Abstract
This study was to investigate the effect of high glucose (HG) on vascular endothelial growth factor (VEGF) expression in bone marrow stem cells and JAK2/STAT-3 signalling. Adult rat bone marrow multipotent progenitor cells (rMAPCs) were cultured to evaluate VEGF expression (both mRNA and protein) with or without exposure to HG for up to 48 hrs using RT-PCR and ELISA. JAK2 and STAT3 phosphorylation in rMAPCs was analysed by Western blotting. With cells in normal media, VEGF mRNA level after 24 hrs of culture was significantly increased by 15 times over baseline (day 0) with detectable level of VEGF protein intracellularly using immunofluorescence staining. Although there was no measurable VEGF in the media after 24 hrs of culture, a significant amount of VEGF was detected in the media after 48 hrs of incubation. VEGF expression was associated with constitutive activation of JAK2 and STAT3 in rMAPCs. However, VEGF mRNA level was significantly reduced without detectable VEGF in the media when rMAPCs exposed to HG for 48 hrs. Tyrosine-phosphorylation of JAK2 and STAT3 and nuclear translocation of phosphorylated STAT3 were significantly decreased in the cells exposed to HG for 48 hrs. When JAK2 and STAT3 phosphorylation was blocked by the selective inhibitor AG490, VEGF mRNA level was significantly decreased in rMAPCs in normal media by 80% with no detectable VEGF in the media. VEGF expression was significantly suppressed in rMAPCs cultured in HG media that was further reduced by AG490. VEGF expression in rMAPCs is impaired by HG possibly through inhibition of JAK2/STAT3 signalling.
Keywords: high glucose, VEGF, JAK2/STAT3, MAPCs
Introduction
Diabetes mellitus (DM) is a major risk factor for the development of cardiovascular diseases that are commonly grouped into microvascular diseases (like retinopathy, nephropathy and neuropathy) and macrovascular disorders (including ischaemic heart disease, peripheral vascular and cerebrovascular diseases) [1–5]. However, the macrovascular complications are the major contributors to the morbidity and mortality in diabetes and are associated with high incidence of cardiovascular diseases including stroke [6], myocardial infarction [7] and diabetic foot ulceration [8]. Although the mechanisms for the development of diabetic macrovasculopathy are complex and remain poorly understood, one of the key components is considered to be endothelial dysfunction and impaired vascular repair and regeneration with inadequate collateral vascular formation [9, 10]. Improving endothelial function and angiogenesis could serve as a new important therapeutic strategy for diabetic macrovascular diseases.
Vascular endothelial growth factor (VEGF) plays a critical role in the function and structural integrity of endothelial cells and angiogenesis [11, 12]. Bone marrow mesenchymal stem cells and other progenitor cells are important sources of VEGF [13, 14]. Recently, bone marrow mesenchymal stem cells including multipotent adult progenitor cells (MAPCs) have been shown to promote vascular regeneration, enhance neovascularization and improve myocardial function in the setting of ischaemic injury at least partially through their paracrine/autocrine actions including secretion of VEGF after cellular transplantation [15, 16]. Signal transducer and activator of transcription 3 (STAT3) is a member of the STATs family and is expressed in a variety of normal cells (such as B cells, endothelial cells, cardiomyctes and bone marrow stem cells) and cancer cells (like M1 leukaemia cells) [17–19]. STAT3 is activated when it is phosphorylated at tyrosine 705 by janus-activated kinase (JAK) like JAK2, leading to the dimerization of STAT3 followed by translocation into nucleus and initiation of various biological responses [20, 21]. STAT3 signalling is important in the regulation of VEGF expression and function in vascular endothelial cells and cardiac myocytes as well as cancer cells [22]. On the other hand, existing evidence suggests that VEGF also promotes the activation of STAT3 in vascular endothelial cells [23, 24], indicating an interactive relationship between STAT3 and VEGF. Recently, it is reported that STAT3 signalling mediates VEGF production in mouse bone marrow stem cells [19]. VEGF expression in bone marrow mesenchymal stem cells is also reported to be enhanced when Akt is overexpressed in the cells [25].
Adult bone marrow multipotent progenitor cells (MAPCs) are a type of special cells that are isolated and cultured from postnatal human and rodent tissues including bone marrow, muscle and brain [26–28]. These cells have been well characterized and are able to differentiate into multiple cell lineages including endothelial cells and neurons [27, 28]. MAPCs have been shown to secret VEGF and significantly contribute to the angiogenesis and revascularization in the setting of ischaemia [29]. In the present study, experiments were designed to investigate the effects of high glucose (HG) on VEGF expression in bone marrow stem cells and JAK2/STAT3 signalling. Rat MAPCs (rMAPCs) were used as the source of bone marrow stem cells. We found for the first time that VEGF expression in rMAPCs was dramatically decreased by high level of D-glucose, but not by mannitol. The decreased VEGF expression was associated with significant suppression of JAK2/STAT3 phosphorylation in these cells. The selective inhibitor of JAK2/STAT3 signalling AG490 significantly suppressed JAK2 and STAT3 phosphorylation as well as VEGF expression. These data indicate that VEGF expression in rMAPCs is significantly impaired by HG very likely via inhibition of JAK2/STAT3 signalling pathway.
Materials and methods
Dulbecco’s Modified Eagle medium (DMEM) and streptomycin were purchased from Gibco BRL (Carlsbad, CA, USA). MCDB-201, 1× insulin-transferrin-selenium (ITS), 1× linoleic-acid-bovine-serum-albumin (LA-BSA), dexamethasone, ascorbic acid 3-phosphate, fibronectin (FN), mouse epidermal growth factor (EGF), D-glucose, mannitol and JAK2 inhibitor AG490 were obtained from Sigma Chemicals (St Louis, MO, USA). Human platelet-derived growth factor (h-PDGF) was from R&D Systems (Minneapolis, MN, USA). Foetal calf serum (FCS) was from Hyclone Laboratories (Logan, UT, USA). Mouse leukaemia inhibitory factor (LIF) was purchased from Chemicon (Temecula, CA, USA). Anti-phospho-specific (Tyr705) STAT3 monoclonal antibody, anti-phospho-specific (Tyr1007/1008) JAK2 monoclonal antibody, anti-phospho-specific (Ser473) Akt monoclonal antibody, anti-STAT3 monoclonal antibody, anti-JAK2 monoclonal antibody, anti-Akt monoclonal antibody, anti-rabbit horseradish peroxidase (HRP) antibody were from Cell Signaling Technology (Beverley, MA, USA), and anti-mouse horseradish peroxidase (HRP) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). TACS Annexin V-FITC Apoptosis Detection kit and ELISA Kit materials for VEGF measurement were from R&D systems (Minneapolis, MN, USA). Cell proliferation ELISA Brdu (colorimetric) kit was obtained from Roche Applied Science (Penzberg, Germany). Supersignal west pico chemiluminescent substrate kit and BCA protein assay kit were from Pierce (Rockford, IL, USA). Rat spleen total RNA was obtained from Ambion (Austin, TX, USA).
Cell culture
Rat MAPCs (rMAPCs) were isolated and cultured as previously described [28]. After isolation and purification, the cells were cultured in expansion media consisted of 60% DMEM and 40% MCDB-201 with 1× ITS, 1× LA-BSA, 10−9 M dexamethasone, 10−4 M ascorbic acid 3-phosphate, 100 units of penicillin, 100 units of streptomycin, 2% FCS, 10 ng/mL human PDGF, 10 ng/ml mouse EGF, and 1000 units/ml mouse LIF in FN-coated plates. The cells were kept at 37°C, 5% O2 and 95% N2 at a density of 2–4 × 102 cells/cm2 in 10-cm petrie dish, and split every 48 hrs. To determine the level of VEGF expression and JAK2/STAT3 phosphorylation, the cells were reseeded at 4.5 × 104 cells/cm2 in 24-well plate or 6-well plate without growth factors or LIF in the media. To investigate the effects of high level of D-glucose or AG490 on VEGF expression and JAK2/STAT3 signalling in rMAPC, D-glucose (15 and 30 mM, final concentration) or AG490 (50 μM, final) was added into the culture media. Of note, the concentration of D-glucose in normal culture media was 5.5 mM. Mannitol (24.5 mM) was used as a control to rule out any effect of hyperosmolarity (HO) on the cells. The cells and media were collected at 24 and 48 hrs after high-density culture for measurement of VEGF mRNA and protein, and analysis of total and phosphorylated JAK2, STAT3 and Akt as described below.
Apoptosis assay
The rate of cell apoptosis was determined by using the TACS Annexin V-FITC apoptosis detection kit according to the manufacturer’s protocol. The cells were harvested after 48 hrs of incubation and washed with cold phosphate-buffered saline (PBS) with 2% BSA. The rMAPCs were then collected by centrifugation and gently resuspended in the Annexin V incubation reagent containing 1 μl annexin V-FITC and 10 μl propidium iodide in a total volume of 100 μl with cell density of 1 × 105 cells/100 μl. The cells were then incubated in dark for 15 min. at room temperature, and mixed with 400 μl binding buffer. The preparations were analysed by flow cytometry within 1 hr for maximal signal. The cells were separated into three distinct populations: (i) viable cells that were negative for both annexin V and propidium iodide; (ii) apoptotic cells that were labelled only with annexin V-FITC and (iii) necrotic cells that were positive for both annexin V and propidium iodide.
Proliferation assay
BrdU incorporation ELISA immunoassay was performed to assess the proliferation of rMAPCs as per the manufacturer’s protocol. All the samples were prepared in quadruplet. OD was measured at 370 nm (reference wavelength 492 nm) using VERSAmax Microplate Reader (Molecular Devices, Sunnyvale, CA, USA).
Quantitative RT-PCR analysis for VEGF expression
VEGF gene expression in rMAPCs was assessed after 24 and 48 hrs of culture. Total RNA was extracted from rMAPCs with RNeasy Micro kit (Qiagen; Valencia, CA, USA) as per manufacturer’s instruction and quantified by spectrophotometry at 260 nm. Reverse transcription was performed using a TaqMan Reverse Transcription Reagents Kit (Applied Biosystems; Branchburg, NJ, USA), and the cDNA underwent 40 rounds of amplification (ABI PRISM 7900; Perkin Elmer/Applied Biosystems) with the following reaction conditions: 40 cycles of a two-step PCR (95°C for 15 sec., 60°C for 60 sec.) after initial denaturation (95°C for 10 min.) with 1 μl cDNA solution and 2× SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK). The primers used were: for VEGF: 5′-AAAAACGAAAGCGCAAGAAA-3′; 5′-TTTCTCCGCTCTGAACAAGG; and for GAPDH: 5′-TGCACCACCAACTGCTTAG-3′; 5′-GATGCAGGGATGATGTTC-3′. The mRNA levels were normalized using GAPDH as housekeep gene.
VEGF measurement using ELISA
The rMAPCs-conditioned media were collected and stored at –80°C until analysis. VEGF concentration in the rMAPCs-conditioned media was determined by enzyme-linked immunosorbent assay (ELISA) using a commercially available ELISA kit as per manufacturer’s instructions. All samples and standards were prepared in duplicate.
Western immunoblot analysis for Akt, JAK2 and STAT3 phosphorylation
Western immunoblot analysis was performed to determine the phosphorylated and total levels of JAK2, STAT3 and Akt proteins as described in detail previously [30]. Briefly, cell lysates (40 μg protein/lane) were loaded on a 10% gradient SDS-PAGE gel and transferred to a polyvinylidene difluoride membrane after electrophoresis. The membranes were incubated in 5% non-fat milk for 1 hr and then exposed to the primary antibodies (Abs, 1:1000) against phosphorate-JAK2 (Tyr1007/1008), JAK2, phosphorate-STAT3 (Tyr 705), STAT3, phosphorate-Akt (Ser 473) and Akt overnight at 4°C. The preparations were washed with TBST, and blocked in 5% milk for 1 hr, followed by incubation with horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse IgG secondary Ab (1:3000). The protein levels were determined using supersignal west pico chemiluminescent substrate kit. The films were developed using Kodak Biomax film by Kodak X-OMAT 2000A processor; and the band density was analysed using Scion Image software (Scion, Frederick, MD, USA).
Immunofluorescence staining
Intracellular VEGF and the nuclear translocation of phosphorylated STAT3 were analysed with immunofluorescence staining. Rat MAPCs were cultured in the media with normal glucose (NG), HG and HO (mannitol) for 24 hrs, then fixed with 2% paraformaldehyde for 10 min. followed by membrane permeabilization with 0.1% triton X-100 at room temperature for 10 min. Cells were washed three times with PBS, and blocked with 1.5% goat serum in PBS for 1 hr at room temperature. The preparations were then incubated with the primary Abs (1:100) in blocking solution overnight at 4°C. After washing three times with PBS, the cells were exposed to the second FITC-conjugated Ab (1:200) in blocking solution for 60 min. at room temperature in dark. The preparations were then examined under Nikon Eclipse TE 2000-S fluorescence microscope (Nikon Instruments, Melville, NY, USA).
Data analysis
All data were expressed as mean ± S.E. and analysed by paired Student’s t-test or one-way ANOVA using SPSS 13.0 software (SPSS, Chicago, IL, USA). The results were representative of four independent experiments. The difference was considered to be significant when P < 0.05.
Results
Effects of HG on VEGF expression in rMAPCs
We first evaluated the transcriptional expression of VEGF gene as reflected by mRNA level in rMAPCs with RT-PCR under normal culture condition. As shown in Fig. 1A, the transcriptional expression of VEGF was readily detectable in rMAPCs after 24 hrs of culture in normal media with a D-glucose concentration of 5.5 mM. The VEGF mRNA level was over 15 times (15.5 ± 1.7 versus 1, n= 4, P < 0.05) that of control (d0). When the cells were incubated with 30 mM D-glucose, VEGF mRNA level was significantly decreased by 49% to 7.9 ± 1.6 (n= 4, P < 0.01). There was no change in VEGF mRNA level in the cells when incubated in the media with 15 mM D-glucose (data not shown), suggesting that the suppression of VEGF expression by HG is dose-dependent. However, when the cells were cultured in high osmolarity media (24.5 mM manntiol + 5.5 mM D-glucose), no significant change in VEGF mRNA was observed in rMAPCs (18.4 ± 0.7, n= 4, P > 0.05 versus normal media), indicating that the decreased VEGF gene expression by HG in rMAPCs was independent of HO.
Figure 1.
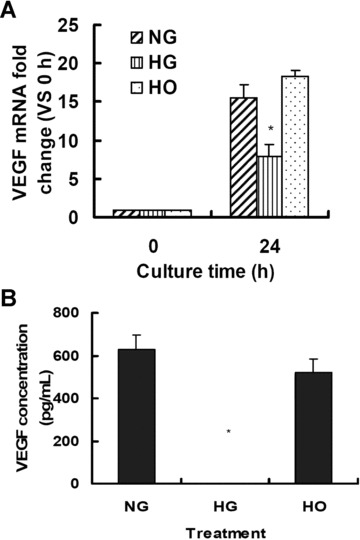
High glucose suppresses VEGF expression in rMAPCs independent of hyperosmolarity. (A) VEGF mRNA level was significantly decreased in rMAPCs when exposed to 30 mM D-glucose for 24 hrs as analysed using real-time PCR. (B) The amount of VEGF protein released in the rMAPCs-conditioned media was dramatically reduced to undetectable level as measured by ELISA after 48 hrs of incubation with 30 mM D-glucose. NG: rMAPCs were incubated in the media with 5.5 mM D-glucose (normal media); HG: rMAPCs were incubated in the media with 30 mM D-glucose; and HO: rMAPCs were incubated in the media with 24.5 mM mannitol (hyperosmalarity). *P < 0.01 compared with NG (5.5 mM D-glucose), n= 4.
VEGF protein released from rMAPCs was then determined in the conditioned media using ELISA. As shown in Fig. 1B, a significant amount of VEGF protein (627.7 ± 67.7 pg/ml, n= 4) was detected in the media with normal D-glucose level after 48 hrs of culture. No detectable VEGF was released in the media after 24 hrs of incubation (data not shown). However, detectable intracellular VEGF was present in rMAPCs after 24 hrs of culture with immunofluorescence staining (Fig. 2A). When rMAPCs were cultured in the media with 30 mM D-glucose, the VEGF production was dramatically decreased with a VEGF concentration below the detectable level by ELISA (P < 0.001) in the media after 48 hrs of incubation. The intracellular VEGF level was also significantly decreased on immunofluorescence staining in the cells exposed to HG media after 24 hrs of incubation (Fig. 2B). As expected, VEGF production in rMAPCs was not affected by mannitol with a VEGF concentration of 519.8 ± 64.3 pg/ml (n= 4, P > 0.05 versus normal media) in the conditioned media after 48 hrs of incubation. The presence of intracellular VEGF was also confirmed by immunofluorescence staining in the cells after 24 hrs of culture in the media with mannitol (Fig. 2C). These data suggested that suppression of VEGF production by high level of D-glucose was not secondary to HO.
Figure 2.
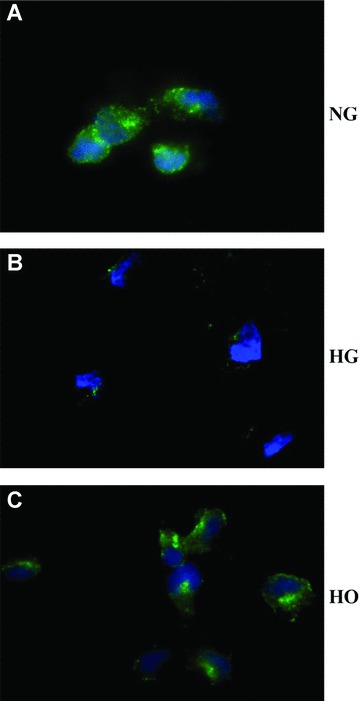
Effect of high glucose on intracellular production of VEGF in rMAPCs. Intracellular accumulation of VEGF in rMAPCs was significantly decreased by high glucose independent of hyperosmolarity as evaluated by immunofluorescence staining after 24 hrs of incubation with 30 mM D-glucose. NG: rMAPCs were incubated in the media with 5.5 mM D-glucose (normal); HG: rMAPCs were incubated in the media with 30 mM D-glucose; and HO: rMAPCs were incubated in the media with 24.5 mM mannitol.
Effects of HG on apoptosis and proliferationof rMAPCs
To rule out the possibility that suppression of VEGF production was due to a reduction of viable cell number in the setting of HG level, the effects of elevated D-glucose on cell apoptosis and proliferation of rMAPCs were evaluated. As shown in Fig. 3A, there was a low level of cell apoptosis (5.5 ± 0.4%, n= 4) when the cells were cultured in the normal expansion media with D-glucose concentration of 5.5 mM. The apoptosis level of rMAPCs was not changed when cultured in the media with D-glucose concentration of 30 mM for 48 hrs (5.3 ± 0.4%, n= 4, P > 0.05). The proliferation rate of rMAPCs was also not affected by 30 mM D-glucose or mannitol (Fig. 3B). However, when D-glucose concentration was increased to 50 mM or above, the proliferation rate of rMAPCs was significantly decreased as shown in Fig. 3C. These data indicate that HG or mannitol is not toxic to rMAPCs under our experimental conditions when their concentrations are below 50 mM, and that the reduction of VEGF production by HG is indeed not due to reduced cell proliferation or increased cell apoptosis.
Figure 3.
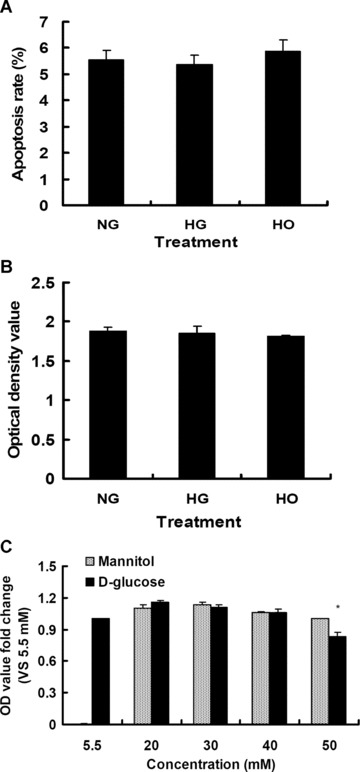
Effect of high glucose on the apoptosis and proliferation of rMAPCs. The rates of apoptosis (A) and proliferation (B) of rMAPCs were not affected by high glucose (30 mM D-glucose) or hyperosmolarity after 48 hrs incubation as evaluated by flow cytometry and BrdU colorimetric immunoassay, respectively. However, the cell proliferation rate was significantly decreased when the cells were incubated with 50 mM D-glucose (C). NG: rMAPCs were incubated in the media with 5.5 mM D-glucose; HG: rMAPCs were incubated in the media with 30 mM D-glucose; and HO: rMAPCs were incubated in the media with 24.5 mM mannitol.
Effects of HG on Akt phosphorylation in rMAPCs
To determine whether Akt phosphorylation in rMAPCs was affected by HG, the cells were cultured in the media with NG (5.5 mM) or HG (30 mM) or high osmolarity with mannitol (30 mM). Western immunoblot analysis demonstrated that the total and phosphorylated Akt levels in rMAPCs were the same for all three groups as shown in Fig. 4A and B, suggesting that Akt expression and activation were not affected by HG or HO.
Figure 4.
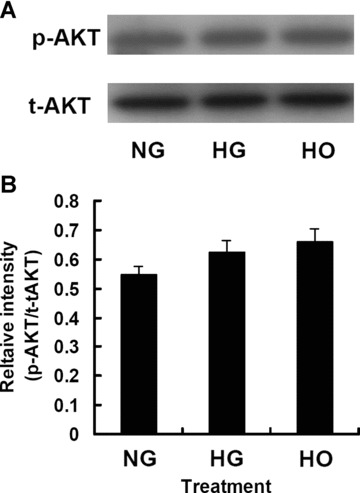
The expression and activation of Akt in rMAPCs were not altered by high glucose or hyperosmolarity after 48 hrs incubation as analysed by Western blot. (A) Representative Western blot for Akt (both total and phosphorylated). (B) Bar graph showing the relative band intensity of the tyrosine-phosphorylated Akt. Results represented the mean ± S.E. of 4 independent experiments. NG: rMAPCs were incubated in the media with 5.5 mM D-glucose; HG: rMAPCs were incubated in the media with 30 mM D-glucose; and HO: rMAPCs were incubated in the media with 24.5 mM mannitol.
Effects of HG on phosphorylationof JAK2 and STAT3 in rMAPCs
As shown in Fig. 5, constitutive activation of JAK2 (Tyr-1007/1008) and STAT3 (Tyr-705) was observed in rMAPCs cultured in normal media with D-glucose concentration of 5.5 mM for 24 hrs. The tyrosine phosphorylation of both JAK2 (Fig. 5A and C) and STAT3 (Fig. 5B and D) were dramatically inhibited in rMAPCs when cultured in the media with high D-glucose concentration of 30 mM by 70% and 68%, respectively (P < 0.05). No changes in the tyrosine phosphorylation of either JAK2 or STAT3 were observed in rMAPCs when exposed to mannitol, indicating that HG inhibits the activation of JAK2/STAT3 signalling pathway independent of HO.
Figure 5.
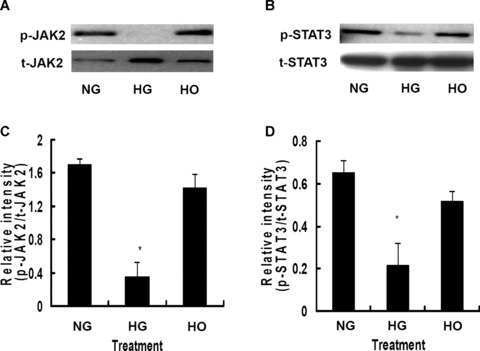
High glucose significantly inhibited the phosphorylation of JAK2 and STAT3 in rMAPCs after 24 hrs of incubation. (A) Tyrosine phosphorylation of JAK2 was dramatically suppressed by high glucose independent of hyperosmolarity as evaluated by Western blot analysis. The bar graph (C) showed the relative band intensity of phosphorylated JAK2. (B) Phosphorylation of STAT3 was markedly blocked by high glucose independent of hyperosmolarity as evaluated by Western blot analysis. The bar graph (D) showed the relative band intensity of phosphorylated STAT3. NG: rMAPCs were incubated in the media with 5.5 mM D-glucose; HG: rMAPCs were incubated in the media with 30 mM D-glucose; and HO: rMAPCs were incubated in the media with 24.5 mM mannitol. *P < 0.01 compared with NG (5.5 mM D-glucose), n= 4.
Effects of HG on nuclear translocation of phosphorylated STAT3 in rMAPCs
STAT3 phosphorylation at tyrosine 705 followed by nuclear translocation is required to initiate various biological responses [20, 21]. Rat MAPCs cultured in normal media with D-glucose concentration of 5.5 mM for 24 hrs showed the presence of significant amount of phosphorylated STAT3 (skyblue) in the cytoplasma and nuclei (blue) as shown in Fig. 6A, confirming that the phosphorylated STAT3 was successfully translocated into the nuclei. The level of phosphorylated STAT3 in the cytoplasma and nuclei was not altered in the cells when cultured in the media with mannitol as expected (Fig. 6C). However, the phosphorylated STAT3 protein content was dramatically decreased in the cytoplasma and nuclei of rMAPCs cultured in the media with high D-glucose of 30 mM (Fig. 6B), further confirming that STAT3 phosphorylation and its subsequent nuclear translocation were suppressed by HG.
Figure 6.
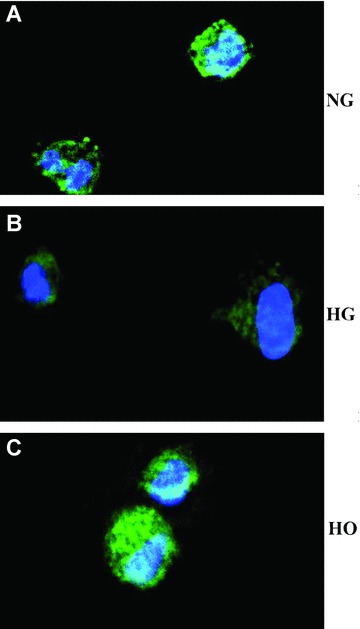
Effect of high glucose on nuclear translocation of phophorylated STAT3 in rMAPCs. Phosphorylated STAT3 was present in both cytoplasma and nuclei of rMAPCs after 24-hr culture in normal media as evaluated by immunofluorescence staining with antibody against phosphor-STAT3 (FITC, green) in combination with DAPI (blue) to stain the nuclei (A). The phosphorylated STAT3 in the cytoplasma and in the nuclei was significantly decreased in the cells after incubation in the media with 30 mM D-glucose (B). However, the phosphorylated STAT3 in the cytoplasma and in the nuclei was not affected in the cells cultured in the media with mannitol (C).
Effects of AG490 on JAK2/STAT3 signalling and VEGF expression in rMAPCs
To refine the relationship between VEGF expression and JAK2/STAT3 signalling pathway in rMAPCs, the cells were pretreated with the selective JAK2 phosphorylation inhibitor AG490 (50 mM) for 2 hrs. As expected, AG490 significantly inhibited STAT3 tyrosine phosphorylation in the cells cultured in normal media (5.5 mM D-glucose) by 80.9% (P < 0.01), and in the cells in the media with mannitol (24.5 mM) by 44.4% (P < 0.05) as shown in Fig. 7A and B. There was a significant decrease in STAT3 phosphorylation in the cells incubated in the media with HG (30 mM) by 57.1% compared to that in normal media (P < 0.05) (Fig. 7A and B). When pre-treated with AG490, there was a trend of further reduction in STAT3 phosphorylation in the cells cultured in HG media, but the change was not significant statistically (P > 0.05).
Figure 7.
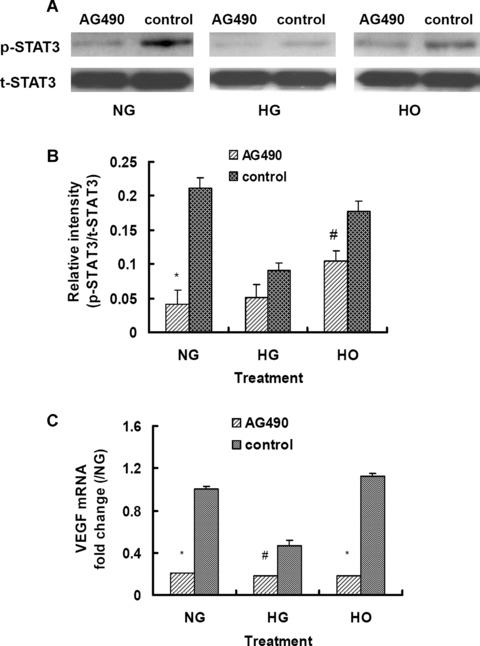
Effect of the specific JAK2 phosphorylation inhibitor AG490 on STAT3 phosphorylation and VEGF expression in rMAPCs. (A) Tyrosine phosphorylation of STAT3 was significantly decreased as evaluated by Western blot analysis in rMAPCs cultured either in normal or high glucose or mannitol media in the presence of AG490. (B) Bar graph showed the relative band intensity of phosphorylated STAT3. (C) The transcriptional expression of VEGF was significantly decreased in rMAPCs by AG490 in all three groups after 24 hrs of incubation as analysed using real-time PCR. *P < 0.01; #P < 0.05 compared with control (without AG490), n= 4. NG: rMAPCs were incubated in the media with 5.5 mM D-glucose; HG: rMAPCs were incubated in the media with 30 mM D-glucose; and HO: rMAPCs were incubated in the media with 24.5 mM mannitol. AG490: cells were treated with AG490 (50 uM).
In parallel to a decrease in STAT3 phosphorylation, pretreatment of the cells with AG490 significantly suppressed VEGF gene expression as indicated by a dramatic reduction in mRNA levels as demonstrated in Fig. 7C. The VEGF mRNA was significantly decreased by 67% and 87% in the cells cultured in normal media and in the media with mannitol in the presence of AG490 (P < 0.01), respectively. The VEGF gene expression was suppressed in the cells cultured in HG media (30 mM D-glucose) with 50% reduction in mRNA level (P < 0.05) as expected. The VEGF mRNA was further reduced significantly by 62% when the cells were pre-treated with AG490 (P < 0.05) as shown in Fig. 7C. No measurable VEGF protein was released in the conditioned media after 24 and 48 hrs of culture in the presence of AG490 (50 mM) using ELISA in all the cells cultured in normal media or with HG or mannitol (data not shown). These data strongly suggest that VEGF expression in rMAPCs is mediated via JAK2/STAT3 signalling pathway.
Discussion
Cardiovascular dysfunction is a major and yet challenging complication in the patients with DM with reduction in coronary collateral vessel formation and angiogenesis [31, 32]. The mechanisms are complex and still poorly defined. VEGF plays an essential role in the processes of angiogenesis and maintenance of endothelial integrity (both structurally and functionally) [11, 12]. In the present study, we demonstrated for the first time that HG suppressed VEGF expression and inhibited JAK2/STAT3 signalling pathway in rMAPCs.
VEGF is expressed in many different cell types including cardiomyocytes, fibroblasts, retinal percytes and bone marrow mesenchymal stem cells as well as human bone marrow-derived progenitor cells [13, 19, 33–35]. In the present study, we showed that rMAPCs produced a significant amount of VEGF in culture that was confirmed by both mRNA transcription and protein expression. These cells may represent an important source of VEGF especially in the setting of cellular therapy for ischaemic diseases when bone marrow stem cells are delivered in the ischaemic areas like myocardial infarction [14, 36]. This may provide a molecular explanation (at least in part) for the beneficial effects of bone marrow stem cell transplantation in experimental ischaemic animal models [15, 16, 37]. Indeed, detectable VEGF protein was present in the areas surrounding the injection sites with mouse MAPCs in a mouse ischaemic limb model [38]. The increased vascularity and improved left ventricular function after myocardial infarction was not due to differentiation and direct contribution of mouse MAPCs to the vascular or cardiomyocyte compartment after cell transplantation. Instead, the beneficial effects were largely contributed by secreting vascular growth factors like VEGF and TGF-b by the stem cells, resulting in increased angiogenesis and cardioprotection [39]. In addition to bone marrow, MAPCs were present in many tissues including fat tissue, muscle and brain [40]. The initial release of VEGF from the local ischaemic tissue (like muscle) may be important to tissue regeneration and repair since VEGF helps to recruit bone marrow-derived progenitor cells to ischaemic tissue [41]. Once the bone marrow progenitor cells arrive at the ischaemic area, they produce more VEGF, and in return more stem cells are attracted to the area. This positive reinforcement process will continue until the local ischaemia resolves.
The regulation of VEGF expression or production is complex and involves many factors, including (but not limited to) hypoxia, nitric oxide and TGF-b [26, 42, 43]. STAT3 is an important regulator for the expression of VEGF in cardiac myocytes, bone marrow mesenchymal stem cells and the majority of malignant cells [19, 44, 45]. Activation of STAT3 enhances the transcription of VEGF promoter in cancer cells [46], thus leading to an increased production of VEGF and promotion of endothelial cell proliferation, migration and vascular formation [44]. Interestingly, VEGF also stimulates tyrosine phosphorylation of STAT3 rapidly and induces its nuclear translocation in endothelial cells [23, 24]. Thus, bi-directional interactions are present between STAT3 and VEGF.
Plasma VEGF is elevated in DM patients in general [47]. However, the vascular responses to hyperglycemia in diabetic microvascular diseases and macrovascular disorders are totally opposite (increased neovascularization versus decreased vascular formation) [1–5]. This mystery is explained at least partially by the finding that VEGF and its receptors are differentially expressed in different organ systems in DM [35]. Thus, the microenvironment is the crucial determinant for the development of diabetic vasculopathy. One important finding in the present study is that HG significantly inhibits VEGF gene expression and production in rMAPCs. There is adequate evidence suggesting that bone marrow stem cells like MAPCs are important contributors to angiogenesis and vascular re-endothelialization after injury [26], and likely local production of VEGF. It has been reported that HG leads to deficient endothelial cell proliferation [48] and impaired survival [49], thus leading to reduced cell number. To rule out the possibility that the reduced VEGF production was due to reduced number of rMAPCs, we observed that the rates of cell apoptosis and proliferation of rMAPCs were not affected by D-glucose when its concentration was below 50 mM. Our data may serve as a molecular explanation for the development of macrovascular diseases in diabetic patients. These results may also have important clinical impact on the selection of patients for cell therapy with bone marrow stem cells especially diabetic patients with poor glucose control.
To investigate the role of STAT3 in the regulation of VEGF expression in rMAPCs, we monitored the status of activation of JAK2/STAT3 signalling pathway in these cells with or without HG. We observed that the expression of VEGF in rMAPCs was associated with phosphorylation of both JAK2 and STAT3 as well as nuclear translocation of phosphorylated STAT3. Suppression of VEGF expression in these cells by HG was associated with significant inhibition of both JAK2 and STAT3 phosphorylation and the nuclear translocation of phosphorylated STAT3, suggesting a close relationship between VEGF expression and STAT3 signalling in these cells. To further define the role of STAT3 signalling in VEGF expression in rMAPCs, the activation of JAK2/STAT3 was blocked with the selective inhibitor AG490. As expected, VEGF expression was significantly suppressed in the cells when the JAK2/STAT3 signalling pathway was inhibited by AG490 to a level that is comparable to that in the setting of HG exposure. These results support the rationale that HG suppresses VEGF expression in rMAPCs very likely through the blockage of JAK2/STAT3 signalling pathway. Our finding that JAK2/STAT3 signalling in rMAPCs is impaired by HG is consistent with a previous report that STAT3 signalling is defective in diabetic mice [50]. However, further studies using STAT3 overexpression are needed to further confirm the involvement of this pathway in VEGF expression in rMAPCs. It is also interesting to investigate the mechanism(s) involved in the regulatory control of JAK2/STAT3 signalling by HG in these cells in the future. Of note, the inhibitory effects of HG on JAK2 and STAT3 phosphorylation and VEGF expression in rMAPCs were achieved at a glucose concentration that was seen clinically in patients with DM. Another potential mechanism for the regulation of VEGF expression is PI3k/Akt signalling [51]. However, in our study no change in Akt phosphorylation was observed by HG in rMAPCs, suggesting that PI3K/Akt signalling is not essential for VEGF expression in rMAPCs.
In summary, our data showed that HG suppressed VEGF expression in rMAPCs in association with inhibition of JAK2/STAT3 signalling pathway. These results may offer an explanation for the development of macrovasculopathy in diabetes. Future in vivo studies are needed to investigate the effects of HG on bone marrow stem cells.
Acknowledgments
This work was supported by NIH K08 HL075410 (ZGL), HL63744 (JLZ), and Davis/Bremer Medical Research Grant (ZGL) at the Ohio State University Medical Center. The authors thank Mr. Dennis E Mathias for his expertise graphic assistance.
References
- 1.Cooper ME, Bonnet F, Oldfield M, et al. Mechanisms of diabetic vasculopathy: an overview. Am J Hypertens. 2001;14:475–86. doi: 10.1016/s0895-7061(00)01323-6. [DOI] [PubMed] [Google Scholar]
- 2.Cull CA, Jensen CC, Retnakaran R, et al. Impact of the metabolic syndrome on macrovascular and microvascular outcomes in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study 78. Circulation. 2007;116:2119–26. doi: 10.1161/CIRCULATIONAHA.107.733428. [DOI] [PubMed] [Google Scholar]
- 3.Kanwar YS, Wada J, Sun L, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med. 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 4.Rahman S, Rahman T, Ismail AA, et al. Diabetes-associated macrovasculopathy: pathophysiology and pathogenesis. Diabetes Obes Metab. 2007;9:767–80. doi: 10.1111/j.1463-1326.2006.00655.x. [DOI] [PubMed] [Google Scholar]
- 5.Asakawa H, Tokunaga K, Kawakami F. Comparison of risk factors of macrovascular complications. Peripheral vascular disease, cerebral vascular disease, and coronary heart disease in Japanese type 2 diabetes mellitus patients. J Diabetes Complications. 2000;14:307–13. doi: 10.1016/s1056-8727(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 6.Shah IM, Ghosh SK, Collier A. Stroke presentation in Type 2 diabetes and the metabolic syndrome. Diabetes Res Clin Pract. 2008;79:e1–4. doi: 10.1016/j.diabres.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Klein L, Gheorghiade M. Management of the patient with diabetes mellitus and myocardial infarction: clinical trials update. Am J Med. 2004;116:47S–63S. doi: 10.1016/j.amjmed.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 8.Tapp RJ, Shaw JE, de Courten MP, et al. Foot complications in Type 2 diabetes: an Australian population-based study. Diabet Med. 2003;20:105–13. doi: 10.1046/j.1464-5491.2003.00881.x. [DOI] [PubMed] [Google Scholar]
- 9.Guerci B, Böhme P, Kearney-Schwartz A, et al. Endothelial dysfunction and type 2 diabetes. Part 2: altered endothelial function and the effects of treatments in type 2 diabetes mellitus. Diabetes Metab. 2001;27:436–47. [PubMed] [Google Scholar]
- 10.Pănuş C, Moţa M, Vladu D, et al. The endothelial dysfunction in diabetes mellitus. Rom J Intern Med. 2003;41:27–33. [PubMed] [Google Scholar]
- 11.Bauer SM, Bauer RJ, Liu ZJ, et al. Vascular endothelial growth factor-C promotes vasculogenesis, angiogenesis, and collagen constriction in three-dimensional collagen gels. J Vasc Surg. 2005;41:699–707. doi: 10.1016/j.jvs.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Sakurai MK, Lee S, Arsenault DA, et al. Vascular endothelial growth factor accelerates compensatory lung growth after unilateral pneumonectomy. Am J Physiol Lung Cell Mol Physiol. 2007;292:L742–7. doi: 10.1152/ajplung.00064.2006. [DOI] [PubMed] [Google Scholar]
- 13.Wang M, Crisostomo PR, Herring C, et al. Human progenitor cells from bone marrow or adipose tissue produce VEGF, HGF, and IGF-I in response to TNF by a p38 MAPK-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2006;291:R880–4. doi: 10.1152/ajpregu.00280.2006. [DOI] [PubMed] [Google Scholar]
- 14.Guarita-Souza LC, Carvalho KA, Woitowicz V, et al. Simultaneous autologous transplantation of cocultured mesenchymal stem cells and skeletal myoblasts improves ventricular function in a murine model of Chagas disease. Circulation. 2006;114:I120–4. doi: 10.1161/CIRCULATIONAHA.105.000646. [DOI] [PubMed] [Google Scholar]
- 15.Tang YL, Zhao Q, Qin X, et al. Paracrine action enhances the effects of autologous mesenchymal stem cell transplantation on vascular regeneration in rat model of myocardial infarction. Ann Thorac Surg. 2005;80:229–36. doi: 10.1016/j.athoracsur.2005.02.072. [DOI] [PubMed] [Google Scholar]
- 16.Tse HF, Siu CW, Zhu SG, et al. Paracrine effects of direct intramyocardial implantation of bone marrow derived cells to enhance neovascularization in chronic ischaemic myocardium. Eur J Heart Fail. 2007;9:747–53. doi: 10.1016/j.ejheart.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 17.Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548–56. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 18.Calò V, Migliavacca M, Bazan V, et al. STAT proteins: from normal control of cellular events to tumorigenesis. J Cell Physiol. 2003;197:157–68. doi: 10.1002/jcp.10364. [DOI] [PubMed] [Google Scholar]
- 19.Wang M, Zhang W, Crisostomo P, et al. STAT3 mediates bone marrow mesenchymal stem cell VEGF production. J Mol Cell Cardiol. 2007;42:1009–15. doi: 10.1016/j.yjmcc.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker S, Groner B, Muller CW. Three-dimensional structure of the Stat3beta homodimer bound to DNA. Nature. 1998;394:145–51. doi: 10.1038/28101. [DOI] [PubMed] [Google Scholar]
- 21.Lamb P, Seidel HM, Haslam J, et al. STAT protein complexes activated by interferon-gamma and gp130 signaling molecules differ in their sequence preferences and transcriptional induction properties. Nucleic Acids Res. 1995;23:3283–9. doi: 10.1093/nar/23.16.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wincewicz A, Sulkowska M, Koda M, et al. STAT3, HIF-1alpha, EPO and EPOR – signaling proteins in human primary ductal breast cancers. Folia Histochem Cytobiol. 2007;45:81–6. [PubMed] [Google Scholar]
- 23.Bartoli M, Gu X, Tsai NT, et al. Vascular endothelial growth factor activates STAT proteins in aortic endothelial cells. J Biol Chem. 2000;275:33189–92. doi: 10.1074/jbc.C000318200. [DOI] [PubMed] [Google Scholar]
- 24.Bartoli M, Platt D, Lemtalsi T, et al. VEGF differentially activates STAT3 in microvascular endothelial cells. FASEB J. 2003;17:1562–4. doi: 10.1096/fj.02-1084fje. [DOI] [PubMed] [Google Scholar]
- 25.Gnecchi M, He H, Noiseux N, et al. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. FASEB J. 2006;20:661–9. doi: 10.1096/fj.05-5211com. [DOI] [PubMed] [Google Scholar]
- 26.Reyes M, Dudek A, Jahagirdar B, et al. Origin of endothelial progenitors in human postnatal bone marrow. J Clin Invest. 2002;109:337–46. doi: 10.1172/JCI14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 28.Breyer A, Estharabadi N, Oki M, et al. Multipotent adult progenitor cell isolation and culture procedures. Exp Hematol. 2006;34:1596–601. doi: 10.1016/j.exphem.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 29.Zeng L, Hu Q, Wang X, et al. Bioenergetic and functional consequences of bone marrow-derived multipotent progenitor cell transplantation in hearts with postinfarction left ventricular remodeling. Circulation. 2007;115:1866–75. doi: 10.1161/CIRCULATIONAHA.106.659730. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, Jiang Y, Hao H, et al. Endothelial nitric oxide synthase is dynamically expressed during bone marrow stem cell differentiation into endothelial cells. Am J Physiol Heart Circ Physiol. 2007;293:H1760–5. doi: 10.1152/ajpheart.01408.2006. [DOI] [PubMed] [Google Scholar]
- 31.Abaci A, Oğuzhan A, Kahraman S, et al. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–42. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 32.Rivard A, Silver M, Chen D, et al. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–63. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bian ZM, Elner SG, Elner VM. Thrombin-induced VEGF expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2007;48:2738–46. doi: 10.1167/iovs.06-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kvanta A. Expression and regulation of vascular endothelial growth factor in choroidal fibroblasts. Curr Eye Res. 1995;14:1015–20. doi: 10.3109/02713689508998523. [DOI] [PubMed] [Google Scholar]
- 35.Chou E, Suzuma I, Way KJ, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105:373–9. doi: 10.1161/hc0302.102143. [DOI] [PubMed] [Google Scholar]
- 36.Hou M, Yang KM, Zhang H, et al. Transplantation of mesenchymal stem cells from human bone marrow improves damaged heart function in rats. Int J Cardiol. 2007;115:220–8. doi: 10.1016/j.ijcard.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 37.Davani S, Marandin A, Mersin N, et al. Mesenchymal progenitor cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a rat cellular cardiomyoplasty model. Circulation. 2003;108:II253–8. doi: 10.1161/01.cir.0000089186.09692.fa. [DOI] [PubMed] [Google Scholar]
- 38.Aranguren XL, McCue JD, Hendrickx B, et al. Multipotent adult progenitor cells sustain function of ischemic limbs in mice. J Clin Invest. 2008;118:505–14. doi: 10.1172/JCI31153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pelacho B, Nakamura Y, Zhang J, et al. Multipotent adult progenitor cell transplantation increases vascularity and improves left ventricular function after myocardial infarction. J Tissue Eng Regen Med. 2007;1:51–9. doi: 10.1002/term.7. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Y, Vaessen B, Lenvik T, et al. Multipotent progenitor cells can be isolated from postnatal murine bone marrow, muscle, and brain. Exp Hematol. 2002;30:896–904. doi: 10.1016/s0301-472x(02)00869-x. [DOI] [PubMed] [Google Scholar]
- 41.Grunewald M, Avraham I, Dor Y, et al. VEGF-induced adult neovascularization: recruitment, retention, and role of accessory cells. Cell. 2006;124:175–89. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 42.Bian ZM, Elner SG, Elner VM. Regulation of VEGF mRNA expression and protein secretion by TGF-beta2 in human retinal pigment epithelial cells. Exp Eye Res. 2007;84:812–22. doi: 10.1016/j.exer.2006.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bussolati B, Dunk C, Grohman M, et al. Vascular endothelial growth factor receptor-1 modulates vascular endothelial growth factor-mediated angiogenesis via nitric oxide. Am J Pathol. 2001;159:993–1008. doi: 10.1016/S0002-9440(10)61775-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Niu G, Wright KL, Huang M, et al. Constitutive Stat3 activity up-regulates VEGF expression and tumor angiogenesis. Oncogene. 2002;21:2000–8. doi: 10.1038/sj.onc.1205260. [DOI] [PubMed] [Google Scholar]
- 45.Funamoto M, Fujio Y, Kunisada K, et al. Signal transducer and activator of transcription 3 is required for glycoprotein 130-mediated induction of vascular endothelial growth factor in cardiac myocytes. J Biol Chem. 2000;275:10561–6. doi: 10.1074/jbc.275.14.10561. [DOI] [PubMed] [Google Scholar]
- 46.Jung JE, Lee HG, Cho IH, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–8. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- 47.Kakizawa H, Itoh M, Itoh Y, et al. The relationship between glycemic control and plasma vascular endothelial growth factor and endothelin-1 concentration in diabetic patients. Metabolism. 2004;53:550–5. doi: 10.1016/j.metabol.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Varma S, Lal BK, Zheng R, et al. Hyperglycemia alters PI3k and Akt signaling and leads to endothelial cell proliferative dysfunction. Am J Physiol Heart Circ Physiol. 2005;289:H1744–51. doi: 10.1152/ajpheart.01088.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sheu ML, Ho FM, Yang RS, et al. High glucose induces human endothelial cell apoptosis through a phosphoinositide 3-kinase-regulated cyclooxygenase-2 pathway. Arterioscler Thromb Vasc Biol. 2005;25:539–45. doi: 10.1161/01.ATV.0000155462.24263.e4. [DOI] [PubMed] [Google Scholar]
- 50.Ghilardi N, Ziegler S, Wiestner A, et al. Defective STAT signaling by the leptin receptor in diabetic mice. Proc Natl Acad Sci USA. 1996;93:6231–5. doi: 10.1073/pnas.93.13.6231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jiang BH, Zheng JZ, Aoki M, et al. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–53. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]


