Abstract
Expression of the Bacillus subtilis trp genes is negatively regulated by an 11-subunit trp RNA-binding attenuation protein (TRAP), which is activated to bind RNA by binding l-tryptophan. We used Western blotting to estimate that there are 200 to 400 TRAP 11-mer molecules per cell in cells grown in either minimal or rich medium.
In Bacillus subtilis and several related bacilli, expression of genes involved in tryptophan metabolism is regulated in response to changes in intracellular l-tryptophan levels by an RNA-binding protein called TRAP (trp RNA-binding attenuation protein) (4, 5, 12, 13). TRAP regulates transcription of the trpEDCFBA operon through an attenuation mechanism (6, 18), as well as translation of trpE by altering the trp mRNA structure to sequester the trpE Shine-Dalgarno sequence in a stem-loop (10, 18, 21). TRAP also regulates translation of trpG (pabA) (11, 30), trpP (yhaG) (23, 29), and ycbK (24) through direct competition with ribosomes for binding to these mRNAs. In addition, the activity of TRAP is regulated by the anti-TRAP (AT) protein (26, 27). AT binds to tryptophan-activated TRAP and inhibits it from binding to its RNA targets, thereby increasing expression of the trp genes.
TRAP is composed of 11 identical subunits arranged in a ring structure (3). Each 75-amino-acid subunit is encoded by the mtrB gene (14). TRAP is activated to bind RNA by binding up to 11 molecules of l-tryptophan in pockets between adjacent subunits (20). Single-stranded RNA binds to TRAP by wrapping around the outside of the protein ring (2). The RNA targets of TRAP consist of multiple GAG, UAG, and occasionally AAG repeats, which are separated from each other by several nonconserved spacer nucleotides. The TRAP binding sites in the trp operon and in ycbK contain 11 repeats, whereas there are 9 triplet repeats in the trpG- and trpP-binding sites.
We used Western blotting to estimate the number of TRAP 11-mers per B. subtilis cell to be approximately 200 to 400. This number varies only slightly with growth phase or in the absence or presence of tryptophan in the growth medium.
Cell growth and preparation of protein extracts.
One-liter cultures of B. subtilis BG2087 (argC4) or BG4233 (argC4 ΔmtrB) cells were grown in Luria-Bertania (LB) or minimal medium (28) at 37°C either overnight or to mid-log phase (A600 of 0.8). The number of cells per milliliter of culture was determined by plating 25, 50, or 100 μl of a 1:106 dilution on LB agar plates. Counting of cells microscopically showed no significant differences with viable cell counts. Cells were harvested by centrifugation at 5,000 × g for 10 min. Cell pellets were resuspended in 5 ml of 10 mM Tris-HCl (pH 7.6)-1 mM EDTA, and the cells were broken by three passages through a French pressure cell at 12,000 lb/in2. The cell lysate was cleared by centrifugation at 30,000 × g for 20 min. TRAP is heat stable (7); therefore, the extract was heated at 65°C for 15 min to denature most of the Escherichia coli proteins and cleared by centrifugation as described above. Total protein in the extract was determined by using the Bio-Rad protein assay with bovine serum albumin standards.
Electrophoresis and western blotting.
Protein samples were run on sodium dodecyl sulfate-Tris-Tricine-15% polyacrylamide gels by using a Bio-Rad mini-Protean II system run at 40 mA. Samples were mixed with an equal volume of loading dye (0.1 M Tris HCl [pH 6.8], 25% glycerol, 1% sodium dodecyl sulfate 0.02% Coomassie G-250) at room temperature and loaded onto the gel without heating. Standard curves were generated by mixing 0 to 25 ng of purified B. subtilis TRAP (1) with protein extract from BG4233 (ΔmtrB) cells, which do not produce TRAP. The amount of control extract protein was equivalent to that used for TRAP determination from BG2087.
Proteins were transferred to polyvinglidene difluoride membrane (Amersham Hybond-P) by submerged wet transfer at 40 V for 10 h in 25 mM Tris-glycine-20% methanol. After being blocked with 5% bovine serum albumin, the membranes were incubated for 1 h with rabbit AT antibodies (1:10,000). Blots were then washed and incubated for 1 h with goat anti-rabbit immunoglobin G conjugated with horseradish peroxidase (Cappel). TRAP was visualized on the blots with the use of an Amersham ECL+ kit and a Storm PhosphorImager (Molecular Dynamics) and quantitated with ImageQuant software.
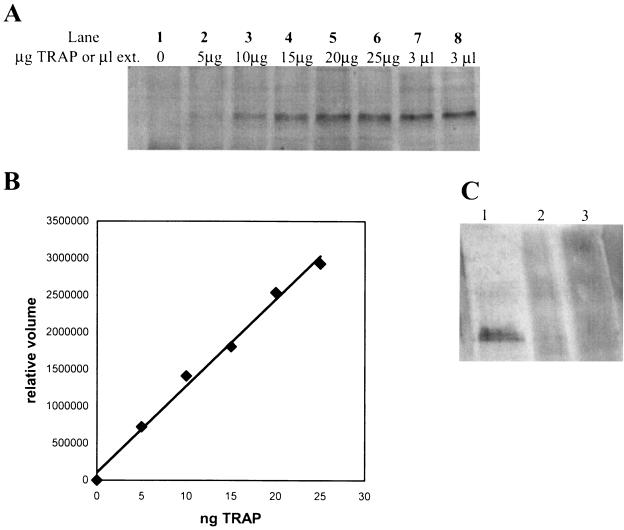
The results of Western blotting to quantitate cellular TRAP are shown in Fig. 1. (B) The standard curve for TRAP was generated from Western blotting data (Fig. 1B). Heat treatment of the lysate does not precipitate TRAP (Fig. 1C).
FIG. 1.
(A and C) Western blots of TRAP from B. subtilis cells. (A) Lanes: 1 to 6, various amounts of purified B. subtilis TRAP mixed with extract from BG4233 (ΔmtrB) cells; 7 and 8, 3 μl of treated BG2087 extract. (B) Standard curve for TRAP generated from the data from lanes 1 to 6 in panel A. In each case, the background calculated from data from lane 1, with no added TRAP, was subtracted from each value. (C) Effect of heat treatment in Western blotting is shown. Lanes 1, 3 μl of treated extract from BG2087; 2 and 3, 2.5 and 5 μl, respectively, of resuspended pellet after the heat treatment of the BG2087 extract.
Table 1 shows the average number of TRAP 11-mers per B. subtilis cell for growth under several conditions. The average number for cells grown in LB harvested at mid-log phase was slightly greater than for that for stationary-phase cells. The number for cells grown overnight in minimal medium without tryptophan was slightly lower than that for cells grown in the presence of tryptophan. In all cases, the differences are less than twofold, although they are significant based on a t test (P ≥ 0.99). Studies have shown that the volume of bacterial cells changes with growth conditions including growth rate (17); therefore, the small changes in TRAP per cell that we observed likely reflect changes in cell volume rather than regulation of mtrB expression. The aqueous volume of an E. coli cell has been measured to be 6.7 × 10−15 liters (22). Because the dimensions of an average B. subtilis cell are approximately 1.5-times larger than those for E. coli (8), or approximately 1 × 10−14 liter the concentration of TRAP for exponentially growing cells in LB is approximately 80 nM.
TABLE 1.
The number of TRAP 11-mer molecules per B. subtilis cella
| Growth medium | No. of TRAP 11-mers/cell after growth
|
|
|---|---|---|
| Overnight | Mid-log phase | |
| LB | 340 ± 169 (22) | 488 ± 193 (17) |
| Minimal medium | ||
| −Trp | 222 ± 108 (14) | |
| +Trp | 378 ± 152 (13) | |
B. subtilis (BG2087) was grown in LB broth either overnight or to mid-log phase (A600 of 0.8) or Vogel-Bonner minimal medium (28) in the absence and presence of 10 μg of tryptophan/ml. (−Trp and +Trp, respectively). Data are the average numbers of TRAP 11-mer molecules per B. subtilis cells ± standard deviations, are shown. The number of replicate determinations are given in parentheses. In each case, the amount of TRAP (nanograms) per microliter of treated extract was determined by comparison with the standard curve. We also determined the volume of treated extract obtained per milliliter of original cell culture and the number of cells per milliliter of culture. From these values, we calculated the amount of TRAP per cell. Using the molecular weight of 91,575 of a TRAP 11-mer, we calculated the number of molecules per cell.
The level of TRAP protein in vivo is higher than those previously determined for several DNA-binding repressor proteins, including λ repressor (19) and the trp repressor from E. coli (15). This finding is appropriate given that TRAP binds to at least four different binding sites in B. subtilis (5). Moreover, regulatory mechanisms based on an RNA-binding protein introduce additional issues beyond those relevant to DNA-binding proteins. Multiple copies of each mRNA are produced from each promoter, and these levels may vary depending on the physiological conditions of the cell. By contrast, the DNA copy number varies by at most a factor of two. One of TRAP's target mRNAs, trp mRNA, is produced from a strong promoter, and the level of this mRNA is highly variable, resulting in the number of copies of trp mRNA per cell varying. Our estimate of 200 to 400 TRAP 11-mer copies per cell would appear to provide sufficient TRAP to cope with all eventualities. However, since the TRAP protein requires tryptophan for activation, the fraction of active TRAP molecules available for mRNA binding will vary, depending on the intracellular concentration of tryptophan. In addition, the availability of active TRAP is also regulated by the AT protein (26, 27).
When an RNA-binding regulatory protein is bound to an mRNA, it is not available to bind other copies of this mRNA, or to other target mRNAs, until the bound protein is released. Recently, it was shown that mRNA degradation plays a role in recycling TRAP molecules to maintain the cellular levels of free protein necessary to regulate the trp operon (9). Cells lacking the degradative RNase polynucleotide phosphorylase overexpress the trp operon structural genes when the cells are grown in the presence of excess tryptophan, apparently due to insufficient free TRAP to bind to newly synthesized trp mRNA. This effect was not observed in cells with active RNase polynucleotide phosphorylase. This issue may be a common concern regarding availability of RNA-binding proteins (16, 25). It may be dealt with by predisposing specific mRNAs that would be affected to susceptibility to attack by nucleases that free the RNA-binding protein.
The antibodies used in this work were generated at the Department of Laboratory Animal Resources Core facility, which is supported in part by the Roswell Park Cancer Institute, National Cancer Institute-funded Cancer Center Support Grant CA16056. This work was supported by grants GM62750 from the National Institutes of Health and MCB 9982652 from the National Science Foundation.
REFERENCES
- 1.Antson, A. A., A. M. Brzozowski, E. J. Dodson, Z. Dauter, K. S. Wilson, T. Kurecki, and P. Gollnick. 1994. eleven-fold symmetry of the trp RNA-binding attenuation protein (TRAP) from Bacillus subtilis determined by X-ray analysis. J. Mol. Biol. 244:1-5. [DOI] [PubMed] [Google Scholar]
- 2.Antson, A. A., E. J. Dodson, G. G. Dodson, R. B. Greaves, X.-P. Chen, and P. Gollnick. 1999. Structure of the trp RNA-binding attenuation protein, TRAP, bound to RNA. Nature 401:235-242. [DOI] [PubMed] [Google Scholar]
- 3.Antson, A. A., J. B. Otridge, A. M. Brzozowski, E. J. Dodson, G. G. Dodson, K. S. Wilson, T. M. Smith, M. Yang, T. Kurecki, and P. Gollnick. 1995. The three dimensional structure of trp RNA-binding attenuation protein. Nature 374:693-700. [DOI] [PubMed] [Google Scholar]
- 4.Babitzke, P. 1997. Regulation of tryptophan biosynthesis: Trp-ing the TRAP or how Bacillus subtilis reinvented the wheel. Mol. Microbiol. 26:1-9. [DOI] [PubMed] [Google Scholar]
- 5.Babitzke, P., and P. Gollnick. 2001. Posttranscriptional initiation control of tryptophan metabolism in Bacillus subtilis by the trp RNA-binding attenuation protein (TRAP), anti-TRAP, and RNA structure. J. Bacteriol. 183:5795-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babitzke, P., and C. Yanofsky. 1993. Reconstitution of Bacillus subtilis trp attenuation in vitro with TRAP, the trp RNA-binding attenuation protein. Proc. Natl. Acad. Sci. USA 90:133-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baumann, C., J. Otridge, and P. Gollnick. 1996. Kinetic and thermodynamic analysis of the interaction between TRAP (trp RNA-binding attenuation protein) and trp leader RNA from Bacillus subtilis. J. Biol. Chem. 271:12269-12274. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan, R., and N. Gibbons (ed.). 1974. Bergey's manual of determinate bacteriology, 8th ed. Williams and Wilkins, Baltimore, Md.
- 9.Deikus, G., P. Babitzke, and D. H. Bechhofer. 2004. Recycling of a regulatory protein by degradation of the RNA to which it binds. Proc. Natl. Acad. Sci. USA 101:2747-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Du, H., and P. Babitzke. 1998. trp RNA-binding attenuation protein-mediated long distance RNA refolding regulates translation of trpE in Bacillus subtilis. J. Biol. Chem. 273:20494-20503. [DOI] [PubMed] [Google Scholar]
- 11.Du, H., R. Tarpley, and P. Babitzke. 1997. The trp RNA-binding attenuation protein regulates TrpG synthesis by binding to the trpG ribosome binding site of Bacillus subtilis. J. Bacteriol. 179:2582-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gollnick, P. 1994. Regulation of the Bacillus subtilis trp operon by an RNA-binding protein. Mol. Microbiol. 11:991-997. [DOI] [PubMed] [Google Scholar]
- 13.Gollnick, P., P. Babitzke, E. Merino, and C. Yanofsky. 2002. Aromatic amino acid metabolism in Bacillus subtilis, p. 233-244. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, D.C.
- 14.Gollnick, P., S. Ishino, M. I. Kuroda, D. Henner, and C. Yanofsky. 1990. The mtr locus is a two gene operon required for transcription attenuation in the trp operon of Bacillus subtilis. Proc. Natl. Acad. Sci. USA 87:8726-8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelley, R. L., and C. Yanofsky. 1982. Trp aporepressor production is controlled by autogenous regulation and inefficient translation. Proc. Natl. Acad. Sci. USA 79:3120-3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kisselev, L. L., and R. H. Buckingham. 2002. Translational termination comes of age. Trends Biochem. Sci. 25:561-566. [DOI] [PubMed] [Google Scholar]
- 17.Kubitschek, H. 1990. Cell volume increase in Escherichia coli after shifts to Richer media. J. Bacteriol. 172:94-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuroda, M. I., D. Henner, and C. Yanofsky. 1988. cis-Acting sites in the transcript of the Bacillus subtilis trp operon regulate expression of the operon. J. Bacteriol. 170:3080-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levine, A., A. Bailone, and R. Devoret. 1979. Cellular levels of the prophage lambda and 434 repressors. J. Mol. Biol. 131:655-61. [DOI] [PubMed] [Google Scholar]
- 20.Li, P. T. X., and P. Gollnick. 2002. Using heter-11-mers composed of wild type and mutant subunits to study tryptophan binding to TRAP and its role in activating RNA binding. J. Biol. Chem. 277:35567-35573. [DOI] [PubMed] [Google Scholar]
- 21.Merino, E., P. Babitzke, and C. Yanofsky. 1995. trp RNA-binding attenuation protein (TRAP)-trp leader RNA interactions mediate translational as well as transcriptional regulation of the Bacillus subtilis trp operon. J. Bacteriol. 177:6362-6370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neidhardt, F. C., and H. E. Umbarger. 1996. Chemical composition of Escherichia coli, p. 13-16. In F. C. Neidhardt, R. Curtiss III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed, vol. 1. ASM Press, Washington, D.C. [Google Scholar]
- 23.Sarsero, J. P., E. Merino, and C. Yanofsky. 2000. A Bacillus subtilis gene of previously unknown function, yhaG, is translationally regulated by tryptophan-activated TRAP and appears to be concerned with tryptophan transport. J. Bacteriol. 182:2329-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarsero, J. P., E. Merino, and C. Yanofsky. 2000. A Bacillus subtilis operon containing genes of unknown function senses tRNATrp charging and regulates expression of the genes for tryptophan biosynthesis. Proc. Natl. Acad. Sci. USA 97:2656-2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Staley, J. P., and C. Guthrie. 1998. Mechanical devices of the spliceosome: motors, clocks, springs, and things. Cell 92:315-326. [DOI] [PubMed] [Google Scholar]
- 26.Valbuzzi, A., P. Gollnick, P. Babitzke, and C. Yanofsky. 2002. The anti-trp RNA-binding attenuation protein (Anti-TRAP), AT, recognizes the tryptophan-activated RNA binding domain of the TRAP regulatory protein. J. Biol. Chem. 277:10608-10613. [DOI] [PubMed] [Google Scholar]
- 27.Valbuzzi, A., and C. Yanofsky. 2001. Inhibition of the B. subtilis regulatory protein TRAP by the TRAP-inhibitory protein, AT. Science 293:2057-2059. [DOI] [PubMed] [Google Scholar]
- 28.Vogel, H. J., and D. M. Bonner. 1956. Acetylornithinase of Escherichia coli: partial purification and some properties. J. Biol. Chem. 218:97-106. [PubMed] [Google Scholar]
- 29.Yakhnin, H., H. Zhang, A. V. Yakhnin, and P. Babitzke. 2004. The trp RNA-binding attenuation protein of Bacillus subtilis regulates translation of the tryptophan transport gene trpP (yhaG) by blocking ribosome binding. J. Bacteriol. 186:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang, M., A. de Saizieu, A. P. G. M. van Loon, and P. Gollnick. 1995. Translation of trpG in Bacillus subtilis is regulated by the trp RNA-binding attenuation protein (TRAP). J. Bacteriol. 177:4272-4278. [DOI] [PMC free article] [PubMed] [Google Scholar]