Abstract
Tight junction (TJ) disruptions have been demonstrated both in vitro and more recently in vivo in infection. However, the molecular basis for changes of TJ during ischaemia-reperfusion (I/R) injury is poorly understood. In the present study, intestinal damage was induced by I/R in an animal model. As assessed by TUNEL and propidium iodide uptake, we showed that I/R injury induced apoptosis as well as necrosis in rat colon, and the frequency of apoptotic and necrotic cells reached the maximum at 5 hrs of reperfusion. Immunofluorescence microscopy revealed that claudins 1, 3 and 5 are strongly expressed in the surface epithelial cells of the colon; however, labelling of all three proteins was present diffusely within cells and no longer focused at the lateral cell boundaries after I/R. Using Western blot analysis, we found that distribution of TJ proteins in membrane microdomains of TJ was markedly affected in I/R injury rats. Occludin, ZO-1, claudin-1 and claudin-3 were completely displaced from TX-100 insoluble fractions to TX-100 soluble fractions, and claudin-5 was partly displaced. The distribution of lipid raft marker protein caveolin-1 was also changed after I/R. I/R injury results in the disruption of TJs, which characterized by relocalization of the claudins 1, 3 and 5 and an increase in intestinal permeability using molecular tracer measurement. I/R injury altered distribution of TJ proteins in vivo that was associated with functional TJ deficiencies.
Keywords: ischaemia/reperfusion injury, permeability, tight junction, claudins, intestinal barrier function
Introduction
The intestinal epithelium functions as a major local defence barrier. In healthy individuals the intestinal barrier is constituted of an intact layer of epithelial cells, which are tightly connected by membrane fusions of tight junction (TJ) [1]. The intercellular TJ plays crucial roles for the intestinal epithelium. TJs provide the protective barrier between the lumen and paracellular space. It acts as a physical and functional barrier against the penetration of bacteria and dynamically regulates the exchange of solutes and ions [2]. Impairment of epithelial barrier function could therefore result in an increased susceptibility to infection and inflammation [3–5]. Ischaemia-reperfusion (I/R) injury is a common clinical event, which is manifested as mucosal damage and a breakdown of the intestinal barrier function. Several reports have indicated that I/R could induce apoptosis in rat intestine [6, 7]. And increased epithelial apoptosis led to increase in paracellular permeability [8]. However, the molecular basis for barrier dysfunction in I/R is not understood in detail.
Primary components of TJs are transmembrane elements such as occludin [9, 10] and the claudins [11] as well as peripheral membrane proteins ZO-1 [12]. Impaired intestinal TJ has been described in the pathological conditions such as stress, burn injury and inflammatory bowel disease [13–17]. Alterations in TJ architecture was mainly attributed to redistribution of the TJ proteins [1, 18]. Changes in expression and distribution of claudins lead to discontinuous TJs in active Crohn’s disease (CD) [19]. I/R injury leads to destruction of the intestinal epithelial barrier function in part due to the release of pro-inflammatory cytokines [20, 21]. Our previous study demonstrated alteration of TJ protein expression by pro-inflammatory cytokines [22]. Since impaired intestinal barrier function is often the result of changes in TJ protein expression, such a mechanism may also be assumed for I/R injury. To date, some studies have reported changes of TJ protein expression after I/R injury. Localized expression of claudin-2 and claudin-4 was altered [23]. The differences in distribution of occludin were noted during I/R injury [24]. Previous studies have demonstrated that claudins 1, 3 and 5 are present in the colon [25, 26]. Based on these findings, we assessed apoptosis in the colon of I/R injury rats with TUNEL and paracellular permeability using small molecular tracer biotin. Immunofluorescence microscopy was used to investigate the distribution of claudin-1, 3 and 5 in colon sections, and subcellular localization of these TJ proteins by detergent resistant membrane (DRM) analysis. The influence of I/R injury on the ultrastructure of TJ was also examined with electron microscopy. I/R injury altered localization of TJ proteins in vivo resulting in functional TJ deficiencies.
Materials and methods
Chemicals and reagents
EZ-link Sulfo-NHS-Biotin was obtained from Pierce Chemical Co. (IL, USA). Antibodies to claudin-1, 3 and 5 were from Zymed Laboratories Inc. (San Francisco, CA, USA). Secondary antibodies conjugated to horseradish peroxidase were purchased from Amersham (Piscataway, NJ, USA). All secondary antibodies conjugated to Alexa fluorochromes, the Alexa 488-conjugated streptavidin and propidium iodide (PI) were purchased from Molecular Probes (Eugene, OR, USA). The ApopTag Fluorescein in situ Apoptosis Detection kit was purchased from Chemicon International Inc. (Temecula, CA, USA).
Animal model
This study was approved by the Institutional Animal Care and Use Committee of the Nanjing University and the Principles of Laboratory Animal Care (NIH publication No. 86–23, revised in 1985) were followed. The experiments were carried out on male Wistar rats weighing between 250 to 300 g. After being acclimatized for 1 week to our laboratory condition, rats were fasted overnight but allowed free access to water. All animals were anesthetized with an intraperitoneal injection of 50 mg/kg/body weight of sodium pentobarbital. Then, abdominal incision was performed. As described by Megison et al.[27], the superior mesenteric artery (SMA) was exposed and occluded by an atraumatic vascular artery clamp. Afterwards, ischaemia of intestine was initiated for 60 min. and the laparotomy incision was closed. At the end of ischaemia, the abdomen was reopened and reperfusion was allowed by the removal of the vascular clamp, and reperfusion of the mesenteric vasculature was confirmed by the return of pulsation to the vascular arcade [28]. The incision was closed again, and all animals were maintained supine and kept warm with radiant lamps. Seven rats of each group were killed at 1, 3 and 5 hrs after reperfusion, respectively. Sham animals underwent the same operative procedure with the exception of the SMA clamping served as control.
Histopathological examination
Tissue specimens were fixed in 10% formalin, embedded in paraffin, sectioned, and stained with haematoxylin and eosin. Damage to the intestinal specimens was assessed in a blinded manner by an experienced pathologist according to the histopathological scoring system of Paola et al.[29]. The score was graded as: 0, no damage; 1, focal epithelial oedema and necrosis; 2, diffuse swelling and necrosis of the villi; 3, necrosis with presence of neutrophil infiltrate in the submucosa; score 4, widespread necrosis with massive neutrophil infiltrate and haemorrhage.
Barrier permeability measurements
EZ-link Sulfo-NHS-Biotin (0.443 kD) was used as a small molecular tracer. It was diluted to 2 mg/ml in PBS containing 1 mM CaCl2- just prior to use. The rats were killed by cervical dislocation, abdomen was opened and the most caudal region of the colon was cut. Biotin was slowly added into the lumen of the colon for 3.5 min. via syringe attached to a 22G blunt end needle. The region of colon just rostral to the area contacting the syringe was removed (1 cm) and placed into 3.7% paraformaldehyde in phosphate buffered saline (PBS) pH 7.3 for 3 hrs. The tissue was then washed three times in PBS and sectioned by Wax-It Histology Services. Tissue sections were incubated with a 1:500 dilution of streptavidin conjugated to Alexa 488 for 30 min. and imaged by a Leica TCS SP2 confocal scanning microscope (Leica Microsystems, Heidelberg GmbH, Mannheim, Germany).
Tissue immunolocalization
Tissue was fixed in 3% paraformaldehyde and 5 μm sections were cut by Wax-It Histology Services, Inc., and treated with 0.2% Triton X-100 in PBS buffer. The samples were washed extensively in PBS and blocked with 5% normal goat serum in PBS containing 0.05% Tween-20 and 0.1% bovine serum albumin. Primary antibodies of rabbit anti-claudin-1 and anti-claudin-5, and mouse anti-claudin-3 antibodies were used at a concentration of 5 μg/ml and incubated overnight at 4°C and then washed and incubated with the secondary antibodies conjugated to Alexa 635 for 60 min. The slides were again washed extensively and stained with DAPI (4′, 6′-diamidino-2-phenylindole). The tissue was visualized using a Leica TCS SP2 confocal scanning microscope (Leica Microsystems).
Isolation of detergent resistant membranes and Western blot analysis
The DRMs of TJs were isolated according to the previous method [16]. Mucosa samples were homogenized in lysis buffer (50 mM Tris, 25 mM KCl, 5 mM MgCl2-.6H2-O, 2 mM ethylenediaminetetraacetic acid, 40 mM NaF, 4 mM Na3-VO4-, pH 7.4) containing 1% Triton X-100 and protease inhibitor mixture solution. The resulting extract was then brought to 40% sucrose by addition of an equal volume of 80% sucrose. The mixture was placed in the bottom of an ultracentrifuge tube and overlayered with 2 ml of 30% sucrose then 25%, 20% and 5% sucrose (2 ml each). All sucrose solutions were prepared in lysis buffer (without detergent). The gradients were then centrifuged at 250,000 ×g, 4°C for 18 hrs in a Ti90 rotor with an Optima L-80XP ultracentrifuge (Beckman Coulter Inc., Fullerton, CA, USA). After centrifugation, 10 fractions (1 ml each) were collected from top to bottom of the tube one by one and stored at –80°C.
For Western blotting, the extracted fractions were separated on SDS-PAGE gels and electrophoretically transferred onto polyvinylidene difluoride membranes (Bio-Rad, Hercules, CA, USA). The membranes were then incubated with the appropriate primary antibodies overnight at 4°C, and then with the secondary antibodies for 1 hr at room temperature. The bound proteins were subsequently detected with the enhanced chemiluminescent detection system (Amersham Biosciences, Chalfont St Giles, UK). Quantification of the blots was achieved densitometrically using Quantity One 1-D analysis software (Bio-Rad).
Transmission electron microscopy
The specimens from control and I/R injury rats were fixed with 4% glutaraldehyde for 2 hrs and post-fixed with 1% OsO4-. Subsequently, the tissue samples were dehydrated by graded ethanol and embedded in Epon 812. Fragments were cut and stained with uranyl acetate and lead citrate. Ultrathin sections were examined in a Hitachi H-600 (Tokyo, Japan) transmission electron microscope at 75 kV at a magnification of 20,000. From each animal an average of 100 consecutive TJ were observed in a blinded manner. TJs were considered to have increased permeability when the electron-dense marker penetrated into the junctional complex [30].
TUNEL assay
TUNEL staining was performed with an Apoptag Fluorescein in situ Apoptosis Detection kit according to the manufacturer’s instructions. Briefly, the tissue cryosections were fixed in 1% paraformaldehyde for 10 min. at room temperature. After rinsing with PBS, sections were incubated with terminal deoxynucleotidyl transferase enzyme mixture for 60 min. at 37°C in a humidified chamber in the dark. The nucleotides in the enzyme mixture are digoxigenin labelled. After then the anti-digoxigenin antibody conjugated to fluorescein was applied to the tissues and incubated for 30 min. in the dark. Tissues were viewed by laser confocal microscopy. Necrosis was identified by the uptake of PI according to the previous method [31] with minor modification. Briefly, the 10 μm cryopreserved tissue sections were incubated with 0.1 μg/ml PI for 3 min. and the nuclei was counterstained with DAPI. And then necrosis was detected by laser confocal microscopy.
Statistical analysis
Statistical analysis was performed by one-way anova statistical test using SigmaStat software. Turkey’s multiple comparison test was used for post hoc analysis. And P < 0.05 was considered statistically significant. All data were presented as mean ± S.E.M.
Results
I/R injury caused mucosa damage
The intestinal tissue damage induced by I/R injury was examined with light microscopy. As shown in Fig. 1A, I/R caused significant tissue damage, which ranged from villous denudation to focal necrosis, ulceration, haemorrhage and architectural disintegration. I/R resulted in an increase in the histopathological score from a mean of 0.57 ± 0.22 in sham-operated rats to 3.0 ± 0.31 (P < 0.001) after 5 hrs reperfusion (Fig. 1B). In addition, the degree of histological destruction increased as the reperfusion period was prolonged (Fig. 1A and B).
Figure 1.
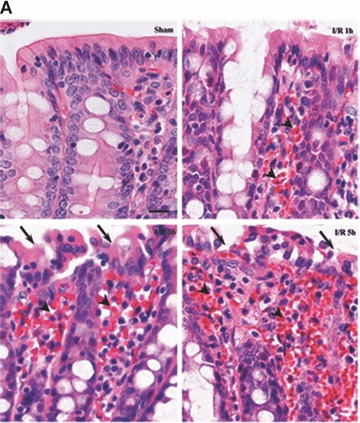
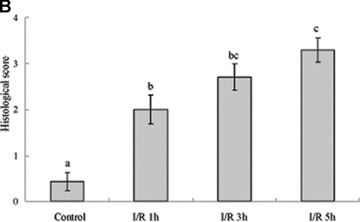
Histopathological findings of haematoxylin & eosin-stained sections of the rat colon (A) (×400). Control group showing normal histopathology. I/R group animals at 1, 3 and 5 hrs after reperfusion demonstrating denuded villi and architectural disintegration. Seven rats were involved in each group. Arrows, denuded villi; arrow heads, haemorrhage. Bar = 5 μm. Histopathological scoring of colon tissue sections (B). The mucosal injury of the HE-stained colon tissues were evaluated by a pathologist. Note that there is a significant increase in the injury score after I/R. Data were mean ± S.E.M., and seven rats were involved in each group. Different letters indicate significant difference by Turkey’s multiple comparison test at 0.05 leve1.
Impairment of barrier function by I/R injury
We incubated the colon of I/R injury rats with a molecular tracer (biotin) to determine if the epithelial barrier in the colon was functionally altered in I/R injury. The biotin tracer lined the luminal boundary of the colonic epithelium and did not penetrate into the tissue in the control (Fig. 2A and B). This staining pattern was markedly different and biotin/streptavidin staining was noted deep to the epithelial surface at 1 hr of reperfusion (Fig. 2A and C). And fluorescent labelling was observed within and between many colonic epithelial cells at 3 hrs of reperfusion (Fig. 2A and D). It produced a vibrant signal that extended well into the lamina propria and resulted in staining throughout the tissue following 5 hrs I/R injury (Fig. 2A and E). This indicates that the epithelial barrier was significantly disrupted by I/R injury.
Figure 2.
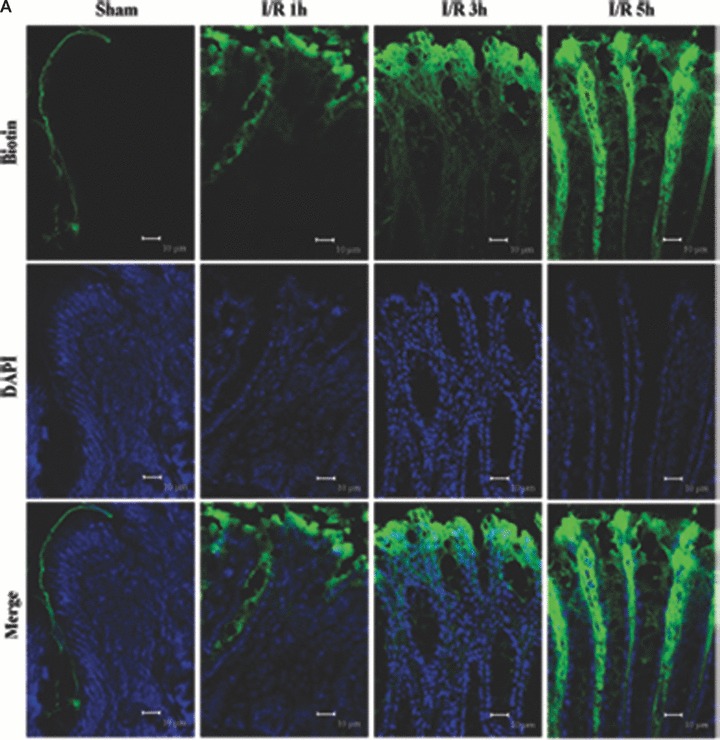
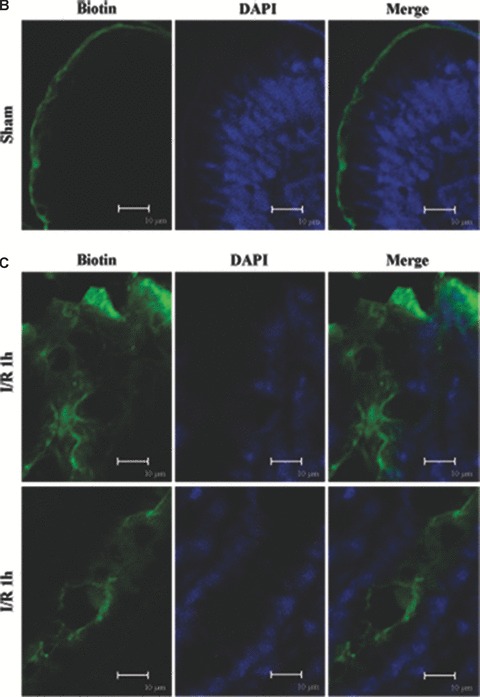
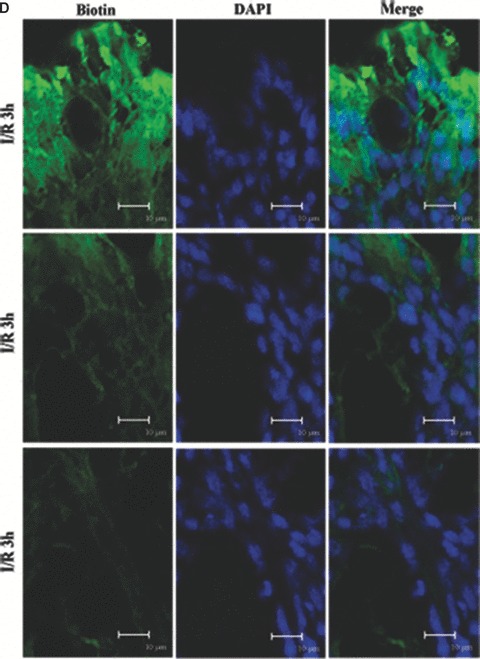
Barrier permeability micrographs of I/R injury. (A) Low magnification confocal projections of a biotin tracer to assess barrier permeability using control and I/R rat tissue. (B–E) Higher magnification of biotin- and DAPI-treated colon tissue of control and I/R rat. Biotin is held to the luminal border in control tissue (A, B). Biotin staining was deep to the epithelial surface at 1 hr of reperfusion (A, C). The fluorescent labelling was observed within and between many colonic epithelial cells after 3 hrs (A, D). Biotin signal extended into the lamina propria and resulted in staining throughout the tissue following 5 hrs I/R injury (A, E) reperfusion. Biotion was seen in green colour and nuclei were stained with DAPI (blue). The merging image of green biotin and blue nuclei was also presented. Images shown are representative of three experiments. Bars = 10 μm.
As increase of epithelial apoptosis and necrosis affect epithelial barrier function [8], we examined whether I/R could induce apoptosis and/or necrosis in colon tissues of the rats. The TUNEL assay was carried out by immunofluorescence detection. As shown in Fig. 3A, TUNEL+ cells was few in rat colon of the sham-operated group. The number of TUNEL+ cells in the I/R rat colon was significantly increased at 3 and 5 hrs of reperfusion, reaching a maximum at 5 hrs of reperfusion (Fig. 3A). And the apoptotic frequency in 3 and 5 hrs I/R injury rats were 5.3% and 13%, respectively (Fig. 3C). To determine whether the permeability of biotin was due to loss of barrier function of TJ and not the loss of barrier function (and viability) of the cells, we examined necrosis in colon tissues by the uptake of PI. In the colon of control rats, there is homogeneous staining of nuclei with DAPI and no PI staining (Fig. 3B). PI+ necrotic cells were shown after I/R injury, especially at 3 and 5 hrs of reperfusion. The percentage of the necrotic cells reached the maximum of 4% at 5 hrs of reperfusion (Fig. 3D). The results demonstrated that I/R injury induced apoptotic damage as well as necrosis in rat colon tissues. And the damage was severe at 5 hrs of reperfusion. It indicates that the increased frequency of apoptotic and necrotic cells as a mechanism of the I/R-induced intestinal barrier defect. And the I/R-induced apoptosis and necrosis in colon is paralleled by an increase in paracellular permeability.
Figure 3.
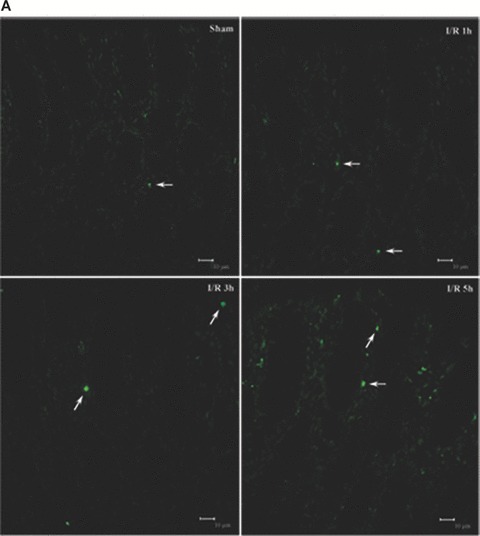
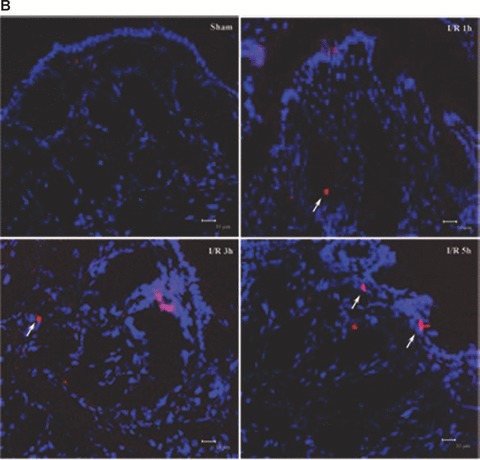
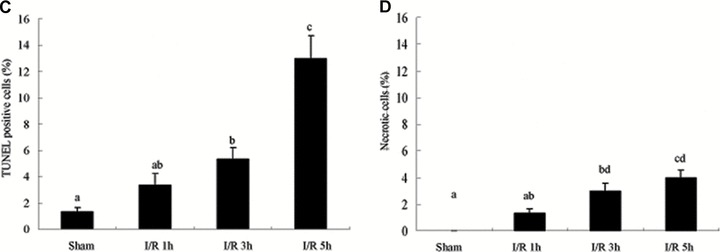
Analysis of apoptosis and necrosis in colons of I/R injury rats. (A) Representative TUNEL stains in colon tissues obtained from sham-operated and I/R rats. Apoptotic cells were shown as bright green fluorescence staining. (B) Detection of necrosis with PI staining. The DAPI (blue) and PI (red) fluorescence were shown. Quantitative analysis of TUNEL+ cells (C) and necrotic cells (D) in the colon at various time-points of reperfusion after 1 hrs ischaemia. White arrows depict TUNEL+ cells (A) and necrotic cells (B). Bars = 10 μm.
TJs play an important regulatory role in barrier function. TJs are major determinants in the maintenance of barrier permeability. We performed transmission electron microscopy analysis to further investigate the impact of I/R on the morphology of TJ. In control group, the TJ and desmosomes were intact, and the microvillus was regularly aligned in intestinal epithelium (Fig. 4). In I/R injury rats, alteration of TJ ultrastructure was observed. Less electron-dense materials were present between the adjoining cells near the brush border, which indicated the disruption of normal TJ morphology. And the amounts of microvillus decreased and the length and arrangement were irregular (Fig. 4).
Figure 4.
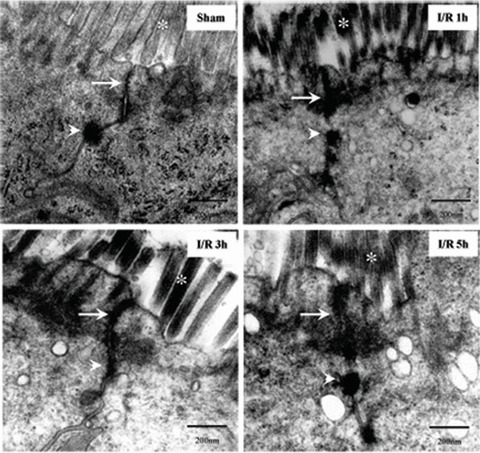
Transmission electron micrograph of mucosa subjected to I/R injury. In control, TJ and desmosome are intact. While, alteration of TJ ultrastructure was observed in I/R group animals at 1, 3 and 5 hrs after reperfusion. Less electron-dense materials were present between the adjoining cells near the brush border, which indicated the disruption of normal TJ morphology. And the amounts of microvillus decreased and the arrangement were irregular. Arrows, TJ; Arrow heads, desmosome; Asterisks, microvilli. Bars = 200 nm.
Confocal laser scanning microscopy of tight junction proteins
To assess TJ disruption in vivo caused by I/R injury, we compared claudin-1, -3 and -5 localizations in tissue sections from I/R injury rats as well as control. The characteristic staining pattern of claudin-1, 3 and 5 localization remained along the lateral cell boundaries in the control (Fig. 5A–C). Following I/R injury, localization of claudin-1, -3 and -5 in tissue sections altered compared with control (Fig. 5A–C). A significant alteration of the disruption of immunofluorescence signal for claudin-1, -3 and -5 was observed in tissue sections of I/R injury rats. An irregular distribution pattern of claudin-1, -3 and -5 was found following I/R injury (Fig. 5A–C). Claudin-1 showed a tight junctional staining in controls (Fig. 5A) and it stained weakly in the upper part of the crypt (Fig. 5A) in tissue sections of I/R injury. Claudin-3 was redistributed from the crypts and surface to the lateral plasma membrane (Fig. 5B) and claudin-5 showed a diffuse cytoplasmic staining (Fig. 5C) following I/R injury. Claudin-3 expression in the tips of the villi was decreased at 5 hrs after I/R. In control claudin-5 showed a regular expression on the tips of the villi, whereas in I/R groups the staining pattern of claudin-5 was partly disrupted and irregular at the apical surfaces. The expression of claudin-5 was decreased in the tips of the villi and strong expression was observed in the basal part of the villi. These results further confirm our findings that TJ was disrupted in the presence of I/R injury. Moreover, the observed alteration of claudins localization seemed to be correlated with intestine barrier permeability.
Figure 5.
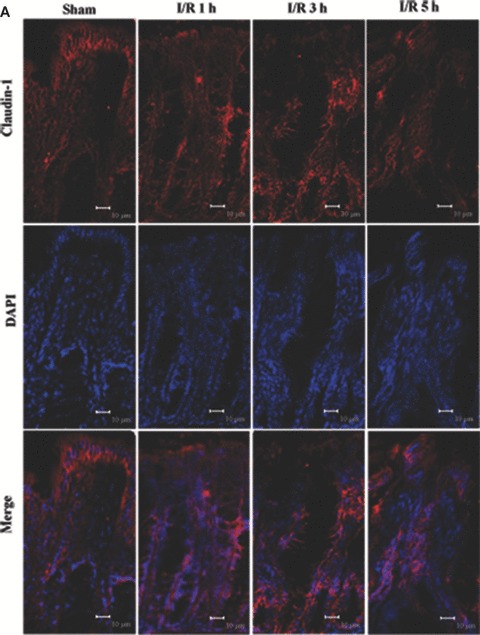
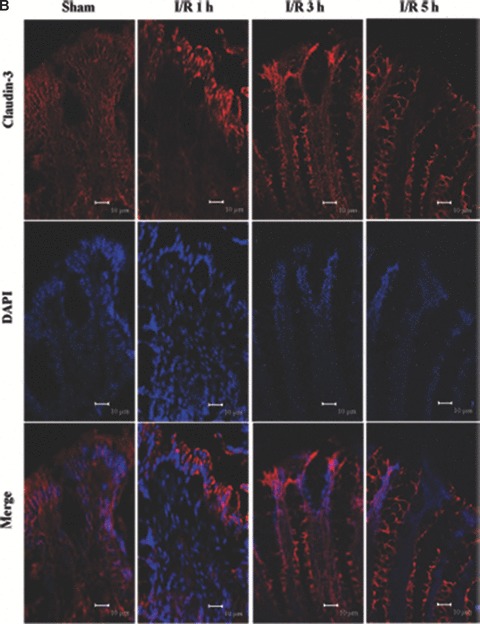
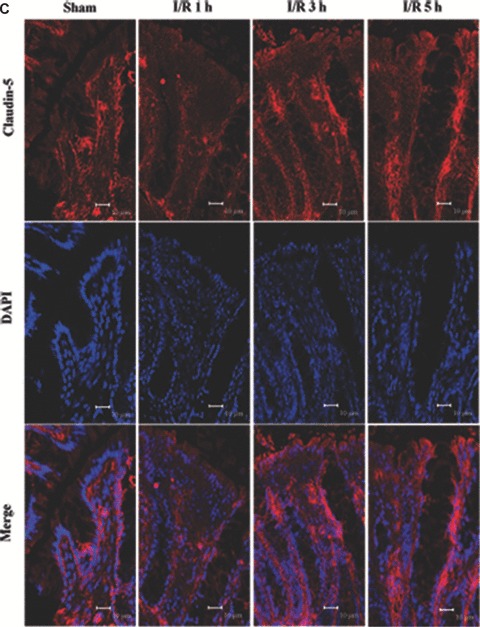
Altered localization of claudin-1, 3 and 5 following I/R injury. The respective claudins (red) and nuclei (blue) images were obtained by immunofluorescence analysis of tissue sections of control rat colon and I/R injury (n= 7). In controls, claudin-1, 3 and 5 exhibited the characteristic lateral membrane staining in the surface epithelial cells. At 1, 3 and 5 hrs after reperfusion, labelling of all three proteins was present diffusely within cells rather than along the lateral cell boundaries. (A) Claudin-1 distribution; (B) claudin-3 distribution; (C) claudin-5 distribution. Bars = 10 μm.
I/R injury changed the expression and distribution of TJ proteins in membrane microdomains of TJ
To assess the effect of I/R injury on the expression and distribution of TJ proteins in membrane microdomains of TJ, we utilized discontinuous sucrose density gradients ultracentrifugation to isolate the DRMs of TJs, and the expression of claudins, occludin and ZO-1 in detergent-insoluble and detergent-soluble fractions was determined. To confirm the localization of DRMs within the sucrose gradients, we used caveolin-1 as a marker. In control rats, caveolin-1 was found mainly (92.6%) in TX-100 insoluble fractions (fractions 3–5), However, after I/R treatment it was displaced from TX-100 insoluble fractions to TX-100 soluble fractions (fractions 6–9) (Fig. 6A and B). And only 2.6% of caveolin-1 was present in TX-100 insoluble fractions. The distribution of occludin (Fig. 6C and D), ZO-1 (Fig. 6E and F) and claudin isoforms (claudin-1, claudin-3 and claudin-5) (Fig. 6G–L) in TJ membrane fractions was analysed and densitometric results were calculated. The distribution of these proteins examined was marked influenced in I/R injury rats. Occludin, ZO-1, claudin-1 and claudin-3 were completely displaced from TX-100 insoluble fractions to TX-100 soluble fractions, while claudin-5 was partly displaced from DRMs.
Figure 6.
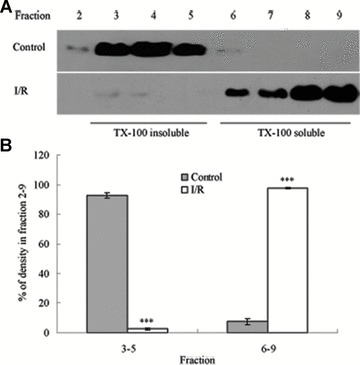
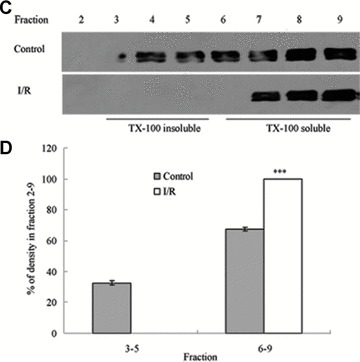
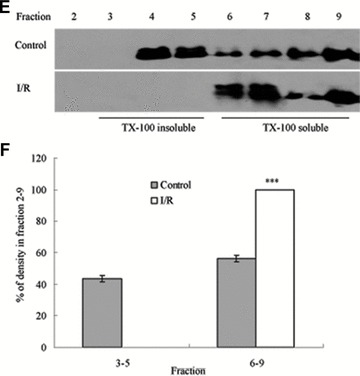
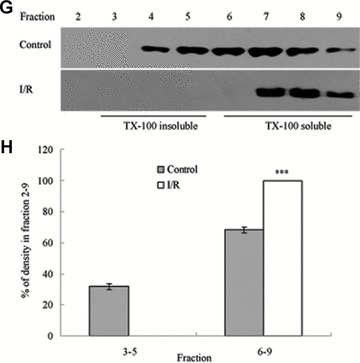
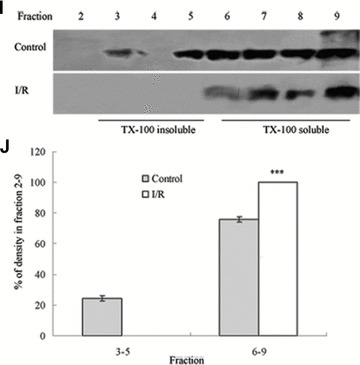
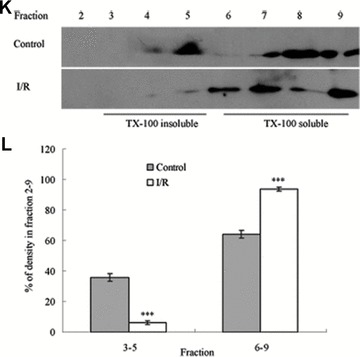
Western blotting analysis of the lipid raft marker protein caveolin-1 and TJ protein Occludin, ZO-1 and claudins 1, 3 and 5. Tissues were homogenized and subjected to discontinuous sucrose density gradient ultracentrifugation. The resulted fractions were analysed by immunoblotting. Blots were probed with antibodies and analysed quantitatively by densitometry with Quantity One 1-D analysis software. The proteins examined were found to translocate from TX-100 insoluble fractions to TX-100 soluble fractions. The blots shown are representative of three experiments. (A, B) Caveolin-1; (C, D) Occludin; (E, F) ZO-1; (G, H) claudin-1; (I, J) claudin-3; (K, L) claudin-5. Data presented were mean ± S.E.M. (***P < 0.001).
Discussion
Ischaemia causes mucosal injury with a subsequent increase of mucosal permeability and these are often fatal conditions in patients that can result in sepsis and multiple organ failure. Mesenteric I/R has been considered to be a pivotal mechanism in the pathogenesis of multiple organ failure. Increased intestinal permeability is one manifestation of mesenteric I/R injury that has been documented in high-risk patients after burns, sepsis, and shock [13, 14]. Although recent studies allow more insight into the molecular aspects of TJs, much less is known about TJ disruption in vivo and alteration in localization and expression of TJ proteins during I/R injury. Here, we demonstrated that I/R injury induced apoptosis which was accompanied by an increase in barrier permeability. The TJ proteins claudins 1, 3 and 5 were redistributed and occludin, ZO-1 and the claudins were displaced from membrane microdomains of TJ to detergent soluble fraction. Moreover, the morphology of TJ was changed. TJs were less apparent at the electron microscopic level compared with controls following I/R injury. Our findings demonstrated that I/R injury disrupted multiple TJ proteins in vivo resulting in a functionally deficient epithelial cell barrier. The results of the present study show that altered expression and distribution of TJ proteins as the structural correlate of barrier dysfunction in I/R injury.
Because disruption of the TJ may be a relatively major part of the pathology of ischaemia through altered TJ permeability, we examined intestinal barrier after ischaemia/refusion. Permeability studies were performed with a low-molecular weight tracer (Fig. 2). During I/R injury, a significant increase of the permeability was detected which indicated that the permeability increase occurs most likely via increased paracellular diffusion between epithelial cells. The ultrastructure observation further supported the increased permeability by representing leaky TJs after reperfusion treatment. This suggests a dysfunction of the interepithelial tight junctional complex.
I/R is a complicated pathophysiological process and it may result in necrotic tissue damage. As increase of epithelial apoptosis and necrosis affect epithelial barrier function, we examined whether I/R could induce apoptosis and/or necrosis in colon tissues of the rats. In this study, we found that I/R induced necrosis in the colon. PI+ necrotic cells were shown after I/R injury, especially at 3 and 5 hrs after reperfusion. The percentage of the necrotic cells reached the maximum of 4% at 5 hrs of reperfusion (Fig. 3D). The results showed that after 3 and 5 hrs I/R injury, the number of apoptotic cells was increased significantly. And the apoptotic cells at 5 hrs of reperfusion were more than that at 1 and 3 hrs of reperfusion. Our results were different from the previous report [6] that apoptotic cells was nearly 18% after 1 hr ischaemia followed by 3 hrs reperfusion and maximumed at 3 hrs of reperfusion in rat jejunum. But the apoptotic index was 30% in the rat ileum at 3 hrs of reperfusion [7].The different results from these reports even in the same condition varied depending on different segment of the intestine. In our study, TUNEL stains were observed in rat colon and maybe the colon was less sensitive to I/R injury than the small intestine. Our results demonstrated that I/R injury induced apoptotic damage as well as necrosis in rat colon tissues. The increased frequency of apoptotic and necrotic cells may be a mechanism of the I/R-induced intestinal barrier defect.
Damage of cell membrane is a characteristic for necrosis, which results in leaky membranes. Thus, membrane leakage of the cells might be a factor of I/R-induced barrier impairment. Increased barrier permeability could be caused by an increase of apoptotic and necrotic cells [8], and by an increased permeability of the epithelial TJs. Therefore, it seems reasonable to conclude that TJ structural alterations and the increased frequency of apoptotic and necrotic cells were the predominant factors in barrier impairment in I/R injury in our study. The alterations of TJ protein expression and localization are the determining structural basis for I/R induced impairment of epithelial barrier function.
The appropriate localization of claudins at the cell boundaries corresponds to intact TJs whereas the redistribution of localization of claudins corresponded to functional TJ deficiencies in the colon by pathogen invasion [32]. Previous studies have demonstrated that claudins 1, 3 and 5 are present in the colon [25, 26]. Based on these findings, we used immunofluorescence microscopy to investigate the distribution and subcellular localization of claudin-1, 3 and 5 on colon sections. The results demonstrated that in control colon sections the expression and subcellular distribution of all claudins showed similar staining patterns. In control rats, the claudins are strongly expressed in the surface epithelial cells of the colon showing the characteristic lateral membrane staining. The staining of claudins focused at the cell apices on the tip of the villi and the crypts. In I/R injury rats, however, labelling of all three proteins was present diffusely within cells rather than along the lateral cell boundaries (Fig. 5). Moreover, expression of claudins followed a differential localization from the tip of the villi to the crypts. I/R caused the expression of claudin-1 and -5 decreased dramatically both in the villi and the crypts (Fig. 5). While claudin-3 expression in the tips of the villi was decreased at 5 hrs after I/R. The results were consistent with previous results showing that claudins were redistributed in response to different pathological conditions such as CD and bacteria invasion [5, 19].
To further investigate the effect of I/R on the distribution of TJ proteins in membrane microdomains of TJ, we isolated the DRMs of TJs and analysed the expression of TJ proteins by Western blot. We found that distribution of TJ proteins in membrane microdomains of TJ was markedly affected in I/R injury rats. Occludin, ZO-1, claudin-1 and claudin-3 were completely displaced from TX-100 insoluble fractions to TX-100 soluble fractions, and claudin-5 was partly displaced from DRMs of TJs. This result was similar with our previous study in experimental colitis induced by TNBS (2,4,6-trinitrobenzenesulfonic acid) in rats, which demonstrated that the expression and distribution of TJ proteins were altered in colitic rats [16].
TJs are one of the major determinants of epithelial barrier function. The disruption of TJs can lead to increased paracellular permeability after intestinal ischaemia [33, 34]. However, the mechanisms by which I/R disrupt intestinal barrier function are not fully characterized. In the present study, redistribution of TJs proteins in membrane microdomains of TJ and increased epithelial permeability was found during I/R. TJs have become increasingly recognized for their role in gut injury. Impaired epithelial barrier function has been reported in many diseases states, including CD [14, 16] and ulcerative colitis [35, 36]. These studies revealed breaks of TJ strands, and the expression and distribution of TJ proteins were changed. In our study, it was shown that the redistribution of TJs proteins might lead to disruption of intestinal barrier function and increased epithelial permeability during I/R. Ischaemia and reperfusion injury is associated with the activation of cytokines [37]. Among these inflammatory cytokines produced in I/R, tumour necrosis factor (TNF)-α plays an important role in reperfusion-induced tissue injury and lethality [38]. In the previous studies we found the altered distribution of TJ proteins in experimental colitis [16]. Pro-inflammatory cytokines induced alteration of the expression of TJ proteins in TJ membrane microdomains by changing the lipid environment of TJ membrane microdomains [22]. There is also evidence to suggest that the changes in paracellular permeability caused by interferon-γ and TNF-α are associated with marked increases in myosin light chain phosphorylation and the cytokines leading to intestinal barrier dysfunction by the myosin light chain kinase pathway [39]. I/R injury led to appearance of aberrant TJs. The disrupted morphology of TJs is often the results of changes in TJ protein expression. These observations indicated that I/R-induced changes in TJ structure and function may be associated with the redistribution of TJ proteins in TJ membrane microdomains. It demonstrated the importance of the altered distribution of TJ proteins in membrane micodomains of TJ in the maintenance of epithelial barrier properties.
Conclusion
Here, we presented evidence that I/R injury induced TJ disruption in vivo. We have shown a significant increase in the frequency of apoptotic and necrotic cells which was accompanied by an increase in barrier permeability. TJ protein expression contributes to this barrier impairment. We demonstrated that redistribution of TJ proteins in TJ membrane microdomains and redistribution of claudins 1, 3 and 5 in epithelial cells of the colon lead to alteration of TJ architecture and TJ barrier dysfunction during I/R injury. Further studies are needed to clarify the molecular basis that leads to changes of TJ proteins and compromised junctional function during I/R injury.
Acknowledgments
We thank the Deutscher Akademischer Austauschdienst Researcher Fellowship (Bioscience Special Program, Germany) for Dr. Q.L. This work was supported by the National Basic Research Program (973 Program) in China (No. 2007CB513005 and 2009CB522405), the Key project of National Natural Science Foundation in China (30830098), the National Natural Science Foundation in China (30672061), Key project of Nanjing Military Command (06Z40)and the Military Scientific Research Fund (0603AM117).
References
- 1.Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–28. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- 2.Hawker PC, McKay JS, Turnberg LA. Electrolyte transport across colonic mucosa from patients with inflammatory bowel disease. Gastroenterology. 1980;79:508–11. [PubMed] [Google Scholar]
- 3.Sandle GI, Higgs N, Crowe P. Cellular basis for defective electrolyte transport in inflamed human colon. Gastroenterology. 1990;99:97–105. doi: 10.1016/0016-5085(90)91235-x. [DOI] [PubMed] [Google Scholar]
- 4.Gassler N, Rohr C, Schneider A, et al. Inflammatory bowel disease is associated with changes of enterocytic junctions. Am J Physiol Gastrointest Liver Physiol. 2001;281:G216–28. doi: 10.1152/ajpgi.2001.281.1.G216. [DOI] [PubMed] [Google Scholar]
- 5.Guttman JA, Fereshte N, Samji FN, et al. Evidence that tight junctions are disrupted due to intimate bacterial contact and not inflammation during attaching and effacing pathogen infection in vivo. Infect Immun. 2006;74:6075–84. doi: 10.1128/IAI.00721-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shucai An, Yoshitaka Hishikawa, Takehiko Koji. Induction of cell death in rat small intestine by ischemia reperfusion: differential roles of Fas/Fas ligand and Bcl-2/Bax systems depending upon cell types. Histochem Cell Biol. 2005;123:249–61. doi: 10.1007/s00418-005-0765-6. [DOI] [PubMed] [Google Scholar]
- 7.Liu KX, Chen SQ, Huang WQ, et al. Propofol pretreatment reduces ceramide production and attenuates intestinal mucosal apoptosis induced by intestinal ischemia/reperfusion in rats. Anesth Analg. 2008;107:1884–91. doi: 10.1213/ane.0b013e3181884bbf. [DOI] [PubMed] [Google Scholar]
- 8.Epple HJ, Schneider T, Troeger H, et al. Impairment of the intestinal barrier is evident in untreated but absent in suppressively treated HIV-infected patients. Gut. 2009;58:220–7. doi: 10.1136/gut.2008.150425. [DOI] [PubMed] [Google Scholar]
- 9.Furuse M, Fujimoto K, Sato N, et al. Overexpression of occludin, a tight junction-associated integral membrane protein, induces the formation of intracellular multilamellar bodies bearing tight junction-like structures. J Cell Sci. 1996;109:429–35. doi: 10.1242/jcs.109.2.429. [DOI] [PubMed] [Google Scholar]
- 10.Furuse M, Hirase T, Itoh M, et al. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Sci. 1993;123:1777–88. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Noda A, Kubo A, Tsukita S. Claudin-based tight junctions are crucial for the mammalian epidermal barrier: a lesson from claudin-1-deficient mice. J Cell Sci. 2002;156:1099–111. doi: 10.1083/jcb.200110122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stevenson BR, Anderson JM, Goodenough DA, et al. Tight junction structure and ZO-1 content are identical in two strains of Madin-Darby canine kidney cells which differ in transepithelial electrical resistance. J Cell Biol. 1988;107:2401–8. doi: 10.1083/jcb.107.6.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassoun HH, Mercer DW, Moody FG, et al. Postinjury multiple organ failure: the role of the gut. Shock. 2001;15:1–10. doi: 10.1097/00024382-200115010-00001. [DOI] [PubMed] [Google Scholar]
- 14.Kompan L, Kompan D. Importance of increased intestinal permeability after multiple injuries. Eur J Surg. 2001;167:570–4. doi: 10.1080/110241501753171155. [DOI] [PubMed] [Google Scholar]
- 15.Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–9. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 16.Li QR, Zhang Q, Zhang M, et al. Effect of n-3 polyunsaturated fatty acids on membrane microdomain localization of tight junction proteins in experimental colitis. FEBS J. 2008;275:411–20. doi: 10.1111/j.1742-4658.2007.06210.x. [DOI] [PubMed] [Google Scholar]
- 17.Marin ML, Geller SA, Greenstein AJ, et al. Ultrastructural pathology of Crohn’s disease: correlated transmission electron microscopy, scanning electron microscopy, and freeze fracture studies. Am J Gastroenterol. 1983;78:355–64. [PubMed] [Google Scholar]
- 18.MacCallum A, Hardy SP, Everest PH. Campylobacter jejuni inhibits the absorptive transport functions of Caco-2 cells and disrupts cellular tight junctions. Microbiology. 2005;151:2451–8. doi: 10.1099/mic.0.27950-0. [DOI] [PubMed] [Google Scholar]
- 19.Zeissig S, Bürgel N, Günzel D, et al. Changes in expression and distribution of claudin-2, -5 and -8 lead to discontinuous tight junctions and barrier dysfunction in active Crohn’s disease. Gut. 2007;56:61–72. doi: 10.1136/gut.2006.094375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grotz MR, Deitch EA, Ding J, et al. Intestinal cytokine response after gut ischemia: role of gut barrier failure. Ann Surg. 1999;229:478–86. doi: 10.1097/00000658-199904000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deitch EA, Xu D, Franko L, et al. Evidence favoring the role of the gut as a cytokine generating organ in rats subjected to hemorrhagic shock. Shock. 1994;1:141–5. doi: 10.1097/00024382-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Li QR, Zhang Q, Wang M, et al. Interferon-γ and tumor necrosis factor-α disrupt epithelial barrier function by altering lipid composition in membrane microdomains of tight junction. Clin Immunol. 2008;126:67–80. doi: 10.1016/j.clim.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Oyamada M, Mitsufuji S, et al. Different changes in the expression of multiple kinds of tight-junction proteins during ischemia-reperfusion injury of the rat ileum. Acta Histochem Cytochem. 2006;39:35–45. doi: 10.1267/ahc.05048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Little D, Dean RA, Young KM, et al. PI3K signaling is required for prostaglandin-induced mucosal recovery in ischemia-injured porcine ileum. Am J Physiol Gastrointest Liver Physiol. 2003;284:G46–56. doi: 10.1152/ajpgi.00121.2002. [DOI] [PubMed] [Google Scholar]
- 25.Rahner C, Mitic LL, Anderson JM. Heterogeneity in expression and subcellular localization of claudins 2, 3, 4, and 5 in the rat liver, pancreas, and gut. Gastroenterology. 2001;120:411–22. doi: 10.1053/gast.2001.21736. [DOI] [PubMed] [Google Scholar]
- 26.Zeissig S, Bojarski C, Buergel N, et al. Downregulation of epithelial apoptosis and barrier repair in active Crohn’s disease by tumour necrosis factor alpha antibody treatment. Gut. 2004;53:1295–302. doi: 10.1136/gut.2003.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Megison SM, Horton JW, Chao H, et al. A new model for intestinal ischemia in the rat. J Surg Res. 1990;49:168–73. doi: 10.1016/0022-4804(90)90257-3. [DOI] [PubMed] [Google Scholar]
- 28.Williams JP, Weiser MR, Pechet TTV, et al. α1-Acid glycoprotein reduces local and remote injuries after intestinal ischemia in the rat. Am J Physiol Gastrointest Liver Physiol. 1997;273:G1031–5. doi: 10.1152/ajpgi.1997.273.5.G1031. [DOI] [PubMed] [Google Scholar]
- 29.Paola RD, Mazzon E, Patel NSA, et al. Beneficial effects of GW274150 treatment on the development of experimental colitis induced by dinitrobenzene sulfonic acid. Eur J Pharmacol. 2005;507:281–9. doi: 10.1016/j.ejphar.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 30.Mazzon E, Cuzzocrea S. Thalidomide treatment reduces the alteration of paracellular barrier function in mice ileum during experimental colitis. Shock. 2006;25:515–21. doi: 10.1097/01.shk.0000209556.31457.e7. [DOI] [PubMed] [Google Scholar]
- 31.Kelly KJ, Sandoval RM, Dunn KW, et al. A novel method to determine specificity and sensitivity of the TUNEL reaction in the quantitation of apoptosis. Am J Physiol Cell Physiol. 2003;284:C1309–18. doi: 10.1152/ajpcell.00353.2002. [DOI] [PubMed] [Google Scholar]
- 32.Guttman JA, Li Y, Wickham ME, et al. Attaching and effacing pathogen-induced tight junction disruption in vivo. Cell Microbiol. 2006;8:634–45. doi: 10.1111/j.1462-5822.2005.00656.x. [DOI] [PubMed] [Google Scholar]
- 33.Schoenberg MH, Beger HG. Reperfusion injury after intestinal ischemia. Crit Care Med. 1993;21:1376–86. doi: 10.1097/00003246-199309000-00023. [DOI] [PubMed] [Google Scholar]
- 34.Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–37. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz H, Barmeyer C, Fromm M, et al. Altered tight junction structure contributes to the impaired epithelial barrier function in ulcerative colitis. Gastroenterology. 1999;116:301–9. doi: 10.1016/s0016-5085(99)70126-5. [DOI] [PubMed] [Google Scholar]
- 36.Gitter AH, Wullstein F, Fromm M, et al. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320–8. doi: 10.1053/gast.2001.29694. [DOI] [PubMed] [Google Scholar]
- 37.Souza DG, Teixeira MM. The balance between the production of tumor necrosis factor-alpha and interleukin-10 determines tissue injury and lethality during intestinal ischemia and reperfusion. Mem Inst Oswaldo Cruz. 2005;100:59–66. doi: 10.1590/s0074-02762005000900011. [DOI] [PubMed] [Google Scholar]
- 38.Souza DG, Soares AC, Pinho V, et al. Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury. Am J Pathol. 2002;160:1755–65. doi: 10.1016/s0002-9440(10)61122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clayburgh DR, Shen L, Turner JR. A porous defense: the leaky epithelial barrier in intestinal disease. Lab Invest. 2004;84:282–91. doi: 10.1038/labinvest.3700050. [DOI] [PubMed] [Google Scholar]


