–Akt and MEK–ERK signalling cascades have distinct roles in the up-regulation of P2X4R expression in microglia at transcriptional and post-transcriptional levels, respectively.
Keywords: P2X4 receptor, microglia, fibronectin, signal transduction, gene expression, post-transcriptional regulation
Introduction
Microglia are a type of immune cell that reside in the central nervous system (CNS) and have been implicated in a wide variety of pathological disorders in the CNS [1–3]. Microglia rapidly respond to adverse physiological conditions (e.g. ischaemia and physical damage) and transform into the activated form following a progressive series of changes in morphology, number and gene expression [3–6]. The expression of multiple cell-surface receptors also change in activated microglia and by responding to extracellular ligands for the receptors, the activated microglia evoke various cellular responses, such as migration towards afflicted sites, secretion of pro-inflammatory factors and phagocytosis of dead cells or dangerous debris [3, 7–9]. A growing body of evidence has indicated that among these cell-surface receptors, purinergic receptors are potential regulators of microglial functions and of the pathogenesis of CNS disorders [10–12]. Purinergic receptors are activated by extracellular nucleotides and have been divided into two groups: one is the ligand-gated cation channel P2X receptors and the other is the metabotropic G-protein-coupled P2Y receptors [10, 13]. Molecular cloning has so far identified seven genes for P2X receptors (P2X1-7R) and eight genes for P2Y receptors (P2Y1, 2, 4, 6, 11-14R). Several purinoceptors have been reported to be expressed in microglial cells [14, 15]. These include P2X4R, P2X7R, P2Y6R and P2Y12R. P2Y12R, which couples to Gi signalling in microglia, is implicated in ATP-induced membrane ruffling and chemotaxis towards a source of ATP [7, 16–19]. Activating P2Y6R, which is up-regulated in activated microglia following neuronal injury, induces phagocytosis of damaged neurons through the Gq/phospholipase C/IP3 pathway [8]. We have shown that P2X4R is up-regulated in activated microglia in the spinal cord after peripheral nerve injury [20]. Stimulation of P2X4R in microglia leads to a release of brain-derived neurotrophic factor (BDNF) [21, 22], which is implicated in neuropathic pain, a debilitating pain condition that occurs after nerve damage [21, 22]. Furthermore, there is evidence that up-regulation of P2X4R expression in activated microglia is found in a model of stroke [23], brain tumour [24], traumatic brain injury [25, 26], spinal cord injury [27] and human acute inflammatory demyelinating polyradiculoneuropathy [28]. Despite rapidly accumulating evidence that the up-regulation of P2X4R in microglia might be an important process in the pathogenesis of CNS disorders, including neuropathic pain [10, 13, 21, 22], the molecular mechanism underlying P2X4R up-regulation in microglia remains unknown.
We have recently demonstrated that fibronectin, an extracellular matrix protein, is a factor that up-regulates P2X4R expression at both mRNA and protein levels in primary cultured microglia in vitro[29]. The level of fibronectin is increased in the spinal cord after nerve injury, and blocking integrins in vitro and in vivo attenuates the augmentation of P2X4R expression and neuropathic pain behaviour [30]. We have recently shown that the up-regulation of P2X4R gene expression in response to fibronectin is suppressed by PP2, an inhibitor of Src-family kinases (SFKs), and is not observed in microglial cells from mice lacking Lyn, a member of the SFKs, implying that Lyn tyrosine kinase in microglia is a key molecule in the signalling pathway that causes the up-regulation of P2X4R expression by fibronectin [31]. However, the intracellular signalling cascade downstream of Lyn responsible for promoting P2X4R expression in microglia remains unknown.
The aim of this study was to elucidate the signal transduction pathway through which fibronectin up-regulates P2X4R expression in microglia. We demonstrate that fibronectin stimulation in microglia divergently activated PI3K–Akt and MEK–ERK signalling downstream of SFKs and that these two signalling pathways have distinct roles in P2X4R gene expression and post-transcriptional control, respectively.
Materials and methods
Microglial cells
Rat primary cultured microglia were prepared according to the method described previously [20, 32]. In brief, mixed glial culture (containing astrocyte and microglia) was prepared from neonatal Wistar rats (Kyudo, Saga, Japan) and maintained for 9–24 days in DMEM with 10% foetal bovine serum in 75-cm2 flasks with a change of medium twice a week. Microglia were obtained as floating cells over the mixed glial culture. The floating cells were collected by a gentle shake of the mixed glial culture flasks at 9–10, 14–15 and 22–24 days in vitro (DIV), and transferred to appropriate dishes or cover-slips coated with or without fibronectin (10 μg/ml; Invitrogen, Carlsbad, CA, USA). PP2 (10 μM; Calbiochem, San Diego, CA) [31, 33], LY294002 (2 μM; Tocris, Bristol, UK) [33, 34], U0126 (10 μM; Tocris) [33, 35], pifithrin-α (10 μM; Biomol, PA) [36, 37], MG-132 (5 μM; Calbiochem) [38, 39] and CGP57380 (30 μM; Calbiochem) [40, 41] were pre-incubated with microglial cells for 1 hr before plating. Nutlin-3 (10 μM; Calbiochem) [42, 43] was pre-incubated with microglial cells for 4 hrs before plating. The mouse microglial cell line MG5 cells (kindly provided by Dr. Shinichi Kohsaka, National Institute of Neuroscience, Tokyo, Japan) were grown in astrocyte-conditioned medium that was obtained from the supernatant of astrocytes cultured overnight in DMEM containing 10% foetal bovine serum [44].
Western blotting
Microglia were lysed in RIPA buffer (50 mM Tris–HCl [pH 7.4], 150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% DOC, protease inhibitors cocktail [Sigma, St. Louis, MO]). All samples were subjected to BCA assay to adjust the loading protein amount and then mixed with the Laemmli sample buffer. The sample was subjected to a 10% polyacrylamide gel (BioRad, Hercules, CA), and the proteins were transferred electrophoretically to nitrocellulose membranes (BioRad) or PVDF membranes (GE Healthcare, Buckinghamshire, UK). The membrane was blocked with 5% non-fat dry milk in Tris-buffered saline containing 0.1% Tween-20. The membrane was incubated with primary antibodies (anti-P2X4R [1:1000; Alomone, Jerusalem, Israel], anti-phospho-p42/44 MAPK [Thr202/Tyr 204][1:1000; Cell Signaling, Danvers, MA], anti-phospho-Akt [Ser473][1:1000; Cell Signaling], anti-p53 [1:500; Cell Signaling], anti-phospho-eIF4E [Ser209][1:1000; Cell Signaling], anti-phospho-MNK1 [Thr197/202][1:1000; Cell Signaling] and anti-β-actin [1:2000; Sigma]) overnight at 4°C. The antibodies were detected using HRP-conjugated anti-rabbit and anti-mouse IgG secondary antibody (GE Healthcare, 1:1000) and visualized with the ECL system (GE Healthcare). Equal amounts of protein loaded on each lane were verified by the band intensity of β-actin. β-Actin was used as the endogenous control to normalize the Western blot data. Each band was quantified using computing software.
Immunocytochemistry
Primary cultured microglia were fixed in 3.7% formaldehyde–PBS. After washing and incubating with blocking buffer (3% normal goat serum–0.3% Triton X-100–PBS) for 15 min. at room temperature, the cells were incubated for 24 hrs at 4°C in primary antibodies (anti-phospho-p42/44 MAPK [Thr202/Tyr 204][1:200; Cell Signaling], anti-phospho-Akt [Ser473][1:200; Cell Signaling] and anti-OX-42 [1:1000; Chemicon, Temecula, CA, USA]). Following incubation, microglial cells were washed and incubated for 1 hr at room temperature in secondary antibody solution (Alexa 488 goat anti-rabbit IgG antibody and Alexa 546 goat anti-mouse IgG antibody, 1:1000, Molecular Probes, Eugene, OR) and analyzed using a LSM510 Imaging System (Carl Zeiss, Oberkochen, Germany).
Real-time quantitative RT-PCR
Microglial cells were plated on fibronectin-coated culture dishes for 1 or 6 hrs. Total cellular RNA was extracted using TRIsure (Bioline, London, UK) according to the manufacturer’s instruction and DNase treatment. Real-time RT-PCR was performed using One Step PrimeScript™ RT-PCR Kit (Takara, Kyoto, Japan). RT-PCR amplification and real-time detection were performed using an Applied Biosystems 7500 Real Time PCR System (Applied Biosystems, Foster City, CA) for 10 min. at 42°C (reverse transcription), 10 sec at 95°C, 40 cycles of denaturation (5 sec at 95°C) and annealing/extraction (34 sec at 60°C). The forward and reverse primer pairs for P2X4R were 5′-TGGCGGACTATGTGATTCCA-3′ (forward) and 5′-GGTTCACGGTGACGATACTG-3′ (reverse). All values were normalized with the 18S ribosomal RNA expression and evaluated the values by comparing 6 hrs with 1 hr.
Semi-quantitative RT-PCR
Microglial cells were plated on fibronectin-coated culture dishes for 30 min. and 1 hr. Total cellular RNA was extracted using TRIsure. For reverse transcription, 100 ng of total RNA was transferred to the reaction with Prime Script reverse transcriptase (Takara). The thermocycle profile for PCR amplification was 10 sec at 98°C, 20 sec at 68°C and 20 sec at 72°C for 25 cycles. The forward and reverse primer pairs for p53 were 5′-CATCGAGCTCCCTCTGAGTC-3′ (forward) and 5′-CTTCGGGTAGCTGGAGTGAG-3′ (reverse). All values were normalized with the β-actin expression. The forward and reverse primer pairs for β-actin were 5′-AGGGAAATCGTGCGTGACAT-3′ (forward) and 5′-TCCTGCTTGCTGATCCACAT-3′ (reverse).
Statistical analyses
Statistical analyses of the results were evaluated using the Tukey’s multiple comparison test after one-way anova.
Results
Distinct roles of PI3K–Akt and MEK–ERK signalling pathways in fibronectin-induced up-regulation of P2X4R expression
Our previous study has shown that fibronectin increases the expression of P2X4R protein in microglia via Lyn kinase [31]. Signalling via Lyn and other SFKs plays a role in fibronectin-stimulated PI3K and ERK activation [45–47]. Thus, we first tested an involvement of these two kinases using LY294002, a PI3K inhibitor, and U0126, an MEK inhibitor. When primary cultured microglia were pre-treated with either LY294002 or U0126 before stimulation with fibronectin, an increase in P2X4R protein levels by fibronectin was markedly suppressed (LY294002, P < 0.05; U0126, P < 0.05; Fig. 1A). Pre-treatment of microglia with Akt inhibitor IV (1 μM), a specific inhibitor of Akt, also reduced P2X4R protein expression (data not shown). Microglial cells exposed to fibronectin consistently showed increased levels of phosphorylated Akt and ERK, effects that were inhibited by LY294002 and U0126, respectively (Fig. 1B). However, LY294002 did not influence ERK phosphorylation, and nor did U0126 affect Akt phosphorylation. Similar results were obtained in immunocytochemical analyses: an increased phosphorylated Akt immunofluorescence in individual microglia, in response to fibronectin, was abolished by LY294002 but not by U0126, whereas increased ERK phosphorylation was blocked by U0126 but not by LY294002 (Fig. 1B). On the other hand, the increase in the levels of phosphorylated Akt and ERK in microglia was prevented when cells were pre-treated with the SFK inhibitor PP2 (data not shown). These results indicate that up-regulation of P2X4R expression in fibronectin-stimulated microglia requires PI3K–Akt and MEK–ERK signalling, which occurs divergently downstream of SFKs.
Figure 1.
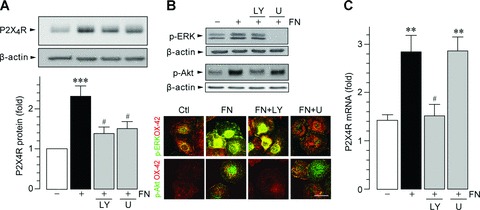
Microglial P2X4R up-regulation by fibronectin is mediated through two distinct signalling pathways. (A) Whole-cell lysate from primary cultured microglial cells (isolated 9–10, 14–15 and 22–24 DIV), cultured for 24 hrs on non-coated and fibronectin (FN; 10 μg/ml)-coated dishes, was subjected to Western blot analyses. The PI3K inhibitor LY294002 (LY; 2 μM) or the MEK inhibitor U0126 (U; 10 μM) was pre-incubated with microglia for 1 hr before plating the cells on dishes. The average (mean ± S.E.M. of six separate experiments) intensity ratio of the bands corresponding to P2X4R and β-actin in each group are shown. (B) Western blot (upper) and immunocytochemical (lower) analyses of ERK and Akt phosphorylation (p-ERK and p-Akt) after 30-min. fibronectin stimulation in primary cultured microglia (15 DIV). Green immunofluorescence: p-ERK or p-Akt; red immunofluorescence: microglial marker OX-42. Scale bars, 20 μm. (C) Real-time quantitative RT-PCR analysis of P2X4R mRNA in total RNA extracts from primary cultured microglia cells (isolated 9–10, 14–15 and 22–24 DIV) that had plated on non-coated (Ctl) or fibronectin (FN; 10 μg/ml)-coated dishes for 6 hrs. LY294002 (LY; 2 μM) and U0126 (U; 10 μM) were pre-incubated with microglia before plating the cells on dishes. The levels of P2X4R mRNA were normalized by the value of 18S mRNA (mean ± S.E.M. of five separate experiments) (**P < 0.01, ***P < 0.001 compared with non-treated microglia, #P < 0.05 compared with fibronectin-treated microglia, by one-way anova).
We next determined the role of these two signalling pathways in P2X4R gene expression induced by fibronectin using real-time quantitative RT-PCR analyses. As shown in Fig. 1C, an increase in the expression of P2X4R mRNA in fibronectin-stimulated microglia was markedly suppressed by pre-treatment with LY294002 (P < 0.05) but, interestingly, was not influenced by U0126. These results provide evidence that PI3K–Akt signalling is necessary for fibronectin-induced regulation of the P2X4R gene in microglia.
Role of p53 degradation via MDM2 in PI3K–Akt signalling-dependent P2X4R expression
MG5, a microglial cell line that was established from p53-deficient mice, is a useful tool for studying microglial function [44, 48]. We used this cell line in our preliminary experiments and obtained unexpected results: when MG5 cells were stimulated by fibronectin, the levels of P2X4R protein were not increased (1.13 ± 0.18-fold over control MG5 cells). This indicates that p53 could be an important transcription factor for P2X4R up-regulation, and therefore, we further investigated the role of p53 using primary cultured microglial cells. As shown in Fig. 2A and B, we found that primary cultured microglia pre-incubated with an inhibitor of p53, pifithrin-α, showed increased expression of P2X4R at both mRNA (P < 0.05) and protein levels (P < 0.05). In addition, when primary cultured microglial cells were treated with fibronectin and pifithrin-α, further enhancement of the up-regulation of P2X4R protein was not observed (Fig. 2C). These observations suggest that p53 is a negative regulator of P2X4R expression in microglia. Consistent with this notion, the level of p53 protein in primary cultured microglia exposed to fibronectin was markedly decreased (P < 0.05, Fig. 3A) without affecting the level of p53 mRNA at 30 min. and 1 hr after fibronectin treatment (30 min., 1.03 ± 0.02-fold over control; 1 hr, 1.02 ± 0.07-fold over control). As p53 is targeted for rapid degradation through the ubiquitin–proteasome pathway [49], we tested the effect of MG-132, a proteasome inhibitor. Pre-treatment of microglial cells with MG-132 resulted in suppression of the down-regulation of p53 protein by fibronectin (P < 0.01, Fig. 3A). The effect of fibronectin on p53 protein expression was attenuated in the presence of either PP2 (P < 0.01) or LY294002 (P < 0.01) but was not attenuated by U0126 (Fig. 3B). Because p53 degradation via the proteasome involves mouse double minute 2 (MDM2), an E3-ubiquitin ligase that binds to p53 [50], we tested the effect of nutlin-3, a novel small-molecule inhibitor of MDM2. As shown in Fig. 4A, we found that nutlin-3 abolished both the fibronectin-induced decrease in p53 protein and the subsequent increase in P2X4R expression (Fig. 4B). Nutlin-3 did not change the basal levels of p53 and P2X4R. Therefore, these results indicate that in microglia an MDM2-dependent degradation of p53 by fibronectin, through the PI3K–Akt pathway, contributes to the increase in P2X4R gene expression.
Figure 2.
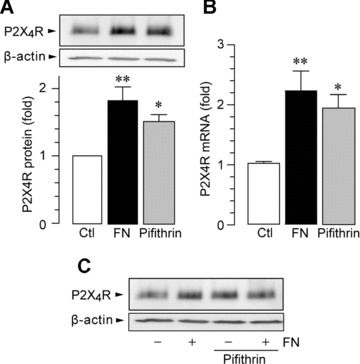
p53 inhibition results in increased P2X4R expression in primary cultured microglia. (A) Western blot analysis of P2X4R protein (24 hrs after plating). (B) Real-time quantitative RT-PCR analysis of P2X4R mRNA (6 hrs after plating). The p53 inhibitor pifithrin-α (10 μM) was pre-incubated with microglia for 30 min. before plating the cells on dishes. Data are mean ± S.E.M. of six to seven separate experiments (*P < 0.05, **P < 0.01 compared with non-treated microglia, by one-way anova). (C) Western blot analysis of P2X4R protein in microglia treated with fibronectin and pifithrin-α (24 hrs after plating). Pifithrin-α (10 μM) was applied to microglia immediately before plating the cells on dishes coated with fibronectin (FN; 10 μg/ml).
Figure 3.
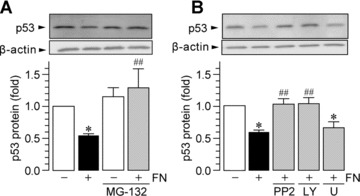
Fibronectin induces p53 degradation through the ubiquitin-proteasome system via the SFK–PI3K pathway. Western blot analysis of p53 protein in whole-cell lysate from primary cultured microglial cells cultured for 1 hr on non-coated and fibronectin (FN; 10 μg/ml)-coated dishes. MG-132 (5 μM; A), PP2 (10 μM; B), LY294002 (LY; 2 μM; B) and U0126 (U; 10 μM; B) were pre-incubated with microglia for 1 hr before plating the cells. The average (mean ± S.E.M. of four to five separate experiments) intensity ratio of the bands corresponding to p53 and β-actin in each group are shown (*P < 0.05 compared with non-treated microglia, #P < 0.05, ##P < 0.01 compared with fibronectin-treated microglia, by one-way anova).
Figure 4.
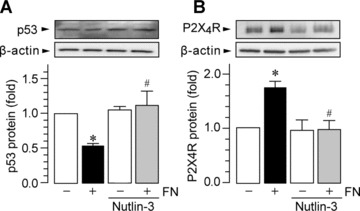
MDM2 is involved in p53 degradation by fibronectin. Western blot analysis of p53 (A) and P2X4R protein (B) in whole-cell lysate from primary cultured microglial cells cultured for 1 hr (A) and 24 hrs (B) on non-coated and fibronectin (FN; 10 μg/ml)-coated dishes. Nutlin-3 (10 μM) was pre-incubated with microglia for 4 hrs before plating the cells. The average (mean ± S.E.M. of three separate experiments) intensity ratio of the bands corresponding to p53 or P2X4R, and β-actin in each group are shown.
Activation of a translational factor through the MEK–ERK pathway regulates P2X4R expression
Based on our findings that inhibiting MEK–ERK signalling suppresses P2X4R protein levels without changing P2X4R mRNA levels, we investigated the role of this signalling in post-transcriptional regulation, focussing on the eukaryotic translation initiation factor 4E (eIF4E), which is a key protein in the translation-initiation process and requires signalling through the MEK–ERK pathway for its activation [51]. As shown in Fig. 5A, microglial cells stimulated by fibronectin showed enhanced levels of eIF4E phosphorylation as early as 15 min. after fibronectin stimulation, and that persisted for 3 hrs. The eIF4E phosphorylation was reduced in microglia pre-treated with either PP2 (P < 0.01) or U0126 (P < 0.01) but not with LY294002 (Fig. 6B), implying an involvement of MEK–ERK signalling. Consistent with evidence that MAPK-interacting protein kinase-1 (MNK1) is a serine/threonine kinase that phosphorylates eIF4E [52], the fibronectin-induced phosphorylation of eIF4E was inhibited by the specific inhibitor of MNK1, CGP57380 (P < 0.05, Fig. 6A). The level of phosphorylated MNK1 in response to fibronectin was abolished in microglia pre-treated either with PP2 or U0126 but not with LY294002 (Fig. 6B). To explore the functional relevance of MNK1-eIF4E signalling, we pre-treated microglia with CGP57380 and found that the fibronectin-induced P2X4R up-regulation was significantly attenuated by CGP57380 (P < 0.05, Fig. 6C). Therefore, the MEK–ERK signalling pathway controls P2X4R expression through activation of the translational factor eIF4E.
Figure 5.
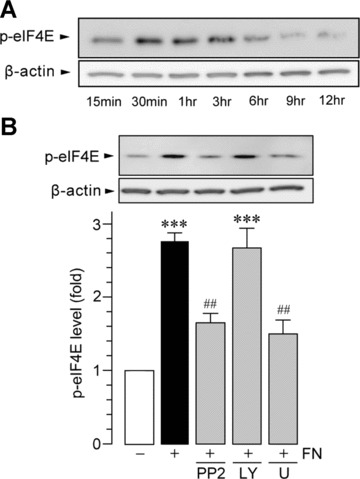
Fibronectin activates eIF4E via ERK. (A) Time course of eIF4E phosphorylation (p-eIF4E) after fibronectin stimulation (FN; 10 μg/ml) in primary cultured microglia. Equal amounts of protein loaded on each lane were verified by the band intensity of β-actin. (B) Effects of kinase inhibitors on fibronectin-induced phosphorylation of eIF4E. Whole-cell lysate from primary cultured microglial cells cultured for 30 min. on non-coated and fibronectin (FN; 10 μg/ml)-coated dishes were prepared. PP2 (10 μM), LY294002 (LY; 2 μM) and U0126 (U; 10 μM) were pre-incubated with microglia for 1 hr before plating the cells. The average (mean ± S.E.M. of four separate experiments) intensity ratio of the bands corresponding to p-eIF4E and β-actin in each group are shown (***P < 0.001 compared with non-treated microglia, ##P < 0.01 compared with fibronectin-treated microglia, by one-way anova).
Figure 6.
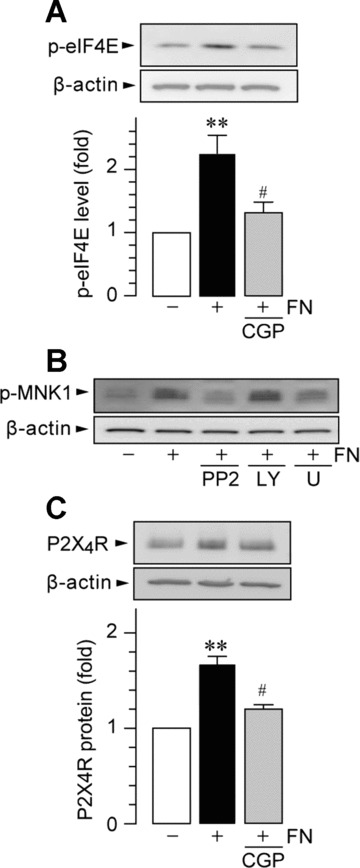
Inhibition of MNK1-eIF4E signalling reduces fibronectin-induced P2X4R protein expression. Western blot analysis of eIF4E phosphorylation (A, B) and P2X4R protein (C) in whole-cell lysate from primary cultured microglial cells cultured for 1 hr (A, B) and 24 hrs (C) on non-coated and fibronectin (FN; 10 μg/ml)-coated dishes. CGP57380 (CGP; 30 μM, A, C), PP2 (10 μM; B), LY294002 (LY; 2 μM; B) and U0126 (U; 10 μM; B) were pre-incubated with microglia for 1 hr before plating the cells. Data are mean ± S.E.M. of four to five separate experiments (**P < 0.01 compared with non-treated microglia, #P < 0.05 compared with fibronectin-treated microglia, by one-way ANOVA).
In addition, in the present study, we used primary cultured microglial cells isolated at 9–10, 14–15 and 22–24 DIV as described in the methods, but similar results were observed in each of the experiments and no substantial differences were found between microglia isolated at these DIV.
Discussion
In the present work, we demonstrated, for the first time, that the fibronectin-stimulated PI3K–Akt and MEK–ERK signalling cascades are important for up-regulating P2X4R expression in microglia. Our previous study identified the microglial SFK, Lyn, as a crucial kinase for the fibronectin-induced P2X4R up-regulation [31]. By showing that the SFK inhibitor PP2 interfered with Akt and ERK activation by fibronectin, PI3K–Akt and MEK–ERK signalling might be mediated by SFKs, presumably Lyn. Of note, these two cascades are likely to diverge at the level of SFKs and, interestingly, play distinct roles in fibronectin-induced regulation of P2X4R expression at the transcriptional level. Consistent with the findings that PI3K–Akt and MEK–ERK signalling did not influence each other’s activities, we revealed that the fibronectin-induced P2X4R gene expression requires PI3K–Akt signalling but not MEK–ERK signalling. To our knowledge, this is the first evidence for these two signalling pathways’ having distinct roles in microglial cells. Furthermore, by using the microglia cell line MG5 that lacks the tumour suppressor protein p53, we provide unexpected evidence that p53 is involved in P2X4R up-regulation by fibronectin. p53 is a transcription factor that induces or represses the expression of a variety of target genes [53, 54]. We found that the p53 inhibitor pifithrin-α caused increases in levels of P2X4R transcripts and protein. Although pifithrin-α has been reported to also show p53-independent effects (such as MAPK inhibition) [55, 56], the increase in P2X4R expression by pifithrin-α might result from the inhibitory effect on p53 because P2X4R up-regulation in fibronectin-treated microglia in which p53 protein had been decreased was not affected by pifithrin-α. From these findings, it is conceivable that p53 may behave as a negative regulator of P2X4R expression in microglia. Our results, showing that fibronectin-stimulated microglia showed decreased levels of p53, also support the repressive role of p53. It is known that cellular p53 levels are tightly controlled via E3 ubiquitin ligases, such as the negative regulator MDM2, which sequesters p53 for proteasomal degradation via ubiquitination [50]. The lack of ability of fibronectin to decrease p53 levels in microglia pre-treated with either the proteasome inhibitor MG-132 or the MDM2 antagonist nutlin-3 indicates that fibronectin stimulates the MDM2-dependent degradation system of p53 in microglia. However, because it has been reported that MG-132 also has an inhibitory effect on lysosomal cysteine protease [57], we cannot exclude a possible involvement of lysosomal degradation.
How fibronectin signals to this system remains unclear, but the data from this study indicate that the degradation of p53 by fibronectin might occur as a downstream event of PI3K–Akt signalling. Indeed, inhibition of PI3K–Akt signalling attenuated the degradation of p53 by fibronectin without affecting Akt activity in fibronectin-stimulated microglia. Moreover, fibronectin-induced p53 degradation via PI3K–Akt signalling has also been reported in other cells [58, 59]. There is evidence that Akt-mediated phosphorylation of MDM2 promotes the nuclear localization of MDM2 and inhibits interaction between MDM2 and p19ARF, one of the first identified MDM2 interacting proteins, thereby decreasing p53 stability [60]. Furthermore, Akt phosphorylation of MDM2 inhibits MDM2 self-ubiquitination [61]. Taking our current findings together with these observations, it seems likely that PI3K–Akt signalling, stimulated by fibronectin, might enhance the ability of MDM2 to reduce the inhibitory effect of p53 in microglia, which in turn leads to the up-regulation of P2X4R gene expression. However, whether P2X4R is a target gene for p53 remains to be determined, and thus, we cannot exclude the possibility that p53 may decrease stabilization of P2X4R transcripts or repress P2X4R indirectly by modulating the expression of an intermediate factor. Further study is needed to clarify this point.
The results of this study provide another important mechanism by which fibronectin promotes P2X4R expression in microglia: a post-transcriptional regulation through MEK–ERK signalling. There is evidence that ERK is crucial for post-transcriptional events, such as transport of nucleocytoplasmic RNA [62] and RNA translation [63]. In the present study, we demonstrated that MEK–ERK signalling induced phosphorylation (at serine 209) of the translational factor eIF4E. eIF4E is a phosphoprotein that binds to the cap structure at the 5’-end of cytoplasmic mRNA and acts to promote mRNA translation with an interaction with eIF4G, a molecule that binds to other translation factors, including eIF4A and eIF3 [51]. Phosphorylation of eIF4E increases its affinity for the 5’-cap4 and facilitates its incorporation into eIF4F complexes, suggesting an increase in translational efficiency [64]. It has been shown that ERK can increase phosphorylation of eIF4E via MNK1 [51, 65]. We used the MNK1-specific inhibitor CGP57380 and demonstrated that MNK1 contributes to fibronectin-induced eIF4E phosphorylation and to P2X4R up-regulation. The fibronectin-induced eIF4E and MNK1 phosphorylation were dependent on MEK–ERK signalling but not on PI3K–Akt signalling. This suggests that activation of MEK–ERK signalling, in response to fibronectin, may lead to stimulation of the signal transduction pathway regulating protein synthesis of P2X4R through MNK1 and eIF4E. However, we cannot exclude the possibility that PI3K–Akt signalling is not involved in an MNK1–eIF4E-independent translational process of P2X4R expression. Indeed, it has been shown in other cells that PI3K–Akt signalling increases protein synthesis through phosphorylation of 4E-BP via mTOR [51, 66, 67].
In summary, we propose the following molecular machinery for the up-regulation of P2X4R expression in microglia in response to fibronectin (Fig. 7). Extracellular fibronectin, by binding to α5β1 integrins on microglial cells, activates the PI3K–Akt and MEK–ERK signalling cascades through SFKs, presumably Lyn tyrosine kinase. The signalling through the PI3K–Akt pathway induces degradation of p53 via MDM2 in a proteasome-dependent manner. The consequence of an attenuated repressive effect of p53 may be associated with enhanced P2X4R gene expression. On the other hand, activated MEK–ERK signalling in microglia exposed to fibronectin enhances eIF4E phosphorylation status via activated MNK1, which may play a role in regulating P2X4R expression at translational levels. The physiological and pathological relevance of these pathways regulating P2X4R expression in microglia in vivo remains to be elucidated, but the role of several proteins in these pathways in CNS diseases has been reported. In neuropathic pain, to which P2X4R critically contributes [10, 13, 20–22], mice lacking Lyn tyrosine kinase exhibit impaired pain behaviour and attenuated P2X4R up-regulation in microglia after nerve injury [31]. Inhibitors of PI3K–Akt signalling and of the proteasome have been reported to attenuate neuropathic pain [68, 69]. Furthermore, ERK activation occurs in microglia after nerve injury, and inhibiting the activation suppresses nerve injury–induced pain [70]. Interestingly, ERK activation in microglia is observed from 2 to 10 days after nerve injury [70], which is similar to the time course of fibronectin up-regulation in the spinal cord, which contributes to initiation of P2X4R expression in microglia [29, 30]. Therefore, the signalling pathways underlying fibronectin-induced up-regulation of P2X4R expression in microglia that we have demonstrated here may provide clues for understanding the molecular machinery for the up-regulation of P2X4R expression in microglia in vivo and for understanding pathological CNS disorders that are associated with microglial P2X4R.
Figure 7.
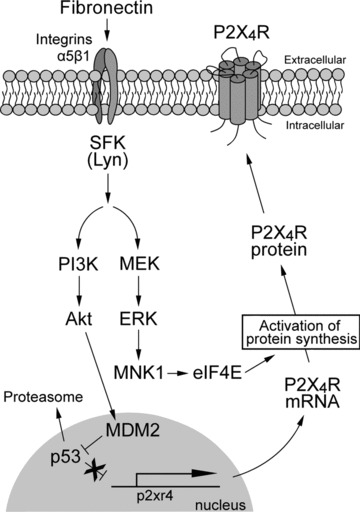
Schematic representation of the hypothetical mechanisms involved in fibronectin-induced P2X4R up-regulation in microglia. Extracellular fibronectin binds to α5β1 integrins on microglial cells, which leads to activation of the PI3K–Akt and MEK–ERK signalling cascades through SFKs, presumably Lyn tyrosine kinase. Signalling through the PI3K–Akt pathway induces degradation of p53 via MDM2 in a proteasome-dependent manner. The reduction of the repressive effect of p53 may enhance P2X4R gene expression. Activated MEK–ERK signalling in microglia exposed to fibronectin enhances eIF4E phosphorylation status via activated MNK1, which may play a role in regulating P2X4R expression at translational levels. SFK, Src-family kinase; PI3K, phosphatidylinositol 3-kinase; MDM2, mouse double minute 2; MEK, mitogen-activated protein kinase kinase; ERK, extracellular signal-regulated kinase; MNK1, MAPK-interacting protein kinase-1; eIF4E, eukaryotic translation initiation factor 4E.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to M.T. and K.I.).
References
- 1.Nakajima K, Kohsaka S. Characterization of brain microglia and the biological significance in the central nervous system. Adv Neurol. 1993;60:734–43. [PubMed] [Google Scholar]
- 2.Streit WJ. Microglia as neuroprotective, immunocompetent cells of the CNS. Glia. 2002;40:133–9. doi: 10.1002/glia.10154. [DOI] [PubMed] [Google Scholar]
- 3.Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–94. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 4.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–8. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 5.Stoll G, Jander S. The role of microglia and macrophages in the pathophysiology of the CNS. Prog Neurobiol. 1999;58:233–47. doi: 10.1016/s0301-0082(98)00083-5. [DOI] [PubMed] [Google Scholar]
- 6.Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog Neurobiol. 1999;57:563–81. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- 7.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injur. in vivo. Nat Neurosci. 2005;8:752–8. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–5. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nasu-Tada K, Koizumi S, Inoue K. Involvement of beta1 integrin in microglial chemotaxis and proliferation on fibronectin: different regulations by ADP through PKA. Glia. 2005;52:98–107. doi: 10.1002/glia.20224. [DOI] [PubMed] [Google Scholar]
- 10.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–90. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 11.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7:423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue K, Koizumi S, Tsuda M. The role of nucleotides in the neuron–glia communication responsible for the brain functions. J Neurochem. 2007;102:1447–58. doi: 10.1111/j.1471-4159.2007.04824.x. [DOI] [PubMed] [Google Scholar]
- 13.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442:527–32. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 14.Inoue K. Microglial activation by purines and pyrimidines. Glia. 2002;40:156–63. doi: 10.1002/glia.10150. [DOI] [PubMed] [Google Scholar]
- 15.Inoue K. The function of microglia through purinergic receptors: neuropathic pain and cytokine release. Pharmacol Ther. 2006;109:210–26. doi: 10.1016/j.pharmthera.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Haynes SE, Hollopeter G, Yang G, et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat Neurosci. 2006;9:1512–9. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 17.Honda S, Sasaki Y, Ohsawa K, et al. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–82. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kurpius D, Nolley EP, Dailey ME. Purines induce directed migration and rapid homing of microglia to injured pyramidal neurons in developing hippocampus. Glia. 2007;55:873–84. doi: 10.1002/glia.20509. [DOI] [PubMed] [Google Scholar]
- 19.Ohsawa K, Irino Y, Nakamura Y, et al. Involvement of P2X4 and P2Y12 receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–16. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 20.Tsuda M, Shigemoto-Mogami Y, Koizumi S, et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature. 2003;424:778–83. doi: 10.1038/nature01786. [DOI] [PubMed] [Google Scholar]
- 21.Coull JA, Beggs S, Boudreau D, et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature. 2005;438:1017–21. doi: 10.1038/nature04223. [DOI] [PubMed] [Google Scholar]
- 22.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: a big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–7. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Cavaliere F, Florenzano F, Amadio S, et al. Up-regulation of P2X2, P2X4 receptor and ischemic cell death: prevention by P2 antagonists. Neuroscience. 2003;120:85–98. doi: 10.1016/s0306-4522(03)00228-8. [DOI] [PubMed] [Google Scholar]
- 24.Guo LH, Trautmann K, Schluesener HJ. Expression of P2X4 receptor in rat C6 glioma by tumor-associated macrophages and activated microglia. J Neuroimmunol. 2004;152:67–72. doi: 10.1016/j.jneuroim.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Guo LH, Trautmann K, Schluesener HJ. Expression of P2X4 receptor by lesional activated microglia during formalin-induced inflammatory pain. J Neuroimmunol. 2005;163:120–7. doi: 10.1016/j.jneuroim.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Zhang Z, Artelt M, et al. Dexamethasone attenuates early expression of three molecules associated with microglia/macrophages activation following rat traumatic brain injury. Acta Neuropathol. 2007;113:675–82. doi: 10.1007/s00401-007-0195-8. [DOI] [PubMed] [Google Scholar]
- 27.Schwab JM, Guo L, Schluesener HJ. Spinal cord injury induces early and persistent lesional P2X4 receptor expression. J Neuroimmunol. 2005;163:185–9. doi: 10.1016/j.jneuroim.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z, Zhang ZY, Fauser U, et al. Mechanical allodynia and spinal up-regulation of P2X4 receptor in experimental autoimmune neuritis rats. Neuroscience. 2008;152:495–501. doi: 10.1016/j.neuroscience.2007.12.042. [DOI] [PubMed] [Google Scholar]
- 29.Nasu-Tada K, Koizumi S, Tsuda M, et al. Possible involvement of increase in spinal fibronectin following peripheral nerve injury in upregulation of microglial P2X4, a key molecule for mechanical allodynia. Glia. 2006;53:769–75. doi: 10.1002/glia.20339. [DOI] [PubMed] [Google Scholar]
- 30.Tsuda M, Toyomitsu E, Komatsu T, et al. Fibronectin/integrin system is involved in P2X(4) receptor upregulation in the spinal cord and neuropathic pain after nerve injury. Glia. 2008;56:579–85. doi: 10.1002/glia.20641. [DOI] [PubMed] [Google Scholar]
- 31.Tsuda M, Tozaki-Saitoh H, Masuda T, et al. Lyn tyrosine kinase is required for P2X(4) receptor upregulation and neuropathic pain after peripheral nerve injury. Glia. 2008;56:50–8. doi: 10.1002/glia.20591. [DOI] [PubMed] [Google Scholar]
- 32.Nakajima K, Shimojo M, Hamanoue M, et al. Identification of elastase as a secretory protease from cultured rat microglia. J Neurochem. 1992;58:1401–8. doi: 10.1111/j.1471-4159.1992.tb11356.x. [DOI] [PubMed] [Google Scholar]
- 33.Suh HS, Kim MO, Lee SC. Inhibition of granulocyte-macrophage colony-stimulating factor signaling and microglial proliferation by anti-CD45RO: role of Hck tyrosine kinase and phosphatidylinositol 3-kinase/Akt. J Immunol. 2005;174:2712–9. doi: 10.4049/jimmunol.174.5.2712. [DOI] [PubMed] [Google Scholar]
- 34.Kim WK, Hwang SY, Oh ES, et al. TGF-beta1 represses activation and resultant death of microglia via inhibition of phosphatidylinositol 3-kinase activity. J Immunol. 2004;172:7015–23. doi: 10.4049/jimmunol.172.11.7015. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki T, Hide I, Ido K, et al. Production and release of neuroprotective tumor necrosis factor by P2X7 receptor-activated microglia. J Neurosci. 2004;24:1–7. doi: 10.1523/JNEUROSCI.3792-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komarov PG, Komarova EA, Kondratov RV, et al. A chemical inhibitor of p53 that protects mice from the side effects of cancer therapy. Science. 1999;285:1733–7. doi: 10.1126/science.285.5434.1733. [DOI] [PubMed] [Google Scholar]
- 37.Bae BI, Xu H, Igarashi S, et al. p53 mediates cellular dysfunction and behavioral abnormalities in Huntington’s disease. Neuron. 2005;47:29–41. doi: 10.1016/j.neuron.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 38.Wang J, Sampath A, Raychaudhuri P, et al. Both Rb and E7 are regulated by the ubiquitin proteasome pathway in HPV-containing cervical tumor cells. Oncogene. 2001;20:4740–9. doi: 10.1038/sj.onc.1204655. [DOI] [PubMed] [Google Scholar]
- 39.Chen Z, Knutson E, Wang S, et al. Stabilization of p53 in human cytomegalovirus-initiated cells is associated with sequestration of HDM2 and decreased p53 ubiquitination. J Biol Chem. 2007;282:29284–95. doi: 10.1074/jbc.M705349200. [DOI] [PubMed] [Google Scholar]
- 40.Andersson K, Sundler R. Posttranscriptional regulation of TNFalpha expression via eukaryotic initiation factor 4E (eIF4E) phosphorylation in mouse macrophages. Cytokine. 2006;33:52–7. doi: 10.1016/j.cyto.2005.11.017. [DOI] [PubMed] [Google Scholar]
- 41.Knauf U, Tschopp C, Gram H. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol Cell Biol. 2001;21:5500–11. doi: 10.1128/MCB.21.16.5500-5511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thompson T, Tovar C, Yang H, et al. Phosphorylation of p53 on key serines is dispensable for transcriptional activation and apoptosis. J Biol Chem. 2004;279:53015–22. doi: 10.1074/jbc.M410233200. [DOI] [PubMed] [Google Scholar]
- 43.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 44.Ohsawa K, Imai Y, Nakajima K, et al. Generation and characterization of a microglial cell line, MG5, derived from a p53-deficient mouse. Glia. 1997;21:285–98. [PubMed] [Google Scholar]
- 45.Giancotti FG, Ruoslahti E. Integrin signaling. Science. 1999;285:1028–32. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- 46.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 47.Playford MP, Schaller MD. The interplay between Src and integrins in normal and tumor biology. Oncogene. 2004;23:7928–46. doi: 10.1038/sj.onc.1208080. [DOI] [PubMed] [Google Scholar]
- 48.Shigemoto-Mogami Y, Koizumi S, Tsuda M, et al. Mechanisms underlying extracellular ATP-evoked interleukin-6 release in mouse microglial cell line, MG-5. J Neurochem. 2001;78:1339–49. doi: 10.1046/j.1471-4159.2001.00514.x. [DOI] [PubMed] [Google Scholar]
- 49.Holmberg CI, Tran SE, Eriksson JE, et al. Multisite phosphorylation provides sophisticated regulation of transcription factors. Trends Biochem Sci. 2002;27:619–27. doi: 10.1016/s0968-0004(02)02207-7. [DOI] [PubMed] [Google Scholar]
- 50.Grossman SR, Perez M, Kung AL, et al. p300/MDM2 complexes participate in MDM2-mediated p53 degradation. Mol Cell. 1998;2:405–15. doi: 10.1016/s1097-2765(00)80140-9. [DOI] [PubMed] [Google Scholar]
- 51.Raught B, Gingras AC. eIF4E activity is regulated at multiple levels. Int J Biochem Cell Biol. 1999;31:43–57. doi: 10.1016/s1357-2725(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 52.Ueda T, Watanabe-Fukunaga R, Fukuyama H, et al. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24:6539–49. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Riley T, Sontag E, Chen P, et al. Transcriptional control of human p53-regulated genes. Nat Rev Mol Cell Biol. 2008;9:402–12. doi: 10.1038/nrm2395. [DOI] [PubMed] [Google Scholar]
- 54.Vousden KH, Lu X. Live or let die: the cell’s response to p53. Nat Rev Cancer. 2002;2:594–604. doi: 10.1038/nrc864. [DOI] [PubMed] [Google Scholar]
- 55.Singh S, Upadhyay AK, Ajay AK, et al. p53 regulates ERK activation in carboplatin induced apoptosis in cervical carcinoma: a novel target of p53 in apoptosis. FEBS Lett. 2007;581:289–95. doi: 10.1016/j.febslet.2006.12.035. [DOI] [PubMed] [Google Scholar]
- 56.Bragado P, Armesilla A, Silva A, et al. Apoptosis by cisplatin requires p53 mediated p38alpha MAPK activation through ROS generation. Apoptosis. 2007;12:1733–42. doi: 10.1007/s10495-007-0082-8. [DOI] [PubMed] [Google Scholar]
- 57.Lee DH, Goldberg AL. Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol. 1998;8:397–403. doi: 10.1016/s0962-8924(98)01346-4. [DOI] [PubMed] [Google Scholar]
- 58.Jeong SJ, Pise-Masison CA, Radonovich MF, et al. Activated AKT regulates NF-kappaB activation, p53 inhibition and cell survival in HTLV-1-transformed cells. Oncogene. 2005;24:6719–28. doi: 10.1038/sj.onc.1208825. [DOI] [PubMed] [Google Scholar]
- 59.Kapila YL, Wang S, Dazin P, et al. The heparin-binding domain and V region of fibronectin regulate apoptosis by suppression of p53 and c-myc in human primary cells. J Biol Chem. 2002;277:8482–91. doi: 10.1074/jbc.M108932200. [DOI] [PubMed] [Google Scholar]
- 60.Zhou BP, Liao Y, Xia W, et al. HER-2/neu induces p53 ubiquitinatio. via Akt-mediated MDM2 phosphorylation. Nat Cell Biol. 2001;3:973–82. doi: 10.1038/ncb1101-973. [DOI] [PubMed] [Google Scholar]
- 61.Mayo LD, Donner DB. The PTEN, Mdm2, p53 tumor suppressor-oncoprotein network. Trends Biochem Sci. 2002;27:462–7. doi: 10.1016/s0968-0004(02)02166-7. [DOI] [PubMed] [Google Scholar]
- 62.Dumitru CD, Ceci JD, Tsatsanis C, et al. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–83. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 63.Kelleher RJ3rd, Govindarajan A, Tonegawa S. Translational regulatory mechanisms in persistent forms of synaptic plasticity. Neuron. 2004;44:59–73. doi: 10.1016/j.neuron.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 64.McKendrick L, Pain VM, Morley SJ. Translation initiation factor 4E. Int J Biochem Cell Biol. 1999;31:31–5. doi: 10.1016/s1357-2725(98)00129-0. [DOI] [PubMed] [Google Scholar]
- 65.Waskiewicz AJ, Flynn A, Proud CG, et al. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–20. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karni R, Gus Y, Dor Y, et al. Active Src elevates the expression of beta-catenin by enhancement of cap-dependent translation. Mol Cell Biol. 2005;25:5031–9. doi: 10.1128/MCB.25.12.5031-5039.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xiao YQ, Freire-de-Lima CG, Schiemann WP, et al. Transcriptional and translational regulation of TGF-beta production in response to apoptotic cells. J Immunol. 2008;181:3575–85. doi: 10.4049/jimmunol.181.5.3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Xu JT, Tu HY, Xin WJ, et al. Activation of phosphatidylinositol 3-kinase and protein kinase B/Akt in dorsal root ganglia and spinal cord contributes to the neuropathic pain induced by spinal nerve ligation in rats. Exp Neurol. 2007;206:269–79. doi: 10.1016/j.expneurol.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 69.Ossipov MH, Bazov I, Gardell LR, et al. Control of chronic pain by the ubiquitin proteasome system in the spinal cord. J Neurosci. 2007;27:8226–37. doi: 10.1523/JNEUROSCI.5126-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhuang ZY, Gerner P, Woolf CJ, et al. ERK is sequentially activated in neurons, microglia, and astrocytes by spinal nerve ligation and contributes to mechanical allodynia in this neuropathic pain model. Pain. 2005;114:149–59. doi: 10.1016/j.pain.2004.12.022. [DOI] [PubMed] [Google Scholar]


