Abstract
Diabetic nephropathy (DN) is a major cause of end-stage renal failure worldwide. Oxidative stress has been reported to be a major culprit of the disease and increased oxidized low density lipoprotein (oxLDL) immune complexes were found in patients with DN. In this study we present evidence, that CXCL16 is the main receptor in human podocytes mediating the uptake of oxLDL. In contrast, in primary tubular cells CD36 was mainly involved in the uptake of oxLDL. We further demonstrate that oxLDL down-regulated α3-integrin expression and increased the production of fibronectin in human podocytes. In addition, oxLDL uptake induced the production of reactive oxygen species (ROS) in human podocytes. Inhibition of oxLDL uptake by CXCL16 blocking antibodies abrogated the fibronectin and ROS production and restored α3 integrin expression in human podocytes. Furthermore we present evidence that hyperglycaemic conditions increased CXCL16 and reduced ADAM10 expression in podocytes. Importantly, in streptozotocin-induced diabetic mice an early induction of CXCL16 was accompanied by higher levels of oxLDL. Finally immunofluorescence analysis in biopsies of patients with DN revealed increased glomerular CXCL16 expression, which was paralleled by high levels of oxLDL. In summary, regulation of CXCL16, ADAM10 and oxLDL expression may be an early event in the onset of DN and therefore all three proteins may represent potential new targets for diagnosis and therapeutic intervention in DN.
Keywords: CXCL16, diabetic nephropathy, ADAM10, podocytes, primary tubular cells
Introduction
The development of diabetic nephropathy (DN) is the leading cause of end-stage renal disease and is clinically characterized by proteinuria and progressive renal insufficiency [1]. It is postulated that localized oxidative stress is a key component in the development of DN [2] and accumulating evidence suggests, that patients with diabetes mellitus have increased rates of lipoprotein oxidation products [3, 4]. The pathogenic role of oxidized low-density lipoprotein (oxLDL) in the kidney has been studied in vitro on a variety of renal cells, like mesangial cells [5–7], endothelial cells [8, 9] and podocytes [10]. Many important roles have been ascribed to oxLDL, which could be involved in the progression of renal diseases. It is well known that oxLDL can induce the production of chemokines and the expression of adhesion molecules on endothelial cells [11]. Furthermore, oxLDL can harm the kidney either directly, by deposition of lipids, or indirectly, by stimulating the generation of reactive oxygen species (ROS) [12, 13]. In addition to this in vitro data, several animal models have documented that chronic exposure to oxLDL promotes collagen synthesis and activates pro-inflammatory pathways [14, 15]. Moreover, oxLDL also promotes fibrosis by stimulating synthesis and expression of TGF-β[16].
Beside the important role of mesangial cells in the onset of DN [17], accumulating data demonstrate that podocytes are functionally and structurally injured very early in the natural history of DN [18]. Therefore, in order to improve the treatment of glomerular diseases like DN the identification of new proteins involved in podocyte injury are of high importance. The loss of podocytes, also known as podocytopenia, is an important characteristic feature in diabetic patients [19–22] and beside pro-inflammatory actions of oxLDL, the cytotoxic effects of oxLDL on podocytes has been recently shown [10]. Scavenger receptors are found on many cell lineages. They are known to bind modified lipoproteins and to promote the transformation of macrophages (MΦ) and smooth muscle cells into foam cells [23, 24]. However, little is known about the regulation and function of scavenger receptors in normal and pathological states of the kidney.
CXCL16 (SR-PSOX) is one of the few scavenger receptors that is found in two distinct forms: membrane bound and soluble. Surface-expressed CXCL16 binds and internalizes oxLDL and promotes adhesion of cells expressing its cognate receptor CXCR6 [25, 26]. In contrast soluble CXCL16 produced by proteolytic cleavage via ADAM10 and ADAM17 [27, 28], acts as a chemotactic factor for CXCR6 expressing cells such as NKT and polarized T helper cells [29, 30]. Importantly, in an animal model of chronic kidney disease elevated CXCL16 levels were accompanied with increased levels of oxLDL in the onset of renal obstruction [31]. We have recently described the expression of CXCL16 and ADAM10 in human podocytes and presented evidence that CXCL16 is involved in the uptake of oxLDL in human podocytes (Gutwein et al., unpublished results). In this study we investigated the functional consequences of CXCL16 mediated uptake of oxLDL in human podocytes. Additionally we characterized CXCL16 and oxLDL expression in kidney sections of streptozotocin- (STZ) induced diabetic mice. Furthermore CXCL16, ADAM10 and oxLDL expression was determined with immunofluorescence staining on biopsies of patients with DN.
Material and methods
Kidney sections
Specimens were taken from healthy parts of renal tissue from six different tumour nephrectomies (obtained from two female and four male patients with ages from 34 to 66 years), which were originally submitted for diagnostic purposes and studied in accordance with national and local ethical principles. The use of human tissue samples has been approved by the local ethics committee (Ref-No. 11/10/04). Tissue sections of four patients diagnosed with DN were obtained from the Department of Urology, Friedrich Alexander University of Erlangen-Nuremberg, Erlangen, Germany. Patients’ characteristics are depicted in Table 1. The investigations of human kidney biopsies were approved by the local ethics committee.
Table 1.
Clinicopathological data of patients analysed by immunofluorescense on kidney biopsies (dm = diabetes mellitus, y = years, S-crea = serum creatinin, m = male, f = female)
| Patient | Sex | Age | Duration of dm | Proteinuria (g/day) | S-crea | Hypertension |
|---|---|---|---|---|---|---|
| 1 | m | 60 | 4 y | 3 | 2.1 | Mild |
| 2 | f | 47 | Short | 5 | 3.4 | Mild |
| 3 | m | 68 | Short | 5 | 2.0 | No |
| 4 | m | 55 | Short | 4.57 | 3.5 | Mild |
Cell culture
Human conditionally immortalized podocytes were isolated and cultivated as previously described [32]. Cells were grown in flasks either at the permissive temperature of 32°C (in 5% CO2) to promote cell propagation as a cobblestone phenotype (undifferentiated) or at the non-permissive temperature of 37°C (in 5% CO2) to inactivate the SV40 T antigen and to allow the cells to differentiate. The growth medium was RPMI 1640 supplemented with non-essential amino acids, foetal bovine serum (10%), Hepes (10 mM) pH 7.4, penicillin (100 U/ml), streptomycin (100 μg/ml), transferrin (5 μg/ml) and sodium selenite (5 ng/ml). Prior to stimulation, cells were incubated for 16 hrs in RPMI 1640 medium, supplemented with 0.1 mg/ml of fatty acid-free bovine serum albumine.
Primary human renal cortical epithelial cells (HRCEpiC) were supplied from Provitro (Berlin, Germany) and cultured according to the manufacturer’s instructions. HRCEpiC were grown at 37°C and 5% CO2 and cultured in epithelial cell medium which consisted of 500 ml of basal medium, 10 ml of foetal bovine serum (2%), 5 ml of epithelial cell growth supplement and 5 ml of penicillin/streptomycin solution (media ingredients were purchased from provitro).
Cytokines, chemicals and antibodies
The blocking anti-human CXCL16 antibody, recombinant human CXCL16, recombinant human TGF-β and the ADAM10 antibody used for immunofluorescence staining were obtained from R&D Systems (Wiesbaden, Germany). Unconjugated rabbit CXCL16 antibody used for immunohistochemistry and immunofluorescence was from PeproTech EC, (London, UK). DiI-oxLDL and oxLDL were purchased from Intracel (Frederick, MD, USA). The blocking anti-CD36 antibody was from Abcam (Cambridge, UK). The monoclonal mouse ADAM10 antibody used in immunofluorescence analysis on tissue sections was from Diaclone (Cologne, Germany). Alexa Fluor 594 phalloidin was purchased from Molecular Probes (Karlsruhe, Germany). Polyclonal rabbit fibronectin antibody and monoclonal CD36 antibody used in immunofluorescense analysis was obtained from Santa Cruz (Heidelberg, Germany). The α3-integrin, ADAM10 and oxLDL antibody were from Chemicon (Hampshire, UK). The 5-(and-6)-chloromethyl-2′7′-dichlorodihydro-fluoroscein diacetate was obtained from Invitrogen (Karlsruhe, Germany).
siRNA
For down-regulation of endogenous ADAM10 expression in human podocytes the following duplex (MWG Biotech AG, Ebersberg, Germany) was used: ADAM10 construct, 5′-AGA CAU UAU GAA GGA UUA UTT-3′ As a negative control an unspecific scrambled siRNA duplex (5′-AGG UAG UGU AAU CGC CUU GTT-3′) was applied.
Protein extraction and western blotting
Cell extracts were prepared 48 hrs after siRNA transfection. PVDF membranes were incubated with antibodies against ADAM10 (Chemicon). Blots were developed using the ECL system (AmershamPharmacia, Buckingshamshire, UK). To confirm equal loading, blots were reprobed with a β-actin antibody (Sigma, Deisenhofen, Germany).
Real-time RT-PCR
One-step RT-PCR was performed with a Taqman (ABI 7000) from PerkinElmer-Cetus (Waltham, MA, USA). The mRNA levels for CXCL-16 and β-actin were determined by using a ‘hot start’ real-time PCR procedure with Quanti-Tec SYBR Green (QIAGEN). The following oligonucleotides were used as Taqman hybridization probes: CXCL-16 forward, 5′-ACGGAGTCTCGCTCTGTCAT-3′; CXCL-16 reverse, 5′-TGGTGAAACCCCGTCTCTAC-3′; β-actin forward, 5′-GGACTTCGAGCAAGAG-ATGG-3′ and β-actin reverse, 5′-AGCACTGTGTTGGCGTACAG-3′. Calculation of relative CXCL-16 mRNA levels was done by using the 2 [-ΔΔC(T)] method [33]. According to this method, the C(T) values of CXCL-16 mRNA level were normalized to the C(T) values of β-actin mRNA in the same sample.
ROS production assay
Human podocytes grown on cover slip were pre-incubated for 1 hr with or without CXCL16 (10 μg/ml) or CD36 (5 μg/ml) blocking antibodies before the cells were treated with oxLDL (100 μg/ml) for 16 hrs. Cells were then washed with Dulbecco’s PBS and incubated with media containing 5 mM 5-(and-6)-chloromethyl-2′7′-dichlorodihydrofluoroscein diacetate acetyl ester (CM-H2DCFDA) for 20 min. in the dark. Culture dishes were transferred to a digital microscope from Keyence (Neu-Isenburg, Germany) to document and analyse ROS generation.
CXCL16 shedding assays
Twenty-four hours before incubation with low glucose (LG) (5 mM) or high glucose (HG) (30 mM) containing media, podoctes were seeded at a density of 0.5 × 105 cells in six-well plates. Cells were washed with PBS and 1 ml of LG or HG containing media was added. The conditioned media were harvested at 24, 72 and 120 hrs and cleared by centrifugation. The presence of soluble CXCL16 in the conditioned media was analysed by ELISA (PeproTech) following the manufacturer′s instructions.
Immunofluorescence analysis of cells
Cells plated on cover slips were fixed with methanol/ 0.01% ethylenediaminetetraacetic acid (EDTA) for 20 min. at –20°C. After fixation, cells were blocked for 15 min. at room temperature with 0.1% Triton X-100/ PBS containing 5% bovine serum albumin (BSA), washed with PBS and incubated overnight with the primary antibody (diluted in 5% BSA/PBS/0.1% Triton X-100) as indicated. Following washing, bound antibodies were detected by Alexa 488 conjugated goat anti-rabbit (Molecular Probes) or goat antimouse Cy3 (Sigma) secondary antibodies. To stain the nuclei, tissue sections were incubated with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) and slides were mounted in Fluoromount G (Biozol, Eching, Germany). Evaluation was performed by fluorescence microscopy (Keyence).
FACS analysis
Human podocytes were left untreated or treated for 48 hrs with 100 μg/ml oxLDL. Cells were trypsinized and washed twice with PBS. To investigate α3-integrin expression, cells were incubated for 30 min. with the monoclonal antibody against α3-integrin (Chemicon) followed by FITC-conjugated antimouse secondary antibody. After washing twice in PBS, stained cells were analysed with an FACS cell analyser (Becton Dickinson, Heidelberg, Germany) using Cellquest Software (Becton Dickinson).
Immunohistochemistry
Paraffin tissue sections were deparaffinized in xylene, rehydrated through a graded ethanol series and washed in 10 mM phosphate-buffered 150 mM saline, pH 7.4. Antigen retrieval was performed by incubating the tissue sections for 20 min. in 0.01 M sodium citrate buffer, pH 6.0, in a microwave oven (500 W). Tissue sections were treated with 3% H2O2 in methanol for 30 min. at room temperature. After blocking the sections with PBS containing 10% horse serum, 1% BSA for 1 hr, the sections were incubated with an Avidin/Biotin Blocking Kit (Linaris, Wertheim, Germany) following the manufacturer protocol. The tissue sections were incubated overnight at 4°C with primary antibodies as indicated. After washing the slides, the Universal Quick Kit (Linaris) was used to stain the kidney sections. As a substrate the AEC Substrate Kit from Biozol was used to detect the immune complexes. Slides were counterstained with haematoxylin (Roth, Karlsruhe, Germany). The sections were inspected with a digital microscope from the company Keyence.
Immunofluorescence analysis on tissue sections
Paraffin tissue sections were deparaffinized in xylene, rehydrated through a graded ethanol series and washed in 10 mM phosphate-buffered 150 mM saline, pH 7.4. Antigen retrieval was performed by incubating the tissue sections for 20 min. in 0.01 M sodium citrate buffer, pH 6.0, in a microwave oven (500 W). After incubation with blocking buffer (0.1% Triton X-100/ PBS containing 1% BSA and 10% horse serum) for 1 hr, tissue sections were incubated with the first antibodies (diluted in 1% BSA/10% horse serum/PBS/0.1% Triton X-100) as indicated. Following washing, bound antibodies were detected by Alexa 488 conjugated goat antimouse (Molecular Probes) or goat anti-rabbit Cy3 (Molecular Probes) secondary antibodies. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) and slides were mounted in Fluoromount G (Biozol, Eching, Germany). Evaluation was performed by fluorescence microscopy (Keyence).
Uptake of DiI-labelled oxidized human LDL in podocytes and primary tubular cells
To analyse the uptake of DiI-labelled oxidized human LDL in human podocytes and primary tubular cells, cells were grown on cover slips and pre-incubated for 1 hr either with 10 μg/ml IgG isotype control antibody (R&D Systems), 10 μg/ml CXCL16 blocking antibody (R&D Systems), or 5 μg/ml CD36 antibody (Abcam). Afterwards, 10 μg/ml DiI-oxLDL was added for 4 hrs to the cells, then media were gently removed from cells and cells were washed three to four times with serum-free media. Subsequently, cells were fixed with methanol/ 0.01% EDTA for 20 min. at –20°C, nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma) and slides were mounted in Fluoromount G (Biozol) and analysed with fluorescence microscopy (Keyence).
Animals
Type 1 diabetes mellitus was induced in 2-month-old male C57BL/6 mice (Charles River Laboratories, Sulzfeld, Germany) by three consecutively daily intraperitoneal injections of streptozotocin (Sigma) (45 mg per kg of body weight) dissolved in 100 mmol/l sodium citrate buffer (pH 4.5). Control animals were injected only with citrate buffer. Mice that developed glucosuria received a subcutaneous insulin implant (Linplant) (Linshin, Ontario, Canada) to prevent ketoacidosis. Blood glucose concentrations were measured before the induction of diabetes and thereafter at 10-day intervals (Accu-Chek Sensor, Roche Diagnostics, Mannheim, Germany). Monitoring of diabetes was performed as described [34]. Kidneys (n= 5 per group) were harvested at day 5, 10, 17, 19, 30, 40 and 50 days after induction of diabetes. All animal experimentation was conducted in accordance with the German Animal Protection Act and had been approved by the Ethics Review Committee for laboratory animals of the District Government of Darmstadt, Germany.
Statistical analysis
Data were analysed by unpaired two-tailed t-test or one-way anova with Tukey- Kramer test for multiple comparisons using Graph Pad Instsat-2. A P-value of less than 0.05 was considered to show a significant difference between two groups.
Results
CXCL16 and CD36 function as scavenger receptors for oxLDL in human podocytes and primary tubular cells
Until now, very few data exist about the expression of oxLDL scavenger receptors in the kidney. Constitutive expression of CD36 [35] and CXCL16 [36] has been described in tubular epithelia of the human kidney. In addition, our group has recently described that CXCL16 is expressed in human podocytes (Gutwein et al., unpublished data). To analyse the receptors which mediate oxLDL uptake in podocytes and primary tubular cells, experiments with blocking antibodies were performed. The application of a CXCL16 specific blocking antibody strongly reduced the amount of DiI-oxLDL uptake in human podocytes (Fig. 1A and B). In addition, blocking CD36 receptor function reduced the uptake of DiI-oxLDL but to a much lesser extent (Fig. 1A and B). Blocking of both receptors (CXCL16 and CD36) inhibited the uptake of oxLDL in human podocytes to a similar extent than CXCL16 alone (Fig. 1A and B). In contrast to podocytes, the uptake of oxLDL in tubular cells was mainly mediated via CD36 and only partially involved CXCL16 (Fig. 1C and D). Again, combined blocking of CXCL16 and CD36 inhibited the uptake of oxLDL to a similar extent than CD36 alone (Fig. 1C and D). Weak expression of CD36 in human podocytes was determined by confocal immunofluorescence analysis (Fig. 1E). In contrast to CD36, stronger constitutive CXCL16 expression was detectable in human podocytes (Fig. 1F). Taken this data together, in podocytes CXCL16 mediates mainly the uptake of oxLDL, whereas in tubular cells CD36 seems to be the main receptor for oxLDL.
Figure 1.
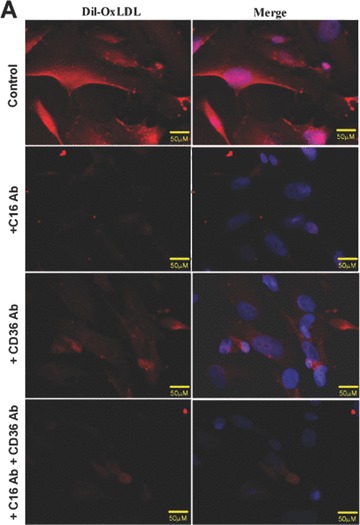
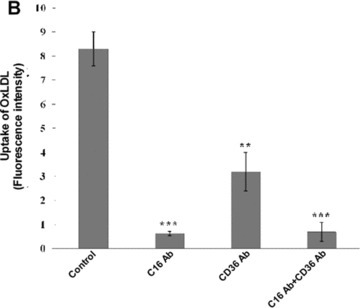
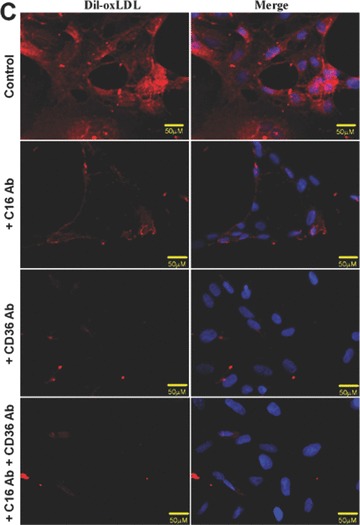
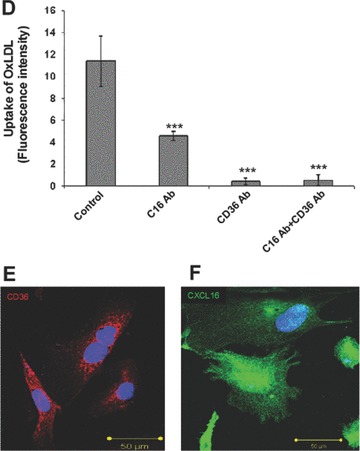
Involvement of CXCL16 and CD36 in the uptake of oxLDL. (A) DiI-oxLDL uptake was analysed after the pre-treatment of podocytes with an IgG control antibody (control), a CXCL16 blocking antibody (+C16 Ab), a CD36 blocking antibody (+CD36 Ab), or both antibodies (+C16 Ab + CD36 Ab). After pre-incubation of human podocytes with the blocking antibodies, cells were incubated for 4 hrs with 100 μg/ml DiI-oxLDL (red) and subsequently washed with PBS and fixed in methanol/0.02% EDTA. In addition, cells were stained with DAPI to visualize the nuclei (blue). (B) Semi-quantitative analysis of the fluorescence intensity of DiI-oxLDL in cells pre-treated with a control IgG antibody (control), a CXCL16 antibody (C16 Ab), a CD36 antibody (CD36Ab) or with a combination of both antibodies (C16AB+CD36Ab). Mean fluorescence of DiI-oxLDL was measured with an image software from Keyence. (C) DiI-oxLDL uptake was analysed after pre-incubation of the primary tubular cell line HRCEpiC with an IgG control antibody (control), a CXCL16 blocking antibody (+C16Ab), a CD36 blocking antibody (+CD36Ab) or the combination of both blocking antibodies (+C16Ab+CD36Ab) and visualized by immunofluorescence microscopy. After pre-incubation of HRCEpiC with the different blocking antibodies, cells were incubated for 4 hrs with 100 μg/ml DiI-oxLDL (red) and subsequently washed with PBS and fixed in methanol/0.02% EDTA. The cells were stained with DAPI to visualize the nuclei (blue). (D) Fluorescence intensity of DiI-oxLDL in HRCEpiC pre-treated with an IgG control antibody (control), a CXCL16 antibody (C16 Ab), a CD36 antibody (CD36Ab) or the combination of both antibodies (C16Ab+CD36Ab) was determined with the BZ-Analyzer software (Keyence). Data represent mean ± S.D. ***P < 0.001; **P < 0.01 considered statistically significant compared to the control. (E) Podocytes were fixed with 4% paraformaldehyde and CD36 expression was investigated using monoclonal CD36 and Cy3 coupled secondary antibodies (red). The cells were stained with DAPI to detect the nuclei (blue) of the cells. Fluorescence analyses were performed with a LSM 510 Meta confocal laser-scanning microscope (Carl Zeiss, Jena, Germany). (F) Podocytes were fixed with methanol and incubated with CXCL16 antibodies followed by Alexa 488 coupled secondary antibodies (green). Cells were than incubated with DAPI to visualize nuclei (blue). Fluorescence analyses were performed with a LSM 510 Meta confocal laser-scanning microscope (Carl Zeiss).
CXCL16 mediated uptake of oxLDL induced the production of ROS and fibronectin in podocytes and reduced the α3 integrin expression
It has been recently shown that alterations in the signalling cascades and the hence ensuing morphological changes in podocytes are mainly responsible for the development of proteinuria [37, 38]. To investigate the influence of oxLDL uptake on the morphology of human podocytes we analysed the expression of the cytoskeleton protein F-actin by phalloidin staining. As shown in Fig. 2A, oxLDL induced morphological changes of podocytes which was accompanied by the reduction of F-actin stress fibres. These effects were almost completely blocked by using a CXCL16 blocking antibody. Application of the CD36 antibody only partially reduced the effect of oxLDL on podocytes morphology (Fig. 2A). The combined treatment with CXCL16 and CD36 blocking antibodies had no additional influence on the F-actin expression in comparison to the CXCL16 blocking antibody alone (Fig. 2A). In addition, the treatment of human podocytes with TGF-β resulted in a strong reduction of F-actin stress fibers (Fig. 2A).
Figure 2.
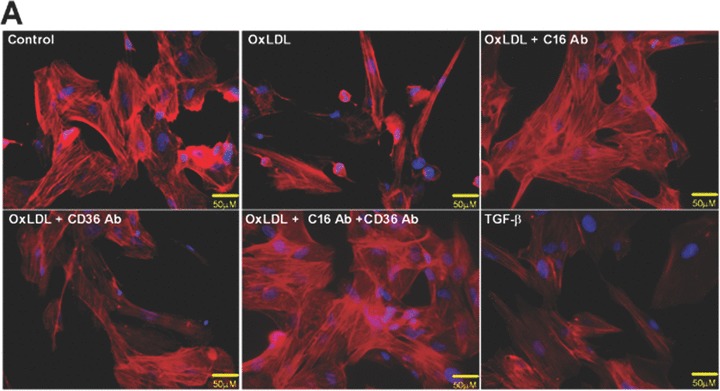
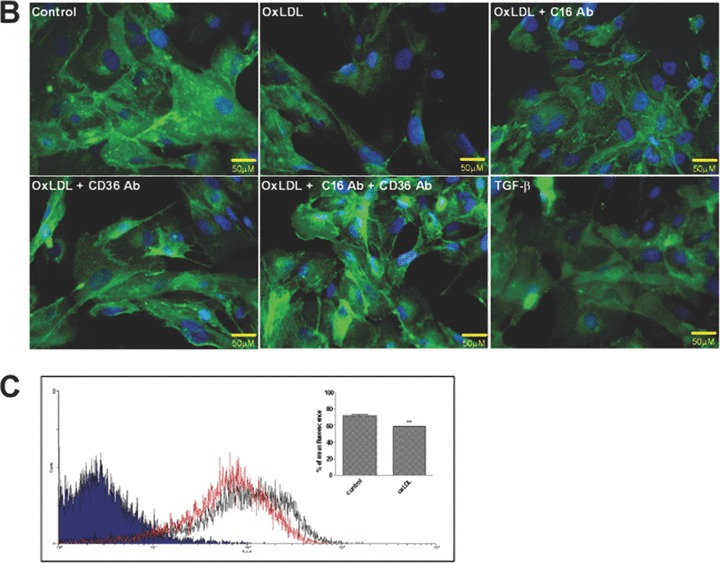
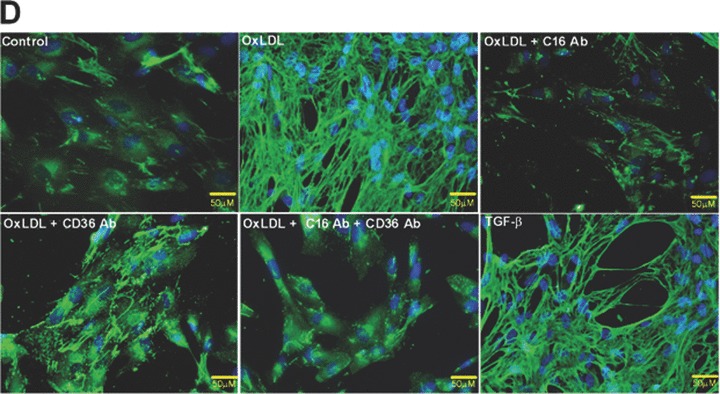
Role of CXCL16 and CD36 in the oxLDL-induced regulation of F-actin, α3-integrin and fibronectin expression in human podocytes. Immunofluorescence analyses of F-Actin stress fibers (A), α3-integrin (B) and fibronectin expression (D) in human podocytes 48 hrs after oxLDL (100 μg/ml) treatment in the presence of 10 μg/ml IgG control antibody (control/ oxLDL), 10 μg/ml CXCL16 blocking antibody (oxLDL+C16Ab), 5 μg/ml CD36 blocking antibody (oxLDL+CD36Ab) or the combination of both antibodies (oxLDL+C16Ab+CD36Ab). Treated human podocytes grown on cover slips were fixed with 4% paraformaldehyde/PBS solution and blocked with 1% bovine serum albumin/0.2 Triton-X-100/PBS. The cells were stained with DAPI to visualize the nuclei, with Alexa 594 conjugated Phalloidin to visualize the F-actin stress fibers (red fluorescence) and with an Alexa 488 coupled secondary antibodies to visualize α3-integrin (green) and fibronectin (green). (C) α3-integrin surface expression was investigated by FACS analysis 48 hrs after the treatment of podocytes with 100 μg/ml oxLDL. Black line shows α3-integrin expression of untreated podocytes, whereas the red line represents the surface expression of α3-integrin after oxLDL treatment. Mean fluorescence of α3-integrin expression of untreated (control) and oxLDL treated podocytes is depicted in a graph.
One main receptor attaching podocytes to the glomerular basement membrane is the integrin α3β1[39]. As documented in Fig. 2B application of oxLDL down-regulated α3-integrin expression. Importantly, blocking surface-expressed CXCL16 significantly restored the expression of α3-integrin (Fig. 2B). In addition the treatment with a CD36 specific blocking antibody led to a recovery of the α3-integrin expression. The most prominent influence on the α3-integrin expression was observed by the combined treatment with a CXCL16 and CD36 antibody (Fig. 2B). Similar to oxLDL treatment the application of TGF-β also reduced α3-integrin expression (Fig. 2B). To quantify the effect of oxLDL on α3-integrin expression, we performed FACS analysis of human podocytes 48 hrs after oxLDL treatment. OxLDL led to a 15% reduction of α3-integrin surface expression (Fig. 2C). In addition, confocal fluorescence analysis revealed, that oxLDL treatment reduced α3-integrin expression at focal contacts, which could be restored by CXCL16 blocking antibody treatment (data not shown).
It is known that increased production of extracellular matrix from podocytes is a hallmark in DN [40]. Therefore the effect of oxLDL mediated uptake on fibronectin production was investigated.
As demonstrated in Fig. 2D, oxLDL dramatically induced fibronectin production in human podocytes. Blocking the uptake of oxLDL with antibodies against CXCL16 or CD36 significantly reduced the fibronectin production. Notably, the strongest inhibitory effect was seen with CXCL16 blocking antibody and with the combined treatment of CXCL16 and CD36 antibodies (Fig. 2D). Again, TGF-β known to induce fibronectin expression was used as positive control.
Blocking of CXCL16 and CD36 reduced oxLDL-mediated ROS production
ROS have been identified as potential major contributors to the pathogenesis of diabetic kidney disease [41]. To investigate the role of oxLDL in ROS production in human podocytes, we analysed the fluorescence intensity of ROS sensitive 5-(and-6)-chloromethyl-2′7′-dichlorodihydro-fluorescein diacetate after oxLDL and TGF-β treatment. As shown in Fig. 3, oxLDL significantly induced ROS production in human podocytes. Importantly, ROS production was significantly inhibited by pre-incubation of podocytes with blocking antibody against CXCL16 (Fig. 3A and B). In addition the oxLDL-induced ROS production was also significantly inhibited by the blockage of CD36 (Fig. 3A and B). Furthermore, TGF-β strongly increased the amount of ROS in human podocytes (Fig. 3A and B).
Figure 3.
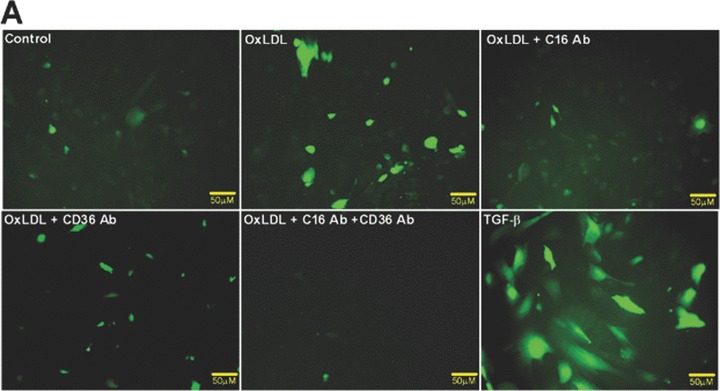
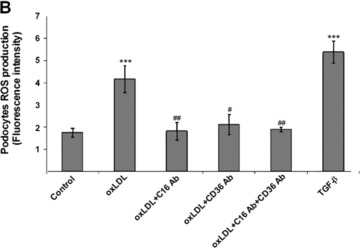
Blocking antibodies against CXCL16 and CD36 inhibit oxLDL-induced ROS production in human podocytes. (A) Human podocytes grown on cover slips were incubated with serum free media or with 100 μg/ml oxLDL (oxLDL) for 1 hr either in the presence of an IgG control antibody (control/oxLDL), a CXCL16 blocking antibody (10 μg/ml) (oxLDL+C16 Ab), a CD36 antibody (5 μg/ml) (oxLDL+CD36 Ab), a CXCL16 (10 μg/ml) and a CD36 (5 μg/ml) blocking antibody (oxLDL+C16 Ab+CD36 Ab) or stimulated with TGF-β (10 ng/ml). Cells were washed with Dulbecco’s PBS and incubated in the dark for 20 min. with 5 mM 5-(and-6)-chloromethyl-2′7′-dichlorodihydrofluorescein diacetate (green fluorescence). (B) Semi-quantitative analysis of fluorescence intensities of ROS production in human podocytes (control) treated with oxLDL in the presence of an IgG antibody (oxLDL), a CXCL16 blocking antibody (oxLDL+C16 Ab), a CD36 blocking antibody (oxLDL+CD36 Ab) or both blocking antibodies (oxLDL+C16 Ab+CD36 Ab), or treated with TGF-β using the BZ-Analyzer software (Keyence). Data represent mean ± S.D. ***P < 0.001 considered statistically significant compared to the control; ##P < 0.01, #P < 0.05 considered statistically significant compared to oxLDL/IgG control antibody treated cells.
Down-regulation of ADAM10 under hyperglycaemic conditions is accompanied by the induction of CXCL16 in human podocytes
Hyperglycaemia is an important determinant in the pathogenesis of diabetic microvascular complications in both type 1 and type 2 diabetes [42]. To analyse the influence of hyperglycaemia on the expression of CXCL16 and ADAM10, human podocytes were cultured in LG (=5 mM) or HG (=30 mM) containing media. As documented in Fig. 4A and B, HG treatment led to a significant induction of cellular CXCL16 in human podocytes 24 and 120 hrs after treatment. Importantly we also noticed a down-regulation of ADAM10 expression after HG treatment at both time-points (Fig. 4A, B and C). In addition, with a CXCL16 specific ELISA we confirmed that hyperglycaemic conditions increased cellular CXCL16 expression (Fig. 4D). To investigate if HG levels induce CXCL16 mRNA expression in podocytes, we performed real-time PCR. As shown in Fig. 4E, treatment of podocytes for 24 and 72 hrs with HG containing media significantly induced CXCL16 mRNA. Furthermore, we provide evidence that hyperglycaemic conditions reduces the release of CXCL16 from podocytes (Fig. 4F). To investigate, if the down-regulation of ADAM10 is involved in the reduced shedding of CXCL16 we performed siRNA experiments against ADAM10. As documented in Fig. 4G, blocking ADAM10 protein synthesis increased cellular CXCL16 and reduced the constitutive cleavage of CXCL16 (Fig. 4H).
Figure 4.
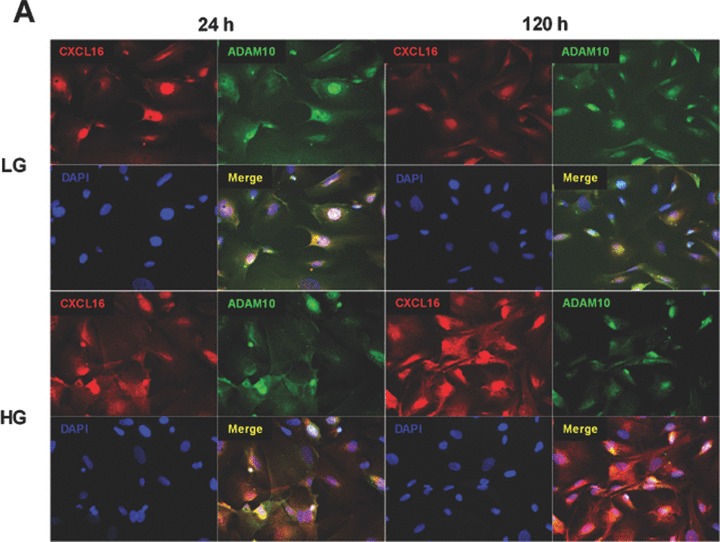
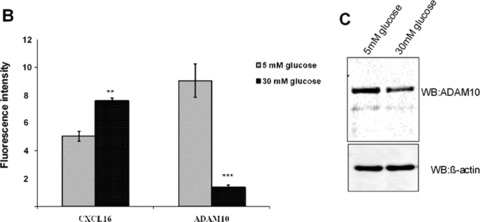
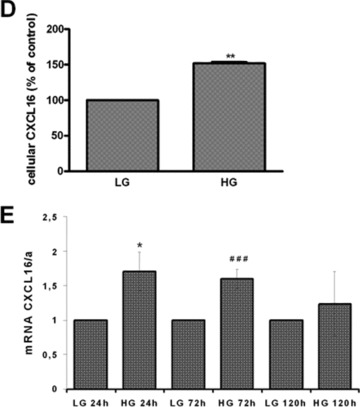
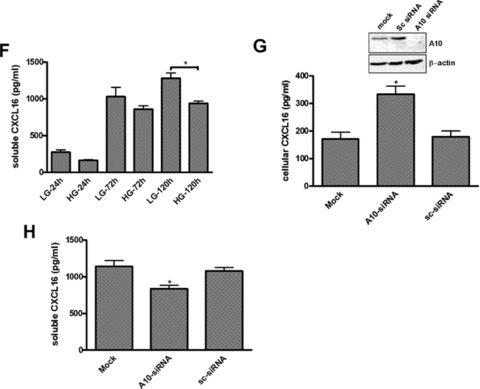
Effect of HG on cellular CXCL16 and ADAM10 expression in human podocytes. (A) Immunofluorescence staining of CXCL16 and ADAM10 expression in human podocytes treated for 24 or 120 hrs with LG (5 mM) or HG (30 mM) containing media. The cells were stained with DAPI to visualize nuclei (blue), and with Alexa Fluor 488 and Cy3 coupled secondary antibodies to visualize the distribution of ADAM10 (green) and CXCL16 (red) proteins. (B) Immunofluorescence results of CXCL16 and ADAM10 expression after 24 hrs glucose treatment using the BZ-Analyzer software (Keyence). Data represent mean ± S.D. ***P < 0.001, **P < 0.01 considered statistically significant compared to cells treated with LG concentration. (C) Podocytes were incubated 72 hrs with 5 or 30 mM glucose containing media. Cells were than lysed and ADAM10 expression was determined by Western blot analysis. β-actin was used to demonstrate equal sample loading. (D) CXCL16 protein expression was measured with a CXCL16 specific ELISA 24 hrs after the treatment of podocytes with 5 mM glucose or 30 mM glucose. Data represent mean ± S.D. ***P < 0.001, **P < 0.01 considered statistically significant compared to cells treated with LG containing media. (E) mRNA from podocytes was isolated at times indicated, transcribed in the cDNA as described in material and methods and real-time PCR was performed. (F) Release of CXCL16 was measured in podocytes incubated for 24, 72 or 120 hrs with LG (5 mM) or HG (30 mM) containing media. Supernatants were prepared as described in material and methods and soluble CXCL16 was measured by CXCL16 specific ELISA. (G-I) ADAM10 expression was down-regulated in human podocytes by ADAM10 specific siRNA (controlled by ADAM10 western blot, depicted above the graph) and cellular (G) and soluble CXCL16 (I) was measured by a CXCL16 specific ELISA. A10 = ADAM10, sc = scrambled.
CXCL16 and oxLDL expression is induced in podocytes and tubular cells of streptozotocin treated mice
Decreased number in podocytes, broadening of the foot processes, and reduction of nephrin expression has been reported in animal models of type 1 diabetes [43, 44]. To investigate the expression of CXCL16 in a STZ-induced mouse model of type 1 diabetes, we performed immunohistochemical analysis of CXCL16 in kidney sections of different time-points after induction. As shown in Fig. 5A and B, 17 days after STZ treatment, CXCL16 expression was significantly induced in podocytes and tubular cells. The glomerular CXCL16 expression increased over time and showed the strongest induction 50 days after STZ injection (Fig. 5A). In addition, immunohistochemical analysis of oxLDL in mouse kidney sections revealed that oxLDL expression was also induced in STZ-treated mice (Fig. 5C). Notably, no oxLDL expression could be seen in kidneys of control mice (Fig. 5C).
Figure 5.
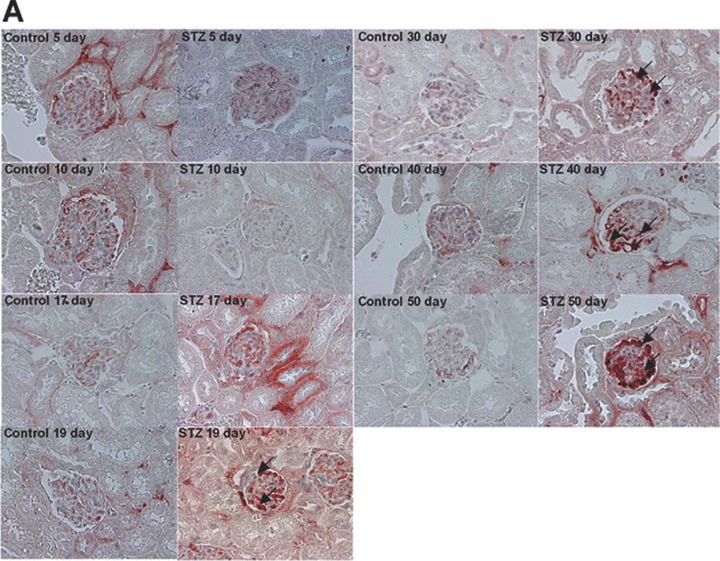
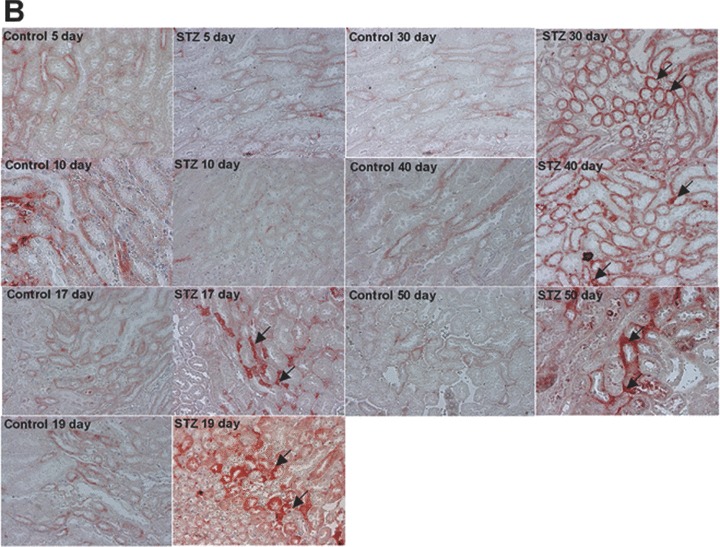
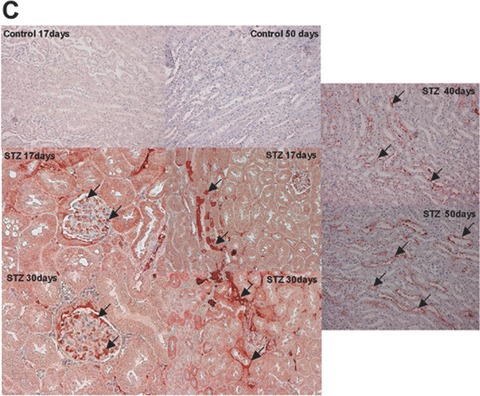
CXCL16 and oxLDL expression is increased in streptozotocin-treated mice Immunohistochemical analysis of CXCL16 (A, B) and oxLDL (C) expression in kidney sections of citrate buffer (control) or streptozotocin (STZ) -treated mice. Representative micrographs show CXCL16 expression in renal glomeruli (A) and tubules (B) 5, 10, 17, 19, 30, 40 and 50 days after STZ injection (examples are marked by black arrows). (C) OxLDL expression was monitored 17, 30, 40 and 50 days after induction of diabetes. Arrows mark oxLDL expressing renal cells.
In biopsies of patients with DN increased glomerular CXCL16 was accompanied by high levels of oxLDL
To further investigate the expression of CXCL16, ADAM10 and oxLDL in kidney sections of patients we DN, we performed immunofluorescence analysis. As documented in Fig. 6 increased CXCL16 expression was mainly found in podocytes (Fig. 6B). Interestingly, increased glomerular CXCL16 expression was accompanied with lower levels of ADAM10 (Fig. 6A and B). In contrast, the amount of ADAM10 in tubuli was not significantly changed between normal kidneys and patients with DN (Fig. 6A). In addition in glomeruli and tubuli of kidney biopsies of patients with DN increased levels of oxLDL were detectable (Fig. 6C and D). To investigate if CXCL16 may be responsible for oxLDL accumulation in glomeruli of DN patients, we performed double immunofluorescense analysis of CXCL16 and oxLDL in biopsies of DN patients. Importantly, co-localization of CXCL16 and oxLDL (Fig. 6E, yellow colour) was observed in glomeruli of DN patients, assuming that CXCL16 represents a scavenger receptor for oxLDL also in vivo.
Figure 6.
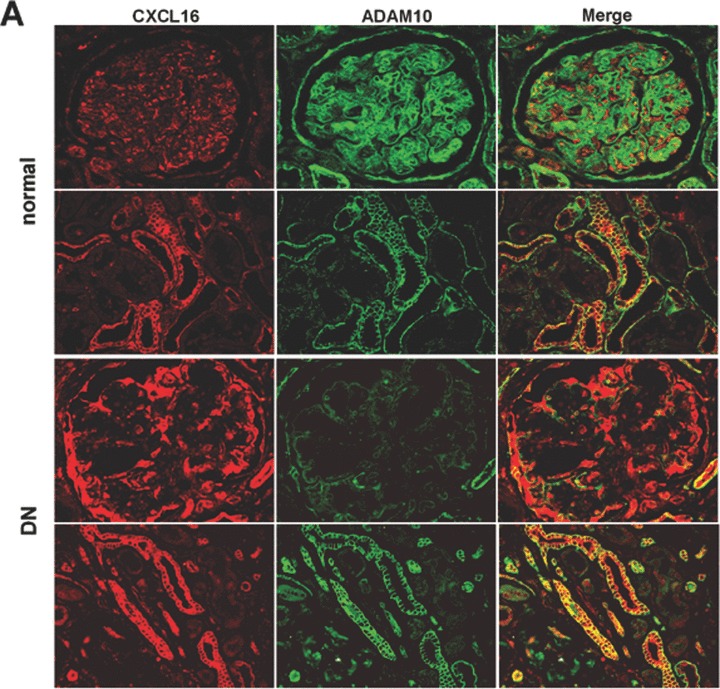
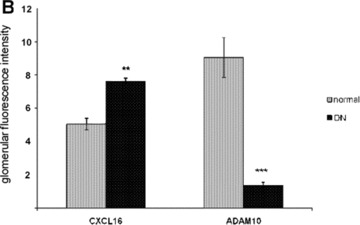
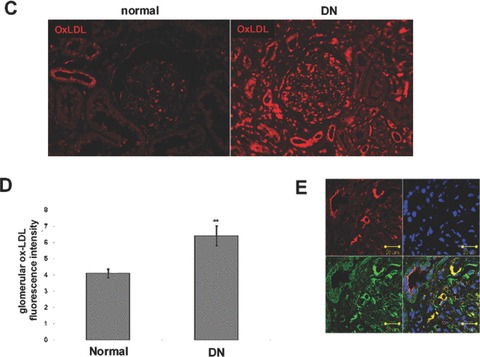
Expression of CXCL16 and ADAM10 in tissue section of normal kidney and patients with DN. (A) Renal tissue of normal kidney (normal) and of patients diagnosed with DN were analysed with double immunofluorescence staining of CXCL16 (red fluorescence) and ADAM10 (green fluorescence) in glomeruli (upper panel) and tubules (lower panel). (B) Semi-quantitative analysis of fluorescence intensities of glomerular CXCL16 (grey bars) and glomerular and ADAM10 (black bars) expression is depicted in a graph. (C) oxLDL expression in renal sections of normal kidney (normal) and patients diagnosed with DN. Tissue sections were stained with oxLDL antibody followed by Cy3 coupled secondary antibody (red). (D) Immunofluorescence results of oxLDL expression in renal tissue of normal kidney and of patients with DN. Data represent mean ± S.D. ***P < 0.001, **P < 0.01 considered statistically significant compared to the normal kidney. (E) Double immunofluorescense analysis of oxLDL (red colour) and CXCL16 (green colour) was performed on kidney biopsies of patients with DN. Tissue section was stained with DAPI to visualize the nuclei of the cells.
Discussion
DN is the leading cause of end-stage renal failure and is clinically characterized by proteinuria und progressive renal insufficiency [45]. Several interventions have been shown to slow down the progression of DN, but unfortunately they cannot prevent the disease [46]. Improved therapeutic treatment of DN is urgently needed but is hampered due to the incomplete understanding of the complex mechanism of the disease. In this context podocytes seem to be an attractive target of research. It is commonly accepted that podocytes are an early target of injury in the onset of DN and therefore may be the major culprit of the disease. Interestingly a link between oxLDL and the injury of podocytes has been already described [10]. In contrast the scavenger receptors responsible for the uptake of oxidized LDL in podocytes have been so far not characterized. We have recently shown that human podocytes constitutively express the scavenger receptor CXCL16 (Gutwein et al., unpublished data). Notably in our current study we identified CXCL16 and CD36 as the main receptors for the uptake of oxLDL in podocytes and in tubular cells respectively.
It is well accepted that the number of podocytes is reduced in animal models of DN [47] and in biopsies of patients with early and late DN [20, 21]. The exact aetiology for the reduction in the number of podocytes in diabetes remains speculative, but two mechanisms have been suggested: apoptosis and cell detachment. It has been recently shown that oxLDL is able to induce apoptosis in murine podocytes [10]. In contrast in our study oxLDL did not lead to apoptosis in human podocytes. The effects induced by oxLDL can differ due to the kind of LDL, its degree of oxidative modification, the amount of oxLDL and on the type of target cell involved [48]. The discrepancy in the above mentioned study and our observations could be explained by an increased sensitivity of mouse podocytes to the cytotoxicity of oxLDL. Furthermore strongly oxidized LDL often exerts a cytotoxic effect, whereas minimally oxidized LDL causes more subtle alteration in vascular cell functions [48]. Currently, we investigate the effects of different forms of oxLDL on cellular responses like survival and proliferation in human podocytes.
Although we did not find in our study any cytotoxic effects of oxLDL in human podocytes, we noticed other important properties of oxLDL in human podocytes. Firstly, we demonstrated that oxLDL led to a down-regulation of α3-integrin, which could be efficiently blocked by pre-incubation with a CXCL16 blocking antibody. α3β1 is known to be a principal protein involved in attaching podocytes to the glomerular basement membrane. Interestingly, altered expression of α3β1 has been detected in podocytes of patients with DN [49]. Therefore down-regulation of α3β1 could lead to the detachment of podocytes, one possible explanation for the decreased number of podocytes found in patients and animal models of DN. In the same line it has been reported that the loss of podocytes in the urine has been associated with the progression of DN. When we investigated pathological factors, which are known to play important role in the development and progression of DN, we found that TNF-α (Gutwein et al., unpublished data) and hyperglycaemic conditions induced CXCL16 expression in human podocytes. Strikingly, HG containing media induced CXCL16 expression but reduced ADAM10 expression in human podocytes. In addition also in patients with DN glomerular ADAM10 expression was dramatically reduced. In the same line, our study demonstrated that ADAM10 knockdown experiments induced cellular CXCL16 and reduced the constitutive cleavage of CXCL16 in human podocytes. In addition, ADAM10 contribute to the processing of many other important cytokines, growth factors, adhesion molecules [50] and it is able to degrade collagen IV [51]. Therefore down-regulation of ADAM10 in vivo could participate in the development of membrane thickening, known to be a characteristic feature of DN [52]. In the same line, long-term incubation of podocytes with HG containing media was shown to reduce the production of MMP-9, one major metalloproteinase involved in the degradation of the extracellular matrix. Furthermore our study presents another important finding. Beside the morphological changes seen after oxLDL treatment (as documented with F-actin immunofluorescence staining), CXCL16 mediated uptake of oxidized LDL induced a dramatic up-regulation of fibronection production. Again interfering with blocking CXCL16 antibodies significantly reduced the fibronection overproduction, assuming an important role of CXCL16 mediated uptake of oxLDL in the progression of glomerular kidney diseases.
It has been postulated that localized tissue oxidative stress is a key component in the development of DN [2]. Oxidative stress (or oxidant-derived tissue injury) occurs when production of oxidants or ROS exceeds local antioxidant capacity [2]. Importantly, overexpression of the antioxidant metallothionein in podocytes reduced the development of DN, highlighting the important role of podocytes in the onset of DN [52]. Beside this our study presents evidence that CXCL16 mediated uptake of oxLDL induced ROS production in podocytes. In addition, it is known that hyperglycaemic conditions can lead to ROS production in podocytes [53] and together with increased CXCL16 expression it could lead to a vicious circle which leads to high levels of ROS production in glomeruli of diabetic patients. Combined treatment of antioxidants and CXCL16 blocking antibodies may be a potential strategy to inhibit the development of DN.
To investigate CXCL16 and oxLDL in DN in vivo we performed immunohistochemical analysis on kidney sections of a STZ-induced diabetic mouse model. Importantly, already 17 days after STZ treatment a strong up-regulation of CXCL16, predominantly in podocytes was observed. This elevated CXCL16 expression was accompanied with increased oxLDL expression. Furthermore we detected increased CXCL16 and oxLDL expression in biopsies of patients with DN which was paralleled by the down-regulation of glomerular ADAM10 protein. Interestingly, in patients with DN increased concentrations of oxLDL immune complexes were associated with abnormal proteinuria [54]. In addition, the preferential binding of oxLDL into rat glomeruli has been reported already a long time ago [55]. Furthermore the accumulation of oxLDL in biopsies of patients with kidney diseases has been also demonstrated [56]. Notably, plasma levels of oxLDL were significantly elevated in patients with DN [57].
In summary our findings, that CXCL16 is a major scavenger receptor for oxLDL in human podocytes and that CXCL16 and oxLDL are early up-regulated in the onset of DN, highlight both molecules as new therapeutic targets in DN.
References
- 1.Gin H, Rigalleau V, Aparicio M. Lipids, protein intake, and diabetic nephropathy. Diabetes Metab. 2000;26:45–53. [Google Scholar]
- 2.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57:1446–54. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 3.Deslypere JP. Modified lipoproteins in diabetes. J Intern Med Suppl. 1994;736:69–74. [PubMed] [Google Scholar]
- 4.Lyons TJ. Oxidized low density lipoproteins: a role in the pathogenesis of atherosclerosis in diabetes. Diabet Med. 1991;8:411–9. doi: 10.1111/j.1464-5491.1991.tb01624.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee HS. Oxidized LDL, glomerular mesangial cells and collagen. Diabetes Res Clin Pract. 1999;45:117–22. doi: 10.1016/s0168-8227(99)00040-6. [DOI] [PubMed] [Google Scholar]
- 6.Nishida Y, Oda H, Yorioka N. Effect of lipoproteins on mesangial cell proliferation. Kidney Int Suppl. 1999;71:S51–3. doi: 10.1046/j.1523-1755.1999.07113.x. [DOI] [PubMed] [Google Scholar]
- 7.Wheeler DC, Chana RS, Topley N, et al. Oxidation of low density lipoprotein by mesangial cells may promote glomerular injury. Kidney Int. 1994;45:1628–36. doi: 10.1038/ki.1994.214. [DOI] [PubMed] [Google Scholar]
- 8.Claise C, Edeas M, Chaouchi N, et al. Oxidized-LDL induce apoptosis in HUVEC but not in the endothelial cell line EA.hy 926. Atherosclerosis. 1999;147:95–104. doi: 10.1016/s0021-9150(99)00170-7. [DOI] [PubMed] [Google Scholar]
- 9.Kamanna VS, Pai R, Ha H, et al. Oxidized low-density lipoprotein stimulates monocyte adhesion to glomerular endothelial cells. Kidney Int. 1999;55:2192–202. doi: 10.1046/j.1523-1755.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- 10.Bussolati B, Deregibus MC, Fonsato V, et al. Statins prevent oxidized LDL-induced injury of glomerular podocytes by activating the phosphatidylinositol 3-kinase/AKT-signaling pathway. J Am Soc Nephrol. 2005;16:1936–47. doi: 10.1681/ASN.2004080629. [DOI] [PubMed] [Google Scholar]
- 11.Witztum JL, Steinberg D. The oxidative modification hypothesis of atherosclerosis: does it hold for humans. Trends Cardiovasc Med. 2001;11:93–102. doi: 10.1016/s1050-1738(01)00111-6. [DOI] [PubMed] [Google Scholar]
- 12.Akiba S, Chiba M, Mukaida Y, et al. Involvement of reactive oxygen species and SP-1 in fibronectin production by oxidized LDL. Biochem Biophys Res Commun. 2003;310:491–7. doi: 10.1016/j.bbrc.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 13.Brune B, Zhou J, von Knethen A. Nitric oxide, oxidative stress, and apoptosis. Kidney Int Suppl. 2003:S22–4. doi: 10.1046/j.1523-1755.63.s84.6.x. [DOI] [PubMed] [Google Scholar]
- 14.Chade AR, Mushin OP, Zhu X, et al. Pathways of renal fibrosis and modulation of matrix turnover in experimental hypercholesterolemia. Hypertension. 2005;46:772–9. doi: 10.1161/01.HYP.0000184250.37607.da. [DOI] [PubMed] [Google Scholar]
- 15.Reddy S, Santanam N, Reddy PP, et al. Interaction of Interceed oxidized regenerated cellulose with macrophages: a potential mechanism by which Interceed may prevent adhesions. Am J Obstet Gynecol. 1997;177:1315–20. doi: 10.1016/s0002-9378(97)70070-x. [DOI] [PubMed] [Google Scholar]
- 16.Ding G, van Goor H, Ricardo SD, et al. Oxidized LDL stimulates the expression of TGF-beta and fibronectin in human glomerular epithelial cells. Kidney Int. 1997;51:147–54. doi: 10.1038/ki.1997.18. [DOI] [PubMed] [Google Scholar]
- 17.Gruden G, Perin PC, Camussi G. Insight on the pathogenesis of diabetic nephropathy from the study of podocyte and mesangial cell biology. Curr Diabetes Rev. 2005;1:27–40. doi: 10.2174/1573399052952622. [DOI] [PubMed] [Google Scholar]
- 18.Kanwar YS, Wada J, Sun L, et al. Diabetic nephropathy: mechanisms of renal disease progression. Exp Biol Med. 2008;233:4–11. doi: 10.3181/0705-MR-134. [DOI] [PubMed] [Google Scholar]
- 19.Dalla VM, Masiero A, Roiter AM, et al. Is podocyte injury relevant in diabetic nephropathy? Studies in patients with type 2 diabetes. Diabetes. 2003;52:1031–5. doi: 10.2337/diabetes.52.4.1031. [DOI] [PubMed] [Google Scholar]
- 20.Pagtalunan ME, Miller PL, Jumping-Eagle S, et al. Podocyte loss and progressive glomerular injury in type II diabetes. J Clin Invest. 1997;99:342–8. doi: 10.1172/JCI119163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steffes MW, Schmidt D, McCrery R, et al. Glomerular cell number in normal subjects and in type 1 diabetic patients. Kidney Int. 2001;59:2104–13. doi: 10.1046/j.1523-1755.2001.00725.x. [DOI] [PubMed] [Google Scholar]
- 22.White KE, Bilous RW, Marshall SM, et al. Podocyte number in normotensive type 1 diabetic patients with albuminuria. Diabetes. 2002;51:3083–9. doi: 10.2337/diabetes.51.10.3083. [DOI] [PubMed] [Google Scholar]
- 23.Kita T, Kume N, Minami M, et al. Role of oxidized LDL in atherosclerosis. Ann N Y Acad Sci. 2001;947:199–205. doi: 10.1111/j.1749-6632.2001.tb03941.x. [DOI] [PubMed] [Google Scholar]
- 24.Zingg JM, Ricciarelli R, Azzi A. Scavenger receptors and modified lipoproteins: fatal attractions. IUBMB Life. 2000;49:397–403. doi: 10.1080/152165400410245. [DOI] [PubMed] [Google Scholar]
- 25.Shimaoka T, Kume N, Minami M, et al. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, SR-PSOX, on macrophages. J Biol Chem. 2000;275:40663–6. doi: 10.1074/jbc.C000761200. [DOI] [PubMed] [Google Scholar]
- 26.Shimaoka T, Nakayama T, Fukumoto N, et al. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J Leukoc Biol. 2004;75:267–74. doi: 10.1189/jlb.1003465. [DOI] [PubMed] [Google Scholar]
- 27.Abel S, Hundhausen C, Mentlein R, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172:6362–72. doi: 10.4049/jimmunol.172.10.6362. [DOI] [PubMed] [Google Scholar]
- 28.Gough PJ, Garton KJ, Wille PT, et al. A disintegrin and metalloproteinase 10-mediated cleavage and shedding regulates the cell surface expression of CXC chemokine ligand 16. J Immunol. 2004;172:3678–85. doi: 10.4049/jimmunol.172.6.3678. [DOI] [PubMed] [Google Scholar]
- 29.Geissmann F, Cameron TO, Sidobre S, et al. Intravascular immune surveillance by CXCR6+ NKT cells patrolling liver sinusoids. PLoS Biol. 2005;3:e113. doi: 10.1371/journal.pbio.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matloubian M, David A, Engel S, et al. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- 31.Okamura DM, Lopez-Guisa JM, Koelsch K, et al. Atherogenic scavenger receptor modulation in the tubulointerstitium in response to chronic renal injury. Am J Physiol Renal Physiol. 2007;293:F575–85. doi: 10.1152/ajprenal.00063.2007. [DOI] [PubMed] [Google Scholar]
- 32.Saleem MA, O’Hare MJ, Reiser J, et al. A conditionally immortalized human podocyte cell line demonstrating nephrin and podocin expression. J Am Soc Nephrol. 2002;13:630–8. doi: 10.1681/ASN.V133630. [DOI] [PubMed] [Google Scholar]
- 33.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer L, Tsalastra W, Babelova A, et al. Decorin-mediated regulation of fibrillin-1 in the kidney involves the insulin-like growth factor-I receptor and mammalian target of rapamycin. Am J Pathol. 2007;170:301–15. doi: 10.2353/ajpath.2007.060497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Susztak K, Ciccone E, McCue P, et al. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med. 2005;2:e45. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schramme A, Abdel-Bakky MS, Gutwein P, et al. Characterization of CXCL16 and ADAM10 in the normal and transplanted kidney. Kidney Int. 2008;74:328–38. doi: 10.1038/ki.2008.181. [DOI] [PubMed] [Google Scholar]
- 37.Li C, Ruotsalainen V, Tryggvason K, et al. CD2AP is expressed with nephrin in developing podocytes and is found widely in mature kidney and elsewhere. Am J Physiol Renal Physiol. 2000;279:F785–92. doi: 10.1152/ajprenal.2000.279.4.F785. [DOI] [PubMed] [Google Scholar]
- 38.Liu G, Kaw B, Kurfis J, et al. Neph1 and nephrin interaction in the slit diaphragm is an important determinant of glomerular permeability. J Clin Invest. 2003;112:209–21. doi: 10.1172/JCI18242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kretzler M. Regulation of adhesive interaction between podocytes and glomerular basement membrane. Microsc Res Tech. 2002;57:247–53. doi: 10.1002/jemt.10083. [DOI] [PubMed] [Google Scholar]
- 40.Li JJ, Kwak SJ, Jung DS, et al. Podocyte biology in diabetic nephropathy. Kidney Int Suppl. 2007:S36–S42. doi: 10.1038/sj.ki.5002384. [DOI] [PubMed] [Google Scholar]
- 41.Ha H, Hwang IA, Park JH, et al. Role of reactive oxygen species in the pathogenesis of diabetic nephropathy. Diabetes Res Clin Pract. 2008;13:S42–5. doi: 10.1016/j.diabres.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 42.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 43.Kelly DJ, Aaltonen P, Cox AJ, et al. Expression of the slit-diaphragm protein, nephrin, in experimental diabetic nephropathy: differing effects of anti-proteinuric therapies. Nephrol Dial Transplant. 2002;17:1327–32. doi: 10.1093/ndt/17.7.1327. [DOI] [PubMed] [Google Scholar]
- 44.Mifsud SA, Allen TJ, Bertram JF, et al. Podocyte foot process broadening in experimental diabetic nephropathy: amelioration with renin-angiotensin blockade. Diabetologia. 2001;44:878–82. doi: 10.1007/s001250100561. [DOI] [PubMed] [Google Scholar]
- 45.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008;4:444–52. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 46.Gurley SB, Coffman TM. The renin-angiotensin system and diabetic nephropathy. Semin Nephrol. 2007;27:144–52. doi: 10.1016/j.semnephrol.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Susztak K, Raff AC, Schiffer M, et al. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–33. [PubMed] [Google Scholar]
- 48.Hofnagel O, Luechtenborg B, Weissen-Plenz G, et al. Statins and foam cell formation: impact on LDL oxidation and uptake of oxidized lipoproteins via scavenger receptors. Biochim Biophys Acta. 2007;1771:1117–24. doi: 10.1016/j.bbalip.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 49.Regoli M, Bendayan M. Alterations in the expression of the alpha 3 beta 1 integrin in certain membrane domains of the glomerular epithelial cells (podocytes) in diabetes mellitus. Diabetologia. 1997;40:15–22. doi: 10.1007/s001250050637. [DOI] [PubMed] [Google Scholar]
- 50.White JM. ADAMs: modulators of cell-cell and cell-matrix interactions. Curr Opin Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 51.Millichip MI, Dallas DJ, Wu E, et al. The metallo-disintegrin ADAM10 (MADM) from bovine kidney has type IV collagenase activity in vitro. Biochem Biophys Res Commun. 1998;245:594–8. doi: 10.1006/bbrc.1998.8485. [DOI] [PubMed] [Google Scholar]
- 52.Zheng S, Carlson EC, Yang L, et al. Podocyte-specific overexpression of the antioxidant metallothionein reduces diabetic nephropathy. J Am Soc Nephrol. 2008;19:2077–85. doi: 10.1681/ASN.2007080967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim NH, Rincon-Choles H, Bhandari B, et al. Redox dependence of glomerular epithelial cell hypertrophy in response to glucose. Am J Physiol Renal Physiol. 2006;290:F741–51. doi: 10.1152/ajprenal.00313.2005. [DOI] [PubMed] [Google Scholar]
- 54.Atchley DH, Lopes-Virella MF, Zheng D, et al. Oxidized LDL-anti-oxidized LDL immune complexes and diabetic nephropathy. Diabetologia. 2002;45:1562–71. doi: 10.1007/s00125-002-0962-y. [DOI] [PubMed] [Google Scholar]
- 55.Coritsidis G, Rifici V, Gupta S, et al. Preferential binding of oxidized LDL to rat glomeruli in vivo and cultured mesangial cells in vitro. Kidney Int. 1991;39:858–66. doi: 10.1038/ki.1991.108. [DOI] [PubMed] [Google Scholar]
- 56.Bosmans JL, Holvoet P, Dauwe SE, et al. Oxidative modification of low-density lipoproteins and the outcome of renal allografts at 1 1/2 years. Kidney Int. 2001;59:2346–56. doi: 10.1046/j.1523-1755.2001.00752.x. [DOI] [PubMed] [Google Scholar]
- 57.Ujihara N, Sakka Y, Takeda M, et al. Association between plasma oxidized low-density lipoprotein and diabetic nephropathy. Diabetes Res Clin Pract. 2002;58:109–14. doi: 10.1016/s0168-8227(02)00134-1. [DOI] [PubMed] [Google Scholar]


