Abstract
Barrett’s oesophagus (BO) is the primary precursor lesion for oesophageal adenocarcinoma (ADC). The natural history of metaplasia-dysplasia-carcinoma sequence remains largely unknown. HER2/neu oncogene results overexpressed/amplified in preneoplastic lesions and in ADC of the oesophagus and it has been associated with poor prognosis. Our aim was to evaluate the role of HER2 overexpression/amplification in predicting the conversion from precursor lesions to ADC. We retrospectively evaluated by univariate analysis of single variables clinical records and histological specimens of 21 patients with a confirmed diagnosis of BO and/or oesophageal dysplasia. Clinical variables included age, gender, alcohol and smoking intake, presence of symptoms (pyrosis, disphagia) and endoscopic features (length). HER2 status was studied by immunohistochemistry and fluorescence in situ hybridization (FISH) on paraffin-embedded tissue. The end-points were the occurrence of progression and the time-to-progression (TTP) from the initial histologic lesion to the worst pathological pattern. Median age at diagnosis was 63 years (range 37–84). BO median length was 4.5 cm. Progression occurred in 11 of 21 patients and median TTP was 24 months. HER2 was overexpressed/amplified in 8 of 21 (38%) patients. HER2 overexpression/ amplification and the presence of dysplasia were statistically associated with progression (P= 0.038). This study provides evidence for a possible role of HER2 in the transition from dysplasia to ADC of the oesophagus. This fact could help in identifying patients at high risk of malignant transformation.
Keywords: adenocarcinoma, Barrett’s oesophagus, dysplasia, HER-2, FISH, immunohistochemistry
Introduction
Adenocarcinoma (ADC) arising in Barrett’s oesophagus (BO) represents the most common oesophageal cancer. Its incidence has dramatically and rapidly increased compared to other malignancies, in both the United States [1, 2] and other western countries [3]. About 60% of newly diagnosed oesophageal cancers are ADC. BO is the only known histologic precursor of oesophageal ADC [4] and represents the major risk factor for the development of cancer. Oesophageal ADC is a highly aggressive neoplasia with disappointingly low survival rates. At the time of diagnosis, more than 50% of patients display metastatic or unresectable disease. Moreover, a high risk for recurrent disease is present after oesophagectomy or radical chemoradiotherapy. Thus, early detection and complete surgical resection represent the only strategy to cure oesophageal cancer [2].
Identification of neoplastic precursor lesions is, therefore, the mainstay of most surveillance and preventive programs. Histopathologic grading of dysplasia in BO is the standard method of risk stratification [5–7]. However, the heterogeneous behaviour of dysplasia must be taken into account; in fact, some cases progress to ADC [8], whereas others regress or remain stable for long periods of time after anti-reflux surgery [9]. For this reason, an accurate and reliable estimate of risk of progression to cancer is difficult to obtain.
The current state of knowledge includes several somatic genetic risk factors that correlate with an increased risk (relative risk > 10) in prospective trials [10], such as LOH in p53 and the presence of aneuploid and tetraploid (4N fraction >6%) cells, all of which provide a detection window of risk stratification extending at least to 5 years for most patients [11, 12]. More recently, a retrospective longitudinal study of epigenetic biomarkers, including methylation of p16, RUNX3 and HPP1, has been reported [13].
The role of the Her2/neu oncogene as a prognostic and predictive factor has been extensively investigated in several human neoplasms [14]. However, less information is available concerning the role of Her2 in the carcinogenesis process. Her2 has been found to be overexpressed/amplified in several dysplastic conditions, including bronchial and cervical dysplasia. In bronchial dysplasia, Her2 increases epithelial proliferation, but it does not seem to have a central role in the progression from dysplasia to invasive carcinoma [15].
Recently, it has been reported that Her2 overexpression/amplification is present in dysplasia of BO [16]. This prompted us to investigate the value of Her2 overexpression/ amplification in the Barrett’s dysplasia-carcinoma sequence (conversion from precursor lesion to ADC in this condition).
Patients and methods
Patient selection, clinical and endoscopic evaluation
We retrospectively analysed clinical records and histological specimens of patients with a confirmed diagnosis of BO or oesophageal dysplasia observed at University of Brescia between 1995 and 2005. The study cohort consisted of 21 patients, 11 of which progressed to a higher-grade lesion and 10 did not. All patients underwent endoscopic surveillance according to suggested surveillance strategy programs [17, 18]. Four biopsies were performed on each centimetre of BO and where visible lesions were present, following standardized instructions for the correct orientation of the biopsies on the filter paper for histopathological assessment, to avoid tissue sampling errors [17]. Endoscopic mucosectomies were carried out according to previously described methods [19, 20] when dysplasia was documented on BO visible lesions, according to commonly accepted criteria [21].
The clinical variables analysed included age (<65/≥65 years), gender, alcohol and smoking intake, presence of symptoms and endoscopic features (BO length <3/≥3 cm).
The Her2 status was studied by immunohistochemistry (IHC) and fluorescence in situ hybridization (FISH), on paraffin-embedded tissue. Numerical alterations of chromosome 17 (CEP17) were also evaluated by FISH.
Pathological evaluation
Immediately after sampling, the specimens were fixed in 10% neutral-buffered formalin for 24 hrs, then were included in paraffin and stained with haematoxylin-eosin (H&E) and Alcian-PAS for routine histological examination. H&E-stained slides from the resection specimens were evaluated for identification of the steps in cancer progression. ADC and precursor lesions (low-grade dysplasia, LGD; high-grade dysplasia, HGD) were diagnosed according to the WHO classification [22], as previously reported [23, 24]. The Her2 status was studied by immunohistochemistry (IHC) and FISH, on paraffin-embedded tissue. Numerical alterations of chromosome 17 (CEP17) were also evaluated by FISH.
Immunohistochemistry
Her2 receptor status was studied using the HercepTest® kit (DAKOCytomation, Carpinteria, CA, USA). Following the manufacturer’s recommendations, tissue sections mounted on slides and stored at room temperature (25°C) were stained within 4–6 weeks from sectioning, in order to maintain the anti-genicity, then the samples were counterstained with Mayer’s haematoxylin. Her2 oncoprotein expression was evaluated by two observers, following the score system suggested by the manufacturer [25] and the FDA guidelines. The immunoreaction was scored as follows: 3+= complete and intense membrane staining of >10% cells; 2+= complete but moderate staining of >10% cells, 1+= weak and incomplete staining in >10% cells; 0 = no membrane staining, or staining in <10% cells.
FISH and score for HER-2/neu
A FDA approved kit (PathVysion HER-2 DNA Probe Kit, Vysis Inc., Downers Grove, IL, USA) was used, according to the manufacturer’s recommendations. The kit consists of directly labelled fluorescent DNA probes specific for the HER-2/neu gene locus (17q11.2-q12) and a DNA probe specific for the alpha satellite DNA sequence at the centromeric region of chromosome 17 (17p11.1-q11.1). Counterstaining of nuclei was performed using DAPI (4,6-diamidino-2-phenylindole). A special amplification pattern as HER-2/neu ‘signal clusters’, usually with more than 10 confluent signals, was observed, as previously described [26, 27]. Although a gene amplification as ‘homogeneously stained regions’ is clearly evident, this pattern does not allow a precise signal enumeration. Thus, the whole area of each neoplastic lesion present in the tissue section was independently evaluated by two investigators (ER, VV) with a fluorescence microscopy (Nikon Optiphot-2, Florence, Italy) equipped with selective filters for the fluorochromes used, in high power fields (HPF; magnification 600×). FISH images were captured and elaborated using Genikon software (Nikon Instruments S.p.A, Florence, Italy). The HER-2/neu gene locus was classified as amplified if there were more than twice the number of red (Spectrum Orange labelling) HER-2/neu signals than green (Spectrum Green labelling) centromere 17 signals (ratio >2:1) per cell nucleus. The presence of more than two nuclear red signals accompanied by the same number of nuclear green signals was considered to be indicative of polisomy of chromosome 17 (ratio 1:1). Following these accepted criteria [28, 29] the cell population of each HPF was classified as displaying a disomy, a polisomy or an amplification for HER-2/neu gene.
Statistical analysis
This study was designed to verify whether Her2 had any influence on progression of Barrett’s dysplasia-carcinoma sequence. Therefore, the occurrence of progression event was considered the statistical end-point and the time during which the progression occurred was identified as the time-to-progression (TTP), and was calculated from the time of initial histologic lesion to the first detection of a worst pathological pattern. We investigated whether clinical and pathological variables were associated with progression. Variables included age (<65/≥65 years), gender, alcohol and smoking intake, presence of symptoms (pyrosis versus pyrosis and dysphagia), histology at diagnosis (BO versus LGD/HGD/ADC), HER2 status according to IHC (0–2 versus 3) and FISH (not amplified versus amplified) and endoscopic features (BO length <3/≥3 cm). The cut-off value of 65 years was chosen according to the median value of this series (median age 63 years) and we adopted the international cut-off value of 3 cm for BO length. These two variables were considered continuous while all the others were categorized. The chi-square test or the t-test were used, where appropriate, to test associations of single variables in two-way tables. P-values of 0.05 or less were chosen to reject the null hypothesis. Statistically significant variables at univariate analysis were then tested for their effect on the TTP, i.e. if they were associated to a faster progression. To do this, TTP curves according to covariates were estimated by the Kaplan–Meier method and differences between curves were analysed using the log-rank test. Statistical analysis was done by means of the (SPSS Inc., Chicago, IL, USA) 12.0 software package.
Ethical considerations
Because this was a retrospective study, no individual patient identification was involved and no study-driven clinical intervention was performed. Thus, a simplified Institutional Review Board approval for retrospective studies was obtained and no patient consent was considered necessary.
Results
Patient population
The study population included 21 patients (16 men and 5 women, median 63 years [range 37–84]) for whom adequate clinical follow-up information and histological samples were available. Demographic, endoscopic and histologic features of these patients are shown in Table 1. BO median length was 4.5 cm (range 2–10); 11/21 (52.4%) patients had a BO length ≥3 cm and 8 of them underwent neoplastic transformation.
Table 1.
Clinical and pathological features and univariate analysis of prognostic factors for TTP
| Feature | n | Chi-square test | |
|---|---|---|---|
| P | |||
| Age | <65 years | 11 | 0.75 |
| >65 years | 10 | ||
| Gender | Male | 16 | 0.90 |
| Female | 5 | ||
| Smoke | Yes | 7 | 0.73 |
| No | 14 | ||
| History of alcohol abuse | Yes | 4 | 0.65 |
| No | 17 | ||
| Symptoms | Pyrosis | 17 | 0.24 |
| Pyrosis + dysphagia | 4 | ||
| BO length | <3 cm | 10 | 0.09 |
| >3 cm | 11 | ||
| HER2 (IHC) | 0−2 | 13 | 0.04 |
| 3+ | 8 | ||
| HER2 (FISH) | Not amplified | 14 | 0.04 |
| Amplified | 7 | ||
| CEP17 | Disomic | 16 | 0.65 |
| Polysomic | 5 | ||
| Histology | BO | 13 | 0.04 |
| LGD+HGD | 8 |
Abbreviations: CEP 17, chromosome enumeration probe
BO, Barrett’s oesophagus
FISH, fluorescence in situ hybridisation
IHC, immunohistochemistry.
All patients were treated with proton pump inhibitors, and 7 of them (33%) underwent laparoscopic fundoplication for symptoms unresponsive to medical treatment. Six of 21 (29%) patients were smokers, while alcohol abuse was documented in 17 of 21 (81%) patients. All patients complained of pyrosis, whereas dysphagia was present in 3 of 21 (14%) patients. After a median follow-up of 36 months (range 12–120), all patients are alive.
Initial presentation included BO in 13 patients, LGD in 7 patients, HGD in 1 patient. Progression occurred in 11 of 21 (52%) patients and median TTP was 24 months (range 12–120). At the evaluated end-point (progression), we recognized 9 BO, 2 LGD, 4 HGD and 6 ADC (Table 2).
Table 2.
Analysis of progression of oesophageal pre-neoplastic lesions in function of time
| Patient # | Initial diagnosis | HER-2 amplification(FISH)/ overexpression (IHC) | Progression (yes/no) | Final diagnosis | HER-2 amplification(FISH)/ overexpression (IHC) | TTP or max follow-up time (months) | Status at follow-up |
|---|---|---|---|---|---|---|---|
| 1 | BO | NA / 0 | Yes | LGD | A / 3+ | 120 | Alive |
| 2 | LGD | A / 3+ | Yes | ADC | A / 3+ | 12 | Alive |
| 3 | LGD | A / 3+ | Yes | HGD | A / 3+ | 24 | Alive |
| 4 | LGD | A / 3+ | Yes | ADC | A / 3+ | 24 | Alive |
| 5 | LGD | A / 3+ | Yes | ADC | A / 3+ | 120 | Alive |
| 6 | BO | NA / 0 | Yes | ADC | NA / 1+ | 48 | Alive |
| 7 | LGD | A / 3+ | Yes | ADC | A / 3+ | 12 | Alive |
| 8 | LGD | A / 3+ | Yes | HGD | A / 3+ | 24 | Alive |
| 9 | BO | NA / 1+ | Yes | ADC | NA / 1+ | 60 | Alive |
| 10 | LGD | NA / 1+ | Yes | HGD | NA / 1+ | 24 | Alive |
| 11 | BO | NA / 1+ | Yes | LGD | NA / 1+ | 36 | Alive |
| 12 | BO | NA / 1+ | No | BO | NA / 1+ | 48 | Alive |
| 13 | BO | NA / 1+ | No | BO | NA / 1+ | 96 | Alive |
| 14 | HGD | A / 3+ | No | HGD | A / 3+ | 12 | Alive |
| 15 | BO | NA / 0 | No | BO | NA / 0 | 36 | Alive |
| 16 | BO | NA / 1+ | No | BO | NA / 1+ | 36 | Alive |
| 17 | BO | NA / 1+ | No | BO | NA / 1+ | 24 | Alive |
| 18 | BO | NA / 0 | No | BO | NA / 0 | 60 | Alive |
| 19 | BO | NA / 2+ | No | LGD | NA / 2+ | 72 | Alive |
| 20 | BO | NA / 1+ | No | BO | NA / 1+ | 48 | Alive |
| 21 | BO | NA / 0 | No | BO | NA / 0 | 72 | Alive |
Abbreviations: BO, Barrett’s oesophagus
LGD, low-grade dysplasia
HGD, high-grade dysplasia
ADC, adenocarcinoma
FISH, fluorescence in situ hybridization
IHC, immunohistochemistry
TTP, time to progression (calculated for those patients who progressed)
maximum follow-up time (calculated for non-progressors patients).
Immunohistochemical and FISH analysis of Her2 expression
Overall, a 100% positive correlation was found between Her2 gene amplification and protein overexpression score 3+. Her2 was overexpressed/amplified in 8 of 21 (38%) cases, whereas CEP17 probe identified an aneusomy in 4 of 21 (19%). Her2 was never found amplified/overexpressed in BO alone. Its amplification/overexpression always characterized dysplasia (both LGD and HGD) and ADC (Figs 1 and 2). Her2 resulted amplified in 7 of 11 patients whose initial dysplastic lesion progressed toward a higher grade and in 1 of 10 patients who did not progress. One out of 11 patients who progressed (patients #1) was negative at the baseline (where it was diagnosed as BO), but after 10 years (120 months, Table 2) of follow-up LGD with HER-2 overexpressed/ amplified was found. Aneusomy of chromosome 17 was detected in 4 of 21 (19%) patients in the whole series, with 3 patients who underwent neoplastic transformation and 1 who did not, respectively.
Figure 1.
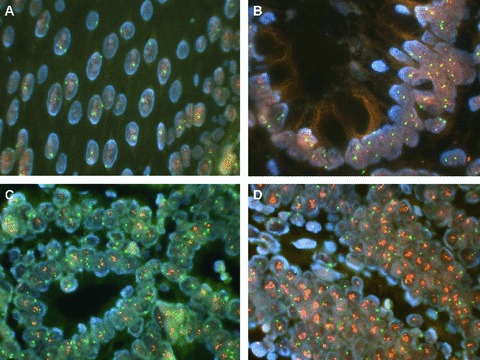
Normal oesophagus (A) and Barrett’s oesophagus (B) display a normal pattern of HER-2/neu protooncogene (red spots) and chromosome 17 probe (green spots). On the other hand, in LGD (C) and in HGD (D) the HER-2/neu gene is amplified (amplification if the ratio between HER-2/neu signals and CEP17 signals is >2) (original magnification, ×40).
Figure 2.
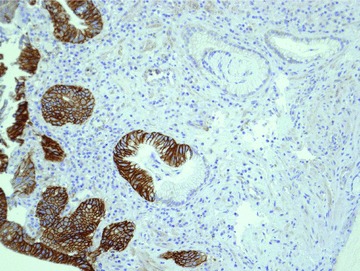
Area of metaplasia (Barrett’s oesophagus) and dysplasia stained for HER-2 by immunohistochemistry. It is possible to recognize the different expression of HER-2: completely negative in BO and overexpressed in the areas of dysplasia. Original magnification, ×40).
Analysis of prognostic factors
At univariate analysis, HER2 overexpression/amplification (P= 0.038) and the existence of LGD or HGD at diagnosis (P= 0.038) were the only variables significantly associated with the occurrence of progression. BO length did not reach statistical significance (P= 0.088) like aneusomy of chromosome 17 (P= 0.652) and age (P= 0.756) (Table 1). Among patients who progressed, the median TTP was shorter (TTP = 24 months) in Her2 positive group than in the Her2- one (TTP = 48 months) although this was not statistically significant (log-rank test P > 0.08).
Discussion
The ultimate goal of cancer surveillance and prevention programs is to identify those patients at high risk of developing a certain cancer, in order to treat the precursor condition, or to identify the invasive cancer at a very early stage. Concerning oesophageal ADC, the identification of intestinal metaplasia, i.e. BO, and dysplasia represents the major risk factor. Patients with BO have an annual incidence of oesophageal carcinoma between 0.5% and 1%. Therefore, the histopathologic classification of the grade of dysplasia in BO is the only currently accepted method for risk stratification of patients. However, progression from one lesion to another and to cancer typically occurs with a long latency [4] and shows considerable to eliminate heterogeneity. Thus, identification of different dysplastic conditions is a poor predictor of which patient and how rapidly he/she will ultimately progress to cancer.
We investigated whether an increased expression of Her2 might contribute to the development and progression of oesophageal dysplasia. Our results show that Her2 amplification/overexpression is correlated to dysplasia (LGD or HGD) and with progression to a more advanced step (HGD or ADC); in fact, we found that TTP was shorter in HER-2 positive group than in HER-2 negative group. Thus, we cannot rule out a definitive causative role for HER2 in the pathogenesis of oesophageal ADC for which further studies and larger series of patients are required.
The median TTP in our series was 24 months; this was shorter than that reported in other countries, 4–8 years [30, 31], even though data from our country suggest that the TTP for the Italian population may be shorter with respect to other populations [7].
Because metaplastic/dysplastic lesions of different grade often coexist in the same histological sample, we selected patients in which only one precursor lesion was present, or at least predominant. This allowed us to identify the role of Her2 in each individual step of the sequence. Consistent with previous reports, we were not able to identify Her2 alterations in BO alone [16], whereas its amplification/overexpression was first detected in LGD patients and was constitutively present up to ADC.
It is worth noting that FISH data overlapped those obtained from IHC 3+ scores (100% positive correlation) at the initial (7/7 cases) and the final (8/8 cases) step. The effect of analysing Her2 status by either FISH or IHC has been extensively reviewed [32–35], confirming that FISH provides a more powerful prog-nostic indicator.
Case #1 represents a peculiarity, because at baseline it was classified as BO negative for HER2 (both IHC and FISH), whereas at the final step (after 10 years) we found HER-2-positive LGD. This case was included because: (1) the patient took time to develop dysplasia, probably because at the beginning BO was HER-2-negative (not overexpressed nor amplified); (2) at present this patient shows LGD positive for HER-2 (overexpressed and amplified), and for this reason we hypothesize that the progression to the subsequent steps (HGD-ADC) could be faster and requires a closer follow-up.
The aneusomy of chromosome 17 did not have influence on BO progression (P= 0.65), probably because of the small size of our series. This was, however, a surprising finding because DNA aneuploidy/aneusomy is a recognized marker of cancer progression [36] and chromosome 17 copy number increases through cancer progression [20]. However, even though in our study chromosome 17 aneusomy was not correlated to a faster development of the neoplasm, these results are not in contrast with literature. Clinically, it is still uncertain whether an increase in Her2 copy number alone or an increase of the Her2 copies/chromosome 17 copies ratio is more useful in predicting the outcome. A recent report in breast cancer suggested that increased Her2 gene dosage may be the most important determinant of Her2 gene expression, whereas that resulting from polysomy 17 alone is rather unlikely to significantly contribute to Her2 overexpression [37].
Among clinical features, neither age nor the presence of dysphagia were significant variables. All patients had a previous history of pyrosis, and the association of dysphagia could represent a clinical indicator of an aggressive evolution. In our series, BO length (a well-recognized risk factor of progression of BO to ADC [38]) did not reach statistical significance but showed a positive correlation with progression.
The clinical significance of Her2 both as a prognostic factor and as a therapeutic molecular target is well-recognized in breast cancer [39, 40]. However, only a few reports exist concerning its role in precursor conditions, including bronchial, cervical and vulvar dysplasia [15, 41, 42]. In these reports, Her2 alterations were not found to be the major pathogenetic hallmark of the condition, but were related to higher proliferation index and contributed to a more malignant phenotype.
This is the first report describing a potential critical role for Her2 in the pathogenesis of oesophageal carcinoma. Although this study reports on a small series and validation is required on a larger analysis, we feel that several points merit attention: (1) Her2 is associated with distinct stages of dysplasia arising in BO; (2) Her2 is associated with the progression of dysplasia and shows a trend toward a shorter TTP.
As other studies (evaluating the role of Her2 in brush cytology from BO patients) are appearing in literature, and are also consistent with our results [43, 44], this should prompt further research into Her2 role in the pathogenesis of oesophageal ADC, to establish whether this is a potential biomarker with prognostic significance and a putative candidate for targeted chemoprevention [45].
Acknowledgments
We wish to thank Professors Vanio Vannini and Cesare Danesino, University of Pavia, tutors of Dr. Elisa Rossi for the PhD in Pathology and Genetics, and Ms Anna Galletti, Ms Lucia Fontana, Ms Monica Brotto, Ms Paola Begni for providing technical support. Dr. Elisa Rossi is the recipient of a grant from Ingenio Finlombarda S.p.a., Regione Lombardia, Italy.
References
- 1.Paulson TG, Reid BJ. Focus on Barrett’s esophagus and esophageal adenocarcinoma. Cancer Cell. 2004;6:11–6. doi: 10.1016/j.ccr.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 2.DeMeester SR. Adenocarcinoma of the esophagus and cardia: a review of the disease and its treatment. Ann Surg Oncol. 2006;13:12–30. doi: 10.1245/ASO.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay Y, et al. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 4.Kelloff GJ, Lippman SM, Dannenberg AJ, et al. Progress in chemoprevention drug development: the promise of molecular biomarkers for prevention of intraepithelial neoplasia and cancer. A plan to move forward. Clin Cancer Res. 2006;12:3661–97. doi: 10.1158/1078-0432.CCR-06-1104. [DOI] [PubMed] [Google Scholar]
- 5.Reid BJ, Levine DS, Longton G, et al. Predictors of progression to cancer in barrett’s esophagus: baseline histology and flow cytometry to identify low- and high-risk patient subsets. Am J Gastroenterol. 2000;95:1669–76. doi: 10.1111/j.1572-0241.2000.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buttar NS, Wang KK, Sebo TJ, et al. Extent of high-grade dysplasia in Barrett’s esophagus correlates with risk of adenocarcinoma. Gastroenterology. 2001;120:1630–9. doi: 10.1053/gast.2001.25111. [DOI] [PubMed] [Google Scholar]
- 7.Conio M, Blanchi S, Lapertosa G, et al. Long-term endoscopic surveillance of patients with Barrett’s esophagus. Incidence of dysplasia and adenocarcinoma: a prospective study. Am J Gastroenterol. 2003;98:1931–9. doi: 10.1111/j.1572-0241.2003.07666.x. [DOI] [PubMed] [Google Scholar]
- 8.Oberg S, Wenner J, Johansson J, et al. Barrett esophagus: risk factors for progression to dysplasia and adenocarcinoma. Ann Surg. 2005;242:49–54. doi: 10.1097/01.sla.0000167864.46462.9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang EY, Morris CD, Seltman AK, et al. The effect of antireflux surgery on esophageal carcinogenesis in patients with Barrett esophagus: a systematic review. Ann Surg. 2007;246:11–21. doi: 10.1097/01.sla.0000261459.10565.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pepe MS, Etzioni R, Feng Z, et al. Phases of biomarker development for early detection of cancer. J Natl Cancer Inst. 2001;93:1054–61. doi: 10.1093/jnci/93.14.1054. [DOI] [PubMed] [Google Scholar]
- 11.Reid BJ, Prevo LJ, Galipeau PC, et al. Predictors of progression in Barrett’s esophagus. II: baseline 17p (p53) loss of heterozigosity identifies a patient subset at increased risk for neoplastic progression. Am J Gastroenterol. 2001;96:2839–48. doi: 10.1111/j.1572-0241.2001.04236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reid BJ, Blount PL, Rabinovitch PS. Biomarkers in Barrett’s esophagus. Gastrointest Endosc Clin N Am. 2003;13:369–97. doi: 10.1016/s1052-5157(03)00006-0. [DOI] [PubMed] [Google Scholar]
- 13.Schulmann K, Sterian A, Berki A, et al. Inactivation of p16, RUNX3 and HPP1 occurs early in Barrett’s-associated neoplastic progression and predicts progression risk. Oncogene. 2005;24:4138–48. doi: 10.1038/sj.onc.1208598. [DOI] [PubMed] [Google Scholar]
- 14.Ross JS, Fletcher JA. The HER-2/neu oncogene: prognostic factor, predictive factor and target for therapy. Semin Cancer Biol. 1999;9:125–38. doi: 10.1006/scbi.1998.0083. [DOI] [PubMed] [Google Scholar]
- 15.Merrick DT, Kittelson J, Winterhalder R, et al. Analysis of c-ErbB1/epidermal growth factor receptor and c-ErbB2/HER-2 expression in bronchial dysplasia: evaluation of potential targets for chemoprevention of lung cancer. Clin Cancer Res. 2006;12:2281–8. doi: 10.1158/1078-0432.CCR-05-2291. [DOI] [PubMed] [Google Scholar]
- 16.Rossi E, Villanacci V, Bassotti G, et al. HER-2/neu in Barrett esophagus. A comparative study between histology, immunohistochemistry, and fluorescence in situ hybridization. Diagn Mol Pathol. 2006;15:125–30. doi: 10.1097/01.pdm.0000213455.22527.f7. [DOI] [PubMed] [Google Scholar]
- 17.Sampliner RE. Practice parameters Committee ACG: update guidelines for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol. 2002;97:1888–95. doi: 10.1111/j.1572-0241.2002.05910.x. [DOI] [PubMed] [Google Scholar]
- 18.Cengia G, Missale G, Minelli L, et al. Screening for and surveillance of Barrett’s esophagus is clinically indicated. Dig Dis. 2007;25:197–202. doi: 10.1159/000103884. [DOI] [PubMed] [Google Scholar]
- 19.Conio M, Repici A, Cestari R, et al. Endoscopic mucosal resection for high-grade dysplasia and intramucosal carcinoma in Barrett’s esophagus: an Italian experience. World J Gastroenterol. 2005;11:6650–5. doi: 10.3748/wjg.v11.i42.6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cestari R, Villanacci V, Rossi E, et al. Fluorescence in situ hybridization to evaluate dysplasia in Barrett’s esophagus: a pilot study. Cancer Lett. 2007;251:278–87. doi: 10.1016/j.canlet.2006.11.033. [DOI] [PubMed] [Google Scholar]
- 21.Lopes CV, Hela M, Pesenti C, et al. Circumferential endoscopic resection of Barrett’s esophagus with high-gradedysplasia or early adenocarcinoma. Surg Endosc. 2007;21:820–4. doi: 10.1007/s00464-006-9187-3. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton SR, Aaltonen LA. WHO pathology and genetics: tumors of digestive system. IARC Press; 2000. Lyon: [Google Scholar]
- 23.Villanacci V, Rossi E, Zambelli C, et al. COX-2, CDX2, and CDC2 immunohistochemical assessment for dysplasia-carcinoma progression in Barrett’s esophagus. Dig Liver Dis. 2007;39:305–11. doi: 10.1016/j.dld.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Villanacci V, Bellone G, Battaglia E, et al. Ski/SnoN expression in the sequence metaplasma-dysplasia-adenocarcinoma of Barrett’s esophagus. Hum Pathol. 2008;39:403–9. doi: 10.1016/j.humpath.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Dako Corp. 2000. Dako Hercep Test: Anti-Her2 IHC System for Immunoenzymatic Staining (package insert. Carpinteria, CA, USA).
- 26.Ishikawa T, Kobayashi M, Mai M, et al. Amplification of the c-erbB-2 (HER-2/neu) gene in gastric cancer cells. Detection by fluorescence in situ hybridization. Am J Pathol. 1997;151:761–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Walch A, Bink K, Gais P, et al. Evaluation of c-erbB-2 overexpression and Her-2/neu gene copy number heterogeneity in Barrett’s adenocarcinoma. Anal Cell Pathol. 2000;20:25–32. doi: 10.1155/2000/947249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ellis IO, Dowsett M, Bartlett J, et al. Recommendations for HER2 testing in the UK. J Clin Pathol. 2000;53:890–2. doi: 10.1136/jcp.53.12.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ellis IO, Bartlett J, Dowsett M, et al. Best Practice No 176: updated recommendations for HER2 testing in the UK. J Clin Pathol. 2004;57:233–7. doi: 10.1136/jcp.2003.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharma P, Falk GW, Weston AP, et al. Dysplasia and cancer in a large multicenter cohort of patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2006;4:566–72. doi: 10.1016/j.cgh.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Lim CH, Treanor D, Dixon MF, et al. Low-grade dysplasia in Barrett’s esophagus has a high risk of progression. Endoscopy. 2007;39:581–7. doi: 10.1055/s-2007-966592. [DOI] [PubMed] [Google Scholar]
- 32.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Oncologist. 1998;3:237–52. [PubMed] [Google Scholar]
- 33.Mitchell MS, Press MF. The role of immunohistochemistry and fluorescence in situ hybridization for HER2/neu in assessing the prognosis of breast cancer. Semin Oncol. 1999;26:108–16. [PubMed] [Google Scholar]
- 34.Pauletti G, Dandekar S, Rong H, et al. Assessment of methods for tissue-based detection of the HER-2/neu alteration in human breast cancer: a direct comparison of fluorescence in situ hybridization and immunohistochemistry. J Clin Oncol. 2000;18:3651–64. doi: 10.1200/JCO.2000.18.21.3651. [DOI] [PubMed] [Google Scholar]
- 35.Ross JS, Fletcher JA, Bloom KJ, et al. Targeted therapy in breast cancer: the HER-2/neu gene and protein. Mol Cell Proteomics. 2004;3:379–98. doi: 10.1074/mcp.R400001-MCP200. [DOI] [PubMed] [Google Scholar]
- 36.Rabinovitch PS, Longton G, Blount PL, et al. Predictors of progression in Barrett’s esophagus III: baseline flow cytometric variables. Am J Gastroenterol. 2001;96:3071–83. doi: 10.1111/j.1572-0241.2001.05261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Downs-Kelly E, Yoder BJ, Stoler M, et al. The influence of polysomy 17 on HER2 gene and protein expression in adenocarcinoma of the breast: a fluorescent in situ hybridization, immunohistochemical, and isotopic mRNA in situ hybridization study. Am J Surg Pathol. 2005;29:1221–7. doi: 10.1097/01.pas.0000165528.78945.95. [DOI] [PubMed] [Google Scholar]
- 38.Weston AP, Sharma P, Mathur S, et al. Risk stratification of Barrett’s esophagus: updated prospective multivariate analysis. Am J Gastroenterol. 2004;99:1657–66. doi: 10.1111/j.1572-0241.2004.30426.x. [DOI] [PubMed] [Google Scholar]
- 39.Ross JS, Fletcher JA. The HER-2/neu oncogene in breast cancer: prognostic factor, predictive factor, and target for therapy. Stem Cells. 1998;16:413–28. doi: 10.1002/stem.160413. [DOI] [PubMed] [Google Scholar]
- 40.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 41.Berchuck A, Rodriguez G, Kamel A, et al. Expression of epidermal growth factor receptor and HER-2/neu in normal and neoplastic cervix, vulva, and vagina. Obstet Gynecol. 1990;76:381–7. [PubMed] [Google Scholar]
- 42.Chang JL, Tsao YP, Liu DW, et al. The expression of type I growth factor receptors in the squamous neoplastic changes of uterine cervix. Gynecol Oncol. 1999;73:62–71. doi: 10.1006/gyno.1998.5301. [DOI] [PubMed] [Google Scholar]
- 43.Rygiel AM, van Baal JW, Milano F, et al. Efficient automated assessment of genetic abnormalities detected by fluorescence in situ hybridization on brush cytology in a Barrett esophagus surveillance population. Cancer. 2007;109:1980–8. doi: 10.1002/cncr.22643. [DOI] [PubMed] [Google Scholar]
- 44.Rygiel AM, Milano F, Ten Kate FJ, et al. Assessment of chromosomal gains as compared to DNA content changes is more useful to detect dysplasia in Barrett’s esophagus brush cytology specimens. Genes Chromosomes Cancer. 2008;47:396–404. doi: 10.1002/gcc.20543. [DOI] [PubMed] [Google Scholar]
- 45.Villanacci V, Rossi E, Grisanti S, et al. Targeted therapy with trastuzumab in dysplasia and adenocarcinoma arising in Barrett’s esophagus: a translational approach. Minerva Gastroenterol Dietol. 2008;54:347–53. [PubMed] [Google Scholar]


