Abstract
The neovascularization of three-dimensional voluminous tissues, such as bone, represents an important challenge in tissue engineering applications. The formation of a preformed vascular plexus could maintain cell viability and promote vascularization after transplantation. We have developed a three-dimensional spheroidal coculture system consisting of human primary endothelial cells and human primary osteoblasts (hOBs) to improve angiogenesis in bone tissue engineering applications. In this study, we investigated the survival and vascularization of the engineered implants in vivo. Endothelial cell spheroids were cocultured with hOBs in fibrin and seeded into scaffolds consisting of processed bovine cancellous bone (PBCB). The cell-seeded scaffolds were evaluated for their angiogenic potential in two different in vivo assays: the chick embryo chorioallantoic membrane (CAM) model and the severe combined immunodeficiency disorder (SCID) mouse model. In both assays, the development of a complex three-dimensional network of perfused human neovessels could be detected. After subcutaneous implantation into immunodeficient mice, the newly formed human vasculature was stabilized by the recruitment of murine smooth muscle α-actin-positive mural cells and anastomoses with the mouse vasculature. We conclude that this endothelial cell spheroid system can be used to create a network of functional perfused blood vessels in vivo. The finding that this process takes place with high efficacy in the presence of co-implanted primary osteoblasts and in an osteoconductive environment provided by the PBCB scaffold, suggests that this system may be suitable for improving vascularization in bone tissue engineering.
Keywords: bone tissue engineering, endothelial cell, osteoblast, spheroid, vascularization, angiogenesis, chick chorioallantoic membrane, SCID mouse
Introduction
Angiogenesis, the sprouting of new blood vessels from pre-existing ones, plays an important role in wound healing, tumour growth, skeletal growth and tissue transplantation. One of the most important limitations for a successful application of ex vivo generated tissue for transplantation purposes is the slow ingrowth of blood vessels, leading to cell loss through hypoxic cell death during the early post-implantational stage [1–3]. Therefore, the construction of blood vessels represents a great challenge for the tissue engineering of voluminous grafts. Most approaches in regenerative medicine to stimulate angiogenesis in hypoxic tissues comprise the use of recombinant angiogenic growth factors such as vascular endothelial growth factor (VEGF) or basic fibroblast growth factor (bFGF) [4–7]. In the meantime, phase II and III clinical trials have been performed, demonstrating the principle efficacy of these angiogenic growth factors, administered either as recombinant proteins or by means of gene therapy, to improve local blood supply in ischemic body regions [8].
Tissue vascularization can also be improved by cell-based approaches using mature endothelial cells or endothelial progenitor cells. In this context, it was shown that Bcl-2-transduced human umbilical vein endothelial cells (HUVECs) seeded in collagen-fibronectin gels form functional microvessels in immunodeficient mice [9]. It was also demonstrated that non-transduced HUVECs are also able to form blood vessels in mice but only when they are co-implanted with mesenchymal precursor cells, which differentiate in vivo into mural cells, thus stabilizing the newly formed blood vessels [10].
Another promising approach to stimulate angiogenesis in tissue engineering is the generation of composite grafts, which contain not only specific cell types characteristic for the respective tissue but also endothelial cells for the rapid creation of microvessels in the newly formed tissue. Such a strategy may be useful for applications in bone tissue engineering, for example to enhance revascularization and ossification in non-healing fractures. In this context, a recent study has shown a direct correlation between angiogenesis and bone repair in various models of bone damage [11].
We have previously described a three-dimensional spheroidal coculture model of human primary endothelial cells and human primary osteoblasts (hOBs), which was designed to improve angiogenesis in bone tissue engineering [12]. In the present study, we demonstrate that this system can be used to create a network of functional perfused blood vessels of human origin in two different in vivo angiogenesis assays, the chick embryo chorioallantoic membrane (CAM) model and the SCID mouse model. The finding that neovascularization originating from implanted endothelial cell spheroids takes place with high efficacy in the presence of co-implanted primary osteoblasts in an osteoconductive environment provided by the PBCB-scaffold implicates that this coculture system may be valuable for bone tissue engineering applications.
Materials and methods
Cell culture
Human osteoblasts (hOBs) were isolated from femoral heads with the informed consent of the patients according to hospital ethic committee guidelines. Isolation of hOBs from bone material was performed as previously described [12]. Osteoblasts were cultured in medium 199 with Earle’s salt (GIBCO, Eggenstein, Germany), supplemented with 10% heat-inactivated foetal calf serum (FCS), 1% L-Glutamine and 1% penicillin/streptomycin at 37°C, 5% CO2. HUVECs were purchased from Promocell (Heidelberg, Germany) and cultured in endothelial cell growth medium (ECGM; Promega, Mannheim, Germany) supplemented with 10% heat-inactivated FCS at 37°C, 5% CO2, in a humidified atmosphere. Only osteoblasts from second passage and HUVECs from second to fifth passage were used for the experiments.
Generation of endothelial spheroids
HUVEC spheroids (1000 cells/spheroid) were generated as previously described [13]. Cells were suspended in culture medium containing 0.25% (w/v) methylcellulose and seeded on plastic dishes in a hanging drop to allow overnight spheroid aggregation. Under these conditions all suspended cells contribute to the formation of a single spheroid per drop of defined size and cell number.
Seeding of processed bovine cancellous bone (PBCB) matrices and 3D culture
Discs of PBCB (diameter 7 mm, thickness 2 mm) were provided by Tutogen Medical (Neunkirchen, Germany). To achieve consistent seeding results, discs were incubated in ECGM supplemented with 10% FCS at 4°C for 24 hrs before seeding. Two hundred HUVEC spheroids along with 2 × 105 hOBs were mixed in 100 μl fibrinogen (final concentration 5 mg/ml, Tissucol-Kit; Baxter, Unterschleißheim, Germany) containing the recombinant angiogenic growth factors VEGF-A and bFGF (500 ng each; R&D Systems, Wiesbaden, Germany). Prior to seeding, thrombin (5 units/ml) was added and discs were immediately seeded with the HUVEC spheroid/hOB-fibrinogen solution. As a negative control, PBCB discs were seeded with fibrin gel immobilized hOBs (2 × 105) only. The cell-seeded constructs were incubated at 37°C for 30 min. to induce polymerization of the fibrin gel. After polymerization, discs were incubated at 37°C, 5% CO2 in a humidified atmosphere in ECGM supplemented with 10% FCS for various time periods. The medium was changed three times per week.
Implantation of PBCB scaffolds into SCID mice
Four- to 6-week-old SCID mice (C.B.-17-SCID, Harlan Winkelmann, Borchen, Germany) served as recipients of the cell-seeded scaffolds. German regulations for care and use of laboratory animals were met at all times. All experiments were approved by the animal care committee of the University of Freiburg. The animals were housed in the veterinary care facility of the University of Freiburg Medical Center. PBCB scaffolds, seeded with HUVEC-spheroids and hOBs or seeded with hOBs only (negative control), were implanted subcutaneously on each side lateral to the dorsal midline of the SCID mice. Three mice were used per experimental group. Twenty-one days after implantation, the mice were killed and the implants were retrieved. Samples were fixed in Schaffer’s solution (37% formaldehyde, 80% ethanol) for 2 days and subsequently decalcified in Tris-ethylenediaminetetraacetic acid (EDTA) solution (260 mm Tris; 270 mm EDTA) for 14 days at 37°C. Samples were then paraffin embedded and sectioned at 5 μm before staining. For perfusion studies, 100 μl FITC-Dextran (70,000 MV, 25 mg/ml anionic, lysine fixable, Molecular Probes, Leiden, Netherlands) was injected into the tail vein of mice 10 min. prior to being killed. Constructs were retrieved and fixed as described above.
Implantation of PBCB scaffolds onto the surface of the chick embryo chorioallantoic membrane
The CAM assay was performed as previously described [14]. In brief, fertilized White Leghorn eggs were incubated at 37.8°C and 65% relative humidity. After 3 days of incubation, a circular window with a diameter of 16 mm was cut into the eggshell. Thereafter, the window was sealed with sellotape and the eggs were returned to the incubator until day 7 of incubation. Implantation of cell-seeded PBCB scaffolds was performed on day 7 of incubation by carefully placing the scaffold onto the surface of the CAM. Afterwards, the window was sealed with sellotape and the eggs were re-incubated for 8 days. After that time, the scaffolds were retrieved, fixed in Schaffer’s solution, decalcified and sectioned at 5 μm
Morphological and immunohistochemical analysis
Histochemical analyses were done with deparaffinized and rehydrated paraffin sections. Sections for immunoperoxidase stainings were treated with 3% H2O2 to inhibit endogenous peroxidase. After washing in PBS, the sections were incubated for 30 min. with blocking solution (goat serum, ready to use, Zymed, CA, USA) followed by incubation with the corresponding monoclonal mouse primary antibodies: anti-human CD34 (Dako, Glostrup, Denmark [1:75]), anti-human CD31 (Dako [1:100]) or anti-vimentin (Dako [1:75]) in a humid chamber at RT for 1 hr. For immunofluorescence, the sections were incubated with the secondary antibody goat antimouse/Alexa 488 (Molecular Probes [1:200]). Afterwards the sections were incubated for 30 min. with anti-smooth muscle α-actin-Cy3 (Sigma, Steinheim, Germany [1:200]). For immunoperoxidase stainings the sections were incubated with biotinylated goat antimouse IgG antibody (Zymed [1:200]), exposed to streptavidin peroxidase (Zymed [ready to use]), developed with diaminobenzidine as substrate, and weakly counterstained with haematoxylin.
Quantification of blood vessels
Each PBCB scaffold was entirely sectioned and three samples were taken from the front, the middle and the back of the scaffold. The selected sections were stained for human CD34 or CD31 (Dako, Glostrup, Denmark [1:75]). Microscopic pictures were taken at 200-fold magnification of three randomized areas per section. To determine the microvascular density (mean number of capillaries per square millimetre) the number of structures with lumen surrounded by CD34− or CD31+ cells was counted manually.
Results
Discs of PBCB (Fig. 1) were seeded with fibrin gel-immobilized hOBs and HUVEC-spheroids. After 24 hrs in vitro, cell-seeded scaffolds were inspected by light microscopy revealing integrity of the spherical cell cluster configuration (Fig. 2A).
Figure 1.
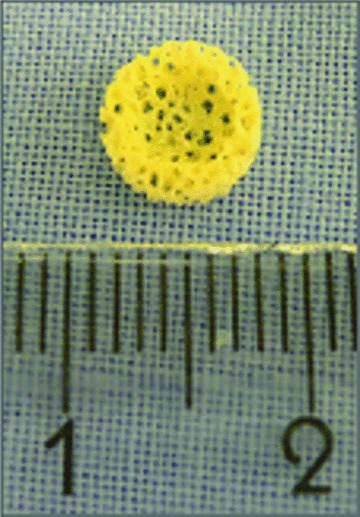
Macroscopic image of the osteoconductive PBCB scaffold.
Figure 2.
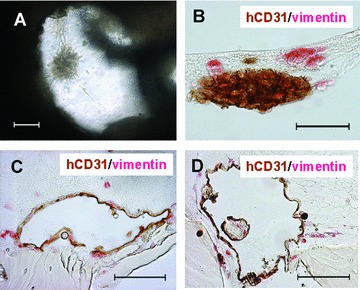
Cell-seeded PBCB scaffold prior to implantation. (A) Phase-contrast microscopy of a single HUVEC spheroid surrounded by co-seeded human osteoblasts. Cells were fibrin gel-immobilized and seeded into the PBCB scaffold (scale bar, 100 μm). (B) hCD31/vimentin double staining of paraffin section of a HUVEC spheroid (brown) surrounded by human osteoblasts (red) 24 hrs after cell seeding (scale bar, 50 μm). (C) hCD31/vimentin double staining of paraffin section after 3 days of in vitro growth showing a large hCD31+ lumenal structure (scale bar, 100 μm). (D) hCD31/vimentin double staining of paraffin section after 14 days of growth in vitro (scale bar, 100 μm).
The distribution of the HUVEC-spheroids and the primary osteoblasts within the scaffold was investigated on histological cross-sections double-stained for human CD31 as a panendothelial marker and human vimentin to visualize cells of human origin. Figure 2B shows a single CD31/vimentin-positive HUVEC-spheroid surrounded by vimentin-positive human osteoblasts within the matrix, 24 hrs after cell seeding. After 3 days, an excessive remodelling of the HUVEC-spheroid was detectable, with a destabilization of the spherical configuration and the development of large human CD31+ lumenal structures (Fig. 2C). These structures were stable in vitro for prolonged time periods, but did not increase in number as shown at day 14 of in vitro growth (Fig. 2D).
In order to assess whether endothelial cell spheroids are able to form functional blood vessels in vivo, HUVEC-spheroid/hOB-seeded scaffolds were investigated in two different in vivo angiogenesis assays: the chick embryo CAM model and the subcutaneous SCID mouse model.
In the CAM assay, the cell-seeded scaffolds were placed on top of the CAM and incubated for 8 days. At this time, a dense network of human neovessels could be detected by using a human-specific antibody against CD31 (Fig. 3A). Double staining with antibodies against human vimentin and human CD31 revealed a homogeneous distribution of human osteoblasts within the human blood vessel network (Fig. 3B). At higher magnification, perfused human CD31+ blood vessels were detectable as demonstrated by the presence of intraluminal haematoxylin-stained avian erythrocytes (Fig. 3C). Quantification of hCD31+ blood vessels in the explanted scaffolds revealed a microvascular density of 50.4 ± 17.3 vessels/mm2 (Fig. 4).
Figure 3.
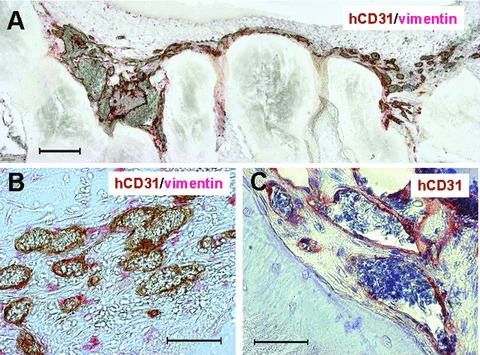
Cell-seeded PBCB scaffolds incubated on top of the chick embryo chorioallantoic membrane for 8 days. (A) hCD31/vimentin double staining of paraffin section of a scaffold seeded with HUVEC spheroids and human osteoblasts at low magnification (scale bar, 200 μm). (B) hCD31/vimentin double staining of cross-section at higher magnification showing human blood vessels (brown) and human osteoblasts (red) dispersed between the blood vessels (scale bar, 50 μm). (C) hCD31 staining of perfused human blood vessels (brown) containing intralumenal haematoxylin-stained avian erythrocytes (pale blue) (scale bar, 50 μm).
Figure 4.
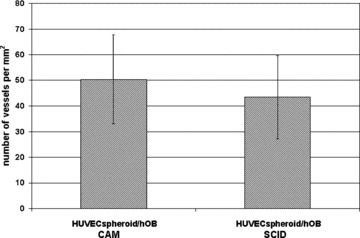
Microvessel densities in HUVEC spheroid/human primary osteoblast implants was quantified by counting hCD31+ vessels after 8 days of growth on the chorioallantoic membrane or by counting hCD34+ vessels after 3 weeks of growth in SCID mice. Values are expressed as number of vessels per square millimetre. Means ± SD from three different sections per group are shown.
Similarly, when HUVEC-spheroid/hOB-seeded scaffolds were implanted subcutaneously into immunodeficient SCID mice and harvested after 21 days, the formation of a dense human neovasculature could be observed as demonstrated by hCD34/vimentin double-staining (Fig. 5A). Quantification of the hCD34+ vessel structures revealed a high microvascular density in the explants with 43.5 ± 16.3 vessels/mm2 (Fig. 4). The newly formed human vasculature formed anastomoses with the mouse vasculature and perfused human vessels, as demonstrated by the presence of intraluminal mouse erythrocytes (Fig. 5B) or injected fluorescently labelled dextran (Fig. 5C), could be detected. hCD34/vimentin double stainings revealed a homogeneous distribution of the vimentin-positive human osteoblasts within the human neovessel network (Fig. 5A, B). Implantation of plain human osteoblasts also revealed a homogeneous distribution of viable cells, but without any sign of neovascularization (Fig. 5D). As expected, implants seeded with human osteoblasts alone failed to form any detectable hCD34+ microvessels (data not shown).
Figure 5.

PBCB scaffolds seeded with HUVEC spheroids and human primary osteoblasts (hOBs) (A–C, E) or with hOBs only (D) were implanted subcutaneously into SCID mice. After 3 weeks in vivo, constructs were explanted and sectioned. (A) Immunostaining using human-specific anti-CD34 (brown) and anti-vimentin (red) antibodies (scale bar, 100 μm). (B) hCD34/vimentin double staining of cross-section at higher magnification showing a perfused human blood vessel (brown) surrounded by hOBs (red) (scale bar, 50 μm). (C) Mice were intravenously injected with FITC/Dextran (green) and the sections were immunofluorescence stained for human CD34 (red) (scale bar, 50 μm). (D) Vimentin staining of a PBCB scaffold seeded with human osteoblasts only (scale bar, 50 μm). (E) Double immunofluorescence staining for human CD34 (grafted HUVEC, green) and α-smooth muscle actin (recruited murine mural cells, red) (scale bar, 100 μm).
Double-staining for human CD34 and smooth muscle α-actin on cross-sections of implants seeded with HUVEC-spheroids and hOBs showed that the newly formed blood vessels were covered with smooth muscle α-actin-positive mural cells, suggesting that the vessels were stabilized by murine pericytes or smooth muscle cells recruited from the surrounding mouse tissue (Fig. 5E).
In summary, we have shown that endothelial cells grown in three-dimensional spheroid culture and co-implanted with hOBs into PBCB-scaffolds, organize in vivo into tubes and form dense functional vessel networks.
Discussion
Construction of stable blood vessels still represents a great challenge in tissue engineering applications. Without the generation of a functional vascular network, implanted cells will undergo apoptosis due to limitations in oxygen and nutrient supply. Multifarious strategies to induce therapeutic angiogenesis are described and comprise the use of various recombinant angiogenic growth factors such as VEGF and bFGF [15–17], gene therapeutic approaches [18, 19] or cell-based therapies using endothelial cells [9, 10]. In this study, we have employed a three-dimensional endothelial cell spheroid system to improve in vivo vascularization in bone tissue constructs. The spheroidal endothelial cell culture model was originally developed as an in vitro angiogenesis assay for studying endothelial cell differentiation and maturation [13, 20]. In addition, this system proved to be suitable as an in vitro assay for the identification of pro- or anti-angiogenic molecules [21]. Because it has been recently demonstrated that endothelial cell spheroids are able to develop lumenized capillary sprouts in vivo[22], we intended to investigate whether this system is also suitable for the in vivo formation of stable blood vessels in bone tissue engineering applications in combination with an osteoconductive PBCB scaffold.
It is well known that tissue engineering of bone substitutes is strongly dependent on vascularization, because no ossification takes place in the absence of blood supply [11, 23]. Therefore, we have used endothelial cell spheroids in combination with hOBs to investigate whether such a blood vessel network can be generated in vivo in a three-dimensional osteoblast environment. Fibrin gel-immobilized HUVEC spheroids and hOBs were co-seeded in PBCB scaffolds and assessed for their angiogenic potential in the CAM assay as well as in a subcutaneous SCID mouse model. In both assays, the development of a complex three-dimensional network of perfused human neovessels could be detected.
In the context of the SCID mouse model, we have also seen that the newly formed human vasculature was covered by smooth muscle α-actin-positive mural cells, indicating that the neovessels were stabilized by murine perivascular cells recruited from the surrounding mouse tissue.
Schechner et al. [9] reported that HUVECs seeded into collagen-fibronectin gels were able to form functional blood vessels in immunodeficient mice. Interestingly, this process was only effective when cells were transduced with a caspase-resistant Bcl-2 protein, which leads to a delay in apoptosis and to the recruitment of perivascular smooth muscle actin-expressing mouse cells. This observation indicates, that the in vivo formation of stable microvessels from implanted HUVECs is in part dependent on the occultation of the newly formed vessels by mural cells. It is well established that new capillaries have to be stabilized by association with pericytes or smooth muscle cells for the formation of mature and long-lasting blood vessels [24–26]. Obviously, this issue seems to be very important for the generation of stable blood vessels in tissue engineering applications as well. Recent work from other groups suggest, that stable blood vessels can only be formed in vivo when endothelial cells are co-seeded with other cell types having the potential to stabilize the newly formed vessel. In this context, it was reported that HUVECs form blood vessels in vivo, when they are co-implanted with mesenchymal precursor cells, which differentiate into mural cells and stabilize the newly formed vessel [10]. Similarly, Levenberg and colleagues [27] have shown in the context of skeletal tissue engineering, that tissue vascularization by implanted HUVECs is strongly dependent on the co-seeding of embryonic fibroblasts which differentiate into smooth muscle cells and stabilize the vessel network. However, as recently reported by Alajati and colleagues [22], when HUVECs were implanted as three-dimensional spheroids in a Matrigel/fibrin matrix, these spheroids were able to develop a stable and perfused vasculature in SCID mice without the need of co-seeding supporting mural cells.
In our study, we have also been able to show that fibrin gel-immobilized spheroidal HUVECs co-seeded with hOBs into a PBCB scaffold were able to form networks of functional perfused blood vessels in mice without the need of co-implanting supporting perivascular cells. However, it was absolutely necessary to initially provide an angiogenic signal for the endothelial cells as a single dose of recombinant VEGF and bFGF, copolymerized into the fibrin matrix. We have used a relatively high concentration of these angiogenic growth factors with 500 ng each per scaffold because this concentration was already proven to be effective in supporting vascularization emerging from implanted HUVEC spheroids [22]. In the absence of these angiogenic growth factors, HUVECs fail to form vascular structures in our in vivo experiments (data not shown).
Recently, Rouwkema and coworkers [28] reported on the in vivo formation of a prevascular network using large cospheroids consisting of HUVECs and human mesenchymal stem cells. These cospheroids were generated in vitro by the application of centrifugal force and consisted of 5 × 105 cells (2% HUVEC; 98% human mesenchymal stem cells) with a diameter of approximately 1 mm. In contrast, the spheroids used in our experiments consisted of only 1000 cells with a diameter of approximately 100 μm and were embedded in growth factor-coated fibrin scaffolds. Although Rouwkema et al. were able to show that the in vitro generated prevascular network was stable and to a certain degree extensible after subcutaneous transplantation into SCID mice, the formation of a dense homogeneously perfused functional microvessel network was not observed. As stated by the authors, perfusion of the vessels was infrequent and only detectable in the periphery of the constructs.
Therefore, our approach using small spheroids embedded in growth factor-activated fibrin scaffolds may be more favourable for the in vivo creation of a dense functional microvessel network.
In our experiments, we have seeded the fibrin gel-immobilized HUVEC spheroid/hOB cocultures into a PBCB scaffold, which represents a clinically established biomaterial for bone replacement. This scaffold, which is already approved for use in human beings [29], is biocompatible [30], osteoconductive [31] and displays biomechanical properties with regard to elasticity and compressive strength that are comparable to autologous bone. The fact that HUVEC spheroid-mediated in vivo neovascularization takes place with high efficacy in the presence of co-implanted primary osteoblasts in an osteoconductive environment provided by the PBCB scaffold implicates that this system may be suitable for improving neovascularization and osteogenesis in bone tissue engineering applications.
Acknowledgments
We thank Beate vom Hoevel for excellent technical assistance and Arash Momeni for critical reading of the manuscript. This work was supported by the competence network Biomaterials Baden-Württemberg and by funding through the Deutsche Forschungsgemeinschaft (STA-472/1–1).
References
- 1.Griffith CK, Miller C, Sainson RC, et al. Diffusion limits of an in vitro thick prevascularized tissue. Tissue Eng. 2005;11:257–66. doi: 10.1089/ten.2005.11.257. [DOI] [PubMed] [Google Scholar]
- 2.Young DM, Greulich KM, Weier HG. Species-specific in situ hybridization with fluorochrome-labeled DNA probes to study vascularization of human skin grafts on athymic mice. J Burn Care Rehabil. 1996;17:305–10. doi: 10.1097/00004630-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Kneser U, Kaufmann PM, Fiegel HC, et al. Long-term differentiated function of heterotopically transplanted hepatocytes on three-dimensional polymer matrices. J Biomed Mater Res. 1999;47:494–503. doi: 10.1002/(sici)1097-4636(19991215)47:4<494::aid-jbm5>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Baumgartner I, Pieczek A, Manor O, et al. Constitutive expression of phVEGF165 after intramuscular gene transfer promotes collateral vessel development in patients with critical limb ischemia. Circulation. 1998;97:1114–23. doi: 10.1161/01.cir.97.12.1114. [DOI] [PubMed] [Google Scholar]
- 5.Schumacher B, Pecher P, von Specht BU, et al. Induction of neoangiogenesis in ischemic myocardium by human growth factors: first clinical results of a new treatment of coronary heart disease. Circulation. 1998;97:645–50. doi: 10.1161/01.cir.97.7.645. [DOI] [PubMed] [Google Scholar]
- 6.Kipshidze N, Chekanov V, Chawla P, et al. Angiogenesis in a patient with ischemic limb induced by intramuscular injection of vascular endothelial growth factor and fibrin platform. Tex Heart Inst J. 2000;27:196–200. [PMC free article] [PubMed] [Google Scholar]
- 7.Henry TD, Rocha-Singh K, Isner JM, et al. Intracoronary administration of recombinant human vascular endothelial growth factor to patients with coronary artery disease. Am Heart J. 2001;142:872–80. doi: 10.1067/mhj.2001.118471. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda S, Yoshii S, Kaga S, et al. Angiogenic strategy for human ischemic heart disease: brief overview. Mol Cell Biochem. 2004;264:143–9. doi: 10.1023/b:mcbi.0000044383.01785.05. [DOI] [PubMed] [Google Scholar]
- 9.Schechner JS, Nath AK, Zheng L, et al. In vivo formation of complex microvessels lined by human endothelial cells in an immunodeficient mouse. Proc Natl Acad Sci USA. 2000;97:9191–6. doi: 10.1073/pnas.150242297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koike N, Fukumura D, Gralla O, et al. Tissue engineering: creation of long-lasting blood vessels. Nature. 2004;428:138–9. doi: 10.1038/428138a. [DOI] [PubMed] [Google Scholar]
- 11.Street J, Bao M, deGuzman L, et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA. 2002;99:9656–61. doi: 10.1073/pnas.152324099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wenger A, Stahl A, Weber H, et al. Modulation of in vitro angiogenesis in a three-dimensional spheroidal coculture model for bone tissue engineering. Tissue Eng. 2004;10:1536–47. doi: 10.1089/ten.2004.10.1536. [DOI] [PubMed] [Google Scholar]
- 13.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–52. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borges J, Tegtmeier FT, Padron NT, et al. Chorioallantoic membrane angiogenesis model for tissue engineering: a new twist on a classic model. Tissue Eng. 2003;9:441–50. doi: 10.1089/107632703322066624. [DOI] [PubMed] [Google Scholar]
- 15.Pieper JS, Hafmans T, van Wachem PB, et al. Loading of collagen-heparan sulfate matrices with bFGF promotes angiogenesis and tissue generation in rats. J Biomed Mater Res. 2002;62:185–94. doi: 10.1002/jbm.10267. [DOI] [PubMed] [Google Scholar]
- 16.Perets A, Baruch Y, Weisbuch F, et al. Enhancing the vascularization of three-dimensional porous alginate scaffolds by incorporating controlled release basic fibroblast growth factor microspheres. J Biomed Mater Res A. 2003;65:489–97. doi: 10.1002/jbm.a.10542. [DOI] [PubMed] [Google Scholar]
- 17.Zisch AH, Lutolf MP, Hubbell JA. Biopolymeric delivery matrices for angiogenic growth factors. Cardiovasc Pathol. 2003;12:295–310. doi: 10.1016/s1054-8807(03)00089-9. [DOI] [PubMed] [Google Scholar]
- 18.Rebar EJ, Huang Y, Hickey R, et al. Induction of angiogenesis in a mouse model using engineered transcription factors. Nat Med. 2002;8:1427–32. doi: 10.1038/nm1202-795. [DOI] [PubMed] [Google Scholar]
- 19.Bouis D, Boelens MC, Peters E, et al. Combination of vascular endothelial growth factor (VEGF) and thymidine phosphorylase (TP) to improve angiogenic gene therapy. Angiogenesis. 2003;6:185–92. doi: 10.1023/B:AGEN.0000021389.49659.31. [DOI] [PubMed] [Google Scholar]
- 20.Korff T, Augustin HG. Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. J Cell Sci. 1999;112:3249–58. doi: 10.1242/jcs.112.19.3249. [DOI] [PubMed] [Google Scholar]
- 21.Haspel HC, Scicli GM, McMahon G, et al. Inhibition of vascular endothelial growth factor-associated tyrosine kinase activity with SU5416 blocks sprouting in the microvascular endothelial cell spheroid model of angiogenesis. Microvasc Res. 2002;63:304–15. doi: 10.1006/mvre.2001.2383. [DOI] [PubMed] [Google Scholar]
- 22.Alajati A, Laib AM, Weber H, et al. Spheroid-based engineering of a human vasculature in mice. Nat Methods. 2008;5:439–45. doi: 10.1038/nmeth.1198. [DOI] [PubMed] [Google Scholar]
- 23.Orban JM, Marra KG, Hollinger JO. Composition options for tissue-engineered bone. Tissue Eng. 2002;8:529–39. doi: 10.1089/107632702760240454. [DOI] [PubMed] [Google Scholar]
- 24.Darland DC, D’Amore PA. TGF beta is required for the formation of capillary-like structures in three-dimensional cocultures of 10T1/2 and endothelial cells. Angiogenesis. 2001;4:11–20. doi: 10.1023/a:1016611824696. [DOI] [PubMed] [Google Scholar]
- 25.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 26.Jain RK. Molecular regulation of vessel maturation. Nat Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 27.Levenberg S, Rouwkema J, Macdonald M, et al. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 28.Rouwkema J, de Boer J, Van Blitterswijk CA. Endothelial cells assemble into a 3-dimensional prevascular network in a bone tissue engineering construct. Tissue Eng. 2006;12:2685–93. doi: 10.1089/ten.2006.12.2685. [DOI] [PubMed] [Google Scholar]
- 29.Gambini A, Mastantuono M, Di Giorgio L, et al. Rehabitation of allograft with bone dehydrated with solvents in reconstruction after removal of bone tumors: MRI evaluation. Chir Organi Mov. 1999;84:359–68. [PubMed] [Google Scholar]
- 30.Trentz OA, Hoerstrup SP, Sun LK, et al. Osteoblasts response to allogenic and xenogenic solvent dehydrated cancellous bone in vitro. Biomaterials. 2003;4:3417–26. doi: 10.1016/s0142-9612(03)00205-9. [DOI] [PubMed] [Google Scholar]
- 31.Kneser U, Stangenberg L, Ohnolz J, et al. Evaluation of processed bovine cancellous bone matrix seeded with syngenic osteoblasts in a critical size calvarial defect rat model. J Cell Mol Med. 2006;10:695–707. doi: 10.1111/j.1582-4934.2006.tb00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


