Abstract
Cyclooxygenase (COX) is a key enzyme in prostanoid synthesis. It exists in two isoforms, COX-1 and COX-2. COX-1 is referred to as a ‘constitutive isoform’, and is considered to be expressed in most tissues under basal conditions. In contrast, COX-2 is referred to as an ‘inducible isoform’, which is believed to be undetectable in most normal tissues, but can be up-regulated during various conditions, many of them pathological. Even though the role of COX in homeostasis and disease in now well appreciated, controversial information is available concerning the distribution of COX isoforms in normal human tissues. There is mounting evidence that it is much more complex than generally believed. Our aim was therefore to analyse the expression and distribution of COX isoforms in normal human tissues, using immunohistochemistry, Western blotting and real-time RT-PCR. Autopsy samples from 20 healthy trauma victims and samples from 48 biopsy surgical specimens were included. COX-1 was found in blood vessels, interstitial cells, smooth muscle cells, platelets and mesothelial cells. In contrast, COX-2 was found predominantly in the parenchymal cells of many tissues, with few exceptions, for example the heart. Our results confirm the hypothesis that the distribution of COX isoforms in healthy tissues is much more complex than generally believed. This and previous studies indicate that both isoforms, not only COX-1, are present in many normal human tissues, and that both isoforms, not only COX-2, are up-regulated in various pathological conditions. We may have to revise the concept of ‘constitutive’ and ‘inducible’ COX isoforms.
Keywords: cyclooxygenase, isoforms, constitutive, inducible, normal tissue, distribution
Introduction
Cyclooxygenase (COX), also known as prostaglandin H synthase or prostaglandin endoperoxide synthase (E.C.1.14.99.1) is the rate-limiting enzyme in the synthesis of prostanoids, potent bioactive lipid messengers with several important functions in physiology and disease. It was discovered in the early 1990s that it exists in at least two distinct isoforms, COX-1 and COX-2 [1]. The purpose of having two COX isoforms has been investigated ever since.
COX isoforms are encoded by two separate genes. The COX-1 gene exhibits the characteristics of a constitutively expressed, housekeeping gene. The COX-2 gene, on the other hand, has the characteristics of an immediate-early gene. Its expression was found to be induced in response to various pro-inflammatory factors, hormones, growth factors and oncogenes, and inhibited by glucocorticoids [1, 2]. Based on the gene expression style of COX isoforms, a distinction into a ‘constitutive isoform’– COX-1 and an ‘inducible isoform’– COX-2 was postulated. Interpretation of this distinction was generalized as the concept of ‘physiological’ and ‘pathological’ COX. COX-1 was considered a housekeeping enzyme, expressed in most tissues under basal conditions and responsible for the production of prostanoids with physiological, protective functions. In contrast, COX-2 was considered to be undetectable in most normal tissues, but up-regulated during various conditions, many of them pathological. This was the rationale for the development of COX-2 selective inhibitors, drugs which would be beneficial in the treatment of pain and inflammation without interfering with physiological processes [3, 4].
Based on this original postulation, the majority of studies during the last decade have been focused on the role of COX-2 in the pathogenesis of various diseases, such as cancer [2], atherosclerosis [5], Alzheimer’s disease and other neurological disorders [6], inflammatory and autoimmune diseases [7]. In contrast, the role of COX-1 in the pathogenesis of various diseases was neglected, as well as the role and distribution of COX isoforms in healthy tissues, particularly in human beings.
However, a growing body of evidence has been emerging, suggesting that the biology of COX isoforms is much more complex, and that the originally postulated division into ‘constitutive’ and ‘inducible’ COX is an oversimplification [8]. Some studies have shown that both isoforms, not only COX-1, are important in the maintenance of homeostasis, and that both isoforms, not only COX-2, are involved in various pathological conditions [8–14].
Even though the role of COX in homeostasis and disease in now well appreciated, scarce and controversial information is available concerning the distribution of COX isoforms in normal human tissues and organs. The aim of our study was, therefore, to analyse the expression and distribution of COX isoforms in presumably normal human tissues and organs, obtained at autopsies and biopsies, using immunohistochemistry, Western blotting and real-time RT-PCR.
Material and methods
Autopsy and biopsy samples
Our study included autopsy samples from various organs and tissues of 20 presumably healthy persons who died accidentally. There were 12 males and 8 females, aged 2 to 50 years. Cases were selected according to the following criteria: absence of disease in the case history, death occurred within 30 min., postmortem delay did not exceed 24 hrs, and there was no macroscopical or microscopical evidence of disease at autopsy.
Samples from 48 biopsy surgical specimens were also included. There were 28 males and 20 females, aged 20 to 70 years. Tissue samples distant from the site of grossly visible pathological processes were collected for further analyses.
Autopsy and biopsy samples to be analysed by immunohistochemistry were fixed in 10% buffered formalin and embedded in paraffin. For Western blotting, fresh tissue was obtained from autopsy and biopsy samples and either snap-frozen in liquid nitrogen and stored at –70°C or stored in RNAlater (Ambion, Austin, TX, USA). For real-time RT-PCR, fresh tissue was obtained from biopsies and stored in RNAlater according to the manufacturer’s instructions.
Prior to further analysis, haematoxylin and eosin slides from all biopsies and autopsies were examined by experienced pathologists to ensure that only samples that were histologically within the normal range were included in the study.
The study was approved by the State Ethics Committee.
Immunohistochemistry
Additional sections were cut at 4 μm from paraffin blocks and deparaffinization was carried out according to standard procedures. Antigen retrieval was optimized for each tested organ. Two antigen retrieval approaches were used. Slides were heated in a microwave oven at maximum power in either EDTA (0.1 M, pH 9) for 20 min. or in citrate buffer (DakoREAL™ Target Retrieval Solution (Dako, Glostrup, Denmark), diluted at 1:10, pH 6) for 10 min. The optimal antigen retrieval method was chosen on the basis of maximum staining intensity and minimum background staining. Immunohistochemical staining was performed in an automatic immunostainer (Nexes, Ventana, Tucson, AZ, USA), using rabbit polyclonal anti-COX-1 (Thermo Fisher Scientific, Fremont, CA, USA, diluted at 1:30) and rabbit monoclonal anti-COX-2 (SP-21, Lab Vision, diluted at 1:100) primary antibodies. Sections were treated with biotinylated secondary antibodies (Ventana) and incubated with peroxidase-conjugated streptavidin. Immunoreactivity was visualized with 3.3′-diaminobenzidine. Sections were counterstained with haematoxylin.
Colon and lung adenocarcinomas were used as positive controls for COX-2. For COX-1, we used vascular endothelial cells as an internal positive control, based on observations from our previous study that endothelial cells are invariably stained [14].
The specificity of the immunoreactions was tested on several levels as follows. Primary antibody was omitted from the automated staining procedure to assess the specificity of secondary antibodies. To assess the sufficiency of endogene biotin blockage, the biotin-streptavidin detection method was replaced with EnVision + Dual Link System–HRP (DAB+) (Dako), performed manually according to the manufacturer’s instructions. To test the specificity of anti-COX-2 primary antibodies, pre-adsorption with COX-2 blocking peptide (SP-21, Lab Vision) was performed.
Western blotting
Expression of COX-2 protein was analysed on autopsy and/or biopsy samples from the brain, lung, liver, hypophysis, thyroid and adrenal glands, spleen, kidney, heart, aorta and coronary artery, stomach, colon, adipose tissue, ovary, uterus, testis and prostate. Samples from five persons were taken for each tested organ.
Tissue samples were lysed in ice-cold RIPA buffer (1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS in 1× PBS, protease inhibitors (Sigma-Aldrich, Saint Louis, MO, USA); 1 mM 4-(2-Aminoethyl) benzenesulfonyl fluoride hydrochloride (AEBSF), 1 mM EDTA, 10 μM E-64, 10 μM Leupeptin hemisulphate, 40 μM bestatin hydrochloride, 1 μM pepstatin A, 770 nM Aprotinin) and mechanically homogenized. After 30 min. incubation on ice, samples were centrifuged at 10,000 × g (10 min., 4°C), the supernatants were collected and centrifuged again to collect supernatants. Protein concentrations in supernatants were evaluated spectrophotometrically by using BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA). Samples containing 30–50 μg of protein were mixed with SDS sample loading buffer and boiled. Proteins were electrophoresed through 10% polyacrilamide gels (Precise™ Protein Gels, Pierce) and transferred onto 0.45-μm nitrocellulose membranes (Pierce). Membranes were blocked overnight at 4°C with 5% non-fat dry milk (NFDM) in TTBS (0.05% Tween 20 in tris-buffered saline (TBS) (10 mM Tris-HCl; pH 7.4, 150 mM NaCl)). Goat policlonal anti-human COX-2 IgG primary antibodies (C-20, Santa Cruz Biotechnology, Santa Cruz, CA, USA), diluted at 1:150 in 5% NFDM in TTBS and HRP-conjugated anti-goat IgG secondary antibodies (Santa Cruz Biotechnology) diluted at 1:2000 were used for immunoblotting. Membranes were incubated with each antibody for 2 hrs at room temperature and subsequently rinsed with TTBS and TBS. Immunolabelling was visualized using Pierce ECL Western blotting substrate (Pierce) and either Hyperfilm™ ECL (GE Healthcare, Little Chalfont, UK) or CL-XPosure™ film (Pierce). RAW 264.7 + LPS/PMA cell lysate (Santa Cruz Biotechnology) and human adenocarcinoma cell lysate (Human cells-13, Lab Vision) were used as positive controls. Chemiluminescent Blue Ranger Marker Mix (Thermo Fisher) and Trail Mix Western Markers (S-protein AP conjugate) (Novagen, EMD Biosciences, San Diego, CA, USA) molecular weight markers were used for protein sizing.
Real-time RT-PCR
Real-time RT-PCR was performed to confirm the expression of COX-1 and COX-2 mRNAs, and to compare their tissue levels. Because an active postmortem transcription of several genes was reported in autopsy samples [15], we only analysed samples from tissues and organs, that were available fresh at biopsy (lung, liver, spleen, stomach, small intestine, colon, thyroid gland and adipose tissue).
Total RNA was extracted from the samples and purified using TRIzol Reagent with the PureLink™ Micro-to-Midi Total RNA Purification System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA purity and yields were evaluated spectrophotometrically and RNA integrity was determined using an Agilent RNA 6000 Nano Assay Kit on the Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Samples with RNA integrity number (RIN) values higher than 6.4 were set as acceptable. Mean RIN value of samples, included in our study was 8.13 and only two samples had RIN values under 7. Total RNA was reversely transcribed using TaqMan reverse transcription reagents (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s instructions. Incubation conditions for reverse transcription were: 10 min. at 25°C, 30 min. at 48°C and 5 min. at 95°C. COX-1 and COX-2 mRNA levels were analysed using quantitative real-time PCR based on the TaqMan fluorescence methodology. Commercially available probes were used for COX-1 (Hs00924803_m1) and COX-2 (Hs00153133_m1) gene expression assays (TaqMan Gene Expression Assays, Applied Biosystems). The 18S rRNA (Eukaryotic 18S rRNA, Applied Biosystems) was used as endogenous control for normalization. From several housekeeping genes analysed (BACT, GAPDH and rRNA), 18S rRNA exhibited the most stable expression levels across different tissue samples. RNA isolated from human lung carcinoma and treated as described above was used as positive control for COX-2 gene expression. Reactions were performed with a TaqMan Universal PCR Master Mix (Applied Biosystems). Quantification was performed on the ABI Prism 7900 sequence detection system (Applied Biosystems). Cycling conditions were: 2 min. at 50°C,10 min. at 95°C and 40 cycles of 15 sec at 95°C and 1 min. at 60°C. Each sample was analysed in triplicate and non-template controls were included. Amplification efficiencies for the target genes and 18S rRNA were determined as follows. Serial dilutions of a cDNA calibrator were amplified by real-time RT-PCR as already described. A plot of threshold cycle (Ct) versus log cDNA dilution was constructed for each target gene and for 18S rRNA. Amplification efficiencies (in percentage) were calculated from the slopes of the plots using the following equation: E= (101/-(slope)– 1) × 100%, where E represents amplification efficiency. Slopes and efficiencies were as follows: COX-1 gene; slope =–3.40 (E= 96.8%), COX-2 gene; slope =–3.41 (E= 96.4%) and 18S rRNA; slope =–3.38 (E= 97.6%). Because the efficiencies of the reactions were determined to be within 5% of each other, a variant of the Livak method for real-time PCR quantification was used [16].
The relative difference in expression of COX-2 and COX-1 within each sample was calculated using the following equation: N= 2ΔCtCOX1 –ΔCtCOX2, where Ct represents the threshold cycle for either COX-1 or COX-2 gene within each sample. N represents the expression ratio of COX-2 over COX-1 within a given sample. Three independent experiments were performed and means were calculated. Final results are given as mean expression ratios of COX-2 over COX-1 for each tested organ.
Statistical analysis
For statistical evaluation of the differences in average expression of COX-2 and COX-1, Student’s t-test was performed on the normalized data using SPSS ver.14 (SPSS, Inc., Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Results
Immunohistochemistry
Positive immunoreaction for both isoforms was seen as a brown cytoplasmic staining and sometimes staining of the nuclear envelope. There was no immunostaining if primary antibody was omitted or blocked with immunoreactive peptide.
Immunohistochemical results are summarized in Table 1, and some examples, representative of all tested samples, are shown in Figs 1–5. COX-1 expression was fairly constant and did not vary significantly between cases. It was found in blood vessels (constantly in endothelial cells and often in smooth muscle cells), platelets, scattered interstitial and supportive cells, resident inflammatory cells, smooth muscle cells and mesothelial cells of all tested organs (Figs. 1A–5A). COX-1 expression was observed in the parenchymal cells in a few organs, for example in the kidney (in collecting ducts), diffuse neuroendocrine system (Fig. 4A), reproductive system (Fig. 5A), and occasionally in endocrine glands and neurons.
Table 1.
Immunohistochemical expression of COX-1 and COX-2 in the normal human organs and tissues
| Organ/tissue | COX-1 | COX-2 |
|---|---|---|
| Central nervous system (n= 20) | Glia (mostly astrocytes) Rarely neurons Blood vessels | Neurons Rarely glia Occasionally blood vessels |
| Peripheral nervous system (n= 20) | Scattered cells in peripheral nerve sheets Sustentacular cells | Ganglion cells |
| Heart (n= 20) | Endocardium Blood vessels (endothelial and smooth muscle cells) Fibroblasts | Occasional cardiomyocytes (increasing with age) |
| Lung (n= 24) | Smooth muscle cells of bronchi and bronchioli Neuroendocrine cells of bronchial and bronchiolar mucosa Macrophages Blood vessels (endothelial and smooth muscle cells) Resident inflammatory cells Pleural mesothelium | Epithelial cells of bronchial and bronchiolar mucosa Macrophages Endothelial cells, mostly in large blood vessels Resident inflammatory cells Type II pneumocytes |
| Liver (n= 23) | Blood vessels and sinusoids Kupffer cells Resident inflammatory cells | Some hepatocytes Rarely epithelial cells of intra-hepatic bile ducts |
| Kidney (n= 20) | Collecting ducts Endothelial cells of blood vessels; pronounced in afferent arterioles at the glomerular entrance Interstitial cells Focally parietal epithelial cells of the Bowman’s capsule | Tubules in the cortex, mostly proximal Rare cells in glomeruli, probably podocytes Focally parietal epithelial cells of the Bowman’s capsule |
| Stomach (n= 15) | Glandular epithelium Majority of cells in lamina propria Smooth muscle cells Blood vessels | Surface and glandular epithelium Rare cells in lamina propria Ganglion cells |
| Colon (n= 10) | Endocrine cells in crypts Majority of cells in lamina propria Smooth muscle cells Blood vessels | Surface and crypt epithelium Rare cells in lamina propria Ganglion cells |
| Adrenal gland (n= 10) | Rare secretory cells in the cortex Blood vessels Cells in the interstitium (fibroblasts, macrophages) | Secretory cells in the cortex (variable) Few secretory cells in the medulla |
| Hypophysis (n= 20) | Some secretory cells in the adenohypophysis Pituicytes in the neurohypophysis Blood vessels | Some secretory cells in the adenohypophysis |
| Thyroid gland (n= 15) | Rare follicular epithelial cells Interstitial cells Blood vessels | Follicular epithelial cells |
| Pancreas (n= 15) | Rare cells in islets of Langerhans Extra-insular endocrine cells Interstitial cells Blood vessels | Some cells in islets of Langerhans Some acinar cells Epithelial cells of interlobular ducts |
| Spleen (n= 15) | Scattered cells in red and white pulp Blood vessels | Scattered cells in red and white pulp Occasionally blood vessels (only endothelial cells) |
| Uterus (n= 8) | Surface epithelium Endometrial glands (focally) Scattered cells in the endometrial stroma Scattered cells in the myometrium Endothelial cells of the blood vessels | Surface epithelium Endometrial glands (focally) Focally endothelial cells of blood vessels |
| Ovary (n= 8) | Surface epithelium Scattered stromal cells Scattered cells in theca of maturing and atretic follicles Corpus luteum Epithelium of rete ovarii | Oocytes Theca and granulosa cells of the periovulatory folicle Degenerating corpus luteum (focally) |
| Testis (n= 12) | Epithelial cells of rete testis Fibromyocytes in the lamina propria of seminiferous tubules Interstitial cells | Seminiferous tubules Rarely interstitial cells Epithelial cells of rete testis (focally) |
| Prostate (n= 12) | Some cells in the glandular epithelium (predominantly basal cells) Smooth muscle cells Interstitial cells (macrophages, fibroblasts) | Rare cells in the glandular epithelium (predominantly secretory cells) Focally smooth muscle cells |
| Miscellaneous | Platelets Adipose tissue Mesothelial cells Endothelial cells and smooth muscle cells in blood vessels | Adipose tissue (variable) Occasionally endothelial cells and smooth muscle cells in blood vessels (for example in the lung and brain) |
The number of analysed samples (n) is given in brackets.
Figure 1.
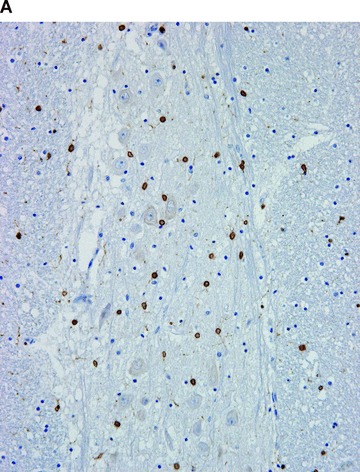
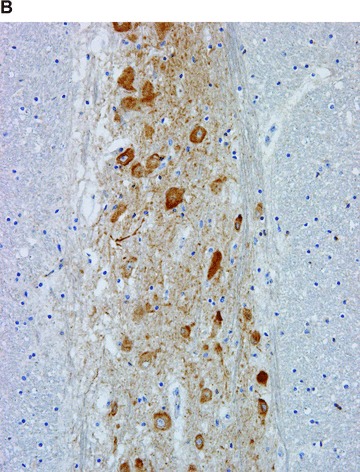
COX in the brain. Positive immunohistochemical reaction for COX-1 in the glial cells (A), and for COX-2 in the neurons (B).
Figure 5.
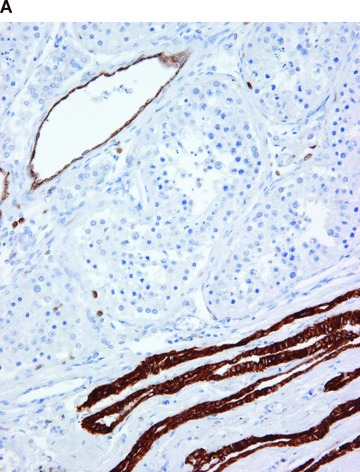
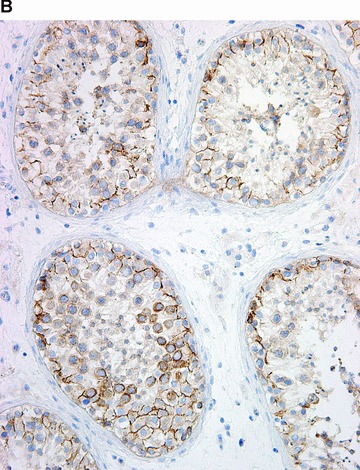
COX in the testis. Positive immunohistochemical reaction for COX-1 in blood vessels, in epithelium of rete testis, and in rare interstitial cells (A). Positive immunohistochemical reaction for COX-2 in the seminiferous tubules (B).
Figure 4.
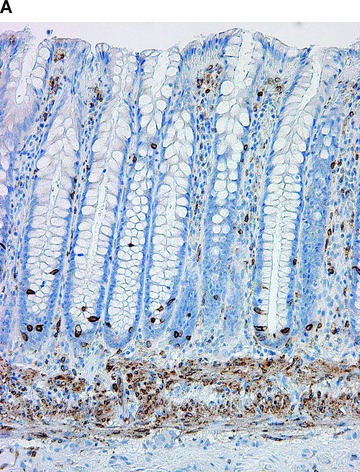
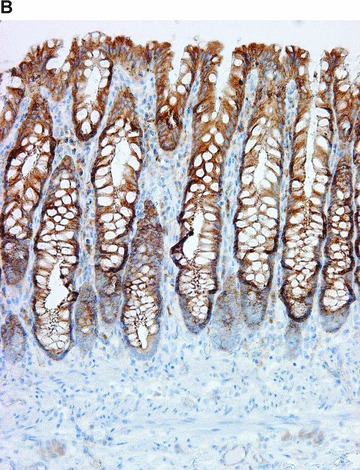
COX in the large bowel. Positive immunohistochemical reaction for COX-1 in the muscularis mucosae, in crypt endocrine cells, and cells in the lamina propria (A). Positive immunohistochemical reaction for COX-2 in the surface and crypt epithelium, and rare cells in lamina propria (B).
Figure 3.
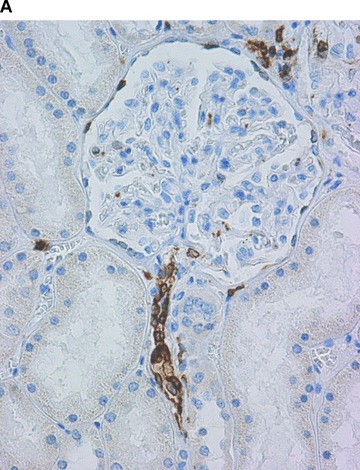
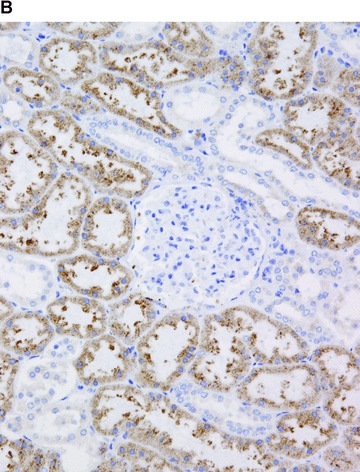
COX in the kidney. Positive immunohistochemical reaction for COX-1 in the terminal portion of afferent arterioles at the glomerular entrance, in interstitial cells and in the parietal epithelial cells of the Bowman’s capsule (A). Positive immunohistochemical reaction for COX-2 in proximal tubules (B).
In contrast, COX-2 was found predominantly in parenchymal cells (Figs. 1B–5B). There were few exceptions, for example the heart. In cardiomyocytes, COX-2 was not expressed in children and adolescents; solitary positives cardiomyocytes appeared after the age of 18 years, and progressively increased in number with age. Resident inflammatory cells, interstitial cells, endothelial cells, mucosal and vascular smooth muscle cells were focally stained in some organs (Figs. 2B and 4B). The intensity and extent of immunoreaction varied considerably between cases in some organs.
Figure 2.
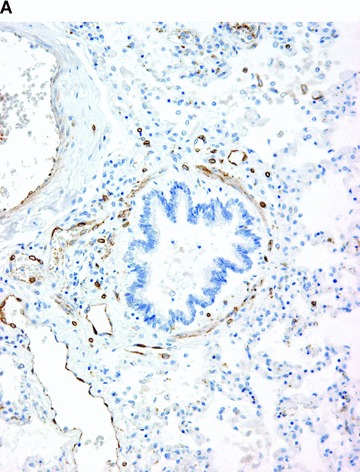
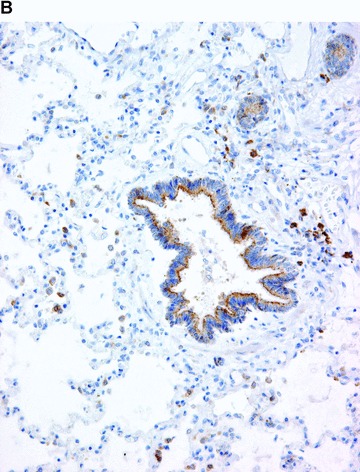
COX in the lung. Positive immunohistochemical reaction for COX-1 in blood vessels and in smooth muscle cells in the bronchiolar wall (A). Positive immunohistochemical reaction for COX-2 in epithelial cells of the bronchiolar wall, and in inflammatory cells (B).
Western blotting
Using Western blotting, we confirmed the expression of COX-2 protein in all organs and tissues that were shown to express COX-2 by immunohistochemistry. It was detected as protein bands of expected size, migrating between 72 and 74 kD. In many samples, clear double bands (at approximately 72 and 74 kD) were observed. Rarely, an additional band at approximately 70 kD was seen (for example in the testis). In the samples from the heart, aorta and coronary artery, scarce bands were only occasionally observed. Variable results were also found in the ovary, prostate and adipose tissue.
Results of Western blotting are presented in Fig. 6. The blots are representative of five out of five samples from the endocrine glands, liver, digestive tract, kidney, brain, lung, spleen, uterus and testis, four out of five samples from the prostate, three out of five samples from the ovary, adipose tissue and heart, two out of five samples from the coronary artery and one out of five samples from the aorta.
Figure 6.
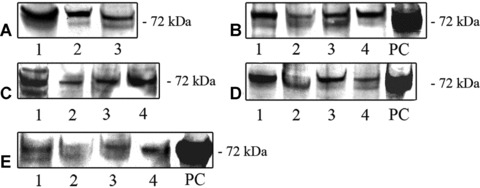
Expression of COX-2 protein in normal human organs and tissues, as detected with Western blot analysis. (A); COX-2 in the endocrine glands: adrenal cortex (1), thyroid gland (2), hypophysis (3). (B); COX-2 in the liver and digestive tract: liver (1), mucosa of the colon (2), mucosa of the stomach – antrum (3), mucosa of the stomach – corpus (4). (C); COX-2 in the reproductive organs: testis (1), prostate (2), uterus (3) and periovulatory-phase ovary (4). (D); COX-2 in the kidney cortex (1), brain (2), lung (3) and spleen (4). (E); COX-2 in the cardiovascular system, where it was only occasionally detected in samples from the heart (1), aorta (2) and coronary artery (3). COX-2 in the subepicardial adipose tissue (4). PC; positive control (RAW 264.7 + LPS/PMA cell lysate).
Real-time RT-PCR
Using real-time RT-PCR, we detected COX-1 and COX-2 mRNAs in all tested samples. Figure 7 represents relative differences in the expression of COX-1 and COX-2 genes at mRNA level. In some organs, for example in the stomach, colon and small intestine, expression of COX-1 mRNA was greater than the expression of COX-2, whereas in other organs, e.g. the lungs, thyroid gland, and spleen the expression of COX-2 mRNA was greater. In the liver, COX-1 and COX-2 mRNAs were equally expressed. The differences in the expression level of the two target genes were statistically significant for the stomach (antrum), spleen, lung and thyroid gland (Student’s t-test; P < 0.05).
Figure 7.
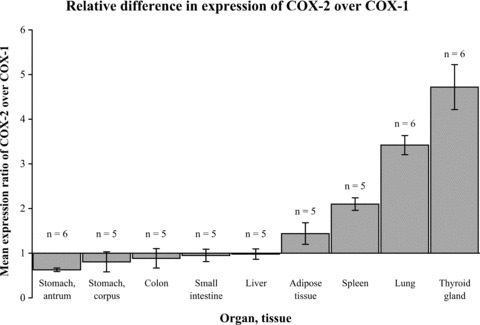
Mean expression ratio of COX-2 over COX-1 (N± S.D.) in normal human organs and tissues as detected by real-time RT-PCR. In some organs (stomach, colon and small intestine), the expression of COX-1 mRNA was greater than the expression of COX-2 (N < 1: expression of COX-1 is 1/N-fold greater). In other organs (lung, thyroid gland, spleen, adipose tissue), the expression of COX-2 mRNA was greater than the expression of COX-1 (N > 1: expression of COX-2 is N-fold greater). In the liver, COX-1 and COX-2 mRNAs were equally expressed (N= 1). The differences in the expression level of the two target genes were statistically significant in the stomach (antrum), spleen, lung and thyroid gland (P < 0.05).
DISCUSSION
Our results indicate that tissue distribution of the two COX isoforms under ‘normal’ conditions is more complex than generally believed. Not only COX-1, but also COX-2 was found to be expressed in many presumably normal human organs and tissues. However, the distribution patterns of the two isoforms were different. COX-1 expression was fairly constant and did not vary significantly among cases. It was found mostly in blood vessels, interstitial and supportive cells, smooth muscle cells, platelets and mesothelial cells, and only rarely in parenchymal cells. In contrast, COX-2 was found predominantly in parenchymal cells, and only occasionally in resident inflammatory cells, interstitial cells, endothelial cells and smooth muscle cells. In some organs, the intensity and extent of immunohistochemical reaction varied considerably between cases.
Despite the fact that COX-1 is widely quoted as a ubiquitously present COX isoform in most organs and tissues under physiological conditions, data in the literature regarding its exact tissue distribution is relatively scarce. The majority of published studies have analysed COX-1 distribution in solitary organs, using variable approaches for in situ protein detection [6, 14, 17–27], and/or methods which do not enable determining the distribution of COX-1, but only confirm the expression of either protein or mRNA in the tissue [9, 11, 28]. Koki et al.[29] did analyse various organs using immunohistochemistry, but reported only the presence of COX isoforms, without describing their exact distribution. Our study, in contrast, included various human organs and tissues, using immunohistochemistry, which enabled the analysis of COX-1 distribution in the tissue. The obtained results are exactly as one would expect for a predominantly ‘constitutive’ or ‘non-early gene’ encoded protein with a proposed role in tissue homeostasis. Its predominant expression in the interstitial and supportive cells might indicate its role in paracrine regulation of parenchymal cell functions. This has been already proposed, for example, for Kupffer cells in the liver [30]. COX-1 was also constantly expressed in blood vessels (in endothelial cells and frequently also in smooth muscle cells), which is in accordance with the suggested role of COX-1 in vascular homeostasis: control of the blood vessel tone, whereas the role of COX-1 in inhibition of thrombosis is still controversial [31]. An interesting finding in our study was fairly constant expression of COX-1 in the smooth muscle cells, for example in the bronchial and gastrointestinal mucosa; even though little is known about the role of mucosal smooth muscles in homeostasis, it has recently been suggested that they might be important in the regulation of absorptive and motor function [32].
In contrast to COX-1, it is generally believed that COX-2 is not present in most normal tissues, with the exception of the brain [6], kidney [18, 33], and female reproductive system [25, 34]. However, some previous studies have detected COX-2 in other normal tissues [9, 11, 17, 19–24, 26, 28, 35–42], but this isoften neglected. Furthermore, there has been no systematic immunohistochemical study on COX-2 distribution in healthy human tissues. Our results indeed confirmed that COX-2 is present in the parenchymal cells of many normal human organs and tissues. There were few exceptions, one of which was the heart [14]. In cardiomyocytes, we did not find COX-2 in children and young adults. However, solitary positive cardiomyocytes appeared after the age of 18 years and progressively increased in number with age. Similar findings have been described in some other studies, both in human beings [11] and experimental animals [43]. Some authors, in contrast, did not find any COX-2-positivity in normal human [44] or animal hearts [45]. The significance of COX-2 in the normal heart has not been elucidated. In rats, COX-2 has been found to increase with age, and this increase was associated with elevations in reactive oxygen species [43]. It has been suggested that COX-2 is the source of the reactive oxygen species which are known to be responsible for oxidative stress characteristic of aging processes [43].
It is widely quoted that COX-2 is the predominant isoform in the normal kidney, but the reported distribution of COX within the kidney is highly variable. We found COX-1 in blood vessels, including the afferent arteriole and collecting ducts, and COX-2 in the proximal tubules and occasionally in podocytes. Our results are similar to some previous reports [18, 46], but differ significantly from those described in some other studies [33, 47]. These variations in the kidneys, as well as in some other organs, can be ascribed to several factors, such as variability among species, different techniques, sources of investigated tissue and tissue sampling [48, 49].
There are important limitations in our study. Firstly, ‘normal’ human tissues can be obtained at autopsies and biopsies but it is questionable whether these tissues are ever entirely normal. There are several factors which might influence COX expression in ‘normal’ tissues. For example, active transcription of several genes has been described in autopsy tissue samples, potentially leading to synthesis of new proteins even after death [15]. Furthermore, human beings are exposed to carcinogens and other noxious agents (e.g. air pollutants, cigarette smoke, alcohol) which are known to affect COX levels [12, 50, 51]. COX levels in tissues might also be influenced by the action of COX inhibitors [52], which are among the most commonly used drugs, and no data was available about drugs taken by trauma victims. Similar limitations can be applied to morphologically normal tissue taken from biopsy specimens. Although we defined inclusion criteria to avoid the aforementioned limitations as much as possible, some of them could not be entirely avoided.
Secondly, we observed that COX-2 immunostaining depends to a certain extent on antigen-retrieval methods. In contrast, COX-1 staining patterns were fairly constant and did not seem to depend on antigen retrieval methods. To confirm the immunohistochemical results, we performed Western blotting and, on selected tissues, real-time RT-PCR. It should be noted, however, that COX-2 mRNA level is probably not always a good measure of COX-2 enzyme level, because COX-2 is highly regulated at the post-transcriptional level (mRNA stability) [53].
Thirdly, we performed no enzyme activity assays, so it cannot be determined whether COX detected in our study was actually active. Furthermore, with Western blotting, we detected two glycoforms of COX-2 in many of the tested samples (shown as 72 and 74 kD protein bands). Recent studies indicate that the activity of COX-2 can be regulated through glycosylation status and the74 kD isoform was shown to be inactive in terms of prostanoid synthesis [54]. The glycosylation status of COX-2 in normal human tissues and its physiological applications would be an interesting topic for further investigation.
Conclusions
Our results confirm the hypothesis that the distribution of COX isoforms in healthy tissues is much more complex than generally believed. This and previous studies indicate that both isoforms, and not only COX-1, are present in many normal human tissues, and that both isoforms, and not only COX-2, are up-regulated in various pathological conditions. We may have to revise the common interpretation of the concept of ‘constitutive’ and ‘inducible’ COX isoforms.
Acknowledgments
We thank Daniel Velkavrh for his excellent technical assistance, and Ema Bostjancic, Vid Mlakar and Mojca Strazisar for their help with Western blotting and PCR.
Part of the study was presented at 21st Congress of Pathology, Istanbul, Turkey, September 8–13, 2007.
References
- 1.Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004;56:387–437. doi: 10.1124/pr.56.3.3. [DOI] [PubMed] [Google Scholar]
- 2.Anderson WF, Umar A, Viner JL, et al. The role of cyclooxygenase inhibitors in cancer prevention. Curr Pharm Des. 2002;8:1035–62. doi: 10.2174/1381612023394935. [DOI] [PubMed] [Google Scholar]
- 3.Seibert K, Masferrer JL. Role of inducible cyclooxygenase (COX-2) in inflammation. Receptor. 1994;4:17–23. [PubMed] [Google Scholar]
- 4.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 5.Cippolone F, Fazia ML. COX-2 and atherosclerosis. J Cardiovasc Pharmacol. 2006;47:S26–36. doi: 10.1097/00005344-200605001-00006. [DOI] [PubMed] [Google Scholar]
- 6.Yermakova A, O’Banion MK. Cyclooxygenases in the central nervous system: implications for treatment of neurological disorders. Curr Pharm Des. 2000;6:1755–76. doi: 10.2174/1381612003398672. [DOI] [PubMed] [Google Scholar]
- 7.Martel-Pelletier J, Pelletier JP, Fahmi H. Cyclooxygenase-2 and prostaglandins in articular tissues. Semin Arthritis Rheum. 2003;33:155–67. doi: 10.1016/s0049-0172(03)00134-3. [DOI] [PubMed] [Google Scholar]
- 8.Smith WL, Garavito RM, De Witt DL. Prostaglandin-endoperoxide H synthases (cyclooxygenases)-1 and -2. J Biol Chem. 1996;271:33157–60. doi: 10.1074/jbc.271.52.33157. [DOI] [PubMed] [Google Scholar]
- 9.O’Neill GP, Ford-Hutchinson AW. Expression of mRNA for cyclooxygenase-1 and cyclooxygenase-2 in human tissues. FEBS Lett. 1993;330:156–60. doi: 10.1016/0014-5793(93)80263-t. [DOI] [PubMed] [Google Scholar]
- 10.Langenbach R, Loftin C, Lee C, et al. Cyclooxygenase knockout mice: models for elucidating isoform-specific functions. Biochem Pharmacol. 1999;58:1237–46. doi: 10.1016/s0006-2952(99)00158-6. [DOI] [PubMed] [Google Scholar]
- 11.Yasojima K, Schwab C, McGeer EG, et al. Distribution of cyclooxygenase-1 and cyclooxygenase-2 mRNAs and proteins in human brain and peripheral organs. Brain Res. 1999;830:226–36. doi: 10.1016/s0006-8993(99)01389-x. [DOI] [PubMed] [Google Scholar]
- 12.Rioux N, Castonguay A. The induction of cyclooxygenase-1 by a tobacco carcinogen in U937 human macrophages is correlated to the activation of NF-kappaB. Carcinogenesis. 2000;21:1745–51. doi: 10.1093/carcin/21.9.1745. [DOI] [PubMed] [Google Scholar]
- 13.Harris RC, Breyer MD. Update on cyclooxygenase-2 inhibitors. Clin J Am Soc Nephrol. 2006;1:236–45. doi: 10.2215/CJN.00890805. [DOI] [PubMed] [Google Scholar]
- 14.Zidar N, Dolenc-Strazar Z, Jeruc J, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the normal human heart and in myocardial infarction. Cardiovasc Pathol. 2007;16:300–4. doi: 10.1016/j.carpath.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Sanoudou D, Kang PB, Haslett JN, et al. Transcriptional profile of postmortem skeletal muscle. Physiol Genomics. 2004;16:222–8. doi: 10.1152/physiolgenomics.00137.2003. [DOI] [PubMed] [Google Scholar]
- 16.Livak KJ, Thomas DS. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 17.Sano H, Kawahito Y, Wilder RL, et al. Expression of cyclooxygenase-1 and -2 in human colorectal cancer. Cancer Research. 1995;55:3785–9. [PubMed] [Google Scholar]
- 18.Komhoff M, Grone HJ, Klein T, et al. Localization of cyclooxygenase-1 and -2 in adult and fetal human kidney: implication for renal function. Am J Physiol. 1997;272:F460–8. doi: 10.1152/ajprenal.1997.272.4.F460. [DOI] [PubMed] [Google Scholar]
- 19.Zimmermann KC, Sarbia M, Schror K, et al. Constitutive cyclooxygenase-2 expression in healthy human and rabbit gastric mucosa. Mol Pharmacol. 1998;54:536–40. doi: 10.1124/mol.54.3.536. [DOI] [PubMed] [Google Scholar]
- 20.Smith TJ, Jennings TA, Sciaky D, et al. Prostaglandin-endoperoxide synthase-2 expression in human thyroid epithelium. Evidence for constitutive expressionin vivo and in cultured KAT-50 cells. J Biol Chem. 1999;274:15622–32. doi: 10.1074/jbc.274.22.15622. [DOI] [PubMed] [Google Scholar]
- 21.Kirschenbaum A, Klausner AP, Lee R, et al. Expression of cyclooxygenase-1 and cyclooxygenase-2 in the human prostate. Urology. 2000;56:671–6. doi: 10.1016/s0090-4295(00)00674-9. [DOI] [PubMed] [Google Scholar]
- 22.Kirschenbaum A, Liotta DR, Yao S, et al. Immunohistochemical localization of cyclooxygenase-1 and cyclooxygenase-2 in the human fetal and adult male reproductive tracts. J Clin Endocrinol Metab. 2000;85:3436–41. doi: 10.1210/jcem.85.9.6780. [DOI] [PubMed] [Google Scholar]
- 23.Jackson LM, Wu KC, Mahida YR, et al. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut. 2000;47:762–70. doi: 10.1136/gut.47.6.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chariyalertsak S, Sirikulchayanonta V, Mayer D, et al. Aberrant cyclooxygenase isozyme expression in human intrahepatic cholangiocarcinoma. Gut. 2001;48:80–6. doi: 10.1136/gut.48.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stavreus-Evers A, Koraen L, Scott JE, et al. Distribution of cyclooxygenase-1, cyclooxygenase-2, and cytosolic phospholipase A2 in the luteal phase human endometrium and ovary. Fertil Steril. 2005;83:156–62. doi: 10.1016/j.fertnstert.2004.06.057. [DOI] [PubMed] [Google Scholar]
- 26.Fornai M, Blandizzi C, Colucci R, et al. Role of cyclooxygenase 1 and 2 in the modulation of neuromuscular functions in the distal colon of humans and mice. Gut. 2005;54:608–16. doi: 10.1136/gut.2004.053322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takano T, Tian GF, Peng W, et al. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9:260–7. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 28.Asano K, Lilly CM, Drazen JM. Prostaglandin G/H synthase-2 is the constitutive and dominant isoform in cultured human lung epithelial cells. Am J Physiol. 1996;271:L126–31. doi: 10.1152/ajplung.1996.271.1.L126. [DOI] [PubMed] [Google Scholar]
- 29.Koki A, Khan NK, Woerner M, et al. Cyclooxygenase-2 in human pathological disease. Adv Exp Med Biol. 2002;507:177–84. doi: 10.1007/978-1-4615-0193-0_28. [DOI] [PubMed] [Google Scholar]
- 30.Kuiper J, Zijlstra FJ, Kamps JA, et al. Cellular communication inside the liver. Binding, conversion and metabolic effect of prostaglandin D2 on parenchymal liver cells. Biochem J. 1989;262:195–201. doi: 10.1042/bj2620195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell JA, Lucas R, Vojnovic I, et al. Stronger inhibition by nonsteroid anti-inflammatory drugs of cyclooxygenase-1 in endothelial cells than platelets offers an explanation for increased risk of thrombotic events. FASEB J. 2006;20:2468–75. doi: 10.1096/fj.06-6615com. [DOI] [PubMed] [Google Scholar]
- 32.Uchida K, Kamikawa Y. Muscularis mucosae – the forgotten sibling. J Smooth Muscle Res. 2007;43:157–77. doi: 10.1540/jsmr.43.157. [DOI] [PubMed] [Google Scholar]
- 33.Harris RC, Breyer MD. Physiological regulation of cyclooxygenase-2 in the kidney. Am J Physiol Renal Physiol. 2001;281:F1–11. doi: 10.1152/ajprenal.2001.281.1.F1. [DOI] [PubMed] [Google Scholar]
- 34.Sirois J, Sayasith K, Brown KA, et al. Cyclooxygenase-2 and its role in ovulation: a 2004 account. Human Reproduction Update. 2006;17:373–85. doi: 10.1093/humupd/dmh032. [DOI] [PubMed] [Google Scholar]
- 35.Ermert L, Ermert M, Goppelt-Struebe M, et al. Cyclooxygenase isoenzyme localization and mRNA expression in rat lungs. Am J Respir Cell Mol Biol. 1998;18:479–88. doi: 10.1165/ajrcmb.18.4.2939. [DOI] [PubMed] [Google Scholar]
- 36.Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Research. 1998;58:3761–4. [PubMed] [Google Scholar]
- 37.McKanna JA, Zhang MZ, Wang JL, et al. Constitutive expression of cyclooxygenase-2 in rat vas deferens. Am J Physiol. 1998;275:R227–33. doi: 10.1152/ajpregu.1998.275.1.R227. [DOI] [PubMed] [Google Scholar]
- 38.Robertson RP. Dominance of cyclooxygenase-2 in the regulation of pancreatic islet prostaglandin synthesis. Diabetes. 1998;47:1379–83. doi: 10.2337/diabetes.47.9.1379. [DOI] [PubMed] [Google Scholar]
- 39.Salmenkivi K, Haglund C, Ristimaki A, et al. Increased expression of cyclooxygenase-2 in malignant pheochromocytomas. J Clin Endocrinol Metab. 2001;86:5615–9. doi: 10.1210/jcem.86.11.8052. [DOI] [PubMed] [Google Scholar]
- 40.Bloomer CW, Kenyon L, Hammond E, et al. Cyclooxygenase-2 (COX-2) and epidermal growth factor receptor (EGFR) expression in human pituitary macroadenomas. Am J Clin Oncol. 2003;26:S75–80. doi: 10.1097/01.COC.0000074163.69381.22. [DOI] [PubMed] [Google Scholar]
- 41.Bernardini N, Colucci R, Mattii L, et al. Constitutive expression of cyclooxygenase-2 in the neuromuscular compartment of normal human colon. Neurogastroenterol Motil. 2006;18:654–62. doi: 10.1111/j.1365-2982.2006.00795.x. [DOI] [PubMed] [Google Scholar]
- 42.Winnall WR, Ali U, O’Bryan MK, et al. Constitutive expression of prostaglandin-endoperoxide synthase 2 by somatic and spermatogenic cells is responsible for prostaglandin E2 production in the adult rat testis. Biol Reprod. 2007;76:759–68. doi: 10.1095/biolreprod.106.053124. [DOI] [PubMed] [Google Scholar]
- 43.Kim JW, Baek BS, Kim YK, et al. Gene expression of cyclooxygenase in the aging heart. J Gerontol A Biol Sci Med Sci. 2001;56:B350–5. doi: 10.1093/gerona/56.8.b350. [DOI] [PubMed] [Google Scholar]
- 44.Abbate A, Santini D, Biondi-Zoccai GG, et al. Cyclo-oxygenase-2 (COX-2) expression at the site of recent myocardial infarction: friend or foe. Heart. 2004;90:440–3. doi: 10.1136/hrt.2003.010280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaPointe MC, Mendez M, Leung A, et al. Inhibition of cyclooxygenase-2 improves cardiac function after myocardial infarction in the mouse. Am J Physiol Heart Circ Physiol. 2004;286:H1416–24. doi: 10.1152/ajpheart.00136.2003. [DOI] [PubMed] [Google Scholar]
- 46.Mungan MU, Gurel D, Canda AE, et al. Expression of COX-2 in normal and pyelonephritic kidney, renal intraepithelial neoplasia, and renal cell carcinoma. Eur Urol. 2006;50:92–7. doi: 10.1016/j.eururo.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 47.Sakurai M, Oishi K, Watanabe K. Localization of cyclooxygenases-1 and -2, and prostaglandin F synthase in human kidney and renal cell carcinoma. Biochem Biophys Res Commun. 2005;338:82–6. doi: 10.1016/j.bbrc.2005.08.194. [DOI] [PubMed] [Google Scholar]
- 48.Khan KN, Venturini CM, Bunch RT, et al. Interspecies differences in renal localization of cyclooxygenase isoforms: implications in nonsteroidal antiinflammatory drug-related nephrotoxicity. Toxicol Pathol. 1998;26:612–20. doi: 10.1177/019262339802600504. [DOI] [PubMed] [Google Scholar]
- 49.Garewal H, Ramsey L, Fass R, et al. Perils of immunohistochemistry: variability in staining specificity of commercially available COX-2 antibodies on human colon tissue. Dig Dis Sci. 2003;48:197–202. doi: 10.1023/a:1021871423154. [DOI] [PubMed] [Google Scholar]
- 50.Sarkar P, Hayes BE. Induction of COX-2 by acrolein in rat lung epithelial cells. Mol Cell Biochem. 2007;301:191–9. doi: 10.1007/s11010-007-9411-z. [DOI] [PubMed] [Google Scholar]
- 51.Knapp DJ, Crews FT. Induction of cyclooxygenase-2 in brain during acute and chronic ethanol treatment and ethanol withdrawal. Alcohol Clin Exp Res. 1999;23:633–43. [PubMed] [Google Scholar]
- 52.Hsueh SF, Lu CY, Chao CS, et al. Nonsteroidal anti-inflammatory drugs increase expression of inducible COX-2 isoform of cyclooxygenase in spinal cord of rats with adjuvant induced inflammation. Brain Res Mol Brain Res. 2004;125:113–9. doi: 10.1016/j.molbrainres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 53.Dixon DA, Kaplan CD, McIntyre TM, et al. Post-transcriptional control of cyclooxygenase-2 gene expression. The role of the 3’-untranslated region. J Biol Chem. 2000;275:11750–7. doi: 10.1074/jbc.275.16.11750. [DOI] [PubMed] [Google Scholar]
- 54.Sevigny MB, Li CF, Alas M, et al. Glycosylation regulates turnover of cyclooxygenase-2. FEBS Lett. 2006;580:6533–6. doi: 10.1016/j.febslet.2006.10.073. [DOI] [PubMed] [Google Scholar]


