Abstract
Inflammation promotes colorectal carcinogenesis. Tumour growth often generates a hypoxic environment in the inner tumour mass. We here report that, in colon cancer cells, the expression of the pro-inflammatory enzyme cyclooxygenase-2 (COX-2) associates with that of the hypoxia response gene carbonic anhydrase-IX (CA-IX). The COX-2 knockdown, achieved by the stable infection of a COX-2 specific short harpin RNA interference (shCOX-2), down-regulates CA-IX gene expression. In colorectal cancer (CRC) cells, PGE2, the main COX-2 gene products, promotes CA-IX gene expression by ERK1/2 activation. In normoxic environment, shCOX-2 infected/CA-IX siRNA transfected CRC cells show a reduced level of active metalloproteinase-2 (MMP-2) that associates with a decreased extracellular matrix invasion capacity. In presence of hypoxia, COX-2 gene expression and PGE2 production increase. The knockdown of COX-2/CA-IX blunts the survival capability of CRC cells in hypoxia. At a high cell density, a culture condition that creates a mild pericellular hypoxic environment, the expression of COX-2/CA-IX genes is increased and triggers the invasive potential of colon cancer cells. In human colon cancer tissues, COX-2/CA-IX protein expression levels, assessed by Western blot and immunohistochemistry, correlate each other and increase with tumour stage. In conclusion, these data indicate that COX-2/CA-IX interplay promotes the aggressive behaviour of CRC cells.
Keywords: COX-2, inflammation, CA-IX, hypoxia, colon cancer
Introduction
Colorectal cancer (CRC) is a leading cause of cancer-related mortality in Western countries [1]. Clinical and experimental data show that inflammation promotes CRC in animal models and human beings [2–4]. Epidemiological reports indicate that the long-term administration of anti-inflammatory drugs decreases the risk of colon carcinoma, as well as induces the regression of polyp growth in familial adenomatous polyposis patients [5]. This phenomenon has been linked to the inhibition of cyclooxygenase-2 (COX-2), the enzyme that synthesizes the pro-inflammatory mediators PGE2[6]. COX-2 and PGE2 levels are increased in colorectal carcinomas and promote invasiveness, angiogenesis and metastatic potential in CRC cells [7–10]. In animal models, COX-2 gene knockout (KO), or COX-2 inhibition by selective drugs reduce tumour incidence, growth rate and tumour multiplicity [11]. COX-2 expression plays an important role in hypoxia survival of colon cancer cells [12]. Hypoxia occurs as a consequence of solid tumours growth and represents a key promoting regulatory factor for cancer growth and metastasis development [13–16]. Carbonic anhydrase-IX (CA-IX) is one of the genes that gained much attention as a possible marker for tumour hypoxia in vivo[17]. CA-IX protein expression also correlates with poor prognosis in solid tumours [18, 19]. Interestingly, CA-IX protein is expressed only in a fraction of CRC hypoxic cells, suggesting that micro-environmental factors, other than hypoxia, up-regulate in vivo CA-IX expression [20]. In fact, CA-IX gene expression is induced by hypoxia-independent mechanisms, such as the activation of ERK pathway [21–23]. Notably, ERK1/2 pathway activation is triggered by COX-2/PGE2 axis up-regulation [24]. We here sought to investigate the interplay between COX-2 and CA-IX genes in CRC cells and we explored the correlation between the expression of COX-2 and CA-IX proteins in CRC tissues.
Methods
Cell culture
Human colon cancer cells HT-29, HCT-116, HCA-7 and Caco-2 were obtained from American Type Culture Collection (Manassas, VA, USA), cultured at 37°C in 5% CO2 in Dulbecco’s modified Eagle’s medium (DMEM, Cambrex Bio Science, Bergamo, Italy) supplemented with 10% heat-inactivated foetal calf serum (FCS). Hypoxia culture condition (2% O2) was generated in a humidified incubator supplied with 95% N2/5% CO2 (Thermoforma, Thermo, Waltham, MA, USA). Desferoxamine (DFX) and dimethylsulphoxide (DMSO) were purchased from Sigma (Sigma Chemical, St. Louis, MO, USA). The selective inhibitor SC-236 was purchased from Calbiochem (Calbiochem, San Diego, CA, USA). Cell death was evaluated by Trypan blue staining (Sigma): both floating and attached cells were assessed for cell death assay.
PGE2 ELISA assay
PGE2 levels were measured by ELISA (Cayman Chemical, Ann Arbor, MI, USA). HT-29 cells (5 × 105 cells/well) were seeded in a 6-well plate in 2 ml of fresh medium. After 48 hrs, supernatants were collected and centrifuged at 400 ×g for 5 min. at room temperature, then 100 μl of supernatant from each sample were used for the test. Samples were measured in triplicate and this experiment was repeated three separate times (n= 3).
COX-2 specific short hairpin RNA (shRNA)
Constructs coding for anti-COX-2 shRNA were prepared as described elsewhere [25]. Forward and reverse sequences for anti-COX-2 shRNA construct were 5′-GATCCCCAACTGCT CAACACCGGAATTTCAAGAG AATTCCGGTGTTGAGCAGTTTTTTTGGAA-3′ and 5′-AGCTTTTCCAAAAAAACTG CTCAACACCGGAATTCT CTTGAAATTCCGGTGTTGA GCAGTTGGG-3′, respectively, 64 nucleotides-containing oligos synthesized and purchased from Proligo (Proligo, Denver, CO, USA). Steps for cloning oligonucleotides into pSUPER.retro vector were made accordingly to pSUPER RNAi system protocol (http://www.oligoengine.com).
CA-IX and COX-2 specific short interfering RNA (siRNA)
CA-IX and appropriate control, scramble, SCR, siRNA were purchased from Invitrogen (Invitrogen, Carlsbad, CA, USA). COX-2/SCR siRNA were purchased from Proligo [25].
Luciferase assay and transfection procedure
Carbonic anhydrase promoter activity was assessed by using a pGL-3 vector containing a luciferase gene under the control of a −174/+63 fragment of the carbonic anhydrase IX promoter (CA−IX Luc, kindly provided by Dr. J. Pastorek, Slovak Academy of Sciences, Slovak Republic, Bratislava). Sixty percent confluent cells, plated on 0.75 cm2 wells, were co-transfected with 500 ng of CA-IX Luc and 20 ng of Thymidine Kinase promoter driven Renilla Luciferase gene reporter vector (TK-Renilla, Promega, Madison, WI, USA) to control for transfection efficiency. All the transfection procedures were performed using Lipofectamine 2000. Luciferase activity was assessed by Dual-Luciferase® Reporter Assay System according the manufacturer’s instructions (Promega, Madison, WI, USA).
Cell invasion assay and gelatin zymography
Invasion assay was performed using Boyden chambers with 8 μm pore polycarbonate membranes (New Technologies Group, Italy). Metalloproteinase-2 (MMP-2) activity was determined by gelatin zymography. Briefly, proteins of collected media were precipitated with 1:4 (vol/vol) ice-cold methanol overnight at −20°C, solubilized with sample buffer in absence of mercaptoethanol (1 M Tris-HCl pH 6.8, 2% SDS, 10% glycerol) and loaded into 10% SDS-polyacrylamide gel containing 1 mg/ml gelatine (Sigma). Gel was then incubated in a developing buffer (100 mM Tris-HCl, 10 mM CaCl2, 20 mM NaCl pH 7.6) overnight at 37°C, stained for 2 hrs with 1% Coomassie Brilliant Blue R-250, and finally de-stained in a solution containing 10% acetic acid and 40% methanol. MMP-2 proteolytic activity was quantified using a semi-automated image analysis system (GelDoc, Biorad, Hercules, CA, USA).
Patients, immunohistochemistry (IHC) procedure and quantitative analysis
Samples from 87 sporadic CRC patients, histologically classified and graded according to WHO guidelines and pTNM (UICC) pathological staging criteria, were assessed in this investigation (Table 1) [26]. Formalin-fixed, paraffin-embedded specimens were available from 82 patients. Tissue samples frozen in liquid nitrogen, immediately after surgical procedure, were available from 17 patients, including 12 belonging to the above 82 cases set. For IHC, tissue sections were stained using polyclonal goat anti-COX-2 (C-20) (Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) and monoclonal mouse anti-CA-IX (M-75, kindly provided by Dr. J. Pastorek) [27, 28]. COX-2 and CA-IX protein expression were evaluated using a semi-quantitative score, called Multiplicative Quickscore, MQ [29]. Briefly, the percentage of immuno-positive cells at each field (100 X) was scored as follows: Percentage ≤1%= Score 0, > 1%≤25%= Score 1, >25%≤50%= Score 2, >50%≤75%= Score 3, >75%= Score 4; Staining intensity was scored as: Weak = Score 1, Moderate = Score 2, Strong = Score 3. The product of percentage and intensity mean values was grouped as follows: <1 = Negative (NEG), ≥1 <4 = LOW, ≥4 <8 = Intermediate (INT), ≥8 = HIGH. Tissues were obtained after patients written informed consent and the use of tissues was approved by the local ethics committee.
Table 1.
Clinico-pathological characteristics of CRC patients
| Features | Patients n= 87 | |
|---|---|---|
| n | % | |
| Age, years | ||
| Mean | 69.3 ± 1.4 | |
| Range | 32–89 | |
| Sex | ||
| Female | 37 | 42.5 |
| Male | 50 | 57.5 |
| Lesion site | ||
| Right colon | 35 | 40.2 |
| Left colon | 52 | 59.8 |
| Tumour diameter (mm) | ||
| Mean | 46.3 ± 1.7 | |
| Range | 5.5–75 | |
| Histological grading | ||
| Poorly differentiated | 8 | 9.2 |
| Moderately differentiated | 64 | 73.6 |
| Well differentiated | 15 | 17.2 |
| Pathological staging | ||
| Stage I | 10 | 11.5 |
| Stage II | 35 | 40.2 |
| Stage III | 35 | 40.2 |
| Stage IV | 7 | 8.1 |
Protein extraction and Western blot analysis
Frozen tissues and cells were homogenized for 15 min. using lysis buffer (50 mM Tris pH 7.4, 150 mM NaCl, 2 mM MgCl2, 1% Triton X-100, 10% glycerol, 2 mM EGTA, 1 mM DTT) containing protease and phosphatase cocktail inhibitors (Sigma). Samples were processed according to standard procedures (see Table S1).
RNA extraction and RT-PCR analysis
Total RNA was isolated using TRIzol Reagent and reverse-transcribed using Superscript II (Invitrogen). Primers and annealing temperatures are described in Table S2. The PCR products were separated through 1.8–2.5% agarose gel.
Statistical analysis
Continuous variables were analysed by anova test (unequal variance assumed) followed by post hoc test adjusted for multiple comparisons. Correlation between variables was assessed by Spearman correlation coefficient. Ordinal and non-normal distributed variables were analysed by non-parametric rank test (Mann–Whitney test). All the statistical analyses were performed using SPSS 10.1 Package (SPSS, Chicago, IL, USA).
Results
COX-2 expression and PGE2 production promote an ERK-dependent CA-IX up-regulation
This investigation started with the observation that high COX-2 expression level is accompanied by the up-regulation of CA-IX protein in colorectal cell lines (Fig. 1A). We therefore sought to investigate the functional relationship between COX-2 and CA-IX gene expression. We purposely examined HT-29 cells sub-population infected with a pSUPER.retro vector, encoding a COX-2 specific (shCOX-2) or control (shCTR) short hairpin RNA. RT-PCR and Western blot (WB) analyses revealed that shCOX-2 HT-29 infected cells showed reduced levels of CA-IX mRNA and protein compared to shCTR infected cells (Fig. 1B). Given that CA-IX gene is regulated by two molecular pathways, namely HIF-1α[30] and MAP kinase ERK1/2 [21–23], we then evaluated the protein levels of HIF-1α, ERK1/2 and phosphorylated ERK1/2 (pERK) in shCTR/COX-2 HT-29 infected cells. We found lower amounts of pERK protein in shCOX-2 HT-29 infected cells compared to shCTR HT-29 infected ones, whereas no HIF-1α expression was found in such a culture condition (Fig. 1C). Moreover, due to the capability of COX-2 and PGE2 activate ERK pathway, we tested the role of ERK1/2 protein in COX-2/CA-IX interplay. We found that the PGE2-dependent up-regulation of CA-IX gene was halted by the administration of the MEK1/ERK inhibitor UO-126 (Fig. 1D). The PGE2/ERK-dependent regulation of CA-IX gene expression was then evaluated by CA-IX gene promoter activity assay (CA-IX Luc) in HT-29 cells. We observed a lower level of CA-IX Luc activity in shCOX-2 HT-29 infected compared to shCTR infected cells, and we found that PGE2 administration triggered an ERK-dependent increase of CA-IX Luc activity (Fig. 1E). Overall, these data indicate that COX-2 gene up-regulation, by means of PGE2/ERK activation, promotes CA-IX gene expression in colon cancer cells.
Figure 1.
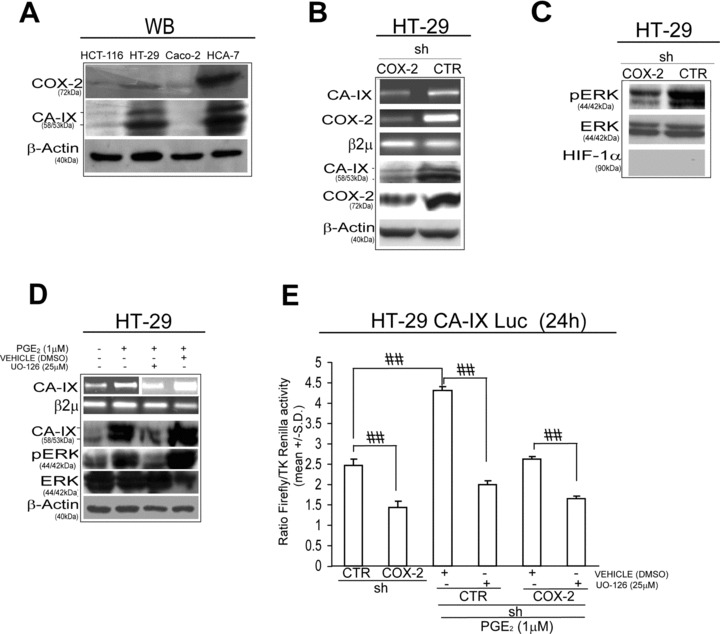
COX-2 up-regulates CA-IX gene expression. (A) Western blot (WB) analysis of CA-IX and COX-2 protein level in 4 colon cancer cell lines: HCT-116, HT-29, Caco-2 and HCA-7. (B) HT-29 cells, stably infected with a pSUPER.retro vector encoding a COX-2 specific (shCOX-2) or control (shCTR) short hairpin RNA: RT-PCR analysis of CA-IX and COX-2 mRNA level, WB analysis of CA-IX, COX-2 and β-Actin. (C) WB analysis of phosphorylated ERK 1/2 (pERK), total ERK and HIF-1α protein level in shCTR/COX-2 HT-29 infected cells (β-Actin is reported in panel B). (D) HT-29 cells administered with PGE2 (1 μM) for 24 hrs in presence/absence of UO-126 (25 μM, 1 hr pre-treatment) or control vehicle DMSO: RT-PCR analysis of CA-IX mRNA level and WB analysis of CA-IX, pERK and ERK protein level. (E) Luciferase assay of −174/+63 fragment of CA-IX promoter (CA-IX Luc) in shCTR/COX-2 HT-29 infected cells, in presence/absence of PGE2 (1 μM) and/or UO-126 (25 μM) or DMSO for 24 hrs. Luciferase activity is expressed as a ratio over Thymidine Kinase (TK) driven Renilla Luciferase activity. Beta-2 microglobulin (β2μ) was assessed as quantitative control for RT-PCR analysis. β-Actin was assessed as quantitative control for WB analysis. Data are expressed as mean ± S.D. of three replicates (n= 3) (anova test, ##P < 0.0001, post hoc test corrected for multiple comparison).
COX-/A-IX interplay promotes invasiveness of colon cancer cells
ERK activation promotes the invasive behaviour of cancer cells [31, 32]. We therefore assessed the role of COX-2/CA-IX interplay in the regulation of the invasive potential of CRC cells. We found that shCOX-2 HT-29 infected cells and HT-29 cells transiently transfected with a CA-IX specific siRNA disclosed a reduced invasive potential compared to matched controls (Fig. 2A). Notably, no cell death was observed in such experimental conditions (see Fig. 2A).
Figure 2.
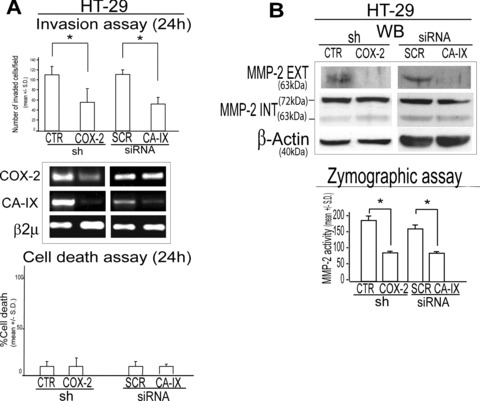
COX-2/CA-IX interplay promotes invasive potential of colon cancer cells. (A) Boyden chamber invasion assay (24 hrs), RT-PCR analysis of COX-2 and CA-IX mRNA level and cell death assay (24 hrs) in shCTR/COX-2 HT-29 infected cells and in HT-29 cells transiently transfected with a CA-IX specific or scramble (SCR) siRNA (1 μg, 48 hrs pre-exposure). (B) WB analysis of active, extracellular (EXT) and inactive, intracellular (INT) protein levels and zymographic assay of MMP-2 in shCTR/COX-2 HT-29 infected cells and SCR/CA-IX siRNA transiently transfected HT-29 cells (1 μg, pre-exposure for 48 hrs). β2μ was assessed as quantitative control for RT-PCR analysis. β-Actin was assessed as quantitative control for WB analysis. Data are expressed as mean ± S.D. of three replicates (n= 3) (anova test, *P= 0.001).
Due to the strict connection between COX-2 and metalloproteinases (MMPs) expression and activity [33, 34], we hypothesized that CA-IX may control the activation of MMPs [35, 36]. Western blot analysis revealed lower levels of extracellular/active, but not intracellular/inactive, MMP-2 protein level in shCOX-2 HT-29 infected cells and in CA-IX siRNA HT-29 transfected cells compared to their matched controls (Fig. 2B). Moreover, zymographic assay showed a reduced activity of MMP-2 enzyme in shCOX-2 HT-29 infected cells and in CA-IX siRNA HT-29 transfected cells, compared to their matched controls (Fig. 2B). These data suggest that the COX-2/CA-IX interplay promotes the invasive capacity of colon cancer cells.
COX-2 gene expression enhances CA-IX-dependent hypoxia survival
CA-IX is a hypoxia survival gene [17, 23]. Recent data show that COX-2 is up-regulated upon hypoxia exposure [12]. We therefore sought to investigate the regulation of the COX-2/CA-IX pathway in presence of hypoxia, by exposing shCTR/COX-2 HT-29 infected cells to the hypoxia mimetic Desferoxamine (DFX, 100 μM) and to hypoxic culture conditions (2% O2) [12]. We found that PGE2 production was increased upon 2% O2 exposure as well as that CA-IX promoter activity and mRNA level was increased upon exposure to DFX 100 μM in shCTR, but not in shCOX-2 HT-29 infected cells (Fig. 3A). ShCOX-2 infected cells disclosed a higher susceptibility to DFX or 2% O2 induced cell death, a phenomenon that was also observed in HT-29 cells transfected with CA-IX siRNA, compared to matched controls (Fig. 3B). PGE2 administration yielded an increase in the survival capacity of HT-29 cells in presence of DFX 100 μM that was hampered by the co-administration of UO-126 (Fig. 3C). The role of COX-2/CA-IX interplay in hypoxia survival of colon cancer cells was confirmed in HCT-116 and HCA-7 colon cancer cells in which we found that the exposure to DFX promotes the expression of CA-IX mRNA in a COX-2-dependent fashion (Fig. S1). Finally, the administration of the COX-2 specific inhibitor SC-236 down-regulated CA-IX mRNA level and triggered cell death in HT-29, HCT-116 and HCA-7 cells exposed to DFX (100 μM, 48 hrs) (Fig. 3D). Notably, no cell death was observed in cells treated with SC-236, in absence of DFX (100 μM for 48 hrs) (data not shown). These data support the argument that COX-2/CA-IX interplay sustains hypoxia survival in CRC cells.
Figure 3.
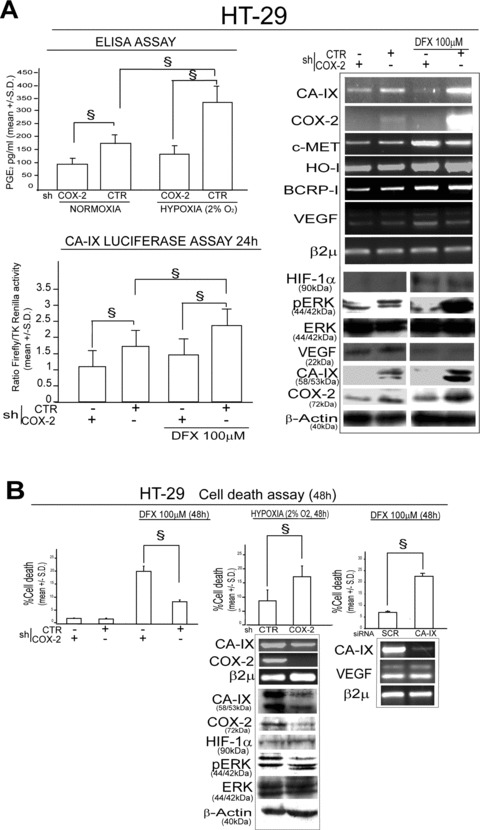
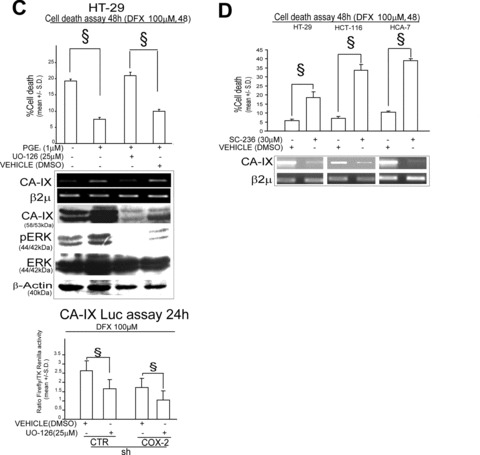
COX-2/CA-IX interplay sustains hypoxia survival in HT-29 cells. (A) shCTR/COX-2 HT-29 infected cells: ELISA assay of PGE2 levels in presence/absence of hypoxic conditions (2% O2); CA-IX Luc assay (24 hrs, Luciferase activity is expressed as a ratio over TK-driven Renilla luciferase activity), RT-PCR analysis of CA-IX, COX-2, c-MET, HO-I, BCRP-I and VEGF mRNA level and WB analysis of HIF-1α, pERK, ERK, VEGF, CA-IX, COX-2 protein level in Desferoxamine (DFX 100 μM, 48 hrs) exposed cells; (B) shCTR/COX-2 HT-29 infected cells in presence/absence of DFX 100 μM or cultured for 48 hrs in hypoxic condition (2% O2): Cell death assay, RT-PCR analysis of CA-IX, COX-2 mRNA level, WB analysis of CA-IX, COX-2, HIF-1α, pERK and ERK protein level. HT-29 cells transiently transfected with SCR/CA-IX siRNA (1 μg, 48 hrs pre-exposure) in presence of 100 μM DFX: Cell death assay (48 hrs), RT-PCR analysis of CA-IX and VEGF mRNA level. (C) HT-29 cells administered with PGE2 (1 μM) for 48 hrs in presence/absence of UO-126 (25 μM, 1 hr pre-treatment) or DMSO and exposed to 100 μM DFX for 48 hrs: Cell death assay 48 hrs and RT-PCR analysis of CA-IX mRNA level, WB analysis of CA-IX, pERK and ERK protein level and CA-IX Luc assay (24 hrs). (D) Cell death assay 48 hrs and RT-PCR analysis of CA-IX mRNA level in HT-29, HCT-116 and HCA-7 cells exposed to 100 μM DFX for 48 hrs in co-presence with COX-2 specific inhibitor SC-236 (30 μM) or vehicle (DMSO). β2μ was assessed as quantitative control for RT-PCR analysis. β-Actin was assessed as quantitative control for WB analysis. Data are expressed as mean ± S.D. of three replicates (n= 3) (anova test, §P < 0.001, post hoc test adjustment for multiple comparison was applied when required).
High-density culture condition up-regulates COX-2/CA-IX interplay
High-density culture conditions associate with CA-IX gene up-regulation, as well as promote pericellular hypoxic environment [21, 37, 38]. We found an increase of COX-2/pERK/CA-IX protein level in HT-29 cells cultured at high (2 × 105cells/cm2) respect to low (0.4 × 105cells/cm2) density (Fig. 4A). We also observed a cell density-dependent increase of the invasive potential in shCTR, but not in shCOX-2 HT-29 infected cells (Fig. 4B). As expected, the increase in the invasive capacity of HT-29 cells cultured at high density was blocked by CA-IX, but not SCR siRNA transfection (Fig. 4C). In the same culture condition, no cell death was observed in shCOX-2 infected or CA-IX siRNA transfected cells (data not shown). Overall, the data reported show that COX-2/CA-IX interplay is up-regulated and modulates invasive behaviour in mild hypoxic conditions.
Figure 4.
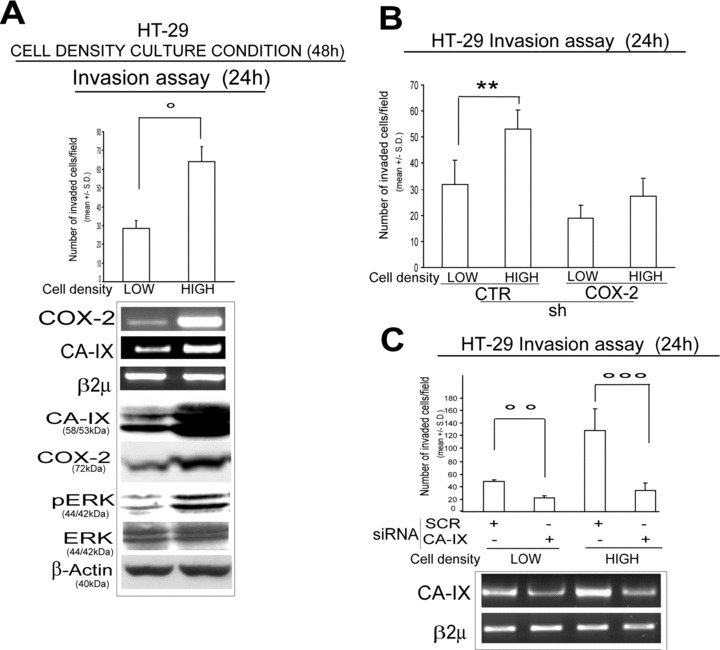
The COX-2/CA-IX up-regulation promotes invasive potential of colorectal cancer cells in high-density culture condition. (A) HT-29 cells cultured at low (0.4 × 105 cells/cm2) and high (2 × 105 cells/cm2) density condition for 48 hrs: Boyden chamber invasion assay (24 hrs), RT-PCR analysis of COX-2 and CA-IX mRNA level and WB analysis of CA-IX, COX-2, pERK, total ERK protein level. (B) Boyden chamber invasion assay (24 hrs) of shCTR/COX-2 HT-29 infected cells cultured at low- and high-density condition. (C) HT-29 cells cultured at low- and high-density condition and transiently transfected with SCR and CA-IX siRNA (1 μg, pre-exposure for 48 hrs): Boyden chamber invasion assay (24 hrs) and RT-PCR analysis of CA-IX mRNA level. β2μ was assessed as quantitative control for RT-PCR analysis. β-Actin was assessed as quantitative control for WB analysis. Data are expressed as mean ± S.D. of three replicates (n= 3) (anova test, **P= 0.006, °P= 0.003, °°P= 0.01, °°°P < 0.001).
High COX-2 and CA-IX protein expression correlates with tumour stage in CRC specimens
The results above suggest us that the COX-2/CA-IX interplay controls malignant features of CRC cells. We then assessed the expression of such genes in tissues from CRC patients, by quantitative evaluation of IHC and WB analyses. IHC analysis revealed a significant correlation between COX-2 and CA-IX protein expression for all stages (Spearman correlation coefficient: ρ= 0.500, P= 0.0005), being the correlation present also when stages were analysed separately (Spearman correlation coefficient: Stage II ρ= 0.640, P= 0.0004 (Fig. 5A); Stage III ρ= 0.366, P= 0.04; Stage IV ρ= 0.847, P= 0.016) except for Stage I (Spearman correlation coefficient: Stage I ρ= 0.307, P= 0.460) (Fig. 5A). WB and IHC analyses revealed an increase of COX-2 and CA-IX protein expression level in stage III-IV CRC compared to stage I-II CRC (Figs. 5B, C and S2). These data indicate that COX-2/CA-IX protein expression in CRC specimens is correlated and increased with tumour stage.
Figure 5.
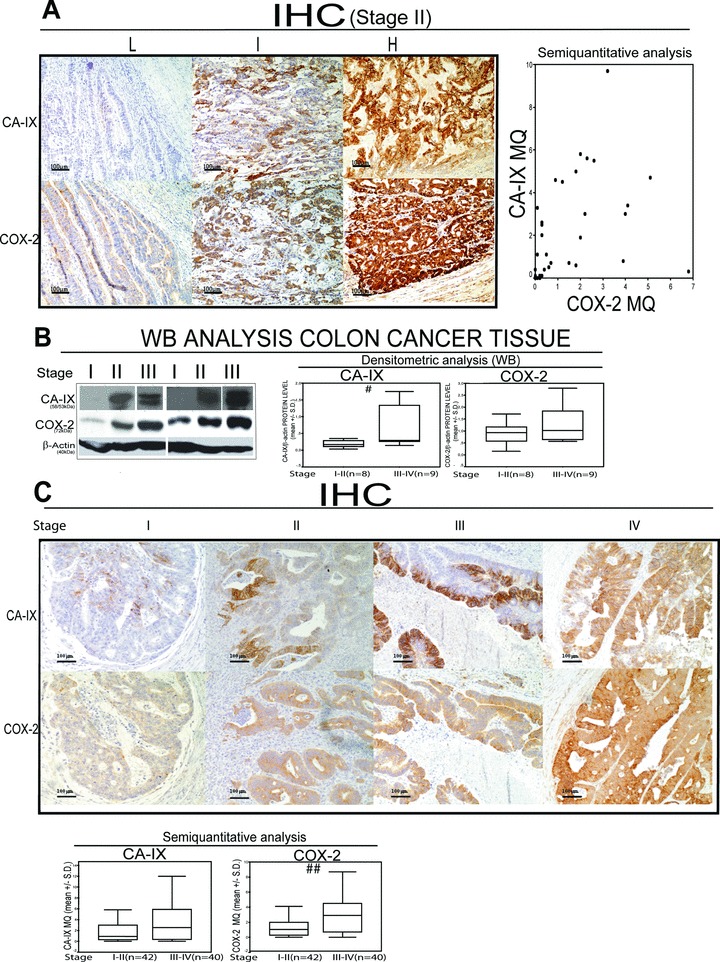
COX-2 and CA-IX protein level correlate with CRC stage. (A) Representative IHC in stage II CRC tissue expressing low (L), intermediate (I) and high (H) CA-IX/COX-2 proteins, scatter plot of immunohistochemical (IHC) semi-quantitative evaluation (Multiplicative Quick score, MQ) of CA-IX and COX-2 protein expression in stage II, CRC (n= 35); (B) WB analysis of CA-IX and COX-2 protein level in 6 representative CRC tumour tissue samples and densitometric analysis of CA-IX and COX-2 protein level assessed by WB analysis in 17 CRC tumour tissue samples (Mann–Whitney test: stage I-II versus stage III-IV: CA-IX #P= 0.042; COX-2 P= 0.48). β-Actin was assessed as quantitative control for WB analysis; (C) Representative IHC analysis of CA-IX and COX-2 protein expression level and IHC semi-quantitative evaluation of CA-IX and COX-2 protein expression level in a cohort of 82 CRC tissues (Mann–Whitney test: CA-IX MQ, P= 0.14; COX-2 MQ, P= 0.0011; stage I-II versus stage III-IV: CA-IX MQ, P= 0.075; COX-2 MQ, ##P= 0.003).
Discussion
In this investigation, we show that, in CRC cells, the pro-inflammatory enzyme COX-2 up-regulates the expression of the hypoxia response gene CA-IX, throughout the PGE2-dependent activation of ERK-1/2. In CRC cells, COX-2/CA-IX axis elicits aggressive features (invasive behaviour and hypoxia survival). Such activities have been previously associated to the expression of these genes in separate experimental models [12, 23]. Here, we provide evidence for the existence of a COX-2/CA-IX axis that promotes malignant behaviour in CRC cells.
CA-IX gene expression has been previously shown to be induced by HIF-1α transcription factor and ERK activation [12, 21, 22]. Here, we show that the up-regulation of COX-2 expression upon hypoxia exposure contributes to the ERK-dependent up-regulation of CA-IX expression. The ERK-dependent regulation of CA-IX gene expression has been observed in cells cultured at high-density culture condition that creates a hypoxia-like environment, by decreasing pericellular pO2[37, 38]. Accordingly, we show that, in dense culture condition, COX-2 up-regulation promotes CA-IX gene expression and invasive behaviour. These data indicate that hypoxia and mild hypoxic environment, generated by cell overgrowth, trigger COX-2/CA-IX axis to enhance malignant features in CRC cells.
CA-IX gene expression contributes to the acidification of the extracellular environment by catalysing the hydration of carbon dioxide to bicarbonate and protons, a phenomenon that induces the activation of extracellular matrix enzymes, MMPs [36]. Noteworthy, PGE2 production and ERK activation have been previously demonstrated to enhance invasiveness and MMPs activity [31, 32]. We here report that COX-2/CA-IX axis up-regulation increases the activity of MMP-2, an enzyme linked to CRC malignancy [39].
CA-IX protein expression is regarded as surrogate marker of hypoxia in vivo[17]. The expression of CA-IX correlates with poor prognosis in several cancers [18, 19]. In CRC tissues, CA-IX gene expression has been previously associated with cell proliferation status [20, 40]. In this investigation, we show that CA-IX expression in CRC tissues correlates with stage, thus suggesting that the enzyme expression increases with the extent of tumour aggressiveness. COX-2 expression has been previously correlated with poor prognosis in CRC [6, 41]. It may be therefore proposed to jointly evaluate COX-2 and CA-IX expression in cohorts of CRC patients in which long-term survival is available, in order to verify whether combination of these markers may identify subsets of patients with different risk of relapse.
COX-2 is a major player of inflammation [6]. Inflammatory molecules play a crucial role in CRC growth and malignancy and inflammatory cells promote an aggressive behaviour in CRC cells [42–44]. Based on the data presented, it may be proposed that the CA-IX-dependent aggressive behaviour is also under the control of inflammation. Such an inflammation-driven CA-IX gene up-regulation may explain the poor in vivo correlation occurring between CA-IX protein expression and the presence of hypoxia in CRC tissues [20]. Speculatively, CA-IX in vivo expression may be a marker of COX-2 activation as a consequence of the exposure of CRC cells to a variety of environmental conditions such as hypoxia, cell overgrowth and inflammation.
COX-2 inhibitors have been proposed as therapeutic tools for CRC patients [45]. A plenty of CA-IX inhibitors have been synthesized so far [46]. Our data suggest that the pharmacological down-regulation of COX-2/CA-IX axis may be a strategy to counteract the aggressiveness of CRC cells [47–49].
In conclusion, the data here presented indicate that the aggressive phenotype of CRC cells is controlled by a pathway, which can be up-regulated by two environmental conditions that have been linked to CRC growth and malignancy, i.e. inflammation (COX-2) and hypoxia (CA-IX). Studies regarding the role of this tight relationship in CRC prognosis and therapy response are warranted.
Acknowledgments
We thank Dr.ssa Lenzi and Prof.ssa C. Fimognari (Department of Pharmacology and Toxicology Bologna) for hypoxia experiments technical support, Prof V. Tomasi (FIRB 2003) Department of Experimental Biology, Bologna. Supports for this research were provided by University of Bologna (ex 60% RFO funds), Cornelia Pallotti and Roberto Pallotti Fundation to M.B., FIRB project RBNE03KZRJ to P.C and Fondazione Cassa di Risparmio of Bologna to GB. We also thank Fondazione Cassa di Risparmio and Fondazione del Monte in Bologna for supporting the Center for Applied Biomedical Research.
No conflicts of interest were declared.
Supporting Information
Fig. S1. COX-2/CA-IX up-regulation sustains hypoxiasurvival in colorectal cancer cells. (A) HCT-116 cellstransiently transfected with CTR/COX-2 siRNA (1 mg, 48 hrspre-exposure) and HCA-7 cells stably infected with shCTR/COX-2pSUPER.retro vector exposed to 100 mM DFX for 48 hrs: Cell deathassay 48 hrs and RT-PCR analysis of CA-IX and COX-2 mRNA level.(B) HCT-116 cells pre-treated with PGE2 1 mM for24 hrs and/or transiently transfected with CA-IX/SCR siRNA (1 mg,24 hrs pre-exposure) and then exposed to DFX 100 mM for 48 hrs:Cell death assay 48 hrs and RT-PCR analysis of CA-IX mRNA level.Data are expressed as mean ± S.D. of three replicates(n = 3) (ANOVA test, §p < 0.001,Post Hoc test adjustment for multiple comparison was applied when required).
Fig. S2. COX-2/CA-IX expression in CRC tissues. Representative immunohistochemical staining of COX-2 and CA-IX protein level in stage I-III-IV. CRC cancer tissue samples (scale bar, 500 mm,100 mm, 20 mm). IHC photos represent both the mass of the tumour (M) and the tumour host interface (THI).
Table S1. Western blot antibodies and conditions. Legends: TBS-TB buffer (20 mmol/L Tris, pH 7.6, 150 mmol/L NaCl, 0.1% Tween 20, 5% bovine serum albumin) TBS-TM buffer (20 mmol/L Tris, pH 7.6, 150 mmol/L NaCl, 0.1% Tween 20, 5% non-fat dry milk).
Table S2. Primers sequences and conditions. Legends:COX-2: cyclooxygenase-2; CA-IX: carbonic Anhydrase-IX;b2m: B-2 microglobulin; C-MET: Hepatocyte Growth Factorreceptor; VEGF: vascular endothelial growth factor; HO-1: Hemeoxygenase-1; BCRP-1: Breast Cancer Resistance Protein-1.
Please note: Blackwell Publishing are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item
References
- 1.Edge SB, Sobin LH, Page DL, et al. Colon cancer survival rates with the new American Joint Committee on Cancer sixth edition staging. J Natl Cancer Inst. 2005;97:463–4. doi: 10.1093/jnci/dji080. author reply 464–5. [DOI] [PubMed] [Google Scholar]
- 2.Taniguchi K, Kakinuma S, Tokairin Y, et al. Mild inflammation accelerates colon carcinogenesis in Mlh1-deficient mice. Oncology. 2007;71:124–30. doi: 10.1159/000100522. [DOI] [PubMed] [Google Scholar]
- 3.Zhang J, Stirling B, Temmerman ST, et al. Impaired regulation of NF-kappaB and increased susceptibility to colitis-associated tumorigenesis in CYLD-deficient mice. J Clin Invest. 2006;116:3042–9. doi: 10.1172/JCI28746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Pei Z. Bacteria, inflammation, and colon cancer. World J Gastroenterol. 2006;14:6741–6. doi: 10.3748/wjg.v12.i42.6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med. 2000;342:1946–52. doi: 10.1056/NEJM200006293422603. [DOI] [PubMed] [Google Scholar]
- 6.Brown JR, DuBois RN. COX-2: a molecular target for colorectal cancer prevention. J Clin Oncol. 2005;23:2840–55. doi: 10.1200/JCO.2005.09.051. [DOI] [PubMed] [Google Scholar]
- 7.Eberhart CE, Coffey RJ, Radhika A, et al. Up-regulation of cyclooxygenase-2 gene-expression in human colorectal adenomas and adenocarcinomas. Gastroenterology. 1994;107:1183–8. doi: 10.1016/0016-5085(94)90246-1. [DOI] [PubMed] [Google Scholar]
- 8.Backlund MG, Mann JR, Dubois RN. Mechanisms for the prevention of gastrointestinal cancer: the role of prostaglandin E2. Oncology. 2005;69:S28–32. doi: 10.1159/000086629. [DOI] [PubMed] [Google Scholar]
- 9.Tsujii M, Kawano S, Tsuji S, et al. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998;93:705–16. doi: 10.1016/s0092-8674(00)81433-6. [DOI] [PubMed] [Google Scholar]
- 10.Sinicrope FA, Gill S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004;23:63–75. doi: 10.1023/a:1025863029529. [DOI] [PubMed] [Google Scholar]
- 11.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc (D716) knockout mice by inhibition of cyclooxygenase 2 (COX-2) Cell. 1996;87:803–9. doi: 10.1016/s0092-8674(00)81988-1. [DOI] [PubMed] [Google Scholar]
- 12.Kaidi A, Qualtrough D, Williams AC, et al. Direct transcriptional up-regulation of cyclooxygenase-2 by hypoxia-inducible factor (HIF)-1 promotes colorectal tumor cell survival and enhances HIF-1 transcriptional activity during hypoxia. Cancer Res. 2006;66:6683–91. doi: 10.1158/0008-5472.CAN-06-0425. [DOI] [PubMed] [Google Scholar]
- 13.Harris AL. Hypoxia-a key regulatory factor in tumor growth. Nat Rev Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 14.Tatum JL, Kelloff GJ, Gillies RJ, et al. Hypoxia: importance in tumor biology, non invasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 15.Graeber TG, Osmanian C, Jacks T, et al. Hypoxia mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 16.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–39. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 17.Swinson DE, Jones JL, Richardson D, et al. Carbonic anhydrase IX expression, a novel surrogate marker of tumor hypoxia, is associated with a poor prognosis in non-small-cell lung cancer. J Clin Oncol. 2003;21:473–82. doi: 10.1200/JCO.2003.11.132. [DOI] [PubMed] [Google Scholar]
- 18.Hussain SA, Ganesan R, Reynolds G, et al. Hypoxia-regulated carbonic anhydrase IX expression is associated with poor survival in patients with invasive breast cancer. Br J Cancer. 2007;96:104–9. doi: 10.1038/sj.bjc.6603530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brennan DJ, Jirstrom K, Kronblad A, et al. CA IX is an independent prognostic marker in premenopausal breast cancer patients with one to three positive lymph nodes and a putative marker of radiation resistance. Clin Cancer Res. 2006;12:6421–31. doi: 10.1158/1078-0432.CCR-06-0480. [DOI] [PubMed] [Google Scholar]
- 20.Goethals L, Debucquoy A, Perneel C, et al. Hypoxia in human colorectal adenocarcinoma: comparison between extrinsic and potential intrinsic hypoxia markers. Int J Radiat Oncol Biol Phys. 2006;65:246–54. doi: 10.1016/j.ijrobp.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Kopacek J, Barathova M, Dequiedt F, et al. MAPK pathway contributes to density- and hypoxia-induced expression of the tumor-associated carbonic anhydrase IX. Biochim Biophys Acta. 2005;1729:41–9. doi: 10.1016/j.bbaexp.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 22.Kaluz S, Kaluzová M, Stanbridge EJ. The role of extracellular signal-regulated protein kinase in transcriptional regulation of the hypoxia marker carbonic anhydrase IX. J Cell Biochem. 2006;97:207–16. doi: 10.1002/jcb.20633. [DOI] [PubMed] [Google Scholar]
- 23.Sansone P, Storci G, Giovannini C, et al. p66Shc/Notch-3 interplay controls self-renewal and hypoxia survival in human stem/progenitor cells of the mammary gland expanded in vitro as mammospheres. Stem Cells. 2007;25:807–15. doi: 10.1634/stemcells.2006-0442. [DOI] [PubMed] [Google Scholar]
- 24.Krysan K, Reckamp KL, Dalwadi H, et al. Prostaglandin E2 activates mitogen-activated protein kinase/Erk pathway signaling and cell proliferation in non-small cell lung cancer cells in an epidermal growth factor receptor-independent manner. Cancer Res. 2005;65:6275–81. doi: 10.1158/0008-5472.CAN-05-0216. [DOI] [PubMed] [Google Scholar]
- 25.Strillacci A, Griffoni C, Spisni E, et al. RNA interference as a key to knockdown overexpressed cyclooxygenase-2 gene in tumour cells. Br J Cancer. 2006;94:1300–10. doi: 10.1038/sj.bjc.6603094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamilton SR, Aaltonen LA. Pathology and genetics of tumours of the digestive system WHO classification of tumours. IARC Press; 2000. Tumors of the colon and rectum; p. 103. In:. Lyon:. [Google Scholar]
- 27.Chrastina A, Zavada J, Parkkila S, et al. Biodistribution and pharmacokinetics of 125I-labeled monoclonal antibody M75 specific for carbonic anhydrase IX, an intrinsic marker of hypoxia, in nude mice xenografted with human colorectal carcinoma. Int J Cancer. 2003;105:873–8. doi: 10.1002/ijc.11142. [DOI] [PubMed] [Google Scholar]
- 28.Zat’ovicova M, Tarabkova K, Svastova E, et al. Monoclonal antibodies generated in carbonic anhydrase IX-deficient mice recognize different domains of tumour-associated hypoxia-induced carbonic anhydrase IX. J Immunol Methods. 2003;282:117–34. doi: 10.1016/j.jim.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 29.Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–8. doi: 10.1136/jcp.48.9.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wykoff CC, Beasley NJ, Watson PH, et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 2000;60:7075–83. [PubMed] [Google Scholar]
- 31.Sheng H, Shao J, Washington MK, et al. Prostaglandin E2 increases growth and motility of colorectal carcinoma cells. J Biol Chem. 2001;276:18075–81. doi: 10.1074/jbc.M009689200. [DOI] [PubMed] [Google Scholar]
- 32.Saxena NK, Sharma D, Ding X, et al. Concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–507. doi: 10.1158/0008-5472.CAN-06-3075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byun JH, Lee MA, Roh SY, et al. Association between cyclooxygenase-2 and matrix metalloproteinase-2 expression in non-small cell lung cancer. Jpn J Clin Oncol. 2006;36:263–8. doi: 10.1093/jjco/hyl024. [DOI] [PubMed] [Google Scholar]
- 34.Lee KW, Kim MS, Kang NJ, et al. H-Ras selectively up-regulates MMP-9 and COX-2 through activation of ERK1/2 and NF-kappaB: an implication for invasive, phenotype in rat liver epithelial cells. Int J Cancer. 2006;119:1767–75. doi: 10.1002/ijc.22056. [DOI] [PubMed] [Google Scholar]
- 35.Svastova E, Hulikova A, Rafajova M, et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 2004;577:439–45. doi: 10.1016/j.febslet.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 36.Swietach P, Vaughan-Jones RD, Harris AL. Regulation of tumor pH and the role of carbonic anhydrase 9. Cancer Metastasis Rev. 2007;26:299–310. doi: 10.1007/s10555-007-9064-0. [DOI] [PubMed] [Google Scholar]
- 37.Uenoyama Y, Seno H, Fukuda A, et al. Hypoxia induced by benign intestinal epithelial cells is associated with cyclooxygenase-2 expression in stromal cells through AP-1-dependent pathway. Oncogene. 2006;25:3277–85. doi: 10.1038/sj.onc.1209359. [DOI] [PubMed] [Google Scholar]
- 38.Jakubickova L, Biesova Z, Pastorekova S, et al. Methylation of the CA9 promoter can modulate expression of the tumor-associated carbonic anhydrase IX in dense carcinoma cell lines. Int J Oncol. 2005;26:1121–7. [PubMed] [Google Scholar]
- 39.Hilska M, Roberts PJ, Collan YU, et al. Prognostic significance of matrix metalloproteinases-1, -2, -7 and -13 and tissue inhibitors of metalloproteinases-1, -2, -3 and -4 in colorectal cancer. Int J Cancer. 2007;121:714–23. doi: 10.1002/ijc.22747. [DOI] [PubMed] [Google Scholar]
- 40.Saarnio J, Parkkila S, Parkkila AK, et al. Immunohistochemical study of colorectal tumors for expression of a novel transmembrane carbonic anhydrase, MN/CA IX, with potential value as a marker of cell proliferation. Am J Pathol. 1998;153:279–85. doi: 10.1016/S0002-9440(10)65569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soumaoro LT, Uetake H, Higuchi T, et al. Cyclooxygenase-2 expression: a significant prognostic indicator for patients with colorectal cancer. Clin Cancer Res. 2004;10:8465–71. doi: 10.1158/1078-0432.CCR-04-0653. [DOI] [PubMed] [Google Scholar]
- 42.Cleven AH, van Engeland M, Wouters BG, et al. Stromal expression of hypoxia regulated proteins is an adverse prognostic factor in colorectal carcinomas. Cell Oncol. 2007;29:229–40. doi: 10.1155/2007/945802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oosterling SJ, van der Bij GJ, Meijer GA, et al. Macrophages direct tumour histology and clinical outcome in a colon cancer model. J Pathol. 2005;207:147–55. doi: 10.1002/path.1830. [DOI] [PubMed] [Google Scholar]
- 44.Petersen VC, Baxter KJ, Love SB, et al. Identification of objective pathological prognostic determinants and models of prognosis in Dukes’ B colon cancer. Gut. 2002;51:65–9. doi: 10.1136/gut.51.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liao Z, Mason KA, Milas L. Cyclo-oxygenase-2 and its inhibition in cancer: is there a role. Drugs. 2007;67:821–45. doi: 10.2165/00003495-200767060-00001. [DOI] [PubMed] [Google Scholar]
- 46.Winum JY, Thiry A, Cheikh KE, et al. Carbonic anhydrase inhibitors. Inhibition of isoforms I, II, IV, VA, VII, IX, and XIV with sulfonamides incorporating fructopyranose-thioureido tails. Bioorg Med Chem Lett. 2007;17:2685–91. doi: 10.1016/j.bmcl.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 47.Dogne JM, Thiry A, Pratico D, et al. Dual carbonic anhydrase-cyclooxygenase-2 inhibitors. Curr Top Med Chem. 2007;7:885–91. doi: 10.2174/156802607780636717. [DOI] [PubMed] [Google Scholar]
- 48.Stubbs M, McSheehy PM, Griffiths JR, et al. Causes and consequences of tumour acidity and implications for treatment. Mol Med Today. 2000;6:15–9. doi: 10.1016/s1357-4310(99)01615-9. [DOI] [PubMed] [Google Scholar]
- 49.Winum JY, Rami M, Scozzafava A, et al. Carbonic anhydrase IX: a new druggable target for the design of antitumor agents. Med Res Rev. 2008;28:445–63. doi: 10.1002/med.20112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting info item
Supporting info item
Supporting info item
Supporting info item
Supporting info item


