Abstract
Curcumin (diferuloylmethane) is an orange–yellow compound from turmeric (Curcuma longa), a spice found in curry powder. Traditionally known for its anti-inflammatory effects, curcumin has established itself in the last two decades to be a potent immunomodulatory agent that can regulate the activation of a variety of immunocytes and the expression of inflammatory factors. Considering that the β-diketone moiety of curcumin may result in its instability and poor metabolic property, we previously designed a series of mono-carbonyl analogues of curcumin with enhanced stability by deleting this moiety. These compounds demonstrate improved pharmacokinetic profiles both in vitro and in vivo. In this study, we reported a total of 44 mono-carbonyl analogues, which have been evaluated for the inhibitory activities against LPS-induced TNF-α and IL-6 release in the macrophages. Based on the screening results of these analogues, five active compounds A01, A03, A13, B18 and C22 were investigated to inhibit TNF-α and IL-6 release in a dose-dependent manner, three of which further demonstrated inhibitory effects on LPS-induced TNF-α, IL-1β, IL-6, MCP-1, COX-2, PGES, iNOS and p65 NF-κB mRNA production. The results indicated that these mono-carbonyl analogues may possess anti-inflammatory activities similar to curcumin despite the absence of the β-diketone. These mono-carbonyl analogues may be a favourable alternative for the development of curcumin-based anti-inflammatory drugs both pharmacokinetically and pharmacologically. We further examined the biological properties of A13, the only hydrosoluble analogue when combined with hydrochloric acid. The results showed a dose-dependent inhibition of LPS-induced cytokine production. These data further indicated that compound A13 may be explored as a promising anti-inflammatory molecule.
Keywords: curcumin analogues, anti-inflammatory activity, SAR, inflammatory factor, COX-2
Introduction
Pro-inflammatory cytokines are involved in the pathogenesis of a large number of disease processes. Interleukin 6 (IL-6) and tumour necrosis factor-α (TNF-α) are two multi-functional pro-inflammatory cytokines that are involved in the pathogenesis of inflammation, cardiovascular diseases, cancer and neurodegenerational disease through a series of cytokine signalling pathways [1, 2]. Hence, inhibition of such cytokines has currently become a major target of drug development. It is, however, important that such potential therapeutic agents demonstrate inhibitory bioactivity with respect to these cytokines [2, 3].
Curcumin (diferuloylmethane) is an orange–yellow compound from turmeric (Curcuma longa), a spice used extensively in curry powder. Traditionally known for its anti-inflammatory effects, curcumin has recently been shown to be a potent immunomodulatory agent. Curcumin modulates the activation of a variety of immunocytes and inflammatory factors, most likely through inactivation of the transcription factor NF-κB [4–6]. Curcumin decreased the UVB-induced over-expression of TNF-α, IL-6 and IL-8 in a dose-dependent manner in the keratinocytes [7]. Curcumin also has been shown to be a potent inhibitor of LPS-induced NF-κB activation as well as TNF-α and IL production in the mouse macrophages [5, 8, 9]. The anti-inflammatory effects of curcumin have further been demonstrated in patients with rheumatoid arthritis, inflammatory eye diseases, inflammatory bowel disease, chronic pancreatitis and cancers in several independent phase I and phase II clinical trials [10].
Although curcumin can be safely used with an oral dose as high as 12 g/day, its clinical applications have been significantly limited by its instability and poor metabolic properties [11–13]. As demonstrated in clinical studies, curcumin possesses poor pharmacokinetic profiles, such as low bioavailability and rapid metabolism. In a recent phase II trial, the curcumin level in plasma reached only up to 22–41 ng/ml even after administering a daily oral dose of 8.0 g/day for 4 weeks [14].
It has been suggested that β-diketone moiety may be responsible for such rapid degradation and poor bioavailability of curcumin. Recent reports have demonstrated the β-diketone moiety in curcumin is a specific substrate for liver aldo-keto reductases. Presence of β-diketone may be one of main factors contributing to the rapid metabolism of curcumin in vivo[15, 16]. In our previous publication, we have demonstrated design and synthesis of a series of curcumin mono-carbonyl analogues without the β-diketone moiety (showed in Fig. 1) [17, 18]. We have also showed that these analogues, without β-diketone, exhibited enhanced stability in vitro and improved pharmacokinetic profiles in vivo[19]. We evaluated a total of 87 analogues for anti-inflammatory properties using LPS-stimulated mouse J774.1A macrophages, 43 of which were reported previously [18]. The purpose of this communication is to examine whether deletion of reactive β-diketone moiety have any effects on the anti-inflammatory activity compared with the lead compound. In this study, we focus on the rest 44 mono-carbonyl analogues of curcumin and report their anti-inflammatory activities. Following initial screening, we further studied three bioactive compounds A01, A13 and B18 with regards to their abilities to prevent LPS-induced inflammatory mRNA expression. We further expanded our studies and characterized bioactivity of only water-soluble compound A13.
Figure 1.
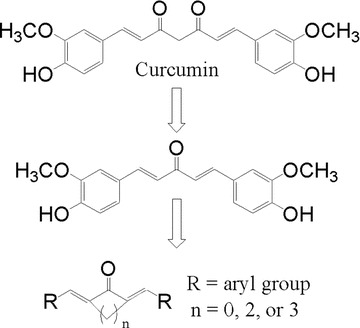
Chemical structure of curcumin and the design of its mono-carbonyl analogues.
Materials and methods
Chemical synthesis
Curcumin (Sigma-Aldrich, St. Louis, MO) and its synthetic analogues were dissolved in DMSO at 20 mmol/l as stock solution. The general procedure of synthesis of the present analogues of curcumin is outlined below. Following the addition of 7.5 mmol ketone to a solution of 15 mmol arylaldehyde in MeOH (10 ml), the solution was stirred at room temperature for 20 min. and was followed by dropwise addition of 20% NaOH (1.5 ml, 7.5 mmol). The mixture was stirred at room temperature and monitored with TLC. Following completion of the reaction, the residue was poured into saturated NH4Cl solution and filtered. The precipitate was washed sequentially with water, cold ethanol, cold acetone and vacuum dried. The solids were purified by chromatography over silica gel using CH2Cl2 /CH3OH as the eluent for compounds 03–21. The synthesis of compounds 01 and 02 has previously been reported by us [17]. A stock solution of A13-HCl at 40 mM was obtained by dissolving 40 μmol A13 in 1 ml of 80 mM HCl solution that was used in appropriate dilution with water for experiments.
Melting points were determined on a Fisher–Johns melting apparatus and are uncorrected. 1HNMR spectra were recorded on a Varian INOVA-400 spectrometer (Varian Medical Systems Inc., Palo Alto, CA, USA). The chemical shifts are presented in terms of parts per million with TMS as the internal reference. Electron-spray ionization mass spectra in positive mode (ESIMS) data were recorded on a Bruker Esquire 3000+ spectrometer (Bruker BioSpin Ltd., Milton, Ontario, Canada). Column chromatography purifications were carried out on Silica Gel 60 (E. Merck, 70–230 mesh). Chemical reagents, structural characterization in 1H NMR and MS, physical properties and molecular formulas of the new derivative compounds are being submitted as supporting information (Supplementary file).
Cell line and reagents
Mouse J774A.1 macrophages were obtained from the Molecular Pharmacology Lab (Department of Microbiology and Immunology, Virginia Commonwealth University, Richmond, VA). Cell culture reagents and NuPAGE Novex Bis–Tris and Tris–acetate Gels were obtained from Invitrogen (Carlsbad, CA). Foetal bovine serum was from Atlanta Biologicals (Norcross, GA) and was heat-inactivated for 30 min. at 65°C. Antibodies against COX-2 and horseradish peroxidase-conjugated donkey anti-goat IgG were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Bio-Rad protein assay reagent, horseradish peroxidase-conjugated goat anti-rabbit IgG and Precision Plus Protein Kaleidoscope Standards were obtained from Bio-Rad (Hercules, CA). BioMax MS film was obtained from Eastman Kodak (Rochester, NY). RNAqueous total RNA isolation kit was purchased from Ambion (Austin, TX). High-Capacity cDNA archive kit and gene expression kits for mouse ATP-binding cassette were bought from Applied Biosystems (Foster City, CA). All other chemical reagents were obtained from Sigma Chemicals (St. Louis, MO).
Cell treatment
Mouse J774A.1 macrophages were maintained in DMEM media supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C with 5% CO2. Human lung cancer H460 cells were cultured in RIPM 1640 medium supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C with 5% CO2. Cells from passages 4–11 were used in these studies. Curcumin and its analogues except A13 were dissolved in DMSO. A13 was dissolved in H2O as a muriate with HCl at twice concentration. Compounds were directly added to culture medium (final concentration, 5–20 μM) and incubated for 1–24 hrs.
Enzyme-linked immunosorbent assay (ELISA)
After treatment, the culture media and cells were collected separately. The levels of TNF-α and IL-6 in the media were determined by ELISA using specific mouse antibody and ELISA Max™ Set Deluxe Kits (Biolegend, San Diego, CA, USA). The tests were performed according to the manufacturer’s instruction. The cells collected were lysated with the total lysis buffer (Tris–HCl, 20 mM; NP40, 1%; NaCl, 150 mM; EDTA, 2 mM; SDS, 0.1%; NaF, 20 mM; Na3VO4, 20mM; H2O). The total protein concentrations of the viable cell pellets were determined using Bio-Rad protein assay reagents. Total amount of the inflammatory factor in the media were normalized to the total protein amount of the viable cell pellets.
RNA isolation and real-time quantitative PCR
Total cellular RNA was isolated from mouse J774A.1 macrophages after treatment with LPS and compounds or vehicle control for 24 hrs, using an Ambion RNAqueous kit. Total RNA (10 μg) was used for first-strand cDNA synthesis using High-Capacity cDNA archive kit (Applied Biosystems). The mRNA levels of TNF-α, IL-1β, IL-6, MCP-1, COX-2 and NF-κB were quantified using the specific gene expression assay kits and primers for mouse TNF-α, IL-1β, IL-6, MCP-1, COX-2 and NF-κB on an iQ5 multi-color real-time PCR detection system. The mRNA values for each gene were normalized to internal control β-actin mRNA. The ratio of normalized mean value for each treatment groups to vehicle control was calculated.
Results
Synthesis of curcumin analogues
Three series of dienones, 1,5-diaryl-1,4-pentadiene-3-ones(B), together with cyclopentanone(A) and cyclohexanone(C) analogues, were designed by displacing β-diketone moiety with a single carbonyl group (shown in Fig. 2). Using the synthetic routes we previously reported [17], compounds 1–19 were obtained by coupling the appropriate aromatic aldehyde with cyclohexanone, cyclopentanone or acetone in an alkaline medium, respectively. The synthetic process of 01 and 02 through hydroxyl protection and deprotection were reported previously [17]. The synthetic yields, melting points, 1HNMR and ESI-MS analysis of unpublished compounds are being described in supplementary file.
Figure 2.
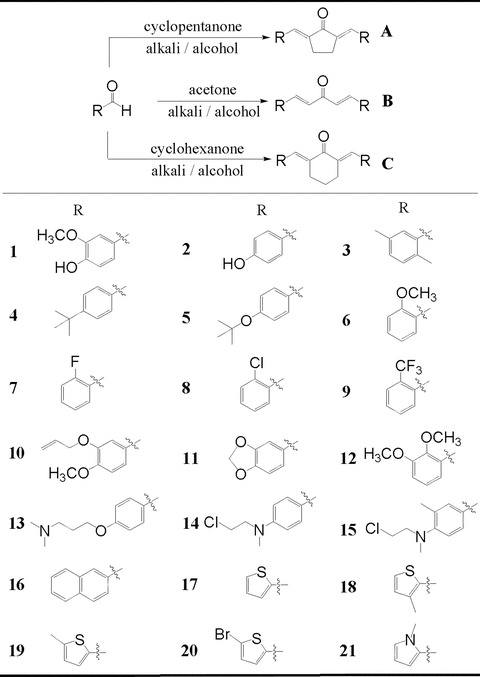
General synthesis and chemical structures of mono-carbonyl analogues of curcumin.
Inhibition of the LPS-induced TNF-α and IL-6 release by curcumin analogues
The stimulation by microbial endotoxins may induce an inflammatory process in the macrophages through cytokine signalling pathways. Lipopolysaccharide (LPS), an endotoxin from the walls of Gram-negative bacteria, is a potent stimulator of inflammatory cytokines in macrophages. Hence, curcumin and its 44 synthetic analogues in the 5-carbon linker series were evaluated for their inhibitory abilities against LPS-induced TNF-α and IL-6 release in the mouse macrophages. Cells were pre-treated with compounds for 2 hrs and then incubated with LPS for 22 hrs. The amount TNF-α and IL-6 in media were detected by ELISA and normalized by protein concentration of cells harvested in the homologous cultural plates. Their inhibitory activities are shown in Fig. 3. The initial screening of these analogs at 10 μM concentration showed that the majority of them inhibited the expression of TNF-α and IL-6 induced by LPS and their inhibitory abilities were comparable to or sometimes more pronounced than that of the leading curcumin at the same concentration.
Figure 3.
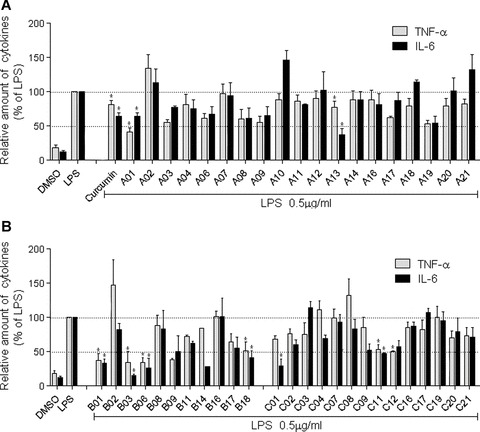
Curcumin and its analogues inhibited LPS-induced TNF-α and IL-6 secretion in J774A.1 macrophages. Macrophages were plated at a density of 1.2 × 106/plate for overnight in 37°C and 5% CO2. Cells were pretreated with curcumin or its analogues (10 μM) for 2 hrs, then treated with LPS (0.5 μg/ml) for 22 hrs. TNF-α and IL-6 levels in the culture media were measured by ELISA and were normalized to the total protein. The results are expressed as percentage of LPS control. Each bar represents mean ± S.E. of three to seven independent experiments.
The results of the anti-inflammation assay of three classes of analogues are shown in Fig. 3A and B, respectively. Among these compounds, 24 compounds were found to be more potent than curcumin in inhibiting LPS-induced TNF-α expression, and 17 compounds showed better inhibitory effects than curcumin did on LPS-induced IL-6 expression. Compounds A01, A08, A13, A19, B01, B03, B06, B09, B11, B17, B18, C01, C02, C11 and C12 exhibited stronger inhibition against both TNF-α and IL-6 than curcumin did. B03, a 2′,5′-dimethyl-substituted compound without the phenolic groups, showed the strongest inhibitory effect on LPS-induced TNF-α and IL-6 release among tested analogues and its inhibitory effects reached 34.3 and 15.9%, respectively.
Active compounds inhibit the TNF-α and IL-6 release in dose-dependent manner
Of the active analogues above, four compounds A01, A03, A13, B18 and one previously reported compound C22 (2,6-bis(4-(allyloxy)benzylidene)cyclohexanone) [18], which demonstrated low cytotoxicities (data not shown) in macrophages, were selected for further assessment of their dose-dependent inhibitory effects against LPS-induced TNF-α and IL-6 release. J774A.1 macrophages were pre-treated with curcumin or its analogues in a series of concentration (2.5, 5.0, 10 and 20 μM) for 2 hrs and were subsequently incubated with LPS (0.5 μg/ml) for 22 hrs. The results (shown in Fig. 4) indicated a dose-dependent inhibition of LPS-induced TNF-α and IL-6 release by curcumin and all five analogues. Compounds A01, A13 and B18 demonstrated comparable inhibitory effects to curcumin. Interestingly, curcumin was found to be more effectively with respect to the inhibition of LPS-induced IL-6 release, whereas A01, A13 and B18 showed better inhibitory activities against TNF-α release. B18, the only heterocyclic ring-containing analogues, was observed to possess, in the concentration of 20 μM, most potent effect in reducing TNF-α release and a comparable IL-6-inhibitory ability reaching a level similar to curcumin.
Figure 4.
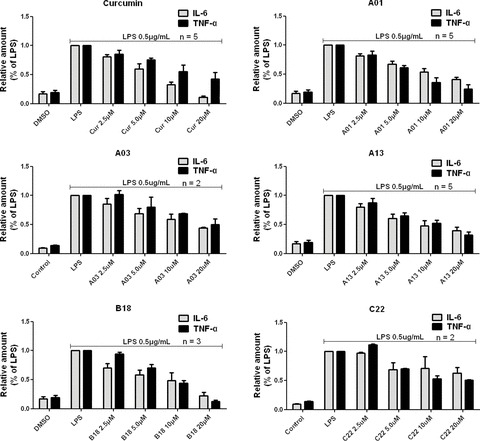
Curcumin, A01, A03, A13, B18 and C22 inhibited LPS-induced TNF-α and IL-6 release in a dose-dependent manner in J774A.1 macrophages. Macrophages were plated at a density of 1.2 × 106/plate for overnight in 37°C and 5% CO2. Cells were pre-treated with specific compounds as indicated for 2 hrs, followed by LPS (0.5 μg/ml) treatment for 22 hrs. TNF-α and IL-6 levels in the culture media were measured by ELISA and were normalized to the total protein amount. The results are expressed as percentage of LPS control. Each bar represents mean ± S.E. of independent experiments in indicated times (n).
A01, A13 and B18 inhibited mRNA expression of inflammatory cytokines induced by LPS
LPS has been reported to augment mRNA expression of inflammatory factors, including TNF-α, IL-6, IL-1β and monocyte chemoattractant protein 1 (MCP-1), which are of importance in the genesis and development of inflammation [20, 21]. Hence, the effects of compounds A01, A13 and B18 were further evaluated with respect to such mRNA expression. The cells were treated with compounds and LPS and total RNA was extracted. Specific mRNAs were detected by real-time RT-PCR (shown in Fig. 5). Compared to the vehicle control, LPS increased the level of the mRNAs of TNF-α, IL-6, IL-1β and MCP-1. When cells were co-incubated with LPS and curcumin or these three analogues at 10 μM, increases in the levels of inflammatory mRNAs were prevented, indicating that A01, A13, B18 and curcumin have inhibitory effects on these mRNAs expression.
Figure 5.
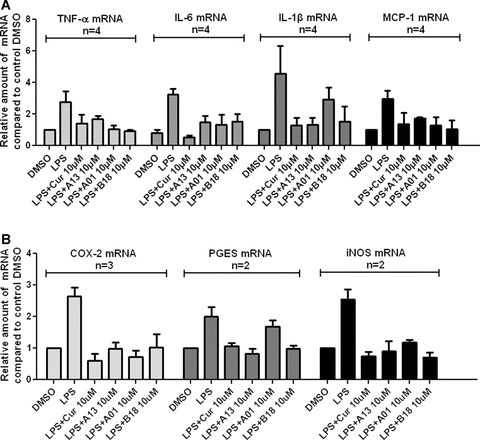
Curcumin, A01, A13 and B18 inhibited LPS-induced inflammatory mRNA expression in J774A.1 macrophages. Cells were pre-treated with compounds at 10 μM or vehicle control for 2 hrs and treated with LPS (0.5 μg/ml) for 22 hrs. The mRNA levels of inflammatory cytokines TNF-α, IL-6, IL-1β and MCP-1 (A), and inflammatory enzymes COX-2, PGES, and iNOS (B) were quantified by real-time quantitative PCR. The mRNA values for each gene were normalized to internal control β-actin mRNA and were expressed as a ratio to DMSO.
Cyclooxygenase 2 (COX-2), prostaglandin E synthase (PGES) and inducible NO synthase (iNOS) have been demonstrated as inflammatory enzymes that are altered in inflammation and in various disease states in humans. They have also been reported to be responsive to the LPS stimulation [9, 20–22]. To determine whether curcumin and its analogs also have any inhibitory effects on the expression of inflammatory enzymes in macrophages, total mRNA was extracted and mRNA of COX-2, PGES and iNOS was, respectively, analyzed by real-time RT-PCR after cells were treated with compounds and LPS for 24 hrs. As shown in Fig. 5B, LPS-induced mRNA expressions of three inflammatory enzymes were significantly reduced by A01, A13, B18 and curcumin at 10 μM concentration. Although the inhibitory effects of analogues were not as pronounced as that of curcumin in COX-2 and iNOS groups, the majority of them reduced LPS-induced expressions of mRNAs below the level of the control group. In PGES group, A13 and B18 exhibited slightly stronger inhibitory effects than curcumin.
Compounds A01, A13 and B18 inhibit the NF-κB p65 mRNA expression
NF-κB is an LPS-inducible transcription factor that plays a central role in the mammalian innate immune response and inflammation. The activation of NF-κB is exerted through the regulation of downstream target genes that encode pro-inflammatory cytokines and inducible enzymes, such as TNF-α, IL-6, IL-1β and COX-2 [23, 24]. A number of studies reported showed that curcumin abrogates NF-κB activation and reduces NF-κB over-expression induced by LPS, TNF-α or H2O2[25, 26]. In this section, we investigated the effects of NF-κB p65 mRNA expression by curcumin and active analogues in order to know about their abilities in the level of transcription factor. As shown in Fig. 6, LPS-induced increase of NF-κB p65 mRNA level were prevented by curcumin and its synthetic analogues at 10 μM concentration. Hence, similar to curcumin, these mono-carbonyl analogues can also inhibit LPS-induced NF-κB expression.
Figure 6.
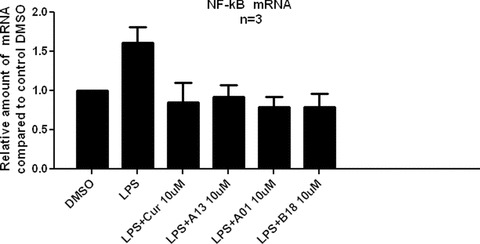
Curcumin, A01, A13 and B18 inhibited LPS-induced NF-κB p65 mRNA expression in J774A.1 macrophages. Cells were pre-treated with compounds at 10 μM or vehicle control for 2 hrs and treated with LPS (0.5 μg/ml) for 22 hrs before harvested. The NF-κB mRNA level was determined by real-time quantitative PCR. The mRNA values were normalized to internal control β-actin mRNA and expressed as a ratio to DMSO control.
Water-soluble compound A13-HCl inhibit the expression of inflammatory cytokines in a dose-dependent manner
Among active analogues above, A13 possesses two N,N-dimethyl groups in its structure, which provides water solubility of A13 as a quaternary ammonium salt when conjugated with two hydrochloric acid molecules. The advantage of hydrosolubility of a drug is of significant importance. Thus, water-soluble A13-HCl was prepared for the study of its dose-dependent effects. As shown in Fig. 7, the quaternary ammonium salt form of A13 inhibited LPS-induced TNF-α, IL-6 and IL-1β release in a dose-dependent manner, indicating that the combination of A13 and HCl dose not decrease the anti-inflammatory ability of A13.
Figure 7.
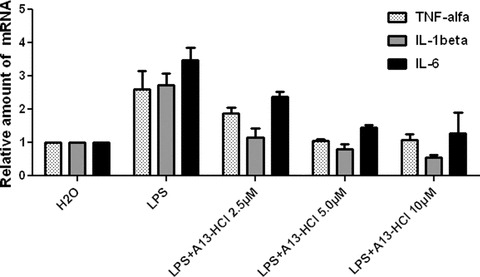
A13-HCl inhibited inflammatory cytokines expression. J774A.1 macrophages were pre-treated with A13-HCl at 10 μM or vehicle H2O for 2 hrs and treated with LPS (0.5 μg/ml) for 22 hrs before harvested. The mRNA levels were determined by real-time quantitative PCR. The mRNA values were normalized to internal control β-actin mRNA and expressed relative to H2O control.
Discussion
The main findings of this study are that we have developed several mono-carbonyl analogues of curcumin with potent anti-inflammatory activities. We have also demonstrated that the water-soluble compound (A13) has dose-dependent anti-inflammatory activity and has the potential to be developed as a therapeutic agent.
As an excellent leading compound, curcumin has been investigated in depth in the field of medicinal chemistry. A number of analogues of curcumin have also been designed and synthesized for the development of new anti-inflammatory and anti-cancer drugs [27–31]. Considering that the presence of β-diketone moiety may result in the instability and poor metabolic properties of curcumin, we have previously designed a series of mono-carbonyl analogues of curcumin with enhanced stability in vitro and improved pharmacokinetic profiles in vivo[17–19]. In the present study, 44 mono-carbonyl analogues were evaluated for the inhibitory activities against LPS-induced TNF-α and IL-6 release in macrophages in an attempt to identify compounds with potent biological activities, which can be targeted for development as pharmaceutical agents.
Curcumin can influence functions of different cells in a variety of ways [32]. Several structural moieties of curcumin, such as the β-diketone and phenolic hydroxyl group, have been reported to be responsible for the bioactivities of this compound [29–31, 33]. As previously reported, the reactive β-diketone was characterized as an important bioactivity-inducing moiety and may contribute to the anti-oxidant properties of curcumin [33, 34]. However, there are no studies that have directly addressed the relationship of β-diketone and anti-inflammatory property of curcumin until now. Compounds 01, which are just derived from the replacement of β-diketone in curcumin by a mono-carbonyl group, exhibited comparable bioactivities to curcumin, suggesting that the β-diketone moiety may not have a role on curcumin’s anti-inflammatory property. According to the screening results, we discussed the possible structure-activity relationship of this kind of analogues.
The present acetone-derived B-class compounds are slightly more effective than cyclopentanone-derived A-class and cyclohexanone-derived C-class compounds, whereas our previous publication has suggested that the C-class compounds showed relatively higher activities than A- and B-class compounds according to the previous 43 compounds [18]. Taken all 87 analogues together, it is indicated that the structure of 5-canbonyl linker may have a role on such activities and acetone (B) and cyclohexanone (C) linkers are more favourable for the anti-inflammatory property than cyclopentanone linker (A). It is interesting to note that the analogues that retained a phenyl substituent showed pronounced biological activities than curcumin. Among the analogues containing heterocyclic ring, only A19, B17 and B18 exhibited higher activities than curcumin. These data indicate that the phenyl structure of curcumin may be necessary to retain its activity. Among the 2′-halogen–containing analogues 07 (2′-F), 08 (2′-Cl) and 09 (2′-CF3), compounds 09 exhibited stronger inhibitory effects against LPS-induced inflammation than 07, 08 and even compared with curcumin. Thus, these results indicate that the bioactivity of analogues against inflammation induced by LPS is associated with electronegativity of the 2′-substituent.
Among analogues with 4′-phenolic group, the 3′-methoxyl-containing compounds 01 showed the excellent inhibitory activities, whereas compounds 02 demonstrated minimal inhibitory activities or even stimulatory effects, suggesting that the presence of a 3′-methyoxy group is critical to curcumin’s activity. Previous reports [33, 35] have demonstrated the formation of hydrogen bonding between 3′-OCH3 and 4′-OH of curcumin decreases the electron-donating ability of 4′-OH. Thus, our data further confirm that reduction of the electron-donating ability of the 4′-substituent may increase the anti-inflammatory abilities of the mono-carbonyl analogues. Alkylization is a common approach to reduce the electron-donating ability of OH. As demonstrated in Fig. 3, N,N-dimethyl-propyl-alkylized A13 and 3′,4′-(O-CH2-O)-containing B11 showed marked inhibitory activities against LPS-induced TNF-α and IL-6. Compounds 03, 06 and 12, lacking electron-effective moiety in the 4′-position, also exhibited excellent anti-inflammatory properties. We further analyzed the SAR of 4′-position, and showed that, a weak electron-donating substituent at the 4′-position augments the anti-inflammatory activity of the mono-carbonyl analogue, whereas strong electron-donating moiety may reduce or remove such bioactivity.
TNF-α and IL-6 are two versatile pleiotropic cytokines that induce growth stimulation and play a crucial role as an immunostimulant and mediator of host resistance to many infectious agents [1, 2]. The inhibition of TNF-α and IL-6 release by A01, A03, A13, B18 and C22 in dose-dependent manner (Fig. 4) demonstrate their potential to be developed as anti-inflammatory agents. Besides TNF-α and IL-6, curcumin has been reported to exert its therapeutic effects via inhibiting various cytokines such as IL-1β and MCP-1, and key enzymes involved in the inflammatory response such as COX-2, PEGS and iNOS [9, 22–24, 36, 37]. As shown in Fig. 5, three active analogues remarkably reduced LPS-induced mRNAs expressions of TNF-α, IL-1β, IL-6 MCP-1, COX-2, PEGS and iNOS, further establishing their anti-inflammatory properties. They also inhibited mRNA expressions of both inflammatory factors and transcription factor NF-κB p65. Therefore, despite the removal of β-diketone, the mono-carbonyl analogues retained the anti-inflammatory properties at the molecular level. Combined with our previous studies with regards to their stability and pharmacokinetics [19], these data indicate that these mono-carbonyl analogues without β-diketone may lend themselves favourably for the development of curcumin-based anti-inflammatory drug development from both pharmacokinetic and pharmacological standpoints.
The biological properties of A13, the only hydrosoluble analogue when combined with HCl, were further investigated in macrophages. Our results from RT-PCR studies in macrophages confirmed the anti-inflammatory ability of A13-HCl in water solution. Hence, the new analogue A13, in the form of its quaternary ammonium salt with the advantages of both hydrosolubility and stability, may be considered as a promising anti-inflammatory candidate to treat various inflammatory diseases. However, further studies are necessary to establish such notion. Such studies should include testing of these new anti-inflammatory analogues of curcumin in animal models and examination of the underlying molecular mechanisms at the transcriptional or post-transcriptional level.
Acknowledgments
This work was supported by the National Natural Science Funding of China (20802054), the NJUST Innovative Ph.D. Training Project (2007 to GL), China, Zhejiang Provincial Program for the Cultivation of High-level Innovative Health talents (to XKL), China, Wenzhou Medical College 50/10 Grant (to HZ), National Institutes of Health R21 AI068432 and R01 AT004148 (to HZ), Jeffress Memorial Trust grant (to HZ). We also thank Dr. Subrata Chakrabarti in the University of Western Ontario for the review and revision in English writing.
References
- 1.Papadakis KA, Targan SR. The role of chemokines and chemokine receptors in mucosal inflammation. Inflamm Bowel Dis. 2000;6:303–13. doi: 10.1002/ibd.3780060408. [DOI] [PubMed] [Google Scholar]
- 2.Popa C, Netea MG, Van Riel PL, et al. The role of TNF-alpha in chronic inflammatory conditions, intermediary metabolism, and cardiovascular risk. J Lipid Res. 2007;48:751–62. doi: 10.1194/jlr.R600021-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Newton RC, Decicco CP. Therapeutic potential and strategies for inhibiting tumor necrosis factor-alpha. J Med Chem. 1999;42:2295–314. doi: 10.1021/jm980541n. [DOI] [PubMed] [Google Scholar]
- 4.Gautam SC, Gao X, Dulchavsky S. Immunomodulation by curcumin. Adv Exp Med Biol. 2007;595:321–41. doi: 10.1007/978-0-387-46401-5_14. [DOI] [PubMed] [Google Scholar]
- 5.Chan MM. Inhibition of tumor necrosis factor by curcumin, a phytochemical. Biochem Pharmacol. 1995;49:1551–6. doi: 10.1016/0006-2952(95)00171-u. [DOI] [PubMed] [Google Scholar]
- 6.Cho JW, Lee KS, Kim CW. Curcumin attenuates the expression of IL-1beta, IL-6, and TNF-alpha as well as cyclin E in TNF-alpha-treated HaCaT cells; NF-kappaB and MAPKs as potential upstream targets. Int J Mol Med. 2007;19:469–74. [PubMed] [Google Scholar]
- 7.Grandjean-Laquerriere A, Gangloff SC, Le NaourR, et al. Relative contribution of NF-kappaB and AP-1 in the modulation by curcumin and pyrrolidine dithiocarbamate of the UVB-induced cytokine expression by keratinocytes. Cytokine. 2002;18:168–77. doi: 10.1006/cyto.2002.0888. [DOI] [PubMed] [Google Scholar]
- 8.Hsu HY, Chu LC, Hua KF, et al. Heme oxygenase-1 mediates the anti-inflammatory effect of curcumin within LPS-stimulated human monocytes. J Cell Physiol. 2008;215:603–12. doi: 10.1002/jcp.21206. [DOI] [PubMed] [Google Scholar]
- 9.Kang BY, Kang BY, Chung SW, et al. Inhibition of interleukin-12 production in lipopolysaccharide-activated macrophages by curcumin. Eur J Pharmacol. 1999;384:191–5. doi: 10.1016/s0014-2999(99)00690-1. [DOI] [PubMed] [Google Scholar]
- 10.Hsu CH, Cheng AL. Clinical studies with curcumin. Adv Exp Med Biol. 2007;595:471–80. doi: 10.1007/978-0-387-46401-5_21. [DOI] [PubMed] [Google Scholar]
- 11.NCI, DCPC. Clinical development plan: curcumin. J Cell Biochem. 1996;26S:72–85. [PubMed] [Google Scholar]
- 12.Sharma RA, Steward WP, Gescher AJ. Pharmacokinetics and pharmacodynamics of curcumin. Adv Exp Med Biol. 2007;595:453–60. doi: 10.1007/978-0-387-46401-5_20. [DOI] [PubMed] [Google Scholar]
- 13.Sharma RA, McLelland HR, Hill KA, et al. Pharmacodynamic and pharmacokinetic study of oral curcuma extract in patients with colorectal cancer. Clin Cancer Res. 2001;7:1894–900. [PubMed] [Google Scholar]
- 14.Dhillon B, Aggarwal BB, Newman RA, et al. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin Cancer Res. 2008;14:4491–9. doi: 10.1158/1078-0432.CCR-08-0024. [DOI] [PubMed] [Google Scholar]
- 15.Rosemond MJ, St John-Williams L, Yamaguchi T, et al. Enzymology of a carbonyl reduction clearance pathway for the HIV integrase inhibitor, S-1360: role of human liver cytosolic aldo-keto reductases. Chem Biol Interact. 2004;147:129–39. doi: 10.1016/j.cbi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Grogan G. Emergent mechanistic diversity of enzyme-catalysed beta-diketone cleavage. Biochem J. 2005;388:721–30. doi: 10.1042/BJ20042038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang G, Yang S, Jiang L, et al. Synthesis and anti-bacterial properties of mono-carbonyl analogues of curcumin. Chem Pharm Bull. 2008;56:162–7. doi: 10.1248/cpb.56.162. [DOI] [PubMed] [Google Scholar]
- 18.Liang G, Li X, Chen L, et al. Synthesis and anti-inflammatory activities of mono-carbonyl analogues of curcumin. Bioorg Med Chem Lett. 2008;18:1525–9. doi: 10.1016/j.bmcl.2007.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang G, Shao L, Wang Y, et al. Exploration and synthesis of curcumin analogues with improved structural stability as anti-tumor agents. Bioorg Med Lett. 2008 doi: 10.1016/j.bmc.2008.10.044. ; doi: 10.1016/j.bmc.2008.10.044. [DOI] [PubMed] [Google Scholar]
- 20.Dumitru CD, Ceci JD, Tsatsanis C, et al. TNF-a induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–83. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 21.Lund S, Christensen KV, Hedtjärn M, et al. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. J Neuroimmunol. 2006;180:71–87. doi: 10.1016/j.jneuroim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 22.Claria J, Romano M. Pharmacological intervention of cyclooxygenase-2 and 5-lipoxygenase pathways. Impact on inflammation and cancer. Curr Pharm Des. 2005;11:3431–47. doi: 10.2174/138161205774370753. [DOI] [PubMed] [Google Scholar]
- 23.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated, cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 24.Karin M, Cao Y, Greten FR, et al. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–15. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 25.Surh YJ, Chun KS, Cha HH, et al. Molecular mechanisms underlying chemopreventive activities of anti-inflamatory phytochemicals: down-regulation of COX-2 and iNOS through suppression of NF-kappa B activation. Mutat Res. 2001;480:243–68. doi: 10.1016/s0027-5107(01)00183-x. [DOI] [PubMed] [Google Scholar]
- 26.Pan MH, Lin-Shiau SY, Lin JK. Comparative studies on the suppression of nitric oxide synthase by curcumin and its hydrogenated metabolites through down-regulation of IkappaB kinase and NFkappaB activation in macrophages. Biochem Pharmacol. 2000;60:1665–76. doi: 10.1016/s0006-2952(00)00489-5. [DOI] [PubMed] [Google Scholar]
- 27.Breinig M, Schirmacher P, Kern MA. Cyclooxygenase-2 (COX-2)–a therapeutic target in liver cancer. Curr Pharm Des. 2007;13:3305–15. doi: 10.2174/138161207782360627. [DOI] [PubMed] [Google Scholar]
- 28.Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191:125–35. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- 29.Lin L, Shi Q, Nyarko AK, et al. Antitumor agents. 250. Design and synthesis of new curcumin analogues as potential anti-prostate cancer agents. J Med Chem. 2006;49:3963–72. doi: 10.1021/jm051043z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvam C, Jachak SM, Thilagavathi R, et al. Design, synthesis, biological evaluation and molecular docking of curcumin analogues as antioxidant, cyclooxygenase inhibitory and anti-inflammatory agents. Bioorg Med Chem Lett. 2005;15:1793–7. doi: 10.1016/j.bmcl.2005.02.039. [DOI] [PubMed] [Google Scholar]
- 31.Zambre AP, Kulkarni VM, Padhye S, et al. Novel curcumin analogs targeting TNF-induced NF-jB activation and proliferation in human leukemic KBM-5 cells. Bioorg Med Chem. 2006;14:7196–204. doi: 10.1016/j.bmc.2006.06.056. [DOI] [PubMed] [Google Scholar]
- 32.Thangapazham RL, Sharma A, Maheshwari RK. Multiple molecular targets in cancer chemoprevention by curcumin. AAPS J. 2006;8:E443–9. doi: 10.1208/aapsj080352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litwinienko G, Ingold KU. Abnormal solvent effects on hydrogen atom abstraction: resolution of the curcumin antioxidant controversy. The role of sequential proton loss electron transfer. J Org Chem. 2004;69:5888–96. doi: 10.1021/jo049254j. [DOI] [PubMed] [Google Scholar]
- 34.Barclay LRC, Vinqvist MR. On the antioxidant mechanism of curcumin: classical methods are needed to determine antioxidant mechanism and activity. Org Lett. 2000;2:2841–3. doi: 10.1021/ol000173t. [DOI] [PubMed] [Google Scholar]
- 35.Jovanovic SV, Boone CW, Steenken S, et al. How curcumin works preferentially with water soluble antioxidants. J Am Chem Soc. 2001;123:3064–8. doi: 10.1021/ja003823x. [DOI] [PubMed] [Google Scholar]
- 36.Moon Y, Glasgow WC, Eling TE. Curcumin suppresses interleukin 1β-mediated microtonal prostaglandin E syntheses 1 by altering early growth response gene 1 and other signaling pathways. J Pharmacol Exp Ther. 2005;315:788–95. doi: 10.1124/jpet.105.084434. [DOI] [PubMed] [Google Scholar]
- 37.Chan MM, Huang HI, Fenton MR, et al. In vivo inhibition of nitric oxide synthase gene expression by curcumin, a cancer preventive natural product with anti-inflammatory properties. Biochem Pharmacol. 1998;55:1955–62. doi: 10.1016/s0006-2952(98)00114-2. [DOI] [PubMed] [Google Scholar]


